Abstract
The photolithographic fabrication of high-density DNA and RNA arrays on flexible and transparent plastic substrates is reported. The substrates are thin sheets of poly(ethylene terephthalate) (PET) coated with crosslinked polymer multilayers that present hydroxyl groups suitable for conventional phosphoramidite-based nucleic acid synthesis. We demonstrate that by modifying MAS procedures to accommodate the physical and chemical properties of these materials, it is possible to synthesize plastic-backed oligonucleotide arrays with feature sizes as small as 14 × 14 μm and feature densities in excess of 125 000/cm2, similar to specifications attainable using rigid substrates such as glass or glassy carbon. These plastic-backed arrays are tolerant to a wide range of hybridization temperatures, and improved synthetic procedures are described that enable the fabrication of arrays with sequences up to 50 nucleotides in length. These arrays hybridize with S/N ratios comparable to those fabricated on otherwise identical arrays prepared on glass or glassy carbon. This platform supports the enzymatic synthesis of RNA arrays and proof-of-concept experiments are presented showing that the arrays can be readily sub-divided into smaller arrays (or ‘millichips’) using common laboratory-scale laser cutting tools. These results expand the utility of oligonucleotide arrays fabricated on plastic substrates and open the door to new potential applications for these important bioanalytical tools.
Graphical abstract

DNA that has been spatially patterned upon a surface is a cornerstone of modern bioanalysis. The development of high-density DNA arrays has been driven largely by their ability to capture complementary nucleic acids from solution with high specificity.1 This capability has enabled numerous applications including gene expression analysis, genotyping, pathogen detection, enrichment for exome sequencing, and comparative genomic hybridization.2-7 Recent studies have expanded the range of analytes that can be studied with DNA arrays, through assays such as those examining the sequence preferences of transcription factors or the binding of aptamers and their targets.8,9 Arrays synthesized using in situ methods, such as those produced commercially by Nimblegen, Agilent, or Affymetrix, are also now routinely used as sources for complex oligonucleotide pools for gene synthesis.10-12 Such arrays are fabricated using phosphoramidite chemistry to build oligonucleotides base-by-base, enabling the synthesis of specific sequences up to ∼150 nucleotides in length.13
In contrast to ‘spotted’ or ‘top-down’ techniques of array production—where pre-synthesized biomolecules are coupled to a surface—in situ or ‘bottom-up’ methods are advantageous in terms of their affordability and versatility. Nonetheless, all in situ approaches for DNA synthesis are dependent upon precision optics or highly targeted reagent delivery and have conventionally employed rigid and planar substrates.14,15 This technical requirement has prevented high-resolution DNA patterning on surfaces with the diverse formats or non-planar geometries often found in miniaturized hybridization devices.16,17 A conceivable solution to this problem would be to synthesize DNA arrays on flexible substrates that could later be physically manipulated or cut into desired shapes or sizes. Oligonucleotides patterned upon these substrates could, in turn, template the programmable attachment of other species, such as small molecules, proteins, or even cells, and thereby serve as a universal scaffold for high-resolution biomolecule patterning on arbitrary surfaces.18
A proof-of-concept approach to high-density DNA array fabrication has previously been demonstrated on polymer film-coated plastic substrates.19 That study demonstrated that plastic substrates coated with multilayered polymer films were physically and chemically robust enough to withstand the in situ synthesis of short oligonucleotides (∼20 bases long), and that these arrays could be hybridized and de-hybridized with fluorescently-labelled complementary sequences. This report extends this work to i) increase the density of features that is possible using this approach, ii) demonstrate that the resulting plastic-backed arrays are stable to a variety of hybridization temperatures, iii) develop improved synthesis conditions for the production of arrays containing longer oligonucleotides, and iv) demonstrate the laser cutting of the PET substrate into smaller sub-sections. These advances are used to synthesize DNA arrays containing two distinct sequences within a single feature and to fabricate the first high-density RNA array on a flexible plastic substrate. We then explore the use of PET-based arrays as a second-generation substrate for millichip fabrication.20
Experimental Section
Glassy Carbon Functionalization
I) Materials
Sigradur G-type glassy carbon surfaces were purchased from HTW (Thierhaupten, Germany). 9-Decen-1-ol was purchased from Sigma-Aldrich (MO, USA).
II) Methods
Glassy carbon surfaces were hydrogen-terminated in a 13.56 MHz inductively coupled hydrogen plasma. Treatment was conducted at 900°C for 20 minutes (H2 pressure at 50 Torr) and slowly cooled to room temperature while the plasma remained running. 50 μL of 9-decen-1-ol was immediately placed on the surface, which was then covered with a quartz cover slip. The slides were irradiated with a mercury vapor quartz grid lamp (λ = 254 nm, 0.35 mW/cm2) for 16 hours under a nitrogen purge. The surfaces were then rinsed with water and ethanol before being dried under a nitrogen stream.
Fabrication and Functionalization of PEI/PVDMA Multilayers
I) Materials
Branched poly(ethylenimine) (PEI; MW ∼25,000), acetone (ACS grade), ethyl acetate (>99.5%), dimethylsulfoxide (DMSO, 98%), and azobisisobutyronitrile (AIBN, 98%, recrystallized from methanol) were purchased from Sigma Aldrich (Milwaukee, WI). D-Glucamine (>95.0%) was purchased from TCI Chemicals (Philadelphia, PA). Methanol (MeOH; ACS grade) was purchased from Avantor Performance Materials (Center Valley, PA). Ethanol (EtOH, 200 proof) was obtained from Decon Labs (King of Prussia, PA). 2-Vinyl-4,4-dimethylazlactone (VDMA), a kind gift from Dr. Steven M. Heilmann (3M Corporation, Minneapolis, MN), was fractionally distilled under vacuum (B.P. ∼22 °C at ∼500 mTorr; clear mobile liquid at room temperature) and then stored with 500 ppm butylated hydroxytoluene (BHT) and 1000 ppm triethylamine at 0 °C prior to use. Poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) was synthesized by free radical polymerization at 60 °C for 24 hours in ethyl acetate (solvent:monomer ratio 3 mL:1 g) initiated by 1.0 mol% AIBN, as described previously.21 A roll of PET film (thickness = 0.004 inches) was purchased from McMaster Carr (Elmhurst, IL). Water with a resistivity of 18.2 MΩ was obtained from a Millipore filtration system. Compressed air used in drying steps was filtered through a 0.2 μm membrane syringe filter. Chemicals were used without further purification unless otherwise noted above.
II) Methods
Poly(ethylene terephthalate) (PET) substrates were manually cut to the dimensions of a standard glass microscope slide (25 mm × 35 mm). Prior to the fabrication of PEI/PVDMA multilayers, these substrates were rinsed with EtOH and then placed into a solution of PEI (1 mg/mL in MeOH) overnight at 37 °C, and then rinsed with MeOH and dried in a stream of filtered compressed air. PEI/PVDMA multilayers were fabricated on PET substrates using the following general procedure: (i) substrates were submerged in a solution of PVDMA (20 mM in acetone with respect to the polymer repeat unit) for 30 s; (ii) substrates were removed and immersed in an acetone bath for 10 s with gentle agitation and then rinsed with acetone from a squirt bottle for 10 s; (iii) substrates were submerged in a solution of PEI (20 mM in acetone with respect to the polymer repeat unit) for 30 s; and (iv) substrates were removed and rinsed again using the procedure in step (ii). This cycle was repeated four times to fabricate multilayers consisting of 4 PEI/PVDMA layer pairs (referred to hereafter as ‘bilayers’). A final layer of PVDMA was then deposited (using steps (i) and (ii)) on the top surface of the film. Films having this general structure are referred to hereafter as being 4.5 bilayers thick. Films were washed with ∼25 mL of acetone and then dried under a stream of compressed air. These films were functionalized with D-glucamine by incubating film-coated substrates in 20 mg/mL solutions in DMSO for 1 hour. These functionalized films were then rinsed copiously with Millipore water and dried under a stream of filtered compressed air.
Maskless Array Synthesis
I) Materials
DCI activator (4,5-dicyanoimidizaole in acetonitrile), 0.1 M Activator 42 (5-[3,5-bis(trifluoromethyl)phenyl]-1H-tetrazole) and all NPPOC (5′-nitrophenylpropyloxycarbonyl) protected phosphoramidites [5′- NPPOC-dAdenosine (tac) 3′-β-cyanoethylphosphoramidite (NPPOC-dA), 5′-NPPOC-dThymidine 3′-β-cyanoethylphosphoramidite (NPPOC-dT),5′-NPPOC-dCytidine (ib) 3′-β-cyanoethylphosphoramidite (NPPOC-dC), 5′- NPPOC-dGuanosine (ipac) 3′-β-cyanoethylphosphoramidite (NPPOC-dG)], tetrahydrofuran (THF), 2,6-lutidine, dimethylsulfoxide (DMSO), imidazole, acetonitrile, 1-methylimidazole, ethylenediamine, and ethanol were purchased from Sigma-Aldrich. Anhydrous acetonitrile, diluent (acetonitrile), capping reagent A (THF/Pac2O), 0.5 M 10-camphorsulfonyl-oxaziridine (CSO), 5′-dimethoxytrityl-2′-deoxythymidine, 3′-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite (DMT-dT), and deblocking mix (3% dichloroacetic acid in toluene) were purchased from Glen Research (VA, USA). Oxidizer (0.02 M iodine in THF/pyridine/H2O), 5′-dimethoxytrityl-polyethyleneglycol 2000 phosphoramidite, and all 3′-dimethoxytrityl-5′-cyanoethylphosphoramite 2′-O-methyl or 2′-fluoro ribonucleosides were purchased from ChemGenes (MA, USA). 2-Dimethylaminopyridine was purchased from Tokyo Chemical Industry Co. LTD (Tokyo, Japan). Trap Packs were purchased from BioAutomation (TX, USA).
II) Methods
The array synthesis was conducted on a custom-built maskless array synthesizer (MAS) in a modification to previous techniques for gene synthesis and RNA array fabrication.22,23 The primary exception is the removal of the capping step from every cycle of the synthesis. Briefly, this yielded synthesis cycles consisting of coupling, oxidation, and deprotection as depicted in Scheme 1. NPPOC-protected pyrimidine phosphoramidites underwent 80-second coupling steps while NPPOC-protected purine phosphoramidites underwent 90-second coupling steps. All 3′-dimethoxytrityl-5′-cyanoethylphosphoramite 2′-O-methyl and 2′-fluoro ribonucleosides underwent 10-minute coupling steps and 5′-dimethoxytrityl-polyethyleneglycol 2000 phosphoramidite underwent two 15-minute coupling steps. 5′-DMT-dT was coupled for 120 seconds. Activator 42 or DCI activator were used as summarized in Supplementary Table 1. Where I2 was used as the oxidizer, oxidation steps (30 × 16 μL pulses on the MAS Expedite solvent delivery system) were conducted for every 3 or 5 couplings of 5′-NPPOC-protected phosphoramidites. When CSO was used as the oxidizer, 4-minute oxidation steps were conducted for every 3 or 5 couplings of 5′-NPPOC protected phosphoramidites and after the coupling of every DMT-protected phosphoramidite. A UV light dose of 3.5 J/cm2 was used for full removal of the NPPOC on the PET surfaces while a 3.7 J/cm2 dose was used on glassy carbon surfaces. This UV light dosage was determined in a separate experiment to yield features of the highest signal upon hybridization with a fluorescent complement. A 1.2 J/cm2 dose was used for partial NPPOC removal on the PET surfaces for the RNA array fabrication. The exposure solvent was 1% (w/v) imidizole in DMSO and was filtered prior to use. Removal of the DMT groups was conducted with 0.36 M DCA in toluene in three 50-second steps. Amidites which were coupled following a step of partial deprotection (5′-DMT-3′-CE-dT for the dual-sequence-feature array and 2′-F-3′-DMT-rC for the RNA array) underwent two coupling steps. In cases where capping was conducted independently of a coupling cycle (between sequence I and II on the dual-sequence-feature array, between DNA and RNA synthesis on the RNA array, and on the ‘inverse’ image of all the features of the array at the beginning of a synthesis), a capping cycle consisted of three 90-second steps of an equivolume mixture of capping reagent A (THF/Pac2O) and capping reagent B (0.5% 2-dimethylaminopyridine, 2% (v/v) N-methylimidazole and 10% (v/v) 2,6-lutidine in THF), which was mixed in-house. Capping was carried out in the same way for 60 seconds/NPPOC cycle on the glassy carbon array shown in Figure 4. The synthesis conditions are summarized in Supplementary Table 1. After synthesis, the arrays were deprotected in a 50:50 mixture of ethylenediamine:ethanol for 90 minutes at room temperature before being rinsed in ethanol, water, then rapidly dried under a nitrogen stream. The nucleic acid sequences for all arrays fabricated are given in Supplementary Table 2.
Scheme 1.
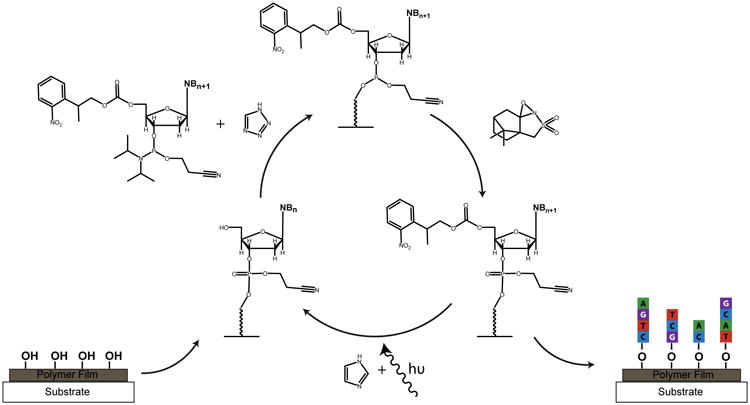
An overview of nucleic acid array fabrication chemistry used in the maskless array synthesis. Phosphoramidites containing a 5′-nitrophenylpropyloxycarbonyl (5′-NPPOC) are coupled to free hydroxyls on a polymer film-coated plastic substrate using a tetrazole-based activator. Oxidation is carried out once every 3 to 5 cycles utilizing camphorsulfyloxoaziridine (CSO) in acetonitrile. A system of digitally controlled micromirrors directs UV light to remove the 5′-NPPOC group in the presence of imidazole to expose a free hydroxyl for the next round of coupling.
Figure 4.

Fabrication of “2-in-1” DNA arrays (containing two sequences per feature) on polymer film-coated PET. A) Scheme depicting the synthesis process to obtain dual-sequence features. Numbers denote the order in which the indicated species was attached to the surface during synthesis. “PEG-2000” indicates polyethylene glycol with a molecular weight of 2 kDa, “DMT-dT” stands for a 5′-dimethoxytrityl protected deoxythymidine phosphoramidite, and “Pac2O” stands for phenoxyacetic anhydride. The “*” indicates the location of the phenoxyacetyl group that terminates the synthesis of sequence I. B) Fluorescence micrograph of hybridization with Cy5 (purple) and Texas Red (yellow) labelled complements to the features; each column of features received a different UV-dose prior to DMT-dT coupling. C) Fluorescence signal intensity of each sequence and the corresponding light doses used at each column depicted in (B). Diamonds indicate signal intensities of sequence I; squares indicate intensities of sequence II.
RNA Array Fabrication
I) Materials
Turbo DNase, 10× Turbo DNase buffer, RNAsecure, 10× phosphate-buffered saline (PBS, pH 7.4), 0.2 μm filtered nuclease free water (not DEPC treated), and 20× SSPE (3.0 M NaCl, 0.2 M NaH2PO4, and 0.02 M EDTA at pH 7.4) were purchased from Ambion (TX, USA). Polyethylene glycol 6000, 10% (w/v) sodium dodecyl sulfate (SDS), and diethylpyrocarbonate (DEPC) were purchased from Sigma Aldrich. RiboLock RNase inhibitor, T7 RNA polymerase, and 5× transcription buffer, Gene Frame gaskets, and all adenosine, cytosine, guanosine, and uradine nucleotide triphosphates were purchased from Thermo Scientific (USA).
II) Methods
After array synthesis and chemical deprotection, a Gene Frame gasket was then applied to surround the array features. 50 μL of annealing buffer consisting of 4× SSPE buffer, 1× RNAsecure reagent, and 9% polyethylene glycol 6000 was applied onto the array and incubated at 60 °C for 20 min in a humid chamber, then slowly cooled down to 37 °C overnight. The surface was then rinsed manually at least three times with 1× transcription buffer (40 mM Tris-HCl, pH 7.9, 6 mM MgCl2, 10 mM DTT, 20 mM NaCl, 2 mM spermidine) before exchange with the RNA extension reaction mixture consisting of 40 mM Tris-HCl (pH = 7.9), 6 mM MgCl2, 10 mM DTT, 20 mM NaCl, 2 mM spermidine, 0.5 mM each NTP, 2 U/μl T7 RNA polymerase, and 1 U/μl RiboLock RNase inhibitor. The extension reaction was incubated at 37 °C for ∼5 hours in a double humid chamber (a humid chamber within a humid chamber). After extension, the array was rinsed extensively with 1× PBS buffer (pH = 7.4) followed by at least three rinsing steps of 1× TurboDNase buffer before exchanging the solution on the surface with Turbo DNase at a concentration of 0.1 U/μL in 1× DNase buffer. The mixture was incubated at 37 °C for ∼6.5 hrs in a double humid chamber and rinsed in 2% SDS in 1× PBS (pH = 7.4) for 10 minutes and followed by extensive rinsing with water and drying under a nitrogen stream. The array was prevented from drying throughout the steps of enzymatic treatment. Water used for rinses and hybridization buffers was DEPC-treated and subsequently autoclaved.
Array Hybridizations
Fluorescently-labelled oligonucleotides were purchased from Integrated DNA Technologies (IA, USA) and Sigma-Aldrich. Sequences are shown in Supplementary Table 3. Hybridization reactions were conducted by incubating the array for at least 30 minutes with a solution consisting of 1 μM complementary oligonucleotide in 4× SSPE buffer and 0.1% Tween-20 in a humid chamber at 37 °C. The arrays were then transferred to a solution of 0.5× SSPE at room temperature for 15 minutes before further rinsing in 0.5× SSPE. A cover slip was then placed over the gasket to keep the surface wet throughout the imaging process, which was performed with a GeneTac UC 4 × 4 microarray scanner. Arrays synthesized on PET were placed onto a microscope slide to ensure that features remained in the focal plane of the scanner. The arrays shown in Figure 2 of the main text were incubated with the labelled oligonucleotides in a humid chamber at the given temperature, then briefly cooled and incubated at 37 °C for 30 minutes before handling as described above. The arrays that were visualized on an Olympus IX70 microscope (details below) were dried rapidly prior to imaging under a nitrogen stream after the 0.5× SSPE rinse step.
Figure 2.
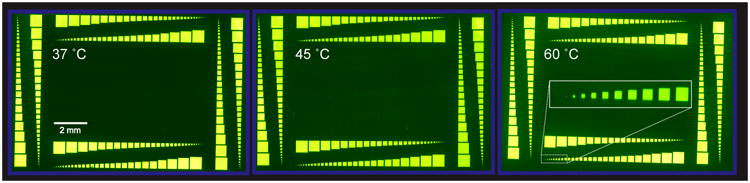
Stability of DNA arrays on polymer-coated PET substrates at various hybridization temperatures. A single array was hybridized at each of the indicated temperatures with a Cy5-labeled complement for 16 hours prior to imaging. After hybridization and imaging at a given temperature, the complement was removed with 8 M urea. The experiments were conducted in order of increasing hybridization temperature. The array layout illustrates that feature quality is consistent across a range of sizes and regions on the surface. Inset of right panel: a magnification of the indicated area, showing that individual features are easily discernible.
Fabrication of Millichips by Laser Cutting
PET-based arrays were cut by laser using an Epilog Helix 12″ × 24″ laser table (Epilog, Golden CO). The CO2 laser (45 watts) had a resolution of 30 μm was set to operate at 30% power and a speed of 50%. The laser cutting guidelines were drawn using Adobe Illustrator (line thickness of 0.001”) and saved as a PDF file for input to the laser table software.
Fluorescence Microscopy
Fluorescence micrographs were obtained using an Olympus IX70 microscope equipped with a Lumen Dynamics XCite 120PC-Q fluorescence source and a Q Imaging EXi Aqua camera with an objective magnification of 20× coupled with a 1.6× magnification booster at an exposure time of 50 ms (Cy3) and 350 ms (Cy5), respectively. Images were obtained in grey scale using MetaMorph Advanced software, version 7.7.8.0 (Molecular Devices, LLC) and subsequently false-colored using ImageJ software (NIH, Bethesda, MD). Where noted, a GeneTac UC 4 × 4 microarray scanner (Genomic Solutions) was also used to visualize arrays. Acquisition settings are summarized in Supplementary Table 4, while false-color palettes used in image preparation are provided in Supplementary Figure 2.
Results and Discussion
Fabrication of High-Density DNA Arrays on PET
Our approach makes use of thin sheets of poly(ethylene terephthalate) (PET) coated with crosslinked polymer multilayers covalently modified to present hydroxyl functionality suitable for conventional phosphoramidite-based nucleic acid synthesis (Scheme 1).24-26 We demonstrated recently that this approach, in combination with conventional methods for in situ maskless array synthesis, could be used to design functional DNA arrays containing oligonucleotides ∼20 bp long.19 We note here that the array synthesis exposes substrates to repeated cycles of phosphoramidite chemistry using photolabile protecting groups and UV-light mediated deprotection (an overview of this MAS strategy is depicted in Scheme 1).27 Further studies (described below) revealed that several procedures and reagents associated with standard array synthesis protocols (e.g., the oxidation step and the acetylation or “capping” step occasionally employed in DNA synthesis, which both involve the use of tetrahydrofuran (THF) as a solvent) can cause the surfaces of film-coated plastic substrates to roughen and promote the delamination of the multilayer film from the underlying PET substrate, ultimately limiting the length of oligonucleotides that can be synthesized. In this study, we sought to address these issues and develop synthetic protocols that would enable the design of high-density plastic-backed arrays with longer probe sequences and more complex features (e.g., array spots containing two distinct DNA features) and arrays with DNA features suitable for the enzymatic synthesis of RNA arrays. We also sought to determine the smallest feature sizes and highest feature densities that could be achieved using these new methods.
Modern DNA arrays used in complex genetic analyses typically contain tens to hundreds of thousands of sequences and thus require that arrays be synthesized with a high feature density.28 To determine whether it was possible to achieve a DNA feature density on PET surfaces that would be comparable to the density obtainable on conventionally used rigid substrates, we synthesized an array consisting of a checkerboard of two sequences, each ∼30 nucleotides long, on polymer film-coated PET substrates. Each feature was synthesized using a single 14 × 14 μm mirror of the DMD so that the area of each feature was as small as the instrumentation could achieve. Features were spaced so that the inter-feature distance between adjacent sites corresponded to a single mirror on the DMD. This “1-in-2” design (where every other mirror is used to synthesize a feature) corresponds to a feature density of 127,667 features/cm2. As shown in Figure 1, the hybridization of labelled complementary oligonucleotides produced fluorescence signals of consistent intensity and high specificity. These results are of comparable quality to those obtained from the same design fabricated upon a rigid substrate (Supplementary Figure 2) and correspond to more than an 80-fold increase in feature density over previous reports,19 suggesting that state-of-the-art genomic analysis experiments could be performed with these flexible DNA arrays.
Figure 1.
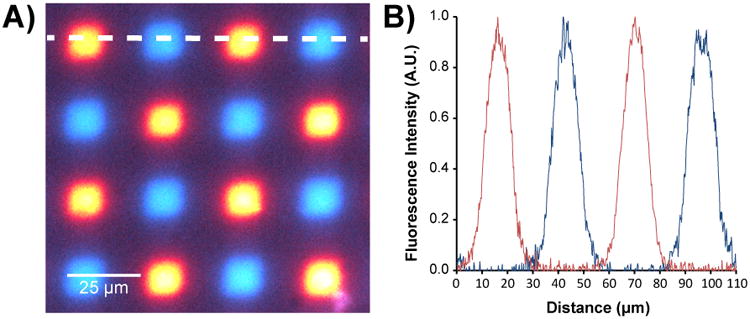
DNA features synthesized from single micromirrors (14 × 14 μm) on polymer-film coated PET. A) Fluorescence micrograph obtained from hybridization of surface-bound DNA to either Cy3 (blue) or Cy5 (red)-labelled oligonucleotides. B) Fluorescence intensity (signal) as measured along the dotted white line shown in (A), from left to right.
Array Stability at Different Temperatures
Oligonucleotide hybridization and capture experiments typically use a range of binding temperatures, particularly in applications such as single-nucleotide polymorphism (SNP) genotyping where a high degree of discrimination between the binding of closely related sequences is required. DNA aptamer arrays are subject to similar considerations, as temperature can be used to control analyte binding or influence the formation of secondary structures on the surface.29 To investigate the stability of the PET arrays fabricated above under different hybridization temperatures, an array was fabricated containing feature sizes from 14 μm to 686 μm and composed of a single sequence of DNA 30 nucleotides long. The array was then incubated overnight at 37, 45, or 60 °C with a fluorescently labelled complementary sequence, cooled to 37 °C (below the Tm of the DNA duplex) to ensure hybridization, and subsequently imaged. Figure 2 shows that there is no discernible physical damage to the surface after such extended incubations at elevated temperature and the ability of the array to bind to the complementary sequence was not negatively affected. The inset in the right panel of Figure 2 depicts the smallest feature size. This feature (measuring 14 ×14 μm) is easily discernible, suggesting that neither large nor small scale degradation is occurring to the oligonucleotides or to the underlying polymer film or plastic substrate. These results compare favorably with previously measured stabilities of arrays made on silanized glass surfaces,30 and demonstrate that PET-based arrays could be used in a variety of assays where elevated temperatures are required.
Improvement of Array Quality Through Oxidizer Optimization
The ability of the polymer-coated PET substrates to withstand the iterative chemical processing steps of array synthesis is an important parameter that governs their utility. The fabrication of arrays containing longer DNA sequences requires a greater number of chemical exposure cycles (the maximum number of cycles is four times the length in nucleotides of the longest feature), thus we examined the suitability of these substrates for the synthesis of arrays with longer sequences. Figure 3 shows fluorescence images after hybridization to the 20 nucleotide termini of a 50 nt long stretch of DNA on a rigid, glassy carbon substrate (3A) and on flexible polymer-coated PET arrays (3B, 3C). Non-complementary control sequences and complementary test sequences were fabricated on the left and right hand sides of the array, respectively. The different colors for the different arrays are false-colored representations of their intensities. Figure 3D shows the signal intensities acquired from each of the arrays under identical imaging conditions. PET arrays fabricated using the standard iodine oxidation conditions (0.02 M I2 in THF/pyridine/H2O, Figure 3B) showed signs of damage to the underlying substrate. This is illustrated by both the high standard deviation of the signal intensities (represented by the error bars in Figure 3D) and the regions of irregular pixel intensity within individual features. This phenomenon was not observed for previous plastic-backed arrays containing shorter sequences that had undergone less cumulative exposure to the tetrahydrofuran (THF).19
Figure 3.
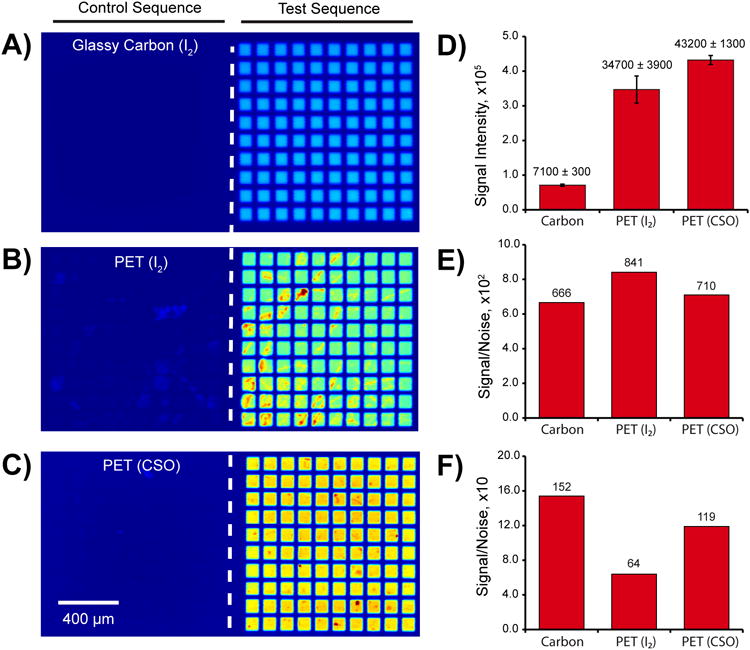
Improved synthesis of longer oligonucleotides. Three arrays consisting of grids containing 100 replicates of 50 nt features were synthesized using different conditions. A)-C) Fluorescence micrographs of arrays hybridized with a Cy5-labelled sequence. The right-hand side contains the test sequences intended to undergo hybridization at their 20 nt termini, while the left-hand side contains the control sequences where no hybridization should occur. A) Hybridization to an array synthesized upon a glassy carbon surface. B) and C) Hybridization to the same array design upon polymer multilayer-coated PET using I2 (B) or CSO (C) as the oxidizing agent during DNA synthesis. D) Feature intensities detected on each array; the standard deviation is given in the plot. The difference in average feature intensity between the carbon and PET surfaces results from omitting the capping step during the PET array synthesis. E) Signal-to-noise ratio measured against the background of the array surface. F) Signal-to-noise ratio measured against the background of the array region containing the control sequences.
To reduce damage to the array and improve the synthesis of longer DNA sequences, the use of THF was eliminated entirely by replacing the oxidation solution with 0.5 M camphorsulfonyl oxazaziridine in acetonitrile (CSO, depicted in Scheme 1).31 Figure 3C shows the resulting improvement in feature uniformity. This is reflected quantitatively in a lower standard deviation without any loss in signal intensity (Figure 3D), although some variation is still evident in the array images. Interestingly, comparison of the signal-to-noise ratio (S/N) across the three conditions implies that there are two modes of surface degradation that occur at high cycle numbers. Figure 3E shows that the S/N values of the three arrays are comparable when the background pixels are selected from a region of the surface where no DNA is synthesized. When the background pixels are selected from regions containing the non-complementary control sequences, the S/N measured on the PET/I2 array is substantially lower than that obtained on the glassy carbon or PET/CSO arrays (Figure 3F). This implies that the majority of the damage is localized to the regions exposed to UV-light. While this was not studied further, a likely interpretation is that the THF may exacerbate low levels of UV damage to the multilayered films and suggests that further development may be required for the synthesis of sequences greater than 50 nucleotides in length.
Fabrication of ‘2-in-1’ DNA Arrays
The improved oxidation conditions developed above were then used to fabricate arrays requiring more complex synthesis protocols. First, a ‘2-in-1’ array was synthesized, where a single feature comprised a mixed population of two different DNA sequences, by using orthogonal deprotection chemistry (Figure 4A). It is notable that such an approach is not possible using an inkjet DNA array synthesizer. A partial dose of UV light was used to remove a fraction of the photolabile 5′-nitrophenylpropyloxycarbonyl (NPPOC) protecting groups early in the synthesis,32 followed by immediate coupling of a 5′-dimethoxytrityl protected (DMT) phosphoramidite to the resultant deprotected hydroxyl groups to yield features now having both NPPOC and DMT-protected oligonucleotides. The remaining NPPOC groups were then removed by UV light, leaving the DMT group unaffected, and allowing for the light-directed synthesis of a sequence to proceed normally at the exposed sites. At the end of the synthesis of this first strand, designated sequence I (21 nt long), the terminal NPPOC was removed to expose a free hydroxyl, which was then capped with phenoxyacetic anhydride to prevent further coupling. The DMT group was then removed with dichloroacetic acid/toluene and the light-directed synthesis of the second strand (sequence II, 22 nt long) was conducted to produce a single feature consisting of two distinct DNA sequences with the same 3′-5′ polarity. Figures 4B and 4C illustrate that it is possible to alter the hybridization signal intensity by varying the dose of UV light prior to the DMT-phosphoramidite coupling. Each column of the array in Figure 4B received a different UV dose, thus altering the ratio of sequence I to sequence II in individual features. The profile of hybridization intensities of sequence I and sequence II across the columns is consistent with the expected NPPOC deprotection behavior.32 A similar approach has been used previously to fabricate DNA sequences of opposite polarity within a single feature.33 This ‘2-in-1’ strategy is key to placing both primer and template strands within a feature to carry out enzymatic reactions.
Fabrication of RNA Arrays on PET
One application that is enabled by the improved oxidation and 2-in-1 DNA synthesis strategies described above is the fabrication of high-density arrays of RNA. A recent report from our group details an enzymatic technique for transforming high-density DNA arrays into arrays of RNA on conventional substrates.23 We evaluated the PET substrates used above to determine their utility in the fabrication of such high-density RNA arrays. To generate the arrays, a partial deprotection step (similar to the one described above) was used to synthesize features containing both template DNA strands (using NPPOC-protected DNA monomers) and short RNA primer strands (utilizing DMT-protected RNA monomers). The two sequences were then annealed together to yield a DNA:RNA duplex that allowed the RNA primer strands to be extended using T7 RNA polymerase. A subsequent DNase treatment left behind only the RNA sequences, which remained localized to the region of the original template DNA. This technique was implemented here to fabricate an array consisting of three different 32-33 nucleotide long RNA features, which was subsequently hybridized with fluorescently-labelled complementary oligonucleotides. Figure 5 shows the localization of each labelled sequence in its intended location with negligible non-specific adsorption to the background between features. The signal-to-noise ratios measured here are comparable to those reported for spotted DNA arrays on the same type of substrate.34 A number of potential applications for RNA arrays, such as the study of RNA binding proteins, aptamers, or the development of new biosensors, depend critically upon minimizing non-specific interactions between the surface and analytes of interest. The ability to synthesize the arrays on a polymeric surface provides an important new avenue for the control of such interactions. These results also demonstrate the chemical robustness of the multilayered polymer films to the various steps required to effect the transition from DNA to RNA arrays, including the annealing at elevated temperatures and the extended incubation times required for the enzymatic reactions.
Figure 5.
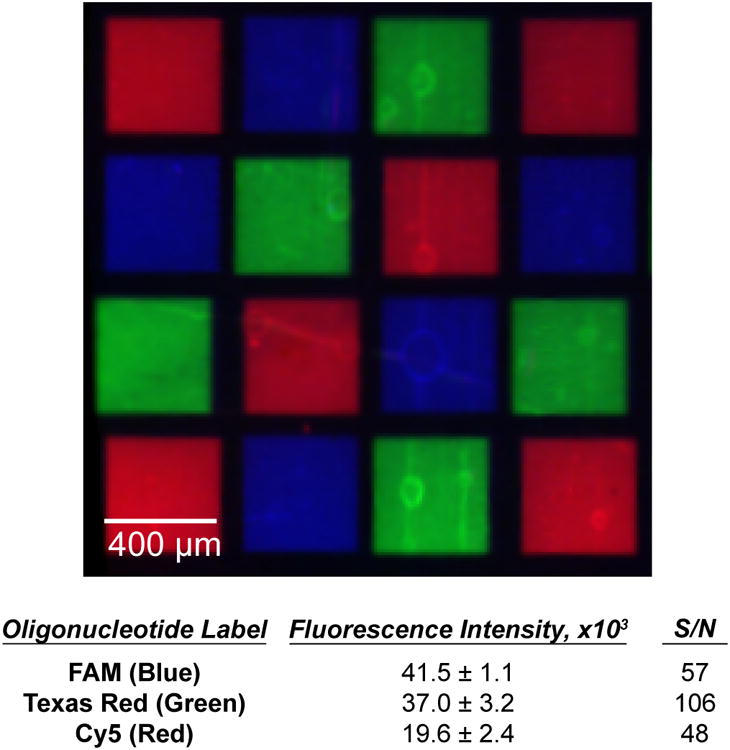
Fluorescence micrograph showing hybridization of three different fluorescently labelled complements to an RNA array fabricated on polymer film-coated PET. The table summarizes the intensity of the fluorescence signals, plus-or-minus one standard deviation and the corresponding signal-to-noise ratio for each of the three sequences.
Millichip Fabrication by Automated Laser Cutting
An important advantage of the plastic-backed arrays relative to arrays fabricated on glass or glassy carbon substrates is that PET is a soft and flexible material that is readily cut to create smaller individual arrays of arbitrary shape.19 A previous study explored the fabrication of DNA ‘millichips’, by synthesizing a DNA array on a pre-scored glass substrate and subsequently physically breaking it into smaller subsections containing arrays that could be used separately.20 This approach has potential to significantly reduce the cost of DNA array fabrication. To explore the potential of this strategy using our plastic-backed arrays, polymer multilayer-coated PET substrates were cut into squares with dimensions of 2.5 × 2.5 mm using an automated laser cutting tool. The cutting procedure did not deleteriously affect the polymer coatings; delamination, peeling, visual hazing, or other physical changes were not observed by visual inspection (Figure 6A).
Figure 6.

A) Digital photograph of polymer film-coated PET cut using a laser into 2.5 × 2.5 mm millichips. B) Fluorescence micrograph of a DNA millichip array after laser cutting and hybridization with fluorescently labelled complements. The white arrow indicates the edge of the millichip, which appears bright after cutting with the laser.
A series of DNA arrays was then synthesized (two different DNA probes, ∼20 nucleotides long) and cut into individual millichips for hybridization with fluorescently labelled complements (Figure 6B). We observed hybridization intensities comparable to those obtained with PET arrays that were not laser cut (e.g. Figure 1) with little non-specific background signal, indicating that the laser cutting process did not negatively impact array quality. Although the coated PET itself appears bright at the cut edges (Figure 6B), cuts can be made very close to the edge of the arrays (e.g. <0.1 mm) without observing damage to the array or the ability of the array to hybridize to complementary oligonucleotides, suggesting that further miniaturization of millichips may be possible. The 2.5 × 2.5 mm millichips used in these proof-of-concept experiments are easy to handle with tweezers and can be readily placed into PCR tubes or 96-well plates for use in experiments.
Conclusions
We describe here the photolithographic fabrication of high-density DNA and RNA arrays on flexible, polymer multilayer-coated plastic substrates. These flexible arrays are compatible with small feature sizes and high feature densities comparable to those of arrays fabricated on rigid substrates such as silanized glass and glassy carbon, and can be imaged and analyzed quantitatively using standard fluorescence imaging techniques. It is shown that the arrays are stable to hybridization at elevated temperatures and are compatible with conditions used for enzymatic procedures such as the in situ synthesis of RNA from DNA oligonucleotides. These flexible and transparent arrays are physically robust, easy to handle, and, because they are fabricated on ‘soft’ polymer films that can be readily cut using a variety of methods (e.g., scissors or laser cutting tools, etc.), can also be used to create smaller sub-arrays (or “millichips”). We anticipate that numerous ‘lab-on-a-chip’-like applications will be enabled by such flexible arrays, including the patterning of oligonucleotides or other molecules in non-planar geometries and the high-throughput production of low-cost millichips for affordable assays with extremely small reagent volumes.
Supplementary Material
Acknowledgments
This work was supported by the NSF (DMR-1121288, PGRP-0801846); the National Institutes of Health (1R01GM108727, 1R01GM109099, and 5T32GM08349); and the Wisconsin Alumni Research Foundation. The work made use of shared facilities supported by the NSF through the UW-Madison Materials Research Science and Engineering Center (DMR-1121288) and Nanoscale Science and Engineering Center (DMR-0832760). M.C.D.C acknowledges the Natural Sciences and Engineering Research Council of Canada for a graduate fellowship.
Footnotes
Author Contributions: All authors have given approval to the final version of the manuscript.
Supporting Information: Experimental details including synthetic conditions, image processing information and identities of oligonucleotides used in the main text. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
prof David M. Lynn, Email: dlynn@engr.wisc.edu.
Prof Lloyd M. Smith, Email: smith@chem.wisc.edu.
References
- 1.Pease AC, Solas D, Sullivan EJ, Cronin MT, Holmes CP, Fodor SP. Proc Natl Acad Sci U S A. 1994;91:5022–5026. doi: 10.1073/pnas.91.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D, Collins C, Kuo WL, Chen C, Zhai Y, Dairkee SH, Ljung Bm, Gray JW, Albertson DG. Nat Genet. 1998;20:207–211. doi: 10.1038/2524. [DOI] [PubMed] [Google Scholar]
- 3.Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, Ghandour G, Perkins N, Winchester E, Spencer J, Kruglyak L, Stein L, Hsie L, Topaloglou T, Hubbell E, Robinson E, Mittmann M, Morris MS, Shen N, Kilburn D, Rioux J, Nusbaum C, Rozen S, Hudson TJ, Lipshutz R, Chee M, Lander ES. Science. 1998;280:1077–1082. doi: 10.1126/science.280.5366.1077. [DOI] [PubMed] [Google Scholar]
- 4.Eisen MB, Brown PO. In: Methods Enzymol. Sherman MW, editor. Academic Press; 1999. pp. 179–205. [DOI] [PubMed] [Google Scholar]
- 5.Call DR. Crit Rev Microbiol. 2005;31:91–99. doi: 10.1080/10408410590921736. [DOI] [PubMed] [Google Scholar]
- 6.Albert TJ, Molla MN, Muzny DM, Nazareth L, Wheeler D, Song X, Richmond TA, Middle CM, Rodesch MJ, Packard CJ, Weinstock GM, Gibbs RA. Nat Methods. 2007;4:903–905. doi: 10.1038/nmeth1111. [DOI] [PubMed] [Google Scholar]
- 7.LaFramboise T. Nucleic Acids Res. 2009;37:4181–4193. doi: 10.1093/nar/gkp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katilius E, Flores C, Woodbury NW. Nucleic Acids Res. 2007;35:7626–7635. doi: 10.1093/nar/gkm922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozers MS, Warren CL, Ansari AZ. In: Micro and Nano Technologies in Bioanalysis. Foote RS, Lee JW, editors. Humana Press; 2009. pp. 637–653. [Google Scholar]
- 10.Kim C, Kaysen J, Richmond K, Rodesch M, Binkowski B, Chu L, Li M, Heinrich K, Blair S, Belshaw P, Sussman M, Cerrina F. Microelectron Eng. 2006;83:1613–1616. [Google Scholar]
- 11.Kosuri S, Eroshenko N, LeProust EM, Super M, Way J, Li JB, Church GM. Nat Biotechnol. 2010;28:1295–1299. doi: 10.1038/nbt.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosuri S, Church GM. Nat Methods. 2014;11:499–507. doi: 10.1038/nmeth.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeProust EM, Peck BJ, Spirin K, McCuen HB, Moore B, Namsaraev E, Caruthers MH. Nucleic Acids Res. 2010;38:2522–2540. doi: 10.1093/nar/gkq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fodor S, Read J, Pirrung M, Stryer L, Lu A, Solas D. Science. 1991;251:767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 15.Hughes TR, Mao M, Jones AR, Burchard J, Marton MJ, Shannon KW, Lefkowitz SM, Ziman M, Schelter JM, Meyer MR, Kobayashi S, Davis C, Dai H, He YD, Stephaniants SB, Cavet G, Walker WL, West A, Coffey E, Shoemaker DD, Stoughton R, Blanchard AP, Friend SH, Linsley PS. Nat Biotechnol. 2001;19:342–347. doi: 10.1038/86730. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Li PCH. Anal Chim Acta. 2011;687:12–27. doi: 10.1016/j.aca.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Zopf A, Raim R, Danzer M, Niklas N, Spilka R, Pröll J, Gabriel C, Nechansky A, Roucka M. Bio Techniques. 2015;58:126–133. doi: 10.2144/000114264. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Li S, Chen N, Yang C, Wang Y. Biomacromolecules. 2013;14:1174–1180. doi: 10.1021/bm400096z. [DOI] [PubMed] [Google Scholar]
- 19.Broderick AH, Lockett MR, Buck ME, Yuan Y, Smith LM, Lynn DM. Chem Mater. 2012;24:938–945. doi: 10.1021/cm202720q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinrich KW, Wolfer J, Hong D, LeBlanc M, Sussman MR. Plant Physiology. 2012;159:548–557. doi: 10.1104/pp.112.195230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck ME, Schwartz SC, Lynn DM. Chemistry of Materials. 2010;22:6319–6327. doi: 10.1021/cm102115e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CH, Lockett MR, Smith LM. Angew Chem Int Ed. 2012;51:4628–4632. doi: 10.1002/anie.201109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu CH, Holden MT, Smith LM. Angew Chem Int Ed. 2014;53:13514–13517. doi: 10.1002/anie.201408747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buck ME, Zhang J, Lynn DM. Adv Mater. 2007;19:3951–3955. [Google Scholar]
- 25.Fredin NJ, Broderick AH, Buck ME, Lynn DM. Biomacromolecules. 2009;10:994–1003. doi: 10.1021/bm900045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buck ME, Lynn DM. Polym Chem. 2012;3:66–80. doi: 10.1039/C1PY00314C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh-Gasson S, Green RD, Yue Y, Nelson C, Blattner F, Sussman MR, Cerrina F. Nat Biotechnol. 1999;17:974–978. doi: 10.1038/13664. [DOI] [PubMed] [Google Scholar]
- 28.Lipshutz RJ, Fodor SPA, Gingeras TR, Lockhart DJ. Nat Genet. 1999;21:20–24. doi: 10.1038/4447. [DOI] [PubMed] [Google Scholar]
- 29.Goda T, Miyahara Y. Biosens Bioelectron. 2011;26:3949–3952. doi: 10.1016/j.bios.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 30.Phillips MF, Lockett MR, Rodesch MJ, Shortreed MR, Cerrina F, Smith LM. Nucleic Acids Res. 2008;36:e7. doi: 10.1093/nar/gkm1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ugi I, Jacob P, Landgraf B, Rupp C, Lemmen P, Verfurth U. Nucleosides and Nucleotides. 1988;7:605. [Google Scholar]
- 32.Chen S, Phillips MF, Cerrina F, Smith LM. Langmuir. 2009;25:6570–6575. doi: 10.1021/la9000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie B, Yang M, Fu W, Liang Z. Analyst. 2015 doi: 10.1039/c5an00349k. [DOI] [PubMed] [Google Scholar]
- 34.Broderick AH, Carter MCD, Lockett MR, Smith LM, Lynn DM. ACS Appl Mater Interfaces. 2013;5:351–359. doi: 10.1021/am302285n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


