Abstract
VEGF165b is an anti-angiogenic form of VEGF165 produced by alternative splicing. The localization of pro-angiogenic VEGF165 and anti-angiogenic VEGF165b was investigated during development of the vasculatures in fetal human eyes from 7 to 21 weeks gestation (WG). The fetal vasculature of vitreous, which includes tunica vasculosa lentis (TVL), had moderate VEGF165 immunoreactivity at 7WG and very little VEGF165b. Both forms were elevated at 12WG. VEGF165 then decreased around 17WG when the TVL regresses but VEGF165b remained elevated. In choroid, VEGF165 was present in forming choriocapillaris (CC) and retinal pigment epithelium (RPE) at 7WG while VEGF165b was present in CC and mesenchymal precursors within the choroidal stroma. By 21WG, both forms were elevated in RPE and choroidal blood vessels but VEGF165b was apical and VEGF165 basal in RPE. Diffuse VEGF165 immunoreactivity was prominent in 12WG innermost retina where blood vessels will form while VEGF165b was present in most CXCR4+ progenitors in the inner neuroblastic layer and migrating angioblasts in the putative nerve fiber layer. By 21WG, VEGF165 was present in nerve fibers and VEGF165b in inner Muller cell process. The localization of VEGF165b was distinctly different from VEGF165 both spatially and temporally and it was often associated with nucleus in progenitors.
Keywords: angioblasts, choroid, endothelial cells, pericytes, retina, vasculogenesis, vascular endothelial growth factor
Introduction
The fetal human eye has several unique vascular systems that form during different stages of development. The three major systems are the hyaloid or fetal vasculature in vitreous, the choroidal vasculature, and the retinal vasculature. During embryonic and early fetal development, the fetal vasculature in vitreous supplies the lens and avascular inner retina with oxygen and nutrients from within the vitreous cavity while the choroidal vasculature supplies the outer retina from the scleral aspect of the eye.
The fetal vasculature of vitreous (FVV) begins forming around 5WG, and consists of the hyaloid artery, which enters from the optic fissure and extends into the primitive vitreous. It's capillaries branch freely to form the vaso hyloidea propria (VHP) in preretinal vitreous and the tunica vasculosa lentis (TVL) adjacent to lens. After the lens has developed, this vascular system begins to regress around 13WG (Zhu et al., 2000). This regression coincides with the initiation of retinal vascularization (Zhu et al., 2000).
The choroidal vasculature begins to develop around 5-6WG by a process called hemo-vasculogenesis, the formation of hematopoietic and erythropoietic cells from a common precursor, the hemangioblast (Hasegawa et al., 2007). The primitive choroidal vasculature forms initially in the posterior region and extends anteriorly with continued development. As it matures, three layers of vessels are established by angiogenic and anastomotic processes (Lutty et al., 2010). Fenestrations, which allow transport of small molecules and solutes between choriocapillaris and RPE, begin to develop around 16WG (Baba et al., 2009).
Primitive retinal vessel development begins around the optic nerve head around 14-16 WG when the FVV starts to regress. The superficial or primary retinal vascular system forms first by a process of vasculogenesis (McLeod et al., 2006; Hasegawa et al., 2008) followed by the development of the deep capillary network by sprouting from pre-existing vessels or angiogenesis. The factors involved in retinal vascular development have been intensely investigated. Vascular endothelial growth factor (VEGF) is one of the major pro-angiogenic factors and its importance during vascular development has been well documented (Ferrara et al., 2003). VEGF production by astrocytes and Muller cells is regulated by hypoxia-induced factor-1 (HIF-1), which is elevated in response to hypoxia. VEGF levels are elevated in avascular retina due to physiological hypoxia (Chan-Ling et al., 1995).
There is considerable evidence that VEGF plays an important role in normal and pathologic vascular development. Recently, it has been reported that VEGF-A has two isoforms VEGFxxx and VEGFxxxb in addition to the known isoforms with varying heparin binding affinities and molecular sizes: VEGF121, 145, 165, 189, 206 (Bates et al., 2002; Ladomery et al., 2007; Qiu et al., 2009). These isoforms are alternatively spliced from one VEGF-A gene. The VEGF gene consists of eight exons and exon 8a can be alternatively spliced generating the VEGFxxxb family. The number of exons (exon 1 to 7b) spliced results in the amino acid number xxx of the VEGF isoforms. VEGF121b, VEGF165b, and VEGF189b have been reported so far. These VEGFxxxb family members are anti-angiogenic and the splicing is stimulated by TGF beta through splicing factor SRp55 (serine/ arginine protein 55) (Nowak et al., 2008). On the contrary, VEGFxxx is pro-angiogenic and splicing to form it is upregulated by IGF1 and TNF alpha through ASF/SF2 (alternative splicing factor/ splicing factor 2) (Nowak et al., 2008; Nowak et al., 2010).
Previous immunohistochemical studies involving VEGF localization in the developing eye may have employed antibodies that recognize both 165 isoforms (David Bates, personal communication). Because VEGFxxx and VEGFxxxb have opposite effects on blood vessels, this study was initiated to demonstrate localization of VEGFxxx versus VEGFxxxb and perhaps suggest the role of both VEGF forms in human ocular vascular development. In this study, we used an antibody specific for the VEGF165b to examine the spatial and temporal localization of this anti-angiogenic isoform of VEGF in the developing fetal human eye. We compared the pattern of VEGF165b staining with VEGF165, which is known to be dominant in most angiogenic states (Bates et al., 2002). Our data show a molecular shift from VEGF165 to VEGF165b in the developing ocular vessels and provides insights regarding expansion and regression of blood vessels during vascular development.
Results
Fetal Vasculature of Vitreous (FVV)
The FVV and TVL were observed in eyes from 7-17WG in this study. It was difficult to see the regressing FVV at 21WG because the size of the eyes was relatively large and the posterior eyecup had to be cut into pieces. Histologically, highly cellular blood vessels with poorly defined lumens were observed at 7WG (Figure 1). The endothelial cell marker CD31 was expressed in 7WG FVV endothelium and labeling was more intense at 10-17WG (Figure 1) but von Willebrand factor (vWf) was not prominent until 12WG (Figure 2). Meanwhile, alpha smooth muscle actin (SMA), which is a marker for pericytes and smooth muscle cells in capillaries and arteries respectively, was positive at 10WG, suggesting mural cell differentiation in hyaloid vessels at that time. Alpha SMA antibody clearly labeled actin filaments in pericytes covering the entire vasculature at 17WG implying pericyte maturation (data not shown). VEGF165b was present in both endothelial cells and perciytes ((Figure 2). VEGF165 was present diffusely in blood vessels at 7WG, increased in staining until 12WG, and decreased by 17WG when FVV was undergoing regression (Figure 1). Conversely, VEGF165b was barely detectable at 7WG, increased steadily thereafter and was associated with nuclei of endothelial cells and pericytes (Figure 1). Double labeling with VEGF165 and VEGF165b antibodies clearly showed different localization of these two VEGF165 forms in the fetal vasculature and that both endothelial cells and pericytes had both forms (Figure 3). While VEGF165 levels were decreased in the fetal vasculature at 17WG, VEGF165b remained elevated (Figure 1). The apparent nuclear localization of the VEGF165b in pericytes and endothelial cells was quite striking at 12-17 WG (Figures 1-3).
Figure 1.
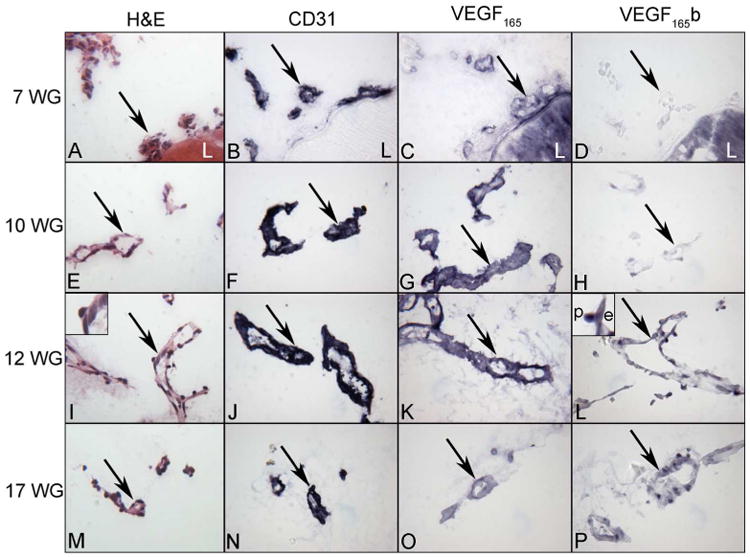
Localization of VEGF165 and VEGF165 b in the human fetal vasculature of vitreous at 7, 10, 12 and 17WG human eyes. Hematoxylin and eosin staining shows the highly cellular and poorly defined lumenal structure of blood vessels at 7 WG which mature into well developed blood vessels by 12 WG. CD31 is expressed until vessels have fully regressed by 21 WG. VEGF165 was present diffusely in blood vessels at 7 WG, increased in staining until 12 WG and decreased by 17 WG. Conversely, VEGF165b was undetectable at 7 WG, increased steadily thereafter, and was associated with nuclei of endothelial cells (e) and pericytes (p) (insets 12 WG). (arrows= blood vessels, L = lens)(APase reaction product)
Figure 2.

Expression of VEGF165 b (green) in endothelial cells (red, vWf) and pericytes (red, aSMA) in the FVV at 12WG. VEGF165b was associated with both cell types.
Figure 3.
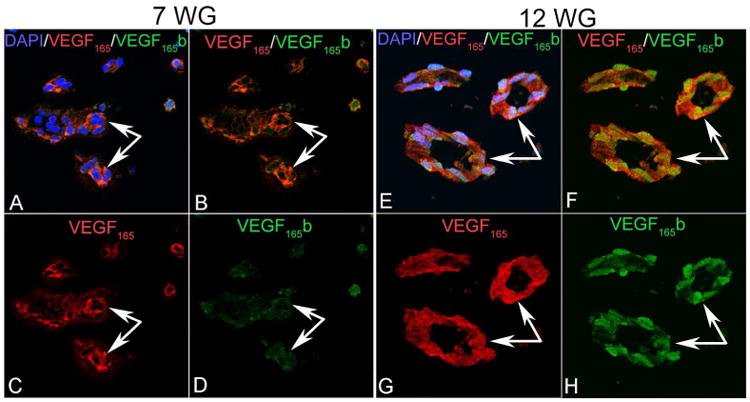
VEGF165 (red) and VEGF165 b (green) in the human fetal vasculature of vitreous at 7 and 12 WG. At 7 WG, moderate VEGF165 immunostaining was present diffusely in blood vessels while very little VEGF165b staining was detected. By 12 WG, VEGF165 immunostaining had increased in endothelial cells and pericytes and VEGF165b labeling was strong and localized to endothelial cell and pericyte nuclei. (arrows indicate blood vessels).
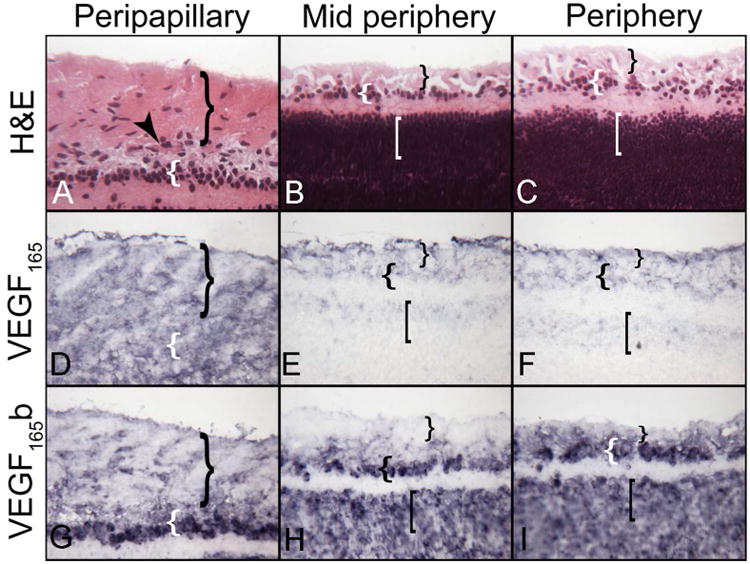
Choroidal vasculature
At 7WG, a single layer of choroidal blood vessels labeled with anti CD31 antibody was observed at the level of the choriocapillaris (CC). VEGF165 staining was present diffusely around the CC at this age and there was no detectable VEGF165b present (data not shown). VEGF165 was also observed around CC at 10WG. On the other hand, VEGF165b was barely detectable around CC and weakly positive in mesenchymal progenitors in choroid (Figure 4).
Figure 4.

Localization of VEGF165 and VEGF165 b in the human choroid at 10, 12, 17 and 21 weeks gestation (WG) eyes. Hematoxylin and eosin staining shows the structure of the choroid and the presence of mesenchymal precursors throughout the forming choroid (single cells at the bottom of the micrograph). CD31 is used as an endothelial cell marker, which demonstrates that only a capillary system is forming at 10WG. VEGF165 staining was present diffusely around the capillaries at 10 WG and increased around all vessels with age. VEGF165b was barely detectable at 10 WG and increased in endothelial cell and mesenchymal precursor nuclei steadily with development. (small white arrowhead = RPE, arrows = choroidal capillaries, large black arrowhead = large vessels)(APase reaction product)
At 12WG, a second layer of choroidal vessels had formed extending from the CC to choroidal stroma (Figure 4). VEGF165 labeling increased around CC but weak staining was associated with the deeper choroidal vessels. VEGF165 was also associated with the basal portion of the RPE. VEGF165b was weakly positive in the CC and mesenchymal cells in choroid at 12WG (Figure 4 and 5).
Figure 5.
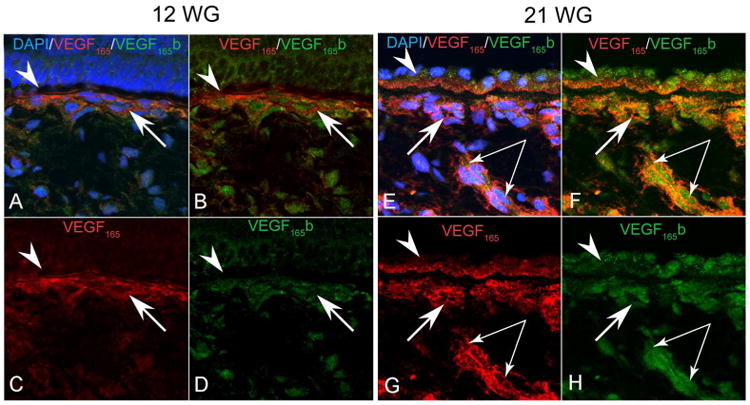
VEGF165 (red) and VEGF165 b (green) localization in the human RPE/Bruch's membrane/choriocapillaris complex at 12 and 21WG. At 12 WG VEGF165 immunostaining was present diffusely in the choriocapillaris (arrows) and weakly associated with the basal portion of the RPE (arrowhead). VEGF165b appeared to have a nuclear association in the choriocapillaris with very little RPE staining. At 21 WG, the choriocapillaris (single arrows), larger choroidal vessels (paired arrows) and RPE all had increased expression of both VEGF165 and VEGF165b. VEGF165b in RPE appeared apical and nuclear while VEGF165 was predominantly basal
At 17WG, the deeper layer of choroidal vessels were more mature (Hasegawa et al., 2007) and showed prominent CD31 immunoreactivity (Figure 4). The choroidal vessels and CC had more intense VEGF165 surrounding them as did the basal portion of the RPE. VEGF165b labeling was seen in the CC but deeper vessels were unstained.
At 21WG, a third layer of choroidal vasculature was present in presumed submacular choroid (Figure 4) and both VEGF165 and VEGF165b were prominent in and around all choroidal vessels. Immunofluorescence showed double labeling of all choroidal vessels with both VEGF165 splice variants (Figure 5). Double labeling with alpha SMA and VEGF165b showed choroidal arterioles and arteries had VEGF165b expression as well as choroidal venules/ CC (data not shown). In arteries, the localization of VEGF165b appeared to be nuclear in endothelial cells and smooth muscle cells as well. At this age, there was a distinctly different localization of these two VEGF isoforms in RPE cells. VEGF165 was localized at the basal portion of the RPE, while VEGF165b was in RPE nuclei and the apical portion of the cells (Figure 5).
Retinal vasculature
VEGF165 was diffusely distributed in innermost retina at 7WG and VEGF165b weakly labeled progenitor cells in the inner neuroblastic layer (data not shown). At 10WG, VEGF165b positive cells were observed migrating inwardly from the inner neuroblastic layer (INL) to the putative nerve fiber layer (NFL). While VEGF165b staining was mainly cell-associated, VEGF165 immunoreactivity was diffuse throughout the inner retina. This was similar to retina at 12WG (results not shown).
At 12WG, regional staining patterns showed the diffuse distribution of VEGF165 in NFL and INL and VEGF165b was associated with inwardly migrating cells from the progenitor pool in the INL, similar to the localization at 7 and 10WG (Figure 6). Migrating cells were most often observed in the peripapillary or posterior pole of retina where blood vessels develop first. Double-labeling and confocal microscopy showed diffuse VEGF165 labeling in NFL and INL and cell-nuclei associated VEGF165b in migrating cells within the NFL and progenitors in the INL (Fig. 7). In mid peripheral retina where the NFL is thinner and the INL is thicker, the localization pattern of VEGF165 was similar to that observed in the posterior pole (Figure 6). In midperiphery, VEGF165b was present in migrating progenitors in the nerve fiber layer, which we previously identified as angioblasts (McLeod et al., 2006; Hasegawa et al., 2008), but was diffuse throughout the inner neuroblastic layer (Figure 6-7).
Figure 6.
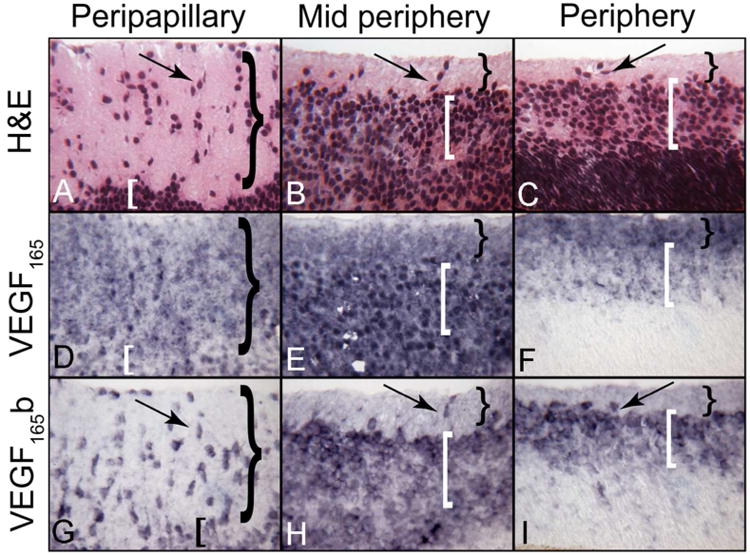
VEGF165 and VEGF165 b localization in different regions of a 12 WG human retina. Structure of developing inner retina at 12 WG showing the nerve fiber layer (NFL= }) and inner neuroblastic layer (INL= [) and cells migrating inwardly (arrows). Both ganglion cells and blood vessels will occupy the inner retina. Regional staining patterns show the diffuse distribution of VEGF165 in both layers while VEGF165b is cell-associated. (APase reaction product)
Figure 7.
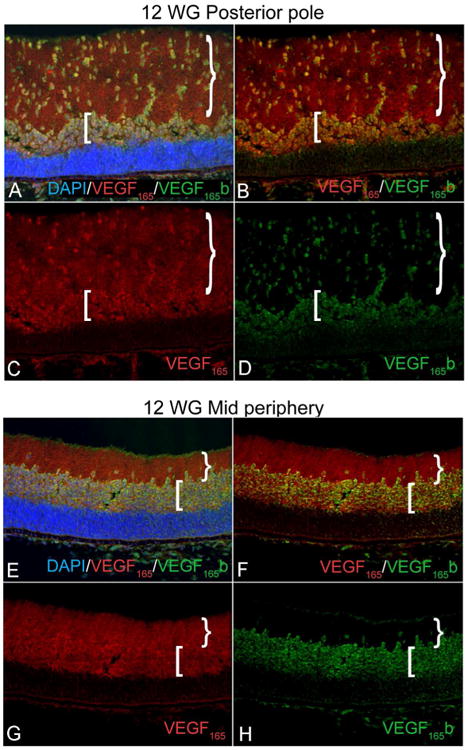
VEGF165 (red) and VEGF165 b (green) in mid peripheral and peripapillary 12WG retina. There is diffuse VEGF165 labeling in the nerve fiber layer (NFL= }) and inner neuroblastic layer (INL= [). VEGF165b is associated with migrating cells of the NFL and progenitors in the INL.(DAPI nuclear stain)
At 17WG, VEGF165 immunoreactivity was diffuse in the nerve fiber and putative ganglion cell layer (GCL). Nerve fibers were prominently labeled in midperipheral retina as well. Cell-associated VEGF165b was in ganglion cells in avascular retina (Figure 8H). VEGF165b was weak around developing vasculature in peripapillary retina (Figure 8G), but many cells were positive in the NFL in the periphery (Figure 8I).
Figure 8.

Three regions in a 17 WG Retina. The inner retina at 17 WG has nerve fiber layer (}) with blood vessels (arrowhead), a putative ganglion cell layer ({), and an inner neuroblastic layer ([). Regional expression patterns show the diffuse distribution of VEGF165 in the nerve fiber and putative ganglion cell layer. Nerve fiber VEGF165 is apparent in (E). Cell-associated VEGF165b is more intense in advance of forming blood vessels and weakest in the vascularized peripapillary area. (APase reaction product)
VEGF165 immunoreactivity at 21WG was weaker in the nerve fiber layer and GCL and it had decreased as vascularization progressed in inner retina. Cell-associated VEGF165b staining was intense in GCL throughout retina (Figure 9). Double-labeled 21WG mid peripheral retina showed VEGF165 labeling of NFL and a few cells in GCL. VEGF165b was more prominent in the GCL than in NFL. Double labeling using endothelial cell markers (vWf or CD31) and VEGF165 splice variants revealed that the developing retinal vessels were associated with VEGF165 and weakly with VEGF165b (data not shown).
Figure 9.
VEGF165 and VEGF165 b localization in different regions of a 21 WG retina. The developing inner retina at 21 WG has a nerve fiber layer (}), blood vessels (arrowhead), a definitive ganglion cell layer ({) and inner neuroblastic layer ([). VEGF165 immunoreactivity in the nerve fiber and ganglion cell layer has decreased as vascularization has occurred in inner retina. Cell-associated VEGF165b immunoreactivity is prominent in the ganglion cell layer ({) throughout retina. (APase reaction product)
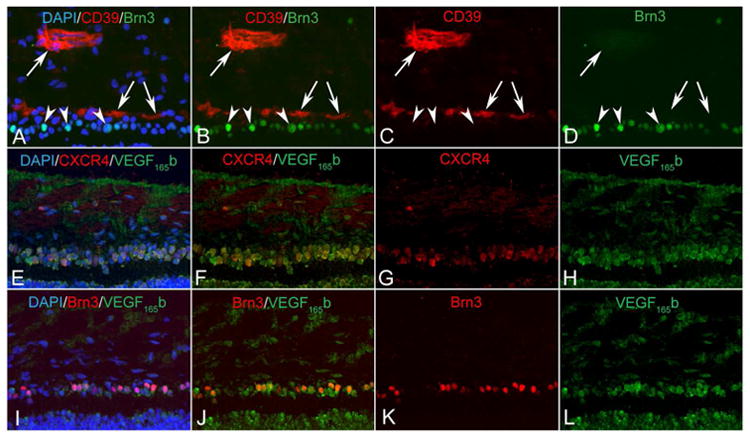
Retinal progenitor cell markers and VEGF165b
The association of VEGF165b with progenitors was striking in retina. VEGF165b+ / CXCR4+ progenitors migrating from the neuroblastic layer into the inner retina represented two distinct populations at 10 WG. Some cells were CD39+ angioblasts, and others were ganglion cell precursors expressing Brn-3B in their nuclei. Double labeling using CD39 and CXCR4 antibodies showed that some of the migrating (VEGF165b+) progenitors were angioblasts (CD39+ / CXCR4+) (Figure 10). Also of note was the apparent nuclear localization in retinal progenitors.
Figure 10.

Markers for angioblasts/endothelial cells (CD39, red) and ganglion cells (Brn3B, green) in a 10 WG retina. VEGF165 b+/CXCR4+progenitors migrating from the neuroblastic layer into the inner retina represent two distinct populations. Some cells are CD39+ angioblasts, and others are ganglion cell precursors expressing Brn3. The migrating VEGF165b progenitors (arrows) were mostly angioblasts (CD39+).
By 21WG, VEGF165b+ / CXCR4+ progenitors have migrated from the neuroblastic layer into the formed GCL and NFL. In regions where vascularization had occurred, CD39+ blood vessels, CD39+ angioblasts and Brn-3B+ ganglion cells were evident. Some undifferentiated CXCR4+ progenitors (CD39- /Brn-3B-) still existed in these regions (Figure 11).
Figure 11.
Angioblasts and endothelial cells (CD39+, red) and ganglion cells (Brn3B+) in inner retina at 21 WG. VEGF165b+(green)/CXCR4+(red) progenitors have migrated from the neuroblastic layer into the formed ganglion cell layer and nerve fiber layer. In regions where vascularization has occurred, CD39+ blood vessels (large arrow), CD39+ angioblasts (small arrows), and Brn-3B+ ganglion cells (arrowheads) are evident. Some undifferentiated CXCR4+ progenitors (CD39- /Brn-3B-) still exist in these regions.
Quantitative real time PCR (qRT-PCR) of VEGF splice variants in the fetal vasculature of vitreous
qRT-PCR was performed to assess VEGF165b and VEGF165 expression in 8WG and 11WG fetal vasculature. VEGF165b RNA was increased 32 fold in the 11WG fetal vasculature compared to that detected in the 8WG fetal vasculature. The fold change was calculated using the ΔCt method after normalizing to β-actin. VEGF165 RNA levels, however, were comparable at 8 and 11WG in the fetal vasculature.
Discussion
The localization of pro-angiogenic VEGF165 and anti-angiogenic VEGF165b was investigated in the developing fetal human eye. VEGF165 in the fetal vasculature of vitreous was expressed early during embryonic developmental and declined after 12 WG while VEGF165b expression appeared after blood vessels had formed and increased with gestational age when this vasculature is regressing. In choroid, VEGF165 was expressed early and throughout development while VEGF165b expression appeared after blood vessels formed and it steadily increased with age. In RPE, VEGF165 was expressed in the basal portion of the RPE throughout development while VEGF165b expression in the nuclei and apical RPE was prominent at 21WG. In retina, VEGF165 was diffusively expressed in inner retina and was most prominent in advance of forming blood vessels. VEGF165b was expressed in nuclei or possibly the nuclear envelope of cells in the INL progenitor pool (CXCR4+) and cells migrating into inner retina from the pool, which were predominantly CD39+/CXCR4+ vascular progenitors or angioblasts (Lutty et al., 1993a; McLeod et al., 2006; Hasegawa et al., 2008). In terms of expression, there appeared to be a distinct balance between VEGF165 and VEGF165b during vascular development. Developing vessels had more VEGF165 associated with them and little or none of the VEGF165b isoform. When vessels had formed and were becoming mature, the intensity of VEGF165 immunoreactivity declined and VEGF165b was upregulated suggesting that VEGF165b is involved in maintaining the established vascular system and possibly preventing further angiogenesis. This was particularly evident in the transient fetal vasculature of the vitreous where the trend was confirmed with qRT-PCR.
VEGF165 and VEGF165b are generated by alternative splicing of the same gene encoding VEGF-A. This splicing system in RPE is known to be regulated by growth factors (Nowak et al., 2008; Nowak et al., 2010). TGF-beta activates p38/MAPK and subsequent Clk/sty kinases. Clk phosphorylates SR protein splicing factors such as SRp55 which is a key regulatory splice factor binding to VEGF pre-RNA in the exon 8b region. SRp55 splices at DSS (distal splice-site selection) and produces VEGF165b. On the other hand, IGF (Insulin-like growth factor 1) activates PKC (protein kinase C) and phosphorylate ASF/SF2 (alternative splicing factor/ splicing factor 2) through the SR protein kinase SRPK1 (serine/ arginine domain protein kinase 1). ASF/SF2 can bind to VEGF pre-RNA around PSS (proximal splice-site selection) and results in VEGF165 expression (Nowak et al., 2008; Nowak et al., 2010). IGF-1 has reported to control VEGF expression via HIF1 alpha (hypoxia induced factor-1 alpha) dependent and independent pathways (Slomiany and Rosenzweig, 2006). Vasculatures develop in physiologically hypoxic tissue where HIF 1 alpha is abundant (Ozaki et al., 1999). Collectively, the growing vessels need VEGF165 and once vessels have developed and oxygen levels are increased, the switch in splicing might occur in response to reduced HIF-1 alpha/IGF-1 and result in increased VEGF165b expression. TGF-β is produced by pericytes and smooth muscle cells in mature blood vessels to maintain endothelial cells in a quiescent state (Orlidge and D'Amore, 1987; Walshe et al., 2009). The fact that differentiated but not dedifferentiated podocytes in human glomerulae secrete significant amounts of VEGF165b protein could underpin this idea (Cui et al., 2004) since podocytes are similar to pericytes in their relationship with endothelial cells.
The down-regulation of VEGF165b has been reported in angiogenic pathologies such as tumor (Bates et al., 2002; Woolard et al., 2004; Pritchard-Jones et al., 2007; Varey et al., 2008), diabetic retinopathy (Perrin et al., 2005) and experimental oxygen-induced retinopathy (Konopatskaya et al., 2006). Those studies showed increased VEGF165 expression and less VEGF165b expression compared to normal conditions where VEGF165b is the dominant splice variant. For example, 64% of total VEGF in the vitreous was VEGFxxxb (this includes VEGF165b, VEGF121b, VEGF 145b, VEGF 183b, VEGF 189b) in non-diabetic patients, whereas only 12.5% of total VEGF was VEGFxxxb in diabetic patients (Perrin et al., 2005). That study showed the switch in splice variants from anti-angiogenic to pro-angiogenic in diabetic retinopathy and suggested that VEGFxxxb might be a potential therapeutic modality for diabetic retinopathy. Hua et al demonstrated that intravitreal administration of VEGF165b inhibited laser-induced choroidal neovascularization in the mouse eye (Hua et al., 2010). Another study using an oxygen-induced retinopathy model as an experimental retinal neovascularization model has shown more direct evidence of anti-angiogenic effect of VEGF165b (Konopatskaya et al., 2006). In that model, intraocularly administrated VEGF165b peptide significantly inhibited the area of retinal neovascularization from 23% of retina to 12% and, interestingly, increased normal vascular area from 62% to 74%. In our study, the developing vasculature in its formative state expressed more VEGF165 and the developed vasculature in stable or anti-angiogenic stage had prominent VEGF165b. This data is coincident with previous reports about decreased VEGF165b in angiogenic status in pathologic condition.
Both VEGF165 and VEGF165b were often associated with endothelial cells in developing blood vessels. In retina, there is substantial VEGF165 diffusely throughout inner retina, including in blood vessels at the older time points. It is not clear from immunohistochemistry, which cells produce it and whether it is bound or diffusable VEGF165. However, in the fetal vasculature of vitreous, VEGF165 and VEGF165b are clearly associated with endothelial cells as well as pericytes (Fig. 2-3) and there is only one non-vascular cell type in close proximity to some vessels, the hyalocyte or dendritic cell of vitreous. So it is possible that the VEGF165 and VEGF165b observed in this vasculature were autocrine in nature, made and used by endothelial cells of the fetal vasculature, or paracrine, produced by pericytes and targeting adjacent endothelial cells. There is precedent for autocrine production of VEGF165 by endothelial cells in vitro (Yamagishi et al., 1999; Bocci et al., 2001) and for pericytes inducing endothelial cell production in vessel fragments (Franco et al., 2011). However, there have been no localization studies of VEGF165b during development so autocrine versus paracrine production of this splice variant is still not known.
VEGF165 in our study was present in the basal portion of the RPE throughout fetal development. Conversely, VEGF165b was not detected in RPE at early ages but was prominent in the apical portion of RPE and their nuclei after the choriocapillaris had formed. Choriocapillaris homeostasis is dependent on VEGF secreted from the basal RPE that development and maintenance of fenestrations in the CC relies on the availability of RPE-derived VEGF (Marneros et al., 2005; Saint-Geniez et al., 2009). Our findings of VEGF165 in the basal portion of the RPE may function to stimulate fenestration formation during development. We have observed contiguous fenestrations in CC at 21WG (Baba et al., 2009). Meanwhile, VEGF165b immunoreactivity in the apical RPE might be important in preventing the choroidal vasculature from invading the subretinal space. Surprisingly, both forms of VEGF165 were seen in 21WG RPE in the same cells. It may suggest the polarity-dependent expression of VEGF splice variants in RPE cells, namely VEGF165b in apical and VEGF165 in basal RPE. However, how two splicing systems can function simultaneously in the same cell is not known. In adult human RPE cells, using the same APase technique used in the current study, we found VEGF165 most prominently in white blood cells in choroid and did not observe it in RPE cells (Bhutto et al., 2006), although Saint-Geniez and associates found VEGF165 mRNA in adult human RPE (Saint-Geniez et al., 2006). VEGF165b also was not associated with RPE in the adult human by APase immunohistochemistry, but was associated with choroidal blood vessels (unpublished data).
The localization of VEGF165b and not VEGF165 to nucleus is also unknown. The alternative splicing does not generate or expose a nuclear translocation sequence (unpublished data). VEGFR-1 is translocated to nucleus in some circumstances (Cai et al., 2006), but both splice variants bind to VEGFR-1 (Bates et al., 2003). It is also not known why the nuclear localization of VEGF165b is so prominent in progenitor cells in retina while it had nuclear localization in fully differentiated endothelial cells and pericytes in established blood vessels in vitreous. VEGF165b from pericytes may serve to stabilize these developed vessels. Recent studies have demonstrated the neuroprotectant potential of VEGF165b (Hua et al, submitted) and VEGF165 is a neuroprotectant as well (Saint-Geniez et al., 2008). Perhaps the presence of diffuse VEGF165 in the inner neuroblastic layer and nuclear VEGF165b in neuronal progenitors in that layer are necessary for survival of neurons during the remodeling stage in retinal development.
In conclusion, this study demonstrates for the first time the localization of VEGF165b spatially and temporally in developing vasculatures of the fetal human eye. VEGF165b and VEGF165 often had distinctly different localizations from each other in the tissues studied: fetal vasculature of vitreous, retina, and choroid. In retina, it appeared that VEGF165 was both extracellular (diffuse) and cell associated in both the confocal (Fig. 7) and APase analysis (Fig. 8-9), while VEGF165b seemed mostly cell associated. We focused on anti-angiogenic form of VEGF, VEGF165b, and found a distinct change in expression during vasculogenesis and hemo-vasculogenesis. VEGF165 was prominently expressed during vascular development and VEGF165b expression was most prominent in developed vasculature. This dramatic molecular shift in splicing of VEGFA from VEGF165 to VEGF165b during vascular development provides insights into a novel system for formation and maintenance of vascular systems by VEGF165b.
Materials and Methods
Age determination and preparation of human fetal tissue
Fetal human eyes from 7 to 21 WG were included in this study. Tissues were provided by Advanced Bioscience Resources Inc. (Alameda, CA) and Stem Express (Placerville, CA) after aspiration abortions in accordance with the guidelines set forth in the Declaration of Helsinki and with the approval by the Joint Commission for Clinical Research at the Johns Hopkins University School of Medicine. The age of each fetus was determined using last menstruation date and/or ultrasonography and fetal foot length as a reliable indicator of gestational age (Mhaskar et al., 1989). The eyes were then fixed within an hour of harvest in 2% paraformaldehyde in 0.1 M sodium phosphate buffer pH 7.2 with 5% sucrose at room temperature for 60 min. The eyes then were washed in 0.1M sodium phosphate buffer with 5% sucrose and shipped overnight at 4°C in the same buffer. For older cryopreserved eyes (≥14 WG), the anterior segments were removed and eye cups were washed in 0.1M sodium phosphate buffer with increasing concentration of sucrose: a 2:1, 1:1, and 1:2 mixture of 5% sucrose: 20% sucrose (Lutty et al., 1993b). The eyecups were kept at room temperature for 2 hrs in 0.1 M sodium phosphate buffer with 20% sucrose and then dissected into four blocks, each containing an arcade of retinal blood vessels in 16 WG and older eyes. For younger eyes (≤12 WG), whole eyes were cryopreserved and frozen by the same method. All tissue was infiltrated at room temperature for 30 min and then embedded in a solution consisting of a 2:1 mixture of 20% sucrose in 0.1 M phosphate buffer: OCT compound (TissueTek, Baxter Scientific Columbia, MD), frozen and stored at -80°C (Lutty et al., 1993a; Hasegawa et al., 2007). Eight μm thick cross sections were cut on Microm HM500M cryostat (Global Medical Instrumentation, Ramsey, MI) at -25°C.
Hematoxylin and eosin staining
After treating cryosections in absolute methanol for 5 min at -20°C and allowing them to air dry, sections were stained with Harris' hematoxylin for 20 seconds. After washing in distilled water, the sections were blued in lithium carbonate, rinsed in distilled water, and then stained in 0.5% alcoholic eosin (Polysciences, Warrington, PA). After dehydration to xylene, the sections were coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ).
Streptavidin alkaline phosphatase (APase) immunohistochemistry on cryopreserved tissue
APase immunohistochemistry was performed on sections of cryopreserved tissue using a nitroblue tetrazolium (NBT) development system that we described previously (Bhutto et al., 2004). In brief, 8-um-thick cryopreserved sections were permeabilized with absolute methanol and blocked with 2% normal goat serum in Tris-buffered saline (TBS; pH 7.4) with 1% BSA. After washing in TBS, the sections were also blocked with avidin-biotin complex (ABC) blocking kit (Vector Laboratories, Inc., Burlingame, CA). Tissues were then incubated overnight at 4°C with one of the primary antibodies listed in Table 1 at the stated titer. Mouse monoclonal IgG1 VEGF165b antibody (Abcam clone MRVL56/1) has been reported previously using immunohistochemistry (IHC) (Pritchard-Jones et al., 2007). Woolard et al (2004) previously demonstrated the specificity of this antibody for VEGF165b in cell lysates using Western blots and more recently Zhao et al (2011) also used Western blots of mouse retinal tissue to show that it recognizes VEGF165b and not VEGF165. After washing in TBS, sections were incubated for 30 minutes at room temperature with the biotinylated secondary antibodies diluted 1:500 (KPL, Gaithersburg, MD). Finally, sections were incubated with streptavidin-alkaline phosphatase (APase)(KPL) and APase activity developed using a BCIP-NBT kit (Vector Laboratories) with the addition of 1 mM (-) Tetramisole HCL (Sigma), yielding a blue reaction product at sites of antibody binding. Melanin in RPE was bleached as previously reported (Bhutto et al., 2004) and coverslips were applied with Kaiser's glycerogel.
Table 1. A summary of antibodies for immunohistochemistry.
| Protein | Function | Marker for | Antibody, manufacturer, titer (APase/Immunofluorescence) | Reference |
|---|---|---|---|---|
| VEGF165 | Pro angiogenic factor | Rabbit anti VEGF Ab-1, Thermo, Waltham, MA, 1:800/ 1:500 | (Bates et al., 2002) | |
| VEGF165b | Anti angiogenic factor | Mouse anti VEGF165b, Abcam, Cambridge, MA, 1:500/ 1:400 | (Bates et al., 2002) | |
| CD31 | Adhesion molecule | Hematopoietic cells and endothelial cells | Mouse anti CD31, Dako, Carpenteria, CA, 1:100/ 1:100 | (Sheibani and Frazier, 1998) |
| vWf | Glycoprotein in plasma, endothelium, megakaryocytes | Vascular endothelial cells or megakaryocytes | Rabbit anti vWf, Dako, Carpenteria, CA, -/ 1:2500 | (Sadler, 1998) |
| aSMA | Actin isoform | Smooth muscle cells and pericytes | Rabbit anti alpha SMA, Abcam, Cambridge, MA, -/ 1:400 | (Herman, 1993; Tomasek et al., 2006) |
| CD39 | Ectoenzyme controlling the extracellular ADP level | Angioblast and endothelial cells | Mouse anti CD39, Ancell, Bayport, MN, -/ 1:400 | (McLeod et al., 2006) |
| CXCR4 | Alpha chemokine receptor for SDF-1 | Angioblasts, embryonic stem cells, hematopoietic progenitors | Rabbit anti CXCR4, Novus, Littleton, CO, -/ 1:250 | (Kucia et al., 2004; McLeod et al., 2006) |
| Brn-3B | Transcription factor | Sensory neural precursors | Goat anti Brn-3B, Santa Cruz, Santa Cruz, CA, -/ 1:250 | (Bain et al., 1995; Fedtsova and Turner, 1995) |
Double label Immunohistochemistry on cryopreserved tissue
Sections were treated with absolute methanol to increase permeability for 5 minutes at -20°C. After washing with TBS, tissues were blocked with 2% goat or rabbit serum for 20 minutes at room temperature. Sections were then incubated with two of primary antibodies (Table 1) for 3 hours at room temperature. After washing, the sections were labeled with one of these combinations, goat anti mouse Cy3 (Jackson Immuno Research, West Grove, PA) 1:500 and goat anti rabbit Cy5 (JIR) 1:500, or rabbit anti goat Cy3 (JIR) and rabbit anti mouse Cy5 (JIR) for 30 minutes. Sections were coverslipped with Dakocytomation (Dako, Carpinteria, CA) and observed using Zeiss confocal microscope meta510 LSM (Carl Zeiss Inc.,Thornwood, NY).
Laser capture of fetal vasculature, RNA preparation and qRT-PCR
Fetal human eyes (three 8WG; three 11WG) were quickly cryopreserved in an increasing gradient of sucrose in 0.1M phosphate buffered saline (prepared in DEPC-treated water) then infiltrated and frozen in a 1:2 mixture of 20% sucrose to OCT. Eight μm sections were cut until the fetal vasculature was visible. At this point, sections were collected on 2.0 μm PEN-Membrane slides (Leica 11505189) and immediately frozen. Sections were brought to room temperature in a closed chamber containing desiccant, rehydrated in ethanol gradients, stained with cresyl violet, and dehydrated in ethanol gradients (all solutions were prepared in DEPC-treated water). Air-dried slides were placed on the Leica LMD6000 Laser Capture Micro-dissection microscope. The fetal vasculature was captured from each section and collected in tubes containing RNAlater. The fetal vasculature captured from a minimum of 25 sections was pooled for each eye. RNA was isolated according to the manufacturer's instructions using the RNeasy Plus micro kit (Qiagen, Valencia, CA). Total RNA was quantified with a Nanodrop ND-1000 spectrophotometer. So that amplification was not necessary, RNA from the three eyes at each age was pooled to perform qRT-PCR.
PCR amplification was performed utilizing Brilliant II SYBR(R) Green QRT-PCR Master Mix Kit (Stratagene) according to the manufacturer's instructions. Beta-actin was run as the loading control. A two-step cycling protocol included: 30 min at 50°C, 10 min at 95°C, followed by 40 cycles of denaturation for 30 seconds at 95°C combined with annealing/extension at 55°C for 1 min. Primers were obtained from Integrated DNA Technologies (Coralville, Iowa). VEGF165b was amplified with primers VEGF165βF: CAAGATCCGCAGACGTGTAA and VEGF165bR: TCCTGGTGAGAGATCTGCAA. VEGF165 was amplified with primers VEGF165F: GAGCGGAGAAAGCATTTGTT and VEGF165R: CTCGGCTTGTCACATCTGC. Beta-actin was amplified with primers Actinb250F: CATGTACGTTGCTATCCAGGC and Actinβ250R: CTCCTTAATGTCACGCACGAT. All data were analyzed in M×300P real time PCR system using the data assist software (Stratagene) and graphs were plotted using Microsoft Excel. The fold change was calculated using the ΔCt method after normalizing to β-actin as we have reported previously (Ma et al., 2011; Zigler et al., 2011).
Acknowledgments
This work was supported by NIH RO1-EY016151 (G.L.), NIH RO1-EY-09357 (G.L.), EY-01765 (Wilmer), and the Altsheler-Durell Foundation. Takayuki Baba was a JSPS Postdoctoral Fellow for Research Abroad. Gerard Lutty received an RPB Senior Scientific Investigator Award in 2008.
Grant support: NIH grants EY016151 (GL), EY09357 (GL), EY01765 (Wilmer); the Altsheler-Durell Foundation; and an unrestricted gift from RPB (Wilmer). Takayuki Baba was a JSPS Postdoctoral Fellow for Research Abroad. G. Lutty received an RPB senior scientific investigator award in 2008.
References
- Baba T, Grebe R, Hasegawa T, Bhutto I, Merges C, McLeod DS, Lutty GA. Maturation of the fetal human choriocapillaris. Invest Ophthalmol Vis Sci. 2009;50:3503–3511. doi: 10.1167/iovs.08-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- Bhutto IA, Kim SY, McLeod DS, Merges C, Fukai N, Olsen BR, Lutty GA. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1544–1552. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- Bhutto IA, McLeod DS, Hasegawa T, Kim SY, Merges C, Tong P, Lutty GA. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci G, Fasciani A, Danesi R, Viacava P, Genazzani AR, Del Tacca M. In-vitro evidence of autocrine secretion of vascular endothelial growth factor by endothelial cells from human placental blood vessels. Mol Hum Reprod. 2001;7:771–777. doi: 10.1093/molehr/7.8.771. [DOI] [PubMed] [Google Scholar]
- Cai J, Jiang WG, Ahmed A, Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res. 2006;71:20–31. doi: 10.1016/j.mvr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- Cui TG, Foster RR, Saleem M, Mathieson PW, Gillatt DA, Bates DO, Harper SJ. Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am J Physiol Renal Physiol. 2004;286:F767–773. doi: 10.1152/ajprenal.00337.2003. [DOI] [PubMed] [Google Scholar]
- Fedtsova NG, Turner EE. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, McLeod DS, Bhutto IA, Prow T, Merges CA, Grebe R, Lutty GA. The embryonic human choriocapillaris develops by hemo-vasculogenesis. Dev Dyn. 2007;236:2089–2100. doi: 10.1002/dvdy.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular precursors in developing human retina. Invest Ophthalmol Vis Sci. 2008;49:2178–2192. doi: 10.1167/iovs.07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman IM. Actin isoforms. Curr Opin Cell Biol. 1993;5:48–55. doi: 10.1016/s0955-0674(05)80007-9. [DOI] [PubMed] [Google Scholar]
- Hua J, Spee C, Kase S, Rennel ES, Magnussen AL, Qiu Y, Varey A, Dhayade S, Churchill AJ, Harper SJ, Bates DO, Hinton DR. Recombinant human VEGF165b inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:4282–4288. doi: 10.1167/iovs.09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Ladomery MR, Harper SJ, Bates DO. Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm. Cancer Lett. 2007;249:133–142. doi: 10.1016/j.canlet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Hasegawa T, Baba T, Grebe R, Bhutto I, McLeod DS. Development of the human choriocapillaris. Eye (Lond) 2010 doi: 10.1038/eye.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-b in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993a;34:477–487. [PubMed] [Google Scholar]
- Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993b;34:477–487. [PubMed] [Google Scholar]
- Ma B, Sen T, Asnaghi L, Valapala M, Yang F, Hose S, McLeod DS, Lu Y, Eberhart C, Zigler J, Sinha D. βA3/A1-crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/Akt/mTOR and ERK survival pathways. Cell Death Dis. 2011 Oct 13;2:e217. doi: 10.1038/cddis.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneros AG, Fan J, Yokoyama Y, Gerber HP, Ferrara N, Crouch RK, Olsen BR. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Hasegawa T, Prow T, Merges C, Lutty G. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn. 2006;235:3336–3347. doi: 10.1002/dvdy.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaskar R, Agarwal N, Takkar D, Buckshee K, Anandalakshmi, Deorari A. Fetal foot length--a new parameter for assessment of gestational age. Int J Gynaecol Obstet. 1989;29:35–38. doi: 10.1016/0020-7292(89)90126-4. [DOI] [PubMed] [Google Scholar]
- Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, Bates DO. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlidge A, D'Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, Harper SJ, Bates DO. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Hoareau-Aveilla C, Oltean S, Harper SJ, Bates DO. The anti-angiogenic isoforms of VEGF in health and disease. Biochem Soc Trans. 2009;37:1207–1213. doi: 10.1042/BST0371207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47:3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Frazier WA. Down-regulation of platelet endothelial cell adhesion molecule-1 results in thrombospondin-1 expression and concerted regulation of endothelial cell phenotype. Mol Biol Cell. 1998;9:701–713. doi: 10.1091/mbc.9.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318:666–675. doi: 10.1124/jpet.106.104158. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Haaksma CJ, Schwartz RJ, Vuong DT, Zhang SX, Ash JD, Ma JX, Al-Ubaidi MR. Deletion of smooth muscle alpha-actin alters blood-retina barrier permeability and retinal function. Invest Ophthalmol Vis Sci. 2006;47:2693–2700. doi: 10.1167/iovs.05-1297. [DOI] [PubMed] [Google Scholar]
- Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, Harper SJ, Bates DO. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D'Amore PA. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009;4:e5149. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, Shields JD, Whittles CE, Mushens RE, Gillatt DA, Ziche M, Harper SJ, Bates DO. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Kawakami T, Fujimori H, Yonekura H, Tanaka N, Yamamoto Y, Urayama H, Watanabe Y, Yamamoto H. Insulin stimulates the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. Microvasc Res. 1999;57:329–339. doi: 10.1006/mvre.1999.2145. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhao M, Shi X, Liang J, Miao Y, Xie W, Zhang Y, Li X. Expression of pro- and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Exp Eye Res. 2011;93:921–6. doi: 10.1016/j.exer.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Zhu M, Madigan MC, van Driel D, Maslim J, Billson FA, Provis JM, Penfold PL. The human hyaloid system: cell death and vascular regression. Exp Eye Res. 2000;70:767–776. doi: 10.1006/exer.2000.0844. [DOI] [PubMed] [Google Scholar]
- Zigler JS, Zhang C, Grebe R, Sehrawat G, Hackler L, Adhya S, Hose S, McLeod DS, Bhutto I, Barbour W, Parthasarathy G, Zack DJ, Sergeev Y, Lutty GA, Handa JT, Sinha D. Mutation in the βA3/A1-crystallin gene impairs phagosome degradation in the retinal pigment pithelium of rat. J Celln Sci. 2011;124:523–3. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]


