Abstract
This Editorial highlights a study by Gibson et al. (2015) published in this issue of JNeurochem, in which the authors reveal a novel role for the alpha-ketoglutarate dehydrogenase complex (KGDHC) in post-translational modification of proteins. KGDHC may catalyze post-translational modification of itself as well as several other proteins by succinylation of lysine residues. The authors’ report of an enzyme responsible for succinylation of key mitochondrial enzymes represents a big step towards our understanding of the complex functional metabolome.
Post-translational modification of proteins by attachment of groups to residues such as lysine is an emerging area of metabolism research (Choudhary et al. 2014). Lysine side chains are frequently modified by addition of different chain length acyl moieties leading to acetylation, butyrylation, propionylation, succinylation, malonylation, myristoylation, glutarylation or crotonylation, which can change the properties of proteins (Choudhary et al. 2014). Many mitochondrial proteins are modified by acetylation and/or succinylation (He et al. 2012; Park et al. 2013; Choudhary et al. 2014). Much attention to date has been focused on modification by acetylation, with the vast majority of enzymes in glycolysis and the TCA cycle subject to this modification with varying degrees of effect on the resultant enzyme activity. Acetylation has emerged as a distinct control mechanism, with some enzymes, such as acetyl Co-A synthetase (E.C. 6.2.1.1) completely deactivated by attachment of an acetyl group (Hallows et al. 2006), other enzymes, such as citrate lyase (E.C. 2.3.3.8) stabilized by acetylation (Lin et al. 2013) and some apparently unaffected by attachment of multiple acetyl groups (Kim et al. 2006). In eukaryotes a handful of possible candidate enzymes responsible for catalyzing protein acetylation have been identified (Arnesen et al. 2005; Iain et al. 2012) but none have been positively associated with direct acetylation of key metabolic enzymes. Indeed, the process has been postulated to be largely autocatalytic (Ghanta et al. 2013) or possibly chemically catalyzed via acetylphosphate (Choudhary et al. 2014). Fewer studies have focused on the processes mediating succinylation of lysine residues (Park et al. 2013; Choudhary et al. 2014), although many metabolic enzymes including key enzymes in glycolysis, the TCA cycle and fatty acid metabolism are modified by succinylation (Papanicolaou et al. 2014).
The lack of identified candidates for protein acetylation makes the manuscript by Gibson and coworkers (this issue) all the more intriguing in that they have identified an enzyme responsible for succinylation of key mitochondrial enzymes. The authors demonstrate that the α-ketoglutarate dehydrogenase complex (KGDHC) can catalyze the post-translational modification of not only itself, but several other proteins by succinylation of lysine residues. These modifications have significant functional effects and could be argued to constitute a functional metabolome controlled by the succinylation actions of KGDHC.
The authors demonstrate that while succinyl-CoA itself increases succinylation, the succinylation of KGDHC, fumarase and isocitrate dehydrogenase is increased by addition of KGDHC, while the activity of the pyruvate dehydrogenase complex (PDHC) is also altered via changes in acetylation levels (Gibson et al. 2015). The key finding is that while the activity of isocitrate dehydrogenase is decreased, the activities of KGDHC, fumarase and PDHC are increased by succinylation (Gibson et al. 2015). The other enzymes in the same chain as KGDHC and fumarase that are post-translationally regulated include malate dehydrogenase; this enzyme is also activated by acetylation (Guan and Xiong 2010) and succinate dehydrogenase, which has been reported to be activated by succinylation (Park et al. 2013).
Gibson et al. (Gibson et al. 2015) evaluated non-enzymatic succinylation as well as the role of enzymatic succinylation using the KGDHC inhibitor CESP (the carboxy ethyl ester of succinylphosphonate). They demonstrate strong succinylation (and acetylation) of both mitochondrial and cytosolic proteins in neurons, which was decreased when KGCHD activity was inhibited. An important feature of this manuscript is the finding that succinylation increased the activity of pyruvate dehydrogenase, KGDH and fumarase by ~25% to two-fold depending on the conditions used, and significantly decreased the activity of isocitrate dehydrogenase (ICDH). The E2k subunit of KGDHC is a trans-succinylase enzyme which is required for KGDH activity (Gibson et al. 2015). Loss of this enzyme decreases succinylation and the presence of α-ketoglutarate (KG) increases succinylation supporting the concept that the KGDHC is serving as the enzymatic catalyst for succinylation as well as the donor of succinyl groups (Gibson et al. 2015).
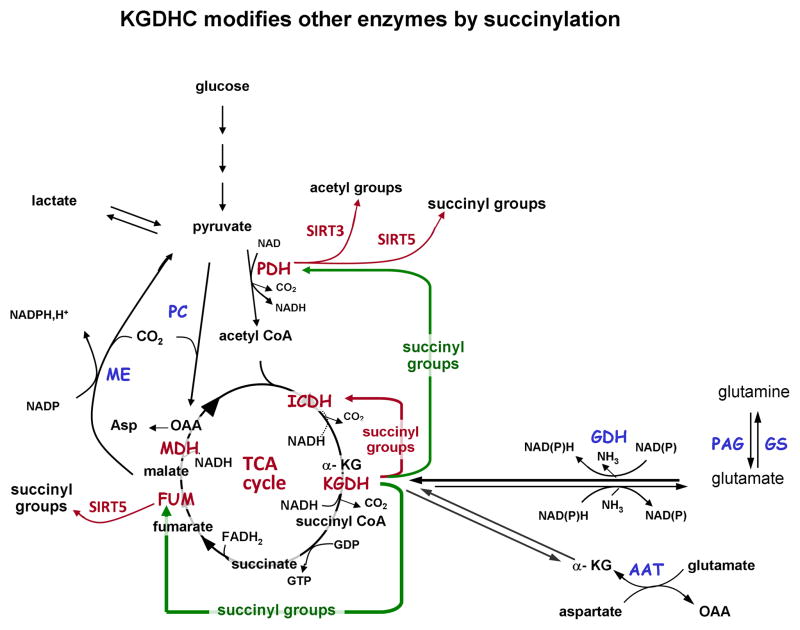
Taken together, this suggests that the activity of a section of the TCA cycle, namely from KGDHC to oxaloacetate, can be increased by succinylation (and acetylation) while the section from isocitrate dehydrogenase to KGDH is decreased, and that these changes are mediated, at least in part, by KGDHC. (Figure 1).
Figure 1.
Schematic illustration of α-ketoglutarate dehydrogenase complex (KGDHC) mediated succinylation of enzymes described by Gibson et al. (Gibson et al. 2015) (this issue). Green arrows point to enzymes fumarase (FUM) and pyruvate dehydrogenase complex (PDH) that have increased activity after succinylation. Gibson et al. (Gibson et al. 2015) also found increased acetylation of PDHC in the presence of KGDHC. The red arrow points to isocitrate dehydrogenase which has decreased activity after succinylation. SIRT5 activity leads to de-succinylation of proteins, and SIRT3 activity leads to deacetylation of proteins. Abbreviations: TCA, tricarboxylic acid; KG, α-ketoglutarate; KGDHC, a-ketoglutarate dehydrogenase complex; FUM, fumarase; ME, MDH, malate dehydrogenase; malic enzyme; GDH, glutamate dehydrogenase; AAT, aspartate aminotransferase; GS, glutamine synthetase; PAG, phosphate activated glutaminase; SIRT3, silent information regulator 3; SIRT5, silent information regulator 5.
Neurons have high glycolysis and TCA cycle activity but they also have high capacity to oxidize glutamine. Sonnewald and coworkers (Waagepetersen et al. 1999) demonstrated that glutamine is extensively metabolized through the neuronal TCA cycle before being converted to glutamate and subsequently GABA in cortical neurons. Adjustment of the relative activity of the TCA cycle by KGDHC-mediated succinylation and activation of KGDHC and fumarase with concomitant loss of activity in isocitrate dehydrogenase would allow accommodation of glutamine oxidation via a partial TCA cycle. Enzyme modification by succinylation is relatively fast, (Gibson et al. 2015), and thus would qualify as a possible mechanism by which TCA cycle oxidative capacity might be regulated in response to cellular demands.
It will be of interest to determine if the KGDHC mediated succinylation of proteins is the same in both glutamatergic and GABAergic neurons. Olstad et al. (Olstad et al. 2007) reported higher metabolism of exogenous glutamate than glutamine in glutamatergic cerebellar granule neurons than in cortical neurons. Considerable glutamate is metabolized for energy via the TCA cycle in astrocytes (McKenna 2012, 2013). It will also be of considerable interest to determine if the KGDHC mediated succinylation and modulation of TCA cycle enzymes is comparable in astrocytes to the effect reported by Gibson et al. (Gibson et al. 2015) in neurons. Succinylation and acetylation of PDHC in astrocytes would increase activity of this enzyme, which could potentially alter the relative flux of pyruvate through pyruvate carboxylase. Since covalent modification of proteins by succinylation would remove succinate from the TCA cycle, depending on the amount of succinylation taking place in neurons (and astrocytes) the process may necessitate increased anaplerosis via pyruvate carboxylase to maintain the level of TCA cycle intermediates.
A caveat to this observation would be to determine the relative effect of succinylation on these enzymes and whether the reported changes in activity result in meaningful changes in flux through the TCA cycle. Succinylation of the KGDHC protein led to an increase in its activity (Gibson et al. 2015) but compared to the theoretical capacity of this enzyme, the reported effect is quite modest. Similarly an increase in fumarase activity of 24.7% is unlikely to have significant effects on TCA cycle flux, given that this enzyme does not contribute greatly to overall metabolic control (Fell 1997). KGDHC has also been reported to be subject to inactivation by glutathionylation (Cooper et al. 2011) which would have a larger impact on metabolism than the modest increase in activity due to succinylation. However, since adding one or more large negatively charged succinyl groups can change the physicochemical properties of proteins, succinylation may significantly alter the ability of enzymes to associate into transient multienzyme complexes that can substantially influence metabolism (McKenna et al. 2000; McKenna et al. 2006; Genda et al. 2011; Bauer et al. 2012; Whitelaw and Robinson 2013; Jackson et al. 2014).
Regulation of succinylation is not yet well understood. It is established that the silent information regulator SIRT 5 has robust de-succinylation activity as well as weak de-acetylation activity (Newman et al. 2012). SIRT5, a Class III deacetylase, is primarily located in mitochondria but considerable cytosolic activity has also been reported (Park et al. 2013). SIRT5 knock out mice have greatly increased levels of protein succinylation along with hyperammonemia due to succinylation and inhibition of carbamoyl phosphate synthase 1 (Nakagawa et al. 2009) but do not display any overt metabolic abnormalities (Yu et al. 2013).
There remains much to learn about succinylation, and little is known about the extent and role of succinylation in brain. Recent studies report that lysine succinylation of lysine residues is a frequent post-translational modification that affecting ~ 26 % of mitochondrial proteins in mammalian cells (He et al. 2012; Park et al. 2013). Careful progress has been made in assessing the impact of acetylation through site-specific study of the effects of each acetyl group. Thus it will be important to identify the overall effects of the succinylation reported here and which succinyl groups are attached by KGDHC. It will also be of interest to determine the effects on other enzymes reported to be modified by succinylation including citrate synthase, malate dehydrogenase, glutamate dehydrogenase 1, aspartate aminotransferase and ATP synthase (He et al. 2012; Park et al. 2013; Papanicolaou et al. 2014). These enzymes have key roles in brain but little is known about modification of their activity by succinylation in neurons and astrocytes. The effect of succinylation on the association and dissociation of multienzyme complexes in another area that needs to be explored.
The observation that the trans-succinylase (E2k) subunit of KGDHC must be active for overall activity of KGDHC suggests that any damage to the E2k subunit could have far reaching effects on metabolism. Studies from Gibson’s group have established that Alzheimer’s patients have decreased KGDH activity (Gibson et al. 2005). Future studies will likely determine if polymorphisms and/or mutations in the E2k subunit of KGDH are more abundant in patients with Alzheimer’s.
Acknowledgments
MCM is an Editor for the Journal of Neurochemistry. Funded in part by NIH grant HD 016596 (MCM). Funded in part by National Health and Medical Research Council (NHMRC) fellowship 630516 (CR).
Abbreviations
- TCA
tricarboxylic acid
- KG
α-ketoglutarate
- KGDHC
a-ketoglutarate dehydrogenase complex
- FUM
fumarase
- ME, MDH
malate dehydrogenase; malic enzyme
- GDH
glutamate dehydrogenase
- AAT
aspartate aminotransferase
- GS
glutamine synthetase
- PAG
phosphate activated glutaminase
- SIRT3
silent information regulator 3
- SIRT5
silent information regulator 5
- CESP
the carboxy ethyl ester of succinylphosphonate
References
- Arnesen T, Anderson D, Baldersheim C, Lanotte M, Varhaug JE, Lillehaug JR. Identification and characterization of the human ARD1-NATH protein acetyltransferase complex. Biochem J. 2005;386:433–443. doi: 10.1042/BJ20041071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Jackson JG, Genda EN, Montoya MM, Yudkoff M, Robinson MB. The glutamate transporter, GLAST, participates in a macromolecular complex that supports glutamate metabolism. Neurochem Int. 2012;61:566–574. doi: 10.1016/j.neuint.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Pinto JT, Callery PS. Reversible and irreversible protein glutathionylation: biological and clinical aspects. Expert Opin Drug Metab Toxicol. 2011;7:891–910. doi: 10.1517/17425255.2011.577738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D. Understanding the control of metabolism. Portland Press; London: 1997. pp. 15–16. [Google Scholar]
- Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci. 2011;31:18275–18288. doi: 10.1523/JNEUROSCI.3305-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta S, Grossmann RE, Brenner C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: chemical and metabolic logic of acetyl-lysine modifications. Crit Rev Biochem Mol Biol. 2013;48:561–574. doi: 10.3109/10409238.2013.838204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Blass JP, Beal MF, Bunik V. The alpha-ketoglutarate-dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Mol Neurobiol. 2005;31:43–63. doi: 10.1385/MN:31:1-3:043. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Xu H, Chen HL, Chen W, Denton TT, Zhang S. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem. 2015 doi: 10.1111/jnc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Xiong Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci. 2010;36:108–116. doi: 10.1016/j.tibs.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Newman JC, Wang MZ, Ho L, Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab. 2012;23:467–476. doi: 10.1016/j.tem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Iain S, Bradley RW, Jian HL, Michael NS. Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem J. 2012;443:655–661. doi: 10.1042/BJ20120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JG, O’Donnell JC, Takano H, Coulter DA, Robinson MB. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci. 2014;34:1613–1624. doi: 10.1523/JNEUROSCI.3510-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Lin RT, Tao R, Gao X, Li TT, Zhou X, Guan KL, Xiong Y, Lei QY. Acetylation stabilizes ATP-citrate lyase to promote lipid biosynthesis and tumor growth. Mol Cell. 2013;51:506–518. doi: 10.1016/j.molcel.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem Res. 2012;37:2613–2626. doi: 10.1007/s11064-012-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC. Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 2013;4:191. doi: 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Stevenson JH, Huang X, Hopkins IB. Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int. 2000;37:229–241. doi: 10.1016/s0197-0186(00)00042-5. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Hopkins IB, Lindauer SL, Bamford P. Aspartate aminotransferase in synaptic and nonsynaptic mitochondria: differential effect of compounds that influence transient hetero-enzyme complex (metabolon) formation. Neurochem Int. 2006;48:629–636. doi: 10.1016/j.neuint.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, He W, Verdin E. Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease. J Biol Chem. 2012;287:42436–42443. doi: 10.1074/jbc.R112.404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olstad E, Qu H, Sonnewald U. Glutamate is preferred over glutamine for intermediary metabolism in cultured cerebellar neurons. J Cereb Blood Flow Metab. 2007;27:811–820. doi: 10.1038/sj.jcbfm.9600400. [DOI] [PubMed] [Google Scholar]
- Papanicolaou KN, O’Rourke B, Foster DB. Metabolism leaves its mark on the powerhouse: recent progress in post-translational modifications of lysine in mitochondria. Front Physiol. 2014;5:301. doi: 10.3389/fphys.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, Lombard DB, Zhao Y. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. Synthesis of vesicular GABA from glutamine involves TCA cycle metabolism in neocortical neurons. J Neurosci Res. 1999;57:342–349. [PubMed] [Google Scholar]
- Whitelaw BS, Robinson MB. Inhibitors of glutamate dehydrogenase block sodium-dependent glutamate uptake in rat brain membranes. Front Endocrinol (Lausanne) 2013;4:123. doi: 10.3389/fendo.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Sadhukhan S, Noriega LG, Moullan N, He B, Weiss RS, Lin H, Schoonjans K, Auwerx J. Metabolic characterization of a Sirt5 deficient mouse model. Sci Rep. 2013;3:2806. doi: 10.1038/srep02806. [DOI] [PMC free article] [PubMed] [Google Scholar]