Abstract
Studies using vitamin D-binding protein (DBP) concentrations to estimate free and bioavailable vitamin D have increased dramatically in recent years. Combinations of two single-nucleotide polymorphisms produce three major DBP isoforms (Gc1f, Gc1s and Gc2). A recent study showed that DBP concentrations quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) did not differ by race, while a widely used monoclonal ELISA quantified DBP differentially by isoform, yielding significantly lower DBP concentrations in black versus white individuals. We compared measurements of serum DBP using a monoclonal ELISA, a polyclonal ELISA, and LC-MS/MS in 125 participants in the Chronic Renal Insufficiency Cohort. Serum free and bioavailable 25OHD were calculated based on DBP concentrations from these three assays in homozygous participants, and race differences were compared. We confirmed that the monoclonal ELISA quantifies DBP differentially by isoform and demonstrated that the polyclonal ELISA is not subject to this bias. While ≤9% of the variability in DBP concentrations quantified using either LC-MS/MS or the polyclonal ELISA was explained by genotype, 85% of the variability in the monoclonal ELISA-based measures was explained by genotypes. DBP concentrations measured by the monoclonal ELISA were disproportionately lower than LC-MS/MS-based results for Gc1f homozygotes [median difference −67%; interquartile range (IQR) −71%, −64%], 95% of whom were black. In contrast, the polyclonal ELISA yielded consistently and similarly higher measurements of DBP than LC-MS/MS, irrespective of genotype, with a median percent difference of +50% [IQR +33%, +65%]. Contrary to findings using the monoclonal ELISA, DBP concentrations did not differ by race, and free and bioavailable 25OHD were significantly lower in black versus white participants based on both the polyclonal ELISA and LC-MS/MS, consistent with their lower total 25OHD. Future studies of DBP and free or bioavailable vitamin D metabolites should employ DBP assays that are not biased by DBP genotype.
Keywords: vitamin D-binding protein, Gc, genotype, bioavailable vitamin D, liquid chromatography-tandem mass spectrometry
2 INTRODUCTION
Vitamin D-binding protein (DBP), originally named “group-specific component” (Gc-globulin),1 is a multifunctional 58 kDa circulating glycoprotein that transports vitamin D metabolites and also contributes to actin scavenging, fatty acid transport, and chemotaxis.2–5 The vast majority of vitamin D metabolites circulate bound to DBP (85–90%) or albumin (10–15%), with <1% circulating in the free form. The binding affinity of DBP for vitamin D metabolites is more than 1000-fold stronger than that of albumin, and therefore, the albumin-bound and free fractions together are considered bioavailable.6–10
Preclinical studies established a role for DBP in regulating the availability of 25OHD and 1,25(OH)2D3 to certain target tissues.11,12 Similarly, numerous investigators hypothesized that free and bioavailable 25OHD concentrations provide superior indices of vitamin D status, compared with the total circulating 25OHD concentration. Assays to measure the free and bioavailable vitamin D are not widely available; however, the concentrations can be estimated using equations that incorporate the 25OHD, DBP and albumin concentrations and the corresponding binding affinity coefficients.
DBP is highly polymorphic, and combinations of two single-nucleotide polymorphisms (SNPs), rs4588 and rs7041, produce three major polymorphic forms (Gc1f, Gc1s and Gc2). The rs7041 polymorphism results in replacement of aspartate with glutamate at amino acid position 416 in Gc1s, while the rs4588 polymorphism results in a lysine substitution for threonine at amino acid position 420 in Gc2. Their glycosylation patterns are also distinct (galactose and sialic acid in Gc1s and Gc1f and galactose only in Gc2).13 The resultant six allelic combinations (1f/1f, 1f/2, 1s/1f, 1s/1s, 1s/2, and 2/2) differ in their affinities for vitamin D metabolites, circulating concentrations, and geographical and racial distribution.14–17
Over the last two years, there has been a dramatic increase in clinical studies using DBP levels to estimate free and bioavailable vitamin D. The majority of studies used a monoclonal antibody enzyme linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN) to measure DBP.18–33 However, the observations that DBP concentrations measured using the R&D Systems assay were markedly lower in black compared to white participants, and that DBP genotype explained 79% of the variation in DBP concentration,34 raised concerns that the assay quantified DBP concentration differentially by DBP isoform.35–37 We recently reported that DBP levels did not vary with race when measured by a polyclonal assay;38 however, that study was limited by lack of a gold standard measure of DBP and lack of genotype data. Henderson, et al recently developed and validated a liquid chromatography-tandem mass spectrometry (LC-MS/MS) DBP assay39 (the manuscript is in press in Clinical Chemistry, and provided in the Appendix). DBP concentrations obtained by LC-MSMS did not differ by race, while concentrations measured using the R&D monoclonal assay demonstrated isoform-specific differences and were significantly lower in black compared with white participants. The study did not include estimates of free or bioavailable vitamin D.
This study comprises a direct comparison of serum DBP measurement by three assays: two widely used commercially available immunoassays, a monoclonal and polyclonal ELISA, and by LC-MS/MS. The objective is to extend the work of Henderson, et al in two important ways. First, this study will assess the accuracy of a polyclonal assay compared with LC-MS/MS, within each genotype. Second, this study will compare race-specific estimates of free and bioavailable 25OHD concentrations based on DBP measures from these three assays.
3 METHODS
We studied a convenience sample of 125 participants in the Chronic Renal Insufficiency Cohort (CRIC) study, examined at their third annual study visit. Each participant had data on their DBP genotype, and sufficient specimen volume to perform all three assays on a single aliquot. CRIC is an NIDDK-sponsored prospective, multicenter observational study of risk factors for cardiovascular disease, chronic kidney disease (CKD) progression, and mortality in adults with CKD.40 A total of 3,939 adults 21–74 years of age with mild-moderate CKD [estimated glomerular filtration rate (eGFR) of 20–70 ml/min/1.73m2] were enrolled from May 2003 through June 2008. The CRIC protocol was approved by the Institutional Review Boards of all participating sites; written informed consent was obtained from all participants.
Serum DBP was measured in duplicate by three assays: 1) Trypsin digestion and LC-MS/MS (Waters Acquity-Xevo TQ-MS) targeting peptides VLEPTLK and ELPEHTVK. The lower limit of quantification (<20% CV) was 71 ug/ml, and this assay was linear from 62 to 434 ug/ml. The total imprecision of the assay, including contributions due to within-day and between-day variability, was determined previously by 5 × 5 analysis to be 7.3%–9.0% CV at 234–266 ug/ml. Recovery was 103–104%.39 2) A monoclonal antibody ELISA (R&D Systems, Minneapolis, MN). As per the manufacturer’s data, the limit of quantification was 0.65 ug/ml. The recovery, intra-assay CV and inter-assay CV were independently verified as 85.2–99.4%, 1.88% and 6.74%, respectively. 3) A polyclonal ELISA (ALPCO Diagnostics, Salem, NH). As per the manufacturer’s data, the limit of quantification was 1.23 ug/ml, and recovery was 99–100%. The intra-assay variation of this ELISA was independently verified as 1.90%. There were no values below the limit of quantification for any assay.
As detailed previously,41 genotyping in CRIC was performed using the HumanCVD BeadChip V2 IBC ITMAT/Broad/CARE (IBC) Array (Illumina, Inc.), a gene-centric SNP array that includes ~50,000 SNPs in ~2,100 candidate genes, at the Children’s Hospital of Philadelphia Center for Applied Genomics. Samples from the CRIC Study were excluded if: sample call rate was <0.97; there was reduced or excess heterozygosity (Inbreeding | F | <0.2); or there was evidence of cryptic relatedness (PI_HAT identity-by-descent <0.2). SNPs were excluded within each race separately if the call rate was <90%, the minor allele frequency was <1%, or if the SNP deviated from Hardy-Weinberg equilibrium (p <0.0001).
Serum total 25OHD (25OHD2 + 25OHD3) was measured by liquid-liquid extraction and LC-MS/MS on a Quattro Micro mass spectrometer (Waters, Milford, USA) (inter-assay CV of 7.9% at 51.9 ng/ml and 10.1% at 10.7 ng/ml). Serum free and bioavailable 25OHD were calculated using total 25OHD, DBP, and albumin concentrations.42 These equations were adapted from corresponding equations for free and bioavailable testosterone43 by replacing testosterone, sex hormone-binding globulin, and albumin and their respective binding constants with those of 25OHD, DBP, and albumin. The equations used to calculate free and bioavailable 25OHD are described in detail in the Supplementary Material of that report42 and summarized below:
Free 25OHD = [−b + √(b2 − 4ac)] / 2a, where
a = Kdbp*Kalb*albumin + Kdbp
b = (Kdbp*DBP) − (Kdbp*25OHD) + (Kalb*albumin) +1
c = −[25OHD]
Kdbp = affinity constant between 25OHD and DBP
Kalb = affinity constant between 25OHD and albumin
Bioavailable 25OHD = (Kalb*albumin + 1)*[free 25OHD]
The calculation of free and bioavailable 25OHD was limited to homozygous participants, so that isoform-specific affinity constants could be applied. The isoform-specific affinity constants of DBP for 25OHD (Kdbp) used were 1.12 × 109 M−1, 0.6 × 109 M−1, and 0.36 × 109 M−1 for Gc1f, Gc1s, and Gc2, respectively.14 Concentrations of albumin, DBP and 25OHD in these equations are in mol/L, and free and bioavailable 25OHD were then converted to pg/ml and ng/ml respectively for ease of interpretation of results. Free 25OHD concentrations were also measured in homozygous participants by immunoassay (Future Diagnostics/DIAsource ImmunoAssays, Belgium). The mean inter-assay CV (14 samples) was 9.6% (range 2.2–25.7%), and the mean intra-assay CV (80 samples) was 2.1% (range 0–5.9%).
Statistical Analysis
All analyses were performed using STATA 13.0 (Stata Corporation, College Station, TX). A two-sided p-value of <0.05 was considered statistically significant. Distributions of all variables were assessed for normality. Descriptive statistics for continuous variables were reported as the median and inter-quartile range (IQR). Correlations were assessed by Spearman’s rank and concordance correlation44 coefficients. The concordance correlation coefficient incorporates measures of both precision and accuracy to determine how far the observed data deviate from the line of perfect concordance (the line at 45 degrees on a square scatterplot). Group differences in continuous measures were determined using the Student’s t-test or Wilcoxon rank-sum test as indicated. Linear regression analysis was used to assess the amount of variability in DBP concentrations explained by genotype.
4 RESULTS
Table 1 shows descriptive characteristics of the study population. Median age was 64 years (inter-quartile range [IQR] 57–68), and 77 (62%) were male. The distribution of race was: 74 (59%) white, 45 (36%) black, and 6 (5%) other. Median eGFR for the 117 participants who had not progressed to end-stage kidney disease was 44.2 ml/min/1.73m2 (IQR 31.1, 55.3). 38% of participants had a total 25OHD concentration <20 ng/ml. Consistent with the established racial distribution of DBP genotype15–17 and with the Powe et al study,34 95% of individuals homozygous for Gc1f were black, while 91% and 77% of those homozygous for Gc1s and Gc2 were white, respectively. We confirmed that the isoform identification of the novel LC-MS/MS assay was 97% (121/125 samples) concordant with genotype, as reported in a different cohort.39
Table 1.
Population characteristics
| Race | |||
|---|---|---|---|
| Black | White | Other | |
| N (%) | 45 (36%) | 74 (59%) | 6 (5%) |
| Age years, median (IQR) | 64 (53, 67) | 64 (56, 69) | 62.5 (60, 68) |
| Male, n (%) | 24 (53%) | 49 (66%) | 4 (67%) |
|
Estimated glomerular filtration rate ml/min/1.73m2, median (IQR) |
40.5 (28.2, 55.2) | 45.2 (31.2, 57.7) | * |
| Genotype | |||
| 1f/1f | 21 (47%) | 1 (1%) | 0 (0%) |
| 1f/2 | 10 (22%) | 9 (12%) | 1 (17%) |
| 1s/1f | 9 (20%) | 11 (15%) | 0 (0%) |
| 1s/1s | 2 (4%) | 20 (27%) | 0 (0%) |
| 1s/2 | 1 (2%) | 16 (22%) | 2 (33%) |
| 2/2 | 2 (4%) | 17 (23%) | 3 (50%) |
| 25-hydroxyvitamin D ng/ml, median (IQR) | 17.2 (10.7, 31.7) | 26.7 (18.5, 32.3) | 19.8 (12.4, 30.1) |
Only 4 values
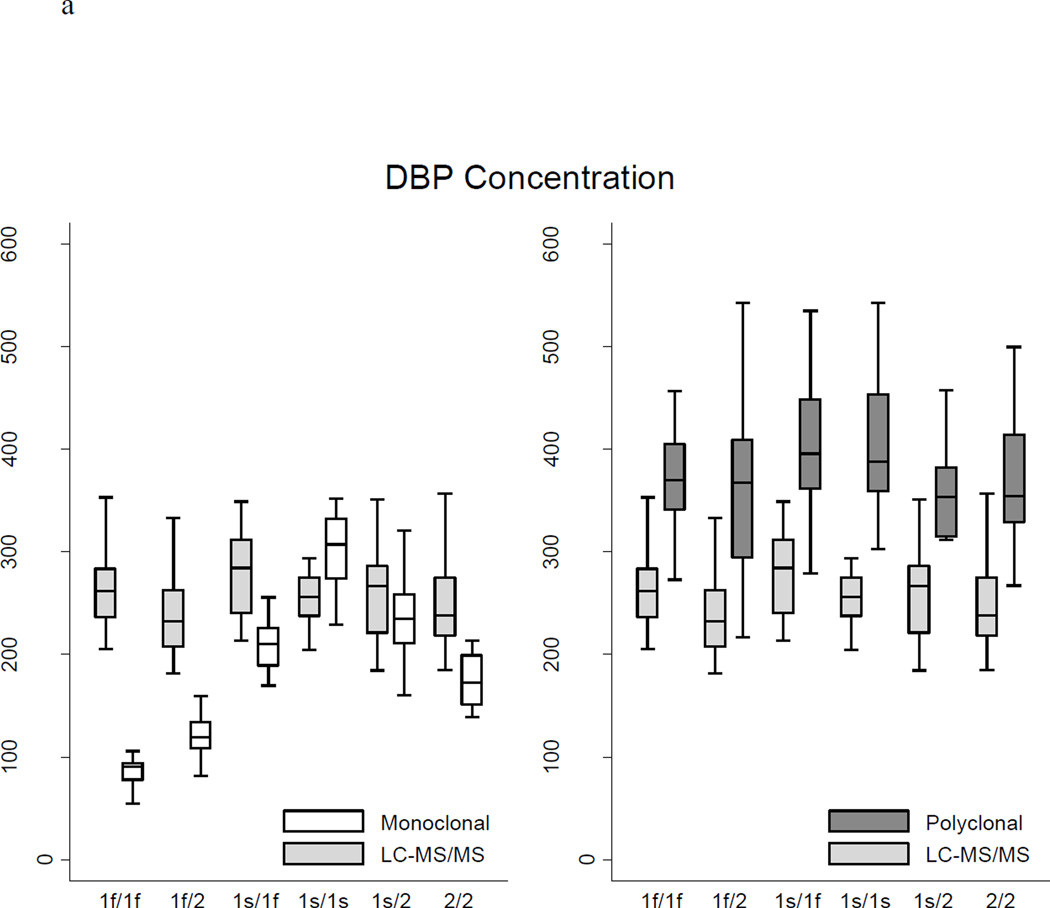
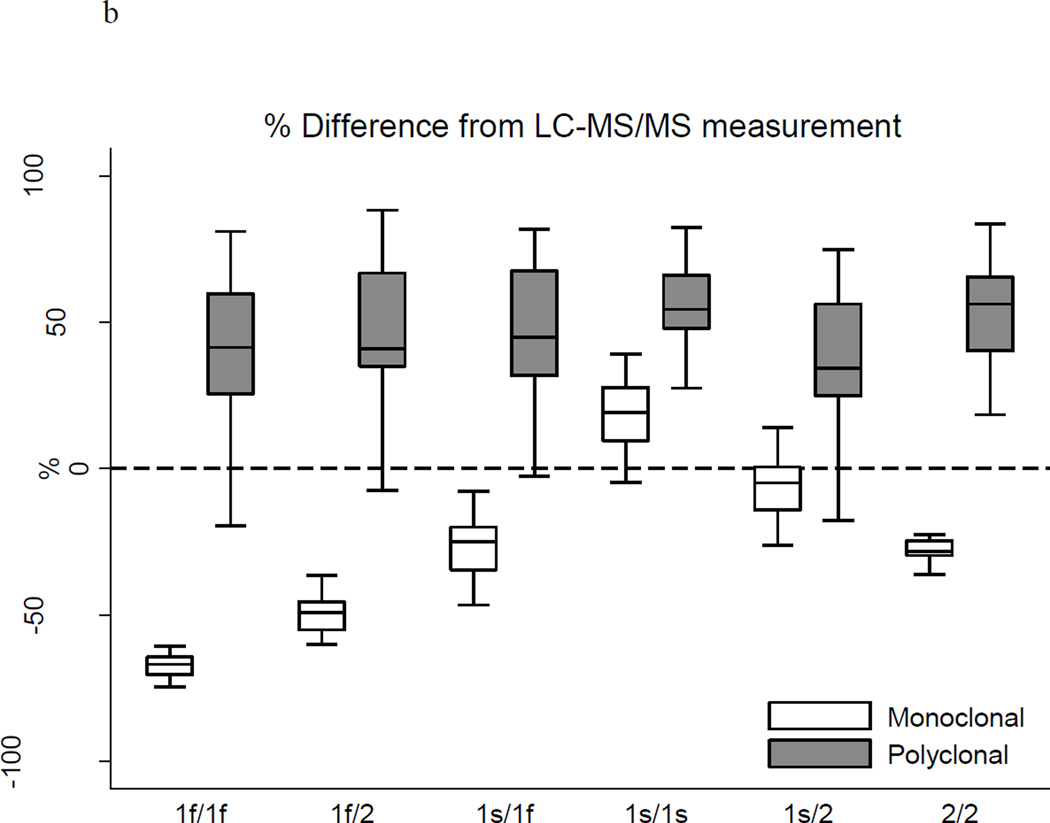
Table 2 and Figure 1 show the distributions of measured DBP concentrations according to the assay method and participant genotype. Overall, DBP concentrations measured by the monoclonal ELISA (median 185.0 ug/ml) were lower than LC-MS/MS measurements (median 253.1 ug/ml, p <0.0001), consistent with the findings of Henderson et al39 (mean 183.9 versus 264.5 ug/ml). DBP concentrations measured by the polyclonal ELISA (median 373.8 ug/ml) were higher compared with LC-MS/MS (p <0.0001). The race-specific distributions of DBP concentration according to the three assays were very consistent with results reported in studies using these assays in non-CKD populations.34,38,39 The LC-MS/MS measurements of DBP concentration differed to some degree across genotypes (Kruskal-Wallis p = 0.02) with the highest concentrations among 1s/1f heterozygotes (median 284.4 ug/ml) and the lowest concentrations among 1f/2 heterozygotes (median 232.5 ug/ml). Measurements of DBP concentration by the monoclonal ELISA differed more than three-fold according to genotype (Kruskal-Wallis p = 0.0001) with the highest median concentration among 1s/1s homozygotes (307.3 ug/ml) and the lowest median concentration among 1f/1f homozygotes (90.9 ug/ml). In contrast, measurements of DBP concentration by the polyclonal ELISA did not differ by genotype (ANOVA p = 0.10). While only 9% or less of the variability in DBP concentrations obtained using either LC-MS/MS or the polyclonal ELISA was explained by genotype, 85% of the variability in the monoclonal ELISA-based results was explained by genotype.
Table 2.
Distribution of vitamin D-binding protein (DBP) measurements according to assay method and genotype
| LC-MS/MS | Monoclonal ELISA | Polyclonal ELISA | ||||
|---|---|---|---|---|---|---|
| N | Concentration ug/ml Median (IQR) Mean ± SD |
Concentration ug/ml Median (IQR) Mean ± SD |
% Difference from LC-MS/MS Median (IQR) |
Concentration ug/ml Median (IQR) Mean ± SD |
% Difference from LC-MS/MS Median (IQR) |
|
| Overall | 125 | 253.1 (227.4, 283.4) 256.3 ± 39.6 |
185.0 (115.5, 248.9) 187.5 ± 79.3 |
−28 (−50, −5) | 373.8 (337.7, 419.5) 377.7 ± 72.6 |
+50 (+33, +65) |
| 1f/1f | 22 | 262.1 (236.2, 283.4) 261.8 ± 36.8 |
90.9 (77.6, 94.3) 85.3 ± 14.0 |
−67 (−71, −64) | 369.7 (341.4, 405.1) 364.7 ± 64.2 |
+41 (+25, +60) |
| 1f/2 | 20 | 232.5 (207.1, 262.4) 241.8 ± 46.0 |
119.2 (108.3, 134.4) 121.0 ± 19.5 |
−49 (−55, −45) | 367.2 (294.4, 409.2) 364.4 ± 87.6 |
+41 (+35, +67) |
| 1s/1f | 20 | 284.4 (240.3, 312.0) 278.5 ± 40.8 |
210.4 (189.1, 226.2) 201.8 ± 41.7 |
−25 (−35, −20) | 395.7 (361.4, 448.5) 406.2 ± 69.2 |
+45 (+32, +67) |
| 1s/1s | 22 | 255.9 (237.2, 274.9) 256.4 ± 22.9 |
307.3 (273.7, 331.9) 303.5 ± 33.8 |
+19 (+9, +28) | 387.6 (359.2, 453.6) 400.9 ± 61.0 |
+54 (+48, +66) |
| 1s/2 | 19 | 266.5 (221.0, 286.3) 254.9 ± 41.9 |
234.9 (210.5, 258.7) 236.6 ± 36.8 |
−5 (−14, 0.5) | 353.5 (314.6, 382.3) 352.0 ± 77.5 |
+34 (+25, +56) |
| 2/2 | 22 | 238.1 (218.0, 275.1) 244.7 ± 40.0 |
172.4 (150.7, 199.2) 179.0 ± 31.9 |
−28 (−30, −25) | 354.0 (328.7, 414.2) 375.6 ± 67.6 |
+56 (+40, +65) |
| ANOVA or Kruskal-Wallis test of difference in DBP concentration by genotype: | ||||||
| P value | 0.02 | 0.0001 | 0.10 | |||
| Variation in DBP concentrations explained by genotype: | ||||||
| R2 | 0.09 | 0.85 | 0.07 | |||
Figure 1.
a Vitamin D-binding protein (DBP) concentrations according to genotype and assay method
b Deviation of ELISA-based measures of DBP from LC-MS/MS measures
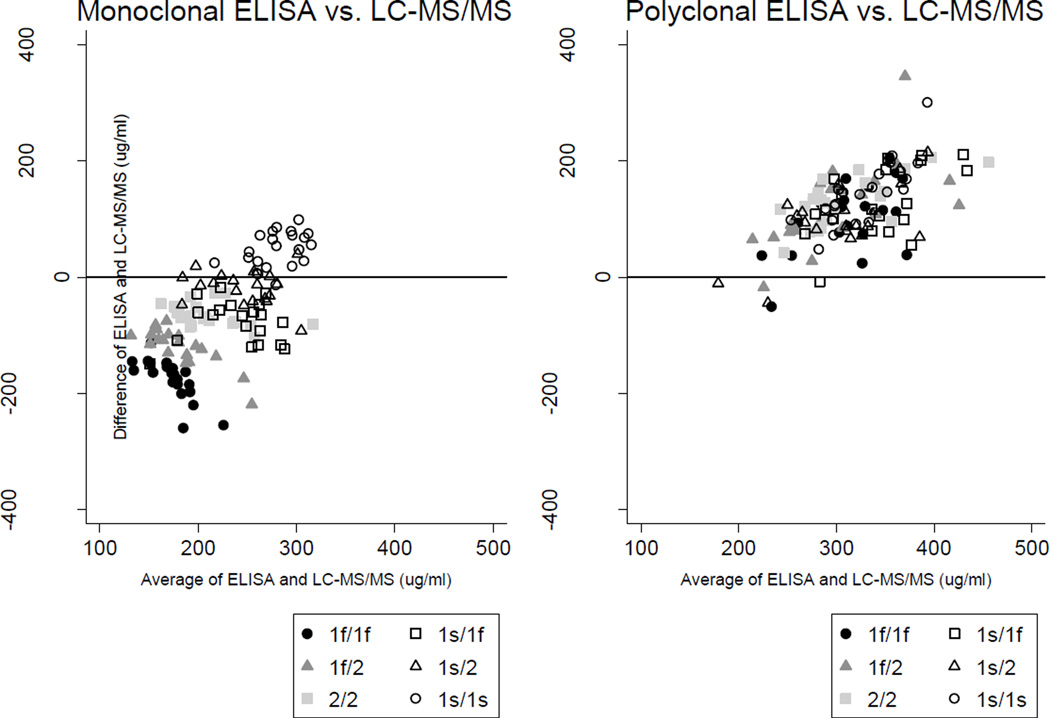
Table 2 and Figure 1 clearly demonstrate that the polyclonal ELISA yielded consistently and similarly higher measurements of DBP than LC-MS/MS, irrespective of genotype, with a median percent difference from the LC-MS/MS-based measurement of +50% overall and +34 to +56% across genotypes. The discrepancies between the measurements obtained using the monoclonal ELISA and LC-MS/MS, however, varied dramatically by genotype, with the 1f/2 heterozygotes and 1f/1f homozygotes having disproportionately lower DBP concentrations by the monoclonal ELISA. Of particular concern, DBP concentrations measured by the monoclonal ELISA were two-thirds lower than LC-MS/MS-based results for the 1f/1f genotype (median −67%; inter-quartile range −71%, −64%). As highlighted in the Bland-Altman plots in Figure 2, the apparent bias of the monoclonal antibody was characterized by binding Gc isoforms 1s > 2 > 1f. Indeed, the nonparametric test for trend across ordered groups (based on this hierarchical binding: 1f/1f, 1f/2, 2/2, 1s/1f, 1s/2, 1s/1s) was significant for the monoclonal ELISA (p <0.001), but not for the polyclonal ELISA or LC-MS/MS.
Figure 2.
Bland-Altman plots of vitamin D-binding protein (DBP) quantified by ELISA versus LC-MS/MS methods
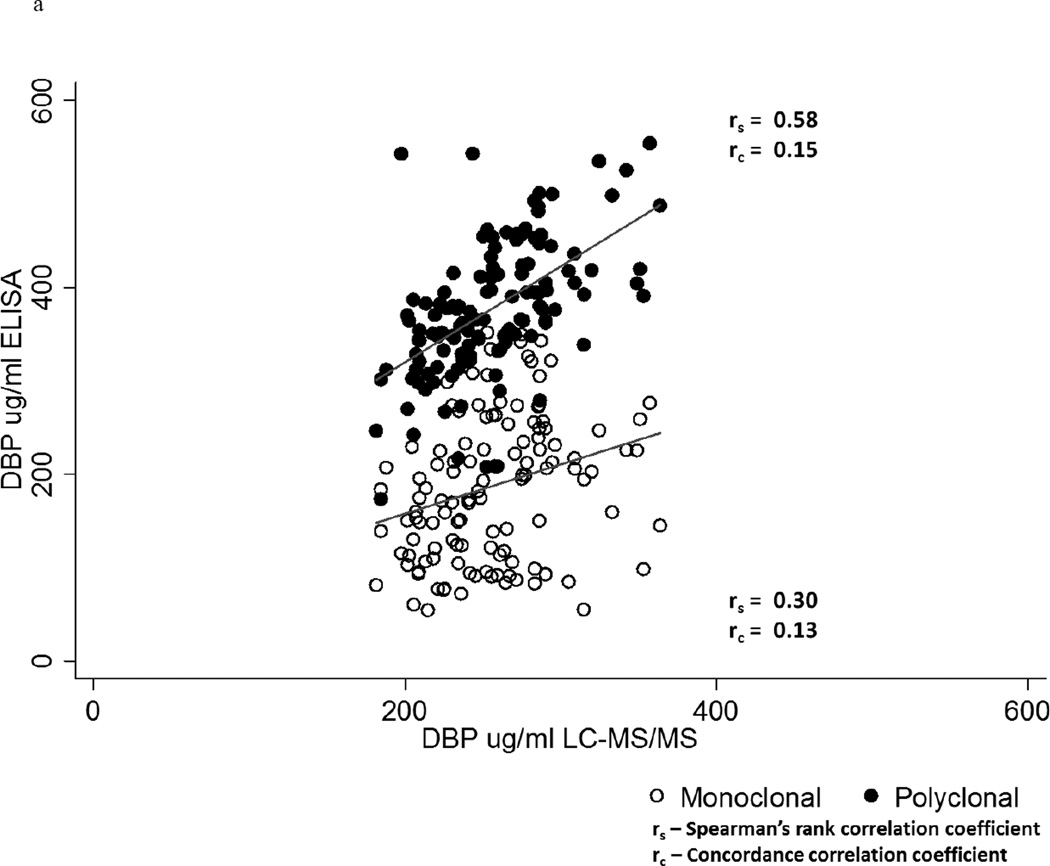
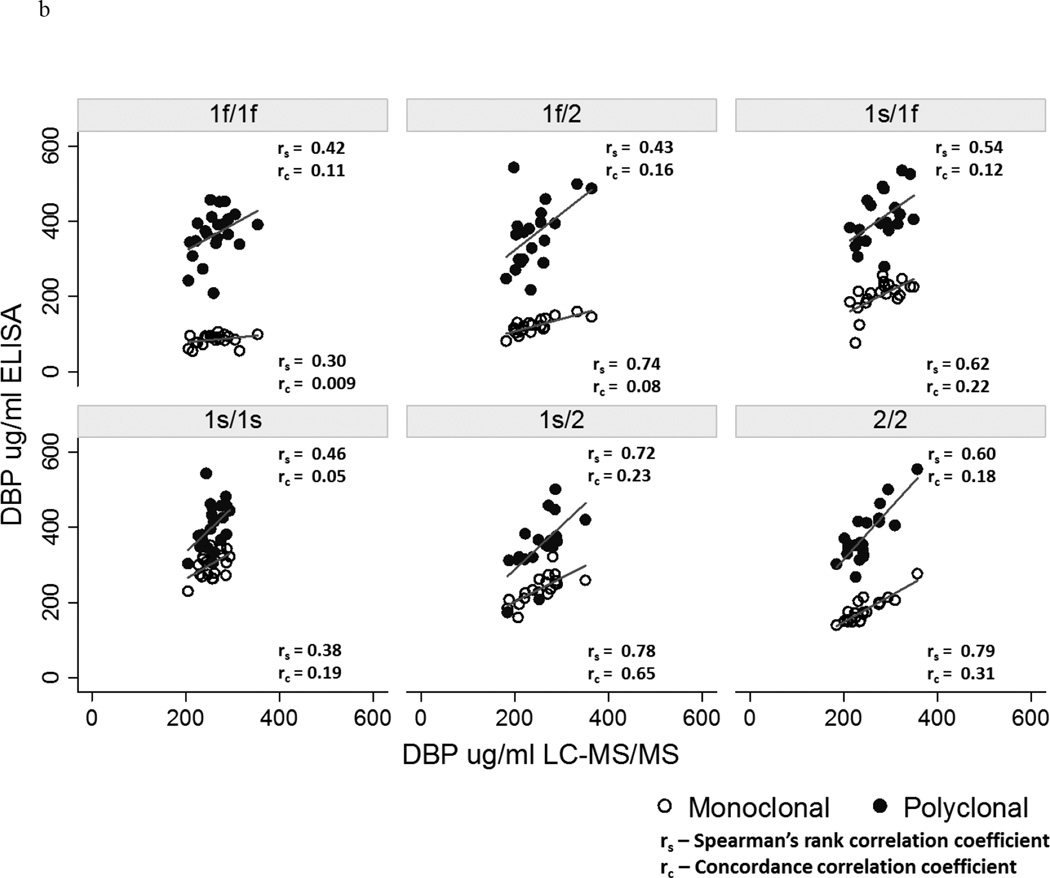
Overall, although the Spearman’s rank correlation (rs) with the LC-MS/MS measurements of DBP was greater for the results obtained using the polyclonal ELISA (rs = 0.58, p <0.0001) compared to the monoclonal ELISA (rs = 0.30, p = 0.0006), both correlations were poor as highlighted by the concordance correlation coefficients (rc) of ≤ 0.15 (Figure 3a). Results from the two ELISA methods were also poorly correlated with each other (rs = 0.32, p = 0.0003), consistent with our prior study.38 Figure 3b compares the Spearman’s rank and concordance correlations of the monoclonal and polyclonal ELISA-based measurements with the LC-MS/MS assay results, stratified on genotype. Neither ELISA had a Spearman’s rank correlation coefficient of ≥ 0.8 within any genotype, and with one exception the concordance correlation coefficients were ≤ 0.31.
Figure 3.
a Correlation of monoclonal versus polyclonal ELISA with LC-MS/MS measures of vitamin D-binding protein (DBP)
b Correlations of monoclonal versus polyclonal ELISA with LC-MS/MS measures of DBP according to genotype
Serum 25OHD levels were significantly lower in black versus white participants (median 17.2 ng/ml, IQR 10.7–31.7 versus 26.7 ng/ml, IQR 18.5–32.3; p = 0.02). Table 3 demonstrates measured DBP concentrations and calculated free and bioavailable 25OHD stratified by race. DBP concentrations measured by the monoclonal ELISA were > 50% lower in black versus white participants (median 105.9 versus 215.4 ug/ml, p <0.0001) participants, while DBP concentrations quantified by the polyclonal ELISA and by LC-MS/MS did not differ by race. Free and bioavailable 25OHD concentrations did not differ by race when based on the monoclonal ELISA measures of DBP. Free 25OHD concentrations measured by immunoassay also did not differ by race (median 6.6 pg/ml, IQR 3.3–8.8 in black participants versus 6.2 pg/ml, IQR 5.0–7.8 in white participants). Using both the polyclonal ELISA and LC-MS/MS measures of DBP, free and bioavailable 25OHD concentrations were significantly lower in black as compared to white participants (all p ≤ 0.0007).
Table 3.
Measured vitamin D-binding protein (DBP) and estimated concentrations of free and bioavailable 25OHD in black versus white participants according to DBP assay method
| Assay Method | DBP ug/ml Median (IQR) N = 119a |
Free 25OHD pg/ml Median (IQR) N = 63b |
Bioavailable 25OHD ng/ml Median (IQR) N = 63b |
|||
|---|---|---|---|---|---|---|
| Black | White | Black | White | Black | White | |
| LC-MS/MS | 261.1 (230.7, 286.8) | 249.5 (230.3, 278.4) | 4.0 (2.9, 6.8) | 10.6 (6.7, 14.0) | 1.8 (1.1, 2.7) | 4.1 (2.2, 5.1) |
| P valuec | 0.24 | <0.0001 | 0.0001 | |||
| Monoclonal ELISA | 105.9 (90.7, 202.7) | 215.4 (169.7, 273.5) | 8.4 (5.8, 17.1) | 10.9 (6.2, 15.0) | 3.4 (2.6, 6.8) | 4.3 (2.1, 6.4) |
| P value | <0.0001 | 0.98 | 0.88 | |||
| Polyclonal ELISA | 379.9 (341.4, 417.5) | 375.8 (333.3, 425.1) | 3.6 (1.9, 4.6) | 6.9 (4.5, 9.5) | 1.3 (0.8, 1.7) | 2.8 (1.5, 3.5) |
| P value | 0.95 | 0.0003 | 0.0007 | |||
Analysis limited to participants of black or white race.
Analysis limited to homozygous participants of black or white race.
Group differences assessed using Student’s t-test or Wilcoxon rank sum test as appropriate.
5 DISCUSSION
This is the first study to directly compare serum DBP measurements and estimates of free and bioavailable 25OHD concentrations based on widely used commercially available monoclonal and polyclonal ELISAs versus a novel LC-MS/MS method, corroborating and expanding upon the recent findings of Henderson, et al in healthy individuals.39 The reproducibility of the comparison between the monoclonal ELISA and LC-MS/MS in a cohort with CKD underscores the robustness of our findings. We confirmed that the monoclonal ELISA quantifies DBP differentially by genotype and demonstrated that the polyclonal ELISA is not subject to this bias. In contrast to the monoclonal ELISA results, DBP concentrations quantified by both the polyclonal ELISA and LC-MS/MS did not differ by race, yielding significantly lower estimated free and bioavailable 25OHD concentrations in black participants. The results of our study in terms of race-specific distributions of 25OHD and DBP (according to assay method) were strikingly similar to those of non-CKD cohorts, including the Atherosclerosis Risk in Communities study of 184 adults by Henderson, et al,39 a cohort of 304 healthy adults enrolled as controls in a study of osteoporosis at the University of Pennsylvania,38 and the Healthy Aging in Neighborhoods of Diversity across the Life Span cohort reported by Powe, et al.34 These comparisons confirm that the renal insufficiency in the CRIC participants had no impact on the conclusions of this study.
The monoclonal antibody of the R&D ELISA binds to a single peptide fragment of DBP and may bind differently to Gc isoforms, yielding underestimated concentrations particularly in individuals homozygous for the Gc1f variant.35,37 DBP concentrations measured using the monoclonal ELISA versus LC-MS/MS methods in our analysis were most discrepant among Gc1f homozygotes, and the median DBP concentration among Gc1f homozygotes of 90.9 ug/ml was less than one-third that of Gc1s homozygotes based on the monoclonal assay. Of note, the mean DBP concentration of Gc1f homozygotes measured by monoclonal ELISA in the Powe et al study34 was very similar (93 ± 2 ug/ml). Commentaries in response to the Powe et al study cited prior studies that did not demonstrate race differences in circulating DBP using polyclonal assays,35,45–47 as well as a study that found the lowest DBP concentrations among Gc2 rather than Gc1f homozygotes using an immunonephelometric method.48 Consistent with these studies, we did not find race or genotypic differences in serum DBP using the polyclonal ELISA. Although the polyclonal assay yielded consistently higher concentrations compared to LC-MS/MS, this was non-differential by genotype. The positive bias of the polyclonal assay may have resulted from the polyclonal antibody cross-reacting with other proteins leading to over-recovery,49,50 from heterophilic antibody interference, or from differences in calibration. Future studies should investigate the source of this positive bias.
The markedly discrepant DBP measures produced discrepant estimates of free and bioavailable 25OHD and consequently, had a substantial impact on interpretation of race differences in free and bioavailable 25OHD. Use of DBP measures from the monoclonal ELISA yielded comparable estimates of free and bioavailable 25OHD in black and white individuals, as concluded in the Powe et al34 study. However, using both the polyclonal ELISA and LC-MS/MS derived measures of DBP, free and bioavailable 25OHD were significantly lower in black versus white individuals, in keeping with their lower total 25OHD concentrations. The results of the free 25OHD immunoassay did not differ by race. Further work is needed to evaluate the effects of race and DBP genotype on free 25OHD, as well as the comparative associations of free versus total 25OHD with clinically relevant outcomes. In both healthy and CKD cohorts, prior studies using the monoclonal ELISA to measure DBP have yielded conflicting results regarding whether free and bioavailable 25OHD provide better indices of vitamin D-related bone health as assessed by parathyroid hormone concentrations and bone mineral density.6,34,38,42,51,52
In summary, our results confirm the differential and biased performance of the monoclonal ELISA according to genotype and therefore race. Inferences from studies that have used this assay should be made cautiously, especially when interpreting reported race differences in vitamin D status. Future studies of DBP and free or bioavailable vitamin D metabolites should employ DBP assays that are not biased by DBP genotype.
Acknowledgments
Dr. Denburg receives/received funding (not related to this work) from NIDDK, PCORI, Genentech, Inc, and Mallinckrodt Pharmaceuticals. Dr. Denburg has a consultancy agreement with Infiniti Medical. Dr. Hoofnagle has received grant funding from Waters and equipment from Waters and Thermo Fisher Scientific. Dr. de Boer receives funding (not related to this work) from NIDDK, NHLBI, and Abbvie, and his consultancy agreements include: Amgen, Bayer, and Janssen. Dr. Leonard receives funding from NIDDK and her consultancy agreements include: Amgen, Inc, Johnson & Johnson, and Novartis.
This project was supported by NIH Grants: K23 DK093556 (MRD), K24 DK076808 (MBL), U01 AG047837 (LJA), U01 DK060990 (HIF, KW), U01 DK061022 (LJA), UL1RR024134 and UL1TR000003 (SS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
The CRIC Principal Investigators are Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, Mahboob Rahman, MD, and Raymond R. Townsend, MD. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
Disclosures: The remaining authors (SS, LJA, JG, KW, HIF) have no disclosures.
AUTHOR CONTRIBUTIONS
Authors’ roles: Study design: MRD, ANH, and MBL. Study conduct: MRD, ANH, SS, KW, and MBL. Data collection: MRD, ANH, SS, KW, and JG. Data analysis: MRD and MBL. Data interpretation: MRD, ANH, IdB, RD, and MBL. Drafting manuscript: MRD and MBL. Revising manuscript content: MRD, ANH, SS, IdB, LJA, RD, HIF, MBL. Approving final version of manuscript:MRD, ANH, SS, JG, IdB, LJA, RD, KW, HIF, MBL. MRD and MBL take responsibility for the integrity of the data analysis.
Contributor Information
Michelle R. Denburg, Pediatrics, The Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, 34th Street and Civic Center Blvd, Philadelphia, PA 19104.
Andrew N. Hoofnagle, Laboratory Medicine, University of Washingon School of Medicine.
Samir Sayed, The Children’s Hospital of Philadelphia.
Jayanta Gupta, Biostatistics and Epideimiology, Paul L. Foster School of Medicine, Texas Tech University.
Ian H. de Boer, Medicine, University of Washingon School of Medicine.
Lawrence J. Appel, Medicine, Epidemiology and International Health, Director, Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins School of Medicine and Bloomberg School of Public Health.
Ramon Durazo-Arvizu, Public Health Sciences, Stritch School of Medicine, Loyola University Chicago.
Krista Whitehead, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine at the University of Pennsylvania.
Harold I. Feldman, Epidemiology and Medicine, Chair, Department of Biostatistics and Epidemiology, Director, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine at the University of Pennsylvania.
Mary B. Leonard, Pediatrics and Medicine, Stanford University School of Medicine.
REFERENCES
- 1.Hirschfeld J. Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta pathologica et microbiologica Scandinavica. 1959;47:160–168. doi: 10.1111/j.1699-0463.1959.tb04844.x. [DOI] [PubMed] [Google Scholar]
- 2.Haddad JG, Hu YZ, Kowalski MA, et al. Identification of the sterol- and actin-binding domains of plasma vitamin D binding protein (Gc-globulin) Biochemistry. 1992;31:7174–7181. doi: 10.1021/bi00146a021. [DOI] [PubMed] [Google Scholar]
- 3.Otterbein LR, Cosio C, Graceffa P, Dominguez R. Crystal structures of the vitamin D-binding protein and its complex with actin: structural basis of the actin-scavenger system. Proc Natl Acad Sci U S A. 2002;99:8003–8008. doi: 10.1073/pnas.122126299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verboven C, Rabijns A, De Maeyer M, Van Baelen H, Bouillon R, De Ranter C. A structural basis for the unique binding features of the human vitamin D-binding protein. Nature structural biology. 2002;9:131–136. doi: 10.1038/nsb754. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Habiel DM, Ramadass M, Kew RR. Identification of two distinct cell binding sequences in the vitamin D binding protein. Biochim Biophys Acta. 2010;1803:623–629. doi: 10.1016/j.bbamcr.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhan I, Powe CE, Berg AH, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61:969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 9.Feldman D, Malloy PJ, Gross C. Vitamin D: Biology, action, and clinical implications. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2nd. Academic Press; 2001. pp. 257–303. [Google Scholar]
- 10.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 11.Bikle DD, Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology. 1989;124:649–654. doi: 10.1210/endo-124-2-649. [DOI] [PubMed] [Google Scholar]
- 12.Chun RF, Lauridsen AL, Suon L, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun A, Bichlmaier R, Cleve H. Molecular analysis of the gene for the human vitamin-D-binding protein (group-specific component): allelic differences of the common genetic GC types. Hum Genet. 1992;89:401–706. doi: 10.1007/BF00194311. [DOI] [PubMed] [Google Scholar]
- 14.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92:183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 15.Constans J, Hazout S, Garruto RM, Gajdusek DC, Spees EK. Population distribution of the human vitamin D binding protein: anthropological considerations. American journal of physical anthropology. 1985;68:107–122. doi: 10.1002/ajpa.1330680110. [DOI] [PubMed] [Google Scholar]
- 16.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986;72:281–293. doi: 10.1007/BF00290950. [DOI] [PubMed] [Google Scholar]
- 17.Speeckaert MM, Speeckaert R, van Geel N, Delanghe JR. Vitamin D binding protein: a multifunctional protein of clinical importance. Advances in clinical chemistry. 2014;63:1–57. doi: 10.1016/b978-0-12-800094-6.00001-7. [DOI] [PubMed] [Google Scholar]
- 18.Anic GM, Weinstein SJ, Mondul AM, Mannisto S, Albanes D. Serum vitamin D, vitamin D binding protein, and lung cancer survival. Lung cancer. 2014;86:297–303. doi: 10.1016/j.lungcan.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anic GM, Weinstein SJ, Mondul AM, Mannisto S, Albanes D. Serum vitamin D, vitamin D binding protein, and risk of colorectal cancer. PLoS One. 2014;9:e102966. doi: 10.1371/journal.pone.0102966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bratke K, Wendt A, Garbe K, et al. Vitamin D binding protein and vitamin D in human allergen-induced endobronchial inflammation. Clinical and experimental immunology. 2014;177:366–372. doi: 10.1111/cei.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman J, Wilson K, Spears R, Shalhoub V, Sibley P. Influence of Vitamin D Binding Protein on Accuracy of 25-Hydroxyvitamin D Measurement Using the ADVIA Centaur Vitamin D Total Assay. International journal of endocrinology. 2014;2014:691679. doi: 10.1155/2014/691679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiemstra TF, Casian A, Boraks P, Jayne DR, Schoenmakers I. Plasma exchange induces vitamin D deficiency. QJM. 2014;107:123–130. doi: 10.1093/qjmed/hct208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda T, Kasai M, Tatsukawa E, et al. A bone substitute with high affinity for vitamin D-binding protein--relationship with niche of osteoclasts. Journal of cellular and molecular medicine. 2014;18:170–180. doi: 10.1111/jcmm.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalousova M, Dusilova-Sulkova S, Zakiyanov O, et al. Vitamin D Binding Protein Is Not Involved in Vitamin D Deficiency in Patients with Chronic Kidney Disease. BioMed research international. 2015;2015:492365. doi: 10.1155/2015/492365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai JC, Bikle DD, Lizaola B, Hayssen H, Terrault NA, Schwartz JB. Total 25(OH) vitamin D, free 25(OH) vitamin D and markers of bone turnover in cirrhotics with and without synthetic dysfunction. Liver international : official journal of the International Association for the Study of the Liver. 2015 doi: 10.1111/liv.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondul AM, Weinstein SJ, Moy KA, Mannisto S, Albanes D. Vitamin D-binding protein, circulating vitamin D and risk of renal cell carcinoma. International journal of cancer Journal international du cancer. 2014;134:2699–2706. doi: 10.1002/ijc.28596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponda MP, McGee D, Breslow JL. Vitamin D-binding protein levels do not influence the effect of vitamin D repletion on serum PTH and calcium: data from a randomized, controlled trial. J Clin Endocrinol Metab. 2014;99:2494–2499. doi: 10.1210/jc.2014-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousseau AF, Damas P, Janssens M, et al. Critical care and vitamin D status assessment: what about immunoassays and calculated free 25OH-D? Clin Chim Acta. 2014;437:43–47. doi: 10.1016/j.cca.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Shoukry A, Bdeer SE, El-Sokkary RH. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Molecular and cellular biochemistry. 2015 doi: 10.1007/s11010-015-2479-y. [DOI] [PubMed] [Google Scholar]
- 30.Smolders J, Peelen E, Thewissen M, Menheere P, Damoiseaux J, Hupperts R. Circulating vitamin D binding protein levels are not associated with relapses or with vitamin D status in multiple sclerosis. Multiple sclerosis. 2014;20:433–437. doi: 10.1177/1352458513500552. [DOI] [PubMed] [Google Scholar]
- 31.Tian XQ, Zhao LM, Ge JP, Zhang Y, Xu YC. Elevated urinary level of vitamin D-binding protein as a novel biomarker for diabetic nephropathy. Experimental and therapeutic medicine. 2014;7:411–416. doi: 10.3892/etm.2013.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RT, Bortner JD, Jr, Roff A, et al. Genetic and environmental influences on plasma vitamin D binding protein concentrations. Translational research : the journal of laboratory and clinical medicine. 2015;165:667–676. doi: 10.1016/j.trsl.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz JB, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99:1631–1637. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouillon R, Jones K, Schoenmakers I. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370:879. doi: 10.1056/NEJMc1315850. [DOI] [PubMed] [Google Scholar]
- 36.Carter GD, Phinney KW. Assessing vitamin D status: time for a rethink? Clin Chem. 2014;60:809–811. doi: 10.1373/clinchem.2013.219386. [DOI] [PubMed] [Google Scholar]
- 37.Hollis BW, Bikle DD. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370:879–880. doi: 10.1056/NEJMc1315850#SA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jemielita TO, Leonard MB, Baker J, et al. Association of 25-hydroxyvitamin D with areal and volumetric measures of bone mineral density and parathyroid hormone: impact of vitamin D-binding protein and its assays. Osteoporos Int. 2015 doi: 10.1007/s00198-015-3296-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson CM, Lutsey PL, Misialek JR, et al. Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D binding protein in blacks and whites. Clin Chem. 2015 doi: 10.1373/clinchem.2015.244541. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 41.Gao Y, Wu K, Xu Y, et al. Characterization of acute renal allograft rejection by human serum proteomic analysis. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2009;29:585–591. doi: 10.1007/s11596-009-0511-8. [DOI] [PubMed] [Google Scholar]
- 42.Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 44.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 45.Bouillon R, van Baelen H, de Moor P. The measurement of the vitamin D-binding protein in human serum. J Clin Endocrinol Metab. 1977;45:225–231. doi: 10.1210/jcem-45-2-225. [DOI] [PubMed] [Google Scholar]
- 46.M'Buyamba-Kabangu JR, Fagard R, Lijnen P, Bouillon R, Lissens W, Amery A. Calcium, vitamin D-endocrine system, and parathyroid hormone in black and white males. Calcif Tissue Int. 1987;41:70–74. doi: 10.1007/BF02555247. [DOI] [PubMed] [Google Scholar]
- 47.Winters SJ, Chennubhatla R, Wang C, Miller JJ. Influence of obesity on vitamin D-binding protein and 25-hydroxy vitamin D levels in African American and white women. Metabolism. 2009;58:438–442. doi: 10.1016/j.metabol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47:753–756. [PubMed] [Google Scholar]
- 49.Christiansen M, Jorgensen CS, Laursen I, Hirschberg D, Hojrup P, Houen G. Protein chemical characterization of Gc globulin (vitamin D-binding protein) isoforms; Gc-1f, Gc-1s and Gc-2. Biochim Biophys Acta. 2007;1774:481–492. doi: 10.1016/j.bbapap.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370:880–881. doi: 10.1056/NEJMc1315850. [DOI] [PubMed] [Google Scholar]
- 51.Denburg MR, Kalkwarf HJ, de Boer IH, et al. Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28:1843–1853. doi: 10.1007/s00467-013-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denburg MR, Tsampalieros AK, de Boer IH, et al. Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab. 2013;98:1930–1938. doi: 10.1210/jc.2012-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]