Abstract
Cardiac hypertrophy is a major risk factor for heart failure, and it has been shown that this increase in size occurs at the level of the cardiac myocyte. Cardiac myocyte model systems have been developed to study this process. Here we focus on cell culture tools, including primary cells, immortalized cell lines, human stem cells, and their morphological and molecular responses to pathological stimuli. For each cell type, we discuss commonly used methods for inducing hypertrophy, markers of pathological hypertrophy, advantages for each model, and disadvantages to using a particular cell type over other in vitro model systems. Where applicable, we discuss how each system is used to model human disease and how these models may be applicable to current drug therapeutic strategies. Finally, we discuss the increasing use of biomaterials to mimic healthy and diseased hearts and how these matrices can contribute to in vitro model systems of cardiac cell biology.
INTRODUCTION
In this review, we discuss the cell biology of heart disease, using the cardiac myocyte as our focus. Although there are many cell types in the heart (myocytes, endothelial cells, fibroblasts, vascular smooth muscle cells), and each cell type contributes to cardiac function, we discuss the contractile myocytes. Myocytes constitute the majority of the heart by mass and have been shown to be major contributors to contractile dysfunction. We discuss various approaches that have been used to study myocyte cell biology in the setting of heart disease and cover recent advances.
For more than three decades, rodent models have been used to study heart disease with many different etiologies; despite divergent causes, cardiac hypertrophy is frequently a common disease indicator (Curtis et al., 1987; Rockman et al., 1991). In fact, pathological cardiac hypertrophy is a strong prognostic indicator of human morbidity and mortality (Kono et al., 2009; Takasaki et al., 2012; Shenasa et al., 2015). It should be mentioned that there is also a type of cardiac hypertrophy that is physiological and caused by stimuli such as exercise and pregnancy, but this type of hypertrophy is distinct and will not be discussed here. Cardiac hypertrophy is largely the result of cardiac myocyte enlargement, and this process can be recapitulated in isolated myocytes in culture by treatment with a number of small molecules and growth factors. Myocytes either contract spontaneously or can be paced to contract and their contractile properties studied. Primary cardiomyocytes are terminally differentiated cells and are difficult to transfect (Naqvi et al., 2009). Therefore some effort has been put into developing permanent cell lines, but these lines suffer from their relevance to bona fide cardiac myocytes (see later discussion). Purification of cardiomyocytes greatly reduces or eliminates other cell types in the heart, including endothelial cells and fibroblasts, making it possible to attribute findings to the cardiac myocytes rather than interactions with other cell types (Diaz and Wilson, 2006). Studying cardiomyocytes offers many inherent advantages over whole-heart models, allowing precise control of experimental conditions, including extracellular matrices, exogenous growth factors, oxygen levels, and electrical stimulation.
In addition to animal models and primary cardiac myocytes from experimental animal models, much recent work has been done to develop human cardiomyocyte model systems. These include differentiation of human embryonic stem cells (ESCs) and human induced pluripotent stem cells (iPSCs) into cardiomyocytes. These models are discussed here, and although they may ultimately be the best model for studying human cardiomyocytes, considerable research is still needed to direct these cells to a fully differentiated cardiomyocyte phenotype.
PRIMARY CARDIAC MYOCYTES
Neonatal rodent ventricular myocytes
Neonatal cardiomyocytes have been the workhorse of in vitro cardiac cell biology. In comparison to adult cardiomyocytes, neonatal myocytes are relatively easy to isolate, survive in culture for relatively long periods of time, and can be transfected using nonviral methods of gene transfer. The first isolation of neonatal rat ventricular myocytes (NRVMs) occurred >50 years ago (Harary and Farley, 1963a, b), and it was clear from the first published report that these spontaneously beating, cultured cells could serve as an invaluable resource for cardiovascular research. Since the initial report, cultured neonatal cardiomyocytes have been used to study myofibrillogenesis and myofibrillar functions and to model cardiac diseases. In fact, live-cell imaging with tagged mutant and wild-type myosins has been used to follow sarcomere distribution in NRVMs, and misaccumulation of mutants has been observed (Buvoli et al., 2012).
Although many protocols exist for isolation of NRVM and neonatal mouse ventricular myocyte (NMVM) isolation, the fundamental aspects of the protocol are essentially the same as described many years ago (Karliner et al., 1985). One of the greatest advantages of using NRVMs and NMVMs is the ability to study cardiac hypertrophic responses using compounds that mimic states seen in heart disease, such as adrenergic stimulation. A number of compounds and growth factors have been shown to induce pathological cardiac myocyte hypertrophy in NRVMs and NMVMs, including phenylephrine (PE; Zobel et al., 2002; Huang et al., 2015; Nakaoka et al., 2015), norepinephrine (NE; Simpson et al., 1982; Bishopric and Kedes, 1991), angiotensin II (Ang II; Menaouar et al., 2014), endothelin-1 (ET-1; Menaouar et al., 2014), and the diacylglycerol mimetic phorbol 12-myristate 12-acetate (PMA; Reid et al., 2016) (see Table 2 later in this paper). Cardiac myocytes can increase in both volume and cell surface area by as much as 150% within 48 h of treatment with PE, for example (Figure 1A; Simpson, 1983; LaMorte et al., 1994). In addition to increases in myocyte size, it has been shown that PE stimulation causes significant (p < 0.05) changes in the expression of between 600 and 3000 genes, so the effects that cause/accompany changes in size are substantial (Frank et al., 2008; Riquelme, Heimiller, Barthel, and Leinwand, unpublished observations). In addition, increases in cell area and volume are positively correlated with sarcomeric organization, determined by sarcomeric disarray, in response to PE (Bass et al., 2012; Buvoli et al., 2012). Further, consistent with what is seen in intact hearts, the cells show increases in the speed and force of contraction when treated with PE (Zobel et al., 2002). This response undoubtedly represents the initial compensatory phase of adrenergic stimulation, and this has limitations in terms of studying the longer decompensation and failure phases. Common downstream readouts of induction of pathological cardiac hypertrophy, both in vivo and in vitro, include reactivation of a “fetal gene program.” Such genes include atrial natriuretic factor (ANF), brain natriuretic peptide, α-skeletal muscle actin, and β-myosin heavy chain (Chien et al., 1991; Harvey and Leinwand, 2011). In addition, activation of Fak and MEK1/2/ERK1/2 signaling pathways are hallmarks of pathological cardiac remodeling (Bass et al., 2012; Huang et al., 2015).
TABLE 2:
Hypertrophic stimuli and markers of cardiac hypertrophy in commonly utilized in vitro model systems.
| Cell type | Pathogenic hypertrophic stimuli | Methodologies used to determine cell hypertrophy | Contractility after hypertrophic stimuli | Fetal gene reexpression with hypertrophic stimuli |
|---|---|---|---|---|
| Neonatal cardiomyocytes (NRVMs/NMVMs) | PE | Coulter counter (diameter and volume) | Action potentials show increases in the speed and force of contraction when treated with PE (Zobel et al., 2002) | Yes |
| NE | Protein content normalized to DNA content | |||
| Ang II | Cell area determined by image analysis | |||
| ET-1 | Diacylglycerol mimetic PMA | |||
| Adult cardiomyocytes (ARVMs/AMVMs) | Ang II | Morphometry | Action potentials show increases in shortening amplitude and force of contraction when treated with ISO ( Jiang et al., 2012) | Yes |
| ISO | (3)H-phenylalanine incorporation | |||
| NE | Total protein content | |||
| PE | Myosin heavy chain content | |||
| Mechanical loading | Time course of activation of protein synthesis | |||
| HL-1 immortalized cardiomyocytes | cAMP | Cell area determined by image analysis | Ang II treatment induces fibrillations (Tsai et al., 2011) | Yes |
| Ang II | ||||
| ET-1 | ||||
| ISO | ||||
| ANF-T-antigen cardiomyocytes | Unknown | Unknown | Unknown | Unknown |
| H2C9 myoblasts | Ang II | Cell area determined by image analysis | Unknown | Yes, brain natriuretic peptide |
| ET-1 | ||||
| ISO | ||||
| Human embryonic and pluripotent stem cells (ESCs) | ISO | Undetermined | Action potentials mimic those seen in isolated human fetal ventricular tissue (Mummery et al., 2003) | Undetermined |
| PE |
Ang II, angiotensin II; ET-1, endothelin-1; ISO, isoproterenol; NE, norepinephrine; PE, phenylephrine; PMA, phorbol 12-myristate 12-acetate.
FIGURE 1:
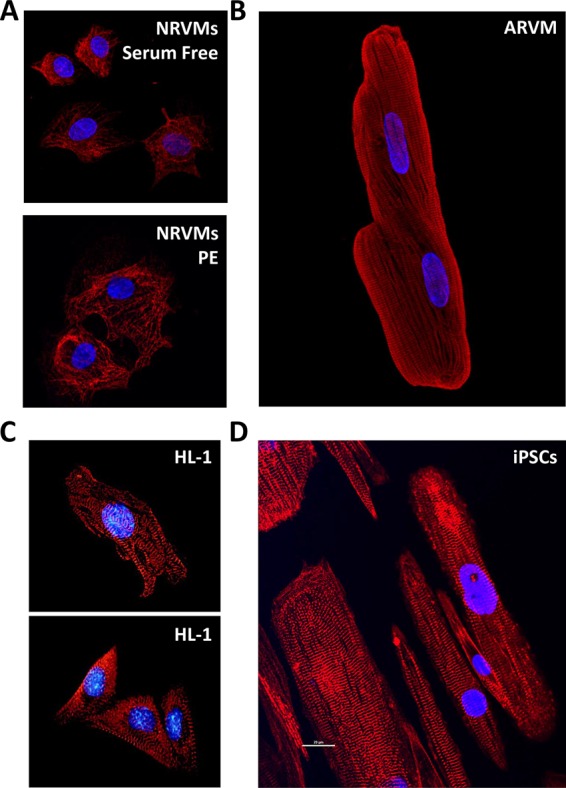
Morphology and sarcomeres in primary cardiac myocytes and the HL-1 cell line. Blue, 4′,6-diamidino-2-phenylindole for nuclei. (A) Mononuclear NRVMs either untreated (top, serum free) or treated with PE (bottom) and stained for myosin heavy chain (red). (B) Binuclear ARVM stained for myosin heavy chain (red). (C) HL-1 cells stained for myosin (top) or titin (bottom). Published with permission from White et al. (2004). (D) iPSC-derived cardiomyocytes cultured on nanopatterned surfaces for 80 to 100 d postdifferentiation induction and stained for α-actinin (image provided by the Michael Regnier laboratory).
Given the desire to develop agents that that blunt or block cardiac cellular hypertrophy, rodent myocytes represent an opportunity to conduct high-throughput screens for agents that block cellular hypertrophy in vitro. Although NRVMs have used utilized in targeted drug screening assays (Dolinsky et al., 2015; von Lueder et al., 2015), the lack of a high-throughput imaging technology that can reproducibly detect cardiac hypertrophy has prohibited the use of these cells in large-scale analysis (Zhang et al., 1999). The use of primary cardiac cells in high- throughput screens could potentially lower the high attrition rates of lead compounds because compounds that cause decreased cell viability could be excluded by this initial assay. An automated, high-throughput NRVM imaging technique has been successfully used to objectively quantify multiple hallmarks of cardiac hypertrophy: myocyte size, elongation, circularity, and sarcomeric organization (Bass et al., 2012). A similar approach was used to quantify both morphology and gene expression profiles of NRVMs in response to 15 hypertrophic agonists (Ryall et al., 2014). A high-throughput screen was recently used to identify microRNAs with the ability to regulate NRVM hypertrophy (Jentzsch et al., 2012). Further expansion of this high-throughput imaging approach was used in a recent study that measured cell size and ANF induction in NRVMs exposed to PE and PMA (Reid et al., 2016). Using this high-content imaging approach, the authors were able to successfully identify compounds that successfully blocked two distinct NRVM hypertrophic models (Reid et al., 2016). This seminal study provides the first example of an in vitro drug-screening assay using primary cardiomyocytes, which, it can be hoped, will reduce the time from drug discovery to in vivo drug trials.
The availability of genetically modified mouse models also provides an opportunity to analyze the structural and functional roles of specific proteins or modifications of those proteins hypothesized to play a role in cardiac disease. Isolation of myocytes from genetically altered rodents allows investigation of sarcomere integrity, force, and rate of contraction ex vivo. Screens for therapeutics could potentially be performed on cardiac myocytes modified to mimic human genetic cardiovascular disease. For example, loss of the muscle LIM protein (MLP) in mice leads to a dilated cardiac phenotype (Arber et al., 1997; Heineke et al., 2010). Isolated NMVMs from these mice show disrupted myofibrillar architecture after 5 d in culture, suggesting that these spontaneously beating myocytes have impaired resistance to mechanical stress. Myofibrillar disruption in MLP-null NMVMs was rescued by transfection of MLP-null NMVMs with an MLP overexpression vector, thus conclusively showing MLP expression is essential for myofibrillar function and resistance to contraction-induced stress in cardiomyocytes. Following the initial murine MLP-knockout study, genetic analysis in a large patient cohort, including patients diagnosed with nonfamilial dilated cardiomyopathy and nonfamilial hypertrophic cardiomyopathy (HCM), uncovered three unrelated HCM patients, each carrying a unique MLP missense mutation. Further analysis revealed cosegregation of clinically affected individuals within each of the families of the index patients with each of the respective mutations in MLP (Geier et al., 2003). Another example in which the study of NMVMs led to insight into the molecular mechanisms behind the development of a cardiac phenotype is the Hspa4-knockout mouse model (Mohamed et al., 2012). As a molecular chaperone, HSPA4 was found to be up-regulated in murine hearts subjected to pressure overload and in failing human hearts. In Hspa4-null mice, cardiac hypertrophy along with enhanced activation of gp130-STAT3, CaMKII, and calcineurin-NFAT signaling pathways was observed in the intact myocardium. Because this was not a cardiomyocyte-specific knockout, it was unclear which cell type within the heart was primarily responsible for these signaling alterations and the development of hypertrophy. NMVMs isolated from neonatal Hspa4-null mice had a significant increase in cross-sectional area and increased expression of hypertrophic markers, suggesting that the hypertrophy of the intact heart was likely the result of primary defects in cardiomyocytes themselves. Hence NMVMs have the advantage of the abundance of genetic alterations in the mouse (Table 1).
TABLE 1:
Advantages and disadvantages of the most commonly used in vitro cardiomyocyte model systems.
| Cell type | Advantages | Disadvantages |
|---|---|---|
| Neonatal cardiomyocytes (NRVMs/NMVMs) | Relatively easy isolation | Immature phenotype |
| Cost-effective | ||
| Greatest ratio of cell number per animal | ||
| Spontaneously beat in culture | ||
| Can be maintained in a serum-free culture medium | ||
| Can be maintained up to 28 d in culture after isolation | ||
| Large number of genetic models available | ||
| Small, circular cells can be analyzed by automated cell systems (fluorescence-activated cell sorting, Coulter, etc.) | ||
| Easily transfectable with lipid or electroporation methodologies | ||
| Respond to hypertrophic stimuli | ||
| Adult cardiomyocytes (ARVMs/AMVMs) | Cost-effective | Technically challenging isolation |
| Mature sarcomeric structure is ideal for patch-clamp/contractility studies | Must be transfected with viral vectors | |
| Presence of mature ion channels are ideal for Ca2+ imaging studies | Can only be maintained for a short time in culture after isolation | |
| Can be maintained in a serum-free culture medium | Do not spontaneously beat in culture | |
| Large number of genetic models available | ||
| Respond to hypertrophic stimuli | ||
| HL-1 cardiomyocytes | Immortalized | Derived from AT-1 atrial tumor cell lineage and do not recapitulate ventricular cells in culture |
| Homogeneous | Have to be maintained in medium containing a cardioprotective agent, a hypertrophic stimulus, and an atrial differentiation factor | |
| Rapid expansion | ||
| Easily manipulated | ||
| Contract spontaneously in ideal culture conditions | ||
| ANF-T-antigen cardiomyocytes | Immortalized | Derived from atrial tumor cell lineage and do not recapitulate ventricular cells in culture |
| Homogeneous | Unknown response to pathological stimuli | |
| Rapid expansion | ||
| Contract spontaneously | ||
| H2C9 myoblasts | Immortalized | Immature |
| Homogeneous | Do not spontaneously beat in culture | |
| Rapid expansion | Media must be supplemented with an atrial differentiation factor in order to differentiate in cardiomyocytes and express cardiomyocyte lineage markers | |
| Easily manipulated | ||
| Ventricular origin | ||
| Respond to hypertrophic stimuli | ||
| Human embryonic and pluripotent stem cells (ESCs) | Immortalized | Expensive |
| Rapid expansion | Technically challenging | |
| Easily manipulated | Numerous protocols for differentiation make it difficult to compare data across different studies | |
| Ventricular origin | ||
| Respond to hypertrophic stimuli | ||
| Contract spontaneously in ideal culture conditions | Immature unless maintained in culture for 12–15 wk | |
| Heterogeneous |
Adult ventricular myocytes
Adult cardiomyocytes have been isolated from many different organisms for the purpose of detailed study of cardiac function and the cellular basis of heart disease. Early studies often used relatively large animals, such as cats or rabbits, yielding many cells from a single animal (Jacobson and Piper, 1986; Mitcheson et al., 1998). Dogs and guinea pigs have also proven to be useful for isolation of adult cardiomyocytes (Horackova et al., 1996; Maltsev et al., 2008). The current standard for adult cardiomyocytes is the adult rat ventricular myocyte (ARVM), in large part due to the relative ease and cost-effectiveness of the rat as an animal model. Even though the first isolation of ARVMs occurred 40 years ago (Powell and Twist, 1976), no single, universal method is currently used for isolation of ARVMs. Instead, investigators follow protocols that are similar to one another in principle (i.e., retrograde perfusion of the heart with an enzymatic solution) but differ widely in details (e.g., apparatus, enzyme(s), dissociation methods; Mitcheson et al., 1998; Louch et al., 2011). Thus the reproducibility of results from studies using ARVMs suffers from this lack of consistency.
Primary cardiomyocytes are commonly prepared from adult mice, as well as from rats (O’Connell et al., 2007 ). Adult mouse ventricular myocytes (AMVMs) have many characteristics in common with ARVMs, with a few important differences. The isolation procedure for AMVMs is much the same as for ARVMs but yields fewer cells per animal and requires different perfusion pressure and cannula size. For pathologies for which transgenic mouse models are available, the decreased efficiency of cell isolation is offset by the opportunity to study these animal models at the cellular level. It is important to note that AMVMs isolated from transgenic or otherwise diseased mice have been exposed to the disease conditions over a period of days to months. Therefore the phenotypes observed may be due to chronic or long-term changes, including altered gene expression, fibrosis, and tissue remodeling. The same can be said for cardiomyocytes isolated from rats and mice on which surgical procedures, such as induction of pressure overload, have been performed (Waring et al., 2014). For example, myocytes isolated from a transgenic mouse model of familiar HCM show numerous structural and functional abnormalities, but these mice also have interstitial fibrosis even at the younger ages studied, and the older mice have systolic dysfunction (Olsson et al., 2004). Thus the cellular phenotypes may be a secondary consequence of the advanced disease progression. For observation of acute effects, in vitro treatment with hypertrophic agonists or infection of ARVMs with viruses (typically adenovirus or adeno-associated virus) carrying genes of interest is more effective (Zhou et al., 2000; Louch et al., 2011). Virus-mediated expression of sarcomeric proteins in ARVMs enables not only the study of the gross cellular phenotype of disease-associated mutations, but also detailed study of sarcomere dynamics (Thompson and Metzger, 2014). The two approaches (surgical/transgenic and drug/virus) can also be combined to analyze signaling pathways downstream of pathogenic hypertrophic stimuli (Métrich et al., 2008; Feest et al., 2014).
The greatest advantage of primary adult cardiomyocytes for cellular modeling of heart disease may be in the similarity of the morphology and behavior of the isolated cells to those of cardiomyocytes in intact tissue (Table 1). ARVMs, AMVMs, and adult cardiomyocytes from other animal models are rod shaped and binucleated and have well-organized sarcomeres throughout the cell body (Figure 1B; Zhou et al., 2000; Louch et al., 2011). Healthy adult cardiomyocytes contract regularly when paced with an electrical stimulus. Pacing the cells in culture not only permits a great deal of control over experimental variables (e.g., duration, intensity, and frequency of stimulation), but also may serve to maintain the cells in more physiological state (Berger et al., 1994; Joshi-Mukherjee et al., 2013). As with any experimental system, the use of isolated adult cardiomyocytes has some significant limitations. The morphology and functional characteristics of adult cardiomyocytes change over time in culture (Banyasz et al., 2008; Hammer et al., 2010; Louch et al., 2011), abrogating one of the key advantages of these cells. Therefore most investigators perform their analyses within 24 h of isolation, although some studies are carried out over the course of several days (Communal et al., 2000; Miyashita et al., 2001; Snabaitis et al., 2005; Mu and Harvey, 2012). This limits the utility of adult cardiomyocytes for investigation of phenotypes other than those that present acutely upon application of the experimental stimulus. Several approaches have been tested to lengthen their usable time in culture, including electrical pacing (Holt et al., 1997; Joshi-Mukherjee et al., 2013), drug treatments (Tian et al., 2012), and low temperatures (Abi-Gerges et al., 2013). The cells are also very difficult to transfect and are not as infectable as other cell types, such as NRVMs or immortalized cell lines (Louch et al., 2011).
As with neonatal cells, many different treatments have been used to cause hypertrophy in primary adult cardiomyocytes. These stimuli include PE (Sowah et al., 2014), NE (Thandapilly et al., 2011), Ang II (Sowah et al., 2014), isoproterenol (Li et al., 2011), and mechanical loading (Clark et al., 1993; Leychenko et al., 2011; Table 2). Similar readouts to hypertrophy of NRVMs are also present, including activation of fetal gene program (Li et al., 2011; Sowah et al., 2014) and increase in the rate of protein synthesis, total protein levels, and abundance of myosin heavy chain protein (Clark et al., 1993; Thandapilly et al., 2011; Sowah et al., 2014). Owing to their elongated shape, adult cardiomyocytes are not compatible with flow-based cell volume measurement methods and are typically measured using image-based morphometric analysis (Clark et al., 1993; Métrich et al., 2008; Thandapilly et al., 2011; Sowah et al., 2014). The shape of cardiomyocytes is closely related to contractile function. NRVMs plated on substrates patterned to constrain the cells in a specific length:width ratio had the best contractile function at ratios similar to those of cells in a healthy adult heart and performed poorly at ratios similar to those of myocytes in failing hearts (Kuo et al., 2012). Unfortunately, the changes observed in the shape of hypertrophic cardiomyocytes in vivo and in vitro are not necessarily consistent. In early stages of hypertrophy, cells become wider, as reflected by an increase in the cross-sectional area (Kehat and Molkentin, 2010). In the later stages of hypertrophy, heart failure is occurring, and cells typically appear elongated. Therefore it is not surprising that in vivo models, in which hypertrophy is chronic, report an increase in left ventricular myocyte length (∼30%; Gerdes et al., 1996), whereas adult cardiomyocytes treated acutely with hypertrophic stimuli in vitro have similar increases in cell width instead (Kehat et al., 2011).
For all of the cell types thus far discussed, in vitro study of the effects of hypertrophy-inducing compounds (e.g., isoproterenol, PE) or potentially antihypertrophic drugs is technically straightforward. The simplicity of treating cells in culture is one of the primary advantages of an in vitro model relative to more physiologically relevant in vivo models. However, concerns have arisen about the applicability of drug screening in animal models (in vitro or in vivo) to human medicine (Tzatzalos et al., 2016). The development of new cellular models that more accurately recapitulate hypertrophic disease in humans has been a focus for the past decade or more.
PERMANENT “CARDIAC” MYOCYTE CELL LINES
Immortalized cardiac myocytes
Because cardiac myocytes are terminally differentiated and thus do not divide in culture, many attempts have been made to derive immortalized cardiac cell lines. Four such lines are AT-1 (Claycomb and Palazzo, 1980; Delcarpio et al., 1991; Lanson et al., 1992; Kline et al., 1993; Yang et al., 1994), HL-1 (Claycomb et al., 1998), ANF-T-antigen (Steinhelper et al., 1990), and H9C2 (Kimes and Brandt, 1976) cells. These cell lines, derived from either mouse atrial cardiomyocyte tumors (AT-1, HL-1, and ANF-T-antigen) or embryonic rat ventricular tissue (H9C2), do not precisely recapitulate the structural and functional physiology of acutely isolated ventricular cardiomyocytes (Figure 1C and Table 2).
Although AT-1, HL-1, and ANF-T-antigen cells have the same origin, they differ in culturing techniques. Unlike AT-1 cells, HL-1 cells can undergo freeze–thaw cycles; however, HL-1 cells need to be maintained in medium containing adenosine (a known cardioprotective agent), retinoic acid (a reagent used to induce or maintain atrial cardiomyocyte differentiation; Zhang et al., 2011), and norepinephrine (a hypertrophic stimulus; Simpson et al., 1982) to maintain a differentiated phenotype and stimulate beating. These requirements vastly limit their usefulness in developing models of cardiac disease (Claycomb et al., 1998). Although HL-1 cells do express connexin 43, the dominant connexin in ventricular cells, they also express high levels of connexin 40, predominantly expressed in atrial cells (Dias et al., 2014). In addition, HL-1 cells do not express connexin 45, which is expressed by both atrial and ventricular cells localized in the atrioventricular node and is essential for cardiac conduction and embryonic development (Kumai et al., 2000). Thus the absence of connexin 45 makes HL-1 cells less myocyte-like compared with primary cell lines. Because these studies did not compare the protein expression of each connexin isoform in HL-1 cells with primary myocytes, it is difficult to determine how closely HL-1 cells recapitulate the connexin expression profile of ventricular myocytes. Hence altered expression of connexins in conjunction with immature or absent sarcomere formation (Claycomb et al., 1998) and an overall heterogeneous cellular phenotype limit the utility of these cells for modeling ventricular or arrhythmogenic cardiac diseases. A recent study found that increased expression of cAMP was sufficient to induce cell hypertrophy and fetal gene expression in HL-1 cells (Fang et al., 2015). However, sarcomeric organization was not thoroughly investigated, nor were the results compared with a primary cardiac myocyte. In a separate study analyzing isoproterenol (ISO)- and ET-1–mediated hypertrophic responses, the authors did not see increases in cell size or fetal gene activation, although they did see some downstream changes in hypertrophic protein profiles in treated cells (Hong et al., 2011).
Similar to HL-1 cells, ANF-T-antigen–derived cardiomyocytes spontaneously beat in culture, can undergo freeze–thaw cycles, and can be passaged. Spontaneously beating cardiomyocytes can be obtained for at least four serial passages without appearing to undergo any dedifferentiation, but serial passaging of these cells does appear to decrease the rapid expansion of the cells. However, because these cells possess the ability to undergo subcutaneous propagation and tumor formation when reintroduced into syngeneic hosts, they may express markers reflective of their tumor lineages. In addition, these cells have yet to be analyzed after treatment with hypertrophic stimuli (Steinhelper et al., 1990). Thus, AT-1, HL-1, and ANF-T-antigen cell lines do spontaneously beat in culture and utilize technically challenging protocols to maintain their contractile properties through serial passages. Combined with their atrial conduction phenotype and their immature sarcomeres, their use in contractility studies and models of ventricular hypertrophy is limited.
Although H9C2 cells were initially isolated from embryonic mouse ventricular tissue, they must undergo a “differentiation” procedure involving the addition of retinoic acid in low-serum (1%) medium in order to express cardiac differentiation markers (Menard et al., 1999). Without the addition of retinoic acid, H9C2 cells will differentiate into an “adult” skeletal muscle phenotype in low-serum media. The cell surface area of H9C2 cells increased approximately threefold in response to Ang II and ET-1 whereas NRVMs increased approximately twofold in the same study (Watkins et al., 2011; Table 1). Fetal gene activation was also present in H9C2 cells at approximately the same induction level as for NRVMs; however, distinct sarcomere organization in H9C2 cells was absent after hypertrophic stimulation (Watkins et al., 2011). Thus, H9C2 cells have been shown to respond to hypertrophic stimuli, but they do not display mature sarcomeric organization, nor do they spontaneously beat in culture as do primary neonatal cardiac myocytes. Therefore, H9C2 cells have limited functionality in contraction-based studies. However, unlike HL-1 cells, H9C2 cells possess β-tubulin II, a mitochondrial isoform of tubulin that plays an important role in mitochondrial function and regulation and may contribute to the decreased cell viability of H9C2 cells in response to hypoxia–reoxygenation injury compared with HL-1 cells (Kuznetsov et al., 2015). However, because this study did not compare HL-1 or H9C2 cells with primary cells, it is difficult to gauge their response to hypoxic environments with primary cells. Although these cells were reportedly isolated from a ventricular origin, their morphology and gene expression patterns are more similar to skeletal muscle myoblasts than to primary cardiomyocytes (Menard et al., 1999). Thus, although these cells are commonly used in place of primary cardiomyocytes, one should use caution when interpreting data obtained from these cells and applying them to other in vitro or in vivo cardiomyopathy model systems.
iPSC/ESC cardiac myocytes
One of the biggest hurdles facing researchers in the pharmaceutical industry is the development of an in vitro assay using human cells to test potential pharmaceutical compounds for cardiotoxicity in a high-throughput manner. For such assays, it is critical that the cardiac cells be as “adult” human as possible, given that the mechanism for cardiac repolarization after contraction can differ greatly among species and levels of maturity (Astashkina et al., 2012). ESCs and iPSCs, particularly those derived from patients with genetic diseases, are increasingly regarded as invaluable tools for disease modeling and drug-screening assays (Drawnel et al., 2014; Hashem et al., 2015). iPSCs taken from patients with a genetic disease are particularly valuable because they capture the precise genetic mutation and background from the individual from whom they are derived. Consequently, the use of iPSCs could pave the way to personalized cardiomyocyte therapeutics in a way that no other cell model has done.
Although directed cell differentiation is still difficult for some cell lineages, several protocols exist for differentiation of both iPSCs and ESCs into cardiomyocytes. For 15 years, ESCs have been used to generate cardiomyocytes (Kehat et al., 2001, 2002; Mummery et al., 2002, 2003). Many early studies examining cardiomyocyte differentiation from ESCs struggled with low cardiomyocyte yields (Kehat et al., 2001). In addition, although the spontaneously beating ESC-derived cardiomyocytes expressed early cardiomyocyte lineage markers, they remained phenotypically immature, appearing more similar to NRVMs than ARVMs. Recent advances in cell culture techniques have increased the percentage of cardiomyocytes in these cultures, even increasing the yield of cardiomyocytes to a level that could be used to potentially regenerate the infarcted human myocardium (Niebruegge et al., 2008; Chong et al., 2014). However, it is clear from studies in which ESC-derived cardiomyocytes were injected into an ischemic primate model that the ESC-derived cardiomyocytes used were not fully mature and increased the incidence of cardiac arrhythmias (Chong et al., 2014). The increased incidence of arrhythmias is not too surprising, given that prior patch-clamp electrophysiology and subsequent adrenergic receptor stimulation, by both PE and ISO, showed that ESC-derived cardiomyocytes displayed action potentials similar to those of human fetal ventricular cells isolated at 16 wk of gestation. In addition, staining for junctional proteins such as connexin 43 showed that ESC-derived cardiomyocytes were immature, although Ca2+ imaging showed electrical coupling between adjacent cells (Mummery et al., 2003). Thus, although ESCs have been used successfully in vitro to mimic contractility responses to PE and ISO (Mummery et al., 2003; Braam et al., 2010), these cells are clearly not fully functional, differentiated cardiomyocytes and need to be viewed with the same limitations as the neonatal animal models.
iPSCs, possibly the most innovative stem cell discovery in the past decade, are generated directly from somatic cells by the introduction of transcription factors (Takahashi and Yamanaka, 2006). Like ESCs, iPSCs can self-renew and differentiate into cellular derivatives of all three germ layers (Takahashi and Yamanaka, 2006; Murata et al., 2010). The first method used for generating iPSC-derived cardiomyocytes was the embryoid body (EB) suspension protocol (Kehat et al., 2001; Fujiwara et al., 2011; Muller et al., 2012). Although the EB method does produce differentiated myocytes, the efficiency is low (Braam et al., 2010), hampering the use of this protocol in high-throughput drug screens. Although directed, growth-factor dependent methods of cardiomyocyte differentiation have been developed (Paige et al., 2010; Kattman et al., 2011), their reliance on recombinant growth factors significantly increases their variability, technical difficulty, and the cost associated with these protocols. Cost-saving protocols using small molecules as opposed to recombinant human cytokines show great promise and may reduce the cost of these cultures substantially (Karakikes et al., 2014; Zanella et al., 2014; Zanella and Sheikh, 2016). Although many improvements have been made regarding cardiomyocyte differentiation in stem cells, the efficiency of proliferation and differentiation remains lower for iPSCs than for ESCs (Mauritz et al., 2008). A study found that complete return to pluripotency might not be necessary in order to generate cardiomyocytes, reducing time to differentiation and thus overall cost (Efe et al., 2011). In addition, commercially available iPSC-derived cardiomyocytes from sources such as Cellular Dynamics (iCell Cardiomyocytes, Madison, WI) and Axolbio (Little Chesterford, Cambridgeshire, UK) increase the accessibility of these cells to labs that lack the funding, resources, or experienced personnel necessary to develop these types of cells de novo. As mentioned earlier, the greatest advantage to using iPSCs over any other cell type mentioned thus far is the ability to use cells from patients with known genetic mutations. As with ESCs, iPSCs have also been used to study PE- and ISO-induced cardiac hypertrophy (Braam et al., 2013; Liang et al., 2013; Table 2). However, unlike ESCs, in which genetic manipulation is necessary in order to recapitulate disease-causing mutations, iPSCs derived from patients with known mutations associated with HCM have been used to recapitulate hallmarks of cardiomyocyte dysfunction associated with cardiac hypertrophy in vitro (Carvajal-Vergara et al., 2010; Moretti et al., 2010; Lan et al., 2013; Ma et al., 2013, 2015; Han et al., 2014). As an example, a study in which iPSC-derived cardiomyocytes generated from a 10-member family cohort carrying a hereditary HCM missense mutation (Arg663His) in the Myh7 gene was able to recapitulate cellular hallmarks of HCM in vitro (Lan et al., 2013). iPSCs carrying this missense mutation exhibited cellular enlargement, contractile arrhythmia at the single-cell level, and dysregulation of calcium cycling. Of interest, pharmacological restoration of calcium homeostasis prevented the development of hypertrophy and electrophysiological irregularities seen in untreated cells. This study highlights the importance of using these cells as both disease models and tools for drug screening.
It is also worth noting that, whereas ESCs and iPSCs remain the best models for human cardiomyocytes, the mechanisms and pathways involved in differentiation are ambiguous, and there are numerous conflicting reports on the purity and maturity of these cells. Most current studies investigating these cells were performed within the first 2–3 wk after differentiation, a time at which these “cardiomyocyte” cells resemble an immature, embryonic-like phenotype (Hartman et al., 2016). More recent studies, aimed at producing more mature cardiomyocytes, have examined cells 12–15 wk after differentiation induction. The cells in these studies are characterized by longer, more rectangular architecture (Snir et al., 2003; Földes et al., 2011; Lundy et al., 2013), the appearance of mature Z-, A-, H-, and I-bands, and more tightly packed, parallel-oriented myofibril arrays (Figure 1D; Kamakura et al., 2013). Combining longer differentiation procedures (80–100 d postdifferentiation) and nanopatterned surfaces has reduced the fragility of iPSC-derived myofibrils, allowing measurements to be performed similar to those conducted in ARVMs (Pioner et al., 2016). Thus, whereas iPSCs and ESCs remain a good in vitro model system for analyzing cardiotoxicity for future therapeutics, the immaturity of these cells at shorter time points and the length of time it takes to make them phenotypically more similar to differentiated cardiomyocytes make them less than ideal for applications in which fully differentiated myocytes are essential. Because other model systems, such as the ARVMs and NRVMs discussed earlier, are easy to establish and cost-effective and have relatively low variability, animal-based cardiomyocyte in vitro model systems remain the most commonly used cells in cardiac research (Table 1).
ALTERNATIVE AND ENGINEERED MATRICES
There is a major thrust in cardiac myocyte biology to develop culture conditions that more closely mimic the native niche in the heart. The development of cardiac tissue in vivo is dependent on a wide range of physical stimuli (Lindsey et al., 2014). Therefore it is not surprising that alterations in the physical environment in which cardiac cells are cultured can have a dramatic effect on their phenotype. The majority of studies on cultured cardiac myocytes are done on tissue culture polystyrene or glass, the elastic modulus of which is 106-fold greater than that of a normal heart. This can affect the structure and function of the cells in many ways. For example, embryonic cardiomyocytes on stiff substrates show a progressive loss of rhythmic beating and have fewer striated myofibrils than cells plated on a softer substrate (Engler et al., 2008). Furthermore, it is well known that the physical environment (e.g., substrates, force loading, electrical stimulation) affects the phenotype and differentiation of stem cells (Discher et al., 2009). Given the importance of these factors for differentiation and maturation of cardiomyocytes in vivo (Turnbull et al., 2014; Zhu et al., 2014), manipulation of substrate elasticity, composition, and geometry is a logical place to begin optimizing differentiation and culture protocols for both primary and iPSC/ESC-derived cardiomyocytes.
For primary neonatal cardiomyocytes, the goals of alternative culture methods include increased longevity in culture, induction of an adult gene expression profile, and organization of myofibrils. Modification of substrate stiffness is a common starting point for cell and tissue engineering, but the effects of alterations in substrate elasticity on cellular phenotype of primary cardiomyocytes are not clear. In contrast to embryonic chick cardiomyocytes, neonatal rat and mouse cardiomyocytes show increased myofibrillar organization on stiff substrates (20–34 kPa or 2 MPa) relative to soft ones (1–5 kPa; Engler et al., 2008; Yahalom-Ronen et al., 2015). The differences could be due to the cell type (e.g., embryonic vs. neonatal) or to the substrates and extracellular matrices (e.g., collagen-coated polyacrylamide vs. fibronectin-coated polydimethylsiloxane). Primary cardiomyocytes are very sensitive to the matrix on which they are plated; cells plated on a surface coated with an integrin-binding arginine-glycine-aspartic acid (RGD) peptide have a less mature phenotype than those plated on whole RGD-containing matrix proteins such as laminin and collagen (LaNasa and Bryant, 2009). One way to create a tissue scaffold with biologically relevant extracellular matrix composition is to use decellularized hearts or heart tissues that can then be repopulated with myocytes or progenitor cells (Ott et al., 2008; Lu et al., 2013). These scaffolds retain the three-dimensional (3D) structure of an intact heart but, like any system, have drawbacks, such as reduced control over the differentiation program of cells seeded within them.
It is important to consider that the cellular environment in vivo is dynamic (Li et al., 2014). Therefore culture conditions that mimic the changes in substrate stiffness or patterning may provide a more physiological environment. For example, hydrogels that stiffen over time promote maturation of embryonic chicken cardiomyocytes, as determined by myofibril orientation and expression of mature cardiac markers such as troponin T (Young and Engler, 2011). The 3D nature of the physiological environment is also a key consideration. The expression of cardiac-specific biomarkers and cellular responses to hypertrophic stimuli have been improved in both primary cardiomyocytes and ESC-derived cardiomyocytes by culture within 3D hydrogels (Shapira-Schweitzer et al., 2009). Multidimensional micromolded gelatin surfaces produce a longer period of active contraction in culture and improved metabolic function in neonatal cardiomyocytes and also increase the longevity of iPSC-derived cardiomyocytes (McCain et al., 2014).
There are many possible approaches to the principal problem of cardiac myocyte maturation with iPSC- and ESC-derived cardiomyocytes (Yang et al., 2014). The simplest approach is time; when iPSC derived cardiomyocytes are kept in culture for a long enough period (days to months), the cells reach a more mature state (Lundy et al., 2013). However, this does not fully recapitulate the phenotype of adult cardiomyocytes and is clearly inefficient. It appears that optimal differentiation occurs on substrates with an elasticity that is similar to that of the intact adult heart (Hazeltine et al., 2014). Of interest, iPSCs are sensitive to this mechanical parameter only during the early specification stage. Improvements in myofibril alignment and mechanical output have been achieved with a combination of tunable polyacrylamide substrates and defined geometry of adhesion (Ribeiro et al., 2015). Alternatively, culture of iPSC-derived cardiomyocytes on a thick Matrigel “mattress” for 5–7 d after initial differentiation improves the contractile function of the cells to a level typically only seen after 80–120 d in culture (Feaster et al., 2015). The contractile kinetics of these cells is similar to that of freshly isolated adult rabbit cardiomyocytes, and there is an elevation in the level of cardiac troponin I. A shift from expression of the slow skeletal troponin I to the cardiac troponin I has been identified as a useful marker of the maturity of iPSC-derived cardiomyocytes (Bedada et al., 2014). The identity of the substrate on which the cells are cultured also has a significant effect on maturity of the resulting phenotype. Screening of a library of combinatorial polymers yielded a substrate that produces cells with improved contractility, mitochondrial function, and, most notably, a switch to expression of cardiac troponin I (Chun et al., 2015).
These pioneering methods produced iPSC- or ESC-derived cardiomyocytes that are similar in structure and function to adult cells, increasing their utility for the study of hypertrophy and other cardiac disorders. They also lengthened the duration of stable phenotypes and increased the maturity of neonatal cardiomyocytes. These approaches are useful for studying responses of individual cells, but many aspects of disease depend on interactions between multiple cells of the same or different cell types. There are many groups devising approaches to culturing cardiomyocytes in a defined architecture to create microtissues (Thavandiran et al., 2013; Tiburcy and Zimmermann, 2014; Tzatzalos et al., 2016; Zhang et al., 2016). A combination of defined architecture or mechanical load and the addition of support cells results in improvements in the organization, electrophysiology, and calcium handling of iPSC-derived cardiomyocytes (Tulloch et al., 2011; Nunes et al., 2013). Engineered heart tissues generated from neonatal rat cardiomyocytes have higher levels of binucleation, improved sarcomere assembly, and a more physiological response to hypertrophy than neonatal cells in standard culture conditions (Tiburcy et al., 2011). Such engineered heart tissues are being used to model HCM and elucidate pathogenic mechanisms of disease-causing mutations (Wijnker et al., 2016). Cardiac microtissues, including those with cocultured fibroblasts and vascular cells, may be able to mimic disease progression and predict drug efficacy more accurately than single-cell assays (Ou et al., 2011; Mathur et al., 2015; Tzatzalos et al., 2016). In this case, the presence of multiple cells and additional cell types is both a benefit, because it is more physiological, and a drawback, due to the limitations in types of assays that can be performed (e.g., difficulty in measuring the size of individual cardiomyocytes) and the confounding effects of multiple cell types.
CONCLUSIONS
The study of cardiac hypertrophy in vitro has yielded a great many insights into the signaling pathways activated on exposure of cardiomyocytes to various hypertrophic stimuli. Although primary cardiomyocytes remain the tool of choice for many cell biologists seeking to uncover the mechanistic underpinnings of cardiac hypertrophy, alternate model systems, including iPSC- and ESC-derived cardiomyocytes, are gaining ground. The advent of engineered culture conditions has vastly improved the maturity of iPSC/ESC-derived cardiomyocytes, and this model has reached the stage at which biologically interesting investigations can be performed. These new methods do not completely abrogate concerns about using induced cells (e.g., purity, toxicity, completeness of differentiation) but do add to the available toolbox. Remaining challenges in optimizing in vitro model systems for understanding cardiac hypertrophy include consistency between in vivo and in vitro models, development of standardized protocols to allow direct comparisons among studies, and refinement of techniques to reduce the technical hurdles associated with culture of primary or induced cardiomyocytes.
Acknowledgments
We thank Christa L. Blenck for ARVM isolation and staining, Ann Robinson for NRVM isolation, Joseph M. Dragavon for ARVM and NRVM confocal image acquisition, Scott Davis for ARVM image construction, and Michael Regnier for the iPSC image. This work is supported by National Institutes of Health Grants GM029090 and HL119937 to L.A.L. M.A.B. is supported by National Institutes of Health Training Grant 2T32HL007822-16.
Abbreviations used:
- α-SMA
alpha-skeletal muscle actin
- AMVM
adult mouse ventricular myocyte
- ANF
atrial natriuretic factor
- Ang II
angiotensin II
- ARVM
adult rat ventricular myocyte
- β-MHC
beta-myosin heavy chain
- BNP
brain natriuretic peptide
- cAMP
adenosine 3,5-cyclic monophosphate
- DCM
dilated cardiomyopathy
- ESC
embryonic stem cell
- ET-1
endothelin-1
- HCM
hypertrophic cardiomyopathy
- iPSC
induced pluripotent stem cell
- ISO
isoproterenol
- MLP
muscle LIM protein
- NE
norepinephrine
- NMVM
neonatal mouse ventricular myocyte
- NRVM
neonatal rat ventricular myocyte
- PE
phenylephrine
- PMA
phorbol 12-myristate 12-acetate.
Footnotes
REFERENCES
- Abi-Gerges N, Pointon A, Pullen GF, Morton MJ, Oldman KL, Armstrong D, Valentin JP, Pollard CE. Preservation of cardiomyocytes from the adult heart. J Mol Cell Cardiol. 2013;64:108–119. doi: 10.1016/j.yjmcc.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Arber S, Hunter JJ, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. 2012;134:82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Banyasz T, Lozinskiy I, Payne CE, Edelmann S, Norton B, Chen B, Chen-Izu Y, Izu LT, Balke CW. Transformation of adult rat cardiac myocytes in primary culture. Exp Physiol. 2008;93:370–382. doi: 10.1113/expphysiol.2007.040659. [DOI] [PubMed] [Google Scholar]
- Bass GT, Ryall KA, Katikapalli A, Taylor BE, Dang ST, Acton ST, Saucerman JJ. Automated image analysis identifies signaling pathways regulating distinct signatures of cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2012;52:923–930. doi: 10.1016/j.yjmcc.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedada FB, Chan SSK, Metzger SK, Zhang L, Zhang J, Garry DJ, Kamp TJ, Kyba M, Metzger JM. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports. 2014;3:594–605. doi: 10.1016/j.stemcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger HJ, Prasad SK, Davidoff AJ, Pimental D, Ellingsen O, Marsh JD, Smith TW, Kelly RA. Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture. Am J Physiol. 1994;266:H341–H349. doi: 10.1152/ajpheart.1994.266.1.H341. [DOI] [PubMed] [Google Scholar]
- Bishopric NH, Kedes L. Adrenergic regulation of the skeletal alpha-actin gene promoter during myocardial cell hypertrophy. Proc Natl Acad Sci USA. 1991;88:2132–2136. doi: 10.1073/pnas.88.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam SR, Tertoolen L, Casini S, Matsa E, Lu HR, Teisman A, Passier R, Denning C, Gallacher DJ, Towart R, et al. Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem Cell Res. 2013;10:48–56. doi: 10.1016/j.scr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:107–116. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Buvoli M, Buvoli A, Leinwand LA. Effects of pathogenic proline mutations on myosin assembly. J Mol Biol. 2012;415:807–818. doi: 10.1016/j.jmb.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YW, Balikov DA, Feaster TK, Williams CH, Sheng CC, Lee J-B, Boire TC, Neely MD, Bellan LM, Ess KC, et al. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials. 2015;67:52–64. doi: 10.1016/j.biomaterials.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WA, Rudnick SJ, LaPres JJ, Andersen LC, LaPointe MC. Regulation of hypertrophy and atrophy in cultured adult heart cells. Circ Res. 1993;73:1163–1176. doi: 10.1161/01.res.73.6.1163. [DOI] [PubMed] [Google Scholar]
- Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb WC, Palazzo MC. Culture of the terminally differentiated adult cardiac muscle cell: a light and scanning electron microscope study. Dev Biol. 1980;80:466–482. doi: 10.1016/0012-1606(80)90419-4. [DOI] [PubMed] [Google Scholar]
- Communal C, Colucci WS, Singh K. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against β-adrenergic receptor-stimulated apoptosis. Evidence for G(i)-dependent activation. J Biol Chem. 2000;275:19395–19400. doi: 10.1074/jbc.M910471199. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Macleod BA, Walker MJ. Models for the study of arrhythmias in myocardial ischaemia and infarction: the use of the rat. J Mol Cell Cardiol. 1987;19:399–419. doi: 10.1016/s0022-2828(87)80585-0. [DOI] [PubMed] [Google Scholar]
- Delcarpio JB, Lanson NA, Jr, Field LJ, Claycomb WC. Morphological characterization of cardiomyocytes isolated from a transplantable cardiac tumor derived from transgenic mouse atria (AT-1 cells) Circ Res. 1991;69:1591–1600. doi: 10.1161/01.res.69.6.1591. [DOI] [PubMed] [Google Scholar]
- Dias P, Desplantez T, El-Harasis MA, Chowdhury RA, Ullrich ND, Cabestrero de Diego A, Peters NS, Severs NJ, MacLeod KT, Dupont E. Characterisation of connexin expression and electrophysiological properties in stable clones of the HL-1 myocyte cell line. PLoS One. 2014;9:e90266. doi: 10.1371/journal.pone.0090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RJ, Wilson GJ. Studying ischemic preconditioning in isolated cardiomyocyte models. Cardiovasc Res. 2006;70:286–296. doi: 10.1016/j.cardiores.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky VW, Soltys CL, Rogan KJ, Chan AY, Nagendran J, Wang S, Dyck JR. Resveratrol prevents pathological but not physiological cardiac hypertrophy. J Mol Med. 2015;93:413–425. doi: 10.1007/s00109-014-1220-8. [DOI] [PubMed] [Google Scholar]
- Drawnel FM, Boccardo S, Prummer M, Delobel F, Graff A, Weber M, Gerard R, Badi L, Kam-Thong T, Bu L, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9:810–821. doi: 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Robinson J, Wang-Hu J, Jiang L, Freeman DA, Rivkees SA, Wendler CC. cAMP induces hypertrophy and alters DNA methylation in HL-1 cardiomyocytes. Am J Physiol Cell Physiol. 2015;309:C425–C436. doi: 10.1152/ajpcell.00058.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, et al. Matrigel mattress: a method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2015;117:995–1000. doi: 10.1161/CIRCRESAHA.115.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feest ER, Korte FS, Tu AY, Dai J, Razumova MV, Murry CE, Regnier M. Thin filament incorporation of an engineered cardiac troponin C variant (L48Q) enhances contractility in intact cardiomyocytes from healthy and infarcted hearts. J Mol Cell Cardiol. 2014;72:219–227. doi: 10.1016/j.yjmcc.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Földes G, Mioulane M, Wright JS, Liu AQ, Novak P, Merkely B, Gorelik J, Schneider MD, Ali NN, Harding SE. Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy. J Mol Cell Cardiol. 2011;50:367–376. doi: 10.1016/j.yjmcc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Brors B, Hanselmann C, Lüdde M, Katus HA, Frey N. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51:309–318. doi: 10.1161/HYPERTENSIONAHA.107.098046. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Yan P, Otsuji TG, Narazaki G, Uosaki H, Fukushima H, Kuwahara K, Harada M, Matsuda H, Matsuoka S, et al. Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PLoS One. 2011;6:e16734. doi: 10.1371/journal.pone.0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, Perrot A, Ozcelik C, Binner P, Counsell D, Hoffmann K, Pilz B, Martiniak Y, Gehmlich K, van der Ven PF, et al. Mutations in the human muscle LIM protein gene in families with hypertrophic cardiomyopathy. Circulation. 2003;107:1390–1395. doi: 10.1161/01.cir.0000056522.82563.5f. [DOI] [PubMed] [Google Scholar]
- Gerdes AM, Onodera T, Wang X, McCune SA. Myocyte remodeling during the progression to failure in rats with hypertension. Hypertension. 1996;28:609–614. doi: 10.1161/01.hyp.28.4.609. [DOI] [PubMed] [Google Scholar]
- Hammer K, Ruppenthal S, Viero C, Scholz A, Edelmann L, Kaestner L, Lipp P. Remodelling of Ca2 +handling organelles in adult rat ventricular myocytes during long term culture. J Mol Cell Cardiol. 2010;49:427–437. doi: 10.1016/j.yjmcc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Han L, Li Y, Tchao J, Kaplan AD, Lin B, Li Y, Mich-Basso J, Lis A, Hassan N, London B, et al. Study familial hypertrophic cardiomyopathy using patient-specific induced pluripotent stem cells. Cardiovasc Res. 2014;104:258–269. doi: 10.1093/cvr/cvu205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harary I, Farley B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963a;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- Harary I, Farley B. In vitro studies on single beating rat heart cells. II. Intercellular communication. Exp Cell Res. 1963b;29:466–474. doi: 10.1016/s0014-4827(63)80009-9. [DOI] [PubMed] [Google Scholar]
- Hartman ME, Dai DF, Laflamme MA. Human pluripotent stem cells: prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv Drug Deliv Rev. 2016;96:3–17. doi: 10.1016/j.addr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PA, Leinwand LA. Cellular mechanisms of cardiomyopathy. J Cell Biol. 2011;194:355–365. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem SI, Perry CN, Bauer M, Han S, Clegg SD, Ouyang K, Deacon DC, Spinharney M, Panopoulos AD, Izpisua Belmonte JC, et al. Brief report: oxidative stress mediates cardiomyocyte apoptosis in a human model of danon disease and heart failure. Stem Cells. 2015;33:2343–2350. doi: 10.1002/stem.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine LB, Badur MG, Lian X, Das A, Han W, Palecek SP. Temporal impact of substrate mechanics on differentiation of human embryonic stem cells to cardiomyocytes. Acta Biomater. 2014;10:604–612. doi: 10.1016/j.actbio.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke J, Wollert KC, Osinska H, Sargent MA, York AJ, Robbins J, Molkentin JD. Calcineurin protects the heart in a murine model of dilated cardiomyopathy. J Mol Cell Cardiol. 2010;48:1080–1087. doi: 10.1016/j.yjmcc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt E, Lunde PK, Sejersted OM, Christensen G. Electrical stimulation of adult rat cardiomyocytes in culture improves contractile properties and is associated with altered calcium handling. Basic Res Cardiol. 1997;92:289–298. doi: 10.1007/BF00788941. [DOI] [PubMed] [Google Scholar]
- Hong HM, Song EJ, Oh E, Kabir MH, Lee C, Yoo YS. Endothelin-1- and isoproterenol-induced differential protein expression and signaling pathway in HL-1 cardiomyocytes. Proteomics. 2011;11:283–297. doi: 10.1002/pmic.201000018. [DOI] [PubMed] [Google Scholar]
- Horackova M, Croll RP, Hopkins DA, Losier AM, Armour JA. Morphological and immunohistochemical properties of primary long-term cultures of adult guinea-pig ventricular cardiomyocytes with peripheral cardiac neurons. Tissue Cell. 1996;28:411–425. doi: 10.1016/s0040-8166(96)80027-9. [DOI] [PubMed] [Google Scholar]
- Huang Q, Huang J, Zeng Z, Luo J, Liu P, Chen S, Liu B, Pan X, Zang L, Zhou S. Effects of ERK1/2/PPARalpha/SCAD signal pathways on cardiomyocyte hypertrophy induced by insulin-like growth factor 1 and phenylephrine. Life Sci. 2015;124:41–49. doi: 10.1016/j.lfs.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Jacobson SL, Piper HM. Cell cultures of adult cardiomyocytes as models of the myocardium. J Mol Cell Cardiol. 1986;18:661–678. doi: 10.1016/s0022-2828(86)80939-7. [DOI] [PubMed] [Google Scholar]
- Jentzsch C, Leierseder S, Loyer X, Flohrschütz I, Sassi Y, Hartmann D, Thum T, Laggerbauer B, Engelhardt S. A phenotypic screen to identify hypertrophy-modulating microRNAs in primary cardiomyocytes. J Mol Cell Cardiol. 2012;52:13–20. doi: 10.1016/j.yjmcc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xu C, Wang Y, Gao L, Yan C, Li D, Sun H. Beta2-adrenoceptor transfection enhances contractile reserve of isolated rat ventricular myocytes exposed to chronic isoprenaline stimulation by improving beta1-adrenoceptor responsiveness. J Recept Signal Transduct Res. 2012;32:36–41. doi: 10.3109/10799893.2011.610107. [DOI] [PubMed] [Google Scholar]
- Joshi-Mukherjee R, Dick IE, Liu T, O’Rourke B, Yue DT, Tung L. Structural and functional plasticity in long-term cultures of adult ventricular myocytes. J Mol Cell Cardiol. 2013;65:76–87. doi: 10.1016/j.yjmcc.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, et al. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77:1307–1314. doi: 10.1253/circj.cj-12-0987. [DOI] [PubMed] [Google Scholar]
- Karakikes I, Senyei GD, Hansen J, Kong CW, Azeloglu EU, Stillitano F, Lieu DK, Wang J, Ren L, Hulot JS, et al. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl Med. 2014;3:18–31. doi: 10.5966/sctm.2013-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karliner JS, Simpson PC, Taylor JE, Honbo N, Woloszyn W. Adrenergic receptor characteristics of cardiac myocytes cultured in serum-free medium: comparison with serum-supplemented medium. Biochem Biophys Res Commun. 1985;128:376–382. doi: 10.1016/0006-291x(85)91689-4. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kehat I, Davis J, Tiburcy M, Accornero F, Saba-el-leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, et al. ERK1/2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91:659–661. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- Kline RP, Sorota S, Dresdner KP, Steinhelper ME, Lanson NA, Jr, Wit AL, Claycomb WC, Field LJ. Spontaneous activity in transgenic mouse heart: comparison of primary atrial tumor with cultured AT-1 atrial myocytes. J Cardiovasc Electrophysiol. 1993;4:642–660. doi: 10.1111/j.1540-8167.1993.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Kono M, Kisanuki A, Takasaki K, Nakashiki K, Yuasa T, Kuwahara E, Mizukami N, Uemura T, Kubota K, Ueya N, et al. Left ventricular systolic function is abnormal in diastolic heart failure: re-assessment of systolic function using cardiac time interval analysis. J Cardiol. 2009;53:437–446. doi: 10.1016/j.jjcc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–3512. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- Kuo PL, Lee H, Bray MA, Geisse NA, Huang YT, Adams WJ, Sheehy SP, Parker KK. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am J Pathol. 2012;181:2030–2037. doi: 10.1016/j.ajpath.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta. 2015;1853:276–284. doi: 10.1016/j.bbamcr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMorte VJ, Thorburn J, Absher D, Spiegel A, Brown JH, Chien KR, Feramisco JR, Knowlton KU. Gq- and ras-dependent pathways mediate hypertrophy of neonatal rat ventricular myocytes following alpha 1-adrenergic stimulation. J Biol Chem. 1994;269:13490–13496. [PubMed] [Google Scholar]
- Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaNasa SM, Bryant SJ. Influence of ECM proteins and their analogs on cells cultured on 2-D hydrogels for cardiac muscle tissue engineering. Acta Biomater. 2009;5:2929–2938. doi: 10.1016/j.actbio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Lanson NA, Jr, Glembotski CC, Steinhelper ME, Field LJ, Claycomb WC. Gene expression and atrial natriuretic factor processing and secretion in cultured AT-1 cardiac myocytes. Circulation. 1992;85:1835–1841. doi: 10.1161/01.cir.85.5.1835. [DOI] [PubMed] [Google Scholar]
- Leychenko A, Konorev E, Jijiwa M, Matter ML. Stretch-Induced hypertrophy activates NFkB-Mediated VEGF secretion in adult cardiomyocytes. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0029055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AH, Liu PP, Villarreal FJ, Garcia RA. Dynamic changes in myocardial matrix and relevance to disease: Translational perspectives. Circ Res. 2014;114:916–927. doi: 10.1161/CIRCRESAHA.114.302819. [DOI] [PubMed] [Google Scholar]
- Li C, Li J, Cai X, Sun H, Jiao J, Bai T, Zhou XW, Chen X, Gill DL, Tang XD. Protein kinase D3 is a pivotal activator of pathological cardiac hypertrophy by selectively increasing the expression of hypertrophic transcription factors. J Biol. Chem. 2011;286:40782–40791. doi: 10.1074/jbc.M111.263046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SE, Butcher JT, Yalcin HC. Mechanical regulation of cardiac development. Front Physiol. 2014;5:318. doi: 10.3389/fphys.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51:288–298. doi: 10.1016/j.yjmcc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T-Y, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:1–11. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- Lundy SD, Zhu W-Z, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Wei H, Lu J, Huang D, Liu Z, Loh LJ, Islam O, Liew R, Shim W, Cook SA. Characterization of a novel KCNQ1 mutation for type 1 long QT syndrome and assessment of the therapeutic potential of a novel IKs activator using patient-specific induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2015;6:39. doi: 10.1186/s13287-015-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Wei H, Zhao Y, Lu J, Li G, Sahib NB, Tan TH, Wong KY, Shim W, Wong P, et al. Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int J Cardiol. 2013;168:5277–5286. doi: 10.1016/j.ijcard.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Maltsev Va, Kyle JW, Mishra S, Undrovinas A. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1.5 antisense inhibition. Am J Physiol Heart Circ Physiol. 2008;295:H667–H676. doi: 10.1152/ajpheart.00111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Ma Z, Loskill P, Jeeawoody S, Healy KE. In vitro cardiac tissue models: current status and future prospects. Adv Drug Deliv Rev. 2015;96:203–213. doi: 10.1016/j.addr.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials. 2014;35:5462–5471. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaouar A, Florian M, Wang D, Danalache B, Jankowski M, Gutkowska J. Anti-hypertrophic effects of oxytocin in rat ventricular myocytes. Int J Cardiol. 2014;175:38–49. doi: 10.1016/j.ijcard.2014.04.174. [DOI] [PubMed] [Google Scholar]
- Menard C, Pupier S, Mornet D, Kitzmann M, Nargeot J, Lory P. Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9C2 cardiac cells. J Biol Chem. 1999;274:29063–29070. doi: 10.1074/jbc.274.41.29063. [DOI] [PubMed] [Google Scholar]
- Métrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc’h F. Epac mediates β-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Takeishi Y, Takahashi H, Kato S, Kubota I, Tomoike H. Role of calcineurin in insulin-like growth factor-1-induced hypertrophy of cultured adult rat ventricular myocytes. Jpn Circ J. 2001;65:815–819. doi: 10.1253/jcj.65.815. [DOI] [PubMed] [Google Scholar]
- Mohamed BA, Barakat AZ, Zimmermann WH, Bittner RE, Muhlfeld C, Hunlich M, Engel W, Maier LS, Adham IM. Targeted disruption of Hspa4 gene leads to cardiac hypertrophy and fibrosis. J Mol Cell Cardiol. 2012;53:459–468. doi: 10.1016/j.yjmcc.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- Mu X, Harvey P. Estrogen differentially affects expression of calcium handling genes in female and male adult cardiomyocytes. J Student Res. 2012;1:31–37. [Google Scholar]
- Muller M, Stockmann M, Malan D, Wolheim A, Tischendorf M, Linta L, Katz SF, Lin Q, Latz S, Brunner C, et al. Ca2+ activated K channels-new tools to induce cardiac commitment from pluripotent stem cells in mice and men. Stem Cell Rev. 2012;8:720–740. doi: 10.1007/s12015-011-9324-9. [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward D, van den Brink CE, Bird SD, Doevendans PA, Opthof T, Brutel de la Riviere A, Tertoolen L, van der Heyden M, Pera M. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat. 2002;200:233–242. doi: 10.1046/j.1469-7580.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Tohyama S, Fukuda K. Impacts of recent advances in cardiovascular regenerative medicine on clinical therapies and drug discovery. Pharmacol Ther. 2010;126:109–118. doi: 10.1016/j.pharmthera.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Nakaoka M, Iwai-Kanai E, Katamura M, Okawa Y, Mita Y, Matoba S. An alpha-adrenergic agonist protects hearts by inducing Akt1-mediated autophagy. Biochem Biophys Res Commun. 2015;456:250–256. doi: 10.1016/j.bbrc.2014.11.067. [DOI] [PubMed] [Google Scholar]
- Naqvi N, Li M, Yahiro E, Graham RM, Husain A. Insights into the characteristics of mammalian cardiomyocyte terminal differentiation shown through the study of mice with a dysfunctional c-kit. Pediatr Cardiol. 2009;30:651–658. doi: 10.1007/s00246-008-9366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebruegge S, Nehring A, Bar H, Schroeder M, Zweigerdt R, Lehmann J. Cardiomyocyte production in mass suspension culture: embryonic stem cells as a source for great amounts of functional cardiomyocytes. Tissue Eng Part A. 2008;14:1591–1601. doi: 10.1089/ten.tea.2007.0247. [DOI] [PubMed] [Google Scholar]
- Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Cardiovasc. Proteomics. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- Olsson MC, Palmer BM, Stauffer BL, Leinwand LA, Moore RL. Morphological and functional alterations in ventricular myocytes from male transgenic mice with hypertrophic cardiomyopathy. Circ Res. 2004;94:201–207. doi: 10.1161/01.RES.0000111521.40760.18. [DOI] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor Da. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Ou DB, He Y, Chen R, Teng JW, Wang HT, Zeng D, Liu XT, Ding L, Huang JY, Zheng QS. Three-dimensional co-culture facilitates the differentiation of embryonic stem cells into mature cardiomyocytes. J Cell Biochem. 2011;112:3555–3562. doi: 10.1002/jcb.23283. [DOI] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioner JM, Racca AW, Klaiman JM, Yang K-C, Guan X, Pabon L, Muskheli V, Zaunbrecher R, Macadangdang J, Jeong MY, et al. Isolation and mechanical measurements of myofibrils from human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Reports. 2016;6:885–896. doi: 10.1016/j.stemcr.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T, Twist VW. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976;72:327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Reid BG, Stratton MS, Bowers S, Cavasin MA, Demos-Davies KM, McKinsey TA. Discovery of novel small molecule inhibitors of cardiac hypertrophy using high throughput, high content imaging. J Mol Cell Cardiol. 2016;97:106–113. doi: 10.1016/j.yjmcc.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AJS, Ang Y-S, Fu J-D, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall KA, Bezzerides VJ, Rosenzweig A, Saucerman JJ. Phenotypic screen quantifying differential regulation of cardiac myocyte hypertrophy identifies CITED4 regulation of myocyte elongation. J Mol Cell Cardiol. 2014;72:74–84. doi: 10.1016/j.yjmcc.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira-Schweitzer K, Habib M, Gepstein L, Seliktar D. A photopolymerizable hydrogel for 3-D culture of human embryonic stem cell-derived cardiomyocytes and rat neonatal cardiac cells. J Mol Cell Cardiol. 2009;46:213–224. doi: 10.1016/j.yjmcc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Shenasa M, Shenasa H, El-Sherif N. Left ventricular hypertrophy and arrhythmogenesis. Card Electrophysiol Clin. 2015;7:207–220. doi: 10.1016/j.ccep.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983;72:732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P, McGrath A, Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982;51:787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- Snabaitis AK, Muntendorf A, Wieland T, Avkiran M. Regulation of the extracellular signal-regulated kinase pathway in adult myocardium: differential roles of Gq/11, Gi and G 12/13 proteins in signalling by α1-adrenergic, endothelin-1 and thrombin-sensitive protease-activated receptors. Cell Signal. 2005;17:655–664. doi: 10.1016/j.cellsig.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–H2363. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- Sowah D, Brown BF, Quon A, Alvarez BV, Casey JR. Resistance to cardiomyocyte hypertrophy in ae3-/- mice, deficient in the AE3 Cl-/HCO3- exchanger. BMC Cardiovasc Disord. 2014;14:89. doi: 10.1186/1471-2261-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhelper ME, Lanson NA, Dresdner KP, Delcarpio JB, Wit AL, Claycomb WC, Field LJ. Proliferation in vivo and in culture of differentiated adult atrial cardiomyocytes from transgenic mice. Am J Physiol. 1990;259:H1826–H1834. doi: 10.1152/ajpheart.1990.259.6.H1826. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takasaki K, Miyata M, Imamura M, Yuasa T, Kuwahara E, Kubota K, Kono M, Ueya N, Horizoe Y, Chaen H, et al. Left ventricular dysfunction assessed by cardiac time interval analysis among different geometric patterns in untreated hypertension. Circ J. 2012;76:1409–1414. doi: 10.1253/circj.cj-11-1369. [DOI] [PubMed] [Google Scholar]
- Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, Zhang S, Wigle J, Kardami E, Zahradka P, Taylor C, et al. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol. 2011;668:217–224. doi: 10.1016/j.ejphar.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Thavandiran N, Dubois N, Mikryukov A, Massé S, Beca B, Simmons CA, Deshpande VS, McGarry JP, Chen CS, Nanthakumar K, et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci USA. 2013;110:E4698–E4707. doi: 10.1073/pnas.1311120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BR, Metzger JM. Cell biology of sacromeric protein engineering: disease modeling and therapeutic potential. Anat Rec. 2014;297:1663–1669. doi: 10.1002/ar.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Pahlavan S, Oleinikow K, Jung J, Ruppenthal S, Scholz A, Schumann C, Kraegeloh A, Oberhofer M, Lipp P, et al. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. J Mol Cell Cardiol. 2012;52:113–124. doi: 10.1016/j.yjmcc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Tiburcy M, Didié M, Boy O, Christalla P, Döker S, Naito H, Karikkineth BC, El-Armouche A, Grimm M, Nose M, et al. Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue. Circ Res. 2011;109:1105–1114. doi: 10.1161/CIRCRESAHA.111.251843. [DOI] [PubMed] [Google Scholar]
- Tiburcy M, Zimmermann WH. Modeling myocardial growth and hypertrophy in engineered heart muscle. Trends Cardiovasc Med. 2014;24:7–13. doi: 10.1016/j.tcm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Tsai CT, Chiang FT, Chen WP, Hwang JJ, Tseng CD, Wu CK, Yu CC, Wang YC, Lai LP, Lin JL. Angiotensin II induces complex fractionated electrogram in a cultured atrial myocyte monolayer mediated by calcium and sodium-calcium exchanger. Cell Calcium. 2011;49:1–11. doi: 10.1016/j.ceca.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, et al. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 2014;28:644–654. doi: 10.1096/fj.13-228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells: macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev. 2016;96:234–244. doi: 10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8:71–78. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2014;35:2722–2731. doi: 10.1093/eurheartj/ehs338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011;47:125–131. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–H829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- Wijnker PJM, Friedrich FW, Dutsch A, Reischmann S, Eder A, Vollert-Mannhardt I, Mearini G, Eschenhagen T, van der Velden J, Carrier L. Comparison of the effects of a truncating and a missense MYBPC3 mutation on contractile parameters of engineered heart tissue. J Mol Cell Cardiol. 2016;97:82–92. doi: 10.1016/j.yjmcc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife. 2015;4:1–18. doi: 10.7554/eLife.07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Wathen MS, Felipe A, Tamkun MM, Snyders DJ, Roden DM. K+ currents and K+ channel mRNA in cultured atrial cardiac myocytes (AT-1 cells) Circ Res. 1994;75:870–878. doi: 10.1161/01.res.75.5.870. [DOI] [PubMed] [Google Scholar]
- Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella F, Lyon RC, Sheikh F. Modeling heart disease in a dish: from somatic cells to disease-relevant cardiomyocytes. Trends Cardiovasc Med. 2014;24:32–44. doi: 10.1016/j.tcm.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella F, Sheikh F. Patient-specific induced pluripotent stem cell models: generation and characterization of cardiac cells. Methods Mol Biol. 2016;1353:147–162. doi: 10.1007/7651_2014_172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. 2016;15:669–678. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y-Y, Wang S-Q, Zhu W-Z, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao R-P. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- Zhu R, Blazeski A, Poon E, Costa KD, Tung L, Boheler KR. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2014;5:117. doi: 10.1186/scrt507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel C, Kassiri Z, Nguyen TT, Meng Y, Backx PH. Prevention of hypertrophy by overexpression of Kv4.2 in cultured neonatal cardiomyocytes. Circulation. 2002;106:2385–2391. doi: 10.1161/01.cir.0000033970.22130.93. [DOI] [PubMed] [Google Scholar]


