Abstract
Age-associated cognitive decline can reduce an individual’s quality of life. As no single neurobiological deficit can account for the wide spectrum of behavioral impairments observed in old age, it is critical to develop an understanding of how interactions between different brain regions change over the life span. The performance of young and aged animals on behaviors that require the hippocampus and cortical regions to interact, however, has not been well characterized. Specifically, the ability to link a spatial location with specific features of a stimulus, such as object identity, relies on the hippocampus, perirhinal and prefrontal cortices. Although aging is associated with dysfunction in each of these brain regions, behavioral measures of functional change within the hippocampus, perirhinal and prefrontal cortices in individual animals are often not correlated. Thus, how dysfunction of a single brain region within this circuit, such as the hippocampus, impacts behaviors that require communication with the perirhinal and prefrontal cortices remains unknown. To address this question, young and aged rats were tested on the interregion dependent object-place paired association task, as well as a hippocampal-dependent test of spatial reference memory. This particular cohort of aged rats did not show deficits on the hippocampal-dependent task, but were significantly impaired at acquiring object-place associations relative to young. These data suggest that behaviors requiring functional connectivity across different regions of the memory network may be particularly sensitive to aging, and can be used to develop models that will clarify the impact of systems-level dysfunction in the elderly.
Keywords: aging, functional connectivity, hippocampus, perirhinal cortex, prefrontal cortex
The majority of individuals over the age of 65 will experience cognitive decline that interferes with their quality of life and ability to maintain independence. Specifically, advanced age is accompanied by deficits in several distinct cognitive domains, such as spatial reference memory (Gallagher, Burwell, & Burchinal, 1993), executive function (Banuelos et al., 2014; Beas, Setlow, & Bizon, 2013; Bizon, Foster, Alexander, & Glisky, 2012), and object recognition (Burke, Ryan, & Barnes, 2012; Burke et al., 2011; Burke, Wallace, Nematollahi, Uprety, & Barnes, 2010). Importantly, age-associated cognitive dysfunction does not occur uniformly across these different domains, in part because there is profound variability amongst individuals in the behaviors that decline (Barense, Fox, & Baxter, 2002; Bizon et al., 2009; Burke et al., 2010; Davidson & Glisky, 2002; Gallagher et al., 1993; Morse, 1993).
Lesion studies have provided insights into the dissociable effects of functional loss within different brain regions on behavior, setting the foundation for hypotheses regarding the neural mechanisms of age-related cognitive decline. For example, the hippocampus (HPC) is critical for spatial reference memory (Morris, Garrud, Rawlins, & O’Keefe, 1982), but not necessary for object recognition memory (Forwood, Winters, & Bussey, 2005), or working memory at delays under 24 s, as measured by the delayed matching to position task (Sloan, Good, & Dunnett, 2006). The perirhinal cortex (PER) is integral to object recognition, but not involved in spatial reference memory (Norman & Eacott, 2005). Finally, the prefrontal cortex (PFC) supports working memory, but is not required for spatial reference memory (Sloan et al., 2006). In line with these lesion data, relationships between age-associated neurobiological alterations within the HPC (for review, see Burke & Barnes, 2010; Rosenzweig & Barnes, 2003; Samson & Barnes, 2013), PFC (Banuelos et al., 2014; Barense et al., 2002), and PER (Burke, Hartzell, Lister, Hoang, & Barnes, 2012; Burke et al., 2014) have been related to deficits in the specific cognitive function that is attributed to each of these brain regions.
Although biochemical and physiological changes within a single brain region correlate with behavioral deficits, no single neurobiological disruption can fully account for the wide spectrum of cognitive abnormalities observed in old age (Ash & Rapp, 2014). Moreover, localized disruptions within one structure plausibly affect higher-level interactions across neural systems either by impairing functional connectivity or provoking compensation. These systems-level interactions are particularly important in the context of age-related memory decline, as episodic and other explicit memories are among the most distributed processes in the brain (e.g., Nadel & Moscovitch, 1997; Squire & Alvarez, 1995). In fact, associating sensory stimuli with a spatial location or context is known to require dynamic interactions between the HPC, PER, and PFC (Barker, Bird, Alexander, & Warburton, 2007; Barker & Warburton, 2015; Jo & Lee, 2010a; Staresina & Davachi, 2008; Vilberg & Davachi, 2013). In this sense, dysfunction within a single locus of the memory network could impact the functional connectivity across brain areas. Thus, it is critical to develop behavioral models that can assay the integrity of the memory network on the systems level.
Recent research, using disconnection lesions, has emphasized the importance of interactions among the HPC, PER, and PFC for supporting the multimodal associations that are a hallmark of explicit memory. For example, in young animals, transient disconnection lesions of the HPC and PER impair the formation of object-place associations (Barker & Warburton, 2015; Jo & Lee, 2010a). Moreover, disconnecting the HPC and PFC leads to deficits in the encoding and retrieval of object-place associations (Barker & Warburton, 2015). These data suggest that quantifying an animal’s ability to form and retain object-place associations across the life span could provide insight regarding memory network interactions in advanced age. In the current experiment, young and aged rats were monitored while they performed the object-place paired association task (OPPA; Jo & Lee, 2010a), which is believed to require HPC-PER-PFC interactions (Jo & Lee, 2010a, 2010b; Lee & Solivan, 2008). Individual aged rats with intact performance on the Morris water maze test of spatial reference memory (Morris, 1984) were significantly impaired on the OPPA task. HPC dysfunction, therefore, cannot fully account for performance declines on a task that requires HPC-PER-PFC interregion communication.
Method
Subjects
A total of 14 young (4–6 months old) and 18 aged (22–26 months old) male rats were cross-characterized on the Morris water maze test of spatial reference memory (Morris, 1984) and the object-place paired association task (OPPA; Jo & Lee, 2010b). Both Fischer 344 (F344; n = 13; four young, nine aged) and Fischer 344 x Brown Norway F1 hybrid (F344xBN; n = 19; ten young, nine aged) rats from the NIA colony at Taconic Farms were used. Each rat was housed individually and maintained on a 12-hr light/dark cycle with behavioral experiments performed exclusively during the dark phase of the cycle. During OPPA behavioral testing, rats were food-restricted on moist chow to 85% of their free-feeding weight, with access to water ad libitum. All experimental procedures were performed in accordance with National Institutes of Health guidelines and were approved by Institutional Animal Care and Use Committees at the University of Florida.
Apparatus and Testing Objects
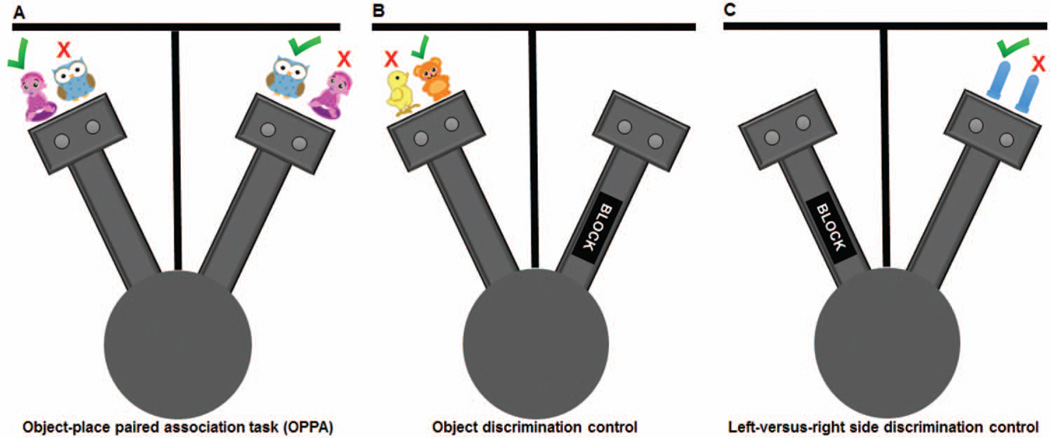
A two-arm maze (see Figure 1), similar to the maze used by Jo and Lee (2010a, 2010b), was constructed from wood and sealed with waterproof black paint. A poster board was used to separate the two arms and ensure that the rat could not see the opposite arm. Distinct salient visual cues were affixed to the divider on each side in order to clearly differentiate the two arms of the maze. The arms radiated off a starting platform that was 48.3 cm in diameter. Each arm was 84 cm long and had a rectangular choice platform (31.75 cm × 24.13 cm) attached at the end. The choice platforms each contained two food wells (2.5 cm in diameter) that were recessed into the maze floor by 1 cm. The arms and choice platforms were contained within 6.4 cm raised walls to prevent the rat from exiting the testing apparatus. To dampen the influence of extraneous noise on behavior, a white noise machine was used during behavioral training and testing.
Figure 1.
Schematic of the object-place paired association (OPPA) task and discrimination control conditions. A two-arm radial maze was used for all testing conditions. (A) During testing on the OPPA task, rats were required to discriminate between two distinct objects (e.g., girl figurine or owl); however, in the left arm the girl figurine was rewarded (green check mark) and not the owl (red X). In the right arm, the rats had to discriminate between the same two objects but the owl was the correct choice and the girl figurine was not rewarded, requiring rats to use an object-in-place rule. The placement of rewarded objects over the left or right food wells varied pseudorandomly across trials. (B) For the object discrimination control task, one arm of the maze was blocked and the rat shuttled between the central circle and choice platforms between discrimination trials. The same object was always rewarded (bear; green check mark). (C) The left-versus-right side discrimination control was carried out in the opposite arm of the maze than that used for object discrimination testing. For each trial, two identical objects covered both food wells and a single well on one side was always rewarded across trials (e.g., left well, green check mark). Whether the left or right well was the correct choice varied between rats. See the online article for the color version of this figure.
Two distinct objects were presented to individual rats during testing on the OPPA task; a plastic turtle and a ceramic baby figurine were used for one cohort, and an owl and a girl figurine were used for a second cohort (see Figure 2). There was no effect of cohort on OPPA task performance, F(1,28) = 0.21, p = .65; nor did cohort interact significantly with age group, F(1,28) = 0.01, p = .98. Both sets of object pairs were distinct in color, shape and texture. The entire maze and both objects were cleaned with 70% ethanol solution between each testing session. The same two-arm maze was used for testing animals on the control object discrimination and left-versus-right side discrimination conditions. For these control conditions, testing occurred within a single arm, while the opposing arm was blocked to prevent the rat from reaching the opposite choice platform (Figure 1B and C).
Figure 2.
Stimulus sets. Object stimuli used for the (A) first and (B) second cohort of rats tested on the object-place paired association task (OPPA), (C) for control object discrimination, and (D) control left-versus-right side discrimination. See the online article for the color version of this figure.
The Morris water maze task was carried out in a pool 1.83 m in diameter that contained a platform 12 cm in diameter. For training trials and probe tests of reference memory, the platform remained in one location, while for cued trials to assess visual function, the platform was moved to one of four different locations. The pool was filled with water at ~27 °C to a depth of 2 cm above the platform. White nontoxic tempera paint (Crayola, Easton, PA) was added to the water to obscure the platform location for reference memory trials. Visual cues were affixed to black curtains surrounding the pool. Water 2100 software (HVS Image, Buckingham, United Kingdom) was used to track the rats’ position throughout training and testing.
Handling and Habituation
Upon arrival to the facility, rats were given 7–14 days acclimation with free access to food. Following this period, rats were handled daily for several days before being tested on the Morris water maze and Spontaneous Object Recognition Task (data not shown). Prior to water maze testing, F344 rats were dyed with ammonia-free black hair colorant (Revlon, New York, NY) to improve tracking in the water maze apparatus. Rats were then handled an additional 1–2 days in the water maze room for further habituation to the testing procedures. All animals were transported to and from testing rooms in ventilated plastic bins.
Morris Water Maze Test of Spatial Reference Memory and Visible Platform Task
Assessment of spatial reference memory followed the procedures of Bizon et al. (2009). Briefly, each rat received three trials a day for 8 consecutive days. For each trial, rats were placed into the water facing the wall of the maze at one of four equally spaced start positions (north, south, east, or west). The start positions were varied in a pseudorandom fashion such that all rats started from each of the locations the same number of times. Rats were allowed to swim for up to 90 s in order to locate the platform. If they failed to find the platform after 90 s, the trial would end, and the experimenter would guide the rat to the goal location where they would remain for 30 s. After each trial, rats were placed in the plastic holding bin for a 30-s intertrial interval. Every sixth trial was a probe test of retrieval in which the platform was lowered to the bottom of the maze for the first 30 s. These probe trials were used to quantify the proximity of the swim path to the target location (cumulative search error; Gallagher et al., 1993). A training block was considered the five trials conducted on 2 consecutive days (three trials and two trials, respectively). The probe trials that were conducted after each training block were used to calculate a Spatial Learning Index, which is a weighted sum of the cumulative search error during probe Trials 2–4 (Spatial Learning Index = probe 2*1.25 + probe 3*1.6 + probe 4*1.7) as previously described (Gallagher et al., 1993).
After completion of the reference memory task, rats were given a single session with six trials of cue training. In this session, rats were trained to escape to a visible platform that was moved to a different maze quadrant for each trial. Rats were given 90 s to reach the platform and were allowed to remain there briefly before a 30-s intertrial interval. These trials were used to control for sensorimotor impairments or poor motivation that could confound the data.
Object-Place Paired Association (OPPA) Task
Following water maze testing, rats were placed on a restricted feeding schedule. Once target weights were reached (over 1–2 weeks), animals were habituated to the OPPA testing maze for 10 min per day for 2 days. Froot Loop pieces (Kellogg Company, Battle Creek, MI) were scattered throughout the maze to encourage exploration of the entire apparatus. Rats were trained to visit alternate arms of the maze by placing a single cereal treat in one random food well on either the left or right arm. Each rat began the OPPA behavioral task once it could successfully alternate arms 32 times within 30 min.
Testing on the OPPA task was carried out as previously described (Jo & Lee, 2010a). The first trial was initiated by placing the two objects over the food wells of one of the radial arms while the rat was placed in the opposite arm. The starting arm was counterbalanced across testing sessions. The same two objects were always presented in both arms of the maze; however, the position of the rewarded object within the choice platform of an arm (left or right) pseudorandomly varied across trials (Figure 1A). Successful completion of the OPPA task required rats to develop an object-in-place rule by learning to associate the different arms with distinct rewarded objects. In other words, one object was always rewarded in one arm while the alternate object was rewarded in the opposite arm. The rat only received a cereal treat if they selected the correct object, which required the displacement of the figurine to access the hidden food well. If an incorrect choice was made, the objects and food reward were immediately removed from the platform preventing the rat from selecting the alternate figurine. Following the completion of a trial, the experimenter moved the objects to the opposite arm. Consecutive trials required alternation between the two arms. If a rat failed to alternate, returning to the previously tested arm, the trial was logged as a working memory error. Criterion performance was achieved once a rat was able to select the correct object for a minimum of 26 per 32 trials, with at least 13 correct choices in each arm on 2 consecutive days. Rats were tested 5 to 6 days a week until reaching criterion for the task. A retention test was administered 48 hr after each rat had successfully achieved criterion performance.
To ensure potential age effects were not confounded by sensorimotor impairments or differences in motivation, upon completion of the OPPA task, all rats were further screened in simple object discrimination (Figure 1B) and left-versus-right side discrimination tasks (Figure 1C). The control discrimination tasks were conducted in a single arm of the radial maze. Prior to testing, the rats were rehabituated to the arena and trained to shuttle back and forth between one choice platform and a reward placed adjacent to a blockade in the central circular platform of the maze. For the object discrimination, two nonidentical novel objects were placed over the food wells in the choice platform. One of the objects, independent of location, covered a rewarded food well. Following an incorrect selection, the objects and reward were removed and the rat was required to exit the testing arm prior to initiating the next trial. Once a correct choice was made for at least 26 out of 32 trials on 2 consecutive days, the rat began testing on the left-versus-right side discrimination task.
Left-versus-right discrimination testing was conducted in the arm not used for the control object discrimination task. In order to disambiguate the control tasks, two identical novel objects were used. The rewarded side was counterbalanced across rats, with approximately half the rats rewarded for selecting the left object while the rest were rewarded for right object selection. Criterion performance was correct selection of the appropriate side (left-vs.-right) 26 out of 32 times on 2 consecutive days.
Statistical Analyses
Prior to acquiring the object-in-place rule, rats often show a response bias for a particular side (Jo & Lee, 2010a, 2010b). Therefore, indices of a bias for choosing a single object (object bias) or a food well (left-vs.-right side bias) were calculated for each rat. The object bias was the absolute value of the (total number of Object 1 choices – total number of Object 2 choices)/ (total number of trials), while the side bias was the absolute value of the (total number of left choices – total number of right choices)/(total number of trials). For the OPPA task, group means of five dependent variables (incorrect trials to criterion, object bias, side bias, working memory errors and percent correct responses during retention testing) were examined using analyses of variance (ANOVA) with the between subjects factors of rat strain (F344, F344xBN), age (young, aged), and task type (OPPA, object control, left-vs.-right side control). The group means of water maze data for the training trials (corrected integrated path length; CIPL), and the Spatial Learning Index (calculated from the cumulative search error; Gallagher et al., 1993) were also examined using ANOVAs. All analyses were performed with Statistical Package for the Social Sciences (SPSS) v22. Statistical significance was considered at p values less than 0.05. Individual group differences were assessed using either the Tukey’s HSD test or planned orthogonal contrasts in cases where specific a priori hypotheses had been made regarding group differences. The relationship between OPPA task performance and spatial reference memory was examined using Pearson’s correlation coefficients.
Results
Object-Place Paired Association (OPPA) Task Performance
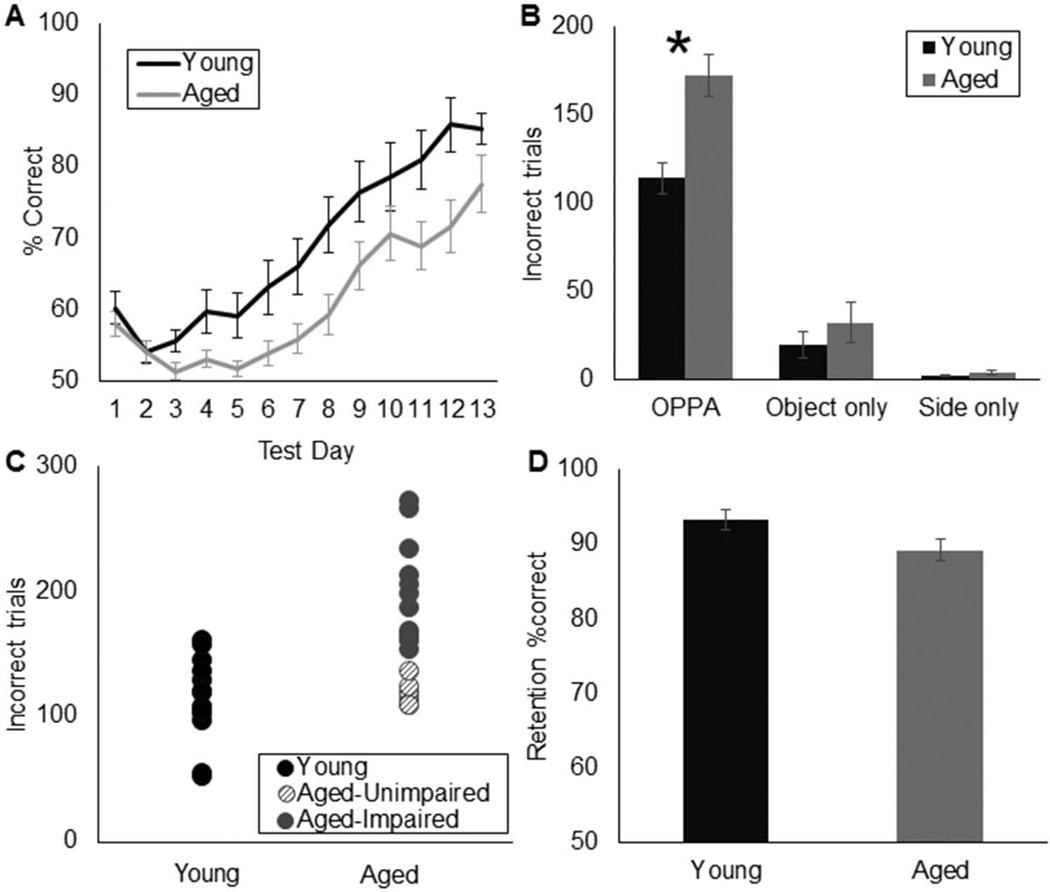
The average number of days it took animals to reach criterion performance on the OPPA task was 11.1 for the young rats (95% confidence interval [8.6, 13.6]) and 14.3 for the aged rats (95% confidence interval [10.8, 17.8]), which differed significantly T(30) = 2.93, p < .01). The mean percent correct responses during the first 13 days of testing for the young (black) and aged (gray) rats is shown in Figure 3A. The mean numbers of incorrect trials made by each age group on the OPPA task, as well as the control object discrimination and left-versus-right side discrimination conditions are presented in Figure 3B. Age had a significant effect on the number of incorrect trials made during the OPPA task, F(1, 28) = 12.17, p < .01, but not during the object discrimination control, F(1, 28) = 0.02, p = .89, or the left versus right side control, F(1, 28) = 0.59, p = .46. Together, these data show that, while aged and young rats perform comparably at discriminating between a pair of objects and the left-versus-right food wells, aged animals are selectively impaired at acquiring the object-in-place rule that is necessary for OPPA task performance. Rat strain (F344 vs. F344xBN) did not significantly affect OPPA performance, F(1, 28) = 0.21, p = .65, nor did the interaction between strain and age reach statistical significance, F(1, 28) = 0.01, p = .98. These data indicate that aged animals of both F344 and F344xBN hybrid strains have similar deficits in forming object-place associations.
Figure 3.
Object-place paired association (OPPA) task performance. (A) Percent of correct responses (Y-axis) on the OPPA task for each of the first 13 days of testing (X-axis) in young (black) and aged (gray) rats. (B) Mean number of incorrect trials made prior to reaching criterion performance (Y-axis) for young and aged rats on OPPA, object discrimination, and left-versus-right side discriminations tasks. Performance between age groups was significantly different for the OPPA task (p< .01*), but not for the control object (p= .89) or left-versus-right side (p= .46) discrimination conditions. (C) Individual performance of young and aged rats on the OPPA task. Four aged rats performed within the normative range of young animals (unimpaired; hashed circles). (D) Percent of correct responses (Y-axis) on the 48-hr delay OPPA retention test was not significantly different between age groups (p= .06; X-axis). Error bars show +/−1 standard error of the mean.
In order to determine if there was a subset of aged rats that were not impaired at acquiring object-place associations, aged rats that performed within 1 SD of young rat performance were identified (Figure 3C; hashed circles). Six of the 18 aged rats were in the normative range of young rat performance. Notably, two aged F344 and four aged F344xBN hybrid rats met this criterion for being aged-unimpaired. The stability of the estimated range of performances across the population of young animals was further validated by bootstrapping the sample of young rat data (randomly resampling the data with replacement) to generate a new sample (n = 14), from which descriptive statistics were recalculated. Aligned with standard Monte Carlo simulations for resampling, this process was repeated 2,000 times to generate a distribution of the estimated population confidence interval and standard deviation (DiCiccio & Efron, 1996; Efron, 1987), which is consistent with the application of this method to behavioral data (Kernan & Mullenix, 1991). Again, six of the 18 aged rats had performance values that fell within the estimated normative range of the young animal bootstrapped data. Importantly, the bootstrapped and raw sample data identified the same six rats as unimpaired.
Following the 48-hr delay, both young and aged rats were able to retain the object-in-place association with aged and young rats having a mean percent correct of 89% and 93%, respectively. Thus, for both age groups retention performance was above the criterion performance of 81.25%. Although there was a trend toward an effect of age on retention, the 4% difference in performances between the age groups did not reach statistical significance (Figure 3D; T(31) = 1.98; p = .06; independent-samples). The observation that aged rats did not drop below criterion performance following a 48-hr delay is consistent with a previous report, which showed that aged animals retained information regarding reward-object stimulus associations similar to young even when acquisition rates are slower (Burke et al., 2011).
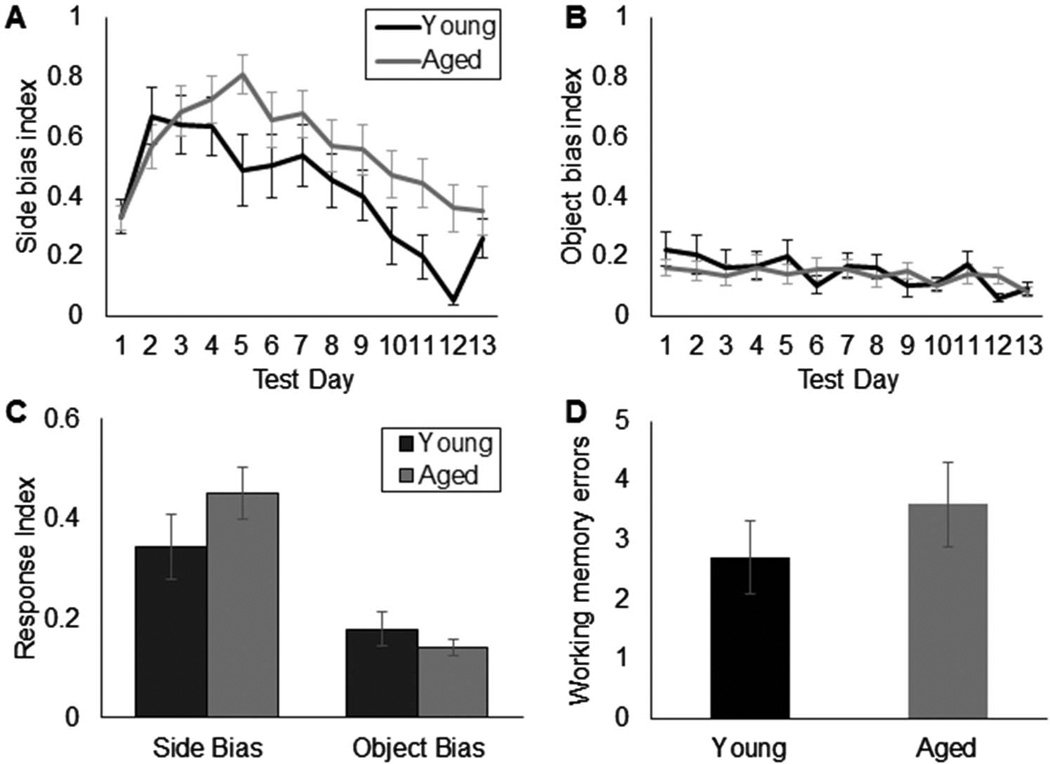
A potential contribution of distinct response biases between age groups to OPPA task performance was determined by examining the mean response index calculated from the left-versus-right side bias and the object bias (see Figure 4). A bias index of 1 would indicate an absolute preference for one stimulus over the other (e.g., the rat only chose the right side in each arm, or chose the same object for each trial), while 0 would be no bias. Both young and aged rats were significantly more likely to display a side bias relative to an object bias prior to learning the object-in-place rule, F(1, 28) = 67.49, p < .001; repeated-measures. The mean side bias as a function of test day for the young and aged rats for the first 13 days of testing in shown in Figure 4A. In both age groups, there was a tendency for the side bias to increase over the first 5 days of testing and then decrease as the animal got closer to reaching criterion. This pattern was not observed for the object bias (Figure 4B), in which a rat’s tendency to select one object over another did not change over testing. Although both age groups were more likely to show a side bias, there was no significant effect of age on the response bias index, F(1, 28) = 2.55, p = .12. Moreover, there was no interaction between age and the type of bias (side vs. object; F(1, 28) = 3.20, p = .1). Together, these data indicate that the difference between young and aged rat OPPA task performance was not due to a perseveration of object or left-versus-right selection in the aged rats. Rather, it appears that aged rats were selectively impaired at forming object-place associations.
Figure 4.
Response bias indices and working memory errors. (A) Mean left-versus-right side bias index (Y-axis) for young (black) and aged (gray) rats as a function of the first 13 test days (X-axis). (B) Mean object bias index (Y-axis) for young and aged rats for each of the first 13 test days (X-axis). (C) Mean response index across all test days (Y-axis) for the left-versus-right side and object biases. Prior to reaching criterion performance, all rats were significantly more likely to respond with a side bias than an object bias, F(1,28) = 67.49, p< .001; repeated-measures, but these response index values did not differ significantly with age, F(1, 28) = 2.55, p= .12. (D) Mean number of working memory errors (Y-axis) made by young (black) and aged (gray) rats before reaching criterion performance. The number of working memory errors did not differ significantly between age groups (T(30)= 0.92, p= .37). Error bars show +/−1 standard error of the mean.
The number of working memory errors during the entire extent of OPPA testing also did not change as a function of age (Figure 4D). Most rats only made two to four of these errors in total before reaching criterion performance, and this did not differ significantly between young and old rats (T(30) = 0.92, p = .37; equal variances not assumed). Thus, both age groups were able to track their previous arm location in order to proceed to the correct arm on a subsequent trial, indicating that aged rats retained an ability to recognize their current location and use that information to guide appropriate behavior.
Morris Water Maze Test of Spatial Reference Memory
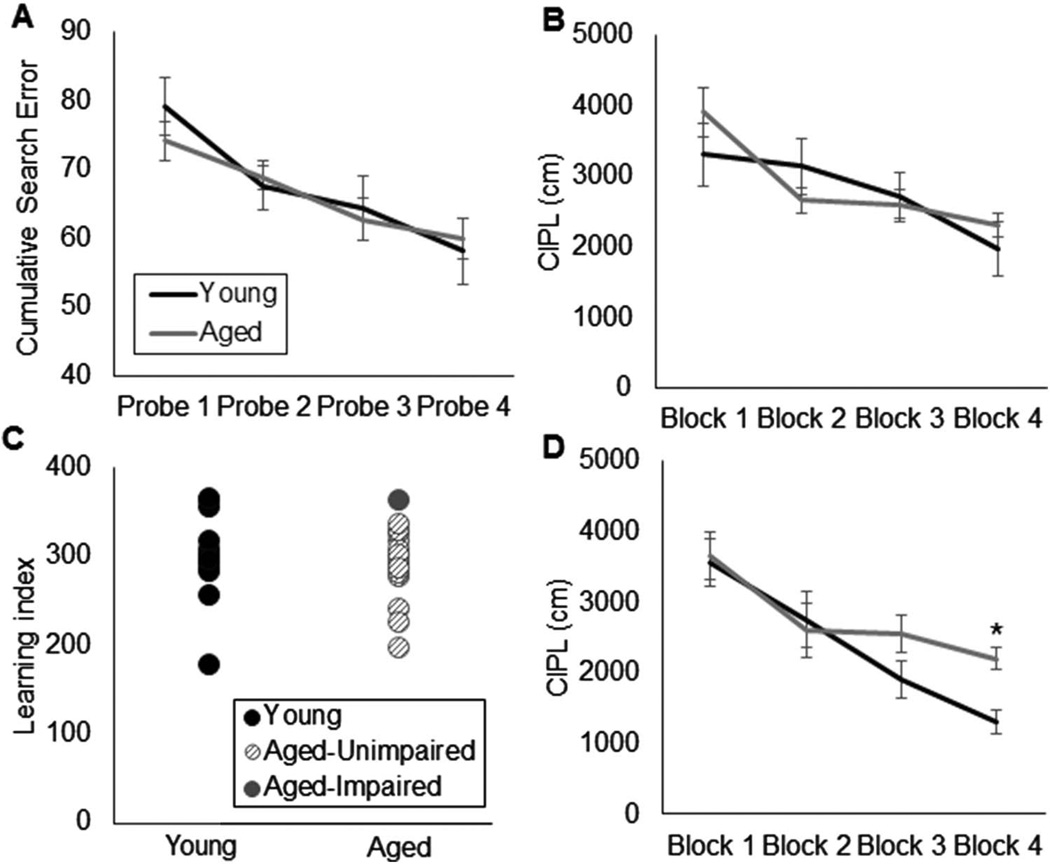
The performance of rats on the spatial reference memory version of the Morris swim task was similar across age groups. Cumulative search error during the four probe trials did not differ significantly between young and aged rats (Figure 5A); F(1, 28) = 0.16, p = .69. Critically, all rats were able to learn the platform location as evidenced by a significant decrease in cumulative search error across probe Trials 1–4, F(1, 28) = 19.03, p < .001, repeated-measures. This observation suggests that the lack of an age effect on water maze performance was not due to an overall inability of all rats to perform the task. In addition to age, the variable of strain did not have a significant effect on cumulative search error, F(1, 28) = 0.67, p = .42. Consistent with these findings, in this cohort of animals, the old rats did not have significantly longer mean corrected integral path length (CIPL) scores during the training trials compared with the young rats (Figure 5B); F(1, 28) = 0.26, p = .87. Moreover, there was a significant effect of testing block on the mean CIPL scores, F(3,84) = 17.44, p < .001 (repeated-measures), indicating that overall both the young and aged rats showed improved performance as a function of training. Planned comparisons revealed that this difference was due to significantly lower CIPL values across each subsequent testing block (p < .05 for all comparisons; repeated contrasts). There was not a significant interaction effect of testing block with age group, F(1, 28) = 0.64, p = .43 (repeated-measures) or with strain, F(1, 28) = 0.02, p = .88 (repeated-measures). Together, these data indicate that young and old rats of both strains showed similar improvement in behavioral performance over the course of water maze training.
Figure 5.
Morris water maze test of spatial reference memory. (A) Cumulative search error (Y-axis) across all probe trials (X-axis) in young (black) and aged rats (gray). Cumulative search error during the 4 probe trials did not differ significantly between age group, F(1,28) = 0.16, p= .69, and all rats showed significantly reduced search errors after training, F(3,84) = 19.03, p< .001; repeated-measures. (B) Mean corrected integrated path length (CIPL) values (Y-axis) for young (black) and (aged) rats across training blocks (X-axis). CIPL values decreased as a function of training block, F(3,84) = 17.44, p< .001 (repeated-measures), which did not differ significantly with age, F(1,28) = 0.64, p= .43. (C) Individual Spatial Learning Index values (Y-axis) for young and aged rats calculated from the cumulative search error on probe Trials 2–4. Learning index also did not significantly differ between age group, F(1,28) = 0.34, p= .56. Moreover, only one aged rat performed outside of the normative range of the young group (impaired, solid circle). (D) CIPL values (Y-axis) for a different cohort of young (black) and (aged) rats across training blocks (X-axis). In this group of rats, which were not tested on the OPPA task, there was a significant effect of age on CIPL values during training Block 4 (T(24)= 2.99, p< .01). Error bars show +/−1 standard error of the mean.
An additional means to evaluate water maze performance is to calculate a Spatial Learning Index (Gallagher et al., 1993). The learning indices of all individual rats are shown in Figure 5C. Only one aged rat performed outside of the normative range of the young animals (> 1 SD). Consistent with the lack of an age effect on CIPL and cumulative search error values, the Spatial Learning Index was also not significantly different between young and aged rats, F(1, 28) = 0.34, p = .56, or between strains, F(1, 28) = 3.48, p = .08. Finally, there was no significant interaction effect between age and rat strain on Spatial Learning Index values, F(1, 28) = 0.10, p = .76.
One prior study has shown that age-associated spatial reference memory deficits may not be evident in F344xBN hybrid rats even at 30 months of age (Hebda-Bauer, Morano & Therrien, 1999), while others show evidence of impairments by 24 months (Markowska & Savonenko, 2002; McQuail & Nicolle, 2015). Moreover, deficits are typically observed in F344 rats by 24 months of age (Shen & Barnes, 1996). The majority of rats in this particular cohort, however, maintained performance on the water maze task. Importantly, when a different cohort of rats were tested on the spatial reference version of the water maze task during the same time frame as the animals in the current study, a significant effect of age was detected for the CIPL score on Block 4 of testing (Figure 5D; T(24) = 2.99, p < .01). These data suggest that the rats in the current study included a disproportionate number of animals that did not have hippocampal impairments.
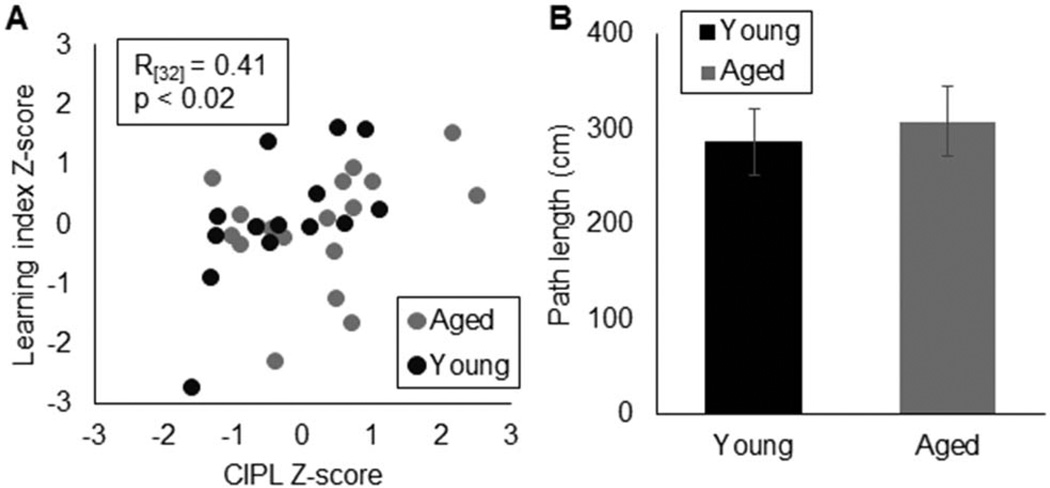
The absence of an age effect on water maze performance for the rats tested on the OPPA task was not due to low internal reliability, as the CIPL scores were significantly correlated with the Spatial Learning Index (Figure 6A; R(31) = 0.41,p < .02; Pearson’s). The sensorimotor function of young and aged rats was also similar, as indicated by the lack of a significant difference in swim path length during the visually cued trials (Figure 6B; T(30) = 0.41, p = .68). Collectively, these data indicate that the aged rats in this study did not display behavioral deficits on a HPC-dependent task, supporting the conclusion that dysfunction within the HPC cannot fully account for age-related impairments on the OPPA task.
Figure 6.
Validity of Morris water maze test data. (A) Relationship between normalized (Z-score transformed) CIPL values for training Block 4 (X-axis) and Spatial Learning Index (Y-axis). These two measures were significantly correlated (R(31)= 0.41, p< .02; Pearson’s), demonstrating the internal reliability of this water maze procedure. (B) Mean path length (Y-axis) of rats during the visually cued version of the water maze was not significantly different between age groups (T(30)= 0.41, p= .68). This indicates that the aged rats did not suffer from gross sensorimotor impairments. Error bars show +/−1 standard error of the mean.
Relationship Between Spatial Reference Memory and Object-Place Paired Association Task Performance
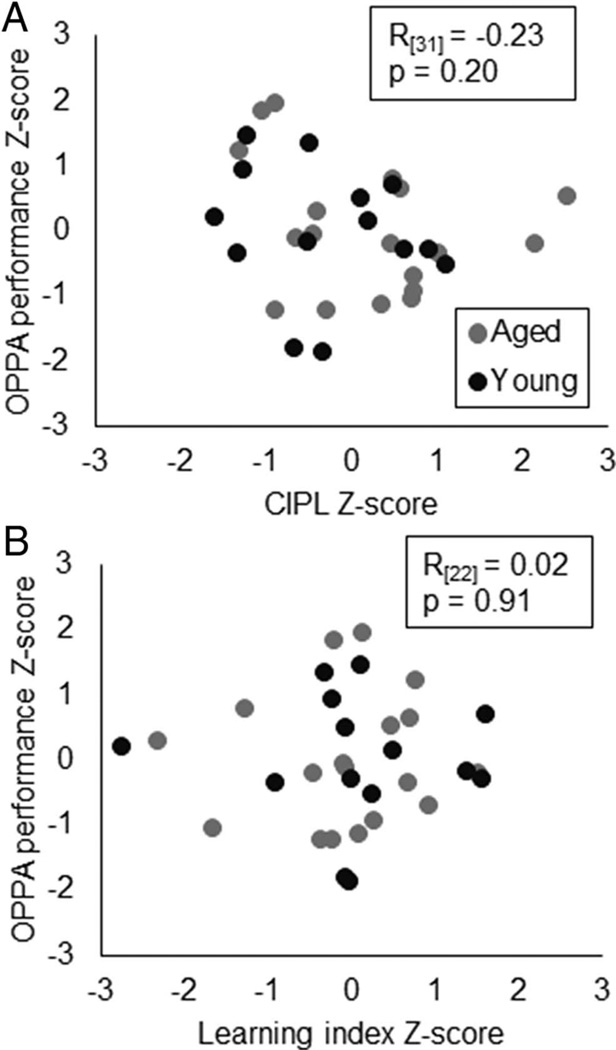
In order to further examine whether or not spatial reference memory was related to OPPA task performance, both of which rely on the HPC, the correlation coefficient between the normalized performance measures (z-scores) on these tasks was obtained. The mean CIPL values during Block 4 did not significantly correlate with the number of incorrect trials that rats made prior to reaching criterion performance on the OPPA task (Figure 7A; R(31) = −0.23, p = .20; Pearson’s). Similarly, the Spatial Learning Index did not significantly correlate with the number of incorrect trials that rats made prior to reaching OPPA criterion (Figure 7B; R(31) = 0.02, p = .91; Pearson’s). Thus, while both spatial reference memory and object-place associations require the HPC, dysfunction in this brain structure alone cannot account for age-associated impairments on the OPPA task.
Figure 7.
Spatial reference memory and object-place paired association (OPPA) task performance. (A) Z-scores of training block 4 CIPL values (Y-axis) did not significantly correlate with z-scores for the number of incorrect trials made on the OPPA task (R(31)= −0.23, p= .20; Pearson’s). (B) Z-score values of the Spatial Learning Index also did not significantly correlate with Z-score values for numbers of incorrect trials on the OPPA task (R(31)= 0.02, p= .91; Pearson’s).
The performance measures on the water maze and the OPPA task were further compared by calculating the effect size of age differences on each task. The effect size for the difference in means between young and aged rats was 0.043 (Cohen’s d) for the Spatial Learning Index of water maze performance and 1.36 (Cohen’s d) for the number of incorrect trials on the OPPA task. The large effect size of age group on the interregion dependent OPPA task, relative to the small effect size of the HPC-dependent water maze test of spatial reference memory suggests that behaviors requiring the HPC, PER, and PFC and functional connectivity among these structures may be more sensitive for detecting age-related cognitive deficits than tasks that assay behaviors more specifically reliant on the function of single brain regions.
Discussion
The current experiments demonstrate that aged animals with intact performance on the Morris water maze test of hippocampal (HPC)-dependent spatial reference memory are impaired in acquiring object-place associations (Figure 3). The dissociation of deficits in acquisition of object-place associations versus intact spatial reference memory in aged animals provides important insights with regards to aging. Lesion data have shown that the prefrontal cortex (PFC; Lee & Solivan, 2008) and the perirhinal cortex (PER; Jo & Lee, 2010a, 2010b) are both necessary for OPPA task, but not water maze, performance (Machin, Vann, Muir, & Aggleton, 2002; Sloan et al., 2006). Thus, these data support the hypothesis that age-associated disruptions in the PER (Burke et al., 2012; Burke et al., 2014) and/or PFC (Banuelos et al., 2014; Barense et al., 2002) are likely contributing to the performance deficits of aged animals on the OPPA task. Future experiments will need to cross-characterize rats on the OPPA task and PER/PFC-dependent behaviors in order to determine the extent to which the functional integrity of these structures contributes to an animal’s ability to form object-place associations.
The current data provide additional insight regarding the ability of different behavioral assays to detect age-related dysfunction. Specifically, in this cohort of rats, age did not have a significant effect on HPC-dependent water maze performance, and the effect size of the difference between means for the young and aged rats was small (0.043). In fact, the observation that only one of 18 aged rats tested in the current experiment performed 1 SD below the mean of the young animals is somewhat surprising. One prior study has shown that age-associated spatial reference memory deficits may not be evident in F344xBN hybrid rats even at 30 months of age (Hebda-Bauer, Morano, & Therrien, 1999). Moreover, it is well documented that aged rats exhibit a wide range of performances on the Morris water maze (e.g., Bizon et al., 2009; Gallagher et al., 1993). Thus, it is conceivable that within the cohort tested here, there was a disproportionate number of spatially unimpaired animals. In support of this idea, when data from a different cohort of rats that were Morris water maze tested in our laboratory during the time frame of the current experiments were analyzed, a significant age effect was detected in the cumulative search error during Block 4 (Figure 5D; p < .01).
In contrast to the Morris water maze test of HPC-dependent spatial reference memory, the effect size for the mean age difference in the number of incorrect trials prior to reaching criteria on the OPPA task was large (1.36). These data indicate that a task requiring interactions between the HPC-PER-PFC memory network may have enhanced sensitivity for identifying age-related impairments relative to a hippocampal-dependent task that does not require the PFC or PER. This observation is perhaps intuitive when one considers the neural circuitry that supports these two behaviors. Specifically, the Morris water maze task requires the HPC (Morris et al., 1982) and input from the medial entorhinal cortex (Brun et al., 2008; Steffenach, Witter, Moser, & Moser, 2005). Conversely, the integration of specific sensory features of an object with a spatial location, a requirement of the OPPA task, necessitates information in the medial entorhinal-HPC circuit to be associated with input from both the PER and the PFC. Thus, an age-associated disruption in any one of these nodes of the memory network, or their ability to interact, will affect behavioral output.
Although a deficit in sensory representations within the PER or PFC, or interactions across the HPC-PER-PFC network would explain the age-related OPPA task deficit, an alternate possibility is that the aged rats were impaired because they used a suboptimal response strategy more than the young animals. While PFC dysfunction in old age is associated with reduced behavioral flexibility (Beas et al., 2013; Moore, Killiany, Herndon, Rosene, & Moss, 2003), this possibility is unlikely. Specifically, in both age groups there was a tendency to develop a side bias over the course of testing, but the young and aged rats shifted their left-versus-right response bias over time to a similar degree (Figure 4).
While it cannot be unequivocally shown with the behavioral approach used in the current experiment, the possibility exists that impairments in OPPA performance arise from declines in the functional connectivity of the HPC-PER-PFC circuit. Such communication deficits could arise either from dysfunction in one or more of these brain areas, or from a reduced ability of these regions to transfer and update information across the memory network. Both of these ideas would be consistent with human imaging data that have used regional fluctuations in the BOLD signal (Cooper, Crow, Walter, & Winter, 1966) to understand functional connectivity in large-scale neural networks (Biswal, Yetkin, Haughton, & Hyde, 1995). Specifically, this experimental approach has established that PER-HPC interactions relate to cognitive performance in young subjects (O’Neil et al., 2012; Vilberg & Davachi, 2013) and that functional connectivity between the HPC and anterior temporal lobe during an associative memory task is reduced in advanced age (Tsukiura et al., 2011). Future research will be necessary to determine the origin of these reductions in interregion communication.
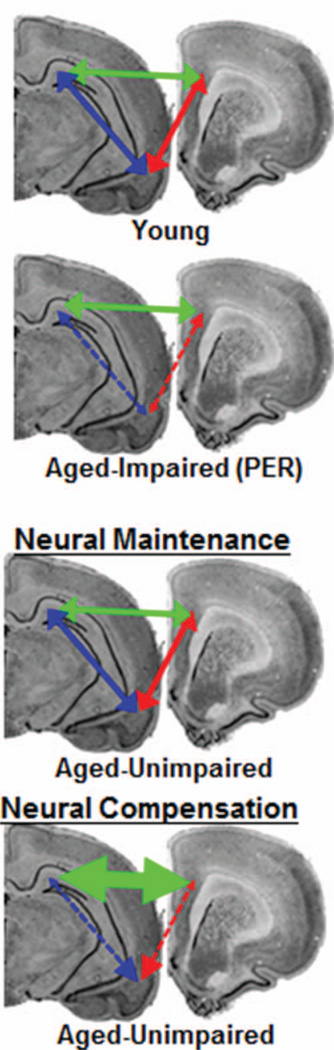
The use of a behavioral task that requires the HPC, PER, and PFC to test cognitive outcomes in old age could provide novel insights regarding the neurobiological explanation for high-performing older animals and the degree to which neural systems reorganize across the life span. One theory contends that variations in cognitive performance among old individuals reflect differences in neural maintenance (Nyberg, Lövdén, Riklund, Lindenberger, & Backman, 2012). In this view, successfully aging animals have intact neural systems resembling those of young, with no dysfunction in any of the structures that comprise the network. Neural maintenance predicts that the four animals without an OPPA task impairment in the current experiment would have normal function across the brain (Neural Maintenance; Figure 8). A second theory proposes that the range of performance across aged individuals is due to differences in the ability to compensate on the network level when one brain area is compromised (Neural Compensation; Figure 8; Cabeza, Anderson, Locantore, & McIntosh, 2002; Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008). This neural compensation view would predict that dysfunction in one brain region could initiate altered network connectivity in associated structures to promote better behavioral outcomes. To date, no neurobiological data are available to reconcile these two ideas. In the current study, the aged rats that had retained a normal ability to form object-place associations also had intact spatial reference memory. Although these data favor a neural maintenance view, future studies will need to cross-characterize animals on the OPPA task along with PFC- and PER-dependent behaviors. The extent to which network interactions reorganize to maintain behavioral output when the HPC, PER, and/or PFC are compromised in age or disease could then be determined. Understanding the relationship between functional connectivity and cognition across the life span will enable future interventional studies to promote memory network interactions that support successful aging.
Figure 8.
Two competing hypotheses of intact cognitive performance in old age: Neural Maintenance versus Neural Compensation. In the scenario presented here, compared to young, aged-impaired animals with perirhinal (PER) dysfunction would have reduced functional connectivity between this structure and adjacent regions. The Neural Maintenance view predicts that functional connectivity across the hippocampal (HPC)-PER-prefrontal cortical (PFC) network is conserved in aged-unimpaired rats, showing the same patterns of interregion communication as young animals. In contrast, the Neural Compensation view predicts that HPC-PER-PFC functional connectivity would change in the face of PER dysfunction to maintain behavior. See the online article for the color version of this figure.
Acknowledgments
This work was supported by the McKnight Brain Research Foundation, the University of Florida Research Seed Opportunity Fund, HHMI Science for Life Program, and the College of Medicine University Scholars Program. Additionally, we thank Amanda Schaerer, Nick Topper, Rodney Ndum, Joseph A. Mc-Quail, and Jennifer L. Bizon for help with completing this article.
References
- Ash JA, Rapp PR. A quantitative neural network approach to understanding aging phenotypes. Ageing Research Reviews. 2014;15:44–50. doi: 10.1016/j.arr.2014.02.001. http://dx.doi.org/10.1016/j.arr.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. The Journal of Neuroscience. 2014;34:3457–3466. doi: 10.1523/JNEUROSCI.5192-13.2014. http://dx.doi.org/10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learning & Memory. 2002;9:191–201. doi: 10.1101/lm.48602. http://dx.doi.org/10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. The Journal of Neuroscience. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: A critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and pre-frontal cortices. Cerebral Cortex. 2015;25:472–481. doi: 10.1093/cercor/bht245. http://dx.doi.org/10.1093/cercor/bht245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Distinct manifestations of executive dysfunction in aged rats. Neurobiology of Aging. 2013;34:2164–2174. doi: 10.1016/j.neurobiolaging.2013.03.019. http://dx.doi.org/10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. http://dx.doi.org/10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL. Characterizing cognitive aging of working memory and executive function in animal models. Frontiers in Aging Neuroscience. 2012;4:19. doi: 10.3389/fnagi.2012.00019. http://dx.doi.org/10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiology of Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. http://dx.doi.org/10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. http://dx.doi.org/10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends in Neurosciences. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. http://dx.doi.org/10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Hartzell AL, Lister JP, Hoang LT, Barnes CA. Layer V perirhinal cortical ensemble activity during object exploration: A comparison between young and aged rats. Hippocampus. 2012;22:2080–2093. doi: 10.1002/hipo.22066. http://dx.doi.org/10.1002/hipo.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Nematollahi S, Uprety A, Wallace JL, Barnes CA. Advanced age dissociates dual functions of the perirhinal cortex. The web of Neuroscience. 2014;34:467–480. doi: 10.1523/JNEUROSCI.2875-13.2014. http://dx.doi.org/10.1523/JNEUROSCI.2875-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Ryan L, Barnes CA. Characterizing cognitive aging of recognition memory and related processes in animal models and in humans. Frontiers in Aging Neuroscience. 2012;4:15. doi: 10.3389/fnagi.2012.00015. http://dx.doi.org/10.3389/fnagi.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-associated deficits in pattern separation functions of the perirhinal cortex: A cross-species consensus. Behavioral Neuroscience. 2011;125:836–847. doi: 10.1037/a0026238. http://dx.doi.org/10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behavioral Neuroscience. 2010;124:559–573. doi: 10.1037/a0020893. http://dx.doi.org/10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. http://dx.doi.org/10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cooper R, Crow HJ, Walter WG, Winter AL. Regional control of cerebral vascular reactivity and oxygen supply in man. Brain Research. 1966;3:174–191. doi: 10.1016/0006-8993(66)90075-8. http://dx.doi.org/10.1016/0006-8993(66)90075-8. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cognitive, Affective & Behavioral Neuroscience. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. http://dx.doi.org/10.3758/CABN.2.2.174. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. http://dx.doi.org/10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCiccio TJ, Efron B. Bootstrap confidence intervals. Statistical Science. 1996;11:189–212. [Google Scholar]
- Efron B. Better bootstrap confidence intervals. web of the American Statistical Association. 1987;82:171–185. http://dx.doi.org/10.1080/01621459.1987.10478410. [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–355. doi: 10.1002/hipo.20059. http://dx.doi.org/10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behavioral Neuroscience. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. http://dx.doi.org/10.1037/0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Morano MI, Therrien B. Aging and corticosterone injections affect spatial learning in Fischer-344 X Brown Norway rats. Brain Research. 1999;827:93–103. doi: 10.1016/s0006-8993(99)01310-4. http://dx.doi.org/10.1016/S0006-8993(99)01310-4. [DOI] [PubMed] [Google Scholar]
- Jo YS, Lee I. Disconnection of the hippocampal-perirhinal cortical circuits severely disrupts object-place paired associative memory. The Journal of Neuroscience. 2010a;30:9850–9858. doi: 10.1523/JNEUROSCI.1580-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.1580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee I. Perirhinal cortex is necessary for acquiring, but not for retrieving object-place paired association. Learning & Memory. 2010b;17:97–103. doi: 10.1101/lm.1620410. http://dx.doi.org/10.1101/lm.1620410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan WJ, Mullenix PJ. Stability and reproducibility of time structure in spontaneous behavior of male rats. Pharmacology, Biochemistry and Behavior. 1991;39:747–754. doi: 10.1016/0091-3057(91)90158-x. http://dx.doi.org/10.1016/0091-3057(91)90158-X. [DOI] [PubMed] [Google Scholar]
- Lee I, Solivan F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learning & Memory. 2008;15:357–367. doi: 10.1101/lm.902708. http://dx.doi.org/10.1101/lm.902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin P, Vann SD, Muir JL, Aggleton JP. Neurotoxic lesions of the rat perirhinal cortex fail to disrupt the acquisition or performance of tests of allocentric spatial memory. Behavioral Neuroscience. 2002;116:232–240. http://dx.doi.org/10.1037/0735-7044.116.2.232. [PubMed] [Google Scholar]
- Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: Implications for genetic variance. Neurobiology of Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. http://dx.doi.org/10.1016/S0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Nicolle MM. Spatial reference memory in normal aging Fischer 344 X Brown Norway F1 hybrid rats. Neurobiology of Aging. 2015;36:323–333. doi: 10.1016/j.neurobiolaging.2014.06.030. http://dx.doi.org/10.1016/j.neurobiolaging.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiology of Aging. 2003;24:125–134. doi: 10.1016/s0197-4580(02)00054-4. http://dx.doi.org/10.1016/S0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. web of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. http://dx.doi.org/10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. http://dx.doi.org/10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morse CK. Does variability increase with age? An archival study of cognitive measures. Psychology and Aging. 1993;8:156–164. doi: 10.1037//0882-7974.8.2.156. http://dx.doi.org/10.1037/0882-7974.8.2.156. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. http://dx.doi.org/10.1016/S0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behavioral Neuroscience. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. http://dx.doi.org/10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends in Cognitive Sciences. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. http://dx.doi.org/10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- O’Neil EB, Protzner AB, McCormick C, McLean DA, Poppenk J, Cate AD, Köhler S. Distinct patterns of functional and effective connectivity between perirhinal cortex and other cortical regions in recognition memory and perceptual discrimination. Cerebral Cortex. 2012;22:74–85. doi: 10.1093/cercor/bhr075. http://dx.doi.org/10.1093/cercor/bhr075. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Progress in Neurobiology. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. http://dx.doi.org/10.1016/S0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Samson RD, Barnes CA. Impact of aging brain circuits on cognition. European web of Neuroscience. 2013;37:1903–1915. doi: 10.1111/ejn.12183. http://dx.doi.org/10.1111/ejn.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Barnes CA. Age-related decrease in cholinergic synaptic transmission in three hippocampal subfields. Neurobiology of Aging. 1996;17:439–451. doi: 10.1016/0197-4580(96)00020-6. http://dx.doi.org/10.1016/0197-4580(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behavioural Brain Research. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. http://dx.doi.org/10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinion in Neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. http://dx.doi.org/10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. web of Cognitive Neuroscience. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. http://dx.doi.org/10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. http://dx.doi.org/10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Sekiguchi A, Yomogida Y, Nakagawa S, Shigemune Y, Kambara T, Kawashima R. Effects of aging on hippocampal and anterior temporal activations during successful retrieval of memory for face-name associations. Journal of Cognitive Neuroscience. 2011;23:200–213. doi: 10.1162/jocn.2010.21476. http://dx.doi.org/10.1162/jocn.2010.21476. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Davachi L. Perirhinal-hippocampal connectivity during reactivation is a marker for object-based memory consolidation. Neuron. 2013;79:1232–1242. doi: 10.1016/j.neuron.2013.07.013. http://dx.doi.org/10.1016/j.neuron.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]