Abstract
PURPOSE
To evaluate reactivation of pediatric uveitis during/following treatment with TNF-alpha inhibition (anti-TNFα).
DESIGN
Retrospective cohort study.
METHODS
We assessed the incidence of uveitis reactivation in children ≤18 years who had achieved uveitis quiescence under anti-TNFα. Survival analysis was used to calculate reactivation rates while still on (primary outcome), and following discontinuation of (secondary outcome), anti-TNFα. Potential predictive factors were assessed.
RESULTS
Among 50 children observed to develop quiescence of uveitis under anti-TNFα, 39 met criteria to be “at risk” of the primary (19 for the secondary) outcome. 60% were female, ~half had Juvenile Idiopathic Arthritis, and most were treated with infliximab. Overall, the estimated proportion relapsing within 12 months was 27.8% (95% confidence interval [CI]: 15.9-45.8%); the estimated probability of reactivation was higher following (63.8% [95% CI: 38.9-87.7%]), than before (21.6% [95% CI: 10.8-40.2%]), anti-TNFα discontinuation. Amongst those who discontinued anti-TNFα, the likelihood of reactivation was higher for those treated with adalimumab vs. infliximab (Hazard Ratio [HR] 13.4, p=0.01, 95% CI: 2.2-82.5) and those with older age at uveitis-onset (HR 1.3, p=0.09, 95% CI: 1.0-1.7). The duration of suppression, on medication, did not significantly affect the likelihood of reactivation when quiescence was maintained for ≥1.5 years.
CONCLUSIONS
Approximately 75% of children remaining on anti-TNFα following achievement of uveitis quiescence remain quiescent at one year. However, most reactivate following anti-TNFα discontinuation. These results suggest that infliximab more often is followed by remission, off medication, than adalimumab. The data do not suggest that maintenance of suppression, for more than 1.5 years decreases the reactivation risk.
INTRODUCTION
Uveitis is an important cause of visual loss. The incidence may range from 7/100,000 - 27/100,000 among children and adolescents (1). In the developed world, the majority of pediatric uveitis is non-infectious, chronic, anterior uveitis, and the preponderance is undifferentiated or associated with Juvenile Idiopathic Arthritis (JIA) (2-6). In most cases, topical corticosteroids are the first line of treatment (7,8). When uveitis is corticosteroid-resistant or requires sufficient corticosteroid therapy to make morbidity likely, conventional immunomodulators, most often methotrexate, are added. If these are inadequate, biologic immunomodulators that block Tumor necrosis factor alpha (anti-TNFα) are often the next treatment choice. A number of studies have demonstrated that the majority of corticosteroid and methotrexate resistant uveitis improved under anti-TNFα (9-14). We previously demonstrated that 75% of children with non-infectious uveitis achieved quiescence within one year of treatment with anti-TNFα (infliximab or adalimumab), and that the children with JIA-associated anterior uveitis were most likely to respond (15). While this study showed that anti-TNFα were useful for uveitis treatment in this setting, it did not address how long we should continue to treat children with anti-TNFα for uveitis.
A common practice amongst pediatric rheumatologists is to maintain JIA-arthritis quiescence for one to two years before discontinuing systemic medications. Similarly, many physicians who care for pediatric uveitis believe that the risk of reactivation is lowered by a more prolonged period of suppression while on medication (suppression). There are scant, conflicting, data on whether a longer duration of suppression results in decreased risk of disease reactivation after withdrawal for either JIA-arthritis or JIA-associated uveitis (16-19). All but one of these studies focused on reactivation following conventional immunomodulator withdrawal (17-19). Whether this is the case in uveitis controlled under anti-TNFα has not been directly examined.
To provide guidance regarding management of children with uveitis while under treatment with anti-TNFα, we provide additional follow-up of patients from our previously published cohort. Here, we report estimates of the risk of uveitis reactivation while patients were still on, and after they had discontinued, anti-TNFα. In addition, we examine potential risk factors for reactivation of uveitis after anti-TNFα discontinuation.
PATIENTS AND METHODS
Study setting and study population
This was a retrospective cohort study of patients managed in the Division of Rheumatology of The Children’s Hospital of Philadelphia between January, 2000- July, 2012 inclusive. This study includes expanded data collection for patients previously described (15) and patients who began care July, 2009-July, 2012. Subjects were identified by searching the electronic medical record (EMR) for ICD-9 codes possibly indicating non-infectious uveitis (ICD-9 363.x, 364.x) (1). The charts of patients thus identified were reviewed to determine if each patient had non-infectious non-traumatic uveitis (uveitis), if ophthalmologic records were available, and if the patient had been treated with an anti-TNFα agent. The study was undertaken after the governing institutional review board (The Children’s Hospital of Philadelphia) granted approval with waiver of informed consent for this retrospective study, which did not involve any contact with patients. HIPAA-compliant procedures were used for the research described herein.
Inclusion criteria
Subjects selected for this analysis had achieved inactive uveitis following treatment with anti-TNFα medications and had ophthalmologic records available for review after achieving quiescence. Inclusion criteria for analysis of achievement initial of quiescence were described previously (15). Briefly, quiescence was defined as achieving either “slightly active” or “inactive” uveitis while on ≤2 drops/day topical corticosteroids and no oral corticosteroids sustained for ≥2 visits spanning ≥28 days. In that analysis, if a patient discontinued medication because of failure or adverse events, a second drug episode could be included per patient, but second rounds of anti-TNFα therapy following initially successful treatment were not included. For this analysis, patients could meet criteria for inactive disease while being on ≤ 2 drops/day of topical corticosteroids. Infliximab and adalimumab were the only anti-TNFα therapies observed. Concomitant treatment with corticosteroids, methotrexate (MTX), and/or mycophenolate mofetil was recorded.
Data collection
Data regarding demographics, treatment, and disease activity were obtained from electronic medical or paper records through the CHOP Division of Rheumatology, the CHOP Department of Ophthalmology, and community ophthalmologist reports available in these records. Data were entered into a custom electronic Microsoft Access 2007 (Microsoft Corporation, Redmond, Washington) database (15). JIA patients were characterized further into subtypes according to the International League of Associations for Rheumatology (ILAR) criteria (20). Age at uveitis was dichotomized (≤6 vs. > 6 years), following the American Academy of Pediatrics ophthalmologic screening guidelines for children with JIA (21).
Definition of disease activity and location
Disease activity at each visit was characterized as an ordered categorical outcome, using the approach the Systemic Immunosuppressive Therapy for Eye Disease Cohort Study has used previously: inactive, slightly active, or active, incorporating information from sites of inflammation including, but not limited to, the anterior chamber (AC) into a single activity variable (including AC cell, vitreous cells, vitreous haze) (21-26). Categories closely parallel the uveitis definitions of the Standardization of Uveitis Nomenclature (SUN) Working Group (27,28). Chart reviewers ascertained disease status from a combination of the physician’s overall assessment at the visit through the use of descriptors such as quiet or quiescent, as well as quantitative descriptors of cell grade and vitreous haze. “Inactive” reflected designations such as no cells, rare cells, no vitreous haze and no corticosteroids; “slightly active” reflected gradings such as trace or fewer AC cells (≤0.5+), and minimal vitreous haze or cells, and “active” reflected higher levels of inflammation. Disease state was recorded by eye; a patient’s disease state was considered as the worse level of the two eyes if both had uveitis or else the level of the single eye with uveitis. Uveitis location was categorized as anterior, intermediate (+/− anterior), posterior, or panuveitis (27).
Outcome definition
The outcome was reactivation, defined as observation of “active” uveitis after achievement of quiescence. The incidence of reactivation was calculated following achievement of quiescence while still on treatment (primary analysis) and following complete discontinuation of anti-TNFα (secondary analysis). When uveitis was inactive, it was termed “suppression” when a patient remained on anti-TNFα and “remission” following discontinuation of anti-TNFα.
Follow-up time
For the primary analysis, follow-up time began with the first visit at which “inactive uveitis” was identified; time-to-reactivation was measured to the first visit at which “active” uveitis reoccurred. Data were not censored when medication was discontinued. For the secondary analysis (time-to-reactivation after stopping anti-TNFα), follow-up time began on the date of the clinic visit at which medication discontinuation was advised. Uveitis information was collected from all available visits to an ophthalmologist, but visit frequency varied in this retrospective, observational study. Follow-up was completed when reactivation occurred, the subject was lost to follow-up without redeveloping “active” uveitis (censoring) or the end of the study (administrative censoring).
Covariates
Demographic and clinical characteristics (summarized in Table 1) were assessed for their relationship to incidence of reactivation of uveitis while continuing anti-TNFα or after discontinuing anti-TNFα therapy. Severity of disease activity was analyzed with a surrogate marker, use of <4 vs. ≥ 4 drops of topical CS daily.
Table 1.
Characteristics of children at risk for uveitis reactivation after achieving quiescence on Tumor Necrosis Factor alpha inhibitors (anti-TNFα)
| Characteristic | Whole Cohort*
(n=39†, PY=64.4‡) |
Subset continuing on anti-TNFα (n=20†, PY=32.1) |
Subset discontinuing anti-TNFα (n=19, PY=13.2) |
|---|---|---|---|
| Sex (% female) | 59.0 | 60.0 | 57.9 |
| Race (% white) | 64.1 | 70.0 | 57.9 |
| Age at uveitis diagnosis (%>6 years) § |
61.6 | 64.7 | 58.8 |
| Median age at uveitis diagnosis (range) § |
6.9 (1.4, 16.3) | 7.4 (2.0, 16.3) | 6.25 (1.4, 13.1) |
| Systemic diagnosis (% JIA) ‡ | 43.6 | 40.0 | 47.4 |
| Uveitis location (% anterior) | 80.1 | 80.0 | 84.2 |
| Anti-TNFα (% infliximab) ¶ | 89.7 | 90.5 | 84.2 |
| Median years from disease diagnosis to initiation anti- TNFα (IQR) ‡ § |
0.6 (0.2, 2.1) | 0.9 (0.3, 2.0) | 0.3 (0.2, 2.1) |
| Median years to achieve uveitis quiescence on anti- TNFα (IQR) |
0.2 (0.1-0.3) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) |
| Median years uveitis quiescence on anti-TNFα to discontinuation (IQR) |
--- | --- | 1.9 (0.6, 2.4) |
The cohort was followed until either reactivation or the end of follow-up data, regardless of whether discontinued anti-TNFα.
One patient with two drug episodes, e.g. 39 patients but 40 drug episodes.
PY, person-years; JIA, juvenile idiopathic arthritis, IQR, interquartile range, anti-TNFα,Tumor Necrosis Factor alpha inhibitors.
Four cases were missing age of uveitis diagnosis (available, n=35).
Percentage of treatment courses, not patients (n=40 for whole cohort).
Data analysis
Data were analyzed using Stata 11.0 (College Station, TX). Differences in categorical demographic and clinical characteristics between subcohorts were assessed using the χ2 test. Nominal statistical significance was defined as a two-tailed p value ≤0.05. Kaplan-Meier methods evaluated time-to-reactivation (failure) after observation of quiescence. As a subject could have more than one anti-TNFα treatment episode, analyses were performed using a robust-variance-estimator to account for clustering by subject (one subject had two treatment episodes). Cox proportional hazards model-derived hazard ratios (HR) estimated the association of each independent patient-level or treatment-level variable with each outcome variable. Being on additional immunomodulatory therapy varied during treatment (e.g. being on one at quiescence did not ensure continued treatment at reactivation); as a time-varying covariate, associations with other variables were analyzed with χ2 testing rather than through regression analysis.
RESULTS
Among 50 children and adolescents observed to develop quiescence of uveitis under anti-TNFα treatment during the period of observation, encompassing 53 drug episodes (i.e., three were treated twice), 39 subjects – observed over 40 drug episodes - met inclusion criteria for assessment of the primary outcome of time-to-reactivation of uveitis while under anti-TNFα treatment (64.4 patient-years at risk). Nineteen subjects discontinued anti-TNFα following achievement of quiescence and met inclusion criteria for the secondary outcome of time to-reactivation of uveitis after cessation of anti-TNFα therapy), covering 13.2 patient-years at risk.
Demographic and Treatment Characteristics of Subjects who Achieved Quiescence
Of the 39 subjects who achieved quiescence, 60.0% were female, 64.1% were white, and 61.6% were diagnosed with uveitis older than six years of age (Table 1). Less than half the cohort, 43.6%, had JIA; the remainder had sarcoidosis (10%) or uveitis without a known systemic association (46%). The majority had anterior uveitis (n=32, 82.1%), one had intermediate uveitis (2.6%), and 6 had posterior uveitis (15.4%). Of those with sarcoidosis, three had anterior uveitis and one panuveitis. Treatment with anti-TNFα was initiated within one year of uveitis onset in 62.9% of the subjects, and 87.5% achieved quiescence within a half a year of starting medication. The majority of patients were treated with infliximab, although five patients were treated with adalimumab (one of whom switched to adalimumab after having an adverse reaction to infliximab). While a greater percentage of patients treated with adalimumab had an underlying diagnosis of JIA (80%) compared to those treated with infliximab (40%), there was no statistically significant association between underlying diagnosis (JIA vs. other) and the anti-TNF-alpha used (p=0.09) (data not shown). Nor was there a statistically significant difference in: the type of uveitis children treated with infliximab had relative to those treated with adalimumab (Anterior Uveitis: 79% vs. 100%, p=0.53); or the underlying disease activity, as reflected by ≥4 drops topical corticosteroids/day, at the onset (more severe uveitis 60% vs. 40%, p=0.4) (data not shown).
Characteristics of Subjects who Continued on anti-TNFα Relative to those who Discontinued
The demographic characteristics of those who continued on and discontinued anti-TNFα were similar: 60.0 vs. 57.9% were female (p=0.8), 70.0 vs. 57.9 were white (p=0.4), 64.7 vs. 58.8% (p=0.9) were diagnosed with uveitis after six years of age, and 80.0% vs. 84.2% (p=0.4) had anterior uveitis (Table 1). Almost half the cohort, 40.0% vs. 47.4% had JIA (p=0.6). Treatment with anti-TNFα was initiated within one year in 38.9 vs. 35.3% of the subjects (p=0.9, data not shown); 90.5 vs. 84.2% were treated with infliximab (p=0.6), and the remainder with adalimumab. Of those who discontinued anti-TNFα, two-thirds (68.4%) were on anti-TNFα for more than 1 year after achieving quiescence, but only one third were on anti-TNFα for more than 2 years after achieving quiescence (36.8%). The median time on anti-TNFα from achievement of quiescence to discontinuation was 1.73 years (IQR: 0.25-2.15). The median total time on anti-TNFα was 2.26 years (IQR: 1.45-3.17) in those who discontinued drug relative to 1.24 (IQR 0.78-2.38) in those who remained on anti-TNFα (p=0.16). By the time of disease reactivation or censoring, 47% (56% infliximab, 0% adalimumab) of the cohort who discontinued anti-TNFα remained on either traditional immunomodulators (methotrexate [n=7] or mycophenolate mofetil [n=2])
Incidence of Uveitis Reactivation
Amongst the 39 subjects at risk of the primary outcome, the estimated proportion of those in whom uveitis reactivated within 12 months of quiescence was 27.8% (95% CI: 15.9-45.8%) (data not shown). The estimated probability of a uveitis reactivation was 2.5% by three months (95% CI: 0%-16.8%), 18.4% by 6 months (95% CI: 9.2-34.9%), and 21.3% by 9 months (95% CI 11.2-38.1%). The median time-to-failure was not observed; the estimated median time-to-failure was 23.5 months.
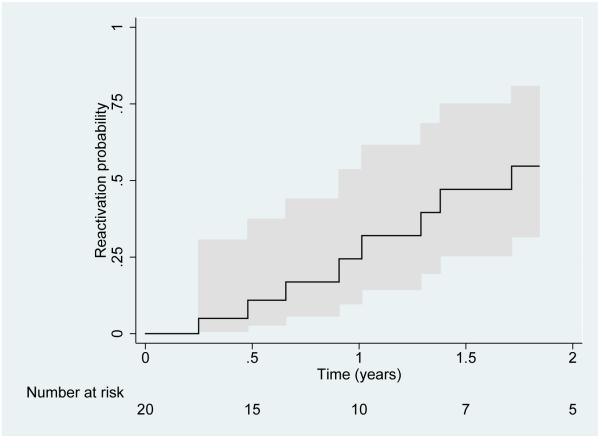
In a sensitivity analysis of the failure rate amongst only those who continued on anti-TNFα, the estimated probability of a uveitis reactivation by 12 months was 24.4% (95% CI 9.7, 53.5%) (Figure 1), and the estimated median time to failure was 20.5 months (32.1 patient-years).
Figure 1.
Time-to-reactivation of pediatric uveitis while remaining on Tumor Necrosis Factor-α inhibitor (anti-TNFα) therapy after achievement of quiescence. The 95% CI is represented by the areas shaded in grey.
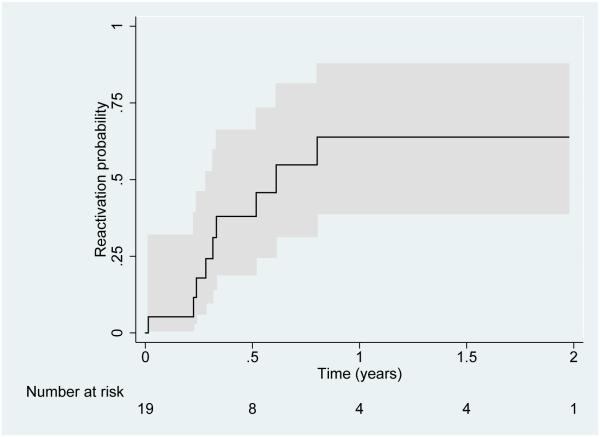
The estimated proportion whose uveitis reactivated within 12 months of discontinuing anti-TNFα was much higher (63.8%, 95% CI: 38.9-87.7%) (Figure 2). The estimated probability of a uveitis reactivation was 17.9% by three months (95% CI: 6.1%-46.6%), 38.0% by 6 months (95% CI: 19.0-66.1%), and 54.8% by 9 months (95% CI 31.4-81.2%); the median time to failure was 3.9 months (range 6.9-23.7 months).
Figure 2.
Time-to-reactivation of pediatric uveitis after discontinuation of Tumor Necrosis Factor-α inhibitor (anti-TNFα) therapy. The 95% CI is represented by the areas shaded in grey.
Factors Associated with Reactivation
Amongst those who discontinued anti-TNFα, the likelihood of failure was significantly higher for those treated with adalimumab vs. infliximab (Hazard Ratio [HR] 13.4, 95% CI: 2.2-82.5) (Table 2). In contrast, there was no statistically significant association between previous infliximab treatment and being on an additional immunomodulatory therapy (methotrexate or mycophenolate mofetil) when disease reactivated (p=1.00, data not shown). Nor was there an association between reactivation and being on an additional immunomodulatory therapy either at the achievement of quiescence or at uveitis reactivation (p=0.16 and p=0.16). Older age at onset of ocular disease was associated with a significantly increased hazard of reactivation (HR 1.3, 95% CI: 1.0-1.7) when analyzed as a continuous variable. The duration of suppression was not significantly associated with the likelihood of failure when it was analyzed as a continuous variable (HR 0.9, 95% CI 0.6, 1.4) or when it was analyzed as a dichotomous variable (more than one vs. more than two years of quiescence until anti-TNFα was discontinued, data not shown) (Table 2). Neither was sex, race, systemic diagnosis, time from diagnosis to drug initiation, or disease severity at drug initiation significantly associated with incidence of failure.
Table 2.
Association of potential risk factors with uveitis reactivation in children after discontinuation of Tumor Necrosis Factor alpha inhibitor (anti-TNFα) therapy
| Variable | HR (95% CI) | P value |
|---|---|---|
| Sex (female vs. male) | 0. 30 (0.07, 1.33) | 0.11 |
| Race (white vs. other) | 5.33 (0.65, 43.53) | 0.12 |
| Age at uveitis diagnosis (per year of age) | 1.32 (1.03, 1.69) | 0.03 |
| Systemic diagnosis (JIA* vs. other) | 0.95 (0.26, 3.55) | 0.94 |
| Time from disease diagnosis to drug initiation (per year) |
1.06 (0.86, 1.32) | 0.57 |
| More severe disease activity at onset † | 0.43 (0.13, 1.43) | 0.17 |
| Years to uveitis quiescence on anti-TNFα * | 0.19 (0.00, 15.20) | 0.46 |
| Treatment with adalimumab | 13.44 (2.19, 82.50) | 0.01 |
| Years of uveitis quiescence on anti-TNFα until discontinuation (per year) |
0.88 (0.55, 1.41) | 0.60 |
JIA, juvenile idiopathic arthritis; anti-TNFα, Tumor Necrosis Factor alpha inhibitors.
As defined by requirement of ≥4 drops per day topical corticosteroids at initiation of anti-TNFα.
DISCUSSION
Our study examined the durability of the response of pediatric uveitis to anti-TNFα by analyzing the risk of reactivation while on medication, risk of reactivation following discontinuation of medication, and factors associated with reactivation. The durability of suppression, while on medication, was moderate (28% risk of reactivation by 12 months). The overall durability of the response, maintenance of remission, was low, as the majority developed reactivation of uveitis within 9 months (64% within 12 months) of discontinuing anti-TNFα. Therefore, while anti-TNFα treatment successfully suppresses disease activity and reduces corticosteroid burden in most cases, it may not be associated with sustained remission in the majority of cases. Neither was a longer duration of suppression prior to withdrawal of therapy associated with a greater likelihood of remission. These data suggest that both medication level factors (infliximab treatment) and patient level (younger age at uveitis diagnosis) factors are associated with increased likelihood of sustained remission.
Few studies have examined the risk of disease reactivation after drug withdrawal. Kalinina reported that uveitis relapses in 46% of JIA-associated uveitis within one year of stopping methotrexate (18). Saboo also examined the likelihood of relapse in children with JIA-associated uveitis, who had maintained a year of inactive disease off immunomodulatory therapy (either conventional and/or anti-TNFα); 43% of children relapsed with an estimated median survival time of 84 months (median follow up of 72 months) (19). This analysis was restricted to children who successfully maintained 1 year of remission off anti-TNFα, suggesting that more than 43% of children would have relapsed after initial anti-TNFα discontinuation. Comparisons of outcomes between our study and these others are difficult because most children were not treated with anti-TNFα in the previous studies. The JIA-associated uveitis relapse rate by 12 months in our study (68%) is similar to that of Basciz (66%) in which the reactivation rate of JIA (arthritis and/or uveitis) was evaluated after withdrawal of anti-TNFα (16). The similarity suggests that among patients who require anti-TNFα treatment, about 2/3 are likely to require persistent, or at least repeat, therapy to maintain disease quiescence. Nevertheless, if a minority of cases can successfully withdraw therapy, it is not unreasonable to try withdrawal in patients with a low reactivation risk profile (see below) under careful observation to identify reactivations early, prior to development of complications of reactivation and with the caveat that intermittent dosing with anti-TNFα may increase the risk of tolerance and adverse reactions (29).
Physicians would like to predict which patients are more likely to remain in remission. Clinical practice currently is guided by the principle that children who maintain a longer period of JIA suppression, while on medication, have a lower likelihood of reactivation after drug discontinuation. In support of this hypothesis, Kalinina reported that the risk of relapse of JIA-associated uveitis after MTX withdrawal was lower in children with a longer duration of inactivity on MTX (>2 years) (18). However, this hypothesis was not supported either by this study or by that of Saboo; in neither of these studies was the duration of treatment with immunomodulatory agents nor the duration of suppression significantly associated with the risk of relapse, at least if the duration of suppression was more than 1.5 years (19). While it is possible that the affect of maintaining a longer suppression, while on medication, varies by agent, Foell, et al, also observed that the risk of relapse in JIA was not associated with the duration of MTX treatment once suppression was achieved, but rather with levels of the pro-inflammatory myeloid related proteins (MRP8/14) during suppression (17).
A potentially important observation in the present study was that the incidence of reactivation after anti-TNFα agent was lower in children treated with infliximab with respect to adalimumab. Although the small number of patients treated with adalimumab limited the comparison’s power, the difference between groups was statistically significant. In interpreting this observation, it is noteworthy that, while the reason for medication choice is not recorded, physicians at this institution select infliximab over adalimumab precisely for more severe uveitis, which would tend to bias the analysis toward more favorable outcomes for adalimumab, the opposite of what was observed. If replicated, this observation would suggest a preference for infliximab over adalimumab for uveitis management in children. Conversely, adalimumab’s lower success might be explained by the fact that: more children treated with adalimumab had a diagnosis of JIA, which is often a chronic uveitis (80% vs. 40%, p=0.09, data not shown); fewer children who had been treated with adalimumab remained on an additional immunomodulatory therapy (0% vs. 56%, data not shown) at the time of reactivation.
Patient-level factors might be associated with the ability to maintain a durable response after drug discontinuation. Evaluating age as potentially such a factor, Kalinina reported that the relapse rates were lower in children who were older than 8 years at methotrexate discontinuation (18). Conversely, our study and that of Saboo found that relapse rates were lower in children who were younger at diagnosis (19). The two populations may not be comparable, as anti-TNFα typically is implemented after failure of methotrexate to adequately control uveitis thus, based on the limited available information, younger age tends to be associated with a better prognosis for sustained remission off of anti-TNFα therapy. Previous studies have shown conflicting associations between age of onset and visual outcomes of uveitis, with either no association or an association of more complications with onset at ≤3 years old Edelsen 2003; Holland 2009; Zulian 2002. It is possible that our results reflect an unmeasured covariate, and this should be evaluated further in a larger cohort.
It has been thought that children who have a shorter period of disease activity prior to suppression may be more likely to have durable remission. Saboo reported a decreased risk of relapse for those treated earlier in disease, but these results were not replicated by either our group or by Basciz (16). It is difficult to evaluate this issue in observational studies from tertiary centers, where both time-to-referral and use of anti-TNFα therapy may be related to severity of underlying disease.
There are several limitations to out study. One is its retrospective nature. Patients had variable follow-up intervals, and multiple ophthalmologists, most of whom were not uveitis specialists, assessed disease activity. However, this problem was mitigated by the grading scale for uveitis being documented in a standardized fashion in the EMR and the fact that there is a high level of interobserver agreement in applying the Standardization of Uveitis Nomenclature (SUN) criteria for cell counts (27,28). Although the authors acknowledge that this interobserver agreement was between uveitis specialists and may be lower between the general pediatric ophthalmologists in this study. Because of its retrospective nature, it is possible that children whose disease remained quiet were more likely to have been lost to follow up; if this had occurred, they would have contributed less time to the survival analysis, thus increasing the estimated risk of reactivation. Another limitation is that the severity of disease activity at the initiation of treatment was not considered as a variable, save as reflected by topical steroid use. However, by targeting a cohort of children considered to have had severe enough uveitis to merit anti-TNFα treatment - rather than analyzing such children together with those treated using any immunomodulatory agent, as in other studies - our cohort should include children at high risk for visual impairment. The cohort was composed largely of Caucasian females – which is a natural byproduct of the majority of patients having JIA. While the skewing towards female and Caucasian was considerably less than that in the underlying JIA population at our clinic (data not shown), this may limit the generalizability of the results. Because of the limited number of subjects, the study was not powered to analyze drug weaning vs. lack of weaning as a time varying covariate, so the likelihood of reactivation during drug weaning could not be assessed. Similarly, the impact of concurrent conventional immunomodulatory therapy at the time of reactivation was unable to be evaluated because the cohort size precluded analyzing time varying covariates. Although the study size was moderately small, it was sufficiently large to detect associations that were clinically and statistically significant. Given the limitations of this cohort of limited size, these results should be validated in larger, prospective studies—preferably restricted to uveitis specialists. Larger cohorts might enable evaluation of the impact of drug dosing and frequency and more precisely evaluate the relative benefit of a particular anti-TNFα agent over another. Because confounding-by-indication complicates comparison of the effectiveness of various drugs in retrospective studies, we did not set out to compare different treatment regimens, biologic vs. non-biologic. Instead, we focused on a single class of treatment and its ability to estimate the risk of reactivation in children treated with anti-TNFα. These results enable us to more clearly characterize the risk of anti-TNFα withdrawal to our patients and their families.
In conclusion, our study shows that the majority of children who achieve quiescence of uveitis under of anti-TNFα treatment will reactivate. Following discontinuation of anti-TNFα, children have a higher incidence of reactivate after discontinuing anti-TNFα than while they remain on treatment. At least in children treated with anti-TNFα for more than 1.5 years, the duration of suppression, while on medication, does not appear to impact their risk of reactivation following anti-TNFα withdrawal. Children are more likely to achieve longer remission if they are younger at diagnosis of uveitis and if they are treated with infliximab rather than adalimumab, which might be related to a higher degree of ongoing use of antimetabolite therapy in the former group. We would benefit from future studies to assess more conclusively whether short vs. longer periods of maintenance of suppression increase the risk of reactivation following medication withdrawal, to establish more conclusively whether infliximab has advantages over adalimumab regarding remission probability, and to identify additional biomarkers that predict the likelihood of remission after suppressive anti-TNFα medication is withdrawn.
ACKNOWLEDGEMENTS
A. Funding/Support: A portion of the work was supported by a Rheumatology Research Foundation Scientist Development Award, Atlanta, GA (M.A.L., 2011-2013). Additional support was provided by: EY014943, National Eye Institute/NIH, Bethesda, MD (J.H.K.); Research to Prevent Blindness, New York, NY (J.H.K.); Paul and Evanina Mackall Foundation, New York, NY (J.H.K.); Lois Pope Life Foundation, New York, NY(J.H.K.); and the Mabel E Leslie Endowed Chair, Division of Ophthalmology, Children's Hospital of Philadelphia, Philadelphia, PA (M.D.M).
B. Financial Disclosures: None of the sponsors had any role in the design and conduct of the report; in collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript. In unrelated work, John H. Kempen is, or has been, a consultant for Alcon (Hünenberg, Switzerland), Allergan (Irvine, CA), Clearside Biomedical (Alpharetta, GA), Can-Fite BioPharma (Petach Tikva, Israel), Lux Biosciences (Jersey City, NJ), Xoma (Berkeley, CA) and Sanofi-Pasteur (Lyon, France).
C. Contributions of Authors: Involved in conception and design of the study (M.A.L.); data collection and management (M.D.L. and M.A.L.); analysis and interpretation of results (all authors); preparation of the initial manuscript (M.A.L.); critical review of the article (all authors); final approval of the article (all authors). The research described in this paper was conducted with the approval of the governing Institutional Review Board (IRB) of the Children’s Hospital of Philadelphia.
D. Other Acknowledgements: None.
Biography
Melissa A. Lerman, MD, PhD, MSCE is an Instructor in Pediatric Rheumatology at The Children’s Hospital of Philadelphia. She completed her PhD in immunology at the University of Pennsylvania, her pediatrics residency and pediatric rheumatology fellowship at The Children’s Hospital of Philadelphia, and her Masters in Clinical Epidemiology at the University of Pennsylvania. While Dr. Lerman treats children with all rheumatic illnesses, she also co-directs the Ophthalmology-Rheumatology Uveitis Coordinated Care Clinic. Her research focuses on understanding the treatment outcomes of children treated with conventional and biologic agents for uveitis.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in northern california; the northern california epidemiology of uveitis study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 2.BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005;89(4):444–8. doi: 10.1136/bjo.2004.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol. 2003;135(5):676–80. doi: 10.1016/s0002-9394(02)02148-7. [DOI] [PubMed] [Google Scholar]
- 4.Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: Clinical characteristics and complications. Am J Ophthalmol. 2009;147(4):667–678. doi: 10.1016/j.ajo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112(7):1287–92. doi: 10.1016/j.ophtha.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116(8):1544–51. doi: 10.1016/j.ophtha.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiligenhaus A, Michels H, Schumacher C, et al. Evidence-based, interdisciplinary guidelines for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatol Int. 2012;32(5):1121–33. doi: 10.1007/s00296-011-2126-1. [DOI] [PubMed] [Google Scholar]
- 8.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 9.Ardoin SP, Kredich D, Rabinovich E, Schanberg LE, Jaffe GJ. Infliximab to treat chronic noninfectious uveitis in children: Retrospective case series with long-term follow-up. Am J Ophthalmol. 2007;144(6):844–9. doi: 10.1016/j.ajo.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher M, Quinones K, Cervantes-Castaneda RA, Yilmaz T, Foster CS. Biological response modifier therapy for refractory childhood uveitis. Br J Ophthlamol. 2007;91(10):1341. doi: 10.1136/bjo.2007.124081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113(5):860–4. doi: 10.1016/j.ophtha.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Simonini G, Zannin ME, Caputo R, et al. Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis. Rheumatology (Oxford) 2008;47(10):1510–4. doi: 10.1093/rheumatology/ken298. [DOI] [PubMed] [Google Scholar]
- 13.Sobrin L, Kim EC, Christen W, Papadaki T, Letko E, Foster CS. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125(7):895. doi: 10.1001/archopht.125.7.895. [DOI] [PubMed] [Google Scholar]
- 14.Zannin ME, Birolo C, Gerloni VM, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the italian registry. J Rheumatol. 2013;40(1):74–9. doi: 10.3899/jrheum.120583. [DOI] [PubMed] [Google Scholar]
- 15.Lerman MA, Burnham JM, Chang PY, et al. Response of pediatric uveitis to tumor necrosis factor-α inhibitors. J Rheumatol. 2013;40(8):1394–403. doi: 10.3899/jrheum.121180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baszis K, Garbutt J, Toib D, et al. Clinical outcomes after withdrawal of anti-tumor necrosis factor α therapy in patients with juvenile idiopathic arthritis: A twelve-year experience. Arthritis Rheum. 2011;63(10):3163–8. doi: 10.1002/art.30502. [DOI] [PubMed] [Google Scholar]
- 17.Foell D, Wulffraat N, Wedderburn LR, et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: A randomized clinical trial. JAMA. 2010;303(13):1266–73. doi: 10.1001/jama.2010.375. [DOI] [PubMed] [Google Scholar]
- 18.Kalinina Ayuso V, van de Winkel EL, Rothova A, de Boer JH. Relapse rate of uveitis post-methotrexate treatment in juvenile idiopathic arthritis. Am J Ophthalmol. 2011;151(2):217–22. doi: 10.1016/j.ajo.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Saboo US, Metzinger JL, Radwan A, et al. Risk factors associated with the relapse of uveitis in patients with juvenile idiopathic arthritis: A preliminary report. J AAPOS. 2013;17(5):460–4. doi: 10.1016/j.jaapos.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: Second revision, edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 21.Cassidy J, Kivlin J, Lindsley C, Nocton J. Section on Rheumatology, Section on Ophthalmology. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 2006;117(5):1843–5. doi: 10.1542/peds.2006-0421. [DOI] [PubMed] [Google Scholar]
- 22.Daniel E, Thorne JE, Newcomb CW, et al. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010;149(3):423–32. doi: 10.1016/j.ajo.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116(11):2188–98. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaçmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117(3):576–84. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009;148(4):500–509. doi: 10.1016/j.ajo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pujari SS, Kempen JH, Newcomb CW, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010;117(2):356–65. doi: 10.1016/j.ophtha.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempen JH, Ganesh SK, Sangwan VS, Rathinam SR. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008;146(6):813–8. doi: 10.1016/j.ajo.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in crohn's disease. Gastroenterology. 2004;126(2):402–13. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]