Short abstract
This article examines the Department of Defense Serum Repository and Defense Medical Surveillance System, identifies gaps, and suggests strategies to improve their management, content, and use to meet current and potential military health needs related to surveillance, outbreak investigation, research, and clinical support.
Abstract
The Army manages the Department of Defense Serum Repository (DoDSR) of over 43 million serum samples and the associated Defense Medical Surveillance System (DMSS) database that links individual service member characteristics to these biological samples. The main mission and use of these resources has been for military health surveillance. The Army turned to RAND Arroyo Center to systematically examine current requirements and capabilities of the DoDSR and DMSS, identify gaps, and suggest strategies to improve their ability to meet current and potential future military health needs, including surveillance, outbreak investigation, research, and clinical support, particularly as these relate to influenza and other infectious disease threats. The research drew information from written documents and interviews with military and civilian experts. The study identified a number of opportunities to improve the management, content, and use of the serum repository and associated database. There were six main recommendations: (1) clarify and communicate the missions of the DoDSR and DMSS both within and beyond the Department of Defense; (2) empower, structure, and resource the organizational oversight of DoDSR and DMSS so that they can fulfill the full range of their missions; (3) create an integrative data plan for comprehensive health surveillance; (4) enhance the utility of specimens; (5) plan for the next repository facility; and (6) raise awareness of and expand access to DoDSR and DMSS.
The Department of Defense Serum Repository (DoDSR) and Defense Medical Surveillance System (DMSS) are longstanding and vital assets to U.S. Armed Forces medical surveillance. The repository contains over 43 million serial blood-derived serum specimens from over 10 million military applicants and active-duty and reserve service members over the course of their service careers; the DMSS database contains serial health data that can be linked to these specimens. Until late February 2008, the Army Medical Surveillance Activity (AMSA) managed both of these systems. On February 26, 2008, the Deputy Secretary of Defense signed a memorandum to create a new organization, the Armed Forces Health Surveillance Center (AFHSC), to oversee DoDSR and DMSS as well as the Global Emerging Infections Surveillance and Response System (GEIS).
In 2006, AMSA recognized that even though the DoDSR and DMSS had grown in response to evolving military health needs, their current and full potential use had not been systematically examined. Mindful of this, AMSA asked RAND to assess the DoDSR and DMSS to help identify ways for Army management to make them available to meet the health needs of the current and future military as fully as possible. The study was carried out between July 2006 and February 2008. The AFHSC now manages these important military assets. Updates since the creation of the AFHSC are outside the scope of this project and article. While RAND understands that some issues raised in this article may have been addressed already by AFHSC, we believe that the findings and recommendations remain relevant.
The DoDSR and the associated DMSS database were originally designed for routine HIV screening purposes, but in recent years they have been assigned additional requirements related to deployment health and the prevention and control of diseases relevant to the military more broadly: force health protection. Over these years, the biological specimen used to fulfill new requirements has remained serum (the liquid component of blood), with serum specimens collected for all purposes archived in the DoDSR. With over 43 million specimens, the DoDSR is by far the largest serum repository in the country, perhaps the world. The associated DMSS database contains demographic and longitudinal service-related data and thus allows for analyses at a given period of time or over time; the ability to link such data with serum specimens creates a valuable surveillance resource for military health and even the broader civilian community, e.g., to the extent that detailed cross-sectional or longitudinal surveillance analyses in military populations reflect disease occurrences in the broader U.S. population.
This article focuses on the current and potential role of the DoDSR and associated DMSS database to support comprehensive health surveillance—referring to surveillance over the career lifetime of a service member and across all locations, epidemiological investigation, research, and clinical management. It describes current requirements and capabilities of both systems, presents findings and gaps, and assesses specific strategies to increase the capabilities of these vital surveillance resources to serve the needs of the U.S. Armed Forces today and into the future. We reviewed DoD policy, doctrine, and other published documents as well as published scientific literature, and we interviewed health experts inside and outside DoD to help identify and assess issues and their potential solutions. We also examined a number of other biological specimen repositories to glean insights potentially relevant to the DoDSR. We constructed a conceptual framework to help identify potential improvements to system elements and to organize the collection, analysis, and presentation of our data related to these potential improvements (Figure 1).
Figure 1.
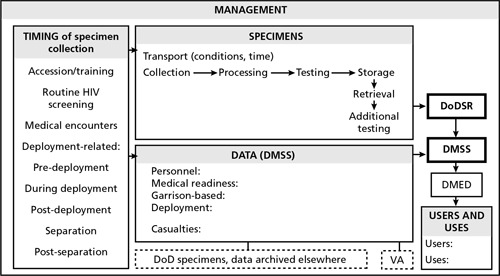
Conceptual Framework to Help Identify Potential Improvements to System Elements
The findings motivated potential improvement strategies addressing the following areas:
Management
Mission (AMSA, DoDSR, DMSS)
Organizational position of AMSA (through January 2008)
Staffing
Transparency for access to specimens
Oversight of access to specimens
Protection of human subjects
Requirements for new repository storage space
DMSS physical infrastructure and backup
HIV and other screening
Timing of specimen collection
Extending specimen collection beyond separation
Specimens
Specimen processing and transport conditions
Timing of specimen shipment to DoDSR
Freeze-thaw cycles
Size of aliquots to be released
Screening beyond HIV
Utility of serum and potential archiving of other blood fractions
Storage conditions
Data
Additional relevant data for DMSS
Connection to other military biological specimen collections
Behavioral risk factor data
DMSS links to classified data
Expanded access to DMSS data
Users and Uses
Awareness of and demand for serum specimens and DMSS data
Enhanced use for deployment health surveillance
Expanded access to DoDSR specimens
Enhanced use of serial specimens
Our recommendations include the following:
Management
1. Clarify and communicate the missions of DoDSR and DMSS both within and beyond DoD.
There is a mismatch between congressional direction for the use of the DoDSR and the DMSS data system as articulated in several enactments of the National Defense Authorization Act and the articulation of the mission and use of the DoDSR and DMSS by AMSA. Clear articulation by the new AFHSC and a common understanding across DoDSR and DMSS users of the full range of uses for these resources and their relative priority—including surveillance, epidemiologic investigation, clinical management, and research related to both infectious and noncommunicable diseases—should lead to their more efficient and robust use within DoD. Further, the mission of DoDSR and DMSS to collect specimens and data could also extend beyond DoD active and reserve populations to include continuation of data and specimen collection on a voluntary basis from separated service members followed in Military Treatment Facilities and/or the Veterans Health Administration system. To harness the full potential of the DoDSR and DMSS resources, AFHSC should establish the relative priority for the different uses and users of these resources and then make these explicit by communicating widely across DoD and into related research and epidemiologic communities if/as appropriate.
2. Empower, structure, and resource the organizational oversight of DoDSR and DMSS so that they can fulfill the full range of missions.
DoD officially established the Armed Forces Health Surveillance Center within CHPPM in late February 2008. This organization is intended to encompass and integrate DoD-wide health surveillance. We hope that the AFHSC will be able to connect the various experts, contracts, and systems that are required not only for its primary surveillance mission but also for the full range of uses (primarily within the military but also extending to the civilian community) for the DoDSR and DMSS resources it manages through its executive agency function, including surveillance, epidemiologic investigation, clinical management, and research. Further, we hope that the chain of command and oversight for this organization will be such that it can receive guidance and resources from policymakers responsible for all of these functions, e.g., the Assistant Secretary of Defense (Health Affairs), Surgeons General, and Army Medical Research and Materiel Command, in order to ensure proper alignment with current Military Health System strategy and resources and medical research and service health priorities as relevant to DoDSR and DMSS. The AFHSC should be configured and staffed to provide the support needed by all users, and especially those within the DoD, supporting execution of the designated missions for DoDSR and DMSS.
Data
3. Create an integrative data plan for comprehensive health surveillance.
Ideally, AFHSC should create an overarching and comprehensive data plan prescribing integration of all relevant heath surveillance data. Such a plan should address issues such as connectivity to occupational and environmental health surveillance systems, both within the garrison and in deployed settings, increasing data collection along the service member's period of service and beyond, and fully realizing policy efforts to facilitate access to surveillance and other data by the Department of Veterans Affairs (VA). Regarding DMSS specifically, several relevant military health datasets remain unconnected, thus limiting the full execution of AFHSC's surveillance mission and limiting the ability of DoD more broadly to take advantage of the full value offered by DMSS. The highest priorities for new data linkages into DMSS relate to deployment health, especially data derived from deployed settings. Current issues related to classified data systems also need to be overcome. We understand that relevant health surveillance data can possibly be made available to DMSS via the unclassified Theater Medical Data Store. For data that cannot be made available via this system, options for linking classified data into DMSS include time-delayed incorporation of declassified location data or near-real-time incorporation of classified data, which would require new secure communications capabilities that DMSS currently does not possess. Other relevant data linkages to consider are to existing DoD biological specimen archives such as isolates and original nasal swab specimens from the DoD Febrile Respiratory Illness surveillance system and pathology and necropsy specimens maintained by the Armed Forces Institute of Pathology in the National Pathology Repository. More robust linkages in both directions between DMSS and the VA health system should also be considered, to the extent that the mission of DoDSR and DMSS are expanded beyond strictly active-duty and reserve populations. Also, consideration should be given to whether and how behavioral risk factor data should be collected and fed into DMSS. Because there are many current data sources that might be tapped for deployment health surveillance, and there may be more in the future, the new AFHSC would be better positioned to fully execute its mission if it were included in the Military Health System information requirements process currently managed at the TRICARE Management Activity.
In addition to DMSS data content and management is the need for better protection of its physical infrastructure and the integrity of the data themselves, i.e., to resist physical or cyber threats to the DMSS database. In addition to assuring adequate housing of the data system, we recommend that strong consideration be given to systematic and frequent offsite backup and even parallel mirroring of the DMSS database, to assure its integrity in response to any threat that may arise, as occurred in late January 2008.
Specimens
4. Enhance the utility of specimens.
The DoDSR serum specimens continue to serve well their original purpose of HIV serosurveillance. However, as early as 1997, the DoD made a decision to continue using serum to meet new requirements related to biological specimens for deployment health surveillance. The sera permit examination of deployment-related exposures to and investigations of infectious agents; they are not particularly useful for time-sensitive environmental exposures for which biomarkers are only fleetingly present. And, as military health research becomes broader and more technologically sophisticated, the limitations of current serum specimens become more apparent: Researchers increasingly recognize the importance of genetic material for current and future research into a range of acute and chronic conditions. Serum specimens as presently stored in the DoDSR at −30°C do not reliably preserve genetic material. The best way to do this is to archive specimens derived from whole blood specimens, e.g., stored in liquid form or as dried blood spots, or storage of buffy coat fractions, in which the quantity of genetic material is substantially greater. Storage requirements for dried blood spots are modest and incrementally the easiest. Storage of both plasma and buffy coat at −80°C reflects current best industry practices for preservation of genetic material and other relevant blood-derived analytes. However, adoption of this alternative would mean costly new repository requirements for future specimens, i.e., walk-in freezers would not be possible for storage at −80°C. Nonetheless, the near-term expiration of the current repository lease and potential relocation provides a timely opportunity for military leadership to think carefully about the needs of the Military Health System into the future and determine whether new kinds of specimens should be archived, to better serve a broader range of mission areas for this valuable military resource.
5. Plan for the next repository facility.
Depending on decisions related to the strategies and recommendations described, DoD should begin already to define the requirements for the next repository, following expiration of the current lease in 2010. Factors to take into consideration include the time horizon for the next repository (e.g., 20 years or more), the annual rate of specimen acquisition (which would increase if specimens are to be collected from members following separation), the types of specimen to be archived (e.g., serum or plasma, buffy coat, whole blood in liquid form or as dried blood spots), and desired storage temperature (e.g., −30°C or −80°C). All of these influence the size and configuration of the future repository and hence the requirements for future repository space.
Users and Uses
6. Raise awareness of and expand access to DoDSR and DMSS.
The use of DoDSR and DMSS resources may be limited because of limited awareness across DoD. For example, military clinicians are apparently largely unaware of these resources in support of clinical management. Broad or targeted “educational campaigns” could be undertaken to raise awareness and use of DoDSR and DMSS. Access also may have been limited because of perceived lack of fully transparent criteria for release of specimens. A remedy for this could include development and dissemination of updated and transparent criteria and procedures for accessing DoDSR specimens and DMSS data. In terms of expanding use, the first priority should probably be for military health users within DoD, followed by more robust use by the VA. DoD should carefully consider whether and how to expand use to civilian researchers, while protecting individual privacy, the overall military health mission, and availability of remaining specimens as more users draw down the number of aliquots from a given specimen. Finally, efforts should be made to take better advantage of the longitudinal nature of the DoDSR inventory, e.g., through clarifying the legitimate use of DoDSR for research and sensitizing military health researchers to the availability of these serial specimens and linked data.
Conclusions
The goal of this study was to help identify opportunities to harness the full value of the DoDSR and DMSS assets—to make even better use of them in addressing military health needs now and into the future. Our analyses uncovered specific opportunities to better fulfill current requirements, especially to close gaps in the content and efficiency of medical surveillance. The largest gap relates to data from deployed settings, which figures prominently within the strategies we describe in the report and our recommendations. The DoDSR and DMSS serve their core surveillance mission; we have identified specific ways to position these resources to better serve the military of the future—planning now for changes that will permit a wider range of uses to improve not only surveillance but also clinical management and research in support of force health protection. Taken as a whole, our recommendations suggest that the DoDSR and DMSS will benefit from improved oversight and management to ensure they function within the strategic goals of the Military Health System, and have access to the needed data systems as well as other resources needed to fulfill the missions assigned to DoDSR and DMSS. Creation of the new AFHSC (after this study was completed) seems to be a good step in that direction, though detailed study of any new directions AFHSC may be taking are beyond the scope of the present study. There are key decisions that need to be made at the Under Secretary of Defense level which will cascade across the recommendations we offer here, affecting the direction of the decisions as well as the magnitude of change.
AMSA was a responsible custodian for the DoDSR and DMSS, characterized by multiple interviewees as “national treasures” whose full potential has yet to be fully harnessed. Creation of the new AFHSC and relocation of the repository offer the opportunity to consider how the DoDSR and DMSS resources can be used to even greater advantage to support military health now and into the future. This study took a systematic approach to analysis of current characteristics and opportunities for improvement. Some of our recommendations are relatively easy, while others are more ambitious. Nonetheless, we feel that implementation of all of these recommendations will allow the AFHSC to better fulfill its current requirements, serve a broader range of legitimate mission areas, and position the DoDSR and DMSS resources for valuable service well into the future.


