Abstract
Study Objectives:
Hypoxia is known to generate sleep-disordered breathing but there is a debate about the pathophysiological responses to two different types of hypoxic exposure: normobaric hypoxia (NH) and hypobaric hypoxia (HH), which have never been directly compared. Our aim was to compare sleep disorders induced by these two types of altitude.
Methods:
Subjects were exposed to 26 h of simulated (NH) or real altitude (HH) corresponding to 3,450 m and a control condition (NN) in a randomized order. The sleep assessments were performed with nocturnal polysomnography (PSG) and questionnaires. Thirteen healthy trained males subjects volunteered for this study (mean ± SD; age 34 ± 9 y, body weight 76.2 ± 6.8 kg, height 179.7 ± 4.2 cm).
Results:
Mean nocturnal oxygen saturation was further decreased during HH than in NH (81.2 ± 3.1 versus 83.6 ± 1.9%; P < 0.01) when compared to NN (95.5 ± 0.9%; P < 0.001). Heart rate was higher in HH than in NH (61 ± 10 versus 55 ± 6 bpm; P < 0.05) and NN (48 ± 5 bpm; P < 0.001). Total sleep time was longer in HH than in NH (351 ± 63 versus 317 ± 65 min, P < 0.05), and both were shorter compared to NN (388 ± 50 min, P < 0.05). Breathing frequency did not differ between conditions. Apnea-hypopnea index was higher in HH than in NH (20.5 [15.8–57.4] versus 11.4 [5.0–65.4]; P < 0.01) and NN (8.2 [3.9–8.8]; P < 0.001). Subjective sleep quality was similar between hypoxic conditions but lower than in NN.
Conclusions:
Our results suggest that HH has a greater effect on nocturnal breathing and sleep structure than NH. In HH, we observed more periodic breathing, which might arise from the lower saturation due to hypobaria, but needs to be confirmed.
Citation:
Heinzer R, Saugy JJ, Rupp T, Tobback N, Faiss R, Bourdillon N, Rubio JH, Millet GP. Comparison of sleep disorders between real and simulated 3,450-m altitude. SLEEP 2016;39(8):1517–1523.
Keywords: sleep disorders, normobaric hypoxia, hypobaric hypoxia
Significance.
Considering the slight physiological differences observed between normobaric and hypobaric hypoxia, we designed a crossover study with the aim to make a direct comparison of sleep disorders in these two hypoxic conditions including a control condition in normobaric normoxia. To assess nocturnal parameters in these three conditions nocturnal sleep polysomnography was used. Our results confirmed that hypoxia generates sleep-disordered breathing, and for the first time that hypobaric hypoxia induces further disordered breathing and sleep structure than normobaric hypoxia. The present findings should be considered when sleeping at altitude is involved during clinical trials or training protocols. Further studies are needed to validate the present results over longer expositions and other hypoxic levels.
INTRODUCTION
Sleep has been recognized as an essential component for athlete preparation and has been suggested to be the single best recovery strategy available to an athlete.1,2 Sleep impairment has been shown to negatively influence the athletes' performance,3 mainly through an impairment of their aerobic performance, which was shown to be directly related to sleep quantity and quality.4,5 A drawback of altitude training might be a lower sleep quality because high-altitude exposure is known for altering sleep quality: West et al.6 reported significant sleep impairment at high altitude based on climbers subjective account and objectively through polysomnography (PSG) recordings. Szymczak et al.7 used two standardized scales (the Pittsburgh Sleep Quality Index and the Athens Insomnia Scale) to assess the subjective sleep quality alterations at high altitude and found significant impairments in general sleep quality and sleep induction.7 However, the precise mechanisms causing sleep disturbances at high altitude remain unclear.
Recreational mountain sports in altitude such as trekking, ski touring, or alpinism are becoming increasingly popular. Altitude/hypoxic training is also commonly used by athletes for enhancing their sea-level performance through an improved oxygen transport capacity as a result of a hypoxia-induced erythropoietic response.8 Hypoxia can be produced by a combination of reduced barometric pressure (PB) and/or a reduced inspired fraction of oxygen (FIO2) resulting in an inspired partial pressure of oxygen (PIO2) less than 150 mmHg.9 There is currently a large debate on the pathophysiological responses to hypoxia in two different types of exposure: hypobaric hypoxia (HH; FIO2 = 20.9%; PB < 760 mmHg) or normobaric hypoxia (NH; FIO2 < 20.9%; PB = 760 mmHg).10 These two types of hypoxia seem to trigger different physiological responses with, for instance, a greater increase in oxidative stress markers11 and a decrease in the nitric oxide (NO) bioavailability in HH, compared to NH condition.12,13 In addition, breathing pattern has been shown to be affected in a different way between HH and NH with a lower tidal volume, lower minute ventilation, a higher physiological dead space, and a higher respiratory frequency during HH exposure.9,14,15 Several previous studies have also demonstrated that the severity of acute mountain sickness (AMS) is higher in HH compared to NH.16–18
During sleep, altitude also seems to have an effect on respiratory physiology in both HH and NH. Oxygen transport has been shown to be reduced in continuous NH19 and intermittent NH20 as well as in HH.21 Moreover, Nespoulet et al. showed that O 2 and CO2 chemosensitivity are closely related to ventilation during NH.22 Kinsman et al.23–25 largely studied respiratory and sleep disturbances during sleep in NH and observed periodic breathing (irregular respiratory pattern marked by alternating periods of rapid and slow respirations and by apneic periods lasting 15 sec or less) at a simulated altitude of 2,650 m. In addition, they also determined that the rate of rapid eye movement sleep (%REM) increased with simulated hypoxic exposure compared to normoxia and that longer exposure time was associated with worse sleep quality. In HH conditions, several authors observed the development of periodic nocturnal breathing with increasing altitude. Moreover, Sargent et al.26 and Roach et al.27 reported a reduction of REM sleep and the presence of periodic breathing during sleep in young athletes after rapid ascent to 3,600 m. This occurrence of nocturnal periodic breathing with accompanying cyclical intermittent hypoxia (IH)19,28 means that the sleeping athlete would experience varying severities of cyclical, intermittent arterial hypoxemia that contain many of the features of maladaptive IH associated with sleep apneas (e.g., reactive oxygen species producing cyclical Hb oxygen desaturation/resaturation, transient arousals, repeated swings in cerebral vascular resistance and blood flow, and intrathoracic pressures coinciding with apneic/hyperpneic phases of the periodic cycles).29 Furthermore, it was speculated30 that these maladaptive effects of the altitude-induced nocturnal IH would persist during the athlete's daily training sessions and negatively affect exercise performance, even in normoxia through elevated sympathetic vasoconstrictor outflow and pulmonary vascular remodeling-reduced plasma volume or high ventilatory drive. Thus, sleeping in altitude would lead to alterations in breathing physiology during sleep and/or subsequently to maladaptive effects altering exercise performance, even at sea level. However, recent studies also suggest that periodic breathing at altitude may be a protective/adaptive mechanism. It has been shown that for a given stimulus (either normobaric or hypobaric) mean nocturnal SpO2 mean can be higher in subjects exhibiting a large amount of apneas compared to those who do not.22 In addition, periodic breathing does not appear to play a predominant role in the pathogenesis of AMS31 and may even have a protective role.22 This supposes a possible disconnection between sleep quality (high number of apneas would be deleterious) and adaptive efficiency (high number of apneas could be positive despite poor sleep quality). This was recently shown in heart failure patients in whom treatment of Cheyne-Stokes breathing with adaptive servoventilation led to a significant increase in cardiovascular mortality.32 To date, sleep pattern has been observed in either NH or HH, but the two conditions have never been directly compared. The purpose of the current study was therefore to compare the magnitude of sleep and breathing disturbances between these two hypoxic conditions in order to better understand the underlying pathophysiological mechanisms.
METHODS
Participants
Thirteen healthy trained male subjects volunteered for this study (mean ± SD; age 34 ± 9 y, body weight 76.2 ± 6.8 kg, height 179.7 ± 4.2 cm). All subjects provided a written informed consent before participation. The experiment was approved by a Medical Ethics Committee (Commission Cantonale Valaisanne d'Ethique Médicale, CCVEM; Agreement 051/09) and performed in accordance with the Declaration of Helsinki.
Protocol
After a first visit to the sleep laboratory, the participants were asked to sleep under three different conditions in random order, 12 to 20 days apart. Two nights were spent in a hypoxic chamber (ATS Altitude, Sydney, Australia) at an altitude of 485 m (Sion, Switzerland). One- night recording was performed in the chamber under normobaric normoxia (NN) conditions with a FIO2 of 20.9% (control night, PB of 718.1 ± 3.8, mmHg PIO2 140.5 ± 0.6 mmHg, a temperature of 23 ± 1°C and a humidity of 42.8 ± 4.4%), and a second night in the chamber under normobaric hypoxia (NH) with a FIO2 of 13.6% to simulate an altitude of 3,450 m (PB of 715.8 ± 3.8 mmHg, PIO2 of 91.0 ± 0.6 mmHg, a temperature of 22.7 ± 0.8°C, and a humidity of 41.0 ± 4.8%). Oxygen level was controlled with an electronic oximeter (GOX 100 oximeter, Greisinger, Regenstauf, Germany). In order to blind the subjects to normobaric hypoxic or normoxic condition, the hypoxic system was also running during the NN night but normoxic airflow was allowed into the chamber. The third nocturnal recording was performed in HH at the Jungfraujoch High Altitude Research Station (3,450 m, FIO2 of 20.9%, PB of 481.8 ± 4.7 mm Hg, PIO2 of 90.9 ± 1.0 mmHg, temperature of 21.3 ± 0.6°C, humidity of 45.1 ± 8.3%). Each session consisted in a 26-h exposure to each condition (NN, NH, HH) in a randomized order. The schedule and activities during the entire sessions were exactly the same for each condition. The bedding conditions were similar among conditions. Sleep hours and conditions were well controlled. Subjects were equipped with the PSG and then immediately went to bed at 22:00. The light were then turned off for the entire night and turned on at 06:00. Thus, the time spent in bed and the night duration were exactly the same among conditions. Moreover, subjects were wearing earplugs and eye masks during the night, to avoid any external (i.e., noise and/or light) perturbations. The travel duration to access the Jungfraujoch High Altitude Research Station by train during the HH sessions was approximately 50 min. This gradual gain in altitude was simulated during the NH and NN sessions. For 45 min before entering the chamber, subjects breathed either hypoxic air (for NH) or room air (for NN) in a blinded fashion, using a mask connected to a three-way valve to an altitude simulation device (Altitrainer, SMTech, Nyon, Switzerland).
The hypoxic chamber is a well-ventilated 30 m3 room (2.4 m × 5.0 m × 2.5 m) with transparent glass panels. The system consists in a compressor storing air in pressurized tanks with serial connection to air filters allowing oxygen reduction (altitude simulation) in the chamber. Temperature inside the chamber was maintained at a mean 23°C (23°C and 22.7°C for NN and NH, respectively) by an internal air conditioning system.
During each session, participants were asked to complete three questionnaires upon awakening in the morning: (1) The ESQ (Environmental Symptoms Questionnaire, divided in two parts: ESQ C for cerebral symptoms and ESQ R for respiratory symptoms, 67 questions33), developed to help researchers quantify symptoms experienced by individuals exposed to extreme environmental conditions; (2) the Lake Louise score questionnaire (LLS), a scoring system developed by the 1991 International Hypoxia Symposium consensus committee, which is currently in widespread use to assess the severity of AMS,34 and (3) the Groningen Sleep Quality Scale,35 which was used to evaluate high altitude sleep disturbances.
Twice per session, 4 h before sleep and 4 h after waking up, the eupneic end-tidal CO2 pressure (PETCO2) was recorded on a breath-by-breath basis by mouth with a Pitot tube (Med-graphics CPX, Loma Linda, CA, USA). This parameter is a good indicator of arterial pressure in CO2 (PaCO2).
Sleep was recorded using PSG. A trained sleep technician equipped the subjects with the PSG recorder (Titanium, Embla Flaga, Reykjavik, Iceland) between 19:00 and 21:00. All sleep recordings included six electroencephalography, two electro-oculography, three surface electromyography (one submental, two for right and left anterior tibialis muscles) channels, electrocardiogram (composed of two electrodes), nasal pressure, thoracic and abdominal belts, body position, oxygen saturation, and pulse rate.
All PSG recordings were scored by a trained sleep technicians (NT) using Somnologica software (Version 5.1.1, by Embla Flaga, Reykjavik, Iceland) and reviewed by certified sleep physicians. Sleep stages, leg movements, and arousals were scored according to the 2007 American Academy of Sleep Medicine (AASM) criteria.36 Apneas/hypopneas were scored according to the AASM 2012 rules.37 The average number of apneas/hypopneas per hour of sleep (apnea-hypopnea index [AHI]) was calculated. The oxygen desaturation indexes represent the number of oxygen saturation drops (≥ 3% and ≥ 4% for oxygen desaturation index [ODI] 3% and ODI 4%, respectively) per hour of sleep.
Statistics
Data are reported as means and standard deviation for all parameters except for AHI and ODI, where the medians and the first and third quartile are reported. Data were tested for equality of variance (Levene test for equality of variances) and for normality (Shapiro-Wilk test). One-way analyses of variance (ANOVAs) with Student-Newman-Keuls post hoc tests for all pairwise comparisons were used to identify differences between conditions (NN, NH, HH) in all respiratory, sleep, and questionnaires data. AHI and ODI were not normally distributed, so ANOVAs were done on log-transformed data and values are presented with medians and confidence intervals. Null hypothesis was rejected at P < 0.05. A two-way ANOVA with repeated measures (condition × time) was used to identify differences in PETCO2 between before and after the night as well as between conditions; Holm-Sidak post hoc test was used for all pairwise comparisons to identify differences between conditions (NN, NH, HH). Pearson correlation was used to determine correlations between SpO2, hypopneas and LLS. All analyses were made using Sigmaplot 11.0 software (Systat Software, San Jose, CA). We estimated that 13 subjects were needed to have a 90% power with and alpha of 0.05 to detect 10 ± 10 respiratory events per hour of sleep between the different conditions.
RESULTS
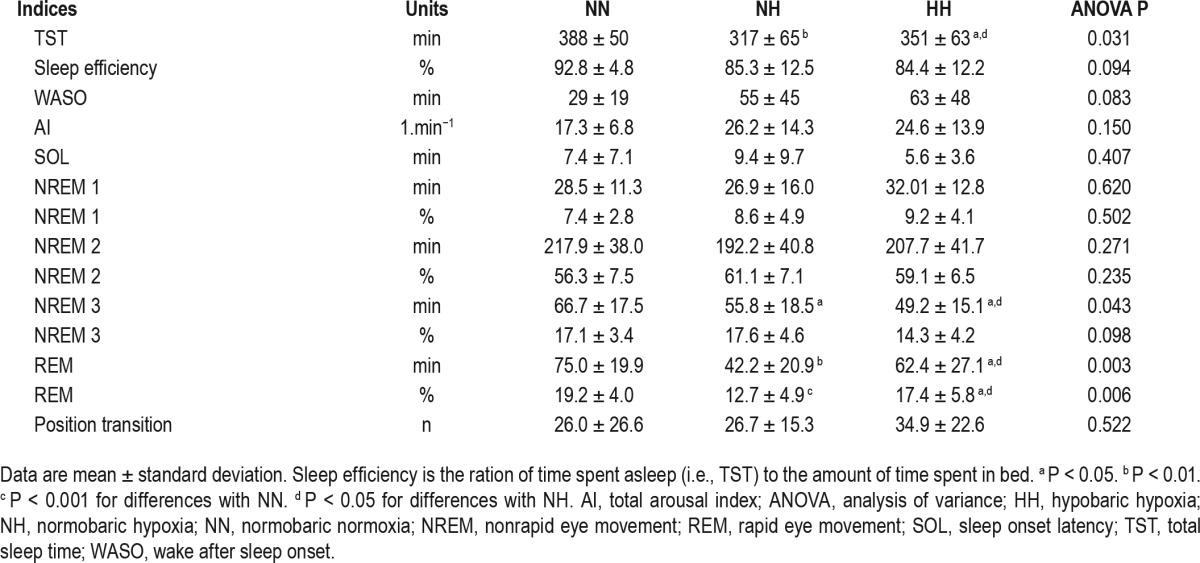
Sleep variables in the three different conditions are presented in Table 1. Total sleep duration was decreased under both hypoxic conditions compared with the normoxic condition. The time spent in deep sleep (N3) and in REM sleep was also reduced in both hypoxic conditions (ANOVA P < 0.05 and P < 0.001, respectively), with an additional reduction in HH compared to NH (P < 0.05 for both).
Table 1.
Sleep indexes and indicators measured with night polysomnography.
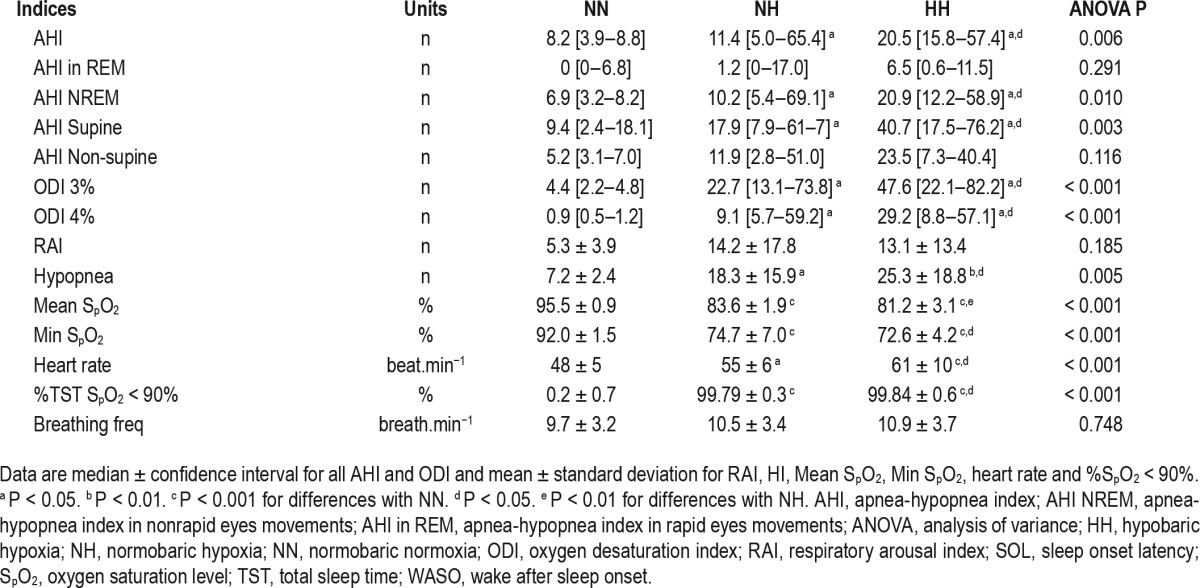
Respiratory parameters during sleep are displayed in Table 2. In HH and NH, there was a decrease in mean nocturnal SpO2 (P < 0.001) and an increase in ODI 3% or ODI 4% compared to NN condition (P < 0.001). We also observed a further significant alteration in these parameters in HH compared to NH (P < 0.05 for all). Similarly, the frequency of respiratory events (AHI) increased in NH compared to NN and was even significantly higher in HH compared to NH (P < 0.05). These differences in AHI were absent in REM sleep. The type of respiratory events observed included most hypopneas, which were almost exclusively central hypopneas. There was no significant difference in the types of apnea (central, obstructive, or mixed) events between the three conditions, which were almost exclusively central apneas. Breathing frequency did not differ between the three conditions.
Table 2.
Respiration indices and indicators measured with the night polysomnography.
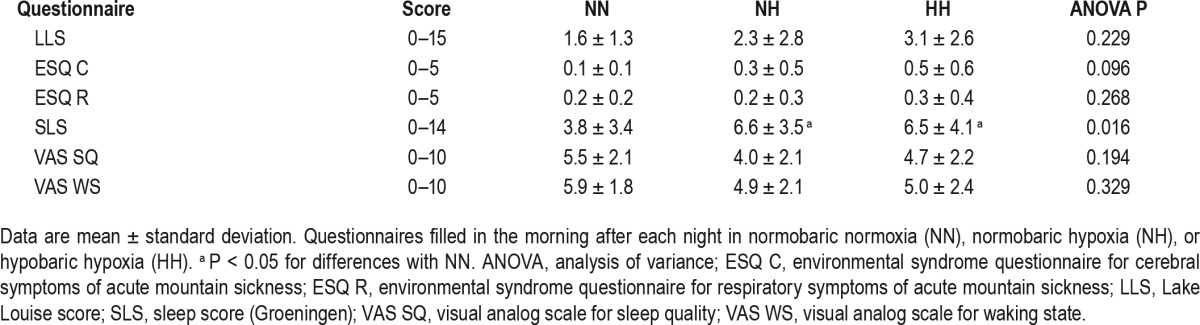
The LLS were not statistically different between conditions (1.6 ± 1.3, 2.3 ± 2.8, and 3.1 ± 2.6 for NN, NH, and HH, respectively), but showed mild AMS (higher than 3) only in HH.
The results of the questionnaires assessing subjective sleep quality and sleep-related complaints are shown in Table 3. Only the Goeningen questionnaire showed a significant decrease in sleep quality in NH and HH compared to NN, without difference between the two hypoxic conditions.
Table 3.
Questionnaires scores.
The PETCO2 before sleep was not different between HH and NH (34.9 ± 5.5 versus 35.1 ± 2.7 mmHg for HH and NH, respectively) but they were both significantly lower compared to NN (38.7 ± 3.2 mmHg, P < 0.05). After sleep, the P ETCO2 remained higher for NN (39.3 ± 3.1 mmHg, P < 0.001) compared to the hypoxic conditions (33.2 ± 2.2 and 33.5 ± 4.8 mmHg for NH and HH, respectively), with both hypoxic conditions being similar (condition × time interaction F = 2.003, P = 0.167).
A negative correlation was noted between SpO2 and the hypopneas (r = −0.442, P = 0.024; NH and HH data pooled). When considering the conditions separately, the relationship was significant only in NH (r = −0.555, P = 0.049 versus R = −0.326, P = 0.277, for NH and HH, respectively). Another correlation was observed between SpO2 and the LLS (r = −0.416, P = 0.035; NH and HH data pooled) but was significant only in HH (r = 0.006, P = 0.984 versus r = −0.659, P = 0.014; for NH and HH, respectively).
DISCUSSION
To our knowledge, this is the first study directly comparing the effects of NH versus HH with matched inspired pressure of oxygen on sleep structure and sleep-disordered breathing in the same group of subjects. Our results show that both hypoxic conditions are associated with decreased sleep quality and increased nocturnal breathing disturbances. The major finding is that the addition of hypobaria to hypoxia (i.e., HH) further alters nocturnal breathing and sleep quality compared to hypoxia alone (i.e., NH).
The decrease in mean SpO2 found in both hypoxic conditions were expected but we observed a further decrease in HH compared to NH despite a similar oxygen pressure level (PIO2 91.0 ± 0.6 mmHg versus 90.9 ± 1.0 for NH and HH). These data are in line with data from previous studies.15,18,38 Although differences in physiological dead space between NH and HH have been previously suggested,14 this mechanism remains highly speculative. In the current study, breathing frequency was not significantly higher in HH than in NH, but differences in breathing pattern between the two hypoxic conditions already have been reported15,39 with a higher breathing frequency in HH, even if this point is still debated.39
The lower SpO2 in HH compared to NH could also be due to a decrease in NO bioavailability, yielding a pulmonary capillary vasoconstriction and impaired alveolar/capillary gas exchange and modifying O2 diffusion by decreasing the pressure gradient.9,40,41 Barometric pressure per se can modify the fluid circulation (e.g., pulmonary lymph node) and the transalveoli-capillary membrane flux.40 It may also influence the N2 and O 2 concentration in the cerebrospinal fluid and therefore partly change the central regulation of ventilation.9 Moreover, it has been speculated that apnea/hypopneas have a protective effect during sleep at altitude by preserving a better oxygenation, probably due to a more pronounced hyperventilation following apneic events.22 It was not the case in the current study: the lower the SpO2, the higher the number of hypopneas. In addition, the relationship between SpO2 and hypopneas was significant only in NH, suggesting that the difference in mean nocturnal SpO2 between NH and HH was not only due to the difference in the ventilatory events.
The increase in respiratory events (central apneas and hypopneas) found in both hypoxic conditions are believed to be related to a hypoxia-induced increase in chemoreceptor sensitivity (higher loop gain).42 When loop gain increases, the ventilatory response to mild increases in arterial CO2 level generated by hypopneas or apneas tends to be excessive during sleep. This ventilatory “overshoot” at the end of the respiratory events will in turn generate a drop in PaCO2 level (and re-increase SpO2) in the following seconds. During sleep, ventilation drive is highly dependent on the blood CO2 level: when P aCO2 drops below a certain level called the apnea threshold, breathing slows down or stops, generating a hypopnea or an apnea until PaCO2 builds up and stimulates breathing again, and so on.
Another factor influencing the increase in respiratory events could be the decreased mean SpO2, which increases the amplitude of the oxygen desaturations (ODI 3% and ODI 4%) for a given decrease in PaO2, because the hemoglobin dissociation curve is much steeper for the SaO2 values of 81% to 84% that we observed in hypoxic conditions. A decrease in arousal threshold (increased arousability) associated with hypoxia could also induce respiratory instability and increase the frequency of nocturnal respiratory events. However, we do not believe that this was a major factor because the arousal index was only mildly increased in hypoxic conditions compared with normoxia.
The decrease in respiratory events found during REM sleep is a well-known phenomenon occurring at low and high altitude due to a lesser CO2 sensitivity in this sleep stage. The increase in sleep-disordered breathing observed in HH compared to NH is, however, more difficult to explain. The possible increase in physiological dead space in HH is probably not the only cause because previous studies by our group and others showed that an increase in dead space (using a face mask) could significantly decrease altitude-induced central respiratory events43,44 (even though this experimental increase in dead space was probably much larger). Altered environmental conditions (comfort, temperature) at high altitude compared with simulated altitude could play a role but the arousal index was not different between both conditions. Of importance was that the environmental factors were strictly controlled, with particular attention on humidity, temperature, and sleep conditions (bedtime and rise, noise and light conditions) and, obviously, inspired oxygen fractions. A measurement of end-tidal CO2 during sleep in both conditions could help to better understand these differences.
We also found decreased sleep duration and a lower proportion of deep sleep (N3) and REM sleep in both hypoxic conditions compared with normoxic condition. These sleep structure alterations have been reported before in young athletes at high altitude (3,600 m) with a resumption after 2 weeks, except for lower deep sleep.26 The same pattern was also reported in mountaineers at higher altitude (4,559 m) with an early improvement after 3 nights.31 The most likely explanation for these sleep structure alterations is the increase in sleep-disordered breathing, which increases sympathetic nervous system activity. Our results do not support direct effect of environmental conditions such as temperature or discomfort of the bed since we saw the same decrease in REM sleep, slow wave sleep, and total sleep time in both hypoxic conditions; whereas NN recordings took place in the exact same chamber as NH, with FIO2 being the only difference. Surprisingly, the sleep duration was more impaired in NH condition than in HH condition despite a lower AHI in NH. We could speculate that hypobaric conditions (HH) may be more exhausting for the brain and could thus generate a greater sleep need than in NH but this hypothesis would need to be confirmed in a specific study.
Despite the fact that no statistical difference in LLS was reported between conditions, a score higher than 3 was only present in HH, showing a mild AMS in this condition only. HH seems to induce more severe AMS than NH, which is in line with a recent study from DiPasquale et al.18 Moreover, the relationship between SpO2 and LLS was significant in the pooled data of NH and HH, but only in HH and not in NH. The lower the SpO2, the higher the AMS, in line with previous studies.
The larger “maladaptive response”30 of sleeping in HH (i.e., induced by lower SpO2 and positive LLS), when compared to NH, is of practical importance for altitude training in athletes. First, it confirms that the severity of hypoxia is higher in HH. Second, it might explain that these detrimental effects may partly counteract the expected positive ones: recently, we reported that the increase in hemoglobin mass45 and the performance enhancement46 were similar following 18 days of “Live High Train Low” (where athletes live and sleep at altitudes between 2,200 and 2,500 m and train under 1,200 m) in NH or HH, despite a more severe hypoxic stimulus (larger sleeping desaturation) and longer hypoxic dose (300 versus 220 h) in HH. However, the current study was performed at real and simulated altitude of 3,450 m and it is likely that the physiological consequences of various degrees of hypoxia might differ. Therefore, the conclusions from the current study might not apply to studies performed at lower or higher altitudes. Further studies at different altitudes are needed to assess the potential sleep differences between NH and HH. Many fields are concerned by the differences between NH and HH: national and international teams and athletes are now using altitude or hypoxic training to improve their preparation47; several military forces are also using preacclimatization strategies to prepare for high-altitude missions or assess the effect of HH versus NH for space and aviation applications.48,49
CONCLUSIONS
Our results demonstrate for the first time that HH (e.g., real altitude) has a greater effect on nocturnal breathing and sleep structure than NH (e.g., simulated altitude) conditions. Primarily, HH induces lower nocturnal oxygen saturation and more AHI compared with NH. The main differences between these conditions could be NO metabolism altering pulmonary capillaries vasodilation or an increased physiological dead space due to hypobaria, but these hypotheses will need to be confirmed in further studies. Additional research is required to determine individually the duration and severity of inspired PO2 (i.e., the degree of hypoxia) for achieving an optimal combination of positive (erythropoietic and peripheral) effects without significantly inducing maladaptive consequences for recovery and performance. However, the current study explores only one (the first) night. A valuable perspective would be to extend the comparison between conditions over longer duration, in order to assess the different adaptations during and after the acclimatization period. Another perspective would be to extend the comparison at lower or higher altitudes, as the current results are only applicable at the tested altitude of 3,450 m.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by grants from the Federal Office of Sport (FOSPO; Switzerland). Dr. Heinzer has consulted for the Nightbalance Company. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Professor Olivier Dériaz and the staff at the Clinique Romande de Réadaptation (Sion, Switzerland) as well as Claudine Frieden and the custodians from the High Alpine Research Station of Jungfraujoch (Jungfraujoch, Switzerland) for their invaluable assistance and access to facilities.
REFERENCES
- 1.Halson S. Nutrition, sleep and recovery. Eur J Sport Sci. 2008;8:119–26. [Google Scholar]
- 2.Fullagar HH, Skorski S, Duffield R, Hammes D, Coutts AJ, Meyer T. Sleep and athletic performance: the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015;45:161–86. doi: 10.1007/s40279-014-0260-0. [DOI] [PubMed] [Google Scholar]
- 3.Cook CJ, Crewther BT, Kilduff LP, Drawer S, Gaviglio CM. Skill execution and sleep deprivation: effects of acute caffeine or creatine supplementation - a randomized placebo-controlled trial. J Int Soc Sports Nutr. 2011;8:2. doi: 10.1186/1550-2783-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver SJ, Costa RJ, Laing SJ, Bilzon JL, Walsh NP. One night of sleep deprivation decreases treadmill endurance performance. Eur J Appl Physiol. 2009;107:155–61. doi: 10.1007/s00421-009-1103-9. [DOI] [PubMed] [Google Scholar]
- 5.Davenne D. Sleep of athletes - problems and possible solutions. Biol Rhythm Res. 2008;40:45–52. [Google Scholar]
- 6.West JB, Schoene RB, Milledge JS, Ward MP, editors. High altitude medicine and physiology. London: Hodder Arnold; 2007. [Google Scholar]
- 7.Szymczak RK, Sitek EJ, Slawek JW, Basinski A, Sieminski M, Wieczorek D. Subjective sleep quality alterations at high altitude. Wilderness Environ Med. 2009;20:305–10. doi: 10.1580/1080-6032-020.004.0305. [DOI] [PubMed] [Google Scholar]
- 8.Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP. Combining hypoxic methods for peak performance. Sports Med. 2010;40:1–25. doi: 10.2165/11317920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Conkin J, Wessel JH., 3rd Critique of the equivalent air altitude model. Aviat Space Environ Med. 2008;79:975–82. doi: 10.3357/asem.2331.2008. [DOI] [PubMed] [Google Scholar]
- 10.Millet GP, Faiss R, Pialoux V. Point: hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J Appl Physiol. 2012;112:1783–4. doi: 10.1152/japplphysiol.00067.2012. [DOI] [PubMed] [Google Scholar]
- 11.Debevec T, Pialoux V, Saugy J, et al. Prooxidant/antioxidant balance in hypoxia: a cross-over study on normobaric vs. hypobaric “Live High-Train Low”. PloS One. 2015;10:e0137957. doi: 10.1371/journal.pone.0137957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly J, Cowan DC, Yeoman DJ, et al. Exhaled nitric oxide and pulmonary artery pressures during graded ascent to high altitude. Respir Physiol Neurobiol. 2011;177:213–7. doi: 10.1016/j.resp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Faiss R, Pialoux V, Sartori C, Faes C, Deriaz O, Millet GP. Ventilation, oxidative stress, and nitric oxide in hypobaric versus normobaric hypoxia. Med Sci Sports Exerc. 2013;45:253–60. doi: 10.1249/MSS.0b013e31826d5aa2. [DOI] [PubMed] [Google Scholar]
- 14.Savourey G, Launay JC, Besnard Y, Guinet A, Travers S. Normo- and hypobaric hypoxia: are there any physiological differences? Eur J Appl Physiol. 2003;89:122–6. doi: 10.1007/s00421-002-0789-8. [DOI] [PubMed] [Google Scholar]
- 15.Saugy JJ, Schmitt L, Cejuela R, et al. Comparison of “Live High-Train Low” in normobaric versus hypobaric hypoxia. PloS One. 2014;9:e114418. doi: 10.1371/journal.pone.0114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richard NA, Sahota IS, Widmer N, Ferguson S, Sheel AW, Koehle MS. Acute mountain sickness, chemosensitivity, and cardiorespiratory responses in humans exposed to hypobaric and normobaric hypoxia. J Appl Physiol. 2014;116:945–52. doi: 10.1152/japplphysiol.00319.2013. [DOI] [PubMed] [Google Scholar]
- 17.Roach RC, Loeppky JA, Icenogle MV. Acute mountain sickness: increased severity during simulated altitude compared with normobaric hypoxia. J Appl Physiol. 1996;81:1908–10. doi: 10.1152/jappl.1996.81.5.1908. [DOI] [PubMed] [Google Scholar]
- 18.DiPasquale DM, Strangman GE, Harris NS, Muza SR. Hypoxia, hypobaria, and exercise duration affect acute mountain sickness. Aerosp Med Hum Perform. 2015;86:614–9. doi: 10.3357/AMHP.4266.2015. [DOI] [PubMed] [Google Scholar]
- 19.Berssenbrugge A, Dempsey J, Iber C, Skatrud J, Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J Physiol. 1983;343:507–24. doi: 10.1113/jphysiol.1983.sp014906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamisier R, Gilmartin GS, Launois SH, et al. A new model of chronic intermittent hypoxia in humans: effect on ventilation, sleep, and blood pressure. J Appl Physiol. 2009;107:17–24. doi: 10.1152/japplphysiol.91165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloch KE, Latshang TD, Turk AJ, et al. Nocturnal periodic breathing during acclimatization at very high altitude at Mount Muztagh Ata (7,546 m) Am J Respir Crit Care Med. 2010;182:562–8. doi: 10.1164/rccm.200911-1694OC. [DOI] [PubMed] [Google Scholar]
- 22.Nespoulet H, Wuyam B, Tamisier R, et al. Altitude illness is related to low hypoxic chemoresponse and low oxygenation during sleep. Eur Respir J. 2012;40:673–80. doi: 10.1183/09031936.00073111. [DOI] [PubMed] [Google Scholar]
- 23.Kinsman TA, Gore CJ, Hahn AG, et al. Sleep in athletes undertaking protocols of exposure to nocturnal simulated altitude at 2650 m. J Sci Med Sport. 2005;8:222–32. doi: 10.1016/s1440-2440(05)80013-1. [DOI] [PubMed] [Google Scholar]
- 24.Kinsman TA, Townsend NE, Gore CJ, et al. Sleep disturbance at simulated altitude indicated by stratified respiratory disturbance index but not hypoxic ventilatory response. Eur J Appl Physiol. 2005;94:569–75. doi: 10.1007/s00421-005-1368-6. [DOI] [PubMed] [Google Scholar]
- 25.Kinsman TA, Hahn AG, Gore CJ, Martin DT, Chow CM. Sleep quality responses to atmospheric variation: case studies of two elite female cyclists. J Sci Med Sport. 2003;6:436–42. doi: 10.1016/s1440-2440(03)80269-4. [DOI] [PubMed] [Google Scholar]
- 26.Sargent C, Schmidt WF, Aughey RJ, et al. The impact of altitude on the sleep of young elite soccer players (ISA3600) Br J Sports Med. 2013;47(Suppl 1):i86–92. doi: 10.1136/bjsports-2013-092829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roach GD, Schmidt WF, Aughey RJ, et al. The sleep of elite athletes at sea level and high altitude: a comparison of sea-level natives and high-altitude natives (ISA3600) Br J Sports Med. 2013;47(Suppl 1):i114–20. doi: 10.1136/bjsports-2013-092843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinsman TA, Hahn AG, Gore CJ, Wilsmore BR, Martin DT, Chow CM. Respiratory events and periodic breathing in cyclists sleeping at 2,650-m simulated altitude. J Appl Physiol. 2002;92:2114–8. doi: 10.1152/japplphysiol.00737.2001. [DOI] [PubMed] [Google Scholar]
- 29.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempsey JA, Morgan BJ. Humans in hypoxia: a conspiracy of maladaptation?! Physiology (Bethesda) 2015;30:304–16. doi: 10.1152/physiol.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erba P, Anastasi S, Senn O, Maggiorirni M, Bloch KE. Acute mountain sickness is related to nocturnal hypoxemia but not to hypoventilation. Eur Respir J. 2004;24:303–8. doi: 10.1183/09031936.04.00006504. [DOI] [PubMed] [Google Scholar]
- 32.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampson JB, Kobrick JL, Johnson RF. Measurement of subjective reactions to extreme environments: the Environmental Symptoms Questionnaire. Mil Psycholo. 1994;6:215–33. [Google Scholar]
- 34.Sutton JR, Coates G, Houston CS, editors. Hypoxia and Mountain Medicine. Burlington, VT: Queen City Printers; 1992. The Lake Louise Consensus on the Definition and Quantification of Altitude Illness. [Google Scholar]
- 35.Jafarian S, Gorouhi F, Taghva A, Lotfi J. High-altitude sleep disturbance: results of the Groningen Sleep Quality Questionnaire survey. Sleep Med. 2008;9:446–9. doi: 10.1016/j.sleep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 37.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiPasquale DM, Strangman GE, Harris NS, Muza SR. Acute mountain sickness, hypoxia, hypobaria and exercise duration each affect heart rate. Int J Sports Med. 2015;36:609–14. doi: 10.1055/s-0034-1398623. [DOI] [PubMed] [Google Scholar]
- 39.Coppel J, Hennis P, Gilbert-Kawai E, Grocott MP. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: a systematic review of crossover trials. Extrem Physiol Med. 2015;4:2. doi: 10.1186/s13728-014-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine BD, Kubo K, Kobayashi T, Fukushima M, Shibamoto T, Ueda G. Role of barometric pressure in pulmonary fluid balance and oxygen transport. J Appl Physiol. 1988;64:419–28. doi: 10.1152/jappl.1988.64.1.419. [DOI] [PubMed] [Google Scholar]
- 41.Loeppky JA, Roach RC, Maes D, et al. Role of hypobaria in fluid balance response to hypoxia. High Alt Med Biol. 2005;6:60–71. doi: 10.1089/ham.2005.6.60. [DOI] [PubMed] [Google Scholar]
- 42.Edwards BA, Sands SA, Owens RL, et al. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol. 2014;592:4523–35. doi: 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovis A, De Riedmatten M, Greiner D, et al. Effect of added dead space on sleep disordered breathing at high altitude. Sleep Med. 2012;13:663–7. doi: 10.1016/j.sleep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Patz DS, Patz MD, Hackett PH. Dead space mask eliminates central apnea at altitude. High Alt Med Biol. 2013;14:168–74. doi: 10.1089/ham.2012.1111. [DOI] [PubMed] [Google Scholar]
- 45.Hauser A, Schmitt L, Troesch S, et al. Similar hemoglobin mass response in hypobaric and normobaric hypoxia in athletes. Med Sci Sports Exerc. 2016;48:734–41. doi: 10.1249/MSS.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 46.Saugy JJ, Rupp T, Faiss R, Lamon A, Bourdillon N, Millet GP. Cycling time trial is more altered in hypobaric than normobaric hypoxia. Med Sci Sports Exerc. 2015 Nov 10; doi: 10.1249/MSS.0000000000000810. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Millet GP, Faiss R, Brocherie F, Girard O. Hypoxic training and team sports: a challenge to traditional methods? Br J Sports Med. 2013;47(Suppl 1):i6–7. doi: 10.1136/bjsports-2013-092793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muza SR. Military applications of hypoxic training for high-altitude operations. Med Sci Sports Exerc. 2007;39:1625–31. doi: 10.1249/mss.0b013e3180de49fe. [DOI] [PubMed] [Google Scholar]
- 49.Self DA, Mandella JG, Prinzo OV, Forster EM, Shaffstall RM. Physiological equivalence of normobaric and hypobaric exposures of humans to 25,000 feet (7620 m) Aviat Space Environ Med. 2011;82:97–103. doi: 10.3357/asem.2908.2011. [DOI] [PubMed] [Google Scholar]


