Abstract
Study Objectives:
To examine associations between cognitive performance and polysomnographic measures of obstructive sleep apnea in patients with multiple sclerosis (MS).
Methods:
Participants underwent a comprehensive MS-specific cognitive testing battery (the Minimal Assessment of Cognitive Function in MS, or MACFIMS) and in-laboratory overnight PSG.
Results:
In adjusted linear regression models, the oxygen desaturation index (ODI) and minimum oxygen saturation (MinO2) were significantly associated with performance on multiple MACFIMS measures, including the Paced Auditory Serial Addition Test (PASAT; 2-sec and 3-sec versions), which assesses working memory, processing speed, and attention, and on the Brief Visuospatial Memory Test-Revised, a test of delayed visual memory. The respiratory disturbance index (RDI) was also significantly associated with PASAT-3 scores as well as the California Verbal Learning Test-II (CVLT-II) Discriminability Index, a test of verbal memory and response inhibition. Among these associations, apnea severity measures accounted for between 12% and 23% of the variance in cognitive test performance. Polysomnographic measures of sleep fragmentation (as reflected by the total arousal index) and total sleep time also showed significant associations with a component of the CVLT-II that assesses response inhibition, explaining 18% and 27% of the variance in performance.
Conclusions:
Among patients with MS, obstructive sleep apnea and sleep disturbance are significantly associated with diminished visual memory, verbal memory, executive function (as reflected by response inhibition), attention, processing speed, and working memory. If sleep disorders degrade these cognitive functions, effective treatment could offer new opportunities to improve cognitive functioning in patients with MS.
Commentary:
A commentary on this article appears in this issue on page 1489.
Citation:
Braley TJ, Kratz AL, Kaplish N, Chervin RD. Sleep and cognitive function in multiple sclerosis. SLEEP 2016;39(8):1525–1533.
Keywords: cognitive dysfunction, MS, multiple sclerosis, sleep apnea, sleep disorders
Significance.
This is the first study to examine relationships between sleep disordered breathing and objective measures of cognition in multiple sclerosis, which provides new insight on potential sleep-based targets that may contribute to cognitive dysfunction in this population. Future studies that build on these findings to demonstrate cause-and-effect relationships between sleep disorders and their treatment on cognitive dysfunction in multiple sclerosis could identify new pathways to ameliorate one of the most common and consequential symptoms of this debilitating disorder.
INTRODUCTION
Multiple sclerosis (MS) is an autoimmune disease that causes inflammation and destruction of the brain and spinal cord. This debilitating neurologic disorder affects nearly half a million Americans, and is the leading cause of nontraumatic disability among young adults.
Cognitive dysfunction is one of the most common and consequential symptoms of MS. Impairments in processing speed, working memory, learning, executive function, visuospatial processing, and language function affect an estimated 42% to 70% of individuals with MS,1–3 and constitute a major cause of loss of employment and reduced quality of life.4 Despite its high prevalence and effect, there are no definitive treatments for cognitive dysfunction in MS, and the identification of appropriate strategies to improve cognition in MS is hindered by a poor understanding of modifiable factors that may influence its severity.
In addition to neurological morbidity, MS is associated with a disproportionately high prevalence of sleep disorders, which are associated with poor functional status.5,6 Furthermore, recent data suggest that obstructive sleep apnea (OSA) is more prevalent in patients with MS than in the general population.7,8 Growing evidence also suggests that OSA and other sleep disturbances are modifiable risk factors for cognitive dysfunction in the general population.9–15 However, relationships between gold-standard measures of cognitive function, sleep apnea, and other objective measures of sleep disturbance remain virtually unstudied in patients with MS, and the potential role for sleep-based interventions to improve cognitive functioning in MS has yet to be fully explored. The purpose of this study was to examine the associations between objective measures of OSA and cognitive function in patients with MS. Based on previous literature, which has linked nocturnal hypoxia to structural changes in brain regions germane to cognitive domains that are affected in MS,16 we hypothesized that OSA, and OSA-associated hypoxia in particular, would be associated with poorer performance on cognitive tests of verbal fluency, executive functioning, attention, processing speed, verbal memory, working memory, and visual memory.
METHODS
Study Overview
Study procedures were approved by the University of Michigan Institutional Review Board. Written informed consent was obtained from all participants. Following a baseline visit, subjects underwent a single cognitive testing session, followed by in-laboratory overnight polysomnography (PSG). Cognitive testing was conducted by a licensed clinical psychologist (ALK). Average time lapse between cognitive testing and overnight PSG was 48 days. Half of the subjects completed both testing sessions within 21 days.
Subjects
Between August 2013 and December 2014, 38 adults with clinically definite MS were recruited from the University of Michigan Multiple Sclerosis Center. Patients who asked about their sleep or cognition during routine visits were referred to study personnel by their neurologist. Exclusion criteria included: (1) severe cognitive impairment that would prevent study participation; (2) severe immobility that, in the opinion of the principal investigator (TJB), could limit the feasibility of overnight PSG; (3) evidence of severe depression per clinical records or as indicated by a score ≥ 19 on the Patient Health Questionnaire-9 (PHQ-9)17; (4) visual or hearing impairments that could preclude reliable cognitive testing; (5) current compliant use of medical treatment for such OSA, such as continuous positive airway pressure; (6) history of surgical treatment for OSA; (7) current drug or alcohol dependence; (8) active or serious cardio-pulmonary illnesses; or (9) concomitant neurological disorder that could increase OSA risk (e.g., stroke, Parkinson disease).
Measures
Basic demographic and clinical information regarding disease duration, disease modifying therapy use, MS subtype, and comorbid conditions were collected during baseline visits. Medical charts were reviewed. The Patient Health Questionnaire-9 (PHQ-9)17 was used to assess depressive symptomatology. Subjects rated how often they experienced nine depressive symptoms over a 2-week period, with higher scores indicating more severe depression. Fatigue severity was assessed with the Fatigue Severity Scale18 and subjective sleepiness was assessed with the Epworth Sleepiness Scale (ESS).19 Physical disability level was assessed with the Expanded Disability Status Scale (EDSS),20 by a board-certified neurologist and MS specialist (TJB). Scores range from 0 (normal neurological examination) to 10 (death due to MS).
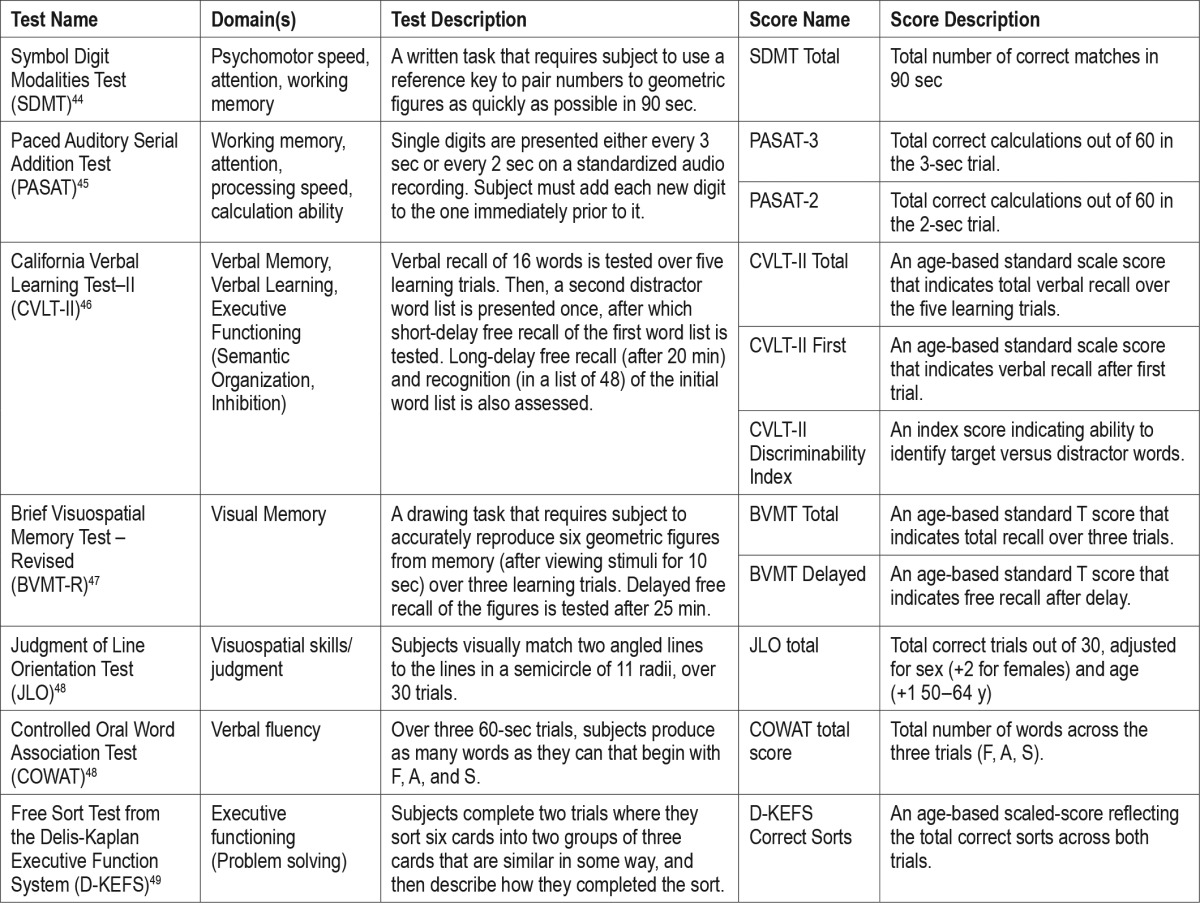
Cognition was assessed with the Minimal Assessment of Cognitive Function in MS (MACFIMS)—a validated, 90-min battery composed of seven standard cognitive tests that assess five domains: processing speed/working memory, learning, executive function, visuo-spatial processing, and language function. Domains of functioning, test descriptions, and meaning of MACFIMS scores most relevant to this study are summarized in Table 1.
Table 1.
Cognitive test and score descriptions.
Polysomnography
Polysomnographic procedures and scoring followed the latest American Academy of Sleep Medicine (AASM) Scoring Rules.21 Standard measures included: total sleep time (TST), sleep efficiency (ratio of time spent asleep to total time in bed), sleep latency (minutes from lights out to first 30-sec epoch of any sleep stage), wake after sleep onset (total time spent awake after sleep onset, and before final awakening time), total arousal index (TAI, average number of electroencephalographic arousals per hour of sleep), % TST spent in stage N1, N2, N3, and rapid eye movement (REM) sleep, the respiratory disturbance index (RDI; total number of apneas, hypopneas, and respiratory-related arousals per hour of sleep), minimum oxygen saturation (MinO2; percentage of sleep time with O2 saturation ≤ 88%), the periodic leg movement index (; number of periodic leg movements per hour of sleep), and the periodic leg movement arousal index (number of periodic leg movements associated with arousals per hour of sleep). Apneas were defined as the cessation of airflow for 10 sec or longer with continued respiratory effort (obstructive apneas) or lack of respiratory effort (central apneas). Hypopneas were defined as a reduction in airflow (≥ 30%) lasting 10 sec or longer, accompanied by either a ≥ 3% oxygen desaturation or an arousal. Respiratory effort-related arousals were defined as the presence of ≥ 10 sec of increasing respiratory effort or flattening of the inspiratory portion of the nasal pressure signal, leading to an arousal from sleep. Given hypotheses regarding the effects of oxygen desaturation frequency on cognitive performance, the oxygen desaturation index was also included (ODI; number of desaturation events ≥ 3% below baseline oxygen saturation levels, per hour of sleep). All PSGs were scored by an experienced, registered polysomnographic technologist, and reviewed by a board-certified sleep specialist blinded to cognitive test scores (NK).
Statistical Methods
Scatterplots and global measures of influence statistics indicated no outliers. Skew and kurtosis values indicated that parametric tests were appropriate except where indicated below. Measures of OSA-related hypoxia (ODI, Min O2) were primary predictor variables of interest. Bivariate correlations (Spearman rho for EDSS correlations, Pearson correlations for all other variables) were examined to identify appropriate adjustment covariates for all regression models, and additional PSG predictor variables for secondary regression analyses.
Separate hierarchical linear regression models were run for each cognitive outcome variable. Age (step 1), disease duration (step 2), PHQ-9 score (step 3), and years of education (step 4) were entered into models before each PSG independent variable (step 5). In analyses that included cognitive variables that were already age-corrected (California Verbal Learning Test-II [CVLT-II], Judgment of Line Orientation Test [JLO], and Brief Visuospatial Memory Test – Revised), age was not included as a covariate.
The Benjamini-Hochberg method, used to correct for multiple testing, indicated a critical value of P = 0.03 with false discovery rate = 0.20.22,23 Relevant variables with P < 0.10 were included in tables. Indicators of effect size were a primary focus; in these models, the R-squared change statistic provided an estimate of the variance accounted for in the outcome by an individual independent variable above and beyond the covariates in the model. All analyses were conducted in IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY: IBM Corp).
RESULTS
Baseline Characteristics
Demographic, cognitive, symptom severity, and PSG measures for subjects are shown in Tables 2 and 3. Mean age was 48.3 y. The majority of subjects (65.8%) carried a diagnosis of relapsing-remitting MS. Half of subjects were receiving disease-modifying therapy at the time of enrollment (n = 5 glatiramer acetate, n = 6 beta-interferon, n = 3 dimethyl fumarate, n = 1 fingolimod, n = 1 natalizumab, n = 2 ocrelizumab, n = 1 mycophenolate mofetil). Thirty-three subjects met criteria for OSA: 29% met criteria for mild OSA, 24% for moderate OSA, and 34% for severe OSA.24 Cognitive test scores for this sample resembled those found in previous studies of the MACFIMS in MS.25
Table 2.
Sample descriptive statistics for demographic and clinical data (n = 38).
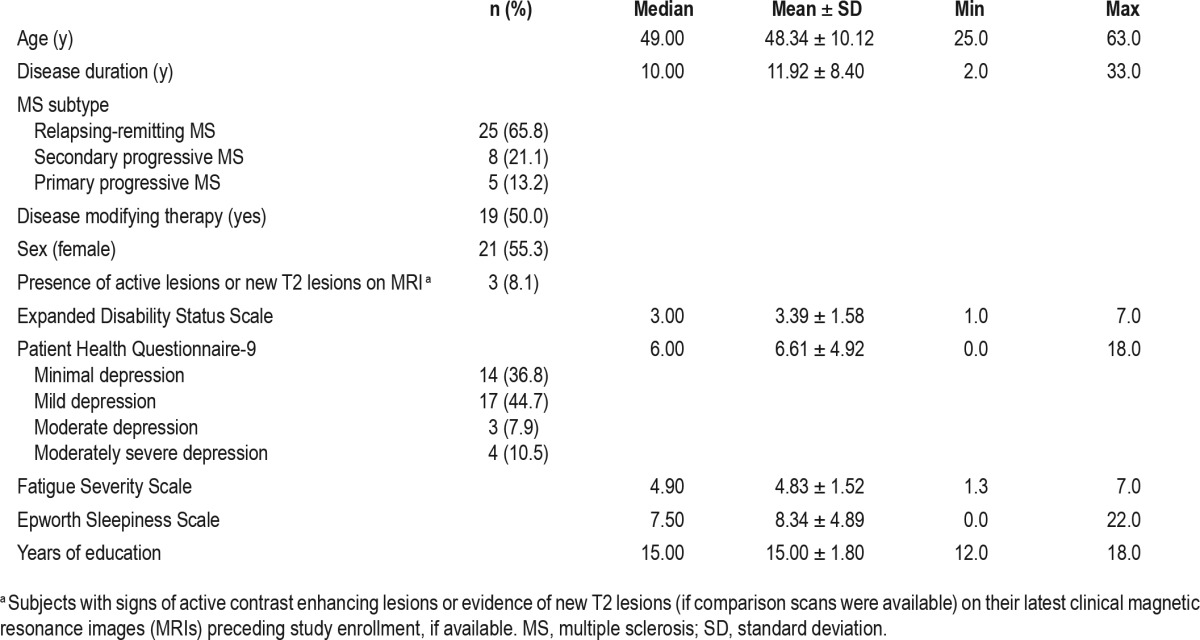
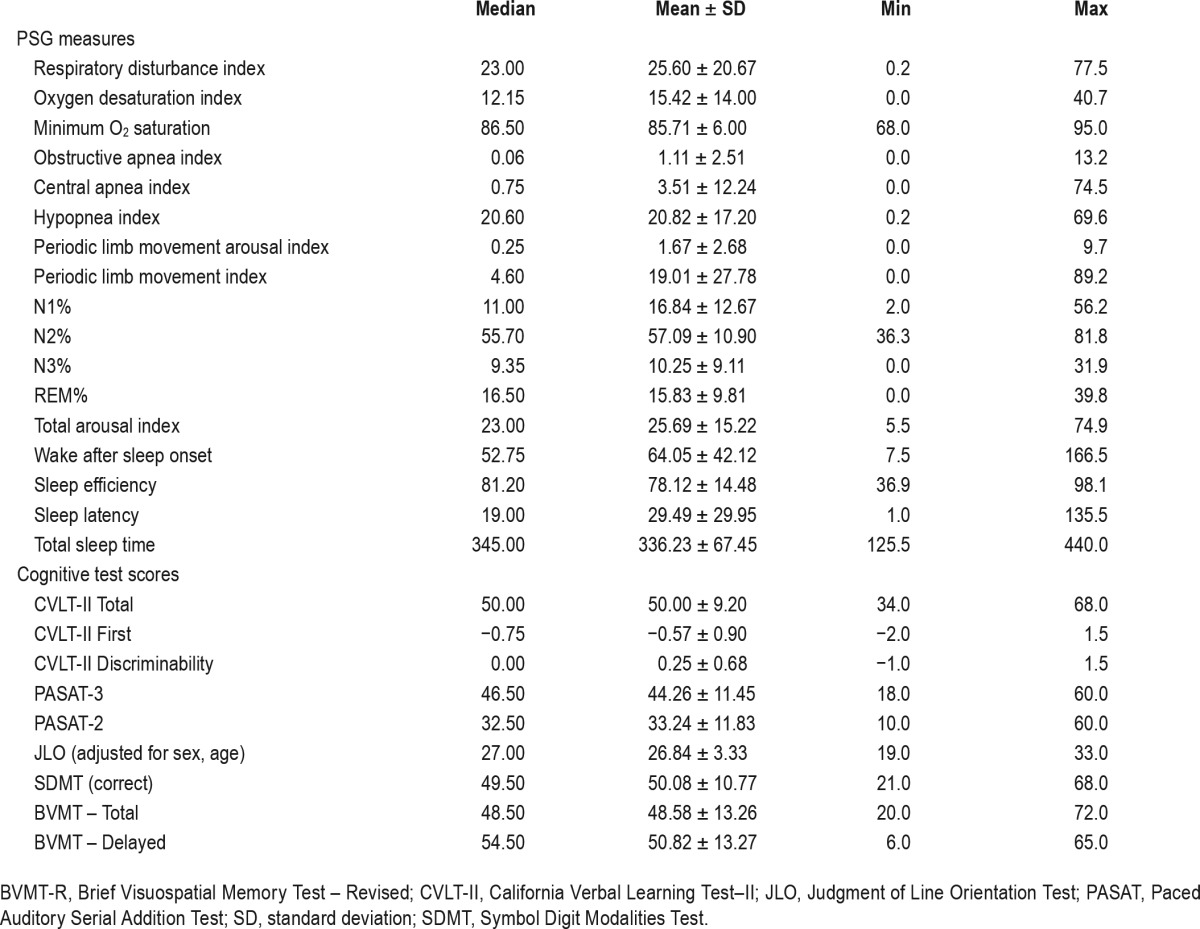
Table 3.
Sample descriptive statistics for polysomnographic measures, and cognitive test scores (n = 38).
Bivariate Analyses
Disability level (EDSS scores) correlated with age (r = 0.44; P < 0.01), depressive symptoms (r = 0.36; P = 0.03), fatigue (r = 0.36, P = 0.03), RDI (r = 0.36; P = 0.03), and TAI (r = 0.46; P < 0.01), but not any cognitive test scores. Age and disease duration were only moderately correlated (r = 0.48, P < 0.01). Fatigue correlated with age (r = 0.32, P = 0.04) and CVLT-II total score only (r = 0.43, P = 0.03). Sleepiness correlated with the CVLT-II Discriminability Index only (r = 0.41, P = 0.03). Fatigue was not associated with sleepiness (r = 0.09, P = 0.58). Depressive symptomatology (PHQ-9) was associated with CVLT-II Discriminability Index (r = 0.36; P = 0.03). As PHQ-9 scores showed associations with measures of fatigue (0.46, P = 0.01), sleepiness (r = 0.44, P = 0.01), and disability (EDSS; r = 0.36, P = 0.03), and in comparison with other measures correlated most consistently with cognitive tests, the PHQ-9 was selected as a key covariate for inclusion in tests of primary study hypotheses. Similarly, education correlated with Paced Auditory Serial Addition Test (PASAT)-3 scores; (r = 0.40; P = 0.01), CVLT-II total scores (r = 0.56; P < 0.001), JLO scores (r = 0.37; P = 0.02), and Symbol Digit Modalities Test (SDMT) scores (r = 0.42; P = 0.01), and therefore was also taken into account in regression analyses.
As expected, PSG measures of sleep quality—TST, SE, wake time after sleep onset, and sleep latency—were highly correlated. TST correlated most highly with other sleep quality measures (e.g. rsleep_efficiency = 0.95) and was therefore identified as a reasonable representative measure to include in other correlation and regression analyses. Measures of sleep quality, as listed previously, were not significantly correlated with OSA measures (r range = −0.03 to 0.29, all P > 0.07), with the exception of the TAI, which correlated strongly with RDI and ODI (r = 0.81 and 0.66, respectively, P < 0.01). The TAI also correlated with the periodic limb movement index and periodic limb movement arousal index (r = 0.42 and 0.49, respectively, P < 0.01).
Higher oxygen desaturation index and lower minimum oxygen saturation values were associated with lower PASAT (r = −0.42, P = 0.002; r = 0.55, P < 0.001, respectively), SDMT (r = −0.33, P = 0.04; r = 0.35, P = 0.03), and Brief Visuospa -tial Memory Test (BVMT)-Delayed Recall scores (r = −0.36, P = 0.03; r = 0.40, P = 0.01). Higher respiratory disturbance index was associated with lower PASAT (r = −0.52, P = 0.001) and SDMT scores (r = −0.39, P = 0.02). TAI was inversely associated with PASAT (r = −0.33, P = 0.04), SDMT (r = −0.38, P = 0.02), and CVLT-II Discriminability Index scores (r = −0.51, P = 0.001). TST positively correlated with CVLT-II Discriminability Index scores (r = 0.40, P = 0.01). Based on these correlations, ODI, minimum oxygen saturation, RDI, TST, and TAI measures were the focus of the primary regression analyses. The Controlled Oral Word Association Test (verbal fluency) and Delis-Kaplan Executive Function System (problem solving) scores were not correlated with any of these sleep measures and were not included in regression analyses.
Regression Models
Results from regression models are shown in Table 4. In multivariate models adjusted for age (when appropriate), disease duration (years), education, and depressive symptoms, measures of apnea severity were significantly associated with performance on multiple cognitive tests. Robust relationships were noted between attention and working memory (PASAT) and the oxygen desaturation index, minimum oxygen saturation, and respiratory disturbance index. Visual memory, as measured by BVMT-Delayed Recall scores, was strongly associated with the ODI and minimum oxygen saturation. CVLT-II Discriminability Index scores, indicative of verbal memory recognition and response inhibition ability, were inversely associated with the RDI. For these noted associations, apnea severity measures accounted for between 12% and 23% of the variance in cognitive test performance. A strong trend also emerged to suggest a positive association between the SDMT (a measure of psychomotor speed, attention, and working memory) and minimum oxygen saturation.
Table 4.
Regression model results examining the association between cognitive test performance (dependent variable) and polysomnographic variables of interest (independent variable) adjusted for age (for tests that are not age-adjusted), disease duration, years of education, and depressive symptoms.
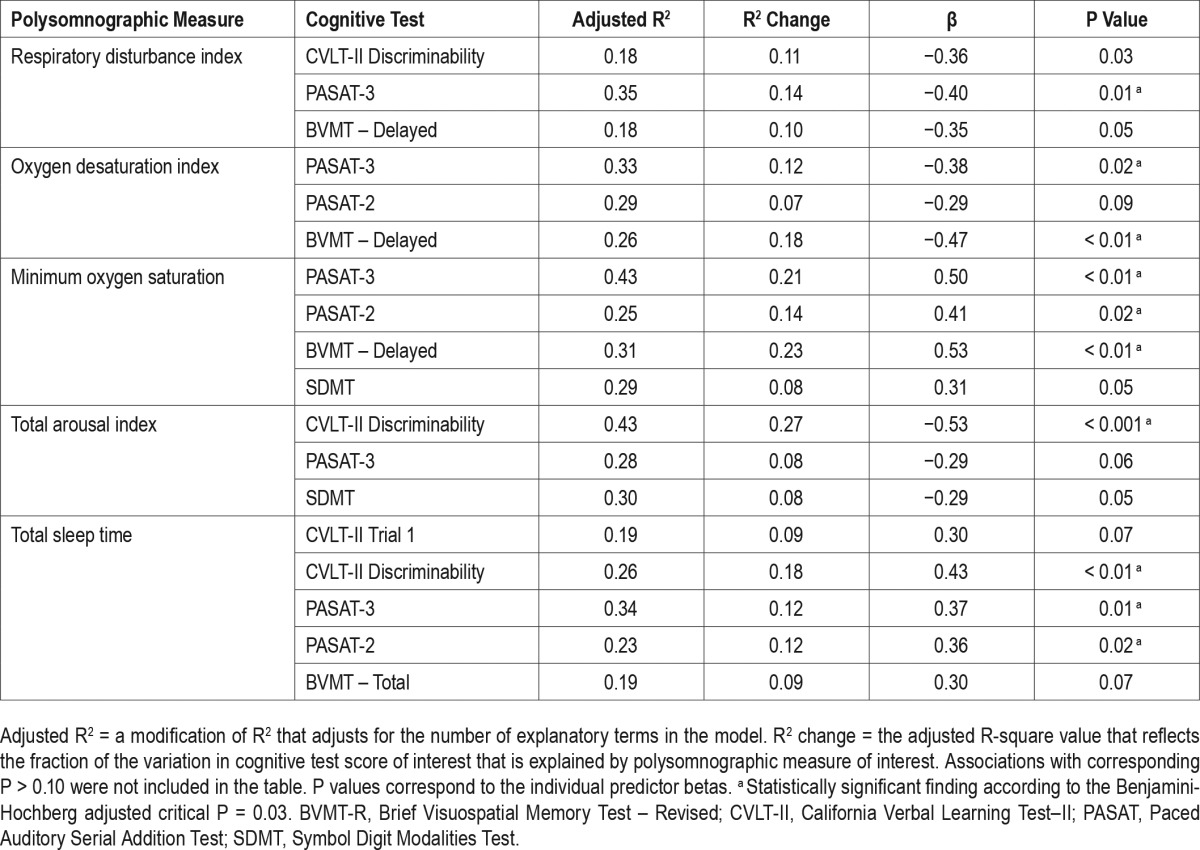
Measures of verbal memory and response inhibition also showed strong associations with sleep quality. Specifically, CVLT-II Discriminability Index scores were associated with the TAI and TST, with 18% to 27% of the variance in CVLTII discriminability performance accounted for by these PSG measures. Although slightly less robust, measures of attention and working memory were also related to sleep quality, with 12% of variability in PASAT scores (both 2-sec and 3-sec versions) accounted for by TST. No significant associations were noted between sleep stages and cognitive test scores in regression models.
DISCUSSION
This study of neuropsychological testing and PSG among patients with MS is the first to show that OSA and sleep disturbances are associated with diminished cognitive performance in persons with MS. Although a causal relationship cannot be demonstrated from these cross-sectional data, our findings highlight a new possibility that, if a causal relationship does exist, strategies to optimize sleep could improve cognitive function in patients with MS.
Our findings offer insight into specific cognitive domains that may be most vulnerable to downstream effects of OSA and other sleep disturbances in MS, and invite comparisons between patients with MS and the general population. Associations between PSG measures and both working memory and attention (as measured by the PASAT and SDMT) in our study are consistent with previous findings linking sleep fragmentation and performance on the PASAT,26 and linking OSA-induced nocturnal hypoxia and sleep fragmentation to impaired memory and attention in the general population.9 Another key finding from our study was the striking association between measures of hypoxia and BVMT-Delayed scores. This is a clinically relevant observation, given that visual memory, and delayed visual memory in particular, are among the most commonly affected domains of cognitive functioning in MS.2 Although data regarding apnea severity and visual memory in the general population have been largely inconclusive,27,28 our findings do suggest a strong connection between OSA and delayed visual memory in MS.
Another noteworthy finding was the association between measures of apnea severity and performance on the CVLT-II Discriminability Index—a measure of ability to correctly identify target words from a list containing distractor words; results therefore reflect both verbal memory and executive functioning (response inhibition) ability.29 These data raise the possibility that apnea severity may differentially affect specific aspects of executive functioning, such as inhibition and semantic organization (CVLT-II subscores), more so than problem solving (D-KEFS) and initiation (Controlled Oral Word Association Test) in persons with MS.
Measures of visuospatial ability (JLO), psychomotor speed, and SDMT-related measures of attention and working memory did correlate with sleep measures, but the significance of some of these associations diminished after controlling for age, education, and depressive symptoms. It is possible that our sample size may have not have been sufficient to identify less robust associations between these variables. The use of the written form of the SDMT test may also have confounded processing speed/attention measurements in patients with diminished upper extremity dexterity, thereby diminishing the robustness of this relationship. Relationships seen between SDMT score and the minimum oxygen desaturation (P = 0.05, Table 4) and other sleep measures may also have been influenced by written form of the SDMT.
Observed associations between apnea measures and both PASAT and BVMT scores also highlight important neuroanatomical and neurophysiological correlates of OSA. Previous research has shown that OSA is associated with reduced gray matter volume,12,30,31 impaired white matter integrity,14 and over-recruitment of specific brain regions in non-MS patients.13 Involved regions include the hippocampus, cingulate cortex, and frontal and parietal lobes. Interestingly, many of these regional changes are seen in cognitively impaired MS patients. Disproportionate reductions in hippocampal volume and white matter integrity involving the superior and inferior longitudinal fasciculi (structures involved in visuospatial function and visual memory) have been reported in cognitively impaired MS patients.32,33 Evidence of increased neuronal vulnerability in these regions allow speculation that potentially detrimental effects of OSA on visual memory experienced by MS patients could exceed those encountered in the general population.
If a relationship between OSA and cognitive impairment in MS can be inferred from these data, the underlying patho-physiological mechanism(s), including relative contributions from OSA-driven hypoxia versus sleep fragmentation, remain speculative. Given the strong associations noted between oxygenation measures (minimum oxygen saturation, in particular) and PASAT and BVMT-Delayed scores, our data support the possibility of a mechanistic contribution from intermittent nocturnal hypoxia. Working, immediate, and delayed memory (PASAT, BVMT, and SDMT) and calculation (PASAT) functions are largely subserved by the hippocampus and prefrontal cortex—regions thought to be susceptible to hypoxic damage from OSA.16 Previous data from non-MS subjects with OSA demonstrate associations between hypoxemia and measures of intelligence, attention, processing speed, and executive function, and therefore also support the hypothesis that OSA-related hypoxemia could explain cognitive impairment in MS patients.15,34–38 If so, the possibility exists that as an alternative to continuous positive airway pressure, oxygen supplementation, though not likely to eliminate OSA, also could ameliorate any effect on cognition. That said, we cannot at this early stage rule out a contribution of sleep fragmentation that may or may not arise from OSA. In our study, approximately half of the TAI variance was accounted for by the RDI and ODI. Given noted associations between the TAI and cognitive measures, it is possible that some of the cognitive impairment found in our sample may be attributable to OSA-induced sleep fragmentation and/or sleep fragmentation that is not OSA related. The sample size of the current study precludes the use of mediation analyses to examine the interrelated contributions of OSA-related hypoxia and sleep fragmentation to cognitive function. However, this important direction for future studies could directly inform interventional decisions. Additionally, the lack of association between apnea measures and remaining PSG sleep quality measures such as TST raises the possibility that cognition in MS could also be affected by other sleep disturbances. The fact that Fatigue Severity Scale and ESS scores were not correlated strongly with cognitive testing scores in this sample raises the possibility that fatigue and sleep are not primary mechanisms to explain why OSA and other sleep disturbances show associations with cognitive functioning in MS.
Cognitive and neuroanatomical changes associated with OSA are potentially reversible with treatment. Positive airway pressure therapy is the first-line treatment for OSA. Positive airway pressure treatment reduces impairments in executive functioning,39 and sustained treatment with positive airway pressure may improve regional hippocampal volume, increase white matter integrity, and normalize brain activation during cognitive tasks in non-MS subjects.12–14 If we assume a similar causal role for OSA and cognitive dysfunction in MS, interventions to treat OSA could offer a new approach to ameliorate cognitive dysfunction in MS patients who suffer from OSA.
Strengths of our study included use of gold-standard measures of cognition and sleep, and adjustment for age, educational level, and depression. Among potential limitations, the time lapse between cognitive testing and PSG may have influenced results; however, PSG and cognitive testing generally are thought to assess traits that are not expected to change substantially in the short term. If a time-lapse effect did occur, it would most likely have led to a conservative error, reducing rather than magnifying observed effect sizes. Recruitment of subjects who inquired about sleep or cognitive function in MS at a single MS specialty clinic may limit generalizability, or explain the high prevalence of OSA in this sample. Although sleep efficiency of subjects in this study was good, 75% of sleep time was spent in stages N1 and N2 sleep, raising the possibility of whether this might have affected daytime wakefulness and attention. Although we cannot discount this possibility, we note that this sleep stage distribution was nearly identical to that which we observed in a previous study among normal controls.40 Moreover, in the current study, ESS scores did not correlate with measures of attention, suggesting that light nocturnal sleep and any consequent daytime sleepiness may not have had a substantial effect on attention.
Although medical charts were reviewed, magnetic resonance imaging reports did not quantify radiographic disease burden (i.e., T2 lesion volume) in a way that could be used in the analyses. We therefore cannot exclude the possibility that radiographic disease burden could have influenced cognitive performance; however, as global T2 lesion burden does not sufficiently account for cognitive impairment in MS,41 nonconventional magnetic resonance measures (tractography, brain parenchymal fraction) would be more useful in future studies. The effects of disease-modifying therapy should also be evaluated as a potential effect modifier in future studies. Some research suggests that certain MS disease-modifying therapies (such as the beta-interferons and natalizumab) may slow or reverse MS-related cognitive dysfunction.42,43 Early data from the principal investigator also suggest that MS patients who are on immunomodulatory therapy have significantly reduced sleep apnea severity than those who are not on treatment, after controlling for other important apnea risk factors including the presence of brainstem lesions, progressive MS subtype, age, and male sex.40 Although the cause of this association is under exploration, the finding suggests that disease modifying therapy use could potentially influence apnea severity as well as cognitive functioning, and the potential mechanistic relationship between these outcomes warrants investigation. To control for disease modifying therapy effect and other MS-specific variables, confirm our current findings, and prospectively evaluate cause-and-effect relationships, a randomized controlled clinical trial is now under way to study the effects of OSA and treatment with positive airway pressure therapy on cognitive function in MS patients with OSA (NCT02544373).
In conclusion, our findings identify for the first time an association between OSA and cognitive functioning in MS. These data provide initial evidence that intermittent hypoxemia and other nocturnal disturbances may contribute to cognitive dys-function in MS. If so, further research on these relationships and demonstration of response to treatment for sleep disorders could lead to novel strategies to alleviate MS-related cognitive dysfunction, one of the most debilitating symptoms of this disease.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported in part by a National Center for Advancing Translational Sciences (CTSA) of the National Institutes of Health Award (award number UL1TR000433) and the University of Michigan Sleep Disorders Center. Dr. Kratz was supported during manuscript preparation by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award number 1K01AR064275). Dr. Braley has received grant support from the National Multiple Sclerosis Society, the American Sleep Medicine Foundation, and the Michigan Translation and Commercialization (MTRAC) for Life Sciences Program. She is principal investigator on a clinical trial that receives material support, but no financial support, from Biogen. She is site principal investigator for several industry-funded studies of MS immunotherapeutics at the University of Michigan (sponsors include Genzyme-Sanofi and Genentech-Roche). She is also named in a provisional patent, held by the University of Michigan, concerning treatment for sleep apnea. Dr. Kratz has received grant support from the NIH, the National Multiple Sclerosis Society, and the Craig H. Neilsen Foundation. Dr. Kaplish has received grant support from the University of Michigan Dr. Chervin has received NIH research grants, University of Michigan research grants, Philips Respironics and Fisher Paykel support to University of Michigan for educational program in sleep biomedical innovation; serves on the Board of Directors of the American Academy of Sleep Medicine, American Sleep Medicine Foundation, American Board of Sleep Medicine, Associated Professional Sleep Societies, International Pediatric Sleep Association; Advisory Board (volunteer), and Sweet Dreamzzz (not-for-profit community organization); consults for MC3, and Zansors; developed a licensed questionnaire from University of Michigan for Zansors; is an editor for UpToDate and book editor for Cambridge University Press; and is named in copyrighted material, patents, and patents pending, held by the University of Michigan, for sleep apnea and sleep disorder-related diagnosis, assessment, and treatments. Statistical analysis was provided by Drs. Kratz and Braley.
ACKNOWLEDGMENTS
The authors thank Judy Rotthoff, RN, Samantha Harrison, BS, and Veena Kutty, MD for their assistance in data collection and management.
REFERENCES
- 1.Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006;245:41–6. doi: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 3.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–91. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 4.Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231:29–34. doi: 10.1016/j.jns.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Boe Lunde HM, Aae TF, Indrevag W, et al. Poor sleep in patients with multiple sclerosis. PloS one. 2012;7:e49996. doi: 10.1371/journal.pone.0049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veauthier C, Gaede G, Radbruch H, Wernecke KD, Paul F. Sleep Disorders Reduce Health-Related Quality of Life in Multiple Sclerosis (Nottingham Health Profile Data in Patients with Multiple Sclerosis) International journal of molecular sciences. 2015;16:16514–28. doi: 10.3390/ijms160716514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braley TJ, Segal BM, Chervin RD. Obstructive sleep apnea and fatigue in patients with multiple sclerosis. J Clin Sleep Med. 2014;10:155–62. doi: 10.5664/jcsm.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brass SD, Li CS, Auerbach S. The underdiagnosis of sleep disorders in patients with multiple sclerosis. J Clin Sleep Med. 2014;10:1025–31. doi: 10.5664/jcsm.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49:1622–7. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 13.Castronovo V, Canessa N, Strambi LF, et al. Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep. 2009;32:1161–72. doi: 10.1093/sleep/32.9.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37:1465–75. doi: 10.5665/sleep.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance--the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2011;34:303–14. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2:461–71. [PubMed] [Google Scholar]
- 17.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 18.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 23.Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. J Educ Behav Stat. 2002;27:77–83. [Google Scholar]
- 24.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 25.Benedict RH, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006;12:549–58. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 26.Martin SE, Engleman HM, Deary IJ, Douglas NJ. The effect of sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1996;153:1328–32. doi: 10.1164/ajrccm.153.4.8616562. [DOI] [PubMed] [Google Scholar]
- 27.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 28.Twigg GL, Papaioannou I, Jackson M, et al. Obstructive sleep apnea syndrome is associated with deficits in verbal but not visual memory. Am J Respir Crit Care Med. 2010;182:98–103. doi: 10.1164/rccm.200901-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill BD, Alosco M, Bauer L, Tremont G. The relation of executive functioning to CVLT-II learning, memory, and process indexes. Applied neuropsychology. Adult. 2012;19:198–206. doi: 10.1080/09084282.2011.643960. [DOI] [PubMed] [Google Scholar]
- 30.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 31.Joo EY, Jeon S, Kim ST, Lee JM, Hong SB. Localized cortical thinning in patients with obstructive sleep apnea syndrome. Sleep. 2013;36:1153–62. doi: 10.5665/sleep.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longoni G, Rocca MA, Pagani E, et al. Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct. 2015;220:435–44. doi: 10.1007/s00429-013-0665-9. [DOI] [PubMed] [Google Scholar]
- 33.Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013;80:1025–32. doi: 10.1212/WNL.0b013e31828726cc. [DOI] [PubMed] [Google Scholar]
- 34.Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13:950–64. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10:254–62. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 36.Naegele B, Thouvard V, Pepin JL, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18:43–52. [PubMed] [Google Scholar]
- 37.Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90:686–90. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- 38.Kotterba S, Rasche K, Widdig W, et al. Neuropsychological investigations and event-related potentials in obstructive sleep apnea syndrome before and during CPAP-therapy. J Neurol Sci. 1998;159:45–50. doi: 10.1016/s0022-510x(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 39.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36:1297–305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braley TJ, Segal BM, Chervin RD. Sleep-disordered breathing in multiple sclerosis. Neurology. 2012;79:929–36. doi: 10.1212/WNL.0b013e318266fa9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245:111–6. doi: 10.1016/j.jns.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Mattioli F, Stampatori C, Bellomi F, Scarpazza C, Capra R. Natalizumab Significantly Improves Cognitive Impairment over Three Years in MS: Pattern of Disability Progression and Preliminary MRI Findings. PloS One. 2015;10:e0131803. doi: 10.1371/journal.pone.0131803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mokhber N, Azarpazhooh A, Orouji E, et al. Cognitive dysfunction in patients with multiple sclerosis treated with different types of interferon beta: a randomized clinical trial. J Neurol Sci. 2014;342:16–20. doi: 10.1016/j.jns.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 44.Smith A. Los Angeles, CA: Western Psychological Services; 1982. Symbol Digit Modalities Test: Manual. [Google Scholar]
- 45.Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and motor skills. 1977;44:367–73. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 46.Delis DC, Kramer JH, Kaplan E, Ober BA. San Antonio, TX: Psychological Corporation; 2000. California Verbal Learning Test manual: Second Edition, Adult version. [Google Scholar]
- 47.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: Studies of normal performance, reliability, and validity. Psychol Assess. 1996;8:145–53. [Google Scholar]
- 48.Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2nd ed. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 49.Delis DC, Kaplan E, Kramer JH. San Antonio, TX: Psychological Corporation; 2001. Delis-Kaplan Executive Function System. [Google Scholar]


