Abstract
Study Objectives:
Intermittent short sleep (ISS) is pervasive among students and workers in modern societies, yet the lasting consequences of repeated short sleep on behavior and brain health are largely unexplored. Wake-activated neurons may be at increased risk of metabolic injury across sustained wakefulness.
Methods:
To examine the effects of ISS on wake-activated neurons and wake behavior, wild-type mice were randomized to ISS (a repeated pattern of short sleep on 3 consecutive days followed by 4 days of recovery sleep for 4 weeks) or rested control conditions. Subsets of both groups were allowed a recovery period consisting of 4-week unperturbed activity in home cages with littermates. Mice were examined for immediate and delayed (following recovery) effects of ISS on wake neuron cell metabolics, cell counts, and sleep/wake patterns.
Results:
ISS resulted in sustained disruption of sleep/wake activity, with increased wakefulness during the lights-on period and reduced wake bout duration and wake time during the lights-off period. Noradrenergic locus coeruleus (LC) and orexinergic neurons showed persistent alterations in morphology, and reductions in both neuronal stereological cell counts and fronto-cortical projections. Surviving wake-activated neurons evidenced persistent reductions in sirtuins 1 and 3 and increased lipofuscin. In contrast, ISS resulted in no lasting injury to the sleep-activated melanin concentrating hormone neurons.
Conclusions:
Collectively these findings demonstrate for the first time that ISS imparts significant lasting disturbances in sleep/wake activity, degeneration of wake-activated LC and orexinergic neurons, and lasting metabolic changes in remaining neurons most consistent with premature senescence.
Citation:
Zhu Y, Fenik P, Zhan G, Somach R, Xin R, Veasey S. Intermittent short sleep results in lasting sleep wake disturbances and degeneration of locus coeruleus and orexinergic neurons. SLEEP 2016;39(8):1601–1611.
Keywords: extended wakefulness, sleep deprivation, sleep loss, noradrenaline, locus coeruleus, orexin, and orexinergic
Significance.
The work dispels a common presumption: that the brain recovers over the weekend after a week of short sleep. Despite extended sleep recovery periods over long weekends, chronic intermittent sleep loss has lasting consequences on locus coeruleus and orexinergic neurons, neurons that are essential for alertness, mood, sleep/wake regulation, brain health, optimal cognitive performance, and goal driven behaviors. Moreover, the work directly demonstrates that this pattern of intermittent short sleep imposes persistent disruptions in sleep/wake patterns.
INTRODUCTION
A repeated pattern of curtailed sleep across the work and/or school week with catch-up sleep on weekends is common in modern societies. Because subjective sleepiness improves with increased sleep over the weekend, it is generally believed that this sleepiness is driven by homeostatic sleep-promoting factors and is readily and fully reversed with recovery sleep.1 Yet, in humans, sleep restriction across 3–5 consecutive days results in impairments in sustained attention that do not completely reverse after 3 full nights of recovery sleep, despite complete recovery of sleep homeostasis.2,3 Delayed or incomplete reversal of impaired vigilance after chronic sleep loss supports the concept that factors other than homeostatic drive, including injury, may underlie residual wake impairments.
Noradrenergic locus coeruleus (LC) and orexinergic (Orex) neurons are essential for sustained attention and wakefulness.4–6 Both groups of neurons display heightened activity across wakefulness and relative quiescence during sleep. Increased or sustained activation of LC neurons can increase mitochondrial oxidant stress.7 Recently, we discovered that young adult mice exposed to short sleep for several consecutive days evidence a reduction in LC neurons, while surviving neurons manifest mitochondrial hyperacetylation and oxidative injury.8 Orexinergic neurons may also be susceptible to extended wakefulness. Specifically, sleep deprivation in mice for 12 h/day for 7 consecutive days may reduce the number of orexinergic neurons; although stereological counts were not performed.9 Whether these wake-activated neurons in response to intermittent short sleep (ISS) simply downregulate their neurotransmitters and/or neurotransmitter synthesizing enzymes for 1–2 days and fully recover or whether they sustain lasting metabolic injuries is not known. As short sleep is commonly repeated week after week, it is critical to discern whether LC and other wake-activated neurons adapt to intermittent short sleep (ISS) or manifest additive or synergistic injury across repeated weeks of exposure.
Having previously identified mitochondrial oxidative stress in LCn in mice exposed to ISS, we hypothesized that ISS might induce lasting chronic metabolic dyshomeostasis in LCn consistent with premature senescence. Lipofuscin is a cytoplasmic aggregate of protein, carbohydrate and lipid and is considered a marker of aging in neurons.10 These composites increase with aging in select neurons, including the locus coeruleus.11,12 Sirtuins 1 and 3 (SirT1 and SirT3) are NAD+ dependent deacetylases that play important roles in metabolic homeostasis in neurons. Previously, we found that SirT3 declines with chronic sleep loss,8 but whether it remains low in recovery was not determined. In addition, we have shown that SirT1 declines with age in LC neurons and that loss of SirT1 in LC neurons promotes a senescence phenotype including vacuolated dendrites and lipofuscin.12 Thus to begin to characterize metabolic and senescence responses in wake-active neurons, we examined LF, SirT1, and SirT3 and NAD+ synthesizing enzyme responses.
In the present series of studies, we hypothesized that weekend recovery sleep is insufficient for LC and Orex neurons to fully recover, so that progressive and lasting injury should ensue across repeated ISS. We further hypothesized that a sleep-active group of neurons, the melanin-concentrating hormone (MCH) neurons, adjacent to Orex neurons, would confer resistance to ISS-mediated degeneration. Here, we report that ISS induces progressive and lasting loss of LC and Orex neurons, as measured by stereological counts. Despite a 4-week recovery opportunity, surviving neurons neither increase in number nor replete the density of their projections into the frontal cortices. Moreover, the remaining LC and Orex neurons evidence altered morphology and a senescent phenotype with increased lipofuscin, loss of SirT1 and SirT3, while MCH neurons are largely unperturbed. Despite the 4-week rest opportunity following ISS, mice exposed to ISS, relative to rested controls, also had disruptions in their sleep/wake patterns, namely increased wake time during the lights-on (sleep) period and reduced wake at the end of the active period, thereby blunting the diurnal ratio of wakefulness. Collectively, the findings support important lasting consequences of chronic ISS that include degeneration of LC and Orex neurons without compensatory axonal sprouting from remaining neurons; a phenotype of senescence in surviving wake neurons; and residual sleep/wake disturbances, all consistent with sleep patterns in advanced aging.
METHODS
Mice and Study Overview
Studies were performed at the University of Pennsylvania with the approval of the Institutional Animal Care and Use Committee and in accordance with the revised National Institutes of Health Office Laboratory Animal Welfare Policy. Male C57BL/6J (Jackson Laboratories) were 8 weeks of age at the start of experimentation. Mice were housed in a light/dark environment with lights on from 06:00 to 18:00 and fed ad libitum standard rodent chow and water throughout experimentation. Ambient temperature and humidity were maintained between 22–24°C and 40% and 60%, respectively.
Intermittent Short Sleep Protocol
Mice were randomized to receive 1, 2, or 4 weeks of intermittent short sleep (ISS1, ISS2, or ISS4) or rested conditions. Subsets of ISS4 mice and their rested controls were further randomized to 4-week unperturbed recovery prior to study. The rested control (Rest) group for all comparisons to ISS4 and ISS4+Rec was age-matched to ISS4+Rec mice (16 weeks of age for sleep, molecular, immunohistochemistry studies). A previously validated enriched, novel environment paradigm was used to promote spontaneous exploratory wakefulness.13–15 On days 1–3 of each week, mice were moved with littermates to an enriched environment (600 × 400 × 600 cm with various climbing toys, water, food, and cage bedding) for 8 h from 09:00 to 17:00 and were then returned to their home cages with littermates and left unperturbed until the next ISS day, as summarized in the schedule in Figure 1. Ambient light (timing and intensity) and temperature were held constant across the novel environments and home cages. Across trials, mice were continuously observed, and when mice were still, new climbing toys, and bedding/nesting materials were introduced. This particular sleep loss paradigm does not influence plasma corticosterone levels.8 On days 4–7 of each week, mice were left undisturbed in their home cages with their littermates. Rest mice were placed into a similar enriched environment for one hour (17:00 to 18:00) on ISS days and then returned to their home cages. The recovery subsets of mice (ISS4+Rec and Rest) received 4 weeks of undisturbed rest in their home cages, with normal lighting, food, and water.
Figure 1.
Intermittent Short Sleep (ISS) Study Paradigm. Adult male mice were randomized to the two groups, ISS (top) and Rested controls (Rest) below. Black and white bars highlight lights-on and lights-off times respectively. For both ISS and Rest mice, red bars highlight times of exploratory wakefulness exposures for ISS mice, from Zeitgeber hours 4–12 (10:00–18:00) and for Rest mice Zeitgeber hour 11–12 (17:00–18:00). At all other times mice are returned to home cages and left undisturbed. Brackets show times of a continuous recovery opportunity for 4 days. This pattern was repeated 3 times and mice were then randomized to recovery or no recovery.
Surgery and Behavioral State Recording and Analysis
Behavioral states were recorded in subsets of ISS4+Rec and Rest mice (n = 8/sleep condition) after 4-week recovery to determine whether ISS4 imparts residual disturbances in sleep/ wake activity. For chronic behavioral state recordings, mice were administered ketamine (90–100 mg/kg) and xylazine (10 mg/kg) anesthesia and then implanted as previously described with parietal electroencephalographic (EEG) electrodes, cerebellar reference and dorsal nuchal electromyographic (EMG) recording wires, and electrode connector.16,17 Following a 1-week postoperative period, mice were connected to a counterweighted recording cable and commutator. Signals were amplified, digitized, and recorded and exported into the Sleep-Sign sleep/wake program (version 3.0, Kissei) for analysis. Wake-sleep states were scored in 4-sec epochs, using recently detailed criteria for state determination.17 A 24-h period for each mouse was analyzed for total wake, non-rapid-eye movement sleep (NREMS), and rapid-eye movement sleep (REMS) times, 12 h lights on and lights off times, hourly percentile wake distribution, sleep/wake bout duration of behavioral state bouts, latencies to sleep in the active period (dark onset period), and the diurnal ratio of wakefulness (wakefulness across lights on: lights off periods).
Immunohistological Studies
Histology was performed to identify both progressive and lasting effects of ISS on cell counts, cortical projections, and molecular markers of senescence.18 Mice were anesthetized with pentobarbital for transcardial perfusion with paraformaldehyde 4%; brains were post-fixed, cryoprotected, and sectioned coronally 60 μm for 1:6 section series.12 Primary antibodies are detailed in Table 1. Secondary antibodies for immunofluorescence were conjugated with Alexa Fluor probes: 488, 594, or 647 (Invitrogen). Imaging was performed with a Leica DM5500B (light microscopy) and Leica SP5/AOBS confocal (double or triple label immunofluorescence). For confocal image acquisition, laser intensities, exposure time, detector gain, amplifier offset, and depth of the focal plane within section were standardized across compared sections.12
Table 1.
Primary antibodies.

For stereological counts of neurons, 1:2 series of brain sections were first confirmed to cover the complete rostralcaudal extent of LC, orexin and MCH neurons. Sections were immunolabeled for tyrosine hydroxylase, orexin, or MCH using Vector Blue (Vector Laboratories) to highlight the targeted neurons using n = 5 mice/condition. This sample size was conservatively based on our previous LC stereology8 to provide 0.80 statistical power for a 25% difference from a mean of 3,000 and a standard deviation (SD) of 500 (higher than our previous SD). Sections were counterstained with Giemsa to visualize nuclei for counts. A Nikon Eclipse 600 microscope equipped with a Stereo Investigator workstation (MicroBrightField) was used following an optical fractionator strategy19 with a 100x oil objective to estimate the number of target neurons with nuclei becoming visible within the counting frames. For each group of neurons, a sampling scheme of 0.25 area sampling frequency with a thickness sampling frequency of 0.80 (allowing 2-μm guard zones on either side) was used. In preliminary studies implementing wild type rested mice, this strategy provided > 150 counts/ mouse and Gundersen coefficients of error < 0.10. Interscorer accuracy was > 90%, and counts were averaged across two counters before analyzing for sleep condition, as described below in statistical analyses. Cell counters were blinded to sleep conditions.
To determine the effects of ISS on LC, Orex, and MCH cortical projections, coronal forebrain sections were selected per mouse between Bregma 0.5 and −0.1 AP. Sections were processed for immunohistochemistry and then incubated with the relevant primary for 72 h at 4°C. Following application of secondary antibodies, Vector Blue was used to label projections. Light microscopy images of the cingulate cortex 1 and 2 and motor cortex 2 were obtained at 5x magnification using a montage across 15 μm. LC projects to all layers. Using NIH ImageJ software images were converted to 8-bit gray scale. A threshold intensity was created to detect all projections into the regions assayed and was held standard across conditions for each axonal target.
The above-threshold area was used to determine the percentage area covered by the measured axonal projections. A similar approach has been validated for LC projection following N-(2-chloroethyl)-N-ethylbromo-benzylamine (DSP-4) lesions of noradrenergic neurons.20 Values were averaged/animal and analyzed per group, as below in statistical analyses.
To determine whether ISS increases LF in wake-activated neurons, we examined LF autofluorescence intensity in LC, Orex, and MCH neurons across Rest, ISS4, and ISS4+Rec conditions.12 Sections (2/target cell type/mouse) were processed free of triton with Alexafluor 488 (green) labeling of target neurons. Lipofuscin autofluorescence was detected using confocal microscopy (SP-5 AOBS, Leica) with 488 nm excitation and bandpass filtering at 605–700 nm.12 To analyze lipofuscin, ImageJ was used to obtain a mean integrated density for lipofuscin/target neuron, relative to neuronal area and background mean gray value. Effects of sleep loss conditions on LC, Orex, and MCH lipofuscin were analyzed with one-way ANOVA for the 3 conditions, Rest, ISS4, and ISS4+Rec, and Bonferroni corrected for 3 comparisons.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed on LC punches to examine lasting effects of ISS on LC mRNA copy numbers for SirT1, SirT3, and NAD+ synthesizing enzymes: NMNAT1, NMNAT3, and NMAPT (n = 5–8/group) using recently detailed methods.12 Primer/probe sets (listed in Table 2) showed excellent sensitivity (detection of > 104 copies/sample) and linearity of Ct vs. log copies (r2 > 0.99). Copy numbers were normalized to 18S ribosomal RNA and then to the average for the rested group prior to analysis as below.
Table 2.
Real time PCR primer probes sets.

Statistical Analysis
All data are presented as mean ± SE. To examine sleep/wake data (wake, NREMS and REMS) for 24 h for 2 groups (ISS+Rec and Rest), we used unpaired t-tests, with Sidak correction for 3 measures, to provide corrected P values. For light and dark period behavioral state % time, bout length and number analyses, two-way ANOVA with repeated measures for light and dark was used and corrected for within group comparisons using Sidak multiple comparisons post hoc analyses. For cell counts, where 3 or more independent groups were measured (sleep and/or recovery conditions) for one dependent value, one-way ANOVA was used with Sidak correction for multiple comparisons. For both the hourly wake percentiles and the mRNA copy numbers in 2 groups, multiple t-test was used with Sidak multiple comparisons correction. Linear regression was performed to define a relationship between ISS duration and LCn cell counts. Axonal projections, lipofuscin, and intraneuronal sirtuin integrated densities were examined for 3 groups (ISS4, ISS4+Rec, and Rest) also using one-way ANOVA with Sidak multiple comparisons. All statistics were performed with GraphPad Prism software (version 7), and corrected P values of 0.05 were considered significant.
RESULTS
Residual Sleep/Wake Disturbances Following ISS
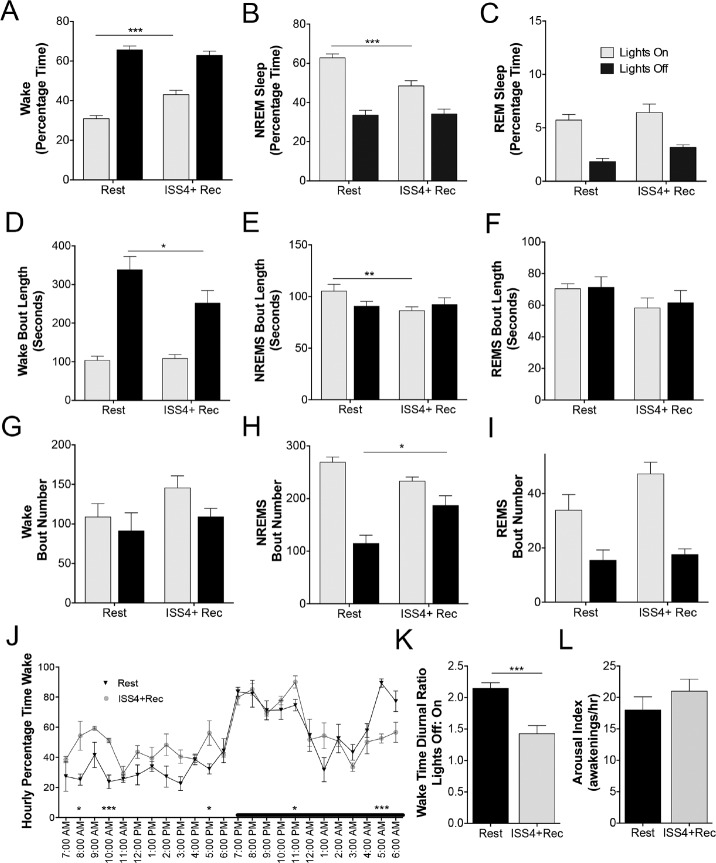
Despite a 4-week opportunity to recover sleep, significant changes were observed in the sleep/wake activity of mice exposed to ISS4. Intriguingly total wake time was increased in ISS4+Rec mice relative to Rest controls (775 min ± 15 vs. 694 ± 22, t = 2.9, P < 0.05). This was attributed to increased wake time in the lights-on period (t = 4.3, P < 0.001) without a change in the 12-h wake time for lights-off period (t = 0.9, N.S., as shown in Figure 2A). The increase in lights-on wake following ISS was accompanied by a reduction in NREM sleep (t = 4.2, P < 0.001) in the lights on period, without a change in REM sleep, as summarized in Figures 2B and 2C. There were also changes in bout length and/or numbers for wake and NREMS. Specifically, wake bout lengths in the dark period were reduced in ISS+Rec mice, t = 2.4, P < 0.05 (Figure 2D), and NREMS bout lengths were shorter in the lights-on period for ISS4+Rec mice, t = 2.4, P < 0.05 (Figure 2E). While there was no effect of ISS on wake bout numbers for either lights-on or lights-off (Figure 2G), there was a greater number of NREMS bouts observed in the ISS4+Rec mice, t = 3.7, P < 0.01 (Figure 2H). In contrast, ISS4+Rec did not affect REM sleep percentage time, bout lengths or numbers (Figures 2F, 2I). Across hourly intervals of the 24-h cycle, there were signifi-cant differences between ISS4+Rec and Rest controls in wake percentages for specific hours, as summarized in Figure 2J. Wake time was reduced in the latter half of the lights-off period and increased for several time points along the lights on period. Thus, the overall effect of ISS on the diurnal ratio of wake activity in the lights-off vs. lights-on period was a marked reduction from 2.1 ± 0.1 (Rest) to 1.4 ± 0.1 (ISS4+Rec), t = 4.6, P < 0.001, as shown in Figure 2K. There was no lasting effect of ISS4 on sleep consolidation, as the arousal indices (arousals/hour sleep) did not vary between ISS4+Rec and Rest controls, t = 1.0, N.S. (Figure 2L). In summary, despite an opportunity of 4 weeks to recover, ISS4 resulted in increased wake time across the inactive period (habitual sleep period) and reduced wake time late in the active period, resulting in a blunting of the diurnal ratio.
Figure 2.
Long-term effects of ISS on sleep/wake activity. (A) Percentage of time spent in wakefulness for the 12-h lights-on period (light gray bars) and the 12-h lights-off period (black bars) for Rest (age-matched) and ISS 4 weeks + recovery 4 weeks (ISS4+Rec). Data are presented as mean ± SE and analyzed with repeated (light and dark) measures ANOVA with Sidak's multiple comparisons correction, ***P < 0.001. (B) Percentage of time spent in non-rapid-eye movement (NREM) sleep for the 12-h lights-on period (light gray bars) and the 12-h lights-off period (black bars) for Rest and ISS4+Rec. Data are presented as mean ± SE and analyzed as in A, ***P < 0.001. (C) Percentage of time spent in rapid-eye-movement (REM) sleep for the 12-h lights-on period (light gray bars) and the 12-h lights-off period (black bars) for Rest and ISS 4 weeks + recovery 4 weeks (ISS4+Rec). Data are presented as mean ± SE and analyzed as above, N.S. (D–F) Mean ± SE bout lengths (seconds) of wake, NREMS and REM for Rest and ISS4+Rec, analyzed as in A, *P < 0.05. (G–I) Mean ± SE bout number (seconds) of wake, NREMS and REM for Rest and ISS4+Rec, analyzed as in A, *P < 0.05. (J) Group hourly wake percentages expressed as means ± SE. Asterisks delineate statistical differences for particular hours corrected (Sidak) for 24 measures using multiple t test *P < 0.05, and ***P < 0.001. (K) Average diurnal ratios for wake time (minutes) in the 12-h lights-off period:12-h lights on period for Rest (black bar) and ISS4+Rec (light gray bar), unpaired t test, P < 0.001. (L) Average arousal index (number of any awakenings, 4 seconds or longer/per hour) for Rest (black bar) and ISS4+Rec (gray bar), unpaired t test N.S.
Lasting Reductions in LC and Orex Neuron Counts Following ISS
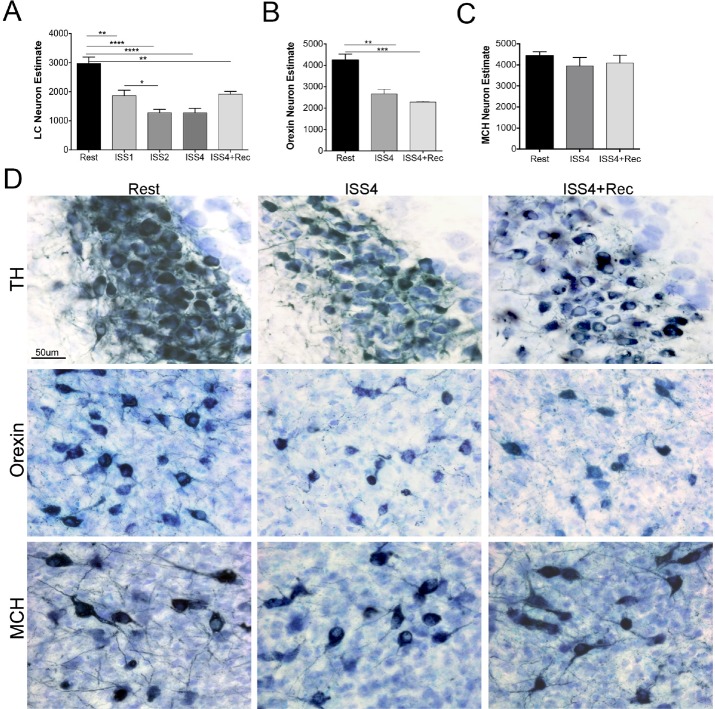
We previously determined that one week of short sleep exposure in the young adult mouse reduced the number of LC neurons by 30%.8 Here we sought to determine whether across repeated weeks of short sleep an adaptive process prevents further cell loss or whether progressive loss of neurons ensues. We also sought to determine whether an extended recovery might unveil LC or Orex neurons that simply down-regulated the identifier protein temporarily, so that after 4 weeks, cell counts would normalize. Mice were subjected to 1, 2, or 4 weeks of extended wakefulness for 3 days with 4 recovery days each week (n = 5/ISS condition). Overall, there were significant effects of ISS on LCn counts, P < 0.0001. Compared to rested (Rest) mice, LCn counts were reduced for all 3 durations ISS1, t = 4.3, P < 0.01; ISS2, t = 6.5, P < 0.0001; ISS4, t = 6.2, P < 0.0001, as summarized in Figure 3A. There was a further reduction in LC counts for ISS1 vs. ISS2, t = 2.7, P < 0.05, without a further decline at ISS4, relative to ISS2 t = 0.0, N.S. Linear regression identified a relationship between ISS durations and LCn count, r2 = 0.5, F = 15.6, P < 0.001. To determine whether the rela -tionship between ISS duration and LCn cell count was based only on the high counts in Rested vs. any duration of ISS, we performed the linear regression exclusively across the 3 ISS conditions and found a weaker but still significant relationship to support a dose response for ISS duration, F = 4.7, r2 = 0.2, P < 0.05.
Figure 3.
Acute and long-term effects of ISS on locus coeruleus (LC), orexin and melanin-concentrating hormone (MCH) cell counts. (A) Optical fractionator LC (bilateral total neuron number) per mouse across conditions: Rest, ISS 1 week (ISS1), ISS 2 weeks (ISS2), ISS4, and ISS4+Rec. Data presented are mean/condition ± SE. Data are analyzed with one-way ANOVA and corrected for multiple groups (n = 5/ condition), *P < 0.05, **P < 0.01, ****P < 0.0001. (B) Orexin neuron counts, also bilateral, as measured for Rest, ISS4 and ISS4+Rec. One-way ANOVA significant differences: **P < 0.01 and ***P < 0.001. (C) Mean ± SE bilateral MCH cell counts/animal, N.S. (D) Representative images of LC (top row) orexin (middle row) and MCH (lower row) neurons (Navy) with Giemsa (light blue/purple) labeled background cells across the 3 conditions.
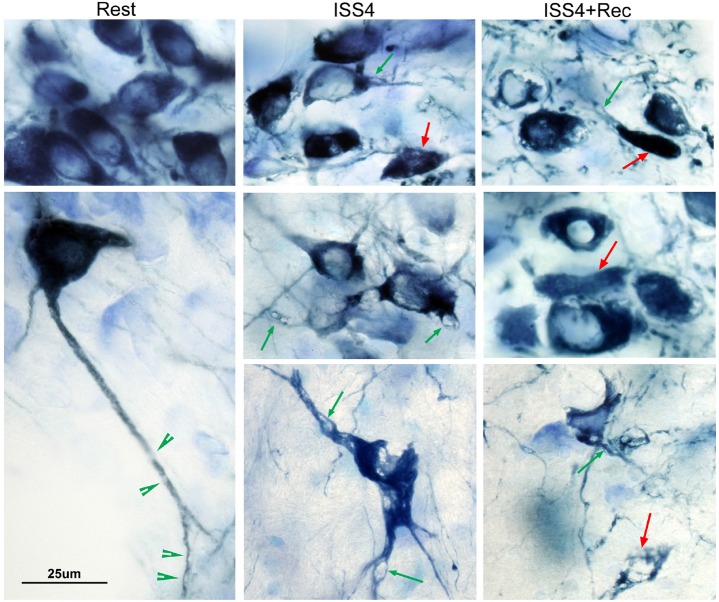
A reduction in tyrosine hydroxylase neurons could be a consequence, not only of neurodegeneration, but also of temporary down regulation of the neuronal marker. Thus, we next performed LC counts on additional groups of mice following 4 weeks of undisturbed rest opportunity (n = 5/condition). LC counts remained significantly reduced after a 4-week recovery opportunity relative to age-matched rested controls, t = 4.1, P < 0.01 (as summarized in Figure 3A), and there was no difference in LCn counts for ISS4+Rec, relative to ISS4 mice, t = 2.7, N.S. In addition to a reduction in the number of TH+LC neurons, there were dramatic effects of ISS4 on LC neuronal morphology. In the young adult mouse, LC neurons typically evidence non-TH-labeled vacuoles within the dendrites (Figure 4). Upon ISS4, the size of the vacuoles markedly increased, resulting in prominent distortion of the somata and dendrites, as observed in Figure 4. Remarkably, the vacuolization of LC neurons persisted despite 4-week recovery opportunity, images also present in Figure 4. Thus, ISS results in persistently reduced numbers of tyrosine hydroxylase-labeled neurons in the LC and lasting morphologic changes in the neurons.
Figure 4.
Morphological changes in LC neurons after ISS. Representative high-power images of locus coeruleus neurons in which tyrosine hydroxylase (TH) is labeled with Vector blue (dark blue) and all nuclei and soma are labeled with Giemsa stain (light blue/purple). Rested control mice (Rest, left images) show homogenous TH labeling in neurons, all of which are > 20 μm in long axis diameter and show both uniform prominent nuclei with condensed chromatin (darker Giemsa) and long dendrites with small vacuoles (green arrowheads). In contrast most neurons in ISS4 and ISS4+Rec evidenced a marked increase in the size of vacuoles within TH+ neurons (green arrows), with vacuolar prominence in somata; smaller cell bodies, typically < 20 μm, with shortened dendrites; and marked distortion of somata. TH+ bodies without a clear nucleus across the focal plane of stereological assessments were not counted. Examples of these TH+ bodies without nuclei are highlighted with red arrows. Scale bar, 25 μm.
Obukuro et al. recently found reduced orexinergic (Orex) neurons following 7 consecutive days of sleep loss, but did not perform unbiased stereology or look at recovery, to distinguish orexin down-regulation from neuronal loss.9 Thus, we next examined Orex neuron counts for Rest, ISS4, and ISS4+Rec groups of mice. Compared to Rest mice, Orex neuron counts were reduced for ISS4, t = 5.0, P < 0.01, as summarized in Figure 3B. Importantly, there was no significant improvement in Orex neuronal counts after the 4-week recovery opportunity, and Orex neuron counts remained significantly reduced relative to Rest, t = 6.2, P < 0.001. In summary, reduced Orex neurons are evident in response to ISS, and 4 weeks of recovery sleep does not increase the numbers of Orex neurons.
We next determined whether ISS degeneration effects are specific to wake-activated populations of neurons, by performing stereological counts on melanin-concentrating hormone neurons (MCH), a group of sleep-active neurons adjacent to Orex neurons. Overall, there were no significant differences across any of the groups, F = 2.8, P = 0.17, as summarized in Figure 3C. Thus, ISS does not impact sleep-active MCH neuron survival in the adult mouse brain.
Effect of Extended Wakefulness on LC, Orex and MCH Projections to the Frontal Cortex
Degeneration within a specific group of neurons can be compensated for, at least in part, through axonal sprouting from remaining neurons, as observed previously for LC neurons exposed to a neurotoxin.21 Thus we next sought to determine whether ISS acutely reduces projections and whether surviving neurons compensate across the extended recovery period to normalize axonal projection densities into the cortex. To relate projection findings to cell count findings, we examined effects of ISS and recovery on LC, Orex, and MCH neurons. Norepinephrine transporter (NET) and dopa-mine β-hydroxylase were used to characterize the effects of ISS on noradrenergic projections. NET cingulate and motor cortex projections (% area covered) were reduced in ISS4 (t = 4.1, P < 0.01) and remained reduced at ISS4+Rec4 (t = 4.6, P < 0.01), as summarized in Figure 5A. Representative images are presented in Figure 5C. To discern whether the ISS effect on NET immunoreactivity was specific for this transporter or similar for other LC projection proteins, we examined DBH, a noradrenaline synthesizing hormone. Effects of ISS on DBH projections were even more pronounced for both ISS4, t = 6.6, P < 0.0001 and ISS4+Rec, t = 4.6, P < 0.0001, as summarized in Figure 5B. ISS also significantly reduced orexinergic projections. Specifically, relative to rested controls, orexinergic projections in the cingulate cortex were reduced in ISS4, t = 6.7, P < 0.0001 and also in ISS4+Rec4, t = 4.9, P < 0.0001. There was no difference in percent area of the cingulate cortex covered with orexinergic axons between ISS4 and ISS4+Rec, t = 1.8, N.S, Figure 5C. Intriguingly there was also a reduction in MCH projections in ISS4 mice, t = 3.2, P < 0.01, yet no difference between Rest and ISS4+Rec (t = 0.8, N.S.), Figure 5D. In summary ISS results in sustained reductions in LC and Orex projections to the cingulate and motor cortices with lesser, reversible effects noted for the MCH sleep-active neurons.
Figure 5.
Acute and long-term effects of ISS on LC, orexin and MCH projections into the cingulate and motor cortices. (A) LC axonal density within the cingulate and motor cortices determined as mean ± SE percentage of sectional area for the cingulate and motor cortices/mouse across conditions Rest, ISS4 and ISS4+Rec. for norepinephrine transporter (NET) immunoreactive fibers (n = 5 mice/group), oneway ANOVA, **P < 0.01. (B) Representative images of NET fibers (Substrate Blue) in superficial layers (I-IV) at the junction of motor cortex 2 and cingulate cortex 1 (top panels) and cingulate cortex 2 (lower panels) in the mouse brain across the three conditions. Calibration bar = 50 μm. (C) Similarly expressed (as in A) dopamine β-hydroxylase projections, across the same 3 conditions, ****P < 0.0001. (D) Similarly expressed orexinergic (Orex) projections, across the same 3 conditions, ****P < 0.0001. (E) Similarly expressed MCH projections, across the same 3 conditions, **P < 0.01.
ISS results in Sustained Lipofuscin in Wake-Activated LC and Orex Neurons
Lipofuscin (LF) is indicative of oxidative stress, and increases gradually across aging. We found a significant effect of sleep condition on LF integrated density (I.D.) for both LC and Orex neurons (F = 32, P < 0.00001 and F = 30, P < 0.0001, respectively). Specifically, relative to Rest conditions, LC neuron LF was increased in both ISS4 (t = 7.1, P < 0.0001) and in ISS4+Rec (t = 6.1, P < 0.001), as summarized in Figure 6A. There was no significant improvement in LF in ISS4+Rec, relative to ISS4 (t = 2.7, N.S.). Similarly, in Orex neurons, LF increased in response to ISS4 relative to Rest controls (t = 8.2, P < 0.0001) and in ISS4+Rec, relative to Rest (t = 4.7, P < 0.001). There was no improvement in comparing ISS4 and ISS4+Rec for Orex neuronal LF (t = 3.1, N.S.), as summarized in Figure 6B. In contrast, with LC and Orex LF responses, there was no effect of sleep condition on MCH neuronal LF, F = 3, N.S., as summarized in Figure 6C. Figure 6D shows representative images of LF within Orex neurons.
Figure 6.
Acute and long-term effects of ISS on lipofuscin (LF) in LC, orexin and MCH neurons. (A) Mean ± SE (n = 5–6/group) autofluorescence integrated density (I.D.) captured in arbitrary (arb) units, relative to background mean gray intensity within LC neuronal somata, ****P < 0.0001. (B) Mean ± SE orexinergic neuron autofluorescence I.D., normalized as in A, one-way ANOVA, ***P < 0.001 and ****P < 0.0001. (C) Mean ± SE MCH neuron autofluorescence I.D., normalized as in A, N.S. (D) Representative images of lipofuscin autofluorescence within LC neurons.
Surviving Wake-Active Neurons Evidence Persistent Loss of Sirtuins Type 1 and 3 and NAD+ Synthesizing Enzymes
Previously we demonstrated a critical role for SirT1 in wake-active neurons, where loss of conditional knock down of SirT1 in young adult mice results in premature senescence phenotype, including increased lipofuscin and loss of projections.12 We first examined LC neuron SirT1 nuclear immunointensity in Rest, ISS4, and ISS4+Rec (n = 8/ condition). Overall, there were significant differences in LC nuclear SirT1 immunointensity, F = 13, P < 0.0001, as summarized in Figure 7A. Specifically, LC nuclear SirT1 was reduced in ISS4 relative to Rest, t = 5.0, P < 0.0001 and in ISS4+Rec relative to Rest, t = 3.8, P < 0.01. SirT1 was unchanged in ISS4+Rec relative to ISS, t = 1.0, N.S. Representative images of SirT1 in LC neurons across the conditions are presented in Figure 7B. We next explored whether SirT1 mRNA showed a similar lasting response to ISS. ISS4 resulted in a persistent reduction in SirT1 mRNA in LC micro-punches (n = 8/group), t = 3.6, P < 0.01. Thus ISS4 results in sustained reductions in SirT1 in LC neurons.
Figure 7.
Acute and long-term LC, orexin and MCH neuronal metabolic responses to ISS. (A) Mean ± SE LC nuclear Sirtuin Type 1 (SirT1) immunoreactivity integrated density (I.D.) across the 3 sleep conditions, one-way ANOVA **P < 0.01, ***P < 0.001. (B) Representative images across Rest, ISS4 and ISS4+Rec conditions showing mid-LC nucleus. LC neurons are identified with tyrosine hydroxylase (TH, Alexafluor 594, red) and SirT1 with Alexafluor 488 (green), where SirT1 localizes in Rest mice almost exclusively to nearly 100% of LC neurons. Very little SirT1 is evident in LC neurons in ISS4 and ISS4+Rec mice. Calib bar, 50 μm. (C) Mean ± SE mRNA copy numbers (n = 5–8/group) by real-time PCR on LC nucleus micropunches across the 2 conditions, Rest (black bars) and ISS4+Rec (gray bars) for SirT1 and SirT3 and NAD+ synthesizing enzymes, NMNAT1 and 3 and NAMPT, *P < 0.05 and **P < 0.01. (D) Mean ± SE SirT3 within LC somata expressed as normalized I.D. across the 3 sleep conditions, ***P < 0.001 and ****P < 0.0001. (E) Mean ± SE SirT3 within orexin neuronal somata expressed as normalized I.D. across the three sleep conditions, ****P < 0.0001. (F) Mean ± SE SirT3 within MCH neuronal somata expressed as normalized I.D. across the three sleep conditions, *P < 0.05, ***P < 0.001, and ****P < 0.0001. (G) Representative images across Rest, ISS4, and ISS4+Rec of SirT3 (green) within LC neurons (TH, red). Calib bar, 50 μm.
We have also found that SirT3 is essential for LC neuronal morphology and survival.8 Thus, we next examined the effect of ISS4 on SirT3 in LC neurons. As with SirT1, ISS4 had a significant effect on LC neuronal SirT3, F = 19, P < 0.0001, as summarized in Figure 7D. Specifically, SirT3 was reduced in ISS4 relative to Rest, t = 6.0, P < 0.0001, and also reduced in ISS4+Rec, relative to Rest, t = 4.3, P < 0.001. There was no difference in ISS4 and ISS4+Rec, t = 2, N.S. Representative images are provided in Figure 7G. We then examined the SirT3 response in Orex neurons, and found an even stronger overall response, F = 30, P < 0.0001. SirT3 in Orex neurons was reduced in ISS4, relative to Rest, t = 7.2, P < 0.0001 and reduced in ISS4+Rec, relative to Rest, t = 4.9, P < 0.0001. In contrast, there was no difference between ISS4 and ISS4+Rec, t = 0.8, N.S., as summarized in Figure 7E. Surprisingly, there was also an overall response in SirT3 in MCH neurons, F = 18, P < 0.001, where ISS4 also resulted in a reduction in SirT3, relative to Rest mice, t = 4.0, P < 0.001. Data are summarized in Figure 7F. However, there was a large increase in SirT3 in ISS4+rec, relative to ISS4, t = 5.8, so that there was also a slight increase in MCH SirT3 in ISS4+Rec, relative to Rest, t = 2.6, P < 0.05. In summary, ISS results in reduced SirT3 in LC, Orex and MCH neurons, but only in the wake groups is the reduction sustained at 4 weeks into recovery. As with SirT1 in the LC, we examined LC SirT3 mRNA was reduced in recovery conditions, t = 5.1, P < 0.01. Both SirT1 and SirT3 are NAD+-dependent enzymes; thus we extended mRNA measures to include NMNAT1, NMNAT3 and NAMPT (n = 5/group). ISS resulted in lasting effects on the mRNA levels for these NAD+ synthesizing enzymes. Both NMNAT3 and NAMPT were reduced in ISS4+Rec vs. Rest mice (t = 3.1, P < 0.05 and t = 3.5, P < 0.05, respectively). Data are summarized in Figure 7C. In summary, ISS4 imparts lasting reductions in SirT1 and SirT3 and key NAD+ synthesizing enzymes.
DISCUSSION
Chronic intermittent sleep loss is pervasive in developed countries, and it is presumed there are no lasting consequences of sleep loss. Using a sleep/wake paradigm to model a pattern of intermittent bouts of short sleep and recovery weekends in young adult mice, we find that 4 weeks of ISS in mice resulted in lasting changes in the 24-h sleep/wake patterns with increased wake time across the habitual sleep period and reduced wake time at the end of the active period. Wake bouts were also of shorter duration in the active period. Importantly, we find sustained reductions in LC and Orex neuron counts and in their axonal projections into the frontal cortices. Surviving neurons showed degenerative morphology and persistent downregulation of SirT1 and 3 and increased lipofuscin, a response most consistent with premature senescence in remaining wake neurons, as discussed below.
Previously we demonstrated that one week of ISS reduced the number of LC neurons. The reduction in LC counts could have been secondary to either a loss of neurons or a down-regulation of LC neuron labeling protein, tyrosine hydroxylase, along with reductions in neuronal soma diameters. Here we demonstrate a progressive reduction in LC neurons across 4-week ISS and show that neuron counts do not improve despite a 4-week recovery opportunity. A similar sustained reduction in counts was confirmed for orexinergic (Orex) neurons. Moreover, there was no compensatory response from surviving wake-activated neurons to re-innervate the frontal cortices across the 4-week recovery. Age-related declines in both LC and orexin neurons have been demonstrated in mice, where > 25% cell loss is not expected before the age of 45 and 100 weeks, respectively.22,23 Thus, the decline we observe in LC and orexinergic neurons at age 16 weeks in ISS4+Rec mice, relative to age-matched Rest control mice, is also consistent with premature senescence of these neurons. Additionally, we observed marked vacuolization of remaining LC neurons. While the etiology of the vacuoles requires further study, this finding is also consistent with aging of neurons, as observed with cholinergic neurons and aging.24 Collectively the work demonstrates for the first time that ISS induces lasting effects on wake-active LC and Orex neuronal numbers, projections and morphology.
While wake disturbances were observed in ISS mice, including shortened wake bouts and times of reduced wakefulness in the lights-off (active) period, the most striking response observed was an increase in wake during the lights-on (sleep-prominent) period and a blunting of the diurnal ratio of wakefulness. Intriguingly, retired shift workers commonly have greater complaints of insomnia than age-matched retired day workers.25 We cannot translate the severity of sleep loss in our ISS paradigm in mice to humans, but our findings support the concept that ISS at some severity can impart lasting changes in sleep/wake activity. Short wake bouts, increased sleep in the active periods, reduced sleep in the inactive period and reduced diurnal ratio for wake are findings consistent with orexin deficiency as in narcolepsy. In contrast with narcolepsy we did not observe shortened REM sleep latencies or abrupt transitions form wakefulness to increase theta activity and loss of muscle tone. A novel model of Orex neuron degeneration suggests that > 95% of Orex neurons must be degenerated before REM sleep onsets and cataplexy develop.26 Whether longer ISS exposures could promote sufficient degeneration in Orex neurons will require further study.
Throughout the body, sirtuins play essential roles in adaptive responses to diverse physiological stimuli and may have even more critical roles in neurons. We found, previously, that SirT3 is essential for the adaptive anti-oxidant response in LC neurons to short-term sleep loss, in that mice lacking SirT3 developed severe oxidative stress in LC neurons in response to just three hours of wakefulness, and transgenic absence of SirT3 manifests as reduced LC neurons and reduced dendritic projections.8 The ISS result in sustained reductions in SirT3 in LC and Orex neurons, suggests that ISS renders these neurons more vulnerable to diverse physiologic challenges, including further short sleep. Previously we discovered striking morphological and metabolic consequences of SirT1 loss in LC neurons, and other wake-activated neurons, including Orex neurons.12 Previously we showed that conditional loss of SirT1in the adult mouse resulted in marked reduction in neurites, vacuolated dendrites, and increased lipofuscin in LC neurons, consistent with premature aging.12 In cultured neurons, SirT1 promotes neurite growth and complexity, while SirT1 inhibition, results in neurite pruning and reduced viability.27 Here we find that ISS results in a sustained reduction in both SirT1 and SirT3 in LC neurons; ISS-induced residual reductions in sirtuins 1 and 3 likely contribute to impaired LC and Orex cortical connectivity. Moreover, key NAD+ synthesizing enzymes (necessary for SirT1 and 3 activity) were also reduced by ISS. SirT1 may regulate SirT3 levels in that a target of SirT3 deacetylation is PGC-1a, which when activated increases SirT3 transcription.28 It would be of interest now to determine whether overexpression of SirT1 or SirT3 in ISS would prevent wake neuron degeneration or might reverse some of the axonopathy.
LF was significantly increased in surviving LC and Orex neurons following ISS, and in both groups remained elevated despite the 4-week recovery opportunity. LF is a marker of protein dyshomeostasis in non-dividing cells such as neurons. Proteins in LF are cross-linked aggregates that have failed both proteasomal degradation and autophagy processing.29 However, LF may also present a vicious cycle for protein homeostasis in that the oxidative aggregates can competitively bind to proteolytic proteins including proteosome 20S,30 and at the same time can impede autophagic clearance by inhibiting lysosomal hydrolases.31 While LF is considered as a marker of aging, there are several instances where LF may be further increased. We have identified increases in LC neuronal LF under conditions of metabolic stress, including loss of SirT1 and long-term sleep fragmentation.12,17 Several lysosomal storage disorders show pronounced lipofuscinoses, where mitochondrial proteins are evident in LF aggregates.32–34 Collectively, persistent LF supports sustained metabolic dyshomeostasis and disturbed autophagy in LC and Orex neurons following exposure to ISS. These findings, too, are consistent with premature cellular senescence.35
It is certainly possible that a far longer recovery opportunity would show improvement in one or more of the behavioral, morphological, or molecular abnormalities we have observed following ISS. Thus, we cannot conclude irreversible changes. In support, a previous study identified delayed restoration (5–12 months) of LC noradrenergic projections after noradrenergic neurotoxin lesions were performed with N-(-2chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) in adult rats.21 Nonetheless, it is critically important to recognize now that sleep loss can have behavioral, molecular, and neuro-anatomic effects lasting at least one month. The present study focused on two select populations of wake-activated neurons, neurons important for attention, alertness, sleep/wake timing, brain health, and memory. It is very likely that other wake-activated neuronal populations are impacted upon by ISS. Future studies will be needed to determine the full extent of neurons susceptible to ISS-induced degeneration. Additionally, studies were performed only in males and should be performed in females to determine relative risk.
The present studies demonstrate that repeated exposures of insufficient sleep interspersed with “recovery” weekends in a mouse model results in persistent sleep/wake disruption and degeneration within at least two groups of wake-activated neurons, without a re-growth of axonal connections to provide compensation for lost neurons. Sleep patterns and the molecular signature of surviving wake neurons following ISS are strikingly similar to findings with advanced age. Lasting injury to the Orex neurons may influence, not only sleep wake stability and timing of sleep, but may also influence anxiety and energy homeostasis.5,36,37 While injury to LC neurons is not expected to influence overall sleep/wake timing, LC dysfunction is expected to impair attention and other aspects of cognition.38 Importantly, LC neurons provide important support for forebrain neurons, glia and vasculature, so that LC injury may jeopardize overall brain health.39 Indeed, in murine models of Alzheimer disease, LC lesioning hastens the temporal progression of plaque formation and cognitive decline.20,40 Clearly, for the millions of individuals who regularly experience intermittent short sleep, it becomes imperative to determine whether sleep loss affects humans as it does mice.
DISCLOSURE STATEMENT
This was not an industry supported study. The studies were performed with support from NIH grants R01 HL123331, HL 124576 and HL096037 to Dr. Veasey. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;109:E2635–44. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 6.Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–54. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Padilla J, Guzman JN, Ilijic E, et al. Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat Neurosci. 2014;17:832–40. doi: 10.1038/nn.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Zhu Y, Zhan G, et al. Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci. 2014;34:4418–31. doi: 10.1523/JNEUROSCI.5025-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obukuro K, Nobunaga M, Takigawa M, et al. Nitric oxide mediates selective degeneration of hypothalamic orexin neurons through dysfunction of protein disulfide isomerase. J Neurosci. 2013;33:12557–68. doi: 10.1523/JNEUROSCI.0595-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem. 2008;106:24–36. doi: 10.1111/j.1471-4159.2008.05385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sladek JR, Jr., Sladek CD. Relative quantitation of monoamine histofluorescence in young and old non-human primates. Adv Exp Med Biol. 1978;113:231–9. doi: 10.1007/978-1-4684-8893-7_14. [DOI] [PubMed] [Google Scholar]
- 12.Panossian L, Fenik P, Zhu Y, Zhan G, McBurney MW, Veasey S. SIRT1 Regulation of wakefulness and senescence-like phenotype in wake neurons. J Neurosci. 2011;31:4025–36. doi: 10.1523/JNEUROSCI.5166-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gompf HS, Mathai C, Fuller PM, et al. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–51. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leger L, Goutagny R, Sapin E, Salvert D, Fort P, Luppi PH. Noradrenergic neurons expressing Fos during waking and paradoxical sleep deprivation in the rat. J Chem Neuroanat. 2009;37:149–57. doi: 10.1016/j.jchemneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–7. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 16.Veasey SC, Valladares O, Fenik P, et al. An automated system for recording and analysis of sleep in mice. Sleep. 2000;23:1025–40. [PubMed] [Google Scholar]
- 17.Zhu Y, Fenik P, Zhan G, Xin R, Veasey SC. Degeneration in arousal neurons in chronic sleep disruption modeling sleep apnea. Front Neurol. 2015;6:109. doi: 10.3389/fneur.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan FC, Hutchison ER, Eitan E, Mattson MP. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology. 2014;15:643–60. doi: 10.1007/s10522-014-9532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 20.Rey NL, Jardanhazi-Kurutz D, Terwel D, et al. Locus coeruleus degeneration exacerbates olfactory deficits in APP/PS1 transgenic mice. Neurobiol Aging. 2012;33:426 e1–11. doi: 10.1016/j.neurobiolaging.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: compensatory response to neurotoxin-induced cell death in the adult rat brain. J Comp Neurol. 1992;321:421–41. doi: 10.1002/cne.903210309. [DOI] [PubMed] [Google Scholar]
- 22.Tatton WG, Greenwood CE, Verrier MC, Holland DP, Kwan MM, Biddle FE. Different rates of age-related loss for four murine monoaminergic neuronal populations. Neurobiol Aging. 1991;12:543–56. doi: 10.1016/0197-4580(91)90086-y. [DOI] [PubMed] [Google Scholar]
- 23.Brownell SE, Conti B. Age- and gender-specific changes of hypocretin immunopositive neurons in C57Bl/6 mice. Neurosci Lett. 2010;472:29–32. doi: 10.1016/j.neulet.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JH, Sampogna S, Morales FR, Chase MH. Age-related changes in cholinergic neurons in the laterodorsal and the pedunculo-pontine tegmental nuclei of cats: a combined light and electron microscopic study. Brain Res. 2005;1052:47–55. doi: 10.1016/j.brainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Monk TH, Buysse DJ, Billy BD, et al. Shiftworkers report worse sleep than day workers, even in retirement. J Sleep Res. 2013;22:201–8. doi: 10.1111/jsr.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabuchi S, Tsunematsu T, Black SW, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34:6495–509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo W, Qian L, Zhang J, et al. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723–36. doi: 10.1002/jnr.22725. [DOI] [PubMed] [Google Scholar]
- 28.Kong X, Wang R, Xue Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohn A, Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013;1:140–4. doi: 10.1016/j.redox.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hohn A, Jung T, Grimm S, Catalgol B, Weber D, Grune T. Lipofuscin inhibits the proteasome by binding to surface motifs. Free Radic Biol Med. 2011;50:585–91. doi: 10.1016/j.freeradbiomed.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Eldred GE. Lipofuscin fluorophore inhibits lysosomal protein degradation and may cause early stages of macular degeneration. Gerontology. 1995;41(Suppl 2):15–28. doi: 10.1159/000213722. [DOI] [PubMed] [Google Scholar]
- 32.Mink JW, Augustine EF, Adams HR, Marshall FJ, Kwon JM. Classification and natural history of the neuronal ceroid lipofuscinoses. J Child Neurol. 2013;28:1101–5. doi: 10.1177/0883073813494268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim JA, Kakhlon O, Li L, Myerowitz R, Raben N. Pompe disease: Shared and unshared features of lysosomal storage disorders. Rare Dis. 2015;3:e1068978. doi: 10.1080/21675511.2015.1068978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim JA, Li L, Kakhlon O, Myerowitz R, Raben N. Defects in calcium homeostasis and mitochondria can be reversed in Pompe disease. Autophagy. 2015;11:385–402. doi: 10.1080/15548627.2015.1009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavilan E, Pintado C, Gavilan MP, et al. Age-related dysfunctions of the autophagy lysosomal pathway in hippocampal pyramidal neurons under proteasome stress. Neurobiol Aging. 2015;36:1953–63. doi: 10.1016/j.neurobiolaging.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Sellayah D, Sikder D. Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology. 2014;155:485–501. doi: 10.1210/en.2013-1629. [DOI] [PubMed] [Google Scholar]
- 37.Giardino WJ, de Lecea L. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol. 2014;29:103–8. doi: 10.1016/j.conb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jardanhazi-Kurutz D, Kummer MP, Terwel D, et al. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem Int. 2010;57:375–82. doi: 10.1016/j.neuint.2010.02.001. [DOI] [PubMed] [Google Scholar]