Cellular sources and secretion mechanisms of IL-36γ in response to recombinant bacterial components and whole bacteria.
Keywords: innate immune response, pneumonia, nonclassic secretion, cytokines
Abstract
Interleukin-36 is a family of novel interleukin-1-like proinflammatory cytokines that are highly expressed in epithelial tissues and several myeloid-derived cell types. Like those of classic interleukin-1 cytokines, the secretion mechanisms of interleukin-36 are not well understood. Interleukin-36γ secretion in dermal epithelial cells requires adenosine 5'-triphosphate, which suggests a nonclassical mechanism of secretion. In this study, murine pulmonary macrophages and human alveolar macrophages were treated with recombinant pathogen-associated molecular patterns (intact bacteria: Klebsiella pneumoniae or Streptococcus pneumoniae). Cell lysates were analyzed for messenger ribonucleic acid by quantitative real-time polymerase chain reaction, and conditioned medium was analyzed for interleukin-36γ by enzyme-linked immunosorbent assay, with or without sonication. In addition, conditioned medium was ultracentrifuged at 25,000 g and 100,000 g, to isolate microparticles and exosomes, respectively, and interleukin-36γ protein was assessed in each fraction by Western blot analysis. Interleukin-36γ mRNA was induced in both murine and human lung macrophages by a variety of pathogen-associated molecular patterns, as well as heat-killed and live Klebsiella pneumoniae and Streptococcus pneumoniae, and induction occurred in a myeloid differentiation response gene 88–dependent manner. Secretion of interleukin-36γ protein was enhanced by adenosine 5'-triphosphate. Furthermore, extracellular interleukin-36γ protein detection was markedly enhanced by sonication to disrupt membrane-bound structures. Interleukin-36γ protein was detected by Western blot in microparticles and exosome fractions isolated by ultracentrifugation. Interleukin-36γ was induced and secreted from lung macrophages in response to Gram-negative and -positive bacterial stimulation. The results suggest that interleukin-36γ is secreted in a non-Golgi–dependent manner by lung macrophages in response to Gram-positive and -negative bacterial challenge.
Introduction
IL-36 is the collective name for 3 recently discovered, novel members of the IL-1 family of cytokines: IL-36α (formerly known as IL-1F6), IL-36β (formerly IL-1F8), and IL-36γ (formerly IL-1F9) [1, 2]. These cytokines share a common receptor (IL-36R, formerly known as IL-1Rrp2 or IL-1RL2), which bears significant homology to the classic IL-1 type I receptor [3]. Binding of agonists to IL-36R recruits IL-1RAcP, a shared accessory protein with IL-1R, activating NF-κB and MAPKs. IL-36Ra (formerly IL-1F5) is an antagonist to the IL-36R, by binding to IL-36R and preventing association with IL-1RAcP [4]. IL-38 (formerly IL-1F10) also binds to IL-36R, with in vitro biologic activity, consistent with IL-36R antagonism, in a magnitude similar to that of IL-36Ra [5]. IL-36 family members are expressed by a variety of cell types, with abundant expression in skin and other epithelial cells [2], as well as monocytes [6, 7]. IL-36 agonists are known to exert proinflammatory effects, best characterized in skin in models of psoriasis [2]. IL-36 is highly expressed in the skin affected by psoriasis [8–11]. In animal models of psoriasis, IL-36 family members have been shown to induce Th17 cytokines, antimicrobial peptides, and other inflammatory cytokines [10–13]. In addition to skin, IL-36 family members have also been shown to have effects on myeloid cells. DCs, bone marrow–derived macrophages, and T cells express IL-36R [14]. Recent data suggest that IL-36 family members can activate DCs and promote skewing of type 1 and Th17 responses [14]. For instance, IL36α and -β promote Th1 polarization of naïve T cells. Moreover, incubation of PBMCs with Aspergillus fumigatus in-vitro induces IL-36γ expression, and blockade of the antagonist IL-36Ra enhances IFN-γ and IL-17 production [15]. In the lung, it has been shown that IL-36 family members are expressed in tracheal and bronchial epithelial cells and fibroblasts in response to a variety of inflammatory cytokines and bacterial and viral PAMPs [16–20]. However, a full determination of IL-36 cell sources, mechanisms of secretion, and IL-36-responsive cell types has yet to be made.
Like classic IL-1 cytokines, IL-36 does not contain known signal sequences, and the mechanism of secretion is not well understood [21, 22]. IL-1β, is released extracellularly in the presence of bacterial toxins [23, 24], ATP [25, 26], or both in combination. ATP-stimulated secretion is mediated by the P2X7 receptor, a ligand-gated ion channel expressed by monocytes and macrophages [26–28]. Stimulation of the P2X7 receptor has been shown to induce plasma membrane blebbing and shedding of membrane components in MPs [29–31]. MPs are 50 nm to 1 μm in size, and in addition to plasma membrane components, contain numerous proteins, mRNAs, microRNAs, organelles, and bioactive lipids [32–35]. Pro-IL-1β, which also lacks a secretory signal sequence, is secreted in response to ATP and is packaged in MP complexed with caspase-1, which is necessary for post-translational processing of IL-1β [25, 26, 36, 37]. IL-1β can also be secreted in smaller membrane structures that are formed intracellularly, referred to as exosomes. P2X7 stimulation can also activate caspase-1, promoting pyroptotic and apoptotic cell death [25, 30, 31]. Secretion of IL-36γ from dermal epithelial cells in response to the TLR3 ligand polyI:C does not require ATP, but is dependent on activation of caspase 1-mediated cell death responses. These findings suggest that IL-36 secretion is complex and poorly understood.
In this study, we examined the induction of IL-36γ in vitro by PMs in response to bacterial stimuli or recombinant PAMPs. We demonstrated that IL-36γ is induced in PMs in response to both the Gram-negative bacterium Klebsiella pneumoniae (Kp) and the Gram-positive organism Streptococcus pneumoniae (Sp). Furthermore, we showed that IL-36γ is secreted by PMs within both MPs and exosomes.
MATERIALS AND METHODS
Animals
Specific pathogen-free age- and sex-matched C57BL/6 mice (WT) and MyD88−/− mice on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed in specific pathogen-free conditions within the animal care facility (Unit for Laboratory Animal Medicine, University of Michigan, Ann Arbor, MI, USA) until euthanasia. Animal studies were reviewed and approved by the University Committee on the Use and Care of Animals (University of Michigan).
Bacterial preparation
Kp and Sp (ATCC, Manassas, VA, USA) were used in our studies. Kp strain 43816, serotype 2 was grown overnight in trypticase soy broth (BD Bioscience, Franklin Lakes, NJ, USA) at 37°C. Sp serotype 3 (6303; ATCC) was grown to mid-log phase in Todd-Hewett broth, washed in PBS, and serially diluted in sterile saline. The concentration of bacteria in broth was determined by measuring the absorbance at 600 nm and then plotting the OD on a standard curve generated by known CFU counts. The bacterial culture was then diluted to the desired in-assay concentration. An aliquot of the inoculated bacterial suspension was serially diluted onto blood agar plates to determine actual dose of bacterial CFU. Heat-killed bacteria were prepared by incubating cultures in a 65°C water bath for 45 min.
Intratracheal bacterial inoculation
For intratracheal injection, mice were anesthetized intraperitoneally with a ketamine and xylazine mixture. Under sterile conditions, the trachea was exposed, and a 30 µl inoculum was administered via a sterile 27-gauge needle. The mice were given either 5 × 103 CFU of Kp or 5 × 104 CFU of Sp. The skin incision was then closed with surgical staples. After 48 h, the mice were euthanized by CO2 asphyxiation. The thoracic cavity was opened under sterile conditions, and the pulmonary vasculature was perfused with 1 ml sterile PBS containing 5 mM EDTA into the right ventricle. Whole lungs and spleen were removed, taking care to dissect the lymph nodes. The organs were then homogenized separately in TRIzol reagent (Thermo Scientific-Invitrogen, Grand Island, NY, USA) with a Tissue-Tearor (Cole-Palmer, Vernon Hills, IL, USA), and mRNA was extracted [38].
Generation of rabbit anti-mouse polyclonal IL-36γ-specific Ab
An anti-IL-36γ Ab was generated in New Zealand white rabbits with recombinant mouse IL-36γ (carrier free; R&D Systems, Minneapolis, MN, USA) [39]. The Ab was purified and titered by ELISA against IL-36γ-coated 96-well plates. Purified IgG from nonimmunized rabbits was used as the control.
Murine PM isolation
PMs consisting of both AMs and interstitial lung macrophages were isolated from dispersed lung digest cells by adherence purification [38]. The purity of these macrophages was >90%, as determined by forward- and side-scatter characteristics and F480 and CD11b staining.
Human AM isolation
Human AMs were isolated from lungs explanted from potential organ donors at the time of organ retrieval. These lungs were deemed not suitable for transplantation and were donated for research purposes, as approved by the University of Michigan Institutional Review Board. Lungs were lavaged with serial aliquots totaling 250 ml of sterile saline. Collected BAL fluid was pooled and passed through sterile gauze to remove any large mucus plugs. Filtrates were centrifuged, and total lung leukocyte pellets were washed with a hypotonic solution to lyse the remaining RBCs. Cytospin centrifuges (Thermo Scientific, Waltham, MA, USA) were prepared with a modified Wright stain, to determine BAL differentials. Lavaged cells consisted of >95% AMs for each of the groups examined (data not shown). Cells were resuspended in RPMI 1640 medium (Thermo Scientific-Gibco, Grand Island, NY, USA) at a concentration of 106 cells/ml in a 12-well cell culture plate (Corning Inc., Tewksbury, MA, USA) and washed with 1 ml RPMI 1640 after 45 min, enabling the isolation of AMs by adherence. Either live or HK Kp was then added to selected wells and incubated for 4 or 18 h. Cell lysates were collected for IL-36γ mRNA analysis by RT-PCR.
MP and exosome isolation
PMs were cultured at a concentration of 5 × 106 cells per tissue culture plate. After the cells were stimulated, the conditioned medium was collected and centrifuged at 1500 g for 30 min at 4°C, to remove cell debris and apoptotic bodies. The supernatant was collected and stored at −80°C until further use, but for a maximum period of 2 wk. Supernatants were then thawed and ultracentrifuged at 25,000 g for 30 min at 10°C (Beckman Coulter Life Sciences, Indianapolis, IN, USA). The pellets containing MPs were resuspended in either RIPA buffer (1% w/w NP-40, 1% w/v sodium deoxycholate, 0.1% w/v SDS, 0.15 M NaCl, 0.01 M sodium phosphate, 2 mM EDTA, and 50 mM sodium fluoride), plus protease and phosphatase inhibitors for immunoblot analysis, or in PBS with 0.5 mM EDTA for ELISA or flow cytometry. For exosome isolation, the remaining supernatants were again ultracentrifuged at 100,000 g for 90 min at 4°C. The exosome-containing pellets were resuspended in RIPA buffer. Supernatants after ultracentrifugation were concentrated by using an Amicon Ultra centrifugal filter with a 3 kDa cutoff (EMD Millipore, Billerica, MA, USA).
Murine ELISAs for IL-36γ protein measurement
Cell-free conditioned medium from PM cultures was analyzed for IL-36γ with a mouse sandwich ELISA developed in our laboratory by using a modified double-ligand method [40]. Some samples were sonicated before ELISA with a Micro-Tip Branson Sonifier twice at 10 s output each (Branson Ultrasonics, Danbury, CT, USA).
Whole-lung–targeted PCR array
WT mice were intraperitoneally anesthetized with a mixture of xylazine and ketamine. The animals were then administered an intranasal dose of either 1 μg of murine recombinant flagellin (R&D Systems) or vehicle. Animals were euthanized, and lungs were removed 6 h after instillation. Whole lungs were homogenized in 2 ml TRIzol Reagent (Thermo Scientific-Invitrogen), with a Tissue-Tearor (Cole-Palmer), and mRNA was extracted [38]. The quantity and purity of RNA were measured with a Thermo Scientific-NanoDrop 1000 Spectrophotometer (NanoDrop Technologies, Berlin, Germany), and the quality of RNA was determined by running aliquots on the 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). Total RNA was reverse transcribed, and the cDNA product was amplified, fragmented, and labeled with the Ovation RNA Amplification and cDNA Biotin System (NuGen, San Carlos, USA). Labeled cDNA was hybridized to RT2 Profiler PCR Array Mouse Innate and Adaptive Immune Responses array plates (Qiagen, Valencia, CA, USA). Plates were processed and analyzed in the University of Michigan Microarray Core Facility.
Statistical analyses of arrays were performed with ArrayStudio, version 5.0 (Omicsoft, Cary, NC, USA). Data were normalized by the robust multichip average method and log2-transformed and assessed by probset intensity, principle component analysis, hierarchical cluster analysis, and sample correlation. General linear model with proper contrast tests were used. Differentially expressed genes were identified with the following criteria: at least a 2-fold change, raw P < 0.01, and estimated least squares mean intensity ≥4 for at least 1 group in the comparison.
qRT-PCR
Measurement of gene expression was performed with the ABI Prism 7000 Sequence Detection System (Thermo Scientific-Applied Biosystems, Foster City, CA, USA) as described in detail elsewhere [38]. Total cellular RNA was isolated from the frozen lungs, reversed transcribed into cDNA, and amplified with specific primers for IL-36γ, with β-actin serving as a control. Specific thermal cycling parameters used with the TaqMan One-Step RT-PCR Master Mix Reagents kit (Thermo Scientific-Applied Biosystems) were as follows: 30 min at 48°C, 10 min at 95°C, and 40 cycles involving denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. Relative quantitation of cytokine mRNA levels was plotted as fold change compared with untreated control cells. All experiments were performed in duplicate.
Western immunoblot analysis
Whole cell lysates were obtained by treating cells with RIPA buffer plus protease and phosphatase inhibitors. Protein concentrations were determined by the Bio-Rad DC protein assay (Bio-Rad Laboratories; Hercules, CA, USA). Samples were electrophoresed in 4–12% gradient SDS-PAGE gels, transferred to nitrocellulose, and blocked with 5% skim milk in PBS. After incubation with primary anti-IL-36γ Abs, blots were incubated with a secondary Ab linked to HRP and bands visualized by ECL (SuperSignal West Pico Substrate, Pierce Biotechnology; Rockford, IL, USA).
Flow cytometry
Isolated MP fractions obtained after ultracentrifugation were resuspended in 100 μl Annexin V Binding Buffer (BD Pharmingen, San Jose, CA, USA) and stained with annexin V-PE, per the manufacturer’s protocol. Stained MPs were then retrieved by ultracentrifugation at 25,000 g for 30 min at 10°C. Flow cytometry samples were run on a MoFlo Astrios (Beckman Coulter Life Sciences) and analyzed with FloJo flow cytometry analysis software (Ashland, OR, USA).
Statistical analysis
Statistical significance was determined by 1-way ANOVA, with P ≤ 0.05 considered significant. All calculations were performed with Prism 6.0 (GraphPad Software, San Diego, CA, USA).
RESULTS
IL-36γ mRNA is induced in whole-lung tissue in response to recombinant bacterial flagellin or live bacteria
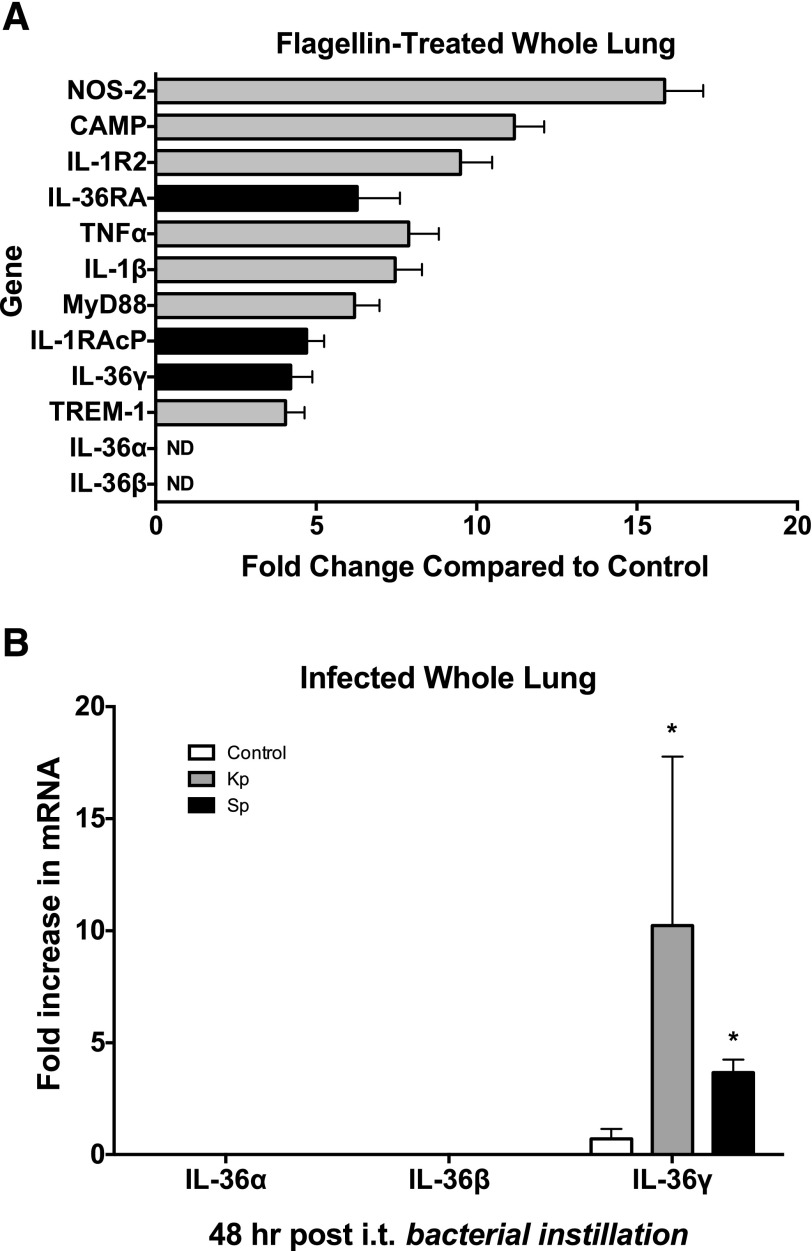
We have demonstrated in a prior study that intranasal administration of flagellin has profound immunostimulatory effects in the lung that confer protection against local bacterial proliferation and dissemination in animal models of bacterial pneumonia [41]. To determine the mediators induced by flagellin that participate in these protective effects, we challenged WT mice with flagellin (1 μg i.n.) or vehicle and then assessed whole lungs for differential gene regulation by using a targeted PCR array to examine a panel of innate and adaptive immune response genes 24 h later (Fig. 1A). Administration of flagellin resulted in significant upregulation of several innate genes, most notably NOS-2 (15.9-fold), cathelicidin antimicrobial peptide (11.1-fold), TNFα (7.9-fold), IL-1β (7.5-fold), MyD88 (6.2-fold), and TREM-1 (4.0-fold). Treatment with flagellin also significantly induced expression of several members of the IL-36 family, including the IL-36R agonist IL-36γ (4.2-fold) and the antagonist, IL-36RA (6.3-fold), as compared to the controls. In addition, IL-1RAcP, the shared accessory protein recruited by both the classic IL-1 receptor and the IL-36 receptor upon ligand binding, was induced in flagellin-treated lungs (4.7-fold). Although IL-36γ was significantly induced, we observed no upregulation of either IL-36α or -β mRNA, at the whole-lung level.
Figure 1. IL-36γ is induced in the lung in vivo by intranasal flagellin.
WT mice were given intranasal purified bacterial flagellin (1 mg). Whole lungs were harvested 2 h later and homogenized and subjected to a targeted RNA microarray analysis. (A) A variety of innate immune response genes were significantly induced in flagellin-treated lungs. Data are means ± sem of 3 mice/group. P < 0.05 for all genes shown, vs. unstimulated controls. (B) Of the IL-36R agonists, only IL-36γ was significantly induced in flagellin-treated lungs (4.2-fold change). No IL-36α or -β mRNA was detected. Data are means ± sem of 3 mice/group. *P < 0.05 vs. controls.
Having shown that the bacterial PAMP flagellin induces IL-36γ, we next assessed whether live bacterial infection would induce IL-36γ. We challenged WT mice with intratracheal inoculation of vehicle, Kp (5 × 103 CFU), or Sp (5 × 104 CFU). Whole lungs were harvested 48 h after infection, and mRNA levels of IL-36 receptor agonists were measured (Fig. 1B). We detected no induction of IL-36α or -β at the whole-lung level. However, we observed significant induction of IL-36γ by both Kp and Sp (10- and 4-fold increase, respectively).
IL-36γ is expressed by PMs in vitro
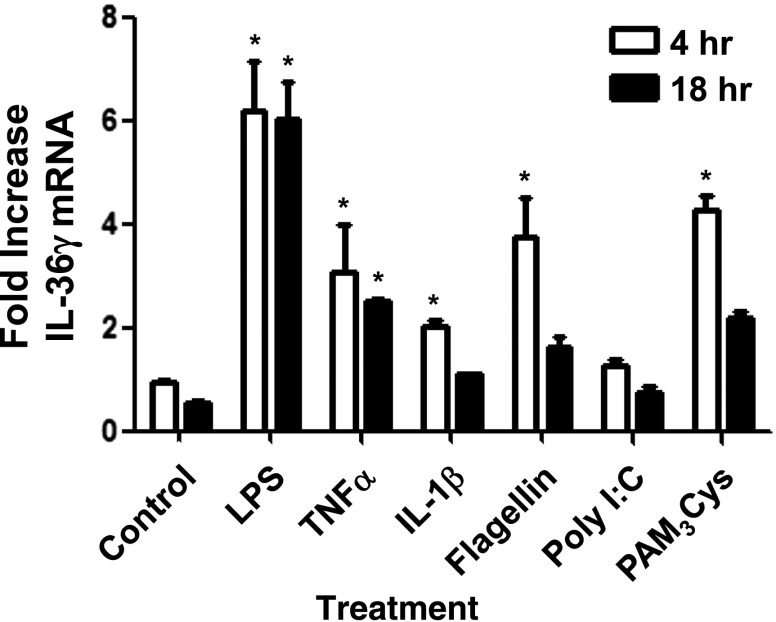
Having demonstrated that IL-36γ mRNA is selectively upregulated in the lung in response to bacterial flagellin, as well as both Gram-positive and -negative bacteria, we next sought to determine cell sources within the lung. We focused our studies on PMs, because these cells are pivotal in the orchestration of lung innate responses. PMs were isolated from dispersed lung and then treated with various bacterial PAMPs, as well as recombinant proinflammatory cytokines in vitro, and IL-36γ mRNA expression was assessed by RT-PCR at 4 and 18 h after stimulation. We observed significant increases in IL-36γ mRNA in response to several bacterial PAMPs, most notably LPS, which demonstrated considerable induction starting at 4 h and persisted at 18 h after stimulation (Fig. 2). Both bacterial flagellin and the TLR 1/2 agonist, PAM3Cys, also induced IL-36γ mRNA at 4 h, with mRNA levels returning to baseline by 18 h. We observed a significant, but lesser, induction of IL-36γ in response to the proximal cytokines TNFα and IL-1β. By comparison, the TLR3 agonist Poly I:C did not result in significant induction of IL-36γ mRNA over control.
Figure 2. IL-36γ mRNA is induced in vitro by PMs in response to TLR ligands and inflammatory cytokines.
WT PM were treated with LPS (100 ng), TNFα (100 μg), IL-1β (100 μg), flagellin (1 μg), Poly I:C (1 μg), or PAM3Cys (1 μg). Cell lysates were collected at 4 and 18 h and assessed for IL-36γ mRNA by RT-PCR. Data are means ± sem of 3 wells/group (106 cells/well). *P < 0.05 vs. control for each time point.
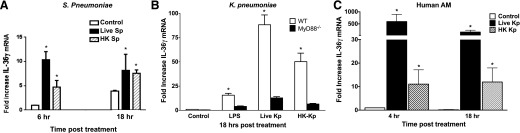
We next determined whether IL-36γ was induced in PMs in response to stimulation with live or HK bacteria. Murine PMs were treated with live or HK Sp (MOI 10:1), and mRNA was harvested at 6 and 18 h after stimulation (Fig. 3A). We observed significant induction of IL-36γ in response to HK Sp as early as 6 h after stimulation and persisting until 18 h. Stimulation with live bacteria resulted in an even greater induction of IL-36γ at 6 h, as compared to induction by HK bacteria (10-fold in response to live bacteria vs. 4.9-fold to HK bacteria at 6 h). However, we did not see a significant difference in induction between live and HK bacteria at 18 h, although both resulted in significant induction as compared to unstimulated cells (7.8-fold in response to live bacteria vs. 7.5-fold to HK bacteria at 18 h).
Figure 3. IL-36γ mRNA is induced in murine PMs and human AMs in vitro in response to Gram-positive and -negative bacteria.
(A) WT PMs were treated with live or HK Sp (MOI 10:1). Cell lysates were collected at 6 and 18 h and assessed for IL-36γ mRNA by RT-PCR. Data are means ± sem of 3 wells/group (106 cells/well). *P < 0.05 vs. control for each time point. (B) WT and MyD88−/− PMs were treated with LPS (100 ng) or with live or HK Kp (MOI 10:1). Cell lysates were collected at 18 h and assessed for IL-36γ mRNA by RT-PCR. *P < 0.05 vs. control. (C) Human AMs were treated with live or HK Kp (MOI 10:1). Cell lysates were collected at 4 and 18 h and assessed for human IL-36γ mRNA by RT-PCR. Data are means ± sem of 4 wells/group (106 cells/well). *P < 0.05 vs. control for each time point.
In addition to stimulation with Sp, WT and MyD88−/− murine PMs were treated with LPS (100 ng/ml) or with live or HK Kp (MOI 10:1; Fig. 3B). As with Sp, we observed significant induction of IL-36γ mRNA in WT PMs treated with LPS and especially with HK Kp at 18 h after stimulation (15.6- and 50.2-fold, respectively). We observed substantially greater induction of IL-36γ in response to live Kp, compared with that induced by HK Kp (88.3-fold). Furthermore, we observed no significant induction of IL-36γ mRNA in PMs isolated from MyD88−/− mice, suggesting that IL-36γ induction is MyD88 dependent.
To assess the relevance of our findings in murine cells to human innate responses, we isolated AMs from human donor lungs, and incubated 1 × 106 cells with live or HK Kp (Fig. 3B). As with murine macrophages, we observed substantial induction of human IL-36γ in response to both HK and especially to live Kp at both 4 and 18 h after treatment, as compared to unstimulated cells. The magnitude of mRNA induction in response to live bacteria was considerably greater than that observed in murine PMs (600-fold vs. 10-fold change at 4 h, and 200-fold vs. 12-fold change at 18 h).
IL-36γ protein is secreted by PMs in response to bacterial stimulation
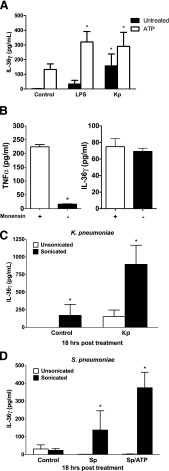
We have shown that IL-36γ mRNA is induced in PMs in response to both PAMPs and intact bacterial stimulation (both live and HK). We next examined whether IL-36γ protein was secreted from PMs in response to bacterial stimulation in vitro. Furthermore, because ATP has been shown to enhance secretion of IL-36 family members [42, 43], we assessed its impact on IL-36γ secretion from PMs. Heat-killed bacteria were chosen rather than live bacteria for these experiments to reduce any potential effect of live bacteria on PM viability at later time points, as well as possible effects on IL-36γ protein stability caused by secretion of active bacterial proteases. Isolated PMs were treated with LPS or HK Kp. Then, after 18 h in culture, ATP (5 mM) or vehicle was added, and conditioned medium was collected 20 min after ATP stimulation. IL-36γ protein was quantified by an IL-36γ sandwich ELISA developed in our laboratory with our polyclonal rabbit anti-mouse IL-36γ Ab (Fig. 4A). We observed a modest increase in extracellular IL-36γ in response to HK Kp alone. However, we found a significant increase in IL-36γ secretion in response to LPS+ATP and an even greater release of IL-36γ in response to the combination of HK Kp+ATP.
Figure 4. IL-36γ protein detection in conditioned medium of PMs is increased after ATP stimulation or sonication.
(A) WT PMs were treated with LPS (100 ng) or HK Kp (MOI 10:1), with or without the addition of ATP (5 mM), during the last 20 min of incubation. Conditioned medium was collected at 18 h, and IL-36γ protein was assessed by ELISA. Data are means ± sem of 4 wells/group (106 cells/well). *P < 0.05 vs. control. (B) WT PMs were treated with HK Kp (MOI 10:1 for 4 h) and ATP (5 mM for the last 20 min), with or without monensin (25 μM) for the last hour of incubation. Conditioned medium was collected and analyzed for TNFα and IL-36γ by ELISA. Data are means ± sem of 3 samples per group. *P < 0.001 vs. control. (C) WT PMs were treated with or without HK Kp (MOI 10:1). Conditioned medium was collected at 18 h and sonicated (10 s output twice) or not. IL-36γ protein was assessed by ELISA. (D) WT PMs were treated with HK Sp (MOI 10:1), with or without addition of ATP (5 mM) during the last 20 min of incubation. Conditioned medium was collected at 18 h and was sonicated (10s × 2 output) or was not sonicated. IL-36γ protein was assessed by ELISA. (C, D) Data are means ± sem of 3 wells/group (106 cells/well). *P < 0.05 vs. control.
Stimulation of purogenic receptors by ATP has been shown to trigger nonclassic secretion, in part by inducing blebbing of the plasma membrane and release of membrane-bound vesicles [25, 26, 36, 37]. The observation that secretion of IL-36γ could be enhanced with the addition of ATP suggested the possibility that IL-36γ is secreted in a nonconventional, Golgi-independent manner. Monensin is a Na+/H+ ionophore that blocks intracellular transport in both the trans-Golgi and Golgi compartments [44, 45]. To elucidate the secretory pathway of IL-36γ, we examined the influence of the ER/Golgi-dependent secretion inhibitor, monensin on IL-36γ release from PMs stimulated with HK Kp+ATP (Fig. 4B). In response to Kp+ATP stimulation, we observed significant increases in the classically secreted cytokine, TNFα, as well as IL-36γ. In the presence of monensin, we observed a >95% reduction in TNFα secretion (P < 0.001), indicating suppression of Golgi-mediated secretion. However, we observed no change in the secretion of IL-36γ in the presence of monensin.
Unconventional secretion mechanisms include secretion of proteins packaged within membrane-bound vesicles, such as MPs or exosomes. To test whether IL-36γ is secreted in such membrane-bound structures, we again stimulated PMs with HK Kp (Fig. 4C) or HK Sp (Fig. 4D) and collected conditioned medium 18 h after stimulation. Aliquots of each sample were sonicated to disrupt any membrane-bound structures, and the IL-36γ protein was quantified in sonicated and nonsonicated samples by ELISA. As compared to nonsonicated samples, detection of extracellular IL-36γ protein was significantly greater after sonication of conditioned medium of Kp- and especially of Sp-treated PMs. This finding suggests that a significant portion of secreted IL-36γ protein is packaged within membrane-bound vesicles.
IL-36γ protein is packaged with MPs and exosomes
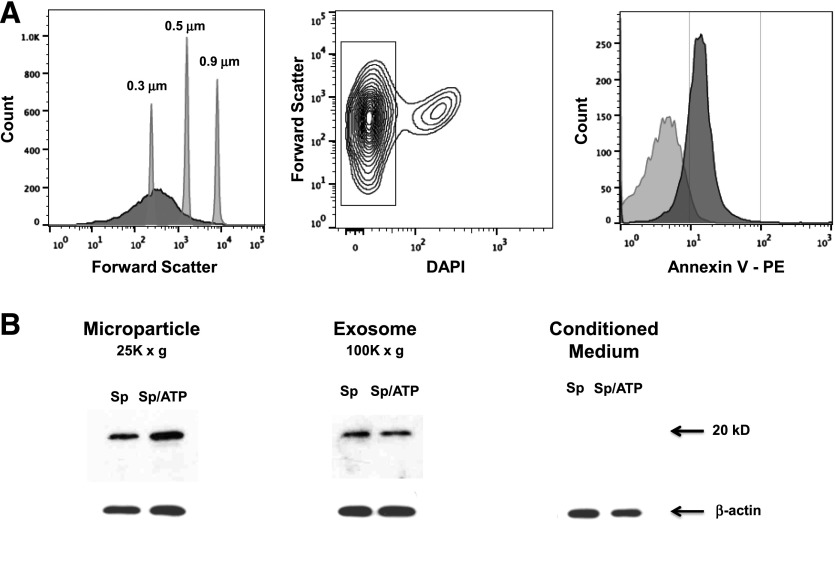
Having shown that IL-36γ may be secreted within membrane-bound structures, we sought to establish whether IL-36γ is indeed packaged in MPs, exosomes, or both. PMs were treated with HK Sp in the presence or absence of ATP, and conditioned medium was collected at 18 h after stimulation. Medium was centrifuged at 1,500 g for 30 min to remove apoptotic bodies and cellular debris, followed by ultracentrifugation at 25,000 g for 30 min to retrieve MP, and again at 100,000 g to isolate exosomes. The particle-free supernatant remaining after ultracentrifugation was then concentrated. To confirm that the particles retrieved in the MP fraction were consistent with viable MPs and were not cellular debris or apoptotic bodies, the MP pellet (after 25,000 g spin) was resuspended in FACS buffer and stained with PE-labeled annexin V, as MP express phosphatidylserine on particle surfaces. The MP fraction was run concurrently with size-calibration beads corresponding to 0.3, 0.5, and 0.9 μm (Fig. 5A). Most of the particles analyzed were within the expected size range (∼0.1–1.0 μm). We then gated on DAPI-negative particles, which represented the intact particle population. As compared to a nonspecific PE-labeled IgGκ isotype Ab, we observed that approximately 80% of our isolated particles stained positively for annexin V.
Figure 5. IL-36γ protein is present in MPs and exosomes of PMs stimulated with Sp, with or without ATP.
(A) WT PMs were treated with HK Sp (MOI 10:1)+ATP (5 mM for 20 min). Conditioned medium was harvested at 6 h and centrifuged at 1500 g for 30 min to remove apoptotic bodies and cell debris. Supernatants were then ultracentrifuged at 25,000 g for 30 min to isolate MPs, assessed by flow cytometry for size relative to submicron calibration beads, and gated to select DAPI-negative particles, corresponding to viable MPs. The MPs were stained for annexin V-PE or a PE-labeled isotype control IgG. Within 95% confidence intervals, as compared to isotype controls, 80% of particles were annexin V positive. (B) WT PMs were treated with HK Sp (MOI 10:1), with or without ATP (5 mM for 20 min). Conditioned medium was collected at 6 h and centrifuged at 1,500 g for 30 min, then ultracentrifuged at 25,000 g for 30 min (MP), followed by 100,000 g for 90 min (exosome). The remaining supernatant was concentrated in a centrifugal concentrator with a 3 kDa cutoff. Pellets from MP and exosome fractions and concentrated conditioned medium were analyzed by Western immunoblot for IL-36γ and IL-1β, with β-actin as the control gene. All lanes were run on a single gel/blot.
Having demonstrated that our ultracentrifugation protocol successfully isolated a significant quantity of viable MPs, MP and exosome fractions and particle-free conditioned medium were analyzed for the presence of IL-36γ protein by Western immunoblot. We observed the presence of IL-36γ protein in both the MP and exosome fractions, but not in the particle-free medium (Fig. 5B). Costimulation with ATP increased the amount of IL-36γ contained within the MPs, but did not increase the quantity of IL-36γ detected within the exosomes.
DISCUSSION
Previous research has shown that IL-36 family members are inducible in bronchial epithelial cells in an experimental asthma model [17, 18]. However, it has also been shown that IL-36 family members are induced in myeloid cell types [6, 7]. In this study, we gained novel insight into the induction of IL-36γ by lung macrophages in response to various infectious stimuli. We first noted that the IL-36R agonist, IL-36γ, was selectively induced in whole-lung tissue of mice stimulated with recombinant bacterial flagellin (Fig. 1). Moreover, IL-36γ was induced in whole-lung tissue of mice challenged with intratracheal Kp or Sp. Given that we did not observe induction of IL-36α or -β at the whole-lung level in response to recombinant PAMPs or live bacteria, we focused our study on IL-36γ. IL-36γ was induced in PMs in vitro in response to diverse bacterial PAMPs, as well as proinflammatory cytokines (Fig. 2). Furthermore, IL-36γ was markedly induced in response to Gram-positive and -negative bacteria in vitro (Fig. 3). We also examined IL-36 family member induction in other cell sources in response to bacterial challenge (unpublished results). Primary alveolar epithelial cells produced IL-36α predominantly, but very little IL-36γ. Bone marrow–derived DCs expressed lesser amounts of both IL-36α and -γ mRNA, with IL-36α again being the predominant agonist. IL-36γ mRNA was induced in lung fibroblasts stimulated with HK bacteria, as well. However, in our studies, induction of IL-36γ in PMs appeared to be more prominent than in other cell types. Given that IL-36γ was the only IL-36 receptor agonist that was significantly observed at the whole-lung level and the crucial role that PMs play in the development of innate immune responses to infection, we focused our experiments on PMs. The induction of IL-36γ in response to Kp was MyD88 dependent, which indicates that both Sp and Kp activated 1 or more MyD88-dependent TLRs. We observed greater induction of IL-36γ mRNA in response to stimulation with Gram-negative bacteria, compared with that induced by Gram-positive bacteria or recombinant PAMPs. A possible explanation for that result is that Kp stimulated more TLRs than Sp in our model, thus promoting heightened IL-36γ induction. In addition to murine lung cells, we observed significant in vitro induction of IL-36γ in human AMs in response to Kp, which suggests that our findings have relevance to human physiology, as well. Induction of IL-36γ by human AMs in response to live Kp was strikingly higher than in our murine samples. Although this finding may be related to differences in the efficiency of our RT-PCR primers and probes for human IL-36γ, as compared to murine IL-36γ, an alternative hypothesis is that IL-36γ plays a more crucial role in the innate responses to Kp in humans than in mice.
Secretion of IL-36 family members has been poorly understood. Lian et al. [42] demonstrated that secretion of IL-36γ by keratinocytes in response to bacterial flagellin was dependent on costimulation with ATP. Similarly, Johnston et al. [10] observed that keratinocytes stimulated with IL-1α or TNFα need ATP for secretion of IL-36γ. In addition, costimulation with LPS and ATP is necessary for IL-36α secretion from bone marrow–derived macrophages [43]. In our study, we confirmed that ATP is essential for optimal IL-36γ secretion from PMs in response to the recombinant PAMP, LPS, or whole bacteria (Fig. 4A).
It has been shown that ATP-mediated stimulation of the P2X7 receptor promotes plasma membrane blebbing and shedding of membrane components in MPs and that this effect represents a non-Golgi-dependent mechanism of IL-1β secretion [25–27, 29–31, 37]. We therefore sought to determine whether IL-36γ secretion also occurs in a nonclassic manner via packaging within MPs. To that end, we observed that treatment of cells with the Golgi-dependent secretion inhibitor monensin did not affect the degree of IL-36γ release from PMs in response to Kp+ATP. In addition, sonication resulted in enhanced detection of IL-36γ protein within conditioned medium (Fig. 4C, D), suggesting that this cytokine is released in the extracellular environment within a membrane-bound structure, such as MPs or exosomes. Moreover, we isolated both MPs and exosomes from PMs that had been incubated with HK Sp, in an ultracentrifugation-based isolation protocol. We first demonstrated that the particles obtained through our ultracentrifugation protocol were viable, appropriately sized particles that stained positively for annexin V, consistent with known characteristics of MPs (Fig. 5A). We observed substantial quantities of IL-36γ protein in both the MP and exosome fractions (Fig. 5B). In contrast, there was no detectable IL-36γ protein in particle-free conditioned medium, suggesting that most IL-36γ is secreted in a nonclassic manner within membrane-bound vesicles. We noted an increase in IL-36γ protein in MPs from PMs stimulated with both Sp and ATP, as compared to those stimulated by Sp alone. However, we observed no increase in IL-36γ proteins, in exosomes from costimulated PMs, as compared to PMs stimulated with Sp alone. ATP has been shown to stimulate exosome release in a P2X7-dependent manner [46]. However, our findings suggest that ATP stimulation plays an important role in blebbing of MPs from the plasma membrane compared with formation of exosomes. As IL-36 receptor agonists are known to be proinflammatory, packaging within MPs and exosomes may provide an efficient means of direct cell-to-cell communication, thereby preventing more extensive tissue damage from excessive induction of inflammatory responses. Alternatively, it is possible that packaging within MPs offers IL-36γ a stable environment for post-translational modification to occur.
In this study, we have shown that IL-36γ is induced in PMs in response to infectious stimuli. Furthermore, we have provided novel evidence that IL-36γ is secreted from PMs, at least in part, in a nonclassic manner via packaging within MPs and exosomes. Secretion within MPs may facilitate intercellular communication. Gaining a more thorough understanding of the induction and secretion of IL-36γ may provide new ways of modulating innate host immune responses to enhance treatment of pulmonary infections.
AUTHORSHIP
M.A.K. conceived the study design, performed experiments and statistical analysis, and drafted the manuscript. B.H.S. assisted with conception of the flow cytometry study design and participated in statistical analysis. M.W.N. performed experiments and assisted with statistical analysis. X.Z. performed experiments and assisted with statistical analysis. T.A.M. assisted with conception of flow cytometry study design. E.S.W. assisted with development of human alveolar macrophage isolation and cadaveric organ procurement. S.L.K. development of rabbit anti-mouse IL-36γ polyclonal Ab. M.P.G. assisted with development of study design. T.J.S. conceived the study design, assisted in data interpretation, and assisted in drafting the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Heart Lung and Blood Institute Grants HL123515, T32 HL007749, and K08 HL121089.The authors thank the University of Michigan Flow Cytometry Core for the use of the core equipment.
Glossary
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- DC
dendritic cells
- HK
heat-killed
- IL-1RAcP
IL-1 receptor accessory protein
- i.n.
intranasal
- Kp
Klebsiella pneumoniae
- MOI
multiplicity of infection
- MP
microparticle
- PAMP
pathogen-associated molecular pattern
- PE
phycoethrin
- PM
pulmonary macrophage
- Sp
Streptococcus pneumoniae
- TREM
triggering receptor expressed on myeloid cells
- WT
wild-type
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Sims J. E., Smith D. E. (2010) The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102. [DOI] [PubMed] [Google Scholar]
- 2.Towne J. E., Sims J. E. (2012) IL-36 in psoriasis. Curr. Opin. Pharmacol. 12, 486–490. [DOI] [PubMed] [Google Scholar]
- 3.Towne J. E., Garka K. E., Renshaw B. R., Virca G. D., Sims J. E. (2004) Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J. Biol. Chem. 279, 13677–13688. [DOI] [PubMed] [Google Scholar]
- 4.Debets R., Timans J. C., Homey B., Zurawski S., Sana T. R., Lo S., Wagner J., Edwards G., Clifford T., Menon S., Bazan J. F., Kastelein R. A. (2001) Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J. Immunol. 167, 1440–1446. [DOI] [PubMed] [Google Scholar]
- 5.Van de Veerdonk F. L., Stoeckman A. K., Wu G., Boeckermann A. N., Azam T., Netea M. G., Joosten L. A., van der Meer J. W., Hao R., Kalabokis V., Dinarello C. A. (2012) IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc. Natl. Acad. Sci. USA 109, 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn E., Sims J. E., Nicklin M. J., O’Neill L. A. (2001) Annotating genes with potential roles in the immune system: six new members of the IL-1 family. Trends Immunol. 22, 533–536. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann M., Scheiermann P., Härdle L., Pfeilschifter J., Mühl H. (2012) IL-36γ/IL-1F9, an innate T-bet target in myeloid cells. J. Biol. Chem. 287, 41684–41696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumberg H., Dinh H., Trueblood E. S., Pretorius J., Kugler D., Weng N., Kanaly S. T., Towne J. E., Willis C. R., Kuechle M. K., Sims J. E., Peschon J. J. (2007) Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 204, 2603–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumberg H., Dinh H., Dean C. Jr., Trueblood E. S., Bailey K., Shows D., Bhagavathula N., Aslam M. N., Varani J., Towne J. E., Sims J. E. (2010) IL-1RL2 and its ligands contribute to the cytokine network in psoriasis. J. Immunol. 185, 4354–4362. [DOI] [PubMed] [Google Scholar]
- 10.Johnston A., Xing X., Guzman A. M., Riblett M., Loyd C. M., Ward N. L., Wohn C., Prens E. P., Wang F., Maier L. E., Kang S., Voorhees J. J., Elder J. T., Gudjonsson J. E. (2011) IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J. Immunol. 186, 2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrier Y., Ma H. L., Ramon H. E., Napierata L., Small C., O’Toole M., Young D. A., Fouser L. A., Nickerson-Nutter C., Collins M., Dunussi-Joannopoulos K., Medley Q. G. (2011) Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J. Invest. Dermatol. 131, 2428–2437. [DOI] [PubMed] [Google Scholar]
- 12.Muhr P., Zeitvogel J., Heitland I., Werfel T., Wittmann M. (2011) Expression of interleukin (IL)-1 family members upon stimulation with IL-17 differs in keratinocytes derived from patients with psoriasis and healthy donors. Br. J. Dermatol. 165, 189–193. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen T. T., Niyonsaba F., Ushio H., Akiyama T., Kiatsurayanon C., Smithrithee R., Ikeda S., Okumura K., Ogawa H. (2012) Interleukin-36 cytokines enhance the production of host defense peptides psoriasin and LL-37 by human keratinocytes through activation of MAPKs and NF-κB. J. Dermatol. Sci. 68, 63–66. [DOI] [PubMed] [Google Scholar]
- 14.Vigne S., Palmer G., Lamacchia C., Martin P., Talabot-Ayer D., Rodriguez E., Ronchi F., Sallusto F., Dinh H., Sims J. E., Gabay C. (2011) IL-36R ligands are potent regulators of dendritic and T cells. Blood 118, 5813–5823. [DOI] [PubMed] [Google Scholar]
- 15.Gresnigt M. S., Rösler B., Jacobs C. W., Becker K. L., Joosten L. A., van der Meer J. W., Netea M. G., Dinarello C. A., van de Veerdonk F. L. (2013) The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur. J. Immunol. 43, 416–426. [DOI] [PubMed] [Google Scholar]
- 16.Bochkov Y. A., Hanson K. M., Keles S., Brockman-Schneider R. A., Jarjour N. N., Gern J. E. (2010) Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 3, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramadas R. A., Ewart S. L., Medoff B. D., LeVine A. M. (2011) Interleukin-1 family member 9 stimulates chemokine production and neutrophil influx in mouse lungs. Am. J. Respir. Cell Mol. Biol. 44, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramadas R. A., Ewart S. L., Iwakura Y., Medoff B. D., LeVine A. M. (2012) IL-36α exerts pro-inflammatory effects in the lungs of mice. PLoS One 7, e45784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vos J. B., van Sterkenburg M. A., Rabe K. F., Schalkwijk J., Hiemstra P. S., Datson N. A. (2005) Transcriptional response of bronchial epithelial cells to Pseudomonas aeruginosa: identification of early mediators of host defense. Physiol. Genomics 21, 324–336. [DOI] [PubMed] [Google Scholar]
- 20.Chustz R. T., Nagarkar D. R., Poposki J. A., Favoreto S. Jr., Avila P. C., Schleimer R. P., Kato A. (2011) Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 45, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S., McDonnell P. C., Lehr R., Tierney L., Tzimas M. N., Griswold D. E., Capper E. A., Tal-Singer R., Wells G. I., Doyle M. L., Young P. R. (2000) Identification and initial characterization of four novel members of the interleukin-1 family. J. Biol. Chem. 275, 10308–10314. [DOI] [PubMed] [Google Scholar]
- 22.Smith D. E., Renshaw B. R., Ketchem R. R., Kubin M., Garka K. E., Sims J. E. (2000) Four new members expand the interleukin-1 superfamily. J. Biol. Chem. 275, 1169–1175. [DOI] [PubMed] [Google Scholar]
- 23.Bhakdi S., Muhly M., Korom S., Schmidt G. (1990) Effects of Escherichia coli hemolysin on human monocytes: cytocidal action and stimulation of interleukin 1 release. J. Clin. Invest. 85, 1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariathasan S., Weiss D. S., Newton K., McBride J., O’Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232. [DOI] [PubMed] [Google Scholar]
- 25.Perregaux D., Gabel C. A. (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin: evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203. [PubMed] [Google Scholar]
- 26.Ferrari D., Chiozzi P., Falzoni S., Dal Susino M., Melchiorri L., Baricordi O. R., Di Virgilio F. (1997) Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 159, 1451–1458. [PubMed] [Google Scholar]
- 27.Ferrari D., Pizzirani C., Adinolfi E., Lemoli R. M., Curti A., Idzko M., Panther E., Di Virgilio F. (2006) The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 176, 3877–3883. [DOI] [PubMed] [Google Scholar]
- 28.Grahames C. B., Michel A. D., Chessell I. P., Humphrey P. P. (1999) Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br. J. Pharmacol. 127, 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubyak G. R. (2012) P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell. Microbiol. 14, 1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahlenberg J. M., Dubyak G. R. (2004) Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 286, C1100–C1108. [DOI] [PubMed] [Google Scholar]
- 31.Verhoef P. A., Estacion M., Schilling W., Dubyak G. R. (2003) P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J. Immunol. 170, 5728–5738. [DOI] [PubMed] [Google Scholar]
- 32.Chandler W. L., Yeung W., Tait J. F. (2011) A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J. Thromb. Haemost. 9, 1216–1224. [DOI] [PubMed] [Google Scholar]
- 33.Grant R., Ansa-Addo E., Stratton D., Antwi-Baffour S., Jorfi S., Kholia S., Krige L., Lange S., Inal J. (2011) A filtration-based protocol to isolate human plasma membrane-derived vesicles and exosomes from blood plasma. J. Immunol. Methods 371, 143–151. [DOI] [PubMed] [Google Scholar]
- 34.Hugel B., Martínez M. C., Kunzelmann C., Freyssinet J. M. (2005) Membrane microparticles: two sides of the coin. Physiology (Bethesda) 20, 22–27. [DOI] [PubMed] [Google Scholar]
- 35.Nickel W., Rabouille C. (2009) Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148–155. [DOI] [PubMed] [Google Scholar]
- 36.Hogquist K. A., Nett M. A., Unanue E. R., Chaplin D. D. (1991) Interleukin 1 is processed and released during apoptosis. Proc. Natl. Acad. Sci. USA 88, 8485–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKenzie A., Wilson H. L., Kiss-Toth E., Dower S. K., North R. A., Surprenant A. (2001) Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15, 825–835. [DOI] [PubMed] [Google Scholar]
- 38.Deng J. C., Cheng G., Newstead M. W., Zeng X., Kobayashi K., Flavell R. A., Standiford T. J. (2006) Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J. Clin. Invest. 116, 2532–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evanoff H. L., Burdick M. D., Moore S. A., Kunkel S. L., Strieter R. M. (1992) A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol. Invest. 21, 39–45. [DOI] [PubMed] [Google Scholar]
- 40.Braun K. M., Cornish T., Valm A., Cundiff J., Pauly J. L., Fan S. (1998) Immunotoxicology of cigarette smoke condensates: suppression of macrophage responsiveness to interferon gamma. Toxicol. Appl. Pharmacol. 149, 136–143. [DOI] [PubMed] [Google Scholar]
- 41.Yu F. S., Cornicelli M. D., Kovach M. A., Newstead M. W., Zeng X., Kumar A., Gao N., Yoon S. G., Gallo R. L., Standiford T. J. (2010) Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J. Immunol. 185, 1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lian L. H., Milora K. A., Manupipatpong K. K., Jensen L. E. (2012) The double-stranded RNA analogue polyinosinic-polycytidylic acid induces keratinocyte pyroptosis and release of IL-36γ. J. Invest. Dermatol. 132, 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin U., Scholler J., Gurgel J., Renshaw B., Sims J. E., Gabel C. A. (2009) Externalization of the leaderless cytokine IL-1F6 occurs in response to lipopolysaccharide/ATP activation of transduced bone marrow macrophages. J. Immunol. 183, 4021–4030. [DOI] [PubMed] [Google Scholar]
- 44.Evdokimovskaya Y., Skarga Y., Vrublevskaya V., Morenkov O. (2012) Release of the glucose-regulated protein 94 by baby hamster kidney cells. Cell Biochem. Funct. 30, 558–562. [DOI] [PubMed] [Google Scholar]
- 45.Bourdonnay E., Zasłona Z., Penke L. R., Speth J. M., Schneider D. J., Przybranowski S., Swanson J. A., Mancuso P., Freeman C. M., Curtis J. L., Peters-Golden M. (2015) Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J. Exp. Med. 212, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu Y., Franchi L., Nunez G., Dubyak G. R. (2007) Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925. [DOI] [PubMed] [Google Scholar]