Administration of AAV-IL-27 blocks autoimmune colitis via novel mechanisms.
Keywords: IL-30, autoimmune colitis, PD-L1, Th1/17, Tregs
Abstract
IL-27 is a heterodimeric cytokine that is composed of two subunits, i.e., EBV-induced gene 3 and IL-27p28 (also known as IL-30). Although the role of endogenous IL-27 in the pathogenesis of autoimmune colitis, an experimental model of human inflammatory bowel disease, remains controversial, IL-27 local delivery has been shown to inhibit autoimmune colitis. IL-30 has been shown to inhibit Th1 and Th17 responses and is considered a potential therapeutic for certain autoimmune diseases. In this study, we have compared the therapeutic efficacy of adeno-associated viral vector-delivered IL-27 and IL-30 in a murine model of autoimmune colitis. We found that 1 single administration of adeno-associated viral vector-delivered IL-27, but not adeno-associated viral vector-delivered IL-30, nearly completely inhibited autoimmune colitis. Adeno-associated viral vector-delivered IL-27 administration inhibited Th17 responses and induced T cell expression of IL-10, programmed death ligand 1, and stem cell antigen 1. Intriguingly, adeno-associated viral vector-delivered IL-27 treatment enhanced Th1 responses and inhibited regulatory T cell responses. Experiments involving the adoptive transfer of IL-10-deficient T cells revealed that adeno-associated viral vector-delivered IL-27-induced IL-10 production was insufficient to mediate inhibition of autoimmune colitis, whereas anti-programmed death 1 antibody treatment resulted in the breaking of adeno-associated viral vector-delivered IL-27-induced T cell tolerance. Thus, systemic delivery of IL-27 inhibits Th17 responses and induces multiple inhibitory pathways, including programmed death ligand 1 in T cells, and adeno-associated viral vector-delivered IL-27, but not IL-30, may have a therapeutic potential for the treatment of human inflammatory bowel disease.
Introduction
IL-27 is a heterodimeric cytokine that is composed of 2 subunits, i.e., EBV-induced gene 3 and IL-27p28 (also known as IL-30). IL-27 is produced by activated APCs, such as dendritic cells and macrophages [1–3], and signals through a heterodimeric receptor (IL-27R), consisting of the WSX-1 and gp130 subunits, which is expressed in a variety of cell types, including T lymphocytes [4]. Previous studies have revealed that IL-27 differentially regulates T cell responses: IL-27 inhibits Th2 and Th17 responses via blocking the expression of transcription factors GATA-3 (Th2) and retinoic acid receptor-related orphan receptor γt (Th17) [5, 6]. IL-27 is also a potent inducer of IL-10 [7–9] and PD-L1 [10] expression in T cells. These functional activities suggest that IL-27 is an immune-suppressive cytokine that inhibits autoimmune diseases [11–13]. Indeed, IL-27 has shown therapeutic efficacy in animal models of multiple sclerosis [9, 14] and collagen-induced arthritis [15], suggesting that IL-27 may be a potential therapeutic for human autoimmune diseases. IL-30 has been shown to block IL-6-mediated Th17 [16] and IL-12-mediated Th1 [17] differentiation and inhibits autoimmune inflammation in the CNS and eye [18], suggesting that IL-30 may also be a potential therapeutic for autoimmune diseases.
Two previous studies [19, 20] have revealed that exogenous IL-27, either delivered systemically or locally by recombinant bacteria LL, can inhibit the murine colitis development. Sasaoka et al. [19] showed that systemic injection of IL-27 inhibits 2,4,6-trinitrobenzene sulfonic acid-induced acute colitis in mice, which was associated with reduced Th17 responses. However, systemic injection of IL-27 is costly, and it is also hard to maintain an effective concentration in the circulation. Hanson et al. [20] showed that mucosal delivery of LL-IL-27 was superior to systemic delivery of the recombinant protein in inhibiting T cell-mediated colitis, and LL-IL-27-induced mucosal IL-10 production is suggested as the protection mechanism. However, the fact [20, 21] that LL-delivered IL-10 is less effective in inhibiting colitis than LL-IL-27 suggests that other IL-27-induced, protective mechanisms may be operative. Thus, the development of a more effective delivery method with reduced cost and a better understanding of IL-27-mediated inhibition of colitis are highly desired.
AAVs are quickly establishing themselves as highly versatile gene delivery agents for gene therapy [22]. AAV vectors can efficiently transfer genes of interest to a broad range of mammalian cell types, leading to high levels of stable and long-term expression after a single application [23]. The relatively low immunogenicity, pathogenicity, and toxicity [24] make AAV arguably the gene-therapy vector of choice for human clinical trials [25, 26]. In this study, we have compared the therapeutic efficacy of AAV-delivered IL-27 (AAV-IL-27) and IL-30 (AAV-IL-30) in T cell-mediated mouse colitis, an experimental model of human IBDs, where the colon inflammation is considered to be mediated by Th1 and Th17 responses [27, 28]. We found that 1 single administration of AAV-IL-27 but not AAV-IL-30 nearly inhibited T cell-mediated colitis completely. AAV-IL-27 administration inhibited Th17 responses and induced T cell expression of IL-10, PD-L1, and Sca-1, whereas it enhanced Th1 responses and inhibited Treg responses. Experiments involving the adoptive transfer of IL-10-deficient T cells revealed that AAV-IL-27-induced IL-10 production was insufficient to inhibit autoimmune colitis, whereas anti-PD-1 antibody treatment resulted in the breaking of AAV-IL-27-induced T cell tolerance.
MATERIALS AND METHODS
Mice
C57BL/6, C57BL/6 mice with Rag1−/−, BALB/c, and IL-10−/−BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). BALB/c mice with Rag2−/− were purchased from Taconic Farms (Germantown, NY, USA). All mice were maintained in Ohio State University laboratory animal facilities that were fully accredited by Institutional Animal Care and Use Committee.
Induction and evaluation of autoimmune colitis
Spleen and lymph node cells from donor mice (C57BL/6, BALB/c, or IL-10−/− BALB/c) were stained with fluorescence-labeled anti-CD4 and anti-CD45RB antibodies, followed by FACS-based high-speed sorting to purify the CD4+CD45RBhigh T cell subsets. Three to 5 × 105 CD4+CD45RBhigh T cells were injected intraperitoneally into each recipient mice. The disease progression was monitored by weighing each mouse every 3 d and recording the signs of disease, such as hunched-over appearance, piloerection of the coat, diarrhea, and blood in the stool. When mice lose ∼20% of their original body weight or at 8 wk post-cell injection, the animals were euthanized and assessed for a clinical score, based on the sum of 4 parameters: hunching and wasting, 0 or 1; colon thickening, 0–3 (0, no colon thickening; 1, mild thickening; 2, moderate thickening; 3, extensive thickening); and stool consistency, 0–3 (0, normal beaded stool; 1, soft stool; 2, diarrhea; an additional point was added if gross blood was noted).
Production of adeno-associated viral vectors and mice treatment
We used a recombinant AAV vector to express IL-27 and IL-30 in vivo. IL-30 expression plasmids [17] were obtained from Dr. Shulin Li (MD Anderson Cancer Center, Houston, TX, USA). In brief, IL-27 or IL-30 cDNA was inserted into an AAV carrier vector under the control of the CMV-chicken β-actin hybrid promoter, which allows highly efficient gene expression [29, 30]. The IL-27 or IL-30 carrier AAV vector was compacted with a helper vector in 293K cells into the AAV serotype 8, which is known to be particularly suitable for expression in muscle cells [31, 32]. A single dose (2 × 1011 DRP/mouse) of AAV vectors was injected into mice intramuscularly.
ELISA
Blood was drawn from mice treated with AAV-IL-27, AAV-IL-30, and AAV-ctrl vectors at various time points after viral injection. Serum was detected for the presence of IL-27 or IL-30 using ELISA kits purchased from eBioscience (San Diego, CA, USA; IL-27) and R&D Systems (Minneapolis, MN, USA; IL-30).
Antibodies and flow cytometry
FITC-, PE-, allophycocyanin-, or PerCP-labeled antibodies to CD4, IL-10, IL-17, IFN-γ, FoxP3, PD-L1, Sca-1, CD11b, Gr-1, Ly6G, Ly6C, and isotype ctrl antibodies were purchased from BD Biosciences (San Jose, CA, USA). For staining of cell surface markers, cells (single cell suspensions of spleen or lymph nodes) were stained with various antibodies in staining buffer (PBS with 1% FCS) on ice for 30 min; after washing with staining buffer, cells were fixed in 1% paraformaldehyde in PBS. Cells were then analyzed on a FACSCalibur flow cytometer. The intracellular cytokine staining procedure was the same as we described [33, 34]. Data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Histopathological analysis
The entire colon, starting from below the cecum to just above the anal opening, was carefully excised from each euthanized mouse. Colons were cleaned from the fecal material, and its length and weight were recorded. Colon tissues were fixed in 10% formalin/PBS, and paraffin-embedded sections (5 μm) were stained with H&E. Inflammation was graded semiquantitatively based on the sum of inflammatory cell infiltration and tissue damage on a scale from 0 (no changes) to 6 (widespread cellular infiltrations and extensive tissue damage). For grading infiltration of inflammatory cells, an infrequent presence of inflammatory cells in the lamina propria was classified as 0; increased numbers of inflammatory cells, including neutrophils, as 1; submucosal presence of inflammatory cell clusters as 2; and transmucosal cell infiltrations as 3. For grading of epithelial damage, a normal mucosal structure was classified as 0, isolated focal epithelial damage was counted as 1, the presence of mucosal erosions/ulcerations was counted as 2, and a score of 3 was given if extensive mucosal damage and extension through deeper structures of the bowel wall were present.
Statistics
Data are expressed as means of individual determinations ± se. Statistical analysis was performed using the unpaired Student’s t test.
RESULTS
AAV-mediated delivery of IL-27 but not IL-30 inhibits autoimmune colitis in mice
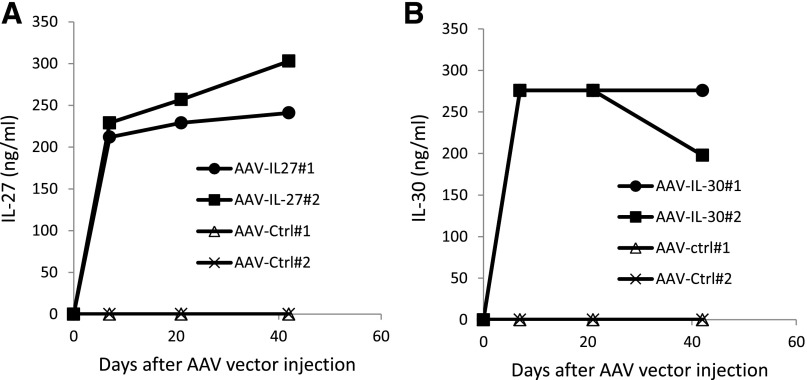
Although the role of endogenous IL-27 in the pathogenesis of autoimmune colitis is contradictory [35–39], studies have revealed that exogenous IL-27 inhibits the development of autoimmune colitis [19, 20]. More recently, IL-27P28 (IL-30), a subunit of IL-27, has been shown to inhibit Th1 and Th17 responses and autoimmune inflammation [18]. However, its roles in autoimmune colitis remain unclear. In this study, we sought to determine if exogenous IL-30 can be used as a potential therapeutic, and if so, what is its efficacy compared with IL-27. Therefore, we tested the efficacy of AAV-delivered IL-30 and IL-27 in inhibiting autoimmune colitis induced by CD45RBhigh T cell transfer, where colitis is mediated by Th1 and/or Th17 cells [40–42]. As demonstrated in Fig. 1, intramuscular injection of 2 × 1011 DRP/mouse of AAV-IL-27 or AAV-IL-30 achieved high concentrations and stable production of IL-27 or IL-30 in the peripheral blood of mice. Thus, we compared the therapeutic effects of AAV-IL-27 with AAV-IL-30 on the development of autoimmune colitis using this dose.
Figure 1. Production of IL-27 or IL-30 in mice treated with AAV-IL-27 or AAV-IL-30 vectors.
A single dose (2 × 1011 DRP/mouse) of AAV-IL-27, AAV-IL-30, or AAV-ctrl viral vectors was injected into each Rag1−/− C57BL/6 mouse intramuscularly. Serum samples were collected at different times after AAV vector injection, and serum IL-27 (A) and IL-30 (B) were detected by ELISA. The experiment is representative of 2 experiments with similar results.
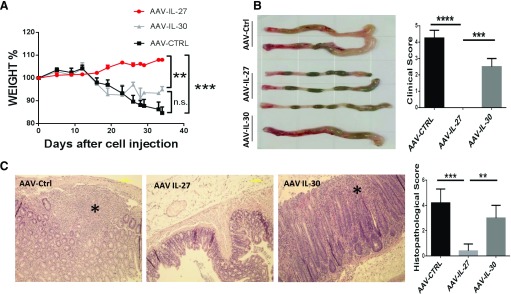
CD45RBhigh T cells purified from the lymph nodes and spleens of C57BL/6 mice were injected into Rag1−/− mice intraperitoneally. Twelve days later, each mouse was treated with 1 single dose, i.e., 2 × 1011 DRPs of AAV-IL-30, AAV-IL-27, or AAV-ctrl viral vectors intramuscularly. AAV-ctrl and AAV-IL-30-treated mice showed signs of wasting diseases, including loss of body weight (Fig. 2A), hunched-over appearance, piloerection of the coat, diarrhea, and blood in the stool, whereas AAV-IL-27-treated mice did not show any signs of wasting diseases. By the end of 5 wk, after T cell transfer, mice were euthanized, and the colons of AAV-IL-27-treated mice remained normal looking, such that normal, beaded stools were seen. Whereas the colons from AAV-ctrl and AAV-IL-30-treated mice were enlarged, beaded stools were absent and gross blood noted, leading to high degrees of clinical scores (Fig. 2B). H&E staining of longitudinal sections of the entire colon revealed massive inflammatory cell infiltration, severe goblet cell loss, as well as significant bowel wall thickening in the mucosa of AAV-ctrl and AAV-IL-30-treated mice, whereas colons from the AAV-IL-27-treated mice showed no significant histopathological changes, such that the differences of the histopathology scores between AAV-IL-27-treated and AAV-ctrl or AAV-IL-30-treated mice were highly significant (Fig. 2C). Thus, AAV-mediated delivery of IL-27 but not IL-30 inhibits autoimmune colitis in mice.
Figure 2. A single dose of AAV-IL-27 but not AAV-IL-30 treatment inhibits the development of autoimmune colitis in mice.
CD45RBhigh T cells were sorted from spleen and lymph node cells from C57BL/6 mice and were injected into Rag1−/− C57BL/6 mice intraperitoneally at a dose of 3 × 105/mouse. At d 12 after T cell transfer, AAV-IL-27, AAV-IL-30, or AAV-ctrl viral vectors were injected into each mouse intramuscularly at a dose of 2 × 1011 DRP/mouse. (A) Mice (n = 4–5/group) were weighted every 3 d and evaluated for the development of wasting disease. (B) Five weeks after T cell transfer, mice were euthanized, and clinical scores of colitis were assigned to each mouse based the macro pathology of the colon, as described in Materials and Methods. (C) Histopathology of each colon tissue samples was analyzed and scored as described in Materials and Methods. Representative micrographs of H&E sections and average scores of each treatment groups are shown. (C, left) *, Areas with severe tissue damage. Data are expressed as means of individual determinations ± se. Statistical analysis was performed using the unpaired Student’s t test. **P < 0.01; ***P < 0.001; ****P < 0.0001.
T cell subsets and the role of IL-10 in AAV-IL-27-induced inhibition of colitis
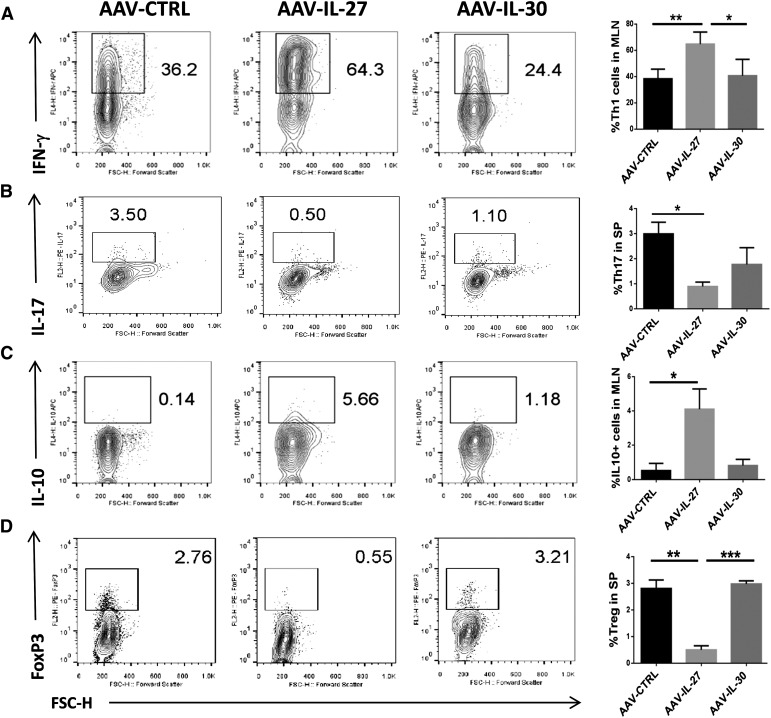
To understand the potential mechanisms of AAV-IL-27-mediated inhibition of autoimmune colitis, we further analyzed T cell responses in mice treated with AAV-IL-27, AAV-IL-30, and AAV-ctrl viral vectors. As shown in Fig. 3, compared with AAV-IL-30 and AAV-ctrl-treated mice, the lymphoid organs contained significantly increased numbers of Th1 (IFN-γ-producing) and IL-10-producing Th cells in AAV-IL-27-treated mice. In contrast, Th17 (IL-17A-producing) and Treg (FoxP3+) responses were significantly reduced in AAV-IL-27-treated mice. The T cell subsets in AAV-IL-30 were similar to that of AAV-ctrl-treated mice.
Figure 3. T cell subsets in the lymphoid organs of mice receiving different treatments.
Lymphocytes were stained with fluorescence-labeled anti-CD4 and anti-IFN-γ, anti-IL-17A, anti-IL-10, or anti-FoxP3, as described in Materials and Methods. FACS analysis was then performed to quantify Th1 (A), Th17 (B), IL-10-producing Tr1 (C), and Treg (D) populations. Representative plots from each group were shown on the left and summarized as means ± sd. Statistical analysis was performed using the unpaired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001. FL2/4-H, Fluorescence 2/4-height; APC, allophycocyanin; FSC-H, forward-scatter-height; SP, spleen.
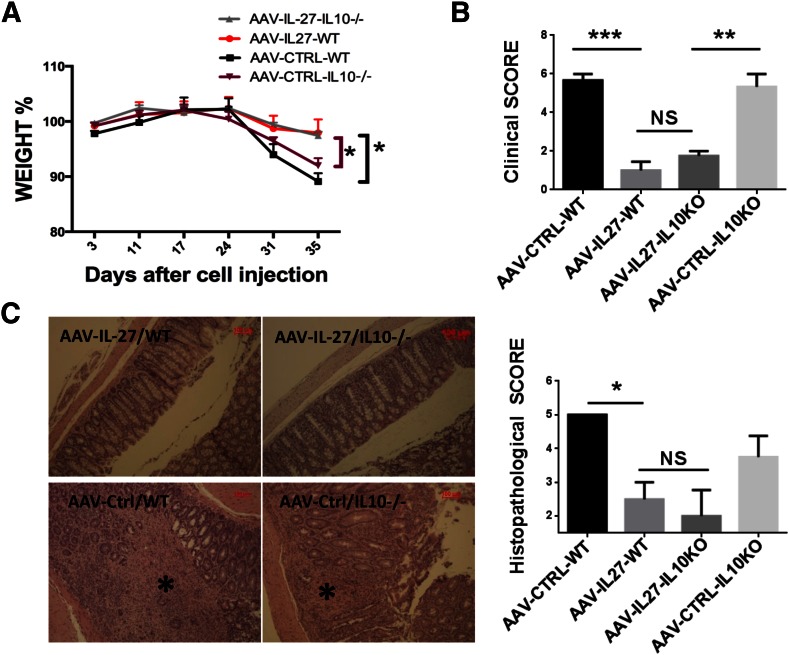
Increased IL-10-producing Th cells suggest that AAV-IL-27 may inhibit autoimmune colitis via induction of IL-10 in T cells. Indeed, a previous study [20] involving the feeding of IL-27-producing bacteria revealed that IL-27 mediated inhibition of autoimmune colitis via induction of IL-10. Therefore, we tested if AAV-IL-27, which induces high levels of systemic IL-27 production, would mediate inhibition of autoimmune colitis via a similar mechanism. Rag2−/− mice receiving CD45RBhigh T cells from BALB/c or IL-10−/− BALB/c mice were treated with AAV-IL-27 or AAV-ctrl viral vectors. Mice treated with AAV-ctrl viral vector exhibited significant body weight loss (Fig. 4A), signs of wasting diseases (Fig. 4B), and massive inflammation/tissue destruction (Fig. 4C) by the end of 5 wk, whereas mice receiving WT or IL-10−/− T cells treated with AAV-IL-27 did not exhibit body weight loss (Fig. 4A), signs of wasting disease (Fig. 4B), and colon tissue inflammation (Fig. 4C). Thus, AAV-IL-27-induced inhibition of autoimmune colitis is not IL-10 mediated.
Figure 4. IL-10 is not essential for AAV-IL-27-mediated inhibition of colitis.
(A) Body weight changes following the adoptive transfer of CD4+CD45RBhigh T cells (2.2 × 105/mouse) from WT or IL-10−/− mice. AAV-IL-27 or AAV-ctrl viral vectors were injected intramuscularly into mice (n = 3–6/group), 3 d after T cell transfer. (B) Mice were euthanized, and clinical scores of colitis were assigned to each mouse based on the macro pathology of the colon, as described in Materials and Methods. KO, Knockout. (C) Representative micrographs of H&E sections and average scores of each treatment groups are shown. Longitudinal sections of the entire colon were stained with H&E. Original scale bars, 100 μm. (C, left) *, Area with severe tissue damage. Histopathology scores were calculated as described in Materials and Methods. Data are expressed as means ± se. Statistical analysis was performed using the unpaired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
AAV-IL-27 induces multiple inhibitory pathways in T cells
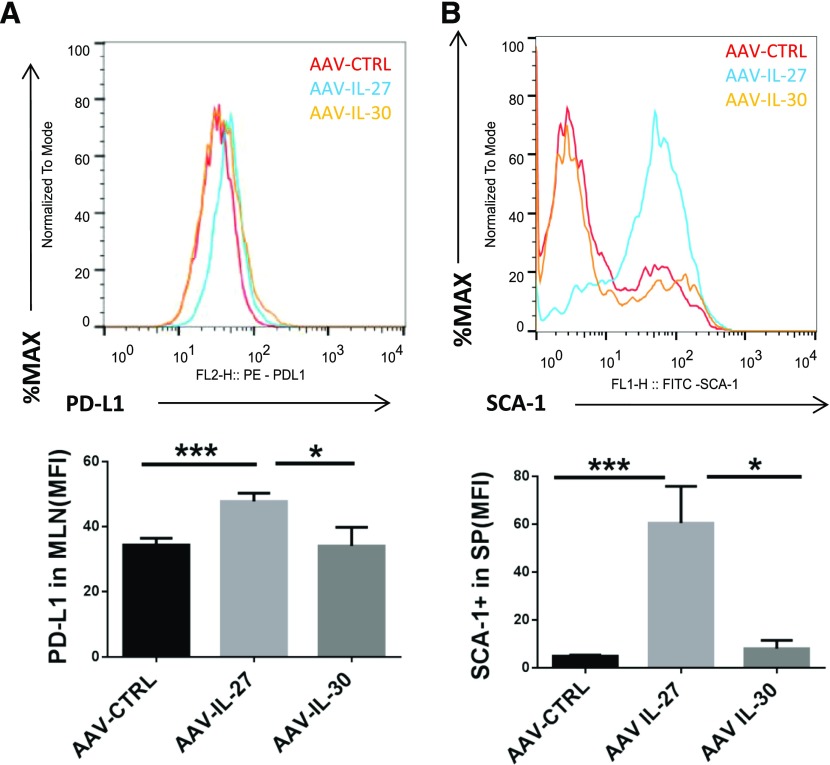
To determine the mechanisms of AAV-IL-27-mediated T cell tolerance, we further evaluated other potential molecular pathways that might be induced by AAV-IL-27. A previous study has revealed that IL-27 can induce T cell expression of PD-L1, which contributed to T cell tolerance in a mouse model of human multiple sclerosis [10]. Recently, we have shown that IL-27 can induce T cell “stemness” and the expression of Sca-1 [43, 44]. Indeed, we found that treatment with AAV-IL-27 but not AAV-IL-30 or AAV-ctrl viruses induced the expression of PD-L1 (Fig. 5A) and Sca-1 (Fig. 5B) in T cells.
Figure 5. AAV-IL-27 treatment induces T cell expression of PD-L1 and Sca-1 in vivo.
(Upper) Representative FACS plots for surface staining of CD4+ T cells in MLN cells for PD-L1 (A) and SCA-1 (B) are shown. (Lower) The mean fluorescence intensities (MFI) of molecular expression are summarized as means ± se. Three to 4 mice/group were included in this experiment. Statistical analysis was performed using the unpaired Student t test. *P < 0.05; ***P < 0.001.
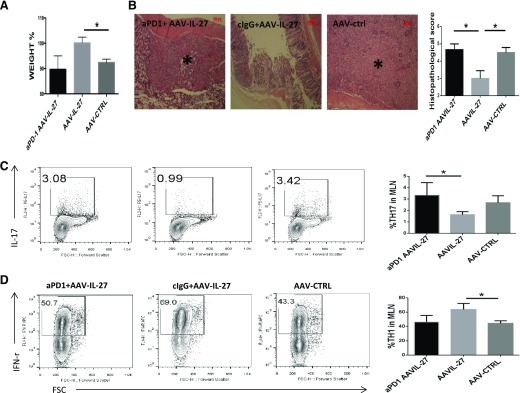
To determine if induction of PD-L1 mediates T cell tolerance, CD4+CD45RBhigh T cells from WT mice were injected into Rag2−/− mice intraperitoneally. Five days after T cell transfer, mice were treated with AAV-IL-27 or AAV-ctrl viral vectors intramuscularly. On d 34, 37, and 40 after T cell transfer, mice receiving AAV-IL-27 treatment were also treated with 300 μg/mouse of anti-PD-1 or an isotype-matched ctrl antibody intraperitoneally. As shown in Fig. 6, mice treated with AAV-ctrl vector or AAV-IL-27 + anti-PD-1 antibody exhibited significant body weight loss (Fig. 6A) and massive colon tissue inflammation/destruction (Fig. 6B) by d 42, whereas mice treated with AAV-IL-27 + ctrl antibody showed no body weight loss (Fig. 6A) and tissue inflammation (Fig. 6B). Anti-PD-1-treatment also significantly increased Th17 (Fig. 6C) but not Th1 (Fig. 6D) responses in AAV-IL-27-treated mice.
Figure 6. PD-1 blockade breaks T cell tolerance in AAV-IL-27-treated mice.
CD4+CD45RBhigh T cells (5 × 105/mouse) from WT mice were injected into Rag2−/− mice intraperitoneally. Five days after T cell transfer, mice (n = 3–4/group) were treated with AAV-IL-27 or AAV-ctrl viral vectors intramuscularly. On d 34, 37, and 40 after T cell transfer, mice receiving AAV-IL-27 treatment were also treated with 300 μg/mouse of anti-PD-1 (aPD-1) or an isotype-matched ctrl antibody intraperitoneally. Disease development was monitored. (A) Body weight changes at d 42 after T cell transfer are shown. (B) Representative images of histology of colon tissues and average scores from each group of mice are shown. Original scale bars, 100 μm. (B, left) *, Severe tissue damage. cIgG, control IgG. Representative FACS plots of CD4+ T lymphocytes expressing IL-17A (C) and IFN-γ (D) are shown. Three to 4 mice/group were included in this experiment. Statistical analysis was performed using the unpaired Student’s t test. *P < 0.05.
DISCUSSION
In this study, we have compared the therapeutic efficacy of AAV-delivered IL-27 (AAV-IL-27) with IL-30 (AAV-IL-30) in a murine model of autoimmune colitis. We found that 1 single administration of AAV-IL-27 but not AAV-IL-30 nearly inhibited autoimmune colitis completely. AAV-IL-27 treatment inhibited the Th17 response and induced multiple inhibitory pathways in T cells. We showed that AAV-IL-27-induced IL-10 production was insufficient to mediate inhibition of autoimmune colitis, whereas anti-PD-1 antibody treatment resulted in the breaking of AAV-IL-27-induced T cell tolerance.
In this study, we have observed that AAV-delivered IL-27 inhibits Th17 responses. This observation is consistent with previous studies [19, 20] using IL-27 as a therapeutic, suggesting that IL-27-mediated inhibition of Th17 responses correlates with attenuation of colitis. However, our current study has also revealed additional, novel insights of how systemically delivered IL-27 inhibits colitis development.
First, we have found that AAV-IL-27-induced IL-10 production by T cells is insufficient for maintaining T cell tolerance. In this study, we observed low but significantly increased numbers of IL-10-producing T cells in MLNs. However, experiments involving the adoptive transfer of IL-10-deficient T cells revealed that AAV-IL-27-induced T cell production of IL-10 was insufficient to mediate inhibition of autoimmune colitis. This observation suggests that AAV-IL-27-mediated inhibition of colitis is mainly through other mechanisms.
Second, we have found that AAV-IL-27-induced PD-L1 expression in T cells is sufficient to mediate T cell tolerance. We found that anti-PD-1 antibody treatment resulted in the breaking of T cell tolerance induced by AAV-IL-27. IL-27-mediated PD-L1 expression in T cells has been shown to be sufficient for inducing T cell tolerance in a mouse model of human multiple sclerosis [10]. Thus, AAV-IL-27-induced PD-L1 expression in T cells could be a major mechanism of AAV-IL-27-induced T cell tolerance and inhibition of colitis.
Third, our recent study [43] has revealed that IL-27 induces T cell expression of Sca-1 in vitro. In this study, we confirm further that IL-27 also induces T cell expression of Sca-1 in vivo. Although the significance of Sca-1 expression in T cell tolerance remains to be investigated, Sca-1-deficient T cells exhibit increased proliferation to antigen [45, 46], suggesting that AAV-IL-27-induced Sca-1 expression in T cells may be an additional inhibitory pathway that contributes to AAV-IL-27-mediated T cell tolerance.
Fourth, although it has been shown that IL-27 inhibits Th1 responses under some circumstances [47], other studies suggest that IL-27 enhances Th1/cytotoxic T cell 1 responses by activating the Stat1-T-bet axis and promotes T cell expression of T-bet, Eomes, IL-12Rβ2, granzyme B, and perforin [48–50]. The role of IL-27 in Treg responses also remains controversial [36, 51–54], whereas in IL-27 transgenic mice, Tregs are deleted [53]. In this study, we have found that AAV-IL-27 treatment enhanced Th1 responses and inhibited Treg responses. Strikingly, increased Th1 responses and reduced Treg responses did not lead to enhanced inflammation, suggesting that IL-27-induced PD-L1 and other inhibitory pathways in Th1 cells could induce exhaustion or senescence [55] of Th1 cells and control their effector functions.
Taken together, we have found that AAV-mediated systemic delivery of IL-27, but not IL-30, significantly inhibits autoimmune colitis. Although AAV-IL-27 treatment enhances Th1 responses and inhibits Treg responses, Th1 cell tolerance can be maintained efficiently by AAV-IL-27-mediated induction of PD-L1 and presumably by inhibition of Th17 responses. Our results suggest that AAV-delivered IL-27 may have a therapeutic potential for the treatment of human IBDs.
AUTHORSHIP
X.Z., Z.L., J.-Q.L., and J. Zhu performed all of the experiments. J. Zhang and J.P.D. prepared AAV vectors. J.C. and J.Y. performed sorting of CD4+CD45RB+ T cells. J. Zhou, M.-S.L., and X.-F.B. designed all of the experiments, wrote the manuscript, and generated funding support to this project.
ACKNOWLEDGMENTS
This study was supported, in part, by U.S. National Institutes of Health National Cancer Institute Grant R21CA198037; and China National Natural Science Foundation Grant 81572805.
Glossary
- AAV
adeno-associate virus
- ctrl
control
- DRP
DNase-resistant particle
- FoxP3
forkhead box P3
- IBD
inflammatory bowel disease
- LL
Lactococcus lactis
- MLN
mesenteric lymph node
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- Rag1/2−/−
targeted mutation of the Rag1/2 gene
- Sca-1
stem cell antigen 1
- Treg
regulatory T cell
- WT
wild-type
DISCLOSURES
The authors declare no financial or commercial conflicts of interest.
REFERENCES
- 1.Murugaiyan G., Mittal A., Weiner H. L. (2010) Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc. Natl. Acad. Sci. USA 107, 11495–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirhonen J., Sirén J., Julkunen I., Matikainen S. (2007) IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J. Leukoc. Biol. 82, 1185–1192. [DOI] [PubMed] [Google Scholar]
- 3.Remoli M. E., Gafa V., Giacomini E., Severa M., Lande R., Coccia E. M. (2007) IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur. J. Immunol. 37, 3499–3508. [DOI] [PubMed] [Google Scholar]
- 4.Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J. F., Phillips J. H., McClanahan T. K., de Waal Malefyt R., Kastelein R. A. (2004) WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231. [DOI] [PubMed] [Google Scholar]
- 5.Lucas S., Ghilardi N., Li J., de Sauvage F. J. (2003) IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 100, 15047–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diveu C., McGeachy M. J., Boniface K., Stumhofer J. S., Sathe M., Joyce-Shaikh B., Chen Y., Tato C. M., McClanahan T. K., de Waal Malefyt R., Hunter C. A., Cua D. J., Kastelein R. A. (2009) IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 182, 5748–5756. [DOI] [PubMed] [Google Scholar]
- 7.Stumhofer J. S., Silver J. S., Laurence A., Porrett P. M., Harris T. H., Turka L. A., Ernst M., Saris C. J., O’Shea J. J., Hunter C. A. (2007) Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371. [DOI] [PubMed] [Google Scholar]
- 8.Awasthi A., Carrier Y., Peron J. P., Bettelli E., Kamanaka M., Flavell R. A., Kuchroo V. K., Oukka M., Weiner H. L. (2007) A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8, 1380–1389. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald D. C., Ciric B., Touil T., Harle H., Grammatikopolou J., Das Sarma J., Gran B., Zhang G. X., Rostami A. (2007) Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 179, 3268–3275. [DOI] [PubMed] [Google Scholar]
- 10.Hirahara K., Ghoreschi K., Yang X. P., Takahashi H., Laurence A., Vahedi G., Sciumè G., Hall A. O., Dupont C. D., Francisco L. M., Chen Q., Tanaka M., Kanno Y., Sun H. W., Sharpe A. H., Hunter C. A., O’Shea J. J. (2012) Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity 36, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batten M., Li J., Yi S., Kljavin N. M., Danilenko D. M., Lucas S., Lee J., de Sauvage F. J., Ghilardi N. (2006) Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7, 929–936. [DOI] [PubMed] [Google Scholar]
- 12.Amadi-Obi A., Yu C. R., Liu X., Mahdi R. M., Clarke G. L., Nussenblatt R. B., Gery I., Lee Y. S., Egwuagu C. E. (2007) TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 13, 711–718. [DOI] [PubMed] [Google Scholar]
- 13.Kastelein R. A., Hunter C. A., Cua D. J. (2007) Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25, 221–242. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald D. C., Fonseca-Kelly Z., Cullimore M. L., Safabakhsh P., Saris C. J., Zhang G. X., Rostami A. (2013) Independent and interdependent immunoregulatory effects of IL-27, IFN-β, and IL-10 in the suppression of human Th17 cells and murine experimental autoimmune encephalomyelitis. J. Immunol. 190, 3225–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niedbala W., Cai B., Wei X., Patakas A., Leung B. P., McInnes I. B., Liew F. Y. (2008) Interleukin 27 attenuates collagen-induced arthritis. Ann. Rheum. Dis. 67, 1474–1479. [DOI] [PubMed] [Google Scholar]
- 16.Stumhofer J. S., Tait E. D., Quinn W. J. III, Hosken N., Spudy B., Goenka R., Fielding C. A., O’Hara A. C., Chen Y., Jones M. L., Saris C. J., Rose-John S., Cua D. J., Jones S. A., Elloso M. M., Grötzinger J., Cancro M. P., Levin S. D., Hunter C. A. (2010) A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 11, 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dibra D., Cutrera J., Xia X., Kallakury B., Mishra L., Li S. (2012) Interleukin-30: a novel antiinflammatory cytokine candidate for prevention and treatment of inflammatory cytokine-induced liver injury. Hepatology 55, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong W. P., Horai R., Mattapallil M. J., Silver P. B., Chen J., Zhou R., Sergeev Y., Villasmil R., Chan C. C., Caspi R. R. (2014) IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. J. Autoimmun. 50, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaoka T., Ito M., Yamashita J., Nakajima K., Tanaka I., Narita M., Hara Y., Hada K., Takahashi M., Ohno Y., Matsuo T., Kaneshiro Y., Tanaka H., Kaneko K. (2011) Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G568–G576. [DOI] [PubMed] [Google Scholar]
- 20.Hanson M. L., Hixon J. A., Li W., Felber, B. K., Anver, M. R., Stewart, C. A., Janelsins, B. M., Datta, S. K., Shen, W., McLean, M. H., Durum, S. K. (2014) Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 146, 210–221.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steidler L., Hans W., Schotte L., Neirynck S., Obermeier F., Falk W., Fiers W., Remaut E. (2000) Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289, 1352–1355. [DOI] [PubMed] [Google Scholar]
- 22.Monahan P. E., Samulski R. J. (2000) Adeno-associated virus vectors for gene therapy: more pros than cons? Mol. Med. Today 6, 433–440. [DOI] [PubMed] [Google Scholar]
- 23.McCarty D. M., Young S. M. Jr., Samulski R. J. (2004) Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 38, 819–845. [DOI] [PubMed] [Google Scholar]
- 24.Kaplitt M. G., Feigin A., Tang C., Fitzsimons H. L., Mattis P., Lawlor P. A., Bland R. J., Young D., Strybing K., Eidelberg D., During M. J. (2007) Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 369, 2097–2105. [DOI] [PubMed] [Google Scholar]
- 25.Daya S., Berns K. I. (2008) Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 21, 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aalbers C. J., Tak P. P., Vervoordeldonk M. J. (2011) Advancements in adeno-associated viral gene therapy approaches: exploring a new horizon. F1000 Med. Rep. 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powrie F., Leach M. W., Mauze S., Menon S., Caddle L. B., Coffman R. L. (1994) Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1, 553–562. [DOI] [PubMed] [Google Scholar]
- 28.Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260. [DOI] [PubMed] [Google Scholar]
- 29.Cox J. H., Kljavin N. M., Ramamoorthi N., Diehl L., Batten M., Ghilardi N. (2011) IL-27 promotes T cell-dependent colitis through multiple mechanisms. J. Exp. Med. 208, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim G., Shinnakasu R., Saris C. J., Cheroutre H., Kronenberg M. (2013) A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J. Immunol. 190, 1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda K., Nakamura K., Matsui N., Takahashi M., Kitamura Y., Mizutani T., Harada N., Nawata H., Hamano S., Yoshida H. (2005) T Helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm. Bowel Dis. 11, 1044–1052. [DOI] [PubMed] [Google Scholar]
- 32.Villarino A. V., Artis D., Bezbradica J. S., Miller O., Saris C. J., Joyce S., Hunter C. A. (2008) IL-27R deficiency delays the onset of colitis and protects from helminth-induced pathology in a model of chronic IBD. Int. Immunol. 20, 739–752. [DOI] [PubMed] [Google Scholar]
- 33.Troy A. E., Zaph C., Du Y., Taylor B. C., Guild K. J., Hunter C. A., Saris C. J., Artis D. (2009) IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J. Immunol. 183, 2037–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elson C. O., Cong Y., McCracken V. J., Dimmitt R. A., Lorenz R. G., Weaver C. T. (2005) Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 206, 260–276. [DOI] [PubMed] [Google Scholar]
- 35.Izcue A., Coombes J. L., Powrie F. (2006) Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212, 256–271. [DOI] [PubMed] [Google Scholar]
- 36.Powrie F. (1995) T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity 3, 171–174. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z., Liu J. Q., Talebian F., Wu L. C., Li S., Bai X. F. (2013) IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur. J. Immunol. 43, 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M. S., Liu Z., Liu J. Q., Zhu X., Liu Z., Bai X. F. (2015) The yin and yang aspects of IL-27 in induction of cancer-specific T-cell responses and immunotherapy. Immunotherapy 7, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanford W. L., Haque S., Alexander R., Liu X., Latour A. M., Snodgrass H. R., Koller B. H., Flood P. M. (1997) Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J. Exp. Med. 186, 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito C. Y., Li C. Y., Bernstein A., Dick J. E., Stanford W. L. (2003) Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood 101, 517–523. [DOI] [PubMed] [Google Scholar]
- 41.Villarino A. V., Hunter C. A. (2004) Biology of recently discovered cytokines: discerning the pro- and anti-inflammatory properties of interleukin-27. Arthritis Res. Ther. 6, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q., Ghilardi N., Wang H., Baker T., Xie M. H., Gurney A., Grewal I. S., de Sauvage F. J. (2000) Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature 407, 916–920. [DOI] [PubMed] [Google Scholar]
- 43.Morishima N., Owaki T., Asakawa M., Kamiya S., Mizuguchi J., Yoshimoto T. (2005) Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J. Immunol. 175, 1686–1693. [DOI] [PubMed] [Google Scholar]
- 44.Schneider R., Yaneva T., Beauseigle D., El-Khoury L., Arbour N. (2011) IL-27 increases the proliferation and effector functions of human naïve CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur. J. Immunol. 41, 47–59. [DOI] [PubMed] [Google Scholar]
- 45.Neufert C., Becker C., Wirtz S., Fantini M. C., Weigmann B., Galle P. R., Neurath M. F. (2007) IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur. J. Immunol. 37, 1809–1816. [DOI] [PubMed] [Google Scholar]
- 46.Huber M., Steinwald V., Guralnik A., Brüstle A., Kleemann P., Rosenplänter C., Decker T., Lohoff M. (2008) IL-27 inhibits the development of regulatory T cells via STAT3. Int. Immunol. 20, 223–234. [DOI] [PubMed] [Google Scholar]
- 47.Wojno E. D., Hosken N., Stumhofer J. S., O’Hara A. C., Mauldin E., Fang Q., Turka L. A., Levin S. D., Hunter C. A. (2011) A role for IL-27 in limiting T regulatory cell populations. J. Immunol. 187, 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall A. O., Beiting D. P., Tato C., John B., Oldenhove G., Lombana C. G., Pritchard G. H., Silver J. S., Bouladoux N., Stumhofer J. S., Harris T. H., Grainger J., Wojno E. D., Wagage S., Roos D. S., Scott P., Turka L. A., Cherry S., Reiner S. L., Cua D., Belkaid Y., Elloso M. M., Hunter C. A. (2012) The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye J., Huang X., Hsueh E. C., Zhang Q., Ma C., Zhang Y., Varvares M. A., Hoft D. F., Peng G. (2012) Human regulatory T cells induce T-lymphocyte senescence. Blood 120, 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y., Okada T., Nomoto T., Ke X., Kume A., Ozawa K., Xiao S. (2007) Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp. Mol. Med. 39, 170–175. [DOI] [PubMed] [Google Scholar]
- 51.Song S., Laipis P. J., Berns K. I., Flotte T. R. (2001) Effect of DNA-dependent protein kinase on the molecular fate of the rAAV2 genome in skeletal muscle. Proc. Natl. Acad. Sci. USA 98, 4084–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z., Zhu T., Qiao C., Zhou L., Wang B., Zhang J., Chen C., Li J., Xiao X. (2005) Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 23, 321–328. [DOI] [PubMed] [Google Scholar]
- 53.Qiao C., Li J., Jiang J., Zhu X., Wang B., Li J., Xiao X. (2008) Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum. Gene Ther. 19, 241–254. [DOI] [PubMed] [Google Scholar]
- 54.Wang L., Liu J. Q., Talebian F., Liu Z., Yu L., Bai X. F. (2015) IL-10 enhances CTL-mediated tumor rejection by inhibiting highly suppressive CD4(+) T cells and promoting CTL persistence in a murine model of plasmacytoma. OncoImmunology 4, e1014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z., Liu J. Q., Shi Y., Zhu X., Liu Z., Li M. S., Yu J., Wu L. C., He Y., Zhang G., Bai X. F. (2015) Epstein-Barr virus-induced gene 3-deficiency leads to impaired antitumor T-cell responses and accelerated tumor growth. OncoImmunology 4, e989137. [DOI] [PMC free article] [PubMed] [Google Scholar]