TSP-1 is expressed by DCs and regulates secondary responses of allergic T cells in a mouse model of AED.
Keywords: AED, conjunctivitis, Th2
Abstract
Allergic eye disease, as in most forms of atopy, ranges in severity among individuals from immediate hypersensitivity to a severe and debilitating chronic disease. Dendritic cells play a key role in stimulating pathogenic T cells in allergen re-exposure, or secondary responses. However, molecular cues by dendritic cells underpinning allergic T cell response levels and the impact that this control has on consequent severity of allergic disease are poorly understood. Here, we show that a deficiency in thrombospondin-1, a matricellular protein known to affect immune function, has subsequent effects on downstream T cell responses during allergy, as revealed in an established mouse model of allergic eye disease. More specifically, we demonstrate that a thrombospondin-1 deficiency specific to dendritic cells leads to heightened secondary T cell responses and consequent clinical disease. Interestingly, whereas thrombospondin-1-deficient dendritic cells augmented activity of allergen-primed T cells, this increase was not recapitulated with naïve T cells in vitro. The role of dendritic cell-derived thrombospondin-1 in regulating secondary allergic T cell responses was confirmed in vivo, as local transfer of thrombospondin-1-sufficient dendritic cells to the ocular mucosa of thrombospondin-1 null hosts prevented the development of augmented secondary T cell responses and heightened allergic eye disease clinical responses. Finally, we demonstrate that topical instillation of thrombospondin-1-derived peptide reduces T cell activity and clinical progression of allergic eye disease. Taken together, this study reveals an important modulatory role of dendritic cell-derived thrombospondin-1 on secondary allergic T cell responses and suggests the possible dysregulation of dendritic cell-derived thrombospondin-1 expression as a factor in allergic eye disease severity.
Introduction
Allergy, or atopy, comprises a plethora of IgE-associated conditions that affect a considerable percentage of the population in industrialized nations [1, 2]. Many of these atopic patients suffer from ocular allergy, which can range from mild allergic conjunctivitis (i.e., seasonal and perennial allergic conjunctivitis) to severe AED (i.e., atopic and vernal keratoconjunctivitis) [1–4]. Allergy affecting the eye is characterized by inflammation of the ocular mucosa (i.e., conjunctiva) and eyelid, tearing, mucosal discharge, and in severe cases, corneal damage [1–4]. As in most forms of allergy, AED is comprised of an immediate hypersensitivity reaction that occurs after the allergen has cross-linked IgE on the surface of mast cells, resulting in their degranulation [5–7]. This is followed by a late-phase reaction involving Th2 responses that lead to the recruitment and activation of eosinophils at the level of the conjunctiva [8–13]. Th1 cells also play an appreciable role in AED pathogenesis [12, 14–16].
As a result of the critical role Th cells play in mediating chronic allergic diseases, considerable focus has been placed on better understanding the mechanism of their activation by DCs, the most potent APC population [9, 11, 17, 18]. In the ocular mucosa, as in other sites, cDCs are characterized by their expression of CD11b or CD103, with some exception in the gut [19]. DCs function by acquiring antigen from peripheral sites and mobilizing to the regional LN through CCR7 [20]. Our lab has shown this process to be relevant in AED [19, 20], where ocular mucosal CD11b+, but not CD103+, cDCs are necessary to stimulate potent allergen-specific T cell responses [17, 19–21]. Thus, elucidating the molecular cues that mediate DC activity in the context of allergic T cell stimulation may be pertinent to identifying novel targets for effective treatments in allergic diseases, such as in AED.
The matricellular protein TSP-1 is one such molecular component, which is involved in regulating immune responses. Seminal work by Crawford et al. [22] showed that TSP-1 is a potent activator of latent TGF-β and that TSP-1 null mice succumb to multiorgan inflammation. A subsequent study by Doyen et al. [23] showed that autocrine expression of TSP-1 and ligation via CD47 by DCs derived from human peripheral blood monocytes caused these cells to become refractory in the presence of microbial products in vitro. Relatedly, our group has previously shown that expression of TSP-1 by mouse DCs prevented their phenotypic maturation (i.e., up-regulation of MHC II and costimulatory factors, such as CD80/86) and resultant ability to stimulate T cells [24]. In a separate study, Turpie et al. [25] demonstrated that TSP-1 null mice develop spontaneous Sjögren’s syndrome-like autoimmunity, affecting the eye clinically at 24 wk of age. Thus, not only does TSP-1 affect immune function, but this activity also appears to be highly relevant in inflammatory diseases of the ocular mucosa.
This understanding prompted us to examine further the potential role of TSP-1 in the immunopathogenesis of AED. Our data revealed that a TSP-1 deficiency, specifically in DCs, results in a remarkably more severe form of AED clinical disease and that this effect was a consequence of augmented secondary allergic T cell responses. Furthermore, with the use of a TSP-1-derived peptide, we demonstrated that topical application of TSP-1 effectively reduces T cell responses and markedly inhibits AED progression. Therefore, our findings demonstrate the contribution of TSP-1, or the lack thereof, in the pathobiology of AED.
MATERIALS AND METHODS
Animals and anesthesia
Eight-week-old C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). TSP-1 null mice, congenic mice (CD45.1), and OT-II mice (all on a C57BL/6 background), 8 wk old, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed and bred at the Duke University Eye Research Institute Animal Facility (Durham, NC, USA). Anesthesia was used for subconjunctival injections with intraperitoneally administered ketamine/xylazine suspensions (120 and 20 mg/kg, respectively). Euthanasia was performed according to the American Veterinary Medical Association Guidelines for Euthanasia of Animals: CO2 asphyxiation, followed by bilateral thoracotomy to ensure nonrecovery. The Institutional Animal Care and Use Committee approved all procedures, and all animals were treated according to guidelines established by the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of ocular allergy by active immunization
This procedure was performed as described previously [14, 19, 20]. In brief, mice were immunized with an intraperitoneal injection of 10 µg OVA (Sigma-Aldrich, St. Louis, MO, USA) in 300 ng pertussis toxin (Sigma-Aldrich) and 1 mg aluminum hydroxide (Thermo Fisher Scientific, Waltham, MA, USA) in PBS. After a 14-d rest period, mice were given topical application of 250 µg OVA solution once/day to the right eye for at least 7 d after the immunization rest period.
Clinical scoring of AED
The immediate hypersensitivity and late-phase reaction were scored clinically, as described previously [14, 19, 20]. In brief, each of the following parameters—lid swelling, tearing, conjunctival chemosis, and conjunctival redness—were scored on a scale of 1–3 and summed [4]. Scoring was done twice/day, at 20 min and at 6 h after challenge. When specified, mice were treated with 10 µg TSP-1-derived peptide, referred to as 4N1K (KRFYVVMWKK) [26, 27], or control peptide, referred to as 4NGG (KRFYGGMWKK; Sigma-Aldrich) [26], which was added to the topical OVA solution.
Adoptive transfer
As described previously [20], purified OVA-sensitized (primed) T cells were isolated from the spleens of immunized WT mice by negative selection [non-T cells were removed with biotin-conjugated antibodies against CD11b, CD11c, CD19, CD45R (B220), CD49b (DX5), CD105, MHC II, and Ter-119] via a MACS magnetic sorting kit (Miltenyi Biotec, San Diego, CA, USA). Cells (5 × 106) were injected intravenously into the tails of naïve WT or naïve TSP-1 null recipient mice.
Generation of BMDC culture and local transfer
This procedure was performed as described previously [19]. In brief, bone marrow cells were collected from WT congenic or TSP-1 null mice and cultured with GM-CSF (BioLegend, San Diego, CA, USA) for 7 d to generate CD11b+ DCs. DCs (1 × 105), cultured from WT or TSP-1 null mice, were injected subconjunctivally into naïve TSP-1 null mice.
DC pulsing and T cell coculture
BMDCs from TSP-1 null mice and WT or OT-II mice were plated at a concentration of 1 × 106 cells/ml and cultured overnight with 1 mg/ml OVA. Separately, T cells were collected from the spleens of naïve WT (or OT-II) and OVA-immunized WT (or OT-II) mice, as described above, and stained with CFSE (CellTrace CFSE cell proliferation kit; Life Technologies, Thermo Fisher Scientific). DCs were washed and then cocultured with the naïve or OVA-sensitized T cells for 4 h at a 1:4 ratio with 0.2% PMA + I + Golgi block (eBioscience, San Diego, CA, USA).
In vitro stimulation of T cells
This procedure was performed as described previously [19]. In brief, dLNs were collected from euthanized mice, tissue pressed, and run through a 70 μ cell strainer. Cells were washed and resuspended in complete media and then plated at a concentration of 2 × 106 cells/ml. Cells were stimulated 24–48 h with 1 mg/ml OVA, followed by a 4 h culture with 0.2% PMA + I + Golgi block for flow analysis or 0.2% PMA + I for ELISA analysis, or stimulated directly with PMA + I + Golgi block/PMA + I. For flow cytometry, cells were stained for CD4, IL-4, IL-5, IL-13, and/or IFN-γ. For ELISA, the supernatant was collected from the culture and analyzed for the same cytokines using ELISA Ready-SET-Go! kits (eBioscience).
Digestion of conjunctiva
As described previously [20], conjunctival tissue was collected after the final day of challenge and placed in digestion buffer consisting of HBSS with 0.2% collagenase D (Roche Diagnostics, Indianapolis, IN, USA) and 0.01% DNase (Roche Diagnostics). Samples were placed in a 37°C water bath and subjected to quick dissociation periods using the GentleMACS dissociator at 20 min intervals over 1 h. EDTA disodium salt solution (75 μl ; Sigma-Aldrich) was added to stop the digestion reaction.
Antibodies for flow cytometry
The following antibodies were used: CD4 (clone RM4-5, PE-CF594, BD Horizon/BD Biosciences, San Jose, CA, USA), IL-4 (clone 11B11, PE-Cy7; BioLegend), IL-5 (clone TRFK5, PE; BioLegend), IL-13 (clone eBio13A, PE, or clone eBio13A, AF660; eBioscience), IFN-γ (clone XMG1.2, Alexa 488, or clone XMG1.2, Alexa 700; BD PharMingen, San Diego, CA, USA), CD45.1 (clone A20, BV650; BioLegend), CD45.2 (clone 104, BV785; BioLegend), CD11b (clone M1/70, BV510, BD Horizon/BD Biosciences), CD11c (clone HL3, BV711, BD Horizon/BD Biosciences), Siglec F (clone E50-2440, AF647; BD PharMingen), Ly6C (clone HK1.4, Alexa 488; BioLegend), Ly6G (clone 1A8, Alexa 700; BioLegend), F4/80 (clone BM8, PE; BioLegend), and CFSE (CellTrace CFSE cell proliferation kit; Life Technologies, Thermo Fisher Scientific).
Statistics
Statistical significance for the clinical scores and ELISA assays were determined using the Student’s t test, and for flow cytometry, the χ2 test was used. With the χ2 test, the event numbers of populations of interest of both groups (A and C, respectively) and the event numbers of populations of noninterest of both groups (B and D, respectively) from the FACS data were compared as follows: {[(AD − BC)2] ⋅ (A + B + C + D)}/[(A + B) ⋅ (C + D) ⋅ (A + C) ⋅ (B + D)]. The χ2 value was then compared with a corresponding probability of significance (P) using a degree of freedom of 1. Statistical significance is described in figure legends.
RESULTS
Assessing the severity of allergic eye disease in TSP-1 null mice
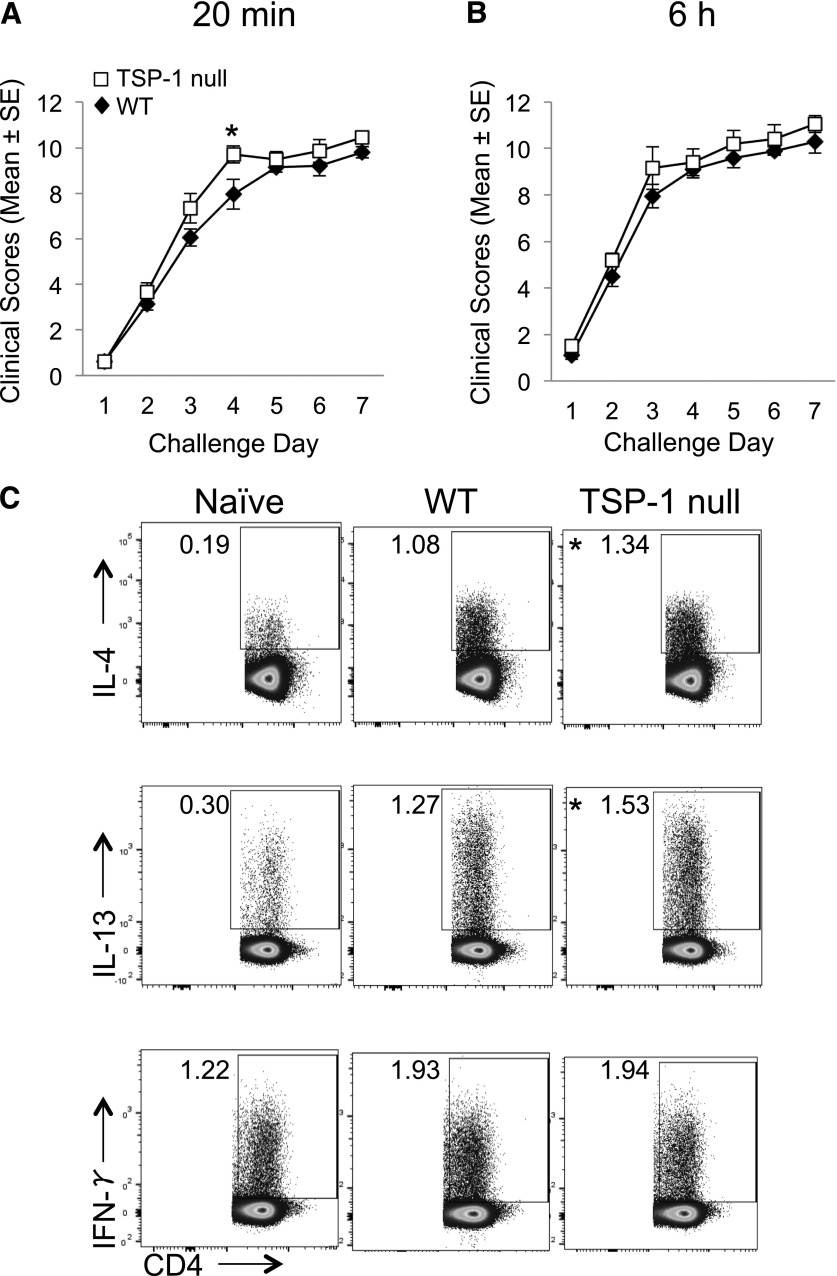
Given that TSP-1 has been established as an immunoregulatory factor [22, 23, 26, 28–30], we wanted to first determine whether the absence of TSP-1 would result in increased severity of AED. We addressed this question by using an established model in which WT and TSP-1 null mice were immunized with OVA and 14 d later, followed by a once/day topical instillation of OVA for 7 d [14, 19, 20]. The mice were scored, as described previously for clinical disease (i.e., tearing, lid swelling, conjunctival chemosis, and conjunctival redness), at 20 min after challenge to assess the immediate hypersensitivity reaction and at 6 h after challenge to assess the late-phase response [4, 6, 10, 20, 31–33]. Interestingly, results indicated that AED severity in TSP-1 null mice was only marginally increased compared with WT mice (Fig. 1A and B), with only 1 time point reaching statistical significance (challenge d 4, 20 min after challenge).
Figure 1. Active immunization leads to only marginal differences in AED clinical disease and allergic T cell responses in TSP-1 null mice compared with WT counterparts.
(A and B) Only marginal increases in AED clinical disease were observed in actively immunized TSP-1 null versus WT mice. Mice were immunized with OVA and adjuvant and 14 d later, were challenged once/day for 7 consecutive d with topical OVA instillation. On each challenge day, mice were clinically scored in a masked fashion for tearing/discharge, eyelid edema, and conjunctival redness and swelling. Scoring was performed at 20 min after challenge (i.e., for the immediate hypersensitivity), and again at 6 h after challenge (i.e., for the late-phase reaction). (C) Active immunization led to slight increases in allergic T cell responses in TSP-1 null versus WT mice. dLNs were collected after the last challenge and restimulated in vitro. Flow cytometric analysis enumerated frequencies of cytokine-expressing CD4+ T cells. This experiment is representative of 3 independent experiments. *P ≤ 0.05 was determined to be statistically significant.
These findings prompted us to further examine if the marginal increase in the clinical score was reflected in the T cell responses. To do so, dLNs were collected after the final day of challenge in WT and TSP-1 null mice with AED. After a 4 h culture, T cells were analyzed by flow cytometry for IL-4- and IL-13-producing Th2 cells, as well as IFN-γ-producing Th1 cells. As has been reported [9, 11–13], Th1 and Th2 responses were increased in AED mice in WT and TSP-1 null mice compared with naïve mice (Fig. 1C). Regarding TSP-1 null mice, data showed small, but statistical, increases in CD4+IL-4+ and CD4+IL-13+ T cells from TSP-1 null mice compared with WT in the AED setting. However, no difference was observed in CD4+IFN-γ+ T cells between TSP-1 null and WT mice (Fig. 1C). Taken together, these data show that with a global TSP-1 deficiency, there is only a marginal increase in allergen-reactive T cell responses and consequent clinical disease in AED compared with their WT counterparts. As differences in T cell responses were observed in the dLN, differences in the 20 min clinical responses (i.e., immediate hypersensitivity) were relatively marginal, and no notable differences were shown in the percentages of eosinophils between the 2 groups (data not shown), the subsequent experiments focused on the DC–T cell relationship instead of immediate responders, such as mast cells, or downstream effecters, such as eosinophils [34].
TSP-1-deficient DCs directly stimulate heightened secondary T cell responses in vitro
Our lab has shown previously that TSP-1 null DCs differ from WT DCs phenotypically, in that TSP-1 null DCs have heightened surface expression of MHC II, B7 costimulatory molecules, and CCR7 [24]. Thus, the presence of only marginally increased allergic T cell responses in the TSP-1 null mice was somewhat in conflict with the established role of TSP-1, as it has been shown to suppress DC maturation in a manner that leads to reduced T cell stimulation [17, 19–21]. This potential discrepancy led us to examine further the role of DC-derived TSP-1 on allergic T cell responses using a more isolated in vitro system. To accomplish this aim, OVA-pulsed BMDCs, generated from WT or TSP-1 null mice, were cocultured with WT T cells from naïve or OVA-sensitized mice. This approach would isolate the direct role of DC-derived TSP-1 on consequent T cell responses. The frequency of IL-13+ and IFN-γ+CD4+ T cells from OVA-sensitized mice cultured with TSP-1 null DCs was increased significantly compared with the same T cells cultured with WT DCs (Fig. 2A). This same increased frequency of Th2 and Th1 cytokine-expressing cells was confirmed using OVA-sensitized T cells from OT-II mice (Fig. 2B). Interestingly, results using naïve T cells did not yield similar trends, as resultant frequencies of such cytokine-expressing T cells did not differ significantly between WT and TSP-1 null DC stimulation (Fig. 2A). These data indicate that whereas TSP-1-deficient DCs increase the response of primed (i.e., secondary) T cells, the deficiency had no significant effect on naïve T cells in vitro. These findings highlight the possibility that TSP-1 expressed by DCs is relevant for the regulation of secondary T cell responses in AED.
Figure 2. In vitro stimulation with TSP-1 null DCs leads to increased responses with secondary allergic T cells but not naïve T cells.
Splenic T cells from naïve or OVA-sensitized WT (or OT-II) mice were purified and CFSE stained. BMDCs were prepared from WT versus TSP-1 null bone marrow and then pulsed with OVA. DC and T cell cocultures were incubated for 3 d. (A) Total WT T cell proliferation and cytokine-expressing CD4+ T cell proliferation were assessed via flow cytometry. (B) Total OT-II T cell proliferation and cytokine-expressing CD4+ T cell proliferation were assessed via flow cytometry. This experiment is representative of 3 independent experiments. *P ≤ 0.05 was determined to be statistically significant. FSC, Forward-scatter.
Adoptive transfer of OVA-sensitized T cells into TSP-1-deficient mice leads to increased severity of allergic immune responses
We next sought to determine in vivo whether DC-derived TSP-1 is relevant in regulating secondary allergic T cell responses in AED, which would potentially support our in vitro findings. We chose to move to the adoptive transfer model of allergy induction to address this question, as global TSP-1 deficiency affects several cell types, including B [35, 36] and T cells [37], and eosinophils [34]. Therefore, our approach was to adoptively transfer T cells from OVA-sensitized WT mice into naïve WT or TSP-1 null recipient mice [38]. Recipient mice were then challenged topically with OVA instillation and scored for AED. At 20 min after challenge, TSP-1 null mice displayed marginally exacerbated AED compared with WT mice, with only 1 time point (challenge d 4) reaching significance (Fig. 3A). Interestingly, however, at 6 h after challenge, TSP-1 null mice displayed significantly worsened AED compared with the WT mice (Fig. 3B). Thus, adoptive transfer of OVA-sensitized T cells into TSP-1 null mice leads to exacerbated late-phase responses and thereby, suggests a regulatory role for TSP-1 in secondary T cell responses during AED.
Figure 3. Adoptive transfer of WT OVA-sensitized T cells into TSP-1 null mice leads to increases in allergic immune responses.
(A and B) AED clinical disease is consistently more severe at 6 h after challenge in TSP-1 null versus WT mice adoptively transferred with WT OVA-sensitized T cells. OVA-sensitized T cells were purified from WT mice and adoptively transferred into naïve WT versus TSP-1 null mice. Recipient mice were challenged with OVA for 7 d, and the clinical scores were assessed at (A) 20 min after and (B) 6 h after challenge. (C and D) Augmented secondary T cell responses in TSP-1 null versus WT mice adoptively transferred with WT OVA-sensitized T cells. dLNs were collected after the last challenge and restimulated in vitro for subsequent (C) flow cytometric analysis of cytokine-expressing CD4+ T cells and (D) ELISA analysis of culture supernatants. This experiment is representative of 3 independent experiments. *P ≤ 0.05 was determined to be statistically significant.
As allergic late-phase responses are largely T cell mediated, the previous results prompted us to examine T cell cytokine production directly in this same model system. Thus, at the end of the challenge period of WT or TSP-1 null mice that received OVA-sensitized T cells, dLNs were collected and assayed for T cell responses. Results showed that TSP-1 null recipient mice had significantly higher frequencies of IL-4+, IL-5+, IL-13+, and IFN-γ+CD4+ T cells compared with WT recipient mice (Fig. 3C). This same trend was reflected in ELISA assays (Fig. 3D). Taken together, our data suggest that TSP-1 regulates secondary T cell responses and consequent late-phase clinical disease in AED.
DC-derived TSP-1 deficiency leads to exacerbated allergic eye disease
We next reasoned that if our finding of augmented T cell responses and worsened disease seen with adoptive transfer to TSP-1 null mice was indeed caused by the absence of DC-derived TSP-1, then such exacerbated responses may be able to be rescued by addition of TSP-1-sufficient (i.e., WT) DCs to the conjunctiva of these mice. We addressed this possibility through adoptive transfer of T cells from OVA-sensitized WT mice and subconjunctival injection of WT versus TSP-1 null BMDCs into TSP-1 null mice [14, 19]. Our lab has shown previously that CD11b+ BMDCs are phenotypically and functionally similar to conjunctival and lung cDCs in the AED setting [19, 20]. Thus, BMDCs adequately reflect conjunctival DC biology. In our experiments here, DC-injected mice were challenged with OVA, and AED was scored clinically. Results showed that TSP-1 null mice, which received TSP-1 null DCs, displayed significantly worsened clinical AED compared with mice that received WT DCs at both 20 min and 6 h after challenge—albeit, the largest differences were seen during late-phase responses (Fig. 4A and B). Consistent with this finding, TSP-1 null mice that received subconjunctival TSP-1 null DCs had a remarkable increase in recruited T cells to their conjunctivae (Supplemental Fig. 1).
Figure 4. DC-derived TSP-1 deficiency is responsible for augmented secondary T cell responses and increased AED severity.
(A and B) TSP-1-deficient DCs in the conjunctiva lead to exacerbated AED clinical disease. OVA-sensitized T cells were purified from WT mice and adoptively transferred into TSP-1 null mice. Recipients were subconjunctivally injected with TSP-1 null or WT BMDCs and subsequently challenged with OVA for 7 d. Clinical scores were assessed at (A) 20 min after and (B) 6 h after challenge. (C) TSP-1-deficient DCs in the conjunctiva trigger augmented secondary T cell responses that exacerbated AED clinical disease. dLNs were collected after the last challenge and restimulated in vitro for subsequent (C) flow cytometric analysis of cytokine-expressing CD4+ T cells and (D) ELISA analysis of culture supernatants. This experiment is representative of 3 independent experiments. *P ≤ 0.05 and **P ≤ 0.01 were determined to be statistically significant.
Analysis of the T cell responses in the dLNs revealed that mice that received TSP-1 null DCs showed a significant increase in the frequency of IL-4+, IL-5+, IL-13+, and IFN-γ+CD4+ T cells compared with mice that received WT DCs (Fig. 4C). The corresponding ELISA data were consistent with this trend (Fig. 4D). Taken together, these data led us to conclude that the absence of TSP-1 expression by DCs in the conjunctiva triggers heightened responses of T cells and in turn, leads to exacerbated clinical responses in AED.
Topical administration of TSP-1-derived peptide has a therapeutic effect in allergic eye disease
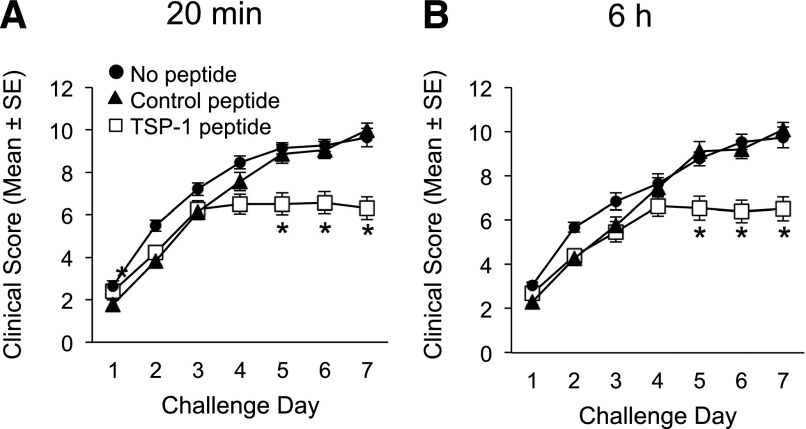
Our data strongly suggest that TSP-1 expression by DCs in the conjunctiva plays an important role in regulating allergic T cell responses and consequent clinical disease in AED. Therefore, we asked whether topical administration of exogenous TSP-1-derived peptide [28, 39] could augment the in vivo modulatory effect of this factor on DC-mediated T cell responses and consequent allergy. WT mice were actively immunized with OVA, as TSP-1-sufficient (WT) DCs can still mature and stimulate T cells in response to allergen [19, 20]. Our approach in the current experiment was to apply TSP-1 peptide (4N1K) topically [26] during the subsequent OVA-challenge period to target the DC population before migration to the dLNs, as DCs are activated and begin to migrate to the dLNs within several hours of allergen challenge [20]. In addition, we included a group that was treated with a control peptide (4NGG), as well as another control group that received no peptide during the OVA-challenge period. Strikingly, our data showed that mice treated topically with TSP-1 peptide displayed a significant reduction in the clinical AED response compared with mice that received control or no peptide (Fig. 5A and B). Furthermore, flow analysis of the dLNs showed that the TSP-1 peptide-treated group had reduced production of IL-4 and IL-5 cytokines by CD4+ T cells to levels compared with naïve mice (data not shown). In addition, there was marked reduction of IL-13 and IFN-γ cytokines by CD4+ T cells compared with control peptide- and no peptide-treated mice (Supplemental Fig. 2). Thus, our data suggest that topical administration of exogenous TSP-1-derived peptide can augment the in vivo modulatory effect of this factor on T cell responses and consequent allergy.
Figure 5. Topical administration of TSP-1-derived peptide prevents clinical disease progression in AED.
WT mice were immunized and 14 d later, were challenged once/day for 7 consecutive d. In conjunction with the OVA challenge, mice were administered topically with TSP-1-derived peptide (4N1K [26]) or control peptide (4NGG [26]) or left untreated. Mice were scored in a masked fashion at (A) 20 min after challenge and (B) 6 h after challenge. This experiment is representative of 3 independent experiments. *P ≤ 0.05 was determined to be statistically significant.
DISCUSSION
Secondary responses by allergen-reactive T cells contribute to the pathobiology of AED and other forms of allergy by promoting persistent IgE production relevant in immediate hypersensitivity, secreting proinflammatory cytokines, and by mediating eosinophil activity significant in late-phase and chronic disease. Allergic reactions in humans vary in severity among individuals, ranging from self-limiting immediate hypersensitivity to chronic and debilitating allergic disease. It is conceivable that by controlling the level of secondary T cell responses, DCs play some role in dictating such disease severity. However, whereas it is known that DCs are central to activating secondary T cell responses [40], how DCs modulate their ability to stimulate such T cells in ocular allergy is poorly understood. Data from the current study have allowed us to conclude that DC-derived TSP-1 reduces the level of secondary allergic T cell responses in AED and that this process can be augmented therapeutically by topical application of the TSP-1-derived peptide 4N1K [26].
We present 3 distinct lines of evidence, allowing us to conclude that DC-derived TSP-1 regulates secondary allergic T cell responses and subsequent clinical disease in AED. First, we show that TSP-1 null DCs lead to increased stimulation of OVA-primed T cells in vitro, as indicated by heightened proliferation of allergen-reactive IL-13 and IFN-γ T (CD4+) cells. This observation was made with OVA-sensitized T cells but not naïve T cells. Second, we show in vivo that adoptive transfer of OVA-primed WT T cells into TSP-1 null mice results in increased responses of allergen-reactive IL-13 and IFN-γ+ (CD4+) T cells, as well as increased consequent clinical disease in AED, compared with adoptive transfer of OVA-primed WT T cells into WT mice. This in vivo setting corroborates the in vitro data, as conjunctival CD11b+ cDCs migrate (via CCR7 [20]) to dLNs for stimulation of secondary T cell responses in AED [19, 20]. Thus, in TSP-1 null mice, increased responses of adoptively transferred T cells would be caused by TSP-1-deficient conjunctival cDCs. Indeed, and thirdly, we were able to show that the progression of this exacerbated T cell response and clinical disease following adoptive transfer into TSP-1 null mice can be reversed through the local conjunctival administration of TSP-1-sufficient DCs.
The relevance of DC-derived TSP-1 suppression of adoptively transferred T cells and diminution of clinical disease in AED are linked with the understanding that secondary T cell activity is central to late-phase clinical responses in allergy. Whereas onset of immediate hypersensitivity/mast cell degranulation (i.e., 20 min time point) is largely dependent on immunization-induced IgE production [14, 19], late-phase clinical onset (i.e., 6 h time point) is triggered by secondary Th2 cell responses that mediate recruitment/activation of eosinophils. Notably, as in other forms of allergy [41], Th1 cells also contribute pathologically to AED [12, 14–16], which is corroborated here with the presence of CD4+ IFN-γ+ T cells. Examination of the isolated contribution by secondary T cell activity to certain aspects of AED pathology, namely late-phase clinical disease, was facilitated here by our use of adoptive transfer assays. This approach permits isolation of T cell responses, as it is devoid of immunization-induced IgE production and consequent mast cell degranulation. This concept can be appreciated here by a reduced/delayed onset of fulminant clinical responses at 20 min after challenge in the adoptive transfer mice compared with actively immunized mice. Indeed, resultant T cell responses and clinical disease at the 6 h time point are also somewhat diminished in the adoptive transfer setting relative to the active immunization setting. This may likely be a result of reduced T cell responses that are otherwise promoted by mast cell degranulation [42]. In addition, the adoptive transfer model circumvents a contribution that TSP-1 deficiency on T cells may have on the allergic response, as T cells also express TSP-1 [37, 43, 44].
We can conclude that the effects of TSP-1-deficient DCs on augmenting allergic T cell responses and clinical disease observed here are independent of autoimmunity. The latter is important, as Turpie et al. [25] demonstrated that TSP-1 null mice develop spontaneous Sjögren’s syndrome-like autoimmunity, which has clinical onset affecting the eye at 24 wk of age. For this reason, TSP-1 null mice used in our study were 8 wk of age, i.e., far before the onset of clinical autoimmune disease. Furthermore, TSP-1 null mice responded to allergen challenge in a manner consistent with clinical allergy responses in WT mice, suggesting that pre-onset of Sjögren syndrome-like disease is not likely contributing to our clinical findings. Regarding T cell responses, OVA-primed T cells from WT mice were used for adoptive transfer into TSP-1 null hosts. Furthermore, antigen-recall assays were used that involved stimulating T cells with OVA, thereby stimulating allergy-specific T cell responses. Likewise, these data from adoptive transfer and recall assays match our in vitro experiments, wherein BMDCs (from WT vs. TSP-1 null mice) were cocultured with OVA-primed WT T cells. Lastly, in another experiment, we demonstrated that the addition of exogenous TSP-1 to WT mice led to reduced T cells responses in an allergy model (AED). Thus, collectively, our approach in these various experiments allows us to conclude that the observed role of DC-derived TSP-1 on allergic T cell responses is not dependent on autoimmune disease.
The prevention of DC maturation by TSP-1 may be playing the important role mechanistically, as our data showed that DC-derived TSP-1 diminishes secondary T cell responses in AED. Phenotypic maturation endows DCs with the optimal machinery to stimulate T cells via increased expression of costimulatory molecules and MHC II [45]. Our group has shown previously that TSP-1 inhibits DC maturation and the ability to stimulate T cells in the setting of transplantation [24]. The inhibition of maturation in this study may have occurred via TSP-1 interaction with CD47 on DCs, an autocrine mechanism that has been demonstrated previously to suppress activation of these cells [23]. Relatedly, we show that the down-regulation of T cell responses and marked decrease in AED clinical disease in the active immunization setting of WT mice were achieved via administration of 4N1K, the C-terminal domain peptide fragment derived from the of TSP-1 known to interact with CD47 [26]. Consistent with such a regulatory pathway, Sarfati and colleagues [46] showed that administration of signal regulatory protein α Fc, which also interacts with CD47, led to reduced allergic immune responses in the airway hyper-reactivity model. An alternative, but not mutually exclusive manner by which DC-derived TSP-1 may have inhibited maturation in our experiments, is via activation of TGF-β [30, 47, 48]. Although we did not assess TGF-β in the current study, TSP-1 is a potent activator of this immunomodulatory cytokine [22].
Taken together, we show that DC-derived TSP-1 plays a central role in modulating secondary responses by allergen-reactive T cells, which results in a reduced severity of clinical disease in a mouse model of AED. Future work is required to determine whether DC-derived TSP-1 dysregulation is involved during initial allergy sensitization (i.e., naïve T cell responses). Our data have also revealed that this modulatory activity can be augmented via eye-drop instillation of TSP-1-derived peptide 4N1K to the ocular surface, which leads to reduced T cell responses and marked diminution of clinical disease. The reasons for why this approach worked may likely be a result of reduced DC maturation at the level of the conjunctiva. Our work highlights the potential for use of the TSP-1-derived peptide as a therapeutic in AED. To this end, the challenge for future studies is to verify the relevance of this regulatory system in humans, i.e., whether TSP-1 expression by conjunctival DCs is inversely correlated with disease severity in human AED. Indeed, a role for TSP-1 in human ocular surface immunity and inflammation is supported by Contreras-Ruiz et al. [49] and others [50].
AUTHORSHIP
R.E.S. and N.J.R. performed experiments, data analysis, and manuscript writing. P.K. and S.L.S. performed experiments and data analysis. H.S.L. was involved in designing and performed experiments and data analysis. S.M. did the experimental design, data analysis and interpretation, and manuscript writing. D.R.S. secured funding for the work, led the team in experimental design, and did the data analysis and interpretation and manuscript writing.
Supplementary Material
ACKNOWLEDGMENTS
Funding sources were provided by the U.S. National Institutes of Health National Eye Institute (Bethesda, MD, USA; R01EY021798 to D.R.S. and P30EY005722) and Research to Prevent Blindness (New York, NY, USA; to D.R.S.). The authors thank Dr. Darlene Dartt (Harvard Medical School) for excellent discussions that contributed to this manuscript.
Glossary
- AED
allergic eye disease
- BMDC
bone marrow-derived dendritic cell
- cDC
classical dendritic cell
- DC
dendritic cell
- dLN
draining lymph node
- I
ionomycin
- LN
lymph node
- MHC II
MHC class II
- TSP-1
thrombospondin-1
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.La Rosa M., Lionetti E., Reibaldi M., Russo A., Longo A., Leonardi S., Tomarchio S., Avitabile T., Reibaldi A. (2013) Allergic conjunctivitis: a comprehensive review of the literature. Ital. J. Pediatr. 39, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ono S. J., Abelson M. B. (2005) Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 115, 118–122. [DOI] [PubMed] [Google Scholar]
- 3.Chen J. J., Applebaum D. S., Sun G. S., Pflugfelder S. C. (2014) Atopic keratoconjunctivitis: a review. J. Am. Acad. Dermatol. 70, 569–575. [DOI] [PubMed] [Google Scholar]
- 4.Magone M. T., Chan C. C., Rizzo L. V., Kozhich A. T., Whitcup S. M. (1998) A novel murine model of allergic conjunctivitis. Clin. Immunol. Immunopathol. 87, 75–84. [DOI] [PubMed] [Google Scholar]
- 5.Ballow M., Mendelson L. (1980) Specific immunoglobulin E antibodies in tear secretions of patients with vernal conjunctivitis. J. Allergy Clin. Immunol. 66, 112–118. [DOI] [PubMed] [Google Scholar]
- 6.Galatowicz G., Ajayi Y., Stern M. E., Calder V. L. (2007) Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. Clin. Exp. Allergy 37, 1648–1656. [DOI] [PubMed] [Google Scholar]
- 7.Irani A. M., Butrus S. I., Tabbara K. F., Schwartz L. B. (1990) Human conjunctival mast cells: distribution of MCT and MCTC in vernal conjunctivitis and giant papillary conjunctivitis. J. Allergy Clin. Immunol. 86, 34–40. [DOI] [PubMed] [Google Scholar]
- 8.Abu El-Asrar A. M., Struyf S., Al-Kharashi S. A., Missotten L., Van Damme J., Geboes K. (2000) Chemokines in the limbal form of vernal keratoconjunctivitis. Br. J. Ophthalmol. 84, 1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calder V. L., Jolly G., Hingorani M., Adamson P., Leonardi A., Secchi A. G., Buckley R. J., Lightman S. (1999) Cytokine production and mRNA expression by conjunctival T-cell lines in chronic allergic eye disease. Clin. Exp. Allergy 29, 1214–1222. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi A., DeFranchis G., Zancanaro F., Crivellari G., De Paoli M., Plebani M., Secchi A. G. (1999) Identification of local Th2 and Th0 lymphocytes in vernal conjunctivitis by cytokine flow cytometry. Invest. Ophthalmol. Vis. Sci. 40, 3036–3040. [PubMed] [Google Scholar]
- 11.Metz D. P., Hingorani M., Calder V. L., Buckley R. J., Lightman S. L. (1997) T-cell cytokines in chronic allergic eye disease. J. Allergy Clin. Immunol. 100, 817–824. [DOI] [PubMed] [Google Scholar]
- 12.Stern M. E., Siemasko K., Gao J., Duong A., Beauregard C., Calder V., Niederkorn J. Y. (2005) Role of interferon-gamma in a mouse model of allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 46, 3239–3246. [DOI] [PubMed] [Google Scholar]
- 13.Uchio E., Ono S. Y., Ikezawa Z., Ohno S. (2000) Tear levels of interferon-gamma, interleukin (IL) -2, IL-4 and IL-5 in patients with vernal keratoconjunctivitis, atopic keratoconjunctivitis and allergic conjunctivitis. Clin. Exp. Allergy 30, 103–109. [DOI] [PubMed] [Google Scholar]
- 14.Lee H. S., Schlereth S., Khandelwal P., Saban D. R. (2013) Ocular allergy modulation to hi-dose antigen sensitization is a Treg-dependent process. PLoS One 8, e75769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magone M. T., Whitcup S. M., Fukushima A., Chan C. C., Silver P. B., Rizzo L. V. (2000) The role of IL-12 in the induction of late-phase cellular infiltration in a murine model of allergic conjunctivitis. J. Allergy Clin. Immunol. 105, 299–308. [DOI] [PubMed] [Google Scholar]
- 16.Reyes N. J., Saban D. R. (2014) T helper subsets in allergic eye disease. Curr. Opin. Allergy Clin. Immunol. 14, 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohbayashi M., Manzouri B., Flynn T., Toda M., Ikeda Y., Nakamura T., Ono S. J. (2007) Dynamic changes in conjunctival dendritic cell numbers, anatomical position and phenotype during experimental allergic conjunctivitis. Exp. Mol. Pathol. 83, 216–223. [DOI] [PubMed] [Google Scholar]
- 18.Saban D. R. (2014) The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation. Ocul. Surf. 12, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khandelwal P., Blanco-Mezquita T., Emami P., Lee H. S., Reyes N. J., Mathew R., Huang R., Saban D. R. (2013) Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PLoS One 8, e64193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlereth S., Lee H. S., Khandelwal P., Saban D. R. (2012) Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am. J. Pathol. 180, 2351–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieu M. C., Vanbervliet B., Vicari A., Bridon J. M., Oldham E., Aït-Yahia S., Brière F., Zlotnik A., Lebecque S., Caux C. (1998) Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford S. E., Stellmach V., Murphy-Ullrich J. E., Ribeiro S. M., Lawler J., Hynes R. O., Boivin G. P., Bouck N. (1998) Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93, 1159–1170. [DOI] [PubMed] [Google Scholar]
- 23.Doyen V., Rubio M., Braun D., Nakajima T., Abe J., Saito H., Delespesse G., Sarfati M. (2003) Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 198, 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saban D. R., Bock F., Chauhan S. K., Masli S., Dana R. (2010) Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J. Immunol. 185, 4691–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turpie B., Yoshimura T., Gulati A., Rios J. D., Dartt D. A., Masli S. (2009) Sjögren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 175, 1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallejo A. N., Mügge L. O., Klimiuk P. A., Weyand C. M., Goronzy J. J. (2000) Central role of thrombospondin-1 in the activation and clonal expansion of inflammatory T cells. J. Immunol. 164, 2947–2954. [DOI] [PubMed] [Google Scholar]
- 27.Leclair P., Lim C. J. (2014) CD47-independent effects mediated by the TSP-derived 4N1K peptide. PLoS One 9, e98358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demeure C. E., Tanaka H., Mateo V., Rubio M., Delespesse G., Sarfati M. (2000) CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J. Immunol. 164, 2193–2199. [DOI] [PubMed] [Google Scholar]
- 29.Masli S., Turpie B., Streilein J. W. (2006) Thrombospondin orchestrates the tolerance-promoting properties of TGFbeta-treated antigen-presenting cells. Int. Immunol. 18, 689–699. [DOI] [PubMed] [Google Scholar]
- 30.Schultz-Cherry S., Lawler J., Murphy-Ullrich J. E. (1994) The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J. Biol. Chem. 269, 26783–26788. [PubMed] [Google Scholar]
- 31.Fukushima A., Ozaki A., Fukata K., Ishida W., Ueno H. (2003) Ag-specific recognition, activation, and effector function of T cells in the conjunctiva with experimental immune-mediated blepharoconjunctivitis. Invest. Ophthalmol. Vis. Sci. 44, 4366–4374. [DOI] [PubMed] [Google Scholar]
- 32.Fukushima A., Ozaki A., Jian Z., Ishida W., Fukata K., Ueno H., Liu F. T. (2005) Dissection of antigen-specific humoral and cellular immune responses for the development of experimental immune-mediated blepharoconjunctivitis in C57BL/6 mice. Curr. Eye Res. 30, 241–248. [DOI] [PubMed] [Google Scholar]
- 33.Niederkorn J. Y. (2008) Immune regulatory mechanisms in allergic conjunctivitis: insights from mouse models. Curr. Opin. Allergy Clin. Immunol. 8, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Immunological Genome Project Consortium (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H. P., Wu Y., Liu J., Jiang J., Geng X. R., Yang G., Mo L., Liu Z. Q., Liu Z. G., Yang P. C. (2013) TSP1-producing B cells show immune regulatory property and suppress allergy-related mucosal inflammation. Sci. Rep. 3, 3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang G., Geng X. R., Liu Z. Q., Liu J. Q., Liu X. Y., Xu L. Z., Zhang H. P., Sun Y. X., Liu Z. G., Yang P. C. (2015) Thrombospondin-1 (TSP1)-producing B cells restore antigen (Ag)-specific immune tolerance in an allergic environment. J. Biol. Chem. 290, 12858–12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talme T., Bergdahl E., Sundqvist K. G. (2014) Regulation of T-lymphocyte motility, adhesion and de-adhesion by a cell surface mechanism directed by low density lipoprotein receptor-related protein 1 and endogenous thrombospondin-1. Immunology 142, 176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes N. J., Mayhew E., Chen P. W., Niederkorn J. Y. (2010) NKT cells are necessary for maximal expression of allergic conjunctivitis. Int. Immunol. 22, 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandyopadhyay G., Bandyopadhyay S., Bankey P. E., Miller-Graziano C. L. (2014) Elevated postinjury thrombospondin 1-CD47 triggering aids differentiation of patients’ defective inflammatory CD1a+ dendritic cells. J. Leukoc. Biol. 96, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Rijt L. S., Jung S., Kleinjan A., Vos N., Willart M., Duez C., Hoogsteden H. C., Lambrecht B. N. (2005) In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201, 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen G., Berry G., DeKruyff R. H., Umetsu D. T. (1999) Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelburne C. P., Nakano H., St John A. L., Chan C., McLachlan J. B., Gunn M. D., Staats H. F., Abraham S. N. (2009) Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe 6, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Futagami Y., Sugita S., Vega J., Ishida K., Takase H., Maruyama K., Aburatani H., Mochizuki M. (2007) Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. J. Immunol. 178, 6994–7005. [DOI] [PubMed] [Google Scholar]
- 44.Li S. S., Ivanoff A., Bergström S. E., Sandström A., Christensson B., van Nerven J., Holgersson J., Hauzenberger D., Arencibia I., Sundqvist K. G. (2002) T lymphocyte expression of thrombospondin-1 and adhesion to extracellular matrix components. Eur. J. Immunol. 32, 1069–1079. [DOI] [PubMed] [Google Scholar]
- 45.Mellman I., Steinman R. M. (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258. [DOI] [PubMed] [Google Scholar]
- 46.Raymond M., Rubio M., Fortin G., Shalaby K. H., Hammad H., Lambrecht B. N., Sarfati M. (2009) Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. J. Allergy Clin. Immunol. 124, 1333–1342. e1. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro S. M., Poczatek M., Schultz-Cherry S., Villain M., Murphy-Ullrich J. E. (1999) The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J. Biol. Chem. 274, 13586–13593. [DOI] [PubMed] [Google Scholar]
- 48.Schultz-Cherry S., Chen H., Mosher D. F., Misenheimer T. M., Krutzsch H. C., Roberts D. D., Murphy-Ullrich J. E. (1995) Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 270, 7304–7310. [DOI] [PubMed] [Google Scholar]
- 49.Contreras-Ruiz L., Ryan D. S., Sia R. K., Bower K. S., Dartt D. A., Masli S. (2014) Polymorphism in THBS1 gene is associated with post-refractive surgery chronic ocular surface inflammation. Ophthalmology 121, 1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winton H. L., Bidwell J. L., Armitage W. J. (2014) Thrombospondin-1 polymorphisms influence risk of corneal allograft rejection. Invest. Ophthalmol. Vis. Sci. 55, 2115–2120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.