Review on microfluidic tools at the interface between clinical and basic research, of direct relevance to leukocyte biology.
Keywords: nanotechnology, inflammation, neutrophils, dendritic cells, monocytes
Abstract
Inflammation is an indispensable component of the immune response, and leukocytes provide the first line of defense against infection. Although the major stereotypic leukocyte behaviors in response to infection are well known, the complexities and idiosyncrasies of these phenotypes in conditions of disease are still emerging. Novel tools are indispensable for gaining insights into leukocyte behavior, and in the past decade, microfluidic technologies have emerged as an exciting development in the field. Microfluidic devices are readily customizable, provide tight control of experimental conditions, enable high precision of ex vivo measurements of individual as well as integrated leukocyte functions, and have facilitated the discovery of novel leukocyte phenotypes. Here, we review some of the most interesting insights resulting from the application of microfluidic approaches to the study of the inflammatory response. The aim is to encourage leukocyte biologists to integrate these new tools into increasingly more sophisticated experimental designs for probing complex leukocyte functions.
Introduction
Once associated primarily with the response to injury and infection, inflammation is now known to underpin the pathology of many common diseases, including heart disease, arthritis, and asthma. Discovery of unexpected roles for the inflammatory microenvironment in cancer progression, such as promotion of tumor mutation, growth, vascularization, and subsequent metastasis [1–3], and in several chronic disorders that are the leading causes of death and disability worldwide [4–8] has led to resurgence in interest regarding innate immune cell behavior and function (Fig. 1A). As we learn more about the intricacy of leukocyte responses in various conditions, beyond stereotypic functions in infections, it becomes more and more apparent that we must look for new technologies to interrogate increasingly complex behaviors and disease-specific phenotypes. Ideally, such methods would allow both single-cell resolution and large-scale experiments in well-controlled conditions and master the time and space of relevant leukocyte interactions and responses.
Figure 1. Microfluidics and innate immunity, two fields of exponential growth.
(A) Graph shows PubMed citations/yr in response to the search terms “Cellular innate immunity” (magenta columns) and “Microfluidic” (green columns). Invention of the Boyden chamber corresponds to a surge in research in the field, peaking in the mid-1980s. Since its resurgence in the early 2000s, the field of leukocyte biology continues to expand exponentially. Following the first microfluidic prototypes for biomedical applications in the early 2000s, this area of study also exhibits exponential growth. (B) Graph shows PubMed citations/yr in response to the search term “Leukocyte” in combination with “Microfluidic” (magenta columns). Use of microfluidic assays to measure leukocyte behavior is becoming increasingly common, with ∼60% of publications emerging in the last 5 yr.
Several technologies today are pushing these limits and integrating advances in signaling and imaging. New fluorescent imaging techniques, including 2-photon and light-sheet microscopy techniques [9, 10], now allow direct observation of leukocyte behavior in vivo in response to injury or infection [11, 12]. Intravital imaging has led to identification of several unexpected leukocyte behaviors, including neutrophil reverse migration [13], roles for macrophages in mycobacterial growth and dissemination [14], and identification of “patrolling” neutrophils in microcirculation [15]. However, leukocyte behavior and activity vary widely among species [16–19], and the relevance of observations made in model organisms must ultimately be validated in human cells to establish evolutionary conservation of biology. Increasingly sophisticated molecular, genomic, and proteomic tools allow rapid identification of specific molecular defects underlying inherited and malignant disease [20, 21]. These provide molecular targets that could be specifically targeted by new drugs to improve patient outcomes [22] or genome-editing techniques, such as zinc finger nucleases [23], transcription activator-like effector nucleases [24], and CRISPR/CRISPR-associated protein 9 [25]. Leukocyte responses to injury and infection stimuli have been characterized at the transcriptomic and proteomic level [26–29]. However, in vitro molecular biology approaches for studies of human leukocyte biology are hampered by difficulty in leukocyte primary culture, particularly of neutrophils. This restricts the use of genetic techniques and increases reliance on differentiated, malignant cell lines, such as the “neutrophil-like” HL-60 line [30–32], which carry many of their own inherent biologic caveats and display activities divergent from their primary counterparts [31, 33].
One area of accelerating progress is represented by studies of primary human leukocytes. These studies are important for testing which leukocyte phenotypes are conserved between model systems and humans and for a better understanding of the role human leukocytes play in diverse pathologies. Currently, studies of human leukocyte behavior ex vivo rely heavily on established technologies, such as the parallel plate, for imaging leukocyte–endothelial interactions under flow [34], the Boyden chamber, for basic assessment of chemotaxis [35], flow cytometry approaches for measurement of phagocytosis and killing [36, 37], and devices for observation of TEM across layers of cultured cells [38]. Devices allowing more detailed assessment of leukocyte migration in response to chemokine gradients, such as the Zigmond (and later Dunn) “bridge chambers” [39, 40] and pipette tip assays [41], are low throughput and generate short-lived gradients that evolve unpredictably. Although current tools provide robust results, they are limited in their potential for customization, thus limiting applicability to the study of new phenotypes [42].
In overcoming these limitations, microfluidic approaches represent a mature, integrative technology that enables highly customizable studies of human leukocytes ex vivo. Increasingly, researchers are embracing microfluidic tools to interrogate leukocyte biology (Fig. 1B). They use small volumes, thereby reducing use of expensive reagents, and provide extensively customizable geometries and stepwise integration of assays, allowing unprecedented control of spatial and temporal experimental aspects. The basic principles of the microfabrication technologies have been perfected by the electronics industry, which makes them also amenable to massive parallelization, thus allowing increased throughput and improving the statistical power of observations. These features can be combined into highly integrated devices, which could perform complex functions for sample preparation, separation, detection, and/or analysis, without the need for an expert operator. Microfluidic tools are already dramatically redefining the landscape of biomedical research in cancer [43, 44], neurosciences [45], tissue engineering [46], microbiology [47], cell biology [48, 49], and others and are poised to accelerate research in inflammation and leukocyte biology.
The aims of this review are to highlight a number of recent studies that have used microfluidic devices to study complex leukocyte behaviors and to outline some of the success stories where microfluidic approaches have advanced the field of leukocyte biology. We will also discuss some promising directions of development for this technology, as a useful translational tool for ex vivo interrogation of leukocyte behavior and function. We will “brush over” the engineering details of these new tools, as they are already covered in several technology-focused reviews [50, 51].
INFLAMMATION-ON-A-CHIP
In response to insult, the cellular arm of the immune system coordinates a highly regulated and choreographed sequence of actions that are essential for successful and appropriate immune response, neutralization of the threat, and return to normal physiology. Recently, microfluidic approaches have contributed to our understanding of how the events in this sequence are coordinated and helped study the details of their regulation.
Activation, adhesion, and transmigration
Leukocyte activation is a common prerequisite for adhesion and transmigration in response to insult. The interactions between leukocytes and endothelial cells are complex, and new features are still emerging. Whereas all leukocytes adhere and roll on vessel surfaces before transmigration, neutrophils are capable of doing this at considerably higher shear stress than other leukocytes [52], likely facilitated by “membrane tethers,” which have been shown to form in vitro between the moving neutrophils and P-selectin-coated surfaces [53]. With the addition of these early observations in macroscale flow chambers, microfluidic perfusion make tight control of shear stress conditions possible. For example, in combination with total internal reflection fluorescence imaging, microfluidic experimental systems enable high-resolution observation of individual neutrophils, which helped to probe the role of PSGL1/P-selectin interactions in modulating this process [54]. An unexpected “sling” mechanism was uncovered, in which tethers from the rear of the cell flip over the cell during rolling to form long membrane extensions (“slings”) at the leading edge of the cell. The slings bind to endothelia via patches of PSGL1 along the sling, and the sequential failure of these adhesive patches under shear stress results in a step-wise peeling of slings, which ultimately controls rolling of cells along the surface [55].
Activated neutrophils in disease have been implicated in promotion of microvascular occlusion, resulting in increased peripheral resistance [56]. For these types of studies, microfluidic devices enable the study of leukocyte–endothelial interactions and the quantification of pressure build-up upstream. Such experimental systems mimic in vivo interactions and also provide precise control of shear stress conditions. Applications of such tools include quantification of the effect of leukocyte–endothelial interactions on blood flow through capillary networks of dividing channels coated with laminin or E-selectin following neutrophil activation [57], relevant to the pathologic context of sickle cell disease [58]. In these studies, microfluidic devices facilitate not only the detailed study of leukocyte–endothelial cell interactions but are also significantly easier to use compared with traditional tools. For example, one device allowed repeated assembly by using a vacuum to seal the microchannels on top of standard 6-well plates. This allows endothelial cells to be cultured by conventional procedures on flat surfaces, avoiding the challenges of culturing cells in channels. Detailed kinetic studies of neutrophil adhesion and rolling behaviors over an endothelial monolayer in this device clearly demonstrated that activation with CXCL8 (IL-8), starting at picomolar concentrations, significantly enhances neutrophil adhesion [59]. More recent microfluidic studies of neutrophils transiting through narrow constrictions demonstrate a role for actin cytoskeletal organization, which directly affects the friction with capillary walls [60].
The detection of higher activation status of circulating neutrophils could potentially be a useful diagnostic tool for early identification of infections. A microfluidic device was designed recently to isolate activated neutrophils from whole blood, based on their lower PSGL1 expression. For this, microfluidic chambers were patterned with parallel, angled stripes of P-selectin, on which only unactivated neutrophil could adhere. Activated neutrophils would pass unaffected, as a result of their lower PSGL1 expression levels. Simply by measuring the flux of neutrophils over the stripes, the device provides a quantitative measure of the neutrophil activation status [61]. A simpler microfluidic design, with uniformly coated P-selectin surfaces, was also used to isolate neutrophils from whole blood before chemotactic analysis using integrated gradient generators [62]. Studies using this device found that cell capture was increased on P-selectin (as well as E-selectin and fibronectin) following stimulation with fMLP [62], suggesting that PSGL1 may actually be up-regulated on activated neutrophils. Neutrophils from chronically inflamed mice [63] and asthmatic patients [64] displayed enhanced adhesion to P-selectin-coated surfaces and may provide useful information for the diagnostic of these conditions. Other practical applications of leukocyte adhesion inside microfluidic devices include label-free separation. For example, the sorting and counting of CD4 lymphocytes from 1 drop of blood were used to diagnose AIDS in HIV-positive patients. The precisely controlled shear stress inside microfluidic channels enabled differentiation between CD4-expressing monocytes and lymphocytes binding to surfaces with immobilized anti-CD4 antibody, based on differences in cell size and CD4 expression levels [65]. More recently, imprinted antibody spots have been used for immobilization of monocytes from minimally processed blood, which were then stimulated with TLR agonists and subsequent cytokine production assayed using integrated cytokine arrays [66].
Extravasation via TEM through cultured endothelial cells has been modeled in microfluidic devices in response to a number of inflammatory stimuli [67]. A microfluidic model of TEM involved development of a 3D structure mimicking the BBB, consisting of a tubular layer of endothelial cells with confirmed tight and adherent junctions after culture under flow inside a rounded microfluidic channel [68]. Application of chemokine gradients demonstrated the ability of the BBB to block leukocyte chemotactic responses, whereas the positive effects of neuroprotective drugs in recovery from vascular oxidative stress were also verified [68]. Integration of microvascular surfaces with devices that induce mechanical stress holds promise for mimicking various tissue microenvironments, including the lung alveoli [69], gut epithelia [70], and human placenta [71].
Cellular motility and chemotaxis
Once inside the tissues, leukocytes must respond efficiently and migrate directionally, following complex and often-conflicting gradients of chemokines originating from infecting microbes, injured tissue, and other leukocytes, e.g., macrophages [72] and neutrophils [73]. Leukocyte motility in response to chemical gradients (chemotaxis) is thus a key prerequisite for all subsequent functions in tissues. The biologic importance of this process and the relative complexity emerging from the need for chemical gradients make this topic of great interest for microfluidic studies.
A range of microfluidic devices have been specifically designed to study particular aspects of immune cell motility and chemotaxis. These include: FCCs to mimic localized injury (Fig. 2); simple mazes to interrogate pathways influencing reverse migration (Fig. 3), and more complex mazes to study cellular navigation decision making (Fig. 4). Subcellular imaging techniques have also been integrated to directly visualize proteins important for motility and chemotaxis (Fig. 5).
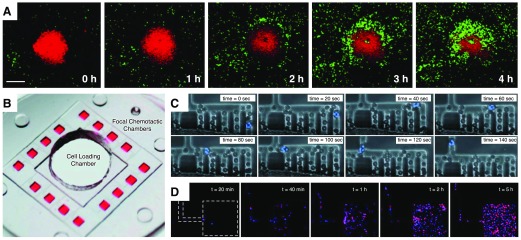
Figure 2. Models of focal leukocyte chemotaxis.
(A) Spinning disk confocal intravital microscopy of mouse neutrophils (lys-EGFP green) responding to a localized thermal injury (propidium iodide; red) on the surface of the liver from the Kubes lab. [Reproduced from McDonald, B., Pittman, K., Menezes, G. B., Hirota, S. A., Slaba, I., Waterhouse, C. C., Beck, P. L., Muruve, D. A., Kubes, P. (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366. Reprinted with permission from the American Association for the Advancement of Science (AAAS).] (B) A microfluidic model of focal chemotaxis allows parallel imaging of leukocyte chemotaxis from a central cell-loading chamber toward 16 identical chemokine reservoirs (red dye) on a single device. [Reproduced from Jones, C. N., Dalli, J., Dimisko, L., Wong, E., Serhan, C. N., Irimia, D. (2012) Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci USA 109, 20560–20565.] (C) Integration of a RBC filter facilitates use of whole-blood samples. Neutrophils (blue nuclei, Hoechst) are able to navigate the filter and migrate toward the chemokine source. [Reproduced from Hoang, A. N., Jones, C. N., Dimisko, L., Hamza, B., Martel, J., Kojic, N., Irimia, D. (2013) Measuring neutrophil speed and directionality during chemotaxis, directly from a droplet of whole blood. Technology 1, 49–57. Electronic version of an article published as Technology 1, 49–57 doi: 10.1142/S2339547813500040, copyright World Scientific Publishing Company, www.worldscientific.com.] (D) Coordinated recruitment of multiple cell types, such as neutrophils (blue) and macrophages (red) in response to complex signals, can be studied under defined conditions. [Reproduced from Jones, C. N., Dalli, J., Dimisko, L., Wong, E., Serhan, C. N., Irimia, D. (2012) Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci USA 109, 20560–20565.]
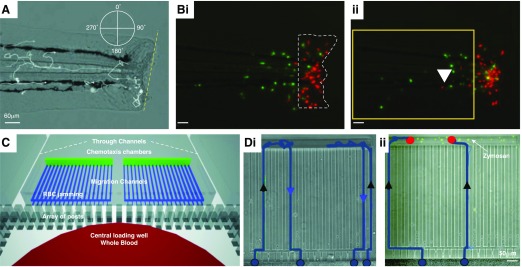
Figure 3. Modeling of inflammation resolution by neutrophil reverse migration.
(A) A transgenic zebrafish model of sterile inflammation. Neutrophils are labeled by GFP expression, driven by the myeloperoxidase promoter, allowing in vivo tracking of neutrophil migration relative to the wound (dashed yellow line) during recruitment and resolution phases. (B, i) Reverse-migrating neutrophils can be more easily visualized by spatially restricted photoconversion (from green to red) of wound-associated neutrophils (dashed white outline) that express the fluorescent protein Kaede. (ii) Reverse migration of neutrophils can then be monitored by identifying those cells that return to the tissue (white arrowhead). [A and B reproduced from Robertson, A. L., Holmes, G. R., Bojarczuk, A. N., Burgon, J., Loynes, C. A., Chimen, M., Sawtell, A. K., Hamza, B., Willson, J., Walmsley, S. R., Anderson, S. R. (2014) A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med 6, 225ra29–225ra29. Reprinted with permission from AAAS.] (C) A microfluidic device to measure neutrophil recruitment and reverse migration from a drop of whole blood. (D, i) Neutrophils (blue tracks) are recruited (black arrowheads) from the whole blood to the chemotaxis chambers and then reverse migrate (blue arrowheads) down the chemokine gradient and back into the whole-blood reservoir. (ii) In the presence of microbial particles (zymosan; green), neutrophils are retained (red dots) at the chemokine reservoir and show reduced reverse migration. [C and D reproduced from Hamza, B., Irimia, D. (2015) Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab Chip 15, 2625–2633. Reprinted with permission from the Centre National de la Recherche Scientifique (CNRS) and The Royal Society of Chemistry.]
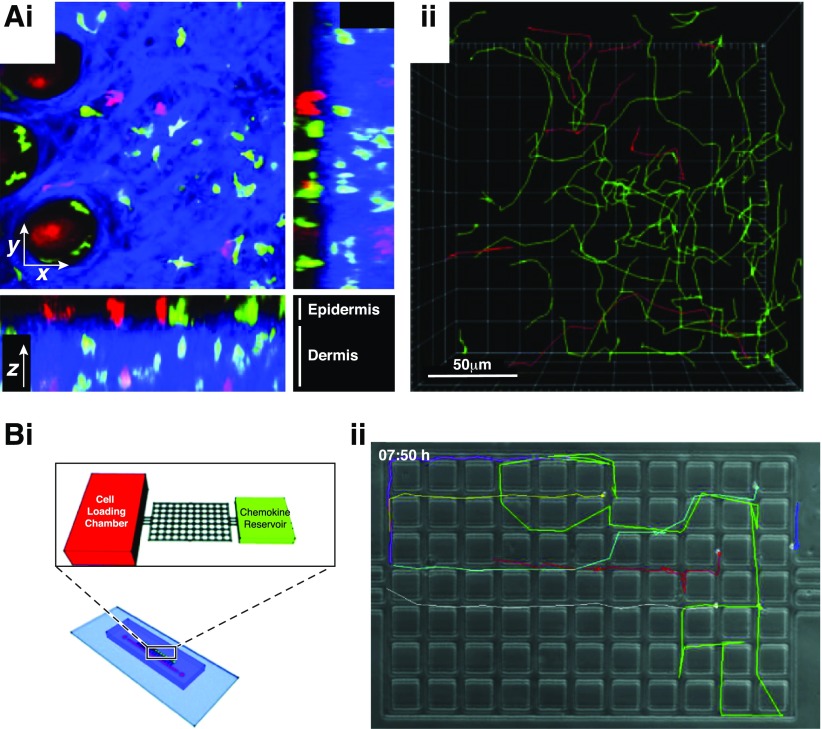
Figure 4. Modeling complex cell migration signatures.
(A, i) Two-photon intravital imaging of CD4 (GFP; green) and CD8 (DsRed; red) T cells in the epidermis (collagen visualized in blue using second-harmonic generation) following infection with HSV. (ii) 3D tracking of cell migration over time demonstrates the complexity of cell migration in vivo. [Reproduced from Gebhardt, T., Whitney, P. G., Zaid, A., Mackay, L. K., Brooks, A. G., Heath, W. R., Carbone, F. R., Mueller, S. N. (2011) Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219, copyright 2011. Reprinted with permission from Macmillan Publishers Ltd., Nature 2011.] (B, i) A microfluidic device for studying complex cell migration signatures. Lymphocytes migrate through a maze of posts toward a chemokine reservoir. (ii) Cell migration (colored tracks) can be tracked at cellular resolution in 2 dimensions, providing rich datasets that reflect complex variations in migration phenotypes. [Reproduced from Jain, N. G., Wong, E. A., Aranyosi, A. J., Boneschansker, L., Markmann, J. F., Briscoe, D. M., Irimia, D. (2015) Microfluidic mazes to characterize T-cell exploration patterns following activation in vitro. Integr Biol 7, 1423–1431. Reprinted with permission from the CNRS and The Royal Society of Chemistry.]
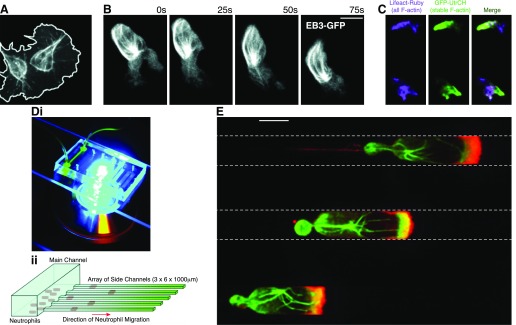
Figure 5. Detailed subcellular imaging.
(A) Transgenic expression of fluorescently tagged proteins allows intravital imaging at subcellular resolution of microtubule dynamics within migrating hemocytes in Drosophila larvae. [Reproduced from Evans, I. R., Wood, W. (2014) Drosophila blood cell chemotaxis. Curr Opin Cell Biol 30, 1–8.] (B) The same approach has been used to image microtubules within neutrophils in zebrafish larvae. (C) Comparison of stable versus all F-actin allows visualization of dynamic F-actin in migrating zebrafish neutrophils. UtrCH, Calponin homology domain of utrophin. [B and C reproduced from Yoo, S. K., Lam, P. Y., Eichelberg, M. R., Zasadil, L., Bement, W. M., Huttenlocher, A. (2012) The role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci 125, 5702–5710. Reprinted with permission from AAAS.] (D, i) Microfluidic devices are highly optically accessible and are well suited to high-power fluorescence imaging. (ii) Cells migrate in the same direction up the chemokine gradient in a single plane, greatly simplifying microscopy. [Reproduced from Butler, K. L., Ambravaneswaran, V., Agrawal, N., Bilodeau, M., Toner, M., Tompkins, R. G., Fagan, S., Irimia, D. (2010) Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS One 5, e11921.] (E) Immunofluorescence micrograph of neutrophils migrating in parallel microfluidic channels. Cells are stained for microtubules (green) and actin (red). [Reproduced from Irimia, D., Charras, G., Agrawal, N., Mitchison, T., Toner, M. (2007) Polar stimulation and constrained cell migration in microfluidic channels. Lab Chip 7, 1783–1790. Reprinted with permission from the CNRS and The Royal Society of Chemistry.]
The first microfluidic devices to be used for the study of neutrophil migration were designed to generate stable gradients of CXCL8 by controlled mixing from 2 starting solutions. These devices were capable of stably generating spatial concentration profiles that were previously unattainable [74]. Observations of cells migrating inside these devices demonstrated that human neutrophils consistently stop at the edge of “cliff” gradients. Surprisingly, when migrating over shallower “hill” gradients, neutrophils reversed direction toward the higher concentration of the chemoattractant [74]. Subsequent microfluidic experiments using similar designs revealed that whereas the concentration range of the gradient was important for directed migration, the steepness of the gradient was not [75]. Interestingly, neutrophils were discovered to exhibit consistent chemorepulsion (fugetaxis), down steep gradients at high concentrations of CXCL8. Although chemoattraction and chemorepulsion shared signaling components, differences in calcium flux were reported to correlate with these distinct behaviors [76]. More sophisticated versions of microfluidic devices, incorporating microscale pneumatic valves, enabled rapid changes in the direction and slope of the CXCL8 concentration gradients. Rapid switches in the direction of the gradients forced the neutrophils to lose polarity transiently before repolarizing in the new direction, a behavior that is clearly distinct from the classic U-turn reported in moving micropipette experiments. Moreover, lowering the concentration resulted in temporary neutrophil depolarization, while increasing the concentration resulted in maintained directional motility [77].
Several unexpected findings have emerged from studies of leukocyte migration in microfluidic channels with micrometer-scale cross-sections [78]. These channels are usually smaller than the leukocytes and comparable in size with the interstitial spaces in most tissues [79]. Interestingly, qualitative and quantitative changes of motility phenotypes emerge for leukocytes confined during migration. For example, the migration speed becomes constant over time, without the usual variations measured during meandering migration on flat surfaces [40]. This ex vivo phenotype better matches the rapid and directional leukocyte migration observed in vivo and reflects the use of efficient, integrin-independent migration pathways [80]. Recent modeling of 3D neutrophil migration by integration of matrigel into microfluidic migration channels further highlighted the different migration modalities used by neutrophils for different environments [81]. These studies identified important roles for integrin regulators RAIM and Talin-1 in protrusion formation on the cells leading edge, even during integrin-independent migration [81]. Moreover, customization of migration channel features, such as addition of mazes or posts, has facilitated a number of unexpected observations regarding neutrophil decisionmaking during chemotaxis. For example, interaction with posts in the middle of the channel resulted in a unique tug-of-war behavior at the leading edge of moving neutrophils. This suggested that chemokine sensing and generation of motility occur locally at the leading edge and coordinated via physical forces in the cytoskeleton rather than via diffusive signaling throughout the cell [82].
The limitations in the directions of movement imposed by channels also enabled precision measurements with single-cell resolution of human neutrophil migration in healthy donors and patients [83]. When bifurcating channels were used, the limited number of directional choices (2) enabled highly accurate measurements for human [82] and rat [84] neutrophils, both highlighting the directional fidelity of healthy neutrophil migration to a chemokine gradient and allowing discrimination of chemotactic (directional) and chemokinetic (nondirectional) stimuli [85].
More complex mazes of microscale channels have been used to characterize lymphocyte migration [86], demonstrating that mitogen activation induces an exploratory, patrolling behavior in lymphocytes compared with unstimulated cells [87] (Fig. 4). This behavior and the independence of migration from the presence extracellular calcium are similar to observations using DCs in vivo [88] and in vitro [80]. During the inflammatory response, both directional cues (chemotactic agents) and modulators of motility (chemokinetic agents) are important for stimulation and regulation of leukocyte responses. Whereas traditional chemotaxis assays do not allow discrimination between chemotactic and chemokinetic responses, a number of microfluidic devices have now been designed for that purpose [85, 89]. The importance of epithelial-derived chemokinetic cytokine thymic stromal lymphopoietin in stimulating DC migration to inflamed tissues has been demonstrated in straight microfluidic channels [90], which allowed precision measurement of cell speed and migration persistence.
The complexity, not only of mechanical but also of the biochemical environment in tissues, is revealed by studies in microfluidic devices. For example, the study of neutrophil migration in response to competing gradients of chemoattractant [42] and prioritization of directional migration responses is 1 area that benefited from the ability to overlap gradients in the same channel. Comparison of neutrophil migration upon exposure to competing chemokine gradients demonstrated a general chemokine hierarchy of fMLP > CXCL8 > CXCL2 > LTB4, with “end-target” chemoattractants, such as C5a and fMLP, taking precedent over endogenously produced chemokines [91, 92]. Whereas prioritization among some chemoattractants is clear cut, others appear to be more complex. Although neutrophils are able to migrate in a step-wise manner along one chemoattractant gradient to another, thereby allowing them to navigate in response to a sequence of cues [93], microfluidic studies have demonstrated that neutrophils may display oscillatory migration when faced with opposing gradients of LTB4 and CXCL8. These results support a model, where rather than relying on sensory adaptation, neutrophils reversibly lock on or off different chemoattractant signals [94]. Furthermore, fMLP was found to activate directed chemotaxis faster than LTB4, resulting in a synergistic enhancement when neutrophils were exposed to the 2 chemokines in combination [95]. Experiments using a flexible microfluidic “jet” device [96] demonstrated that desensitization of neutrophils on a gradient could be modeled, although re-exposure to a spatially shifted gradient of CXCL8 did not reveal any significant desensitization [97].
Dynamic control of chemotactic cues over time has proven useful for unraveling how chemotaxing cells respond to fast [77] and slow [98] changes of gradients or traveling waves of chemoattractants, such as cAMP, and demonstrated that directional persistence corresponded to stability of activated RAS polarity and the strength of directional cues [98]. Rapid exposure of neutrophils to exponential chemokine gradients using microfluidic valves showed that neutrophils could sense subcellular-scale, spatial chemokine gradients, without a requirement for temporal sampling [99]. Most recently, microfluidic “neutrophil treadmill” devices helped clearly decouple spatial and temporal chemokine gradients to reveal that temporal chemoattractant changes are required for persistence during directional migration [100]. To accomplish this decoupling, microfluidic devices and computer-controlled motion compensation strategies were integrated to expose moving neutrophils to a chemokine gradient that was moving at the same speed as the cells.
The ability to image individual chemotaxing cells at cellular and subcellular resolution in microfluidic devices (see Fig. 5E) has enabled measurements of contractile forces during migration on a stiff surface, and demonstrated that these forces are localized to the uropod [101]. This distribution of forces is distinct from that observed in slower moving, anchorage-dependent motile cells, such as fibroblasts, where forces are localized at the leading edge and fillopodia [102], and suggests that fast migration in neutrophils may rely on a posterior RhoA-mDia-dependent actin pool, as has been demonstrated recently for DCs [103]. It is also becoming clear that chemokines are not the only factor guiding directed immune cell migration. T cells showed preferential electrotaxis toward the cathode in a DC electric field in microfluidic devices, even in the presence of a competing chemokine gradient [104], and electrotaxis can be stimulated by anti-CD3/CD28 antibodies [105].
Cell–cell interaction and intercellular communication
In response to injury and infection, signals from tissue resident cells drive recruitment of leukocytes from the bloodstream. Coordinated, simultaneous recruitment of neutrophils and monocytes is integral to correctly regulated modulation of the inflammatory environment [106].
Analysis of leukocyte migration from a mixed neutrophil and monocyte population to microfluidic FCCs (Fig. 2B) demonstrated lineage-specific migration signatures in response to different chemokines, with neutrophil-dominated migration in response to fMLP, LTB4, and CXCL8 and monocyte-dominated recruitment in response to the proresolution lipid mediator LXA4 and MCP1 [85]. Interestingly, comparison of recruitment from homogenous cells versus heterogeneous populations to LTB4 showed that comigration favored increased monocyte recruitment at the expense of neutrophils and reduced neutrophil elastase activity, as measured using an integrated fluorescent reporter assay [85]. Pretreatment of neutrophils and monocytes with nanoparticles containing LXA4 or resolvin D2 significantly reduced recruitment of both lineages, and nanoparticles were trafficked to the FCC by monocytes, suggesting a mechanism by which these proresolving nanoparticles might potentially be targeted to sites of inflammation in vivo [85].
Integration of cytokine arrays into microfluidic cell-capture platforms allows measurement of intercellular communication molecules, such as cytokines and chemokines [66]. The confinement of cells in small chambers limits the physical space in which the cytokines can diffuse, increasing the concentration available for sampling and augmenting the sensitivity of single-cell analysis beyond that of traditional ELISPOT methods [107]. For example, exposure of T cells to media conditioned by LPS-matured DCs induced calcium transients stimulated by an unknown chemical signal [108], whereas both immature and LPS-matured DCs produced chemotactic factors for NK cells that signaled through the CXCR3 receptor [109]. NK immune synapse formation, defined by clustering of MHC I at the cellular interface, was visualized with unprecedented resolution, using cell confinement and controlled spatial positioning to allow manipulation and detailed observation of direct cell–cell contacts [110, 111].
Small extracellular vesicles, such as ectosomes and microvesicles, another pathway for intercellular communication, are currently under heavy scrutiny as potential carriers of important disease biomarkers [112]. Exposure to microvesicles from RBCs infected with malaria induced spontaneous neutrophil migration inside microscale channels, suggesting that malaria may actively modulate and perturb the innate immune system [113]. Phagocytosis of plasmodium-infected RBCs by macrophages has also been modeled using a microfluidic device [114]. Moreover, the modulatory effect of human neutrophil-derived nanoparticles on monocytes has been quantified with single-cell resolution in microfluidic chambers [85]. The suppressive and chemotactic effects of microbial metabolites were tested by diverse microfluidic approaches. Chemotaxis of neutrophils toward infection foci, mimicked by physically compartmentalized bacterial cocultures, was demonstrated [115], whereas coupling of established microfluidic approaches with an in vivo zebrafish larval inflammation assay identified an immunosuppressive action for the fungal metabolite endocrocin, which could be abrogated by deletion of the polyketide synthase gene, encA [116].
Phagocytosis and NETosis
A primary role for professional phagocytes, such as macrophages and DCs, is to capture and present antigen, usually via engulfment of pathogens [117, 118]. By integrating subcellular imaging, phagocytosis, and microfluidic cell migration assays, an antagonistic relationship between antigen capture and processing was recently revealed at multiple steps in the pathway [119]. In immature DCs expressing a myosin IIA reporter, spatial redistribution of myosin IIA from the rear of the cell to the leading edge of the cell during phagocytosis disrupted the front-to-back gradient required for migration, significantly reducing migration speed. Reduced migration speed could be phenocopied by selective application of myosin-disrupting Blebbistatin to the rear of the cell or by application of myosin-enhancing Calyculin to the front of the cell [119]. MHC II-associated invariant chain I (CD74)-dependent myosin enrichment of the front of the cell was demonstrated to regulate macropinosome dynamics and facilitate delivery of antigens to acidic endolysosomes, suggesting a prioritized response that enhances antigen capture and processing at the expense of migration speed [119], building on previous observations that CD74 negatively regulated DC motility [120]. Further microfluidic studies using transgenic reporters of actin polymerization demonstrated that following TLR4-MyD88-induced maturation, DCs again switch locomotion phenotypes. Down-regulation of an anterior Arp 2/3-dependent actin pool, involved in antigen capture, facilitated switching to a posterior RhoA-Dia1-dependent actin pool, allowing accelerated and more directional migration [103], presumably toward lymphatic vessels and lymph nodes producing chemotactic factors, such as CCL-19 [121].
Inside microfluidic chambers, human neutrophil migration, in response to fMLP gradients, significantly enhanced phagocytosis and killing of fungal conidia compared with uniform fMLP concentration [122]. Also in neutrophils, the phagocytosis of zymosan particles significantly suppressed retrotaxis in microscale channels, a phenotype that could be partially alleviated using LXA4 or the antioxidant Tempol [33, 123]. From these studies, it seems likely that the priming of shared cell migration and phagocytosis signaling pathways may act as a key regulatory framework to enhance pathogen clearance and coordinate inflammation resolution via the neutrophil reverse-migration (retrotaxis) pathway.
Originally identified in the context of infections, the release of NETs from overly stimulated neutrophils is a topic of increasing interest in biology and in the clinic. New microfluidic devices are emerging to analyze the effect that NETs have on blood-flow traffic through networks of channels replicating geometrical features of capillary plexuses. Experiments have shown that trapped NETs, occluding the entrance of channels, can decouple the traffic of blood cells from plasma and lead to the formation of large areas void of blood cells. This effect of NETs is independent from the activation of platelets or fibrin clots and when validated in vivo, may represent a new mechanism of tissue hypoxia and organ injury driven by neutrophil activation [124]. Other devices have aimed to miniaturize existing assays and measure NET formation in conjunction with reactive oxygen species production by neutrophils [125]. These devices integrated the neutrophil separation from whole blood in the same assay, before stimulation by PMA or bacteria [125].
Efferocytosis, retrotaxis, and inflammation resolution
Inflammation resolution is generally thought to rely on macrophages switching from proinflammatory to proresolution signaling, stimulated, in part, by efferocytosis of apoptotic cells at the wound, including neutrophils [106, 126].
Following identification of retrotaxis as an alternative path to resolution of neutrophilic inflammation in zebrafish [13], microfluidic studies of human cells provided key evidence that this behavior was conserved in mammals [33]. Studies of neutrophil migration in U-shaped channels containing chemokine gradients showed that >90% of neutrophils retrotaxed, with >60% continuing migration once they reached the highest concentration of chemoattractant at the bend [33]. This proportion could be increased to >85% following treatment with LXA4 or reduced to ∼10% by introduction of high concentrations of fibronectin or zymosan particles. The effect of fibronectin and zymosan could be rescued by pretreatment of neutrophils with LXA4 or the antioxidant Tempol [33].
In response to fMLP, polarization of PI3K signaling (dispensable for neutrophil chemotaxis to fMLP [127]) was maintained during retrotaxis, whereas velocity was increased significantly, suggesting that an efficient, self-perpetuating locomotion mechanism [128, 129] may dominate during retrotaxis. This hypothesis is supported further by similar observations in vivo [130], which show that PI3K signaling is important for neutrophil random migration, which likely facilitates resolution by diffusion in vivo [131]. Redistribution of PI3K signaling to zymosan phagosomes in nonretrotaxing cells supports a role for maintenance of PI3K polarization in retrotaxis. Alternatively, the phagosomal oxidative burst itself, which is regulated, in part, by PI3K [132], may inhibit cell migration, as Tempol significantly increased the distance migrated by neutrophils containing a phagocytosed particle [33].
Microfluidic approaches provide many useful tools to probe pathways that might potentially coordinate retrotaxis, including fugetaxis [76], chemokine receptor densensitization [97], chemokinetic versus chemotactic responses [89], complex and competing chemokine gradients [91, 92, 94], and temporal changes in chemokine gradient [100]. Current approaches aimed at studying resolution by reverse migration directly have suggested that the kinetics of neutrophil influx and efflux from the wound might be most simply regulated by balance between neutralization of microbe-like particles and retrotaxis [123] (Fig. 3D). However, enhancement of reverse migration by lipid mediators and small molecules suggests that this process is more likely modulated by multiple intercellular signals [33, 123, 133]. Insights from studies that incorporate both neutrophils and macrophages in the same assay [85] contribute further to our understanding of interactions that occur between these cells in the context of inflammation and infection.
MICROFLUIDICS FOR LEUKOCYTE ANALYSIS DURING DISEASE
Continual refinement of diagnostic techniques and identification of new biomarkers allow earlier and more accurate diagnosis and measurement of disease progression. However, the contribution of innate immune responses to disease is still emerging and is under increasing scrutiny [134, 135]. The importance of specific leukocyte populations is coming to light, supported by new findings, enabled by new tools. For example, neutrophil phenotype is highly sensitive to changes in blood components, is easily perturbed by a range of diseases, and in many cases, contributes directly to disease pathophysiology [8, 136–139]. In this context, microfluidics assays provide new tools to assess leukocyte biology, which are customized to probe specific functions, easy to use, and complement current technologies.
Microfluidic devices to probe the status of innate immune responses in patients
Analysis of blood biochemical and cellular components remains one of the most widely applicable and versatile tools for disease diagnosis in the clinic. This provides a potentially useful platform for identifying behavioral biomarkers for specific disease states. Recent examples include the following: microfluidic devices for characterization of leukocyte “migration signatures” in response to various stimuli [89]; microfluidic devices to discriminate between allergic rhinitis and asthma based on neutrophil chemotactic velocity in response to fMLP [64]; characterization of abnormal neutrophil behavior in patients with major burn injuries [83], leading to identification of spontaneous migration as a signature of preseptic patients [140]; reduced efficacy of neutrophils from transplant patients in tackling fungal infection [122]; and chemorepulsion of neutrophils in response to sputum from chronic obstructive pulmonary disease patients [141]. The effect of drug treatments in correcting neutrophil behavioral disorders has also been measured with precision in microfluidic devices [84, 85, 122].
An important step in translating use of these devices from the lab to the clinic is development of simple assays of leukocyte functions [142] that can be run at or near the bedside using small volumes of whole blood [62, 63, 123, 143–145]. Devices will ideally be preprimed and disposable and will need to provide highly accurate and robust measurements. Overall, microfluidic assays designed to probe neutrophil behavior represent an attractive clinical tool, not only to diagnose disease and predict outcomes but also as a rapid method of assessing efficacy of potential treatments, facilitating individually tailored care.
Microfluidic assays for ex vivo studies complement in vitro molecular biology approaches and in vivo animal model studies
Advances in intravital labeling and imaging technology over the past decade have vastly improved our understanding of the complex and unexpected behaviors that leukocytes exhibit in vivo [12]. However, current gold-standard in vitro and ex vivo assays of leukocyte behavior continue to rely on robust but inflexible techniques developed more than 2 decades ago [42]. These techniques measure neutrophil migration over flat surfaces, a scenario likely using only a subset of mechanisms required for neutrophil tissue migration [80, 128, 146], or provide an endpoint measurement of cell motility that does not discriminate between chemokinesis and directed chemotaxis.
Although advances are being made in in vitro organoid culture to better mimic in vivo environments [147, 148], these carry many biologic caveats and limited control of the cellular environment and provide nonoptimal imaging conditions.
Microfluidic approaches provide an opportunity to customize assay design to test specific hypotheses. The technology is ideally suited for identifying subtle and complex changes in leukocyte behavior and can be used for analysis of genetically manipulated cells from culture or model organisms, allowing direct comparison with ex vivo human cells under the same experimental conditions (Fig. 6).
Figure 6. Applications of microfluidic technologies to leukocyte research.
(A) Sample preparation for transcriptome analysis and cytokine arrays. (B) Ex vivo leukocyte assays for the study of transmigration, chemotaxis and trafficking, phagocytosis, host-pathogen interaction, etc. (C) Ex vivo assays for leukocytes from patients for diagnosis and monitoring of disease.
An increasingly expanding toolbox of microfluidic techniques is available for probing several key aspects of leukocyte biology (Table 1). Devices are highly customizable toward specific experimental goals, and their design allows for easy integration of multiple technologies. Devices are generally optically accessible (Fig. 5D), providing the opportunity for detailed imaging using existing microscopy techniques. They are also compatible with emerging imaging technologies, e.g., super-resolution approaches, such as stochastic optical reconstruction microscopy [160]. In addition, the ability to parallel assays massively greatly enhances statistical power, aiding detection of subtle phenotypes in a notoriously noisy biologic system. Microfluidics labs are now established at every major university, facilitating local collaborations that will be key to developing and applying these technologies in meaningful ways to biologic problems.
TABLE 1.
Microfluidic toolbox for leukocyte assay design
| Leukocyte activity | Assay | Technological innovation | Representative studies |
|---|---|---|---|
| Activation and TEM | Induced adhesion of leukocytes to substrates | Channel size and geometry comparable with human capillaries | [58, 81, 149] |
| Controlled shear stress for expression and size-based cell sorting | [61, 63, 65] | ||
| Migration of leukocytes through endothelial layer | Generation of chemokine gradients across endothelial cells in capillary-like constructs | [67, 68] | |
| Chemotaxis | Migration in response to chemokine gradients | Stable gradients of precise shape by diffusion mixing under flow | [74, 91] |
| Stable, competing gradients to test signal prioritization | [150–152] | ||
| Geometric control of chemokine diffusion from microscale reservoirs | [143, 145] | ||
| Microfluidic jets | [96, 97] | ||
| Mechanical confinement of moving cells inside microchannels smaller than the cells | [78, 86, 119, 121, 153–155] | ||
| Mazes to test directional persistence and fidelity | [82–85, 87] | ||
| Dissecting the role of spatial and temporal gradients | Rapid actuation of controllable membrane valves | [77, 99] | |
| Microfluidic jets for rapidly changing chemokine exposure | [97] | ||
| Neutrophil treadmill | [100] | ||
| Whole-blood assays | Integrated leukocyte separation and migration | [94, 95] | |
| Intercellular communication | Production of cytokines | Integrated cytokine array | [66, 67, 115] |
| Recipient cell effect in daisy-chain device | [108] | ||
| Imaging of interacting cell pairs | Microwells for imaging spatially constricted cell pairs | [108, 111] | |
| Host defense | Suppression of pathogen growth | Host-pathogen interaction chambers | [122] |
| Effect of phagocytosis on chemotaxis | Integration of microbial particles into chemotaxis assays | [123, 156] | |
| Blood flow obstruction by NETs | Capture of NETs in capillary plexus | [124] | |
| Inflammation resolution | Screening for inflammation resolution mediators | Whole-blood assay using a droplet of blood | [84, 157–159], |
| Reverse migration of neutrophils | Parallel channels for bidirectional leukocyte traffic | [33, 123] |
CONCLUDING REMARKS
Microfluidic tools to study leukocyte biology are emerging as key for ex vivo studies of human cells. These tools can be customized for specific tasks and enable precision measurements by decoupling distinct processes that contribute to complex phenotypes, such as migration, phagocytosis, and cell–cell interactions. These tools can reveal leukocyte phenotypes that are new and more diverse than previously known. Moreover, these tools can help integrate distinct functions in unitary assays with relevance in the clinic. As these tools become more easily accessible and easier to use, biologists should embrace technologies, such as microfluidics, and apply them to identify biologic truths and discern subtle behaviors of the leukocyte lineages.
AUTHORSHIP
Both authors contributed to the systematic review of literature, discussion of the most relevant advances in the field, and the writing of the manuscript.
ACKNOWLEDGMENTS
This work was supported, in part, by the U.S. National Institutes of Health (Grant Nos. GM092804 and EB002503).
Glossary
- 3D
3-dimensional
- BBB
blood brain barrier
- CRISPR
clustered regularly interspaced short palindromic repeats
- DC
dendritic cell
- FCC
focal chemotaxis chamber
- LTB4
leukotriene B4
- LXA4
lipoxin A4
- MHC I/II
MHC class I/II
- NET
neutrophil extracellular trap
- PSGL1
P-selectin-glycoprotein ligand 1
- TEM
transendothelial migration
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Meira L. B., Bugni J. M., Green S. L., Lee C. W., Pang B., Borenshtein D., Rickman B. H., Rogers A. B., Moroski-Erkul C. A., McFaline J. L., Schauer D. B., Dedon P. C., Fox J. G., Samson L. D. (2008) DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 118, 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoppmann S. F., Birner P., Stöckl J., Kalt R., Ullrich R., Caucig C., Kriehuber E., Nagy K., Alitalo K., Kerjaschki D. (2002) Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 161, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komohara Y., Fujiwara Y., Ohnishi K., Takeya M. (2015) Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 99, 180–185. [DOI] [PubMed] [Google Scholar]
- 4.Coussens L. M., Werb Z. (2002) Inflammation and cancer. Nature 420, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willerson J. T., Ridker P. M. (2004) Inflammation as a cardiovascular risk factor. Circulation 109 (21 Suppl 1), II2–II10. [DOI] [PubMed] [Google Scholar]
- 6.McInnes I. B., Schett G. (2011) The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219. [DOI] [PubMed] [Google Scholar]
- 7.Gan W. Q., Man S. F., Senthilselvan A., Sin D. D. (2004) Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59, 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segel G. B., Halterman M. W., Lichtman M. A. (2011) The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 89, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denk W., Strickler J. H., Webb W. W. (1990) Two-photon laser scanning fluorescence microscopy. Science 248, 73–76. [DOI] [PubMed] [Google Scholar]
- 10.Huisken J., Swoger J., Del Bene F., Wittbrodt J., Stelzer E. H. (2004) Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009. [DOI] [PubMed] [Google Scholar]
- 11.Lämmermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmüller W., Parent C. A., Germain R. N. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luu L., Coombes J. L. (2015) Dynamic two-photon imaging of the immune response to Toxoplasma gondii infection. Parasite Immunol. 37, 118–126. [DOI] [PubMed] [Google Scholar]
- 13.Mathias J. R., Perrin B. J., Liu T. X., Kanki J., Look A. T., Huttenlocher A. (2006) Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288. [DOI] [PubMed] [Google Scholar]
- 14.Davis J. M., Ramakrishnan L. (2009) The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillipson M., Kubes P. (2011) The neutrophil in vascular inflammation. Nat. Med. 17, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara T., Miyamoto M., Takayama S., Kato M. (1995) Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J. Pharmacol. Toxicol. Methods 33, 91–100. [DOI] [PubMed] [Google Scholar]
- 17.Rehli M. (2002) Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 23, 375–378. [DOI] [PubMed] [Google Scholar]
- 18.Inflammation and the Host Response to Injury Investigators (2005) Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 12, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mestas J., Hughes C. C. (2004) Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738. [DOI] [PubMed] [Google Scholar]
- 20.Chang V. Y., Basso G., Sakamoto K. M., Nelson S. F. (2013) Identification of somatic and germline mutations using whole exome sequencing of congenital acute lymphoblastic leukemia. BMC Cancer 13, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treon S. P., Xu L., Yang G., Zhou Y., Liu X., Cao Y., Sheehy P., Manning R. J., Patterson C. J., Tripsas C., Arcaini L., Pinkus G. S., Rodig S. J., Sohani A. R., Harris N. L., Laramie J. M., Skifter D. A., Lincoln S. E., Hunter Z. R. (2012) MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N. Engl. J. Med. 367, 826–833. [DOI] [PubMed] [Google Scholar]
- 22.Treon S. P., Tripsas C. K., Meid K., Warren D., Varma G., Green R., Argyropoulos K. V., Yang G., Cao Y., Xu L., Patterson C. J., Rodig S., Zehnder J. L., Aster J. C., Harris N. L., Kanan S., Ghobrial I., Castillo J. J., Laubach J. P., Hunter Z. R., Salman Z., Li J., Cheng M., Clow F., Graef T., Palomba M. L., Advani R. H. (2015) Ibrutinib in previously treated Waldenström’s macroglobulinemia. N. Engl. J. Med. 372, 1430–1440. [DOI] [PubMed] [Google Scholar]
- 23.Urnov F. D., Rebar E. J., Holmes M. C., Zhang H. S., Gregory P. D. (2010) Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 11, 636–646. [DOI] [PubMed] [Google Scholar]
- 24.Joung J. K., Sander J. D. (2013) TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sander J. D., Joung J. K. (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fessler M. B., Malcolm K. C., Duncan M. W., Worthen G. S. (2002) A genomic and proteomic analysis of activation of the human neutrophil by lipopolysaccharide and its mediation by p38 mitogen-activated protein kinase. J. Biol. Chem. 277, 31291–31302. [DOI] [PubMed] [Google Scholar]
- 27.Luerman G. C., Uriarte S. M., Rane M. J., McLeish K. R. (2010) Application of proteomics to neutrophil biology. J. Proteomics 73, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sintsova A., Sarantis H., Islam E. A., Sun C. X., Amin M., Chan C. H., Stanners C. P., Glogauer M., Gray-Owen S. D. (2014) Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog. 10, e1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLeish K. R., Merchant M. L., Klein J. B., Ward R. A. (2013) Technical note: proteomic approaches to fundamental questions about neutrophil biology. J. Leukoc. Biol. 94, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. (1979) Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J. Exp. Med. 149, 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauert A. B., Martinelli S., Marone C., Niggli V. (2002) Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis. Int. J. Biochem. Cell Biol. 34, 838–854. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F., Gallo R. (1979) Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 54, 713–733. [PubMed] [Google Scholar]
- 33.Hamza B., Wong E., Patel S., Cho H., Martel J., Irimia D. (2014) Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr. Biol. (Camb). 6, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence M. B., McIntire L. V., Eskin S. G. (1987) Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood 70, 1284–1290. [PubMed] [Google Scholar]
- 35.Chen H. C. (2005) Boyden chamber assay. Methods Mol. Biol. 294, 15–22. [DOI] [PubMed] [Google Scholar]
- 36.White-Owen C., Alexander J. W., Sramkoski R. M., Babcock G. F. (1992) Rapid whole-blood microassay using flow cytometry for measuring neutrophil phagocytosis. J. Clin. Microbiol. 30, 2071–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjerknes R. (1984) Flow cytometric assay for combined measurement of phagocytosis and intracellular killing of Candida albicans. J. Immunol. Methods 72, 229–241. [DOI] [PubMed] [Google Scholar]
- 38.Furie M. B., Naprstek B. L., Silverstein S. C. (1987) Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J. Cell Sci. 88, 161–175. [DOI] [PubMed] [Google Scholar]
- 39.Zicha D., Dunn G. A., Brown A. F. (1991) A new direct-viewing chemotaxis chamber. J. Cell Sci. 99, 769–775. [DOI] [PubMed] [Google Scholar]
- 40.Zigmond S. H. (1977) Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol. 75, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Servant G., Weiner O. D., Herzmark P., Balla T., Sedat J. W., Bourne H. R. (2000) Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keenan T. M., Folch A. (2008) Biomolecular gradients in cell culture systems. Lab Chip 8, 34–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denais C. M., Gilbert R. M., Isermann P., McGregor A. L., te Lindert M., Weigelin B., Davidson P. M., Friedl P., Wolf K., Lammerding J. (2016) Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley S. O., Mirkin C. A., Walt D. R., Ismagilov R. F., Toner M., Sargent E. H. (2014) Advancing the speed, sensitivity and accuracy of biomolecular detection using multi-length-scale engineering. Nat. Nanotechnol. 9, 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millet L. J., Gillette M. U. (2012) New perspectives on neuronal development via microfluidic environments. Trends Neurosci. 35, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crunkhorn S. (2016) Respiratory diseases: lung airway-on-a-chip. Nat. Rev. Drug Discov. 15, 86.26837593 [Google Scholar]
- 47.Wessel A. K., Hmelo L., Parsek M. R., Whiteley M. (2013) Going local: technologies for exploring bacterial microenvironments. Nat. Rev. Microbiol. 11, 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duncombe T. A., Tentori A. M., Herr A. E. (2015) Microfluidics: reframing biological enquiry. Nat. Rev. Mol. Cell Biol. 16, 554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S., Kim H. J., Jeon N. L. (2010) Biological applications of microfluidic gradient devices. Integr. Biol. (Camb). 2, 584–603. [DOI] [PubMed] [Google Scholar]
- 50.Wu J., Wu X., Lin F. (2013) Recent developments in microfluidics-based chemotaxis studies. Lab Chip 13, 2484–2499. [DOI] [PubMed] [Google Scholar]
- 51.Guo M. T., Rotem A., Heyman J. A., Weitz D. A. (2012) Droplet microfluidics for high-throughput biological assays. Lab Chip 12, 2146–2155. [DOI] [PubMed] [Google Scholar]
- 52.Sundd P., Pospieszalska M. K., Cheung L. S., Konstantopoulos K., Ley K. (2011) Biomechanics of leukocyte rolling. Biorheology 48, 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidtke D. W., Diamond S. L. (2000) Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J. Cell Biol. 149, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sundd P., Gutierrez E., Pospieszalska M. K., Zhang H., Groisman A., Ley K. (2010) Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling. Nat. Methods 7, 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundd P., Gutierrez E., Koltsova E. K., Kuwano Y., Fukuda S., Pospieszalska M. K., Groisman A., Ley K. (2012) ‘Slings’ enable neutrophil rolling at high shear. Nature 488, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmid-Schönbein G. W. (1987) Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed. Proc. 46, 2397–2401. [PubMed] [Google Scholar]
- 57.Akenhead M. L., Horrall N. M., Rowe D., Sethu P., Shin H. Y. (2015) In vitro evaluation of the link between cell activation state and its rheological impact on the microscale flow of neutrophil suspensions. J. Biomech. Eng. 137, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dominical V. M., Vital D. M., O’Dowd F., Saad S. T., Costa F. F., Conran N. (2015) In vitro microfluidic model for the study of vaso-occlusive processes. Exp. Hematol. 43, 223–228. [DOI] [PubMed] [Google Scholar]
- 59.Schaff U. Y., Xing M. M., Lin K. K., Pan N., Jeon N. L., Simon S. I. (2007) Vascular mimetics based on microfluidics for imaging the leukocyte–endothelial inflammatory response. Lab Chip 7, 448–456. [DOI] [PubMed] [Google Scholar]
- 60.Gabriele S., Benoliel A. M., Bongrand P., Théodoly O. (2009) Microfluidic investigation reveals distinct roles for actin cytoskeleton and myosin II activity in capillary leukocyte trafficking. Biophys. J. 96, 4308–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bose S., Singh R., Hanewich-Hollatz M., Shen C., Lee C. H., Dorfman D. M., Karp J. M., Karnik R. (2013) Affinity flow fractionation of cells via transient interactions with asymmetric molecular patterns. Sci. Rep. 3, 2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agrawal N., Toner M., Irimia D. (2008) Neutrophil migration assay from a drop of blood. Lab Chip 8, 2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sackmann E. K., Berthier E., Young E. W., Shelef M. A., Wernimont S. A., Huttenlocher A., Beebe D. J. (2012) Microfluidic kit-on-a-lid: a versatile platform for neutrophil chemotaxis assays. Blood 120, e45–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sackmann E. K., Berthier E., Schwantes E. A., Fichtinger P. S., Evans M. D., Dziadzio L. L., Huttenlocher A., Mathur S. K., Beebe D. J. (2014) Characterizing asthma from a drop of blood using neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA 111, 5813–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng X., Irimia D., Dixon M., Sekine K., Demirci U., Zamir L., Tompkins R. G., Rodriguez W., Toner M. (2007) A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects. Lab Chip 7, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vu T., Rahimian A., Stybayeva G., Gao Y., Kwa T., Van de Water J., Revzin A. (2015) Reconfigurable microfluidic device with integrated antibody arrays for capture, multiplexed stimulation, and cytokine profiling of human monocytes. Biomicrofluidics 9, 044115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han S., Yan J. J., Shin Y., Jeon J. J., Won J., Jeong H. E., Kamm R. D., Kim Y. J., Chung S. (2012) A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab Chip 12, 3861–3865. [DOI] [PubMed] [Google Scholar]
- 68.Cho H., Seo J. H., Wong K. H., Terasaki Y., Park J., Bong K., Arai K., Lo E. H., Irimia D. (2015) Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 5, 15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huh D., Matthews B. D., Mammoto A., Montoya-Zavala M., Hsin H. Y., Ingber D. E. (2010) Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huh D., Kim H. J., Fraser J. P., Shea D. E., Khan M., Bahinski A., Hamilton G. A., Ingber D. E. (2013) Microfabrication of human organs-on-chips. Nat. Protoc. 8, 2135–2157. [DOI] [PubMed] [Google Scholar]
- 71.Lee J. S., Romero R., Han Y. M., Kim H. C., Kim C. J., Hong J. S., Huh D. (2016) Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J. Matern. Fetal Neonatal Med. 29, 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baggiolini M., Clark-Lewis I. (1992) Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 307, 97–101. [DOI] [PubMed] [Google Scholar]
- 73.Afonso P. V., Janka-Junttila M., Lee Y. J., McCann C. P., Oliver C. M., Aamer K. A., Losert W., Cicerone M. T., Parent C. A. (2012) LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Jeon N., Baskaran H., Dertinger S. K., Whitesides G. M., Van de Water L., Toner M. (2002) Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat. Biotechnol. 20, 826–830. [DOI] [PubMed] [Google Scholar]
- 75.Lin F., Nguyen C. M., Wang S. J., Saadi W., Gross S. P., Jeon N. L. (2004) Effective neutrophil chemotaxis is strongly influenced by mean IL-8 concentration. Biochem. Biophys. Res. Commun. 319, 576–581. [DOI] [PubMed] [Google Scholar]
- 76.Tharp W. G., Yadav R., Irimia D., Upadhyaya A., Samadani A., Hurtado O., Liu S. Y., Munisamy S., Brainard D. M., Mahon M. J., Nourshargh S., van Oudenaarden A., Toner M. G., Poznansky M. C. (2006) Neutrophil chemorepulsion in defined interleukin-8 gradients in vitro and in vivo. J. Leukoc. Biol. 79, 539–554. [DOI] [PubMed] [Google Scholar]
- 77.Irimia D., Liu S. Y., Tharp W. G., Samadani A., Toner M., Poznansky M. C. (2006) Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab Chip 6, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irimia D., Charras G., Agrawal N., Mitchison T., Toner M. (2007) Polar stimulation and constrained cell migration in microfluidic channels. Lab Chip 7, 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf K., Te Lindert M., Krause M., Alexander S., Te Riet J., Willis A. L., Hoffman R. M., Figdor C. G., Weiss S. J., Friedl P. (2013) Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 201, 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lämmermann T., Bader B. L., Monkley S. J., Worbs T., Wedlich-Söldner R., Hirsch K., Keller M., Förster R., Critchley D. R., Fässler R., Sixt M. (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55. [DOI] [PubMed] [Google Scholar]
- 81.Yamahashi Y., Cavnar P. J., Hind L. E., Berthier E., Bennin D. A., Beebe D., Huttenlocher A. (2015) Integrin associated proteins differentially regulate neutrophil polarity and directed migration in 2D and 3D. Biomed. Microdevices 17, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ambravaneswaran V., Wong I. Y., Aranyosi A. J., Toner M., Irimia D. (2010) Directional decisions during neutrophil chemotaxis inside bifurcating channels. Integr. Biol. (Camb). 2, 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butler K. L., Ambravaneswaran V., Agrawal N., Bilodeau M., Toner M., Tompkins R. G., Fagan S., Irimia D. (2010) Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS One 5, e11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurihara T., Jones C. N., Yu Y. M., Fischman A. J., Watada S., Tompkins R. G., Fagan S. P., Irimia D. (2013) Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 27, 2270–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones C. N., Dalli J., Dimisko L., Wong E., Serhan C. N., Irimia D. (2012) Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc. Natl. Acad. Sci. USA 109, 20560–20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobelli J., Friedman R. S., Conti M. A., Lennon-Dumenil A. M., Piel M., Sorensen C. M., Adelstein R. S., Krummel M. F. (2010) Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat. Immunol. 11, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jain N. G., Wong E. A., Aranyosi A. J., Boneschansker L., Markmann J. F., Briscoe D. M., Irimia D. (2015) Microfluidic mazes to characterize T-cell exploration patterns following activation in vitro. Integr. Biol. (Camb). 7, 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Renkawitz J., Schumann K., Weber M., Lämmermann T., Pflicke H., Piel M., Polleux J., Spatz J. P., Sixt M. (2009) Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol. 11, 1438–1443. [DOI] [PubMed] [Google Scholar]
- 89.Boneschansker L., Yan J., Wong E., Briscoe D. M., Irimia D. (2014) Microfluidic platform for the quantitative analysis of leukocyte migration signatures. Nat. Commun. 5, 4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandez M. I., Heuzé M. L., Martinez-Cingolani C., Volpe E., Donnadieu M. H., Piel M., Homey B., Lennon-Duménil A. M., Soumelis V. (2011) The human cytokine TSLP triggers a cell-autonomous dendritic cell migration in confined environments. Blood 118, 3862–3869. [DOI] [PubMed] [Google Scholar]
- 91.Kim D., Haynes C. L. (2012) Neutrophil chemotaxis within a competing gradient of chemoattractants. Anal. Chem. 84, 6070–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heit B., Tavener S., Raharjo E., Kubes P. (2002) An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foxman E. F., Campbell J. J., Butcher E. C. (1997) Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 139, 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Byrne M. B., Kimura Y., Kapoor A., He Y., Mattam K. S., Hasan K. M., Olson L. N., Wang F., Kenis P. J., Rao C. V. (2014) Oscillatory behavior of neutrophils under opposing chemoattractant gradients supports a winner-take-all mechanism. PLoS One 9, e85726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cho H., Hamza B., Wong E. A., Irimia D. (2014) On-demand, competing gradient arrays for neutrophil chemotaxis. Lab Chip 14, 972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keenan T. M., Hsu C.-H., Folch A. (2006) Microfluidic “jets” for generating steady-state gradients of soluble molecules on open surfaces. Appl. Phys. Lett. 89, 114103. [Google Scholar]
- 97.Keenan T. M., Frevert C. W., Wu A., Wong V., Folch A. (2010) A new method for studying gradient-induced neutrophil desensitization based on an open microfluidic chamber. Lab Chip 10, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Skoge M., Yue H., Erickstad M., Bae A., Levine H., Groisman A., Loomis W. F., Rappel W. J. (2014) Cellular memory in eukaryotic chemotaxis. Proc. Natl. Acad. Sci. USA 111, 14448–14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herzmark P., Campbell K., Wang F., Wong K., El-Samad H., Groisman A., Bourne H. R. (2007) Bound attractant at the leading vs. the trailing edge determines chemotactic prowess. Proc. Natl. Acad. Sci. USA 104, 13349–13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aranyosi A. J., Wong E. A., Irimia D. (2015) A neutrophil treadmill to decouple spatial and temporal signals during chemotaxis. Lab Chip 15, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jannat R. A., Dembo M., Hammer D. A. (2011) Traction forces of neutrophils migrating on compliant substrates. Biophys. J. 101, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beningo K. A., Dembo M., Kaverina I., Small J. V., Wang Y. L. (2001) Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153, 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vargas P., Maiuri P., Bretou M., Sáez P. J., Pierobon P., Maurin M., Chabaud M., Lankar D., Obino D., Terriac E., Raab M., Thiam H. R., Brocker T., Kitchen-Goosen S. M., Alberts A. S., Sunareni P., Xia S., Li R., Voituriez R., Piel M., Lennon-Duménil A. M. (2016) Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat. Cell Biol. 18, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J., Zhu L., Zhang M., Lin F. (2012) Microfluidic device for studying cell migration in single or co-existing chemical gradients and electric fields. Biomicrofluidics 6, 24121-1–24121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li J., Nandagopal S., Wu D., Romanuik S. F., Paul K., Thomson D. J., Lin F. (2011) Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices. Lab Chip 11, 1298–1304. [DOI] [PubMed] [Google Scholar]
- 106.Ortega-Gómez A., Perretti M., Soehnlein O. (2013) Resolution of inflammation: an integrated view. EMBO Mol. Med. 5, 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. (1983) A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 65, 109–121. [DOI] [PubMed] [Google Scholar]
- 108.Faley S., Seale K., Hughey J., Schaffer D. K., VanCompernolle S., McKinney B., Baudenbacher F., Unutmaz D., Wikswo J. P. (2008) Microfluidic platform for real-time signaling analysis of multiple single T cells in parallel. Lab Chip 8, 1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mahmood S., Nandagopal S., Sow I., Lin F., Kung S. K. (2014) Microfluidic-based, live-cell analysis allows assessment of NK-cell migration in response to crosstalk with dendritic cells. Eur. J. Immunol. 44, 2737–2748. [DOI] [PubMed] [Google Scholar]
- 110.Guldevall K., Vanherberghen B., Frisk T., Hurtig J., Christakou A. E., Manneberg O., Lindström S., Andersson-Svahn H., Wiklund M., Önfelt B. (2010) Imaging immune surveillance of individual natural killer cells confined in microwell arrays. PLoS One 5, e15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Forslund E., Guldevall K., Olofsson P. E., Frisk T., Christakou A. E., Wiklund M., Onfelt B. (2012) Novel microchip-based tools facilitating live cell imaging and assessment of functional heterogeneity within NK cell populations. Front. Immunol. 3, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boukouris S., Mathivanan S. (2015) Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin. Appl. 9, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mantel P. Y., Hoang A. N., Goldowitz I., Potashnikova D., Hamza B., Vorobjev I., Ghiran I., Toner M., Irimia D., Ivanov A. R., Barteneva N., Marti M. (2013) Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Antia M., Herricks T., Rathod P. K. (2007) Microfluidic modeling of cell-cell interactions in malaria pathogenesis. PLoS Pathog. 3, e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gopalakrishnan N., Hannam R., Casoni G. P., Barriet D., Ribe J. M., Haug M., Halaas Ø. (2015) Infection and immunity on a chip: a compartmentalised microfluidic platform to monitor immune cell behaviour in real time. Lab Chip 15, 1481–1487. [DOI] [PubMed] [Google Scholar]
- 116.Berthier E., Lim F. Y., Deng Q., Guo C. J., Kontoyiannis D. P., Wang C. C., Rindy J., Beebe D. J., Huttenlocher A., Keller N. P. (2013) Low-volume toolbox for the discovery of immunosuppressive fungal secondary metabolites. PLoS Pathog. 9, e1003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Banchereau J., Steinman R. M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 118.Unanue E. R. (1984) Antigen-presenting function of the macrophage. Annu. Rev. Immunol. 2, 395–428. [DOI] [PubMed] [Google Scholar]
- 119.Chabaud M., Heuzé M. L., Bretou M., Vargas P., Maiuri P., Solanes P., Maurin M., Terriac E., Le Berre M., Lankar D., Piolot T., Adelstein R. S., Zhang Y., Sixt M., Jacobelli J., Bénichou O., Voituriez R., Piel M., Lennon-Duménil A. M. (2015) Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells. Nat. Commun. 6, 7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faure-André G., Vargas P., Yuseff M. I., Heuzé M., Diaz J., Lankar D., Steri V., Manry J., Hugues S., Vascotto F., Boulanger J., Raposo G., Bono M. R., Rosemblatt M., Piel M., Lennon-Duménil A. M. (2008) Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science 322, 1705–1710. [DOI] [PubMed] [Google Scholar]
- 121.Koria P., Bhushan A., Irimia D., Yarmush M. L. (2012) Microfluidic device for examining directional sensing in dendritic cell chemotaxis. Nano Life 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jones C. N., Dimisko L., Forrest K., Judice K., Poznansky M. C., Markmann J. F., Vyas J. M., Irimia D. (2015) Human neutrophils are primed by chemoattractant gradients for blocking the growth of Aspergillus fumigatus. J. Infect. Dis. 213, 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamza B., Irimia D. (2015) Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab Chip 15, 2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boneschansker L., Inoue Y., Oklu R., Irimia D. (2016) Capillary plexuses are vulnerable to neutrophil extracellular traps. Integr. Biol. (Camb). 8, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moussavi-Harami S. F., Mladinich K. M., Sackmann E. K., Shelef M. A., Starnes T. W., Guckenberger D. J., Huttenlocher A., Beebe D. J. (2016) Microfluidic device for simultaneous analysis of neutrophil extracellular traps and production of reactive oxygen species. Integr. Biol. (Camb). 8, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197. [DOI] [PubMed] [Google Scholar]
- 127.Heit B., Liu L., Colarusso P., Puri K. D., Kubes P. (2008) PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J. Cell Sci. 121, 205–214. [DOI] [PubMed] [Google Scholar]
- 128.Malawista S. E., de Boisfleury Chevance A. (1997) Random locomotion and chemotaxis of human blood polymorphonuclear leukocytes (PMN) in the presence of EDTA: PMN in close quarters require neither leukocyte integrins nor external divalent cations. Proc. Natl. Acad. Sci. USA 94, 11577–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malawista S. E., De Boisfleury Chevance A. (1982) The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J. Cell Biol. 95, 960–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoo S. K., Deng Q., Cavnar P. J., Wu Y. I., Hahn K. M., Huttenlocher A. (2010) Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 18, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holmes G. R., Dixon G., Anderson S. R., Reyes-Aldasoro C. C., Elks P. M., Billings S. A., Whyte M. K., Kadirkamanathan V., Renshaw S. A. (2012) Drift-diffusion analysis of neutrophil migration during inflammation resolution in a zebrafish model. Adv. Hematol. 2012, 792163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hawkins P. T., Stephens L. R., Suire S., Wilson M. (2010) PI3K signaling in neutrophils. Curr. Top. Microbiol. Immunol. 346, 183–202. [DOI] [PubMed] [Google Scholar]
- 133.Robertson A. L., Holmes G. R., Bojarczuk A. N., Burgon J., Loynes C. A., Chimen M., Sawtell A. K., Hamza B., Willson J., Walmsley S. R., Anderson S. R., Coles M. C., Farrow S. N., Solari R., Jones S., Prince L. R., Irimia D., Rainger G. E., Kadirkamanathan V., Whyte M. K., Renshaw S. A. (2014) A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci. Transl. Med. 6, 225ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iwasaki A., Medzhitov R. (2010) Regulation of adaptive immunity by the innate immune system. Science 327, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Irwin M. R., Cole S. W. (2011) Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harbord M. W., Marks D. J., Forbes A., Bloom S. L., Day R. M., Segal A. W. (2006) Impaired neutrophil chemotaxis in Crohn’s disease relates to reduced production of chemokines and can be augmented by granulocyte-colony stimulating factor. Aliment. Pharmacol. Ther. 24, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Capsoni F., Sarzi-Puttini P., Atzeni F., Minonzio F., Bonara P., Doria A., Carrabba M. (2005) Effect of adalimumab on neutrophil function in patients with rheumatoid arthritis. Arthritis Res. Ther. 7, R250–R255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trellakis S., Bruderek K., Dumitru C. A., Gholaman H., Gu X., Bankfalvi A., Scherag A., Hutte J., Dominas N., Lehnerdt G. F., Hoffmann T. K., Lang S., Brandau S. (2011) Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer 129, 2183–2193. [DOI] [PubMed] [Google Scholar]
- 139.Buchanan D. R., Cromwell O., Kay A. B. (1987) Neutrophil chemotactic activity in acute severe asthma (status asthmaticus). Am. Rev. Respir. Dis. 136, 1397–1402. [DOI] [PubMed] [Google Scholar]
- 140.Jones C. N., Moore M., Dimisko L., Alexander A., Ibrahim A., Hassell B. A., Warren H. S., Tompkins R. G., Fagan S. P., Irimia D. (2014) Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS One 9, e114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu J., Hillier C., Komenda P., Lobato de Faria R., Levin D., Zhang M., Lin F. (2015) A microfluidic platform for evaluating neutrophil chemotaxis induced by sputum from COPD patients. PLoS One 10, e0126523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Walsh D. I. III, Lalli M. L., Kassas J. M., Asthagiri A. R., Murthy S. K. (2015) Cell chemotaxis on paper for diagnostics. Anal. Chem. 87, 5505–5510. [DOI] [PubMed] [Google Scholar]
- 143.Moussavi-Harami S. F., Pezzi H. M., Huttenlocher A., Beebe D. J. (2015) Simple microfluidic device for studying chemotaxis in response to dual gradients. Biomed. Microdevices 17, 9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jones C. N., Hoang A. N., Dimisko L., Hamza B., Martel J., Irimia D. (2014) Microfluidic platform for measuring neutrophil chemotaxis from unprocessed whole blood. J. Vis. Exp. e51215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoang A. N., Jones C. N., Dimisko L., Hamza B., Martel J., Kojic N., Irimia D. (2013) Measuring neutrophil speed and directionality during chemotaxis, directly from a droplet of whole blood. Technology (Singap World Sci). 1, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malawista S., de Boisfleury C. A. (1998) [Functions of neutrophil motility of human blood: locomotion and chemotaxis in simplified systems]. Bull. Acad. Natl. Med. 182, 1011–1021; discussion 1022–1014. [PubMed] [Google Scholar]
- 147.Zhang Y. G., Wu S., Xia Y., Sun J. (2014) Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol. Rep. 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giese C., Lubitz A., Demmler C. D., Reuschel J., Bergner K., Marx U. (2010) Immunological substance testing on human lymphatic micro-organoids in vitro. J. Biotechnol. 148, 38–45. [DOI] [PubMed] [Google Scholar]
- 149.Lamberti G., Soroush F., Smith A., Kiani M. F., Prabhakarpandian B., Pant K. (2015) Adhesion patterns in the microvasculature are dependent on bifurcation angle. Microvasc. Res. 99, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Berthier E., Surfus J., Verbsky J., Huttenlocher A., Beebe D. (2010) An arrayed high-content chemotaxis assay for patient diagnosis. Integr. Biol. (Camb). 2, 630–638. [DOI] [PubMed] [Google Scholar]
- 151.Saadi W., Rhee S. W., Lin F., Vahidi B., Chung B. G., Jeon N. L. (2007) Generation of stable concentration gradients in 2D and 3D environments using a microfluidic ladder chamber. Biomed. Microdevices 9, 627–635. [DOI] [PubMed] [Google Scholar]
- 152.Irimia D. (2014) Cell migration in confined environments. Methods Cell Biol. 121, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prentice-Mott H. V., Meroz Y., Carlson A., Levine M. A., Davidson M. W., Irimia D., Charras G. T., Mahadevan L., Shah J. V. (2016) Directional memory arises from long-lived cytoskeletal asymmetries in polarized chemotactic cells. Proc. Natl. Acad. Sci. USA 113, 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Prentice-Mott H. V., Chang C. H., Mahadevan L., Mitchison T. J., Irimia D., Shah J. V. (2013) Biased migration of confined neutrophil-like cells in asymmetric hydraulic environments. Proc. Natl. Acad. Sci. USA 110, 21006–21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilson K., Lewalle A., Fritzsche M., Thorogate R., Duke T., Charras G. (2013) Mechanisms of leading edge protrusion in interstitial migration. Nat. Commun. 4, 2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McLaughlin L. M., Xu H., Carden S. E., Fisher S., Reyes M., Heilshorn S. C., Monack D. M. (2014) A microfluidic-based genetic screen to identify microbial virulence factors that inhibit dendritic cell migration. Integr. Biol. (Camb). 6, 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kasuga K., Yang R., Porter T. F., Agrawal N., Petasis N. A., Irimia D., Toner M., Serhan C. N. (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 181, 8677–8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang R., Fredman G., Krishnamoorthy S., Agrawal N., Irimia D., Piomelli D., Serhan C. N. (2011) Decoding functional metabolomics with docosahexaenoyl ethanolamide (DHEA) identifies novel bioactive signals. J. Biol. Chem. 286, 31532–31541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oh S. F., Dona M., Fredman G., Krishnamoorthy S., Irimia D., Serhan C. N. (2012) Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 188, 4527–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang B., Bates M., Zhuang X. (2009) Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 78, 993–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]