Abstract
Climate change may affect the internal defense system (IDS) of freshwater snails, and as a result their capacity to transmit disease. We examined effects of short-term exposure to supra- and sub-optimal temperatures or starvation on 3 parameters of the IDS of the schistosome-resistant Salvador strain of Biomphalaria glabrata — hemocyte concentrations, cell division in the amebocyte-producing organ (APO), and resistance to infection with Schistosoma mansoni. Adult snails were exposed to 1 of 3 temperatures, 20 °C, 27 °C (controls), or 33 °C, for 1 or 2 weeks, with food. A fourth group was maintained at 27 °C, but without food. Compared to the controls, starved snails had significantly higher hemocyte counts at both 1 and 2 weeks, although mitotic activity in the APO was significantly lower at both time periods. Exposure to 20 °C or 33 °C for 1 or 2 weeks did not affect hemocyte numbers. However, APO mitotic activity in snails exposed to 20 °C was significantly higher at both 1 and 2 weeks, whereas mitotic activity in snails exposed to 33 °C was significantly lower at 1 week but normal at 2 weeks. None of the treatments altered the resistance phenotype of Salvador snails. In a follow-up experiment, exposure to 33 °C for 4–5 h, a treatment previously reported to both induce expression of heat shock proteins (Hsps) and abrogate resistance to infection, caused immediate upregulation of Hsp 70 and Hsp 90 expression, but did not alter resistance, and Hsp expression levels returned to baseline after 2 weeks at 33 °C. Results of this study indicate that abnormal environmental conditions can have both stimulatory and inhibitory effects on the IDS in adult B. glabrata, and that some degree of acclimation to abnormal temperatures may occur.
Keywords: climate change, internal defense system, hemocyte, hemopoiesis, Mollusca
Graphical abstract
Introduction
Changes in global climate may affect the biology of disease-transmitting snails (Mas-Coma et al., 2009), presumably through effects on water temperature, water chemistry, food availability, and aquatic community structure. Several studies have attempted to predict the impact of climate change on schistosomiasis transmission (reviewed by McCreesh and Booth, 2013), and using an agent-based model incorporating effects of increasing temperature on both the snail host and larval parasite, McCreesh et al. (2015) have hypothesized that an increase in prevalence and intensity of schistosomiasis may occur in some parts of eastern Africa over the next several decades.
In Biomphalaria glabrata, an intermediate host of Schistosoma mansoni in the New World, a potential effect of climate change that could impact disease transmission is alteration of the immune or internal defense system (IDS), which largely determines the outcome of invasion by a larval trematode. The IDS of B. glabrata consists of both phagocytic hemocytes and plasma immune factors, and in incompatible B. glabrata–S. mansoni relationships, snail hemocytes recognize, then rapidly encapsulate and kill schistosome sporocysts (reviewed by Coustau et al., 2015). Hemocyte production occurs in a region of the anterior pericardial wall called the amebocyte-producing organ (APO) (Lie et al., 1975; Pila et al., 2016b), and is stimulated by an endogenous growth factor, B. glabrata granulin (Pila et al., 2016a). Hypothetically, environmentally-induced physiological stress could affect hemocytes, plasma factors, or hemopoiesis in the APO, and as a result host vector competence, making B. glabrata either less or more susceptible to infection with S. mansoni as well as microbial snail pathogens. For example, Ittiprasert and Knight (2012) have reported that exposure of 4–6 mm juvenile BS-90 (schistosome-resistant) B. glabrata to high temperature (32 °C) for 4 or more h causes upregulated expression of heat shock proteins (Hsps) 70 and 90. Moreover, this heat treatment abrogates resistance to infection with S. mansoni, apparently due to increased expression of Hsp 90, as shown by reversal of this effect by geldanamycin, an inhibitor of Hsp 90.
The concentration of circulating phagocytic leukocytes is a commonly measured indicator of immune status in animals. For example, in humans prolonged and very low blood concentrations of neutrophils (chronic severe neutropenia) is associated with increased risk of microbial infection, and among several extrinsic causes of this condition are certain types of nutritional deficiencies that impair hemopoiesis (Newburger and Dale, 2013). In marine bivalves, a large number of studies have investigated effects of abnormal environmental conditions, e.g., toxicants, temperature, salinity, and starvation, on hemocyte concentrations, as well as on other parameters of the IDS, including susceptibility to infection with pathogens (partially reviewed by Raftos et al., 2014). Among gastropods, Al-Rawadeh (2010) reported a significant decrease in the number of hemocytes in Helix aspersa after 3 weeks of starvation. Stumpf and Gilbertson (1978) exposed B. glabrata to a range of temperatures, from 12 °C to 36 °C, for 3 days and observed a bell-shaped effect on hemocyte concentrations, with hemocyte numbers increasing from 12 °C to 27 °C and then decreasing at higher temperatures. Suresh et al. (1994) exposed 30 °C-acclimated Lymnaea acuminata and Indoplanorbis exustus to 20 °C, 25 °C, 35 °C, and 40 °C for up to 24 h, and found elevated cell counts at 20 °C and 25 °C after 2 and 12 h in I. exustus and at all time periods in L. acuminata. The highest temperature did not affect hemocyte numbers in I. exustus but in L. acuminata caused an increase after 2 h of exposure and a sharp decrease at 24 h. Hypothetically, the observed changes in hemocyte numbers could result from direct effects on hemocyte survival and/or distribution between hemolymph and tissues, as well as effects on hemocyte production.
The hypothesis for this study is that short-term abnormal environmental conditions will cause physiological stress, broadly defined as a condition that challenges homeostasis (Kagias et al., 2012), and that such stress may affect the IDS of B. glabrata. Relative to “normal” and “abnormal” environmental conditions, B. glabrata, which is endemic to South America, has been raised in the laboratory at temperatures ranging from 20 to 29 °C (Bruce et al., 1971), with an optimal temperature in the range of 24–28 °C (Eveland and Haseeb, 2011). Survival is limited to several days at 7 °C and only 4 h at 42 °C (Maldonado, 1967). Freshwater pulmonate snails normally feed on algae (Palmieri et al., 1978) and decaying plant matter (Rollinson, 2011), but in the laboratory B. glabrata grows and reproduces well on a diet of Romaine lettuce leaves. Young adult snails (10.1–13 mm, shell diameter) are able to survive without food for at least 22 days, although their O2 consumption declines significantly (van Aardt et al., 2003).
In this study, we examined effects of 1- and 2-week exposures to supra- and sub-optimal temperatures or starvation on 3 parameters of the IDS of the schistosome-resistant Salvador (BS-90) strain of B. glabrata, i.e., hemocyte concentrations, mitotic activity in the APO as a measure of hemocyte production, and resistance to infection with S. mansoni.
1. Materials and Methods
2.1. Snails
Schistosome-resistant Salvador (Paraense and Correa, 1963) and schistosome-susceptible M-line (Newton, 1955) Biomphalaria glabrata were reared in aerated aquaria at room temperature, which varied from 22–25 °C during this study, and were fed a diet of Romaine lettuce. Because acquiring sufficient hemolymph from small snails may require pooling samples (Jeong et al., 1980), adult snails, measuring 10.5–13 mm, were used for all experiments in order to obtain individual hemocyte counts.
2.2. Miracidia
Livers were removed from mice experimentally infected with the NMRI strain of Schistosoma mansoni at the Biomedical Research Institute (Rockville, MD), chilled with refrigerant gel cold packs, and shipped overnight to the University of San Francisco (USF). Upon receipt of the livers, miracidia were harvested by a previously described method (Sullivan and Richards, 1981).
2.3. Exposure to different environmental conditions
Salvador snails were individually exposed in 500-ml jars in incubators maintained at 20 °C, 27 °C, or 33 °C for 1 or 2 weeks, with food. Incubators were not equipped with illumination, and consequently snails were exposed in the dark. Water was brought to the appropriate temperature before snails were added. A fourth group was maintained at 27 °C, but without food. A total of 30–31 snails were used for each treatment. For this study, 27 °C with food was considered the normal or control environmental condition.
2.4. Hemocyte and mitotic figure counts
After 1 or 2 weeks of exposure to each treatment, a total hemocyte count was obtained for each snail by counting all adherent cells in a 2-µl sample of hemolymph, as described previously (Sullivan et al., 2016). The bleeding technique involved puncturing the body wall through a hole made in the shell overlying the ventral side of the digestive gland and collecting hemolymph that welled up in the shell depression (Jeong et al., 1980). After snails were bled for hemocyte counts, the pericardial sac was dissected, fixed in 1/3-strength Bouin's fluid for at least 24 h, before being dehydrated in an isopropanol-xylene series and embedded in paraffin. Tissues were then serially sectioned at 7 µm. Sections were mounted on microscope slides, and stained with Delafield’s hematoxylin and eosin. Rows of stained sections were scanned at 100× to locate the section that appeared to possess the most hemopoietic tissue (identifiable by its location and staining), and then mitotic activity was estimated by counting total numbers of mitotic figures in that section and the 2 sections on either side at 1,000× (5 sections/APO). Counts were obtained for 25–30 APOs from each treatment.
2.5. Exposure to miracidia
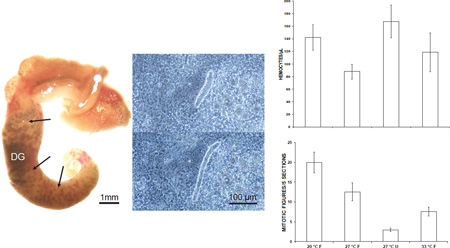
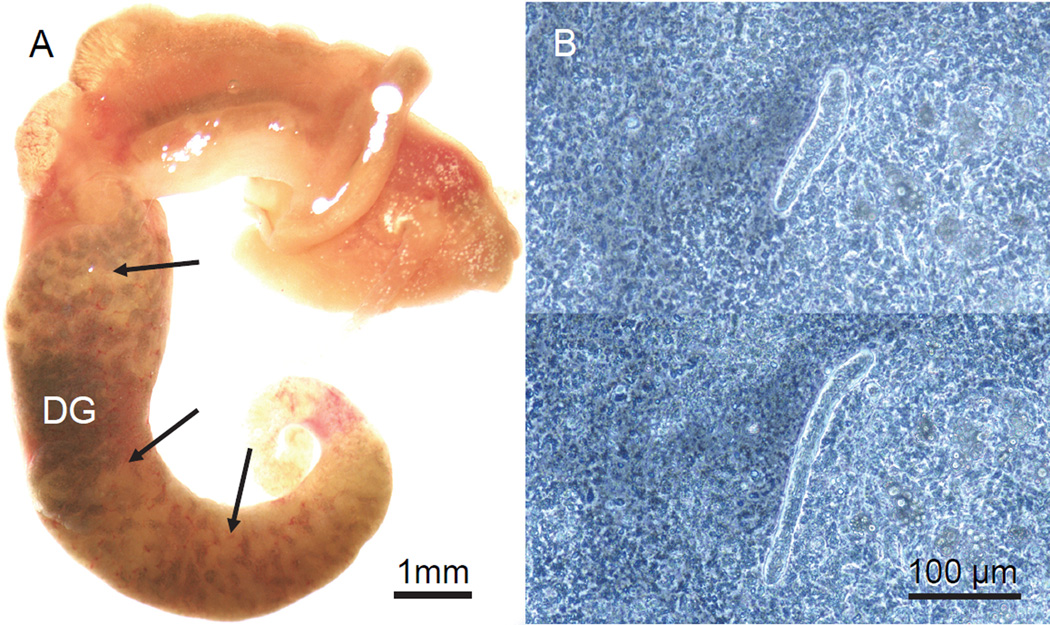
Groups of 11–15 adult B. glabrata, pooled in 2 L of water in covered plastic rectangular containers, were subjected to the above 4 treatments for 1 or 2 weeks, and were then individually challenged with 25 miracidia of S. mansoni. Incubations were set up such that 1-and 2-week treatment groups were exposed simultaneously to the same batch of miracidia. This experiment was conducted on 3 separate occasions, using 3 different batches of miracidia. Infection controls consisted of 3 groups of 15 schistosome-susceptible M-line strain adult snails that had been reared in aquaria prior to miracidial challenge. Two of the 3 control groups were exposed simultaneously with the experimental groups; the third control group was exposed to a separate batch of miracidia. Snails were exposed to miracidia overnight at room temperature in 4 ml of water in 35-mm plastic Petri dishes immediately upon removal from the treatment temperature. Unlike the procedure of Ittiprasert and Knight (2012), we did not incubate the snails overnight in distilled water containing ampicillin beforehand. Following exposure to miracidia, snails were maintained at 27 °C with food, again in the dark, until they were dissected and assessed for the presence of daughter sporocysts. For assessing infection status, 2 methods were employed. M-line snails, and Salvador snails exposed to 27 °C, 20 °C, or starvation, were dissected from their shells, and the digestive gland was observed with a dissecting microscope for folded, cream-colored daughter sporocysts, which usually are apparent in susceptible snails by 21 days post exposure (DPE) to miracidia (Fig. 1A). In addition, all Salvador snails exposed to 33 °C, as well as M-line snails showing no external signs of infection in the digestive gland, were minced into small pieces in 0.3% NaCl, squashed between 2 microscope slides, and examined with a phase contrast microscope at 100× for the presence of motile immature daughter sporocysts (Fig. 1B) (Sullivan and Spence, 1994). A total of 32–36 surviving Salvador snails in each treatment category and 44 M-line snails were examined for infection. All but 3 M-line snails, and approximately 1/3 of Salvador snails in each treatment group were dissected beginning at 21 DPE; the rest were dissected at 28–33 DPE. We used this method because it reliably reveals prepatent infections in susceptible snails when incubated at 27 °C following miracidial exposure (Fig. 1) and avoids the repetitive testing of uninfected snails for cercarial emergence. However, retarded infections such as those described by Lewis et al. (1993), in which secondary sporocysts were first detected in the head foot at up to 10 months after exposure to miracidia, would not be detected by this method.
Figure 1.
Sporocysts of Schistosoma mansoni in Biomphalaria glabrata. A. M-line snail dissected from its shell at 21 days post-exposure (DPE) to miracidia. The digestive gland (DG), which occupies most of the posterior half of the snail and is uniformly dark green in uninfected specimens, is heavily infected with cream-colored masses of secondary sporocysts. This snail also was releasing cercariae prior to dissection. B. Phase contrast images of a motile, immature daughter sporocyst in a squash of head foot tissue from a snail at 22 DPE, showing characteristic extension and retraction of the body.
Once it became apparent that snails maintained at 33°C for 1 or 2 weeks remained uninfected after challenge with miracidia (see below), we decided to employ short-term exposures to elevated temperature, similar to the method of Ittiprasert and Knight (2012). A group of 40 adult snails was incubated at 33 °C for 5–6 h, with food, and then individually exposed to 25 miracidia from a single liver. Snails were assessed for infection by removing them from their shells beginning at 30 DPE and examining the digestive gland with a dissecting microscope as described above.
2.6. Heat shock protein expression
To detect upregulation of heat shock protein expression, adult Salvador snails were incubated at 33 °C in individual 500-ml jars for 4–5 h or 2 weeks with food. After heat treatment, 3 specimens from each group, as well as 3 untreated snails removed from aquaria (controls), were dissected from their shells, snap frozen in liquid N2, ground to a powder, and added to 1 ml TRIzol (Life Technologies) in individual microcentrifuge tubes. Total RNA was isolated according to the manufacturer’s protocol and was reverse transcribed with SuperScript II reverse transcriptase (Invitrogen). cDNA was amplified for both qualitatitive and quantitative RT-PCR using Hsp 90, Hsp 70, and myoglobin gene-specific primers and conditions described previously (Ittiprasert et al., 2009; Ittiprasert and Knight 2012). Real time PCR assays of individual snails were carried out in triplicate, using Brilliant II SYBR Green QRT-PCR Master mix (Stratagene) and a CFX96 Real-Time PCR System (Bio-Rad).
2.7. Statistical analysis
Means of hemocyte concentrations and mitotic figures in snails exposed to 20 °C, 33 °C, and starvation were compared to corresponding means in snails maintained at 27 °C with food (“normal” environmental conditions) with a two-tailed Student’s t test. Fold-differences in expression of Hsp 70 and Hsp 90, normalized for myoglobin expression, in heat shocked snails relative to controls were analyzed for statistical significance by comparing delta Ct values with the Student’s t test (Ittiprasert and Knight, 2012). Differences at P < 0.05 were considered statistically significant.
3. Results
3.1. Hemocyte Counts
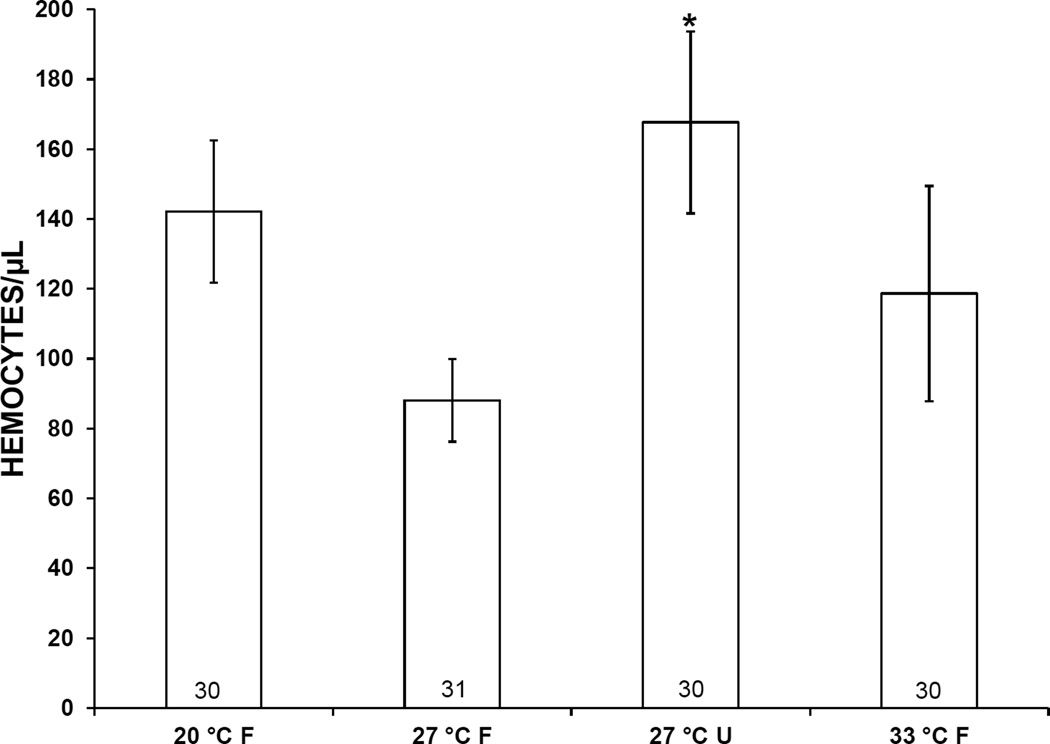
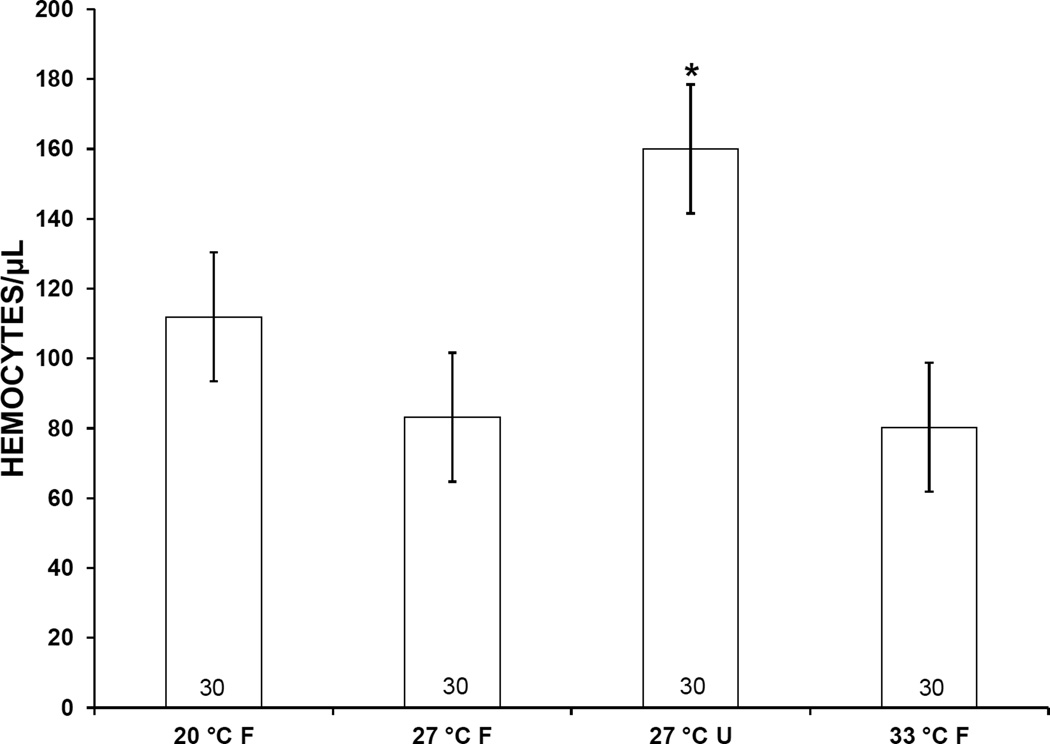
Mean hemocyte counts in snails subjected to 20 °C, 33 °C, or starvation for 1 week were higher than those of controls, i.e., snails incubated at 27 °C with food (Fig. 2). However, this elevation was statistically significant only in starved snails. At 2 weeks, mean hemocyte counts in snails exposed to 20 °C or starvation similarly were higher than those in controls (Fig. 3). Again, a statistically significant difference was observed only in starved snails.
Figure 2.
Concentration of hemocytes in the hemolymph of Biomphalaria glabrata incubated for 1 week at 3 different temperatures and either fed (F) or unfed (U). Error bars indicate standard error. Numbers at bases of bars show sample size. *, mean is significantly different from 27 °C F treatment (P < 0.05).
Figure 3.
Concentration of hemocytes in the hemolymph of Biomphalaria glabrata incubated for 2 weeks at 3 different temperatures and either fed (F) or unfed (U). Error bars indicate standard error. Numbers at bases of bars show sample size. *, mean is significantly different from 27 °C F treatment (P < 0.05).
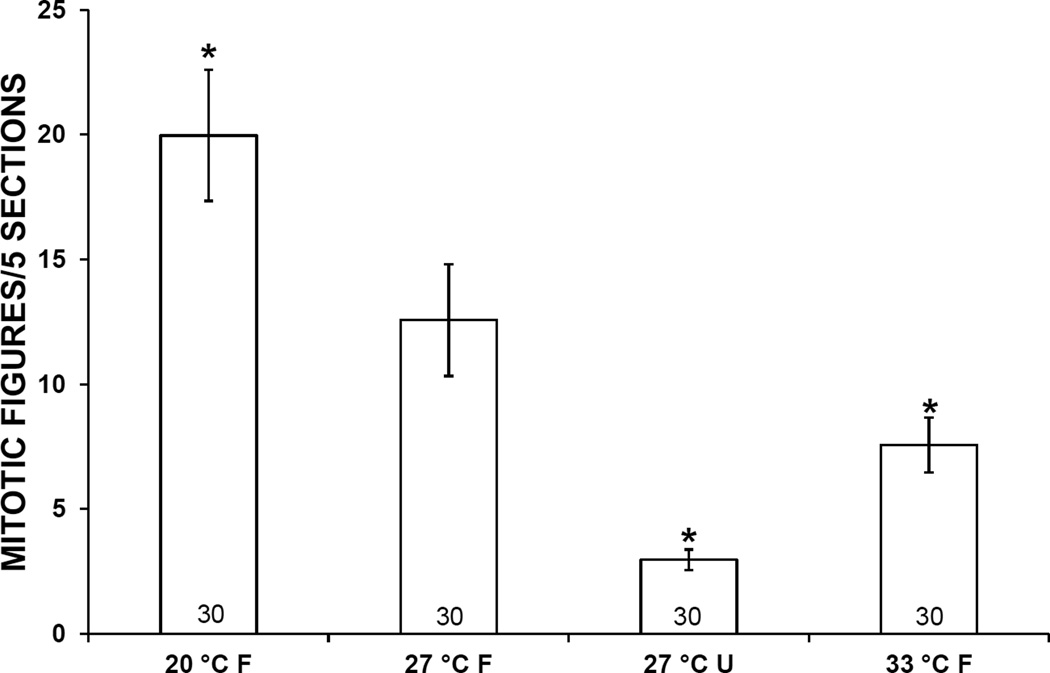
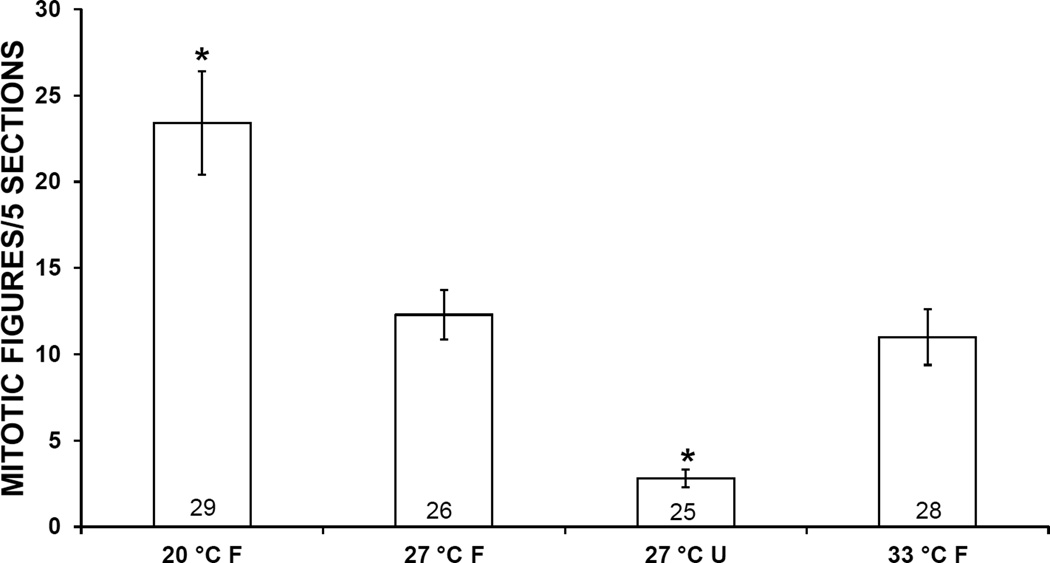
3.2. Mitotic Activity
At both 1 and 2 weeks, mean mitotic activity in the APO of snails exposed to 20 °C was higher than in controls, and mitotic activity in snails exposed to 33 °C or starvation was lower. These differences were statistically significant in snails exposed to 20 °C, 33 °C or starvation for 1 week or to 20 °C or starvation for 2 weeks (Figs. 4, 5)
Figure 4.
Mitotic activity in the amebocyte-producing organ of Biomphalaria glabrata incubated for 1 week at 3 different temperatures and either fed (F) or unfed (U). Error bars indicate standard error. Numbers at bases of bars show sample size. *, mean is significantly different from 27 °C F treatment (P < 0.05).
Figure 5.
Mitotic activity in the amebocyte-producing organ of Biomphalaria glabrata incubated for 2 weeks at 3 different temperatures and either fed (F) or unfed (U). Error bars indicate standard error. Numbers at bases of bars show sample size. *, mean is significantly different from 27 °C F treatment (P < 0.05).
3.3. Schistosome Infection
None of the experimental treatments altered prevalence of infection. Among 317 adult Salvador snails exposed for 1 or 2 weeks to 20 °C, 27 °C, 33 °C, or starvation, or exposed to 33 °C for 5–6 h, prior to challenge with 25 miracidia, only 1 (33 °C × 2 weeks), became infected, versus 36 out of 44 susceptible M-line controls (Table 1). Among the 36 infected M-line snails, 35 showed obvious daughter sporocysts in the digestive gland when removed from the shell and examined with a dissecting microscope between 21 and 33 DPE (Fig. 1A). The remaining infected M-line snail showed no external sign of infection in the digestive gland, but contained motile daughter sporocysts in the head foot when examined by tissue squash at 21 DPE (Fig. 1B).
Table 1.
Prevalence of infection in adult Biomphalaria glabrata incubated at 3 different temperatures, either fed (F) or unfed (U), and then exposed to 25 miracidia each of Schistosoma mansoni. M-line controls were removed from room-temperature aquaria prior to exposure to miracidia.
| Strain | Pre-exposure Treatment |
No. Survivors/ No. Exposed |
No. Infected |
|---|---|---|---|
| M-Line | Control | 44/45 | 36 (82%) |
| Salvador | 27 °C × 1 week (F) | 35/37 | 0 |
| Salvador | 27 °C × 2 weeks (F) | 34/37 | 0 |
| Salvador | 27 °C × 1 week (U) | 35/37 | 0 |
| Salvador | 27 °C × 2 weeks (U) | 35/37 | 0 |
| Salvador | 20 °C × 1 week (F) | 37/37 | 0 |
| Salvador | 20 °C × 2 weeks (F) | 33/37 | 0 |
| Salvador | 33 °C × 1 week (F) | 32/37 | 0 |
| Salvador | 33 °C × 2 weeks (F) | 36/37 | 1 (3%) |
| Salvador | 33 °C × 5–6 hr (F) | 40/40 | 0 |
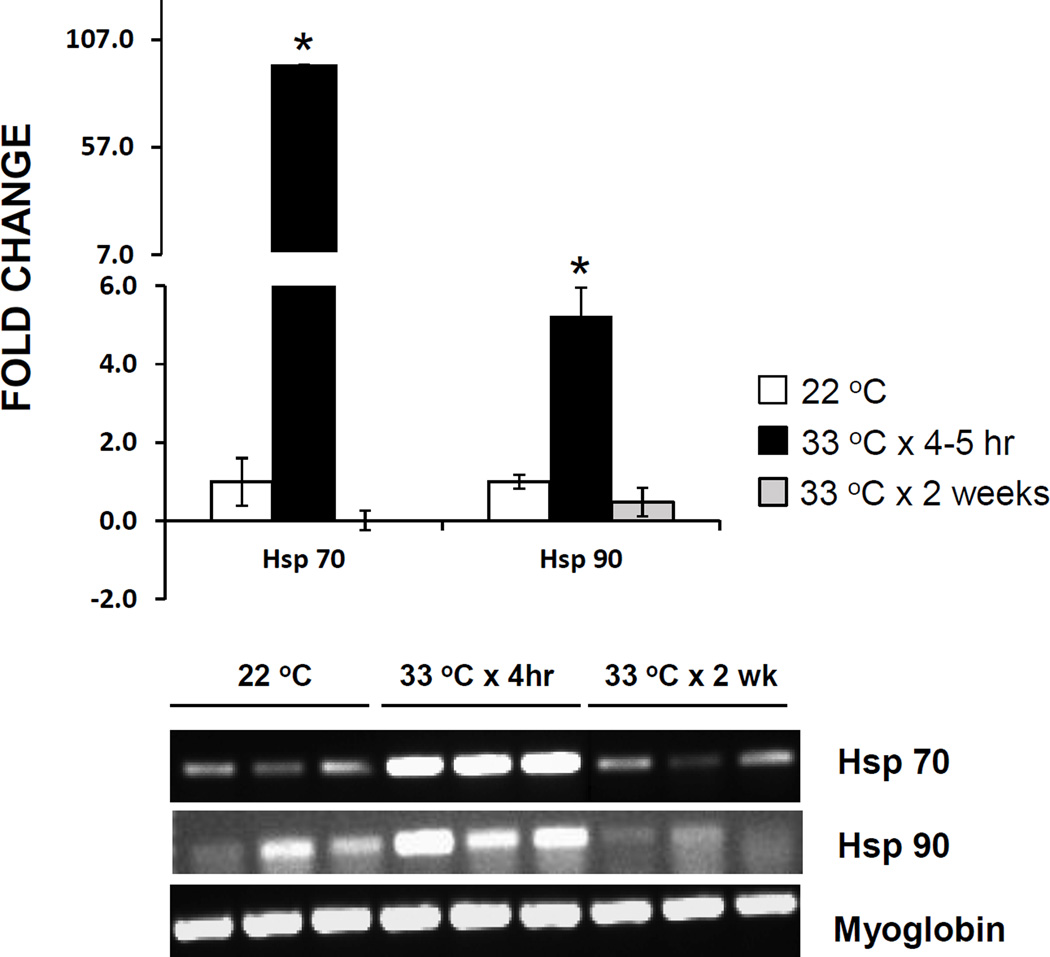
3.4. Hsp Expression
Hsp 70 and Hsp 90 were upregulated approximately 95-fold and 5-fold, respectively, in adult Salvador snails exposed to 33 °C for 4–5 h, relative to control expression (Fig. 6). However, after 2 weeks of exposure to 33 °C, the expression levels of both Hsp 70 and Hsp 90 were below those of controls, although myoglobin expression was unaltered.
Figure 6.
Expression of Hsp 70 and Hsp 90 in Biomphalaria glabrata incubated at 33 °C for 4–5 h or 2 weeks, versus snails removed from room temperature (22 °C) aquaria (controls). Graph depicts mean fold change in expression in 3 snails at each treatment, each snail sample run in triplicate. *, mean differs significantly from that of controls (P < 0.05). Error bars indicate standard deviation. Gel photograph shows results of RT-PCR amplification of Hsp 70, Hsp 90, and myoglobin cDNA in the 3 snails from each treatment category.
4. Discussion
The results of this study demonstrate that future environmental changes may affect the IDS of Biomphalaria glabrata. Specifically, whereas supra- and suboptimal temperatures had no effect on hemocyte concentrations, unexpectedly starvation for 1 or 2 weeks caused significant leukocytosis. The mechanism for this increase in hemocyte numbers, as well as its adaptive significance, are unclear. Higher numbers of circulating hemocytes could be due to release of hemocytes from tissue depots, prolonged hemocyte lifespan, or increased hemopoiesis. The latter possibility seems unlikely, since starvation significantly depresses mitotic activity in the APO at both 1 and 2 weeks, although hemocyte production could be occurring at other sites (see Pila et al., 2016b). Inasmuch as increased numbers of hemocytes are associated with increased resistance to schistosome infection in the 13–16-R1 strain of B. glabrata (Larson et al., 2014), it would be interesting to test the effects of starvation-induced leukocytosis on resistance to infection in susceptible snails.
Exposure to 20 °C for 1 or 2 weeks resulted in a significant increase in the number of mitotic figures in the APO, and yet hemocyte numbers did not increase. This effect could be a result of shorter life span of individual hemocytes or their sequestration in the tissues at the lower temperature. Additionally, rather than an increased production of hemocytes, the increased number of mitotic figures may represent a cold-induced slowdown of the mitotic phase of the cell cycle, with a resulting accumulation of cells progressing through nuclear division. However, we did not observe any obvious increase in the proportion of cells in a particular phase of mitosis, e.g., as occurs in colchicine-treated B. glabrata (Sullivan and Castro, 2005). The lower mitotic activity at 1 week, but not at 2 weeks, in snails exposed to 33 °C may be due to initial stress-related inhibition of cell division followed by eventual acclimation to the elevated temperature.
Results show no effect of treatments with abnormal temperatures or starvation on resistance to infection with S. mansoni. In particular, adult snails subjected to 33 °C for 5–6 h, 1 week, or 2 weeks remained uninfected following challenge with miracidia. In that respect, our results differ from those of Ittiprasert and Knight (2012) and Knight et al. (2015). It is possible that the method we used to assess infection, i.e., examination of the digestive gland or tissue squashes for daughter sporocysts beginning at 21 DPE, instead of cercarial emergence beginning at 4 weeks PE, as measured by Ittiprasert and Knight (2012), underestimated infection prevalences. However, while some immature infections may have been missed, infection was clearly observed in 82% of M-line controls assessed by this method (Fig. 1), and only 1 of 36 M-line snails had a cryptic infection that could not readily be discerned with a dissecting microscope. Also, by necessity we used day-old rather than fresh livers as the source of parasites, perhaps with diminished viability of miracidia. However, 82% of M-line control snails became infected, indicating adequate infectivity at a dose of 25 miracidia. Another consideration is that we used adults rather than juveniles, and as discussed by Lewis et al. (2001), mechanisms of resistance can differ in the 2 age categories of snails. Consequently, effects of heat shock may also differ. Finally, the same strain of B. glabrata from different laboratories can show considerable genetic heterogeneity (Mulvey and Bandoni, 1994), and the isolate of snail used in our study (originally obtained from Dr. Eric S. Loker, University of New Mexico) has been maintained independently from that in other laboratories since 1999, allowing ample time for genetic divergence. Although our method of assessing infection did not show any effect of heat shock on resistance, a histological study of the fate of sporocysts in heat-shocked and non heat-shocked snails may reveal more subtle differences between them.
It is noted that snails were maintained under the various environmental conditions in the dark. While effects of constant darkness on parameters of the IDS prior to miracidial exposure are not known, Steinauer and Bonner (2012) investigated the effect of lighting conditions during the prepatent period, and found no difference between infection prevalence in snails maintained in the dark and those exposed to a 12 h cycle of light and dark, although snails kept under dim light conditions showed a lower prevalence.
Using adult specimens, we were able to confirm prior reports of increased expression of Hsp 70 and Hsp 90 in juvenile snails following a brief exposure to high temperature. Interestingly, by 2 weeks of exposure to 33 °C the expression level of neither protein was elevated, possibly indicating that snails had acclimated to the abnormal temperature.
In conclusion, results of our study indicate that abnormal environmental conditions can have both stimulatory and inhibitory effects on hemocyte numbers and APO mitotic activity. Furthermore, some degree of acclimation to abnormal temperatures may occur. However, although elevated expression levels of heat shock proteins were seen following a 4–5 h exposure to 33 °C, none of the experimental treatments had any effect on resistance to infection with S. mansoni.
Highlights.
Abnormal environmental conditions can have both stimulatory and inhibitory effects on hemocyte numbers and hemopoiesis in adult Biomphalaria glabrata.
Some acclimation occurs with prolonged exposure to abnormal temperature.
Innate resistance to infection with Schistosoma mansoni is unaffected by abnormal temperature or starvation.
Acknowledgments
This study was supported by the Fletcher Jones Foundation, and NIH Grant R15AI097967. The following reagent was provided by the NIAID Schistosomiasis Resource Center for distribution through BEI Resources, NIAID, NIH: Schistosoma mansoni, Strain NMRI, livers from exposed Swiss Webster mice, NR-21963.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Rawadeh A. The role of starvation on selective immunological parameters in land snail Helix aspersa. Adv. Envir. Biol. 2010;4:265–270. [Google Scholar]
- Bruce JI, Radke MG, Davis GM. Culturing Biomphalaria and Oncomelania (Gastropoda) for large-scale studies of schistosomiasis. Biomedical report number; 406th Medical Laboratory, U.S. Army Medical Department Activity; Japan. 1971. p. 161. [Google Scholar]
- Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G. Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: A review of research progress over the last decade. Fish Shellfish Immunol. 2015;46:5–16. doi: 10.1016/j.fsi.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Eveland LK, Haseeb MA. Laboratory rearing of Biomphalaria glabrata snails and maintenance of larval schistosomes in vivo and in vitro. In: Toledo R, Fried B, editors. Biomphalaria Snails and Larval Trematodes. New York: Springer; 2011. pp. 33–55. [Google Scholar]
- Ittiprasert W, Knight M. Reversing the resistance phenotype of the Biomphalaria glabrata snail host Schistosoma mansoni infection by temperature modulation. PLoS Pathog. 2012;8:e1002677. doi: 10.1371/journal.ppat.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittiprasert W, Nene R, Miller A, Raghavan N, Lewis F, Hodgson J, Knight M. Schistosoma mansoni infection of juvenile Biomphalaria glabrata induces a differential stress response between resistant and susceptible snails. Exp. Parasitol. 2009;123:203–211. doi: 10.1016/j.exppara.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KH, Lie KJ, Heyneman D. Leucocytosis in Biomphalaria glabrata sensitized and resensitized to Echinostoma lindoense. J. Invertebr. Pathol. 1980;35:9–13. doi: 10.1016/0022-2011(80)90076-2. [DOI] [PubMed] [Google Scholar]
- Kagias K, Nehammer C, Pocock R. Neuronal responses to physiological stress. Front. Genet. 2012;3:222. doi: 10.3389/fgene.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Elhelu O, Smith M, Haugen B, Miller A, Raghavan N, Wellman C, Cousin C, Dixon F, Mann V, Rinaldi G, Ittiprasert W, Brindley PJ. Susceptibility of snails to infection with schistosomes is influenced by temperature and expression of heat shock proteins. Epidemiology (Sunnyvale) 2015;5:189. doi: 10.4172/2161-1165.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MK, Bender RC, Bayne CJ. Resistance of Biomphalaria glabrata 13–16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes. Int. J. Parasitol. 2014;44:343–353. doi: 10.1016/j.ijpara.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis FA, Patterson CN, Knight M, Richards CS. The relationship between Schistosoma mansoni and Biomphalaria glabrata: genetic and molecular approaches. Parasitology. 2001;123(Suppl):S169–S179. doi: 10.1017/s0031182001007831. [DOI] [PubMed] [Google Scholar]
- Lewis FA, Richards CS, Knight M, Cooper LA, Clark B. Schistosoma mansoni: analysis of an unusual infection phenotype in the intermediate host snail Biomphalaria glabrata. Exper. Parasitol. 1993;77:349–361. doi: 10.1006/expr.1993.1092. [DOI] [PubMed] [Google Scholar]
- Lie KJ, Heyneman D, Yau P. The origin of amebocytes in Biomphalaria glabrata. J. Parasitol. 1975;63:574–576. [Google Scholar]
- Maldonado JF. Schistosomiasis in America. Barcelona: Editorial Cientifico-Medica; 1967. p. 119. [Google Scholar]
- Mas-Coma S, Valero MA, Bargues MD. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet. Parasitol. 2009;163:264–280. doi: 10.1016/j.vetpar.2009.03.024. [DOI] [PubMed] [Google Scholar]
- McCreesh N, Booth M. Challenges in predicting the effects of climate change on Schistosoma mansoni and Schistosoma haematobium transmission potential. Trends Parasitol. 2013;29:548–555. doi: 10.1016/j.pt.2013.08.007. [DOI] [PubMed] [Google Scholar]
- McCreesh N, Nikulin N, Booth M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit. Vectors. 2015;8:4. doi: 10.1186/s13071-014-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M, Bandoni SM. Genetic variability in the M-line stock of Biomphalaria glabrata (Mollusca: Planorbidae) J. Helminth. Soc. Wash. 1994;61:103–108. [Google Scholar]
- Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin. Hematol. 2013;50:198–206. doi: 10.1053/j.seminhematol.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton WL. The establishment of a strain of Australorbis glabratus which combines albinism and high susceptibility to infection with Schistosoma mansoni. J. Parasitol. 1955;41:526–528. [PubMed] [Google Scholar]
- Palmieri MD, Palmieri JR, Sullivan JT. The natural diet of three Malaysian freshwater pulmonate snails. Malayan Nat. J. 1978;31:173–180. [Google Scholar]
- Paraense WL, Correa LR. Variation in susceptibility of populations of Australorbis glabratus to a strain of Schistosoma mansoni. Rev. Inst. Med. Trop. Sao Paulo. 1963;5:15–22. [PubMed] [Google Scholar]
- Pila EA, Gordy MA, Phillips VK, Kabore AL, Rudko SP, Hanington PC. Endogenous growth factor stimulation of hemocyte proliferation induces resistance to Schistosoma mansoni challenge in the snail host. Proc. Natl. Acad. Sci. USA. 2016a;113:5305–5310. doi: 10.1073/pnas.1521239113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pila EA, Sullivan JT, Wu XZ, Fang J, Rudko S, Gordy MA, Hanington PC. Haematopoiesis in molluscs: a review of haemocyte development and function in gastropods, cephalopods and bivalves. Dev. Comp. Immunol. 2016b;58:119–128. doi: 10.1016/j.dci.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftos DA, Kuchel R, Aladaileh S, Butt D. Infectious microbial diseases and host defense responses in Sydney rock oysters. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollinson D. Biomphalaria: natural history, ecology and schistosome transmission. In: Toledo R, Fried B, editors. Biomphalaria Snails and Larval Trematodes. New York: Springer; 2011. pp. 57–79. [Google Scholar]
- Steinauer ML, Bonner KM. Host susceptibility is altered by light intensity after exposure to parasites. J. Parasitol. 2012;98:1052–1054. doi: 10.1645/GE-3109.1. [DOI] [PubMed] [Google Scholar]
- Stumpf JL, Gilbertson DE. Hemocytes of Biomphalaria glabrata: factors affecting variability. J. Invertebr. Pathol. 1978;32:177–181. doi: 10.1016/0022-2011(78)90027-7. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Beltran RV, Cruz BC, Manuel RA, White JK. Hemopoietic tissue volume is a heritable trait in the schistosome-transmittting snail Biomphalaria glabrata. Invertebr. Biol. 2016 [Google Scholar]
- Sullivan JT, Castro L. Mitotic arrest and toxicity in Biomphalaria glabrata (Mollusca: Pulmonata) exposed to colchicine. J. Invertebr. Pathol. 2005;90:32–38. doi: 10.1016/j.jip.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Richards CS. Schistosoma mansoni, NIH-SM-PR-2 strain, in susceptible and nonsusceptible stocks of Biomphalaria glabrata: comparative histology. J. Parasitol. 1981;67:702–708. [PubMed] [Google Scholar]
- Sullivan JT, Spence JV. Transfer of resistance to Schistosoma mansoni in Biomphalaria glabrata by allografts of ameobocyte-producing organ. J. Parasitol. 1994;80:449–453. [PubMed] [Google Scholar]
- Suresh PG, Reju MK, Mohandas A. Factors influencing total hemocyte counts in freshwater gastropods. Comp. Haematol. Int. 1994;4:17–24. [Google Scholar]
- van Aardt WJ, de Kock KN, Naudé K. The respiratory properties of Biomphalaria glabrata exposed to Schistosoma mansoni infection, starvation, CO, and choices of different oxygen concentrations. Exper. Parasitol. 2003;103:93–101. doi: 10.1016/s0014-4894(03)00069-9. [DOI] [PubMed] [Google Scholar]