INTRODUCTION
Humans have had a long and complex relationship with salt. While highly valued in many societies, dietary salt has long been associated with high blood pressure1-3 and, more recently, with other diseases.4-6 Some individuals with hypertension often display salt-sensitive blood pressure changes, which is a condition more prevalent among African-Americans, older persons, and individuals with renal insufficiency or diabetes.7-9 In general, for those with salt-sensitive hypertension, excess sodium intake is associated with higher blood pressure, while a low-salt diet decreases blood pressure.3 In spite of this well-known association, the basic molecular and cellular mechanisms underlying the effects of salt on blood pressure regulation are still not well understood. Furthermore, individuals with high blood pressure are at increased risk for multiple diseases (i.e., coronary artery disease, heart failure, stroke, and renal disease) though at present whether or not a high dietary salt intake can directly lead to these diseases (i.e., in the absence of hypertension) is not known.
Our understanding of the effect of salt on health has grown even more complex recently. Researchers have reported a new connection between salt and autoimmunity: a high-salt diet was shown to accelerate autoimmune activity in a mouse model of multiple sclerosis.10,11 In addition, a close connection between hypertension and the immune system has been revealed.12-16 However, the causal relationships between salt, immunity, and hypertension (e.g., how salt could mediate interactions between the immune system and the vasculature, brain, or kidney to increase blood pressure) are not well understood.
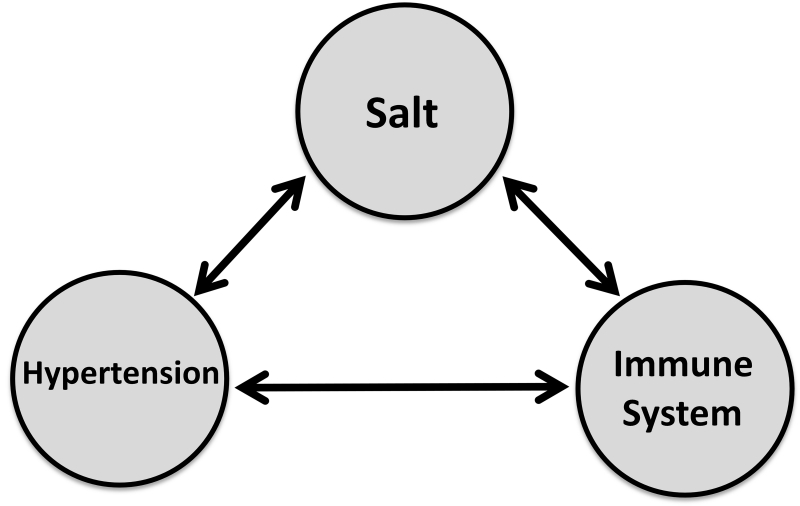
NHLBI convened a Working Group (WG) in 2014 to discuss this new emerging scientific area in hypertension research. The WG brought together experts from diverse backgrounds including hypertension, epidemiology, preeclampsia, cardiovascular disease, kidney disease, and autoimmune diseases. The WG reviewed existing and emerging scientific evidence that connects salt to human diseases, with a focus on hypertension and immune diseases (Fig. 1). The main areas of discussion addressed the bases of relationships between (i) dietary salt and hypertension, (ii) salt, autoimmunity, and cardiovascular diseases, and (iii) hypertension and immunity in various organs and systems (brain, kidney, skin, and vasculature). The WG also discussed the potential role of intrinsic factors (e.g., genetics, sex, and race) and environment on these relationships. Since the WG members came from diverse backgrounds, the meeting provided an excellent opportunity for cross-disciplinary thinking and discussions and highlighted the potential for future collaborations and research topics. In this report, we provide a brief summary of the WG discussion and recent progress in this area.
Figure 1. Connecting the dots: salt, immunity, and hypertension.
An NHLBI working group was held to discuss current scientific evidence and knowledge and to identify new research topics that could be tested experimentally in animal models and early clinical studies to examine the effect of multiple interactions and the direction (cause-effect) of these relationships.
Salt and Human Disease: What is the link?
The discovery of the relationship between salt and human hypertension is credited to Dr. Lewis Dahl.3,17 During the post-world war II era, the US Atomic Energy Commission conducted radiation-related research on populations in specific global research sites. Dr. Dahl took advantage of the varied salt-consuming food habits of these populations and studied the relationship between salt and blood pressure (for review, see Ref. 17). The data obtained were used in one of the first reports that demonstrated a direct relationship between dietary salt and blood pressure levels in humans. In these early studies, Dr. Dahl and colleagues also demonstrated that the blood pressure lowering effect of the Kempner rice-fruit diet, used for the treatment of hypertension, was primarily a result of its low sodium content. Others including Kawasaki et al.18 and later Weinberger et al.19 recognized the heterogeneity of blood pressure response to sodium intake and further developed the concept of sodium-sensitivity in humans.
The definition of salt-sensitivity varies between studies, mostly being defined as a proportional change (i.e., ≥3 to ≥10%) or an absolute change (i.e., ≥3 to ≥10 mm Hg) in mean arterial blood pressure from low to high sodium intake. Despite the variety of definitions and protocols used to test for salt-sensitivity, several findings have been consistently observed. First, sodium-induced changes in blood pressure are normally distributed in populations, and there is no evidence for a bimodal distribution.7 Like defining hypertension, using a cut point to categorize subjects as salt-sensitive or non-sensitive is arbitrary. Second, salt-sensitivity is a common biological phenomenon in human populations. Depending on the definition and measurement methods, salt-sensitivity has been noted in 25-50% of normotensives and 40-75% of hypertensive patients.7-9 Third, older individuals, African-Americans, and persons with obesity and metabolic syndrome are more likely to be salt-sensitive.7-9,20 In addition, salt-sensitivity is reproducible over time and associated with increased risk of cardiovascular and total mortality. Furthermore, both genetic and environmental factors determine an individual’s salt-sensitivity.20,21 Genetic factors may account for 27-42% of the variation in blood pressure salt-sensitivity.21 It should be noted, however, that most of these studies involve short term (several days to weeks) administration or deprivation from salt, and we still do not know if longer term (months to years) administration of salt might lead to blood pressure elevation in all subjects. Future studies should be undertaken to develop a standard protocol and definition to test for salt-sensitivity. It will be also important to identify biomarkers for salt-sensitivity, which can be used for risk classification and prediction.
Currently, the molecular mechanisms underlying salt-sensitivity are not clear. Schmidlin et al.22 reported that only in salt-sensitive but not salt-resistant blacks, salt administration induced increased blood pressure and systemic vascular resistance, which were associated with increased formation of asymmetrical dimethylarginine (ADMA), an endogenous vasodilation inhibitor. Also, there is evidence that high salt intake can enhance local generation of angiotensin II in humans, which may have local effects on vascular tone and function.23 Other mechanisms include impaired sodium excretion resulting in salt retention.24 Further understanding the molecular mechanisms of salt-sensitivity could aid in developing novel treatments for salt-sensitive hypertension.
Salt and Autoimmune Disease
Recent studies have provided evidence for a strong link between dietary salt, inflammation, and defective immune regulation, particularly in the Th17 and Foxp3+ regulatory T-cell (Treg) populations, both of which are part of the CD4+ helper T-cell lineage and have pivotal roles in the induction of autoimmune disease.10,25 The responsible genetic components for autoimmune disease have been studied to show that many immune-responsive genes are shared among autoimmune diseases, but these data fail to fully explain the continued increase in incidence of autoimmune disease over the last several decades, suggesting that environmental factors are also likely involved.26,27
Recent studies have also established a strong correlation between eating a fast food diet and increased circulation of Th17 cells.28 Salt-exposed Tregs adopt a Th1-type Interferon (IFN)-gamma signature that is similar to that observed in Tregs isolated from patients with autoimmune disease, such as those with type 1 diabetes and multiple sclerosis.29 A high-salt was shown to hasten the development of experimental autoimmune encephalomyelitis and to exacerbate the severity of xenogeneic graft versus host disease in mice.29 This study exploring the link between dietary salt and innate immunity also revealed that high-salt significantly enhances inflammatory macrophage function by decreasing the ability of M2 macrophages to suppress T-cell responses. A related study showed that the inflammasome, which is critical for Th17 cellular induction, can induce widespread inflammatory responses following exposure to a high salt environment.30
Thus, recent studies on the immune system have broadened our perspectives on the effects of salt on health since these studies strongly suggest a link between dietary salt and the immune system. Does exposure to high salt exacerbate the induction of autoimmunity in a genetically susceptible individual and if so, can we therapeutically manipulate these dietary salt effects in patients with autoimmune disease? Conversely, is there any benefit of anti-inflammatory or immune-suppressive agents in the treatment of salt-sensitive hypertension? Dietary shifts in sodium intake could have widespread effects on the gut by causing changes in gut architecture and immune profiles. Investigating these and other questions will advance our knowledge of salt and disease and will be aided by sodium magnetic resonance imaging (MRI), a form of MRI that can reveal extracellular and intracellular sodium levels.31 Development of other such diagnostic tools will continue to push this research area forward. Further work is needed to explore whether or not dietary salt interventions provide any benefit to patient outcomes, and more research will enable elucidation of the relationships between genes and environment in the context of immune disease. The greatest challenge we face is carving out a research agenda capable of addressing the many complexities of immunity, inflammation, diet, and disease. In this regard, the DASH (Dietary Approaches to Stop Hypertension) diet - which is rich in fruits, vegetables, and low-fat dairy foods and with reduced saturated and total fat – has been effective in lowering blood pressure32, but its interaction with the immune system has not been studied. Morris and colleagues33 have shown that dietary potassium intake antagonizes the pressor response to dietary sodium in both blacks and whites. In the future, it will be interesting to determine if the DASH diet or increased potassium intake affects the immune system.
Salt and Lymphatic System
Recent evidence suggests that large amounts of sodium are stored in the interstitium.31,34 This unappreciated electrolyte accumulation induces compensatory local regulatory lymphatic clearance mechanisms and parallel mononuclear phagocyte system (MPS) cells and T-cell responses. The tonicity-responsive enhancer-binding protein (TONEBP, also known as NFAT5) was found to be expressed in skin macrophage and has been implicated in modulating lymphatic growth and salt clearance from the skin, which in turn affects blood pressure.34 It is well known that the Lys2-Cre mouse targets genes expressed in resident tissue macrophages and neutrophils.35 At one time, Lys2 was thought to be absent in cells closely related to macrophages, especially in dendritic cells (DCs). However, a thorough analysis of different DC subpopulations revealed Lys2 expression and target gene deletional capacity in Lys2-Cre mice within some DC subsets and circulating monocytes.36 In particular, DCs that utilize the lymphatic vasculature to migrate from non-lymphoid organs like skin or lung to draining lymph nodes (so called migratory DC) are Lys2+ and show reporter expression in Lys2-Cre mice.36 Based on rigorous expression profiling in the Immunological Genome Project, it appears that DCs that migrate from skin to draining lymph nodes have highest levels of NFAT5, far outweighing the low-level of NFAT5 expression in macrophages.37 These observations, which require experimental testing, raise the possible alternative interpretation of published experiments that focused on Lys2-Cre × NFAT5fl/fl mice34; instead of macrophages in the skin regulating response to salt stored therein, the DCs that migrate from the skin to the skin-draining lymph node may play a central role in salt storage and associated lymphangiogenesis. Future research should determine if it is really macrophages that sit in skin or if it is DCs that traffic to lymph nodes to control the lymphatic-salt storage axis, or both.
Recent work has shown that sodium retention can occur in the skin, muscle, and other organs without added water retention.31 Interestingly, dialysis treatment and diuretic drug therapy can mobilize the sodium stored in skin and muscle in humans.38,39 The high sodium concentration under the skin boosts macrophage driven host defense in bacterial skin infections, suggesting that high salt concentrations under the skin are a physiological component of the immunological barrier. Microstructural analysis of sodium distribution in rodents suggested that sodium is concentrated under the keratinocyte layer.40 The resulting electrolyte gradient may result in electrochemical gradient formation. Epithelial sodium channels in human keratinocytes may mediate the directionality of electrolyte gradient formation, suggesting that sodium could be concentrated in the skin by active transport. A possible explanation for the high sodium concentration in the skin has been proposed; active sodium transport, combined with cutaneous blood capillary loops, may create a functional kidney-like counter-current system for concentration of sodium chloride under the skin.40 While the microanatomy of electrolyte distribution and its association with keratinocyte-driven sodium transport and vascular countercurrent loops make the existence of a functional counter current system likely, it is important to note that this hypothesis has not yet been addressed in physiological studies.
Salt and Brain
Studies have demonstrated that acute hypernatremia effectively activates sodium/osmolality sensing neurons in the forebrain and thence neurons downstream in the hypothalamus and brainstem that drive sympathetic nerve activity and raise arterial pressure.41 Thus, people who regularly consume high-salt meals will be challenged to continuously maintain normal sodium and water balance. Whereas neuronal excitatory actions of elevated plasma sodium have been repeatedly demonstrated, studies have yet to firmly establish the extent to which this mechanism contributes to the sustained increase of sympathetic nerve activity that accompanies salt-sensitive forms of arterial hypertension.
An unresolved but pressing question is whether a moderately elevated level of salt intake that does not increase plasma sodium over the long term can nevertheless recruit pro-hypertensive, sympathoexcitatory neural mechanisms. Available evidence suggests that this may be the case, but mechanisms are still under investigation. An attractive hypothesis being tested is that even moderately elevated salt intake over the long term induces a state of “neuroplasticity,” which causes other pro-hypertensive stimuli (e.g., renin-angiotensin II-aldosterone system, RAAS, activation) to elicit an exaggerated increase in sympathetic activity and a heightened hypertensive response. It is worth noting that neural mechanisms which increase sympathetic activity in response to acute hypernatremia could be pertinent to other diseases such as diabetes insipidus (DI) in which the plasma sodium concentration is chronically elevated.42,43 Although rats with neurogenic DI have normal resting blood pressure, they experience a greater fall in mean arterial pressure during complete autonomic blockade compared to AVP (arginine vasopressin) intact controls.42,43 The extent to which this reflects greater resting sympathetic vasomotor tone in DI rats, perhaps driven by their standing hypernatremia, versus their inability to release AVP in response to a fall in blood pressure has not been resolved. Despite lacking the ability to release AVP, rats with neurogenic DI have intact responses to acute renovascular hypertension. Although consistent with AVP having a limited role in renovascular hypertension, exaggerated RAAS and sympathetic activity in the hypernatremic DI rat could potentially compensate for the lack of AVP.
The ability of dietary salt to induce expression of pro-inflammatory and autoimmune cytokines (e.g., interleukin (IL)-17a) among peripheral immune cells (e.g., T helper cells) could be a principle part of salt-induced sympathetic activation.10,11 Recent evidence indicates that activated immune cells can invade regions of the forebrain and hypothalamus and contribute to sympathoexcitatory and hypertensive responses to pro-hypertensive hormones such as angiotensin II and aldosterone.44 Available evidence is also consistent with the view that salt intake can induce an initial wave of pro-inflammatory signals that subsequently triggers persistent microglial activation,45 culminating in a heightened neurogenic contribution to hypertension.
Whereas animal models of hypertension have identified numerous synaptic and intrinsic neuronal alterations that contribute to sympathetic activation, these adaptations have yet to be directly linked to specific upstream cytokine signaling pathways emanating from brain tissue resident peripheral immune cells or from activated microglia. Establishing these functional interactions represents a critical next step toward illuminating how elevated salt intake promotes a neurogenic contribution to arterial hypertension and, potentially, other sympathoexcitatory sodium-retaining diseases such as chronic heart failure.
Salt and Gut Microbiome
Dr. Lewis Dahl addressed the question of whether or not salt causes or contributes to the development of hypertension using rat models he generated from Sprague Dawley rats. Despite feeding 8% NaCl to all the rats, only a subset of them developed hypertension. By applying a selection breeding strategy for divergent blood pressure responses to dietary salt, the Dahl salt-sensitive (SS) and the Dahl salt-resistant (SR) rats were developed. Wide use of these animals in research has not only demonstrated the genetic basis for hypertension but also resulted in the identification of several loci on the rat genome that are linked to blood pressure.17 Recent studies have further demonstrated that the composition of the gut microbiota between the Dahl SS and SR rats are quite different, suggesting a potential connection with salt-sensitivity.46
Gut microbiota are increasingly being recognized as a significant factor influencing several inflammation and immunity related traits such as colitis and inflammatory bowel disease, and also in cardiovascular and metabolic traits such as hypertension, obesity and metabolic syndrome.46,47 Besides the genomic contribution of the host to salt-sensitive hypertension, a recent study utilized the Dahl rats to shed light on the microbiome as yet another important factor contributing to the development of salt-sensitive hypertension.46 The most significant change observed with the lowering of blood pressure was a lower level of the bacteria of the phylum Tenericutes and of the family Veillonellaceae belonging to the phylum Firmicutes. Other changes noted to be associated with a lowering of blood pressure were decreases of Clostridiales and Mollicutes.46 Given the link between salt and the immune system, each of the individual relationships of (i) salt and hypertension, (ii) salt and the immune-response, and (iii) salt-sensitive hypertension genetics and gut-microbiota are being established. Beyond further research into each of these relationships, it will be important to delineate the three-way relationship for the development of hypertension in the context of the extent of dietary salt, the host-microbiotal genetic and epigenetic cross-talk, and the state of the immune system. To this end, it will be necessary to develop animal models that have compromised immune systems and genetic propensity to develop hypertension.
Preeclampsia and Hypertension
Preeclampsia, a pregnancy complication characterized by high blood pressure, is an emerging risk factor for future salt-sensitive hypertension and cardiovascular disease.6 It has been argued that the underlying metabolic milieu of these women (such as obesity and hyperlipidemia) confers risk for both preeclampsia and cardiovascular disease. However, the possibility that preeclampsia-induced vascular damage causes persistent vascular dysfunction that interacts with pre-gestational cardiovascular disease risk factors has emerged as a likely scenario for the etiology of cardiovascular disease in this population.48 The absence of hypertension in the siblings of women with preeclampsia who might be expected to be at similar risk of cardiovascular disease, and the increased risk of hypertension in women with recurrent preeclampsia, suggest that preeclampsia, per se, can directly lead to long term hypertension and cardiovascular disease.
Emerging data from rodent studies suggest that direct vascular injury induced by high levels of circulating anti-angiogenic factors (i.e., sVEGFR-1, also known as sFlt1) that are upregulated in humans with preeclampsia causes long-term changes in the vasculature that promotes cardiovascular disease.6,49 Other studies have revealed that experimental preeclampsia in mice induces long term changes in the global plasma protein profile (proteome) that correlate with changes associated with cardiovascular disease.50 A recent study also suggests alteration in factors regulating lymphangiogenesis in preeclampsia,51 which may lead to altered salt storage handlings in patients with preeclampsia. As cardiovascular disease remains the leading cause of death in women, a greater understanding of the mechanism of post-preeclampsia hypertension and cardiovascular disease will improve screening and suggest novel targets for prevention of cardiovascular disease in this high-risk population of women.
Systemic Autoimmune Disease and Hypertension
An association exits between immune system activation and hypertension. Evidence suggests that patients with primary hypertension have increased circulating levels of autoantibodies, thus implicating autoimmunity as a potential underlying factor.52 Patients with autoimmune disorders including systemic lupus, rheumatoid arthritis, and scleroderma have an increased risk for developing hypertension, although the mechanisms underlying this risk have not been widely studied.14 To date, much of what has been learned about immune function and hypertension has been achieved through studies in experimental models of angiotensin-II induced and salt-sensitive hypertension. While these models will continue to yield new insights, it will be essential to study additional models of immune-mediated dysfunction that develop hypertension as this will further advance our understanding of the mechanistic importance of autoimmunity to hypertension, and help determine whether specific clinical populations can benefit from immune targeting as a component of their effort to control blood pressure. Already, there have been hints that targeting the immune system may be beneficial for blood pressure control. For example, a small clinical study showed that immunosuppression lowers blood pressure in patients with rheumatoid arthritis or sarcoidosis.53,54 Similarly, removing autoantibodies via immunoadsorption in refractory hypertensive patients significantly reduced blood pressure.55
These studies further underscore the need to understand how autoantibodies might contribute to the pathogenesis of hypertension. Using an experimental model of systemic lupus, it has been demonstrated that inhibition of inflammatory cytokines like TNF (tumor necrosis factor)-alpha attenuates the development of hypertension and renal disease.56 Further, it was demonstrated that long-term treatment with an anti-CD20 antibody (to knock down B cells and prevent autoimmunity) protected against the development of hypertension.14 Ultimately, it will be important to delineate the specific immune cells, antibodies and cytokines, and how they impact renal, vascular, and neural function to contribute to the development of hypertension. It will be also critical to examine the role of hormonal factors and environmental factors (i.e., dietary salt) that have immune modulatory roles and may ultimately lead to increased cardiovascular risk.
Psoriasis and Hypertension
Systemic inflammatory disorders such as psoriasis provide a reliable human in vivo model to understand immune activation, inflammation, and effects on the body. Psoriasis is associated with hypertension as well as increased risk of cardiovascular diseases such as myocardial infarction.57,58 As such, psoriasis provides a framework to test hypotheses related to interactions between environmental effects (i.e., diet, exercise, etc.) and immune responses depending on the clinical status of the skin disease. This is especially informative when a patient is observed in a “flared” state and then started on an immune modulating drug such as anti-IL17 or anti-IL12/23 therapy. Interestingly, recent evidence suggests that psoriatic skin contains more renin, which may provide a link between psoriasis and hypertension.59 Drawing on early results from an ongoing cohort study at NIH (NCT:01778569), down-regulation of the immune system with biological therapy appears to improve cardiovascular risk factors including dyslipidemia, dysglycemia, and markers of vascular inflammation (Nehal Mehta, unpublished data). Whether this is a drug effect or disease improvement leading to better health decisions is not yet known.
Hypertension had a variable response to immune modulatory drugs, with some classes increasing blood pressure and others having differing effects by individuals. When skin disease improves, however, it is not well understood whether there is a direct effect on blood pressure. Therefore, a systematic characterization including skin, blood, urine, and blood pressure responses following therapy would facilitate understanding of a potential relationship between immune activation and modulation of blood pressure. These findings potentially may also inform of changes in salt handling in the skin if in fact renin content changes in the skin following therapy.
Role of Immune Cells in Salt-Sensitive Hypertension
Multiple reports have documented the importance of the immune system in hypertension, vascular disease, and renal disease.12-15 In recent years, with the availability of new experimental tools, the fundamental links between immunity and hypertension have been explored. In particular, a seminal study by Guzik et al.16 used an approach with adoptive transfer of immune cells in immune-deficient mice to illustrate the role of T lymphocytes in experimental hypertension. Their study has driven highly increased interest in this area of study.
The role of immune mechanisms in hypertension and renal damage has been studied in Dahl SS rats, that have strong phenotypic similarities with salt-sensitive human hypertension.13 For example, the Dahl SS strain and a subset of the human population exhibit elevated arterial pressure and albuminuria when sodium intake is increased. Also, both Dahl SS fed high-salt and hypertensive humans demonstrate an infiltration of macrophages and CD4+ and CD8+ T-cells in the kidneys.13 These mononuclear cells are found in regions near damaged blood vessels, glomeruli, and tubules. To demonstrate the importance of the infiltrating immune cells in the development of salt-sensitive hypertension and renal disease, experiments were performed in which pharmacological inhibitors of the immune system were administered to Dahl SS rats during the period of high salt intake. The immunosuppressive agents were able to prevent the infiltration of T-cells into the kidneys of the treated animals and attenuate the development of salt-sensitive hypertension and renal damage.13 Subsequent studies utilized zinc finger nuclease (ZFN) technology to delete Recombination Activating Gene 1 (leading to a loss of mature T- and B-cells) and CD247 (leading to a loss of T-cells) in the Dahl SS genetic background. Experiments performed on these mutant rats demonstrated that elimination of the T-cells attenuated the salt-sensitive hypertension and renal damage in the Dahl SS.60 These studies demonstrate the importance of T-cells as amplifiers of salt-sensitive hypertension and renal damage. It has been interpreted that tissue damage, as a result of an initial increase in arterial pressure, triggers the inappropriate immune response in hypertension.13
A link between inflammation and human hypertension has recently been demonstrated in animal models to examine SH2B3 (also known as lymphocyte adaptor protein), a gene linked to human hypertension and renal disease through genome wide association studies (GWAS).61,62 SH2B3 functions as a negative regulator in many signaling pathways, including inflammatory signaling processes. A ZFN-mediated mutation of SH2B3 significantly attenuated the infiltration of leukocytes into the kidneys which was accompanied by a reduction in salt-sensitive hypertension and renal disease in Dahl SS rats.61 A separate report demonstrated that mice lacking SH2B3 have an exaggerated hypertensive response to angiotensin II.62 Taken together, these studies provide further support for the immune system’s role in the pathogenesis of hypertension and end-organ damage.
An exciting recent study has defined a novel mechanism of immune activation in hypertension.63 This study has shown that both angiotensin II- and DOCA-salt hypertension are associated with the formation of gamma ketoaldehydes (isoketals) in DCs. These are lipid oxidation products that rapidly react with lysines of proteins, and these modified proteins seem to be immunogenic. DCs that present isoketal-modified peptides in their MHC potently drive T-cell proliferation and production of cytokines by T-cells. Scavenging isoketals has proven effective in preventing immune activation and lowering blood pressure in these experimental models. How sodium might affect the formation of isoketal-modified proteins in antigen presenting cells is an important direction of future research. It will also be important to perform studies to understand the cell types involved in the response, the stimuli responsible for the activation of the different immune cell subtypes, and the cytokines and other factors mediating the pathogenic effects. As this field progresses, further advances should permit the development of targeted therapies to treat salt-sensitive hypertension and associated end-organ damage more effectively.
Sex-Differences in Hypertension
Sex differences in hypertension are clearly demonstrated by animal and human studies.64-66 Females have lower blood pressure than males across numerous species and in diverse genetic and induced animal models of hypertension. Epidemiological studies show that the onset of hypertension occurs earlier in men than women; and that Caucasian, African-American and Hispanic men have a higher prevalence of hypertension than women up through the fifth decade of life. While these studies clearly show sex differences exist in both blood pressure levels and prevalence of hypertension, little is known about the mechanisms underlying these differences.
Animal studies have shown that the higher arterial blood pressure observed in male compared to female mice after angiotensin II stimulation disappears in the T-cell deficient Rag-1−/− mice,67,68 suggesting that the immune system contributes to sex differences in this model of hypertension. Furthermore, while adoptive transfer of male T-cells restored the magnitude of hypertension induced by angiotensin II in male Rag-1−/− mice, they did not increase arterial pressure in female Rag-1−/− mice,68 which may be due to less T cell-mediated tissue infiltration in the female Rag-1−/− mice. Interestingly, female T-cells did not increase arterial pressure in male Rag-1−/− mice,67 suggesting that sex-specific T cell mechanisms contribute to resistance and susceptibility to hypertension.
Studies on the impact of biological sex on salt effects in hypertension are sparse and controversial. One study reported that women are more sensitive to salt than men. When salt intake was restricted from 15 g/day to less than 3 g/day, blood pressure in women remained sensitive to salt while blood pressure in men did not.69 Furthermore, a recent study showed women exhibited larger changes in blood pressure than men in response to changes from low-to-high or high-to-low salt intake.20 In contrast, in animal studies of salt-sensitivity, male Dahl SS rats had higher blood pressure and more renal injury than female Dahl SS rats in response to a high sodium diet indicating that the males were more sensitive to the sodium than the females.70
At NIH, considering sex as an important biological variable is now mandatory on all grant applications (NOT-OD-15-102) because biological sex can have profound effects on the incidence and progression of disease as well as the response to treatment. In the future, it will be important to investigate the impact of biological sex on mechanisms underlying the effects of salt on blood pressure regulation and end organ damage in diverse animal models of hypertension and salt-sensitivity. Understanding the cause and consequences of sex differences in salt effects could lead to novel therapeutic and perhaps sex-specific approaches through the discovery of new drug targets as a result of comparing mechanisms in both sexes.
Summary and Future Directions.
The WG concluded that the initial research that has implicated salt as a factor in important diseases points to the need to further illuminate the biological mechanisms and pathological processes to which salt may contribute. To guide this research into how salt may connect with human diseases, the WG members identified several scientific gaps and challenges and highlighted some opportunities for scientific inquiry and technical development.
Hypertension and immune disease (including autoimmune diseases), using existing experimental models, sex-specific animal models, and new experimental models.
- Salt-sensitive hypertension; in particular:
-
○The relative importance and interactions of several cytokines (specifically, IL-17, IFN-gamma, TNF-alpha, and IL-6).
-
○The relationships between genes and environment (i.e., epigenetics).
-
○The role of the gut microbiome.
-
○The role of the lymphatic system.
-
○
The underlying mechanisms of salt storage in the skin.
How pro-hypertensive neural inflammatory processes and peripheral immune cell activation by sodium interact to affect health.
Cardiovascular disease development in women with a history of preeclampsia.
The group also identified tools and technologies that are needed to move this research agenda forward:
Standardized protocols to determine salt sensitivity at an individual level.
New technologies to measure sodium concentrations in human tissues and animal models.
The kinds of technologies that would be helpful include methods to measure electrolyte distribution in skin microvasculature and easily accessible sodium MRI. Unlike traditional MRI, sodium MRI can reveal extracellular and intracellular sodium levels, which will help with understanding the role of salt in health and disease. As these technologies, protocols, and diagnostic tests become available, it will be critical that they are adopted. During the general discussion, the WG members also noted that it may be necessary to adopt a new term (e.g., homeostatic immune response) to describe the evolving concept of the physiological role of immunity and inflammation.
ACKNOWLEDGEMENTS
The authors wish to thank NIH staff (Drs. Lawrence Fine, Katarzyna Bourcier, and Chris Ketchum) and Dr. Ralf Dechend, who have participated in the Working Group.
SOURCE OF FUNDING
The proceedings of the “Salt in Human Health and Sickness: Building on the Current Scientific Evidence” Working Group were supported through funds provided by the National Heart, Lung, and Blood Institute.
Footnotes
CONFLICTS OF INTEREST DISCLOSURE(S)
None. This report represents the authors’ views and does not reflect official National Heart, Lung, and Blood Institute positions.
REFERENCES
- 1.O’Donnell M, Mente A, Yusuf S. Sodium intake and cardiovascular health. Circ Res. 2015;116:1046–1057. doi: 10.1161/CIRCRESAHA.116.303771. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Appel LJ, Sacco RL, Anderson CAM, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, Labarthe DR, MacGregor GA, Sacks FM, Stamler J, Vafiadis DK, Van Horn LV. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–2889. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 3.Dahl LK. Salt and hypertension. Am J Clin Nutr. 1972;25:231–244. doi: 10.1093/ajcn/25.2.231. [DOI] [PubMed] [Google Scholar]
- 4.Hucke S, Wiendl H, Klotz L. Implications of dietary salt intake for multiple sclerosis pathogenesis. Mult Scler. 2015;22:133–139. doi: 10.1177/1352458515609431. [DOI] [PubMed] [Google Scholar]
- 5.Vallon V, Thomson SC. Anomalous role for dietary salt in diabetes mellitus? Nat Rev Endocrinol. 2011;7:377–378. doi: 10.1038/nrendo.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, Chen JC, Duan X, Huang JF, Chen CS, Kelly TN, Bazzano LA, Whelton PK. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. doi: 10.1097/hjh.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidlin O, Forman A, Sebastian A, Morris RC., Jr. Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension. 2007;50:1085–1092. doi: 10.1161/HYPERTENSIONAHA.107.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JT, Jr., Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, Islam M, Eissa M, White S, Douglas JG. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–1092. doi: 10.1161/01.HYP.0000101687.89160.19. [DOI] [PubMed] [Google Scholar]
- 10.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol. 2014;307:F499–508. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathis KW, Broome HJ, Ryan MJ. Autoimmunity: an underlying factor in the pathogenesis of hypertension. Curr Hypertens Rep. 2014;16:424. doi: 10.1007/s11906-014-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens. 2010;19:181–186. doi: 10.1097/MNH.0b013e3283360a2e. [DOI] [PubMed] [Google Scholar]
- 16.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joe B. Dr Lewis Kitchener Dahl, the Dahl rats, and the “inconvenient truth” about the genetics of hypertension. Hypertension. 2015;65:963–969. doi: 10.1161/HYPERTENSIONAHA.114.04368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–198. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 19.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373:829–835. doi: 10.1016/S0140-6736(09)60144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu D, Rice T, Wang S, Yang W, Gu C, Chen CS, Hixson JE, Jaquish CE, Yao ZJ, Liu DP, Rao DC, He J. Heritability of blood pressure responses to dietary sodium and potassium intake in a Chinese population. Hypertension. 2007;50:116–122. doi: 10.1161/HYPERTENSIONAHA.107.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC., Jr. Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–385. doi: 10.1161/HYPERTENSIONAHA.111.170175. [DOI] [PubMed] [Google Scholar]
- 23.Boddi M, Poggesi L, Coppo M, Zarone N, Sacchi S, Tania C, Neri Serneri GG. Human vascular renin-angiotensin system and its functional changes in relation to different sodium intakes. Hypertension. 1998;31:836–842. doi: 10.1161/01.hyp.31.3.836. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Mechanisms of disease - Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. New Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 25.Safa K, Ohori S, Borges TJ, Uehara M, Batal I, Shimizu T, Magee CN, Belizaire R, Abdi R, Wu C, Chandraker A, Riella LV. Salt Accelerates Allograft Rejection through Serum- and Glucocorticoid-Regulated Kinase-1-Dependent Inhibition of Regulatory T Cells. J Am Soc Nephrol. 2015;26:2341–2347. doi: 10.1681/ASN.2014090914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotsapas C, Voight BF, Rossin E, Lage K, Neale BM, Wallace C, Abecasis GR, Barrett JC, Behrens T, Cho J, De Jager PL, Elder JT, Graham RR, Gregersen P, Klareskog L, Siminovitch KA, van Heel DA, Wijmenga C, Worthington J, Todd JA, Hafler DA, Rich SS, Daly MJ. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet. 2011;7:e1002254. doi: 10.1371/journal.pgen.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJ, Shishkin AA, Hatan M, Carrasco-Alfonso MJ, Mayer D, Luckey CJ, Patsopoulos NA, De Jager PL, Kuchroo VK, Epstein CB, Daly MJ, Hafler DA, Bernstein BE. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–343. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of “Western Diet” in Inflammatory Autoimmune Diseases. Curr Allergy Asthm R. 2014:14. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, Hafler DA. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125:4212–4222. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ip WK, Medzhitov R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun. 2015;6:6931. doi: 10.1038/ncomms7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 32.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N, DASH Collaborative Research Group A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 33.Morris RC, Jr., Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt sensitivity: effects of race and dietary potassium. Hypertension. 1999;33:18–23. doi: 10.1161/01.hyp.33.1.18. [DOI] [PubMed] [Google Scholar]
- 34.Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Muller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 36.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. Journal of Experimental Medicine. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Immunological Genome Project Consortium . [Accessed February 2, 2016]. [Google Scholar]
- 38.Dahlmann A, Dorfelt K, Eicher F, Linz P, Kopp C, Mossinger I, Horn S, Buschges-Seraphin B, Wabel P, Hammon M, Cavallaro A, Eckardt KU, Kotanko P, Levin NW, Johannes B, Uder M, Luft FC, Muller DN, Titze JM. Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int. 2015;87:434–441. doi: 10.1038/ki.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Garlichs C, Janka R, Cavallaro A, Luft FC, Uder M, Titze J. 23Na Magnetic Resonance Imaging of the Lower Leg of Acute Heart Failure Patients during Diuretic Treatment. PLoS One. 2015;10:e0141336. doi: 10.1371/journal.pone.0141336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmeister L, Perisic S, Titze J. Tissue sodium storage: evidence for kidney-like extrarenal countercurrent systems? Pflug Arch Eur J Phy. 2015;467:551–558. doi: 10.1007/s00424-014-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol. 2010;588:3375–3384. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiwatari M, Nolan PL, Johnston CI. The contribution of vasopressin and angiotensin to the maintenance of blood pressure after autonomic blockade. Hypertension. 1985;7:547–553. doi: 10.1161/01.hyp.7.4.547. [DOI] [PubMed] [Google Scholar]
- 43.Williams JL, Jr., Johnson MD. Sympathetic nervous system and blood pressure maintenance in the Brattleboro DI rat. Am J Physiol. 1986;250:R770–775. doi: 10.1152/ajpregu.1986.250.5.R770. [DOI] [PubMed] [Google Scholar]
- 44.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:178–191. doi: 10.1161/CIRCRESAHA.117.305853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, Shi P. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–316. doi: 10.1161/HYPERTENSIONAHA.115.05333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. Cardiovasc Res. 2014;101:579–586. doi: 10.1093/cvr/cvu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pruthi D, Khankin EV, Blanton RM, Aronovitz M, Burke SD, McCurley A, Karumanchi SA, Jaffe IZ. Exposure to experimental preeclampsia in mice enhances the vascular response to future injury. Hypertension. 2015;65:863–870. doi: 10.1161/HYPERTENSIONAHA.114.04971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bytautiene E, Bulayeva N, Bhat G, Li L, Rosenblatt KP, Saade GR. Long-term alterations in maternal plasma proteome after sFlt1-induced preeclampsia in mice. Am J Obstet Gynecol. 2013;208:388 e381–388 e310. doi: 10.1016/j.ajog.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lely AT, Salahuddin S, Holwerda KM, Karumanchi SA, Rana S. Circulating lymphangiogenic factors in preeclampsia. Hypertens Pregnancy. 2013;32:42–49. doi: 10.3109/10641955.2012.697953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kristensen BO, Andersen PL, Wiik A. Autoantibodies and vascular events in essential hypertension: a five-year longitudinal study. J Hypertens. 1984;2:19–24. doi: 10.1097/00004872-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Ferro CJ, Edwards NC, Hutchison C, Cockwell P, Steeds RP, Savage CO, Townend JN, Harper L. Does immunosuppressant medication lower blood pressure and arterial stiffness in patients with chronic kidney disease? An observational study. Hypertens Res. 2011;34:113–119. doi: 10.1038/hr.2010.193. [DOI] [PubMed] [Google Scholar]
- 54.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 55.Wenzel K, Haase H, Wallukat G, Derer W, Bartel S, Homuth V, Herse F, Hubner N, Schulz H, Janczikowski M, Lindschau C, Schroeder C, Verlohren S, Morano I, Muller DN, Luft FC, Dietz R, Dechend R, Karczewski P. Potential relevance of alpha(1)-adrenergic receptor autoantibodies in refractory hypertension. PLoS One. 2008;3:e3742. doi: 10.1371/journal.pone.0003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeshita J, Wang S, Shin DB, Mehta NN, Kimmel SE, Margolis DJ, Troxel AB, Gelfand JM. Effect of psoriasis severity on hypertension control: a population-based study in the United Kingdom. JAMA Dermatol. 2015;151:161–169. doi: 10.1001/jamadermatol.2014.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, Troxel AB, Hennessy S, Kimmel SE, Margolis DJ, Choi H, Mehta NN, Gelfand JM. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326–332. doi: 10.1136/annrheumdis-2014-205675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:2552–2564. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL. PhysGen Knockout P. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension. 2015;65:1111–1117. doi: 10.1161/HYPERTENSIONAHA.114.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015;125:1189–1202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirabo A, Fontana V, de Faria APC, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen S-c, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 65.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 2013;125:311–318. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension. 2014;64:573–582. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kojima S, Murakami K, Kimura G, Sanai T, Yoshida K, Imanishi M, Abe H, Kawamura M, Kawano Y, Ashida T, Yoshimi H, Kuramochi M, Omae T, Ito K. A Gender Difference in the Association between Salt Sensitivity and Family History of Hypertension. Am J Hypertens. 1992;5:1–7. [PubMed] [Google Scholar]
- 70.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296:F771–779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]