Abstract
Background and Aims
Amoxicillin–clavulanate (AC) is the most frequent cause of idiosyncratic drug-induced injury (DILI) in the US DILI Network (DILIN) registry. Here, we examined a large cohort of AC-DILI cases and compared features of AC-DILI to those of other drugs.
Methods
Subjects with suspected DILI were enrolled prospectively, and cases were adjudicated as previously described. Clinical variables and outcomes of patients with AC-DILI were compared to the overall DILIN cohort and to DILI caused by other antimicrobials.
Results
One hundred and seventeen subjects with AC-DILI were identified from the cohort (n = 1038) representing 11 % of all cases and 24 % of those due to antimicrobial agents (n = 479). Those with AC-DILI were older (60 vs. 48 years, P < 0.001). AC-DILI was more frequent in men than women (62 vs. 39 %) compared to the overall cohort (40 vs. 60 %, P < 0.001). The mean time to symptom onset was 31 days. The Tb, ALT, and ALP were 7 mg/dL, 478, and 325 U/L at onset. Nearly all liver biopsies showed prominent cholestatic features. Resolution of AC-DILI, defined by return of Tb to <2.5 mg/dL, occurred on average 55 days after the peak value. Three female subjects required liver transplantation, and none died due to DILI.
Conclusion
AC-DILI causes a moderately severe, mixed hepatocellular–cholestatic injury, particularly in older men, unlike DILI in general, which predominates in women. Although often protracted, eventual apparent recovery is typical, particularly for men and usually in women, but three women required liver transplantation.
Keywords: Allergy, Amoxicillin, Augmentin, Clavulanic acid, Drug-induced liver injury, Liver toxicity
Introduction
Idiosyncratic drug-induced liver injury (DILI) due to exposure to amoxicillin–clavulanate (AC) is observed relatively frequently. The US Drug Induced Liver Injury Network (DILIN) [1] and the Spanish DILI Registry [2] have identified AC as the most common cause of non-acetaminophen DILI. The incidence of AC-DILI was recently determined in a 2-year prospective study in Iceland where medication prescriptions for the entire population (approximately 250,000) are tracked [3]. Of the 35,000 patients who were treated, 15 cases of AC-DILI were identified, corresponding to 1 in 2350 patients (43 cases per 100,000). If one assumes generalizability to the USA where more than 70 million Americans are treated each year with AC, this would suggest that there may be nearly 30,000 cases of DILI due to AC annually in the USA [4].
The clinicopathologic presentation and natural history of AC-DILI are variable, but, as with other penicillins, the illness is most often a cholestatic liver injury. Symptoms of cholestatic hepatitis (e.g., fatigue, loss of appetite, itching) predominate [2, 5, 6]. Histologically, a spectrum of cholestatic liver injury can occur that includes pure intrahepatic cholestasis, and mixed hepatitic/cholestatic injury, which, occasionally, may evolve into vanishing bile duct syndrome [7, 8]. Jaundice is typical of patients with AC-DILI reported in the literature; most recover fully, although death or need for liver transplant may occur [9, 10]. The US DILIN cohort, established in 2004, now consists of over 1450 cases of DILI, of which 11 % are due to AC. The chief aim of this work is to describe the clinical, laboratory, and histopathological features associated with AC-DILI in the USA. We have focused particularly on characterizing AC-DILI compared to DILI due to other antibiotics. Moreover, as the largest prospective series of AC-DILI to date, we also describe the three cases from our cohort that required liver transplantation and the twelve cases that evolved features of chronic DILI. We contrast features of AC-DILI between men and women, amoxicillin alone, and also discuss histopathological features of the 30 subjects who had liver biopsies. These data advance our current understanding of the clinical phenotype and further highlight unique features of AC-DILI.
Methods
Study Design
The DILIN protocol has been previously described in detail [11]. Each participating center maintains active IRB approval, and all subjects enrolled provide written informed consent. Study subjects must have had exposure to a drug or herbal agent raising suspicion for DILI and must meet pre-specified criteria for severity of liver injury based upon biochemical testing. The onset of DILI is taken to be the date on which subjects had laboratory abnormalities that qualified them for enrollment, as previously described [11]. The pattern of liver injury is assessed by the ratio (R) of the serum ALT to alkaline phosphatase (both expressed as multiples of the ULN): By convention, an R < 2 indicates cholestatic-type injury, an R > 5 hepatocellular, and R = 2–5 as mixed cholestatic-hepatocellular injury. Chronic DILI is defined as laboratory, imaging, or physical examination evidence of continuing liver injury and disease ≥6 months after onset [12]. Liver biopsies, when performed, were initially reviewed in a blinded fashion by a single experienced hepatopathologist [DEK]. A second, unblinded review of biopsies was performed after cases were identified for this series.
Causality
The cases described here were adjudicated between October 2004 and July 2014. The causal relationship between AC and the liver injury event was evaluated in a formal and standardized fashion by the DILIN Causality Committee as described [11, 13]. The likelihood of DILI in individual cases is assessed as definite (≥95 % likelihood), highly likely (75–94 % likelihood), probable (50–74 % likelihood), possible (25–49 % likelihood), or unlikely (<25 % likelihood). In this analysis, we included only the 117 cases adjudicated as being definitely, highly likely, or probably due to AC. The cases adjudicated as possibly (n = 5) and unlikely (n = 3) due to AC were excluded.
Statistics
Descriptive statistics of demographic and clinical data of AC cases in comparison with other agents were generated and analyzed. The data are expressed as mean ± SD, range, frequencies, and percentages. Statistical significance among groups was determined by Wilcoxon rank-sum test for continuous variables, Fisher’s exact for binary variables, and Chi square for categorical variables. The LOESS regression model was used to fit smooth curves (and 95 % confidence intervals of the curves) of the R value at DILIN onset through 275 days of follow-up for men and women ≥50 and <50 years. Differences with a P value <0.05 were considered statistically significantly different. SAS 9.4 [SAS Institute, Cary, NC] was used for all statistical analyses.
Results
Clinical Features of Amoxicillin–Clavulanate (AC) DILI (AC-DILI)
In the DILIN cohort, 117/1038 patients with DILI were determined to be definitely (n = 57), highly likely (n = 51), or probably (n = 9) due to AC (Table 1; Fig. 1). AC constituted the most common implicated medication in DILIN; the 117 patients with AC-DILI represented 24 % of all antimicrobial agents (n = 479) and 11 % of all drugs adjudicated within the entire registry (n = 1038).
Table 1.
Selected demographic and clinical features of subjects with DILI caused by AC in comparison with DILIN due to other antimicrobial agents and to all other DILIN cases
| Clinical features | AC (n = 117) |
Other antimicrobial agents (n = 362) | P value* | Non-antimicrobials (n = 559) | P value** |
|---|---|---|---|---|---|
| Age (mean—years) | 60 | 48 | <0.001 | 47 | <0.001 |
| Male—n (%) | 72 (62) | 130 (36) | <0.001 | 235 (42) | <0.001 |
| Self-reported race | 0.003 | 0.002 | |||
| White or Caucasian—n (%) | 107 (92) | 279 (78) | 435 (78) | ||
| Black or African American—n (%) | 5 (4) | 46 (13) | 70 (13) | ||
| Other/multiracial—n (%) | 5 (4) | 34 (10) | 53 (10) | ||
| Latino—n (%) | 3 (3) | 40 (11) | 0.007 | 68 (12) | <0.001 |
| Time from drug exposure to symptom onset [median—days (IQR)] | 29 (17–37) | 29 (15–65) | 0.19 | 51 (26–115) | <0.001 |
| Symptom onset range by week(s) from drug exposure | <0.001 | <0.001 | |||
| ≤1 week n (%) | 2 (2) | 30 (9) | 14 (3) | ||
| 2–4 weeks n (%) | 53 (47) | 129 (40) | 109 (24) | ||
| 5–12 weeks n (%) | 56 (50) | 98 (30) | 177 (40) | ||
| 13–24 weeks n (%) | 2 (2) | 25 (8) | 70 (16) | ||
| >24 weeks n (%) | 0 (0) | 43 (13) | 78 (17) | ||
| Sign/symptoms at onset | |||||
| Jaundice n (%) | 102 (87) | 240 (66) | <0.001 | 385 (69) | <0.001 |
| Nausea n (%) | 70 (60) | 223 (62) | 0.74 | 336 (60) | 1.0 |
| Fever n (%) | 23 (20) | 126 (35) | 0.002 | 125 (22) | 0.62 |
| Abdominal pain n (%) | 38 (33) | 160 (44) | 0.03 | 243 (43) | 0.03 |
| Rash n (%) | 26 (22) | 105 (29) | 0.19 | 131 (23) | 0.81 |
| Itching n (%) | 96 (82) | 190 (53) | <0.001 | 270 (48) | <0.001 |
| Causality | <0.001 | <0.001 | |||
| Definite n (%) | 57 (49) | 83 (23) | 114 (20) | ||
| Very likely n (%) | 51 (44) | 187 (52) | 298 (53) | ||
| Probable n (%) | 9 (8) | 92 (25) | 147 (26) |
P AC-DILI versus other antibiotics, and
P AC-DILI versus all other agents
Fig. 1.
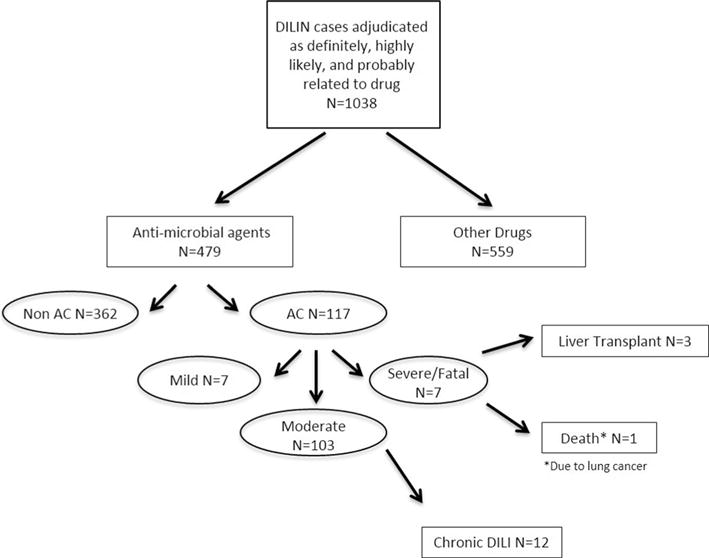
Flow diagram of amoxicillin–clavulanate DILI patients. Patients with DILI from the US DILIN, October 2004–July 2014, with complete adjudication (n = 1038) are shown. The number of patients with DILI due to amoxicillin–clavulanate and the severity of injury with ultimate outcomes are shown
In comparison with other antimicrobial causes of DILI, the mean age of subjects developing AC-DILI was significantly greater (60 vs. 48 years, P < 0.001) (Table 1). Men (72/117, 62 %) more frequently developed AC-DILI than women (45/117, 39 % P < 0.001). This gender distribution was also significantly different than DILI ascribed to other antimicrobials (men = 36 %, P < 0.001). Among men and women, 82 % of cases occurred in persons aged 50 years of age or older (Supplemental Table 1). All other drugs in DILIN had an age and gender distribution similar to other antibiotics and significantly different than the AC-DILI cases (Table 1). Over 90 % of AC-DILI events occurred in study subjects who self-reported their race as Caucasian, whereas the corresponding frequency for the overall study population was 78 % (P = 0.002).
The median number of days to symptom onset after drug initiation was 29, with all but two cases occurring within 12 weeks after initial drug exposure. For other antimicrobials, the corresponding latency was 29 days as well (P = 0.19). For the remaining agents in the DILIN cohort, the latency was 51 days (P < 0.001), (Table 1). Jaundice and pruritus were each reported in over 80 % of patients with AC-DILI. In fact, 23 % of subjects (27/117) underwent baseline evaluation for biliary obstruction with ERCP and/or MRCP. Cholestatic features were significantly less common in all other cases of DILI (jaundice—68 %, P < 0.001; pruritus—50 %, P < 0.001).
Pattern of Laboratory Testing and Evolution Over Time
The results of laboratory tests at the date of onset revealed a cholestatic or mixed cholestatic-hepatocellular liver injury pattern in the majority of patients. The median R value at the time of DILIN onset was 2.7 (Table 2). Only 29/114 (25 %) AC cases had a hepatocellular injury pattern at onset (R value >5). The R value at symptom onset date corresponds to a median serum ALT of 362 U/L, AST of 171 U/L, ALP of 288 U/L, and TB of 6 mg/dL (Table 2). Eighteen percent of subjects with AC-DILI had a positive antinuclear antibody, and 15 % had a positive anti-smooth muscle antibody [ASMA]. More men (15/67, 22 %) than women (1/42, 2.4 %) among the AC-DILI cohort tested positive for ASMA (P < 0.001). Of a total of 32 patients with either a positive ANA or ASMA, 7 subjects received corticosteroids. Of the remaining 85 subjects with AC-DILI, an additional 14 received treatment with corticosteroids in spite of negative serologies.
Table 2.
Selected laboratory features of AC-DILI
| Laboratory feature | At onset date | Range | Peak value from onset to 6 month visit | Range |
|---|---|---|---|---|
| ALT (U/L), [median (IQR)] | 362 (201–528) | 52–3890 | 405 (211–648) | 52–3890 |
| AST (U/L), [median (IQR)] | 171 (102–308) | 33–5990 | 201 (125–371) | 47–7525 |
| Alkaline phosphatase (U/L), [median IQR (SD)] | 288 (210–380) | 123–1005 | 361 (252–551) | 144–1472 |
| Total bilirubin (mg/dL), [median (IQR)] | 6 (4–9) | 0.5–26 | 13 (7–23) | 0.5–45 |
| INR [median (IQR)] | 0.9 (0.9–1.1) | 0.8–4 | 1.1 (1.1–1.2) | 0.8–9 |
| R value at onset [median (IQR)] | 2.7 (1.7–5) | 0.5–44 | ||
| R value <2 n (%) | 37 (33) | |||
| R value >2 and <5 n (%) | 48 (42) | |||
| R value >5 n (%) | 29 (25) | |||
| Positive HCV RNA n (%) | 2 (3 %) | |||
| Positive ANA n (%) | 20 (18 %) | |||
| Positive ASMA n (%) | 16 (15 %) | |||
| Serum IgG (mg/dL) [median (IQR)] | 995 (793–1166) | 478–2840 | ||
| Absolute eosinophil count (/uL) [median (IQR)] | 228 (99–357) | 0–1253 |
Serum ALT and AST levels peaked quickly after onset (4 and 5 days), while ALP and TB values peaked at 17 and 12 days, respectively (Fig. 2). Aminotransferase levels improved on average more quickly than ALP and TB, with a 50 % reduction from peak value occurring within 30 days for both ALT and AST and normalizing on average by day 90. The mean number of days until the total bilirubin improved by 50 % from its peak value was 18, with biochemical resolution, defined as TB <2.5 mg/dL, occurring on average at 55 days. ALP levels were the slowest to resolve with a 50 % improvement by 43 days and normalization on average not until 114 days after onset. LOESS curves of the R value from onset until day 275 for men and women (<50 and ≥50 years) showed that women on average had a higher R value at onset and that the R value decreased in all groups as disease evolved (Supplemental Figure 1).
Fig. 2.
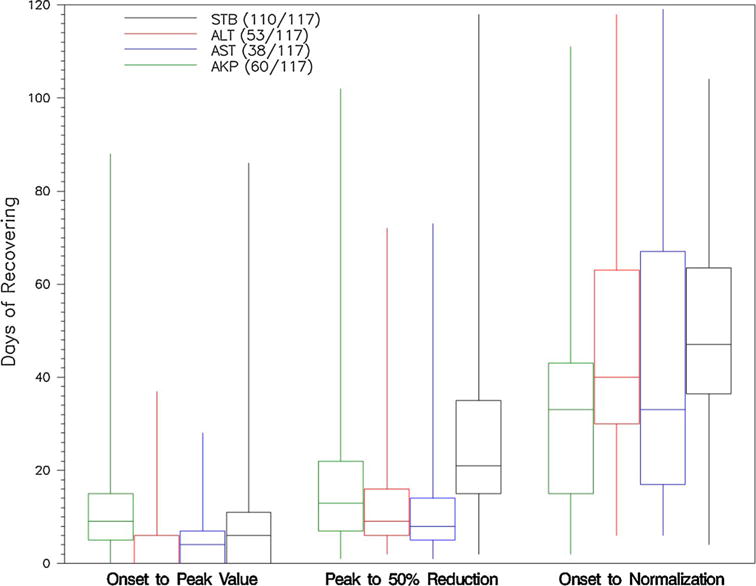
Time course of AC-DILI presented as box and whisker plot: ALT peak must be ≥×5 ULN or ×5 baseline, AST peak must be ≥×5 ULN or ×5 baseline, ALP must be ≥×2 ULN or ×2 baseline, TB peak must be ≥2.5 mg/dL. Biochemical resolution defined as ALT, AST, or ALP below ULN; TB <1.2 mg/dL
Severity and Outcome of Amoxicillin–Clavulanate (AC)-Induced DILI
The majority (88 %) of AC-DILI cases fell into the DILIN severity category of “moderate” liver injury, defined by enzyme elevations and jaundice with (46/117, 39 %) or without (57/117, 49 %) hospitalization (Table 1). Three men, all of whom were ≥50 years old, developed severe DILI, defined by evidence of coagulopathy or encephalopathy. Two fully recovered, while the other evolved features of chronic cholestasis. Three women (3/45, 6.6 %), ranging in age from 49 to 67 years, required liver transplantation, which was nearly statistically different compared to men (0/72, 0 %, P < 0.0545). There were several features that appeared to differentiate these patients from the others in the cohort (Supplemental Table 2). The most important is that patients with severe AC-DILI had evidence of severe hepatocellular injury with markedly elevated aminotransferases and markedly elevated total bilirubin levels at presentation. One patient with AC-DILI died, but this was not ascribed to DILI and rather to metastatic lung cancer after resolution of liver injury. In contrast to all other drugs adjudicated in the DILIN cohort, severe and fatal cases of AC-DILI were less common 6 % (7/117) versus 28 % (262/921), P < 0.001).
Twelve subjects (11 %) developed chronic DILI, and their clinical characteristics are summarized in Table 3. All subjects presented with jaundice, 11 with itching, 5 with nausea, and 3 each with abdominal pain, fever and rash. Ten subjects (83 %) with chronic AC-DILI were male as compared with 62 (59 %) of 105 subjects without chronic DILI, P = 0.13. All other patient characteristics, such as age, race and weight, and liver injury characteristics, including latency, aminotransferases at onset and peak of injury, injury pattern and symptoms, were similar in subjects with and without chronic AC-DILI, with no evident predictors of chronic AC-DILI. Aminotransferases normalized within 2–3 years in 5 subjects with chronic DILI, and 4 subjects had asymptomatic but persistent mild abnormalities in aminotransferases (Table 3). Three subjects did not follow up after the initial study visit, including 2 subjects with asymptomatic mild elevations in aminotransferases 8 months post-DILI onset, and 1 subject who was evaluated for liver transplantation due to DILI who was alive 2 years post-DILI onset with improved but persistently abnormal aminotransferases (no other clinical information was available).
Table 3.
Selected features of chronic AC-DILI
| Age and gender | Latency (days) | ALT at DILI onseta | AST at DILI onseta | ALP at DILI onseta | T.Bili at DILI onseta | Liver injury pattern at DILI onset | Interval from DILI onset to abnormal aminotransferases defining chronic DILI (months) | Abnormal aminotrasnferases defining chronic DILIa | DILI outcomesa |
|---|---|---|---|---|---|---|---|---|---|
| 54 M | 29 | 610 (12.2) | 224 (5) | 239 | 4.4 (4.4) | Hepatocellular | 6 | ALT 86 (1.9), AST 67 (1.5) | Normal liver biochemistries 3 years post-DILI |
| 71 M | 34 | 755 (11.4) | 308 (8.3) | 337 (3.7) | 8.8 (8.8) | Mixed | 7 | ALT 63 (1.4), AST 45 (1.1), T.Bili 2.1 (2.1) | Normal liver biochemistries 2 years post-DILI |
| 71 M | 27 | 202 (3.2) | 143 (3.5) | 178 (2) | 4.6 (3.8) | Cholestatic | 6 | AST 45 (1.1 ULN), T.Bili 1.3 (1.1 ULN) | Normal liver biochemistries 2 years post-DILI, fibroscan suggested fatty liver but no significant fibrosis |
| 60 M | 23 | 387 (8.6) | 134 (3.8) | 286 | 7.7 (7) | Mixed | 8 | ALT 55, AST 41 | Normal liver biochemistries 2 years post-DILI, with fatty appearing liver by ultrasound 1 year post-DILI |
| 32 M | 12 | 375 (5.6) | 11 (1.7) | 198 (1.7) | 4.7 (4.7) | Mixed | 6 | ALT 87 (2.5), AST 49 (1.6),ALP 165 (1.3) | Asymptomatic abnormal ALT 64, with normal liver ultrasound 2 years post-DILI |
| 39 F | 3 | 271 (6.8) | 88 (2.4) | 302 (2.6) | 5.3 (4.4) | Mixed | 7 | ALT 71 (2), AST 92 (2.5), ALP 644 (5.5), T.Bili 1.8 (1.5) | Asymptomatic abnormal ALT 65 (1.4) and T.Bili 1.3 (1.1) 2 years post-DILI |
| 72 F | 33 | 126 (3.6) | 70 (1.9) | 299 (2.6) | 3.8 (3.2) | Cholestatic | 8 | AST 41 (1.2) | Subject did not follow up after first study visit but was asymptomatic at last visit |
| 40 M | 47 | 204 (3.9) | 88 (2.4) | 182 (1.6) | 5.8 (4.8) | Mixed | 8 | ALT 84 (1.6), ALP 139 (1.2) | Subject did not follow up after first study visit |
| 63 M | <14 | 208 (3.2) | 95 (2.3) | 490 (3.6) | 10.2 (10.2) | Cholestatic | 6 | ALT 78 (1.4), AST 100 (2.4), ALP 928 (5.8), T.Bili 17.6 (12.6) | Initial intent to list for liver transplant was limited by cardiac comorbidities. The subject was alive with abnormal ALT 71, AST 48, ALP 263, T.Bili 1.5 2 years after DILI onset |
| 36 M | 29 | 352 (6.3) | 201 (5.7) | 204 (1.6) | 8.2 (6.3) | Mixed | 10 | ALT 102 (1.8), AST 80 (2.3), ALP 142 (1.1) | Asymptomatic abnormal ALT 71 and ALP 189 2 years post-DILI |
| 66 M | 22 | 235 (5.9) | 237 (6.4) | 473 (4.04) | 13.1 (13.1) | Cholestatic | 8 | ALT 51 (1.1), ALP 150 (1.25) | Normal liver biochemistries 2 years post-DILI |
| 63 M | 39 | 977 (18) | 363 (10.7) | 393 (2.6) | 3.5 (2.9) | Hepatocellular | 6 | ALT 54 (1.2), AST 44 (1.05), ALP 274 (2.3) | Asymptomatic abnormal ALT 55 2 years post-DILI |
ALP alkaline phosphatase, DILI drug-induced liver injury, T.Bili total bilirubin, M male, F female
Values of ALT, AST, and ALP reported in U/L with (multiples of upper limit of normal when available), T.Bili reported in mg/dL with (multiples of upper limit of normal when available)
Concomitant Medications and AC-DILI Versus Amoxicillin-DILI
In the 2 months prior to AC-DILI onset, the percentage of subjects taking concomitant medications was 23 % (0–2 meds.), 35 % (3–5 meds.), and 42 % (>5 meds.). Of note, 42 % (49/117) of subjects reported a history of any prior drug allergy. Only one patient reported prior exposure to AC, 2 years prior to the implicated DILI event. An analysis of subjects with AC-DILI with prior AC, PCN, or cephalosporin exposure (n = 5) to cases without documented prior PCN/cephalosporin class exposure (n = 112) did not find any significant differences between the two groups (data not shown). In our DILIN registry, there were 8 cases of DILI attributed to amoxicillin only (Supplemental Table 3). The 8 cases were younger than the corresponding AC-DILI cases (51 vs. 60 years, P = 0.03) and were more likely to present with a rash (63 vs. 22 %, P = 0.02). DILI onset with amoxicillin tended to be abrupt compared to AC (median 17 vs. 29 days, P = 0.07) and resolution of DILI defined by improvement in TB from peak to 50 % reduction from peak was also more rapid (median 5 vs. 8 days, P = 0.02). Three of the eight amoxicillin cases received a severe DILIN Severity score, but none were fatal or required liver transplantation.
Histopathological Findings
Thirty liver biopsies and one explanted liver obtained during the period of acute injury were available for histological review. The biopsies were obtained a median 9 days after protocol-defined onset (range −35 to 35) and a median 38 days after the first dose of AC (range 17–82 days). The one explant was obtained 4 days after onset, but 132 days after the first dose of AC. Supplemental Table 4 shows the distribution of histopathological patterns of injury compared with the biochemical injury at onset. Most of the biopsies showed cholestatic patterns of injury, with 20 showing cholestatic hepatitis (Fig. 3), 3 showing acute cholestasis and 5 chronic cholestasis. Three patients had atypical histopathological patterns; 2 with acute hepatitis and the explant with fulminant hepatitis and extensive necrosis. Visible canalicular and hepatocellular bile accumulation in zone 3 was a common feature, present in 28 cases (90 %) and was sufficiently severe that it was observed at low magnification in 13 cases. Duct injury, often severe, was seen in 25 (83 %) cases. Three cases showed some evidence of duct paucity during this acute injury period, but they did not develop chronic DILI. The inflammation (portal and lobular) was generally mild except in the cases of acute and fulminant hepatitis. Immuno-allergic features (eosinophils and granulomas) were noted in 21 and 28 cases, respectively, and although no cases were classified as granulomatous hepatitis, 4 cases did show large epithelioid granulomas. Although 12 cases showed some degree of periportal fibrosis, none had bridging fibrosis or cirrhosis. Three patients had one or two follow-up biopsies after the acute injury period. Two were taken at 2 months after the first dose of AC revealed cholestatic hepatitis with findings similar to those described above. The remaining two follow-up specimens were from the same patient, taken 108 and 289 days after the first dose of AC. Both revealed chronic cholestasis, with strong staining for copper and pseudoxanthomatous changes in hepatocytes, but normal numbers of bile ducts.
Fig. 3.
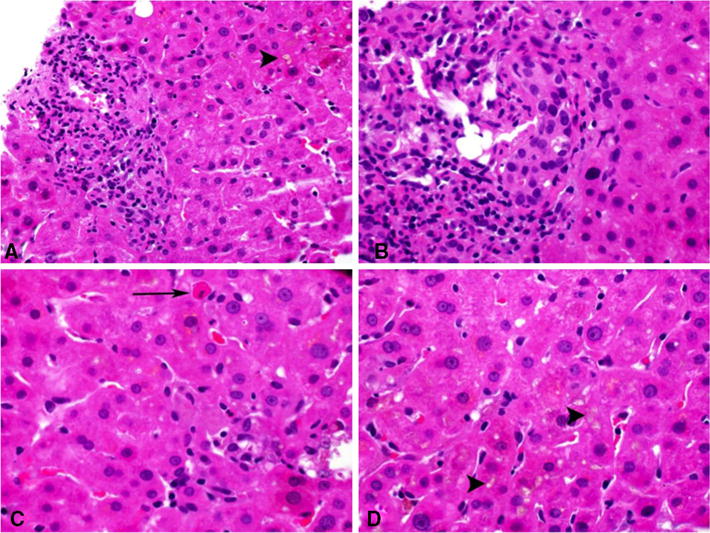
Representative histopathologic findings of cholestatic hepatitis due to amoxicillin–clavulanate. a Portal inflammation with cholestasis (arrowhead). b Portal inflammation with injured duct. c Lobular inflammation and apoptotic hepatocyte (arrow). d Canalicular cholestasis (arrowheads). ALP serum alkaline phosphatase, ALT serum alanine aminotransferase, AST serum aspartate aminotransferase, SD standard deviation, TB serum total bilirubin, ULN upper limit of normal
Discussion
Here, we have demonstrated that AC is the most common cause of idiosyncratic liver injury in the DILIN data set and that, like other penicillins and cephalosporins, it causes predominantly a cholestatic-type liver injury [5, 14]. In addition, DILI due to AC, unlike DILI due to other drugs, appears to preferentially afflict older men. In the Spanish DILI Registry [15], the gender distribution was found to be nearly equal (52 % men and 48 % women). In contrast, earlier published case series from the USA [16], Belgium [17], and France [18] reported men to have predominated (Table 4). In the Spanish cohort, patients ≥55 years old were significantly more likely to develop a cholestatic (R value <2) or mixed (R value >2 but <5) liver injury. Our findings corroborate the Spanish experience since the R value at onset among those 50 years of age or older was 3.3 for men and 4.9 for women in contrast to 7.6 and 6.3, among men and women less than 50 years of age (Supplemental Table 1). We found similar results if we used the age cutoff of 55 years, but chose 50 because this is the average age of menopause for women in the USA. Interestingly, among the 6 severe cases, all three who required liver transplantation were women. On the other hand, 83 % (10/12) of all chronic DILI cases were in men. These differences highlight the possibility that epigenetic changes due to age and gender could play a role in the outcome from AC-DILI. Importantly though, we found that the majority of patients eventually recover fully.
Table 4.
Summary of features of DILI due to AC from our and previous published series with at least 10 subjects
| Author | Type of study and location |
Years of study |
Number of AC-DILI cases |
Age (years, mean, range) |
Male gender (%) |
Latency (days) (mean (range)) |
Presentation with jaundice (%) |
Injury pattern cholestatic/mixed/ hepatocellular |
Duration of injury (days) [mean (range) unless otherwise specified] |
Death or liver transplant |
Chronic injury (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| deLemos et al. | Prospective, USA | 2004–2014 | 117 | 59 (13–87) | 62 | 30 (3–129) | 87 | 32/42/25 % | 52 (2–534) for hyperbilirubinemia* | 3.4 % (1 died, 3 transplant) | 11 |
| Lucena et al. [15] | Prospective, Spain | 1995–2005 | 69 | 56 | 52 | 16 (13–20) | 81 | 31/33/36 % | 77 (10–300) | 2.9 % (1 died, 1 transplant) | 8 |
| De Haan et al. [24] | Retrospective, | 1982–1996 | 40 | 61 | 70 | 22 (max 55) | 90 | 46 (10–168) | None | ||
| Thomson et al. [25] | Retrospective, Australia | 1986–1993 | 34 | 60 | 58 | ||||||
| Bjornssen et al. [3] | Prospective, Iceland | 2010–2011 | 22 of 96 cases of DILI | 40 | None | ||||||
| O’Donohue et al. [26] | Retrospective, Scotland | 1991–1997 | 22 | 59 (43–91) | 45 | 17 to jaundice (3–48) | median 69 for Jaundice to resolve (29–150) | 5.6 % (1 died) | |||
| Garcia-Rodriguez et al. [27] | Retrospective, Spain | 1991–1992 | 21 | 43 | 76 | 75 %/NA/NA | None | ||||
| Reddy et al. [16] | Retrospective, USA | 1984–1987 | 18 | 59 (33–80) | 67 | 27 (2–45) | 89 | 39/33/22 % | None | ||
| Larrey et al. [18] | Retrospective, France | 1987–1990 | 15 | 64 (39–82) | 74 | 34 (7–89) | 100 | 74/13/13 % | 7 (4–16) weeks | None |
The vast majority of our cases showed prominent zone 3 cholestasis and bile duct injury with mild inflammation, as described by others [18]. Granulomas and/or eosinophils, which are often associated with and thought to be consistent with immuno-allergic injury, were relatively common. Three cases, all women, had atypical histological findings, with two cases showing acute hepatitis (without cholestasis) and one explanted liver showing fulminant hepatitis with extensive necrosis.
In assessing the cause of acute liver injury, it is important to consider drugs and chemicals as potential causative agents, but also to exclude other more common causes, such as viral hepatitis, alcohol, hypoxemia/ischemia, or gallstones or other biliary tract disorders. Another confounding factor in causality assessment is the high frequency of polypharmacy, with many subjects receiving multiple drugs and botanical, herbal, and dietary supplements. In the US DILIN, we have developed and refined an approach to causality assessment over several years that takes advantage of expert opinion and of the collective wisdom and accumulated data of the Network. Because of the typical “signature” and high frequency of liver injury due to AC, we likely ascribe relatively high likelihood scores to such cases.
In recent work from DILIN, we have shown that AC and other agents associated with immuno-allergic injury cause hepatitis with infiltration of portal triads by many T cells, most of which are CD8+ cytotoxic T cells [19]. These and other findings suggest that the pathogenesis of the liver injury is primarily an immune-mediated attack on cholangiocytes or perhaps on the apical pole of hepatocytes. The specific antigens against which a cellular immune response is mounted currently remain unknown, but it seems likely that they represent neo-antigens produced by the beta-lactam structures of amoxicillin or clavulanic acid, perhaps, reacting with proteins of the susceptible hosts. While neo-antigens may form in many people who take AC, DILI may occur in only a small subset with specific genetic susceptibilities. Genetic variation at human leukocyte antigen (HLA) class I & II loci has been shown to be associated with AC-DILI [17, 20]. The strongest association thus far identified is at a single nucleotide polymorphism in the gene encoding the class II HLA-DRB1* 1501-DQB1* 0602 allele. A more recent analysis of genotype–phenotype interaction in AC-DILI cases found that the class I alleles A*3002 and B*1801 were more frequently associated with hepatocellular injury compared to controls, while the presence of the DRB1*1501-DQB1*0602 allele was significantly increased in cholestatic/mixed cases [21]. Thus, clinical, histopathological, and genetic data point to the importance of the adaptive immune response in the pathogenesis and course of DILI due to AC. The genetic associations, although striking, are not of sufficient power to provide a priori prediction of who is at high risk to develop DILI due to AC, based solely upon HLA typing. Even in the case of DILI due to another penicillin, flucloxacillin, in which an 80-fold risk has been established for persons with HLA-B*5701, prior screening before administration of the drug is not considered clinically warranted [22]. While the association between genetic variation at HLA loci in AC-DILI has been reproduced, other investigations into the contribution of genetic variation and the risk of DILI have been negative [23].
An uncertainty is whether the DILI due to AC is actually due to amoxicillin or to clavulanate or to both together. The eight cases of DILI due only to amoxicillin showed similar clinical, biochemical, and other features as those of the AC cohort, suggesting that amoxicillin alone, like virtually all penicillins or cephalosporins, is capable of causing cholestatic or mixed-type DILI (Supplemental Table 3). Then, too, clavulanic acid itself also has a beta-lactam ring, and it may give rise to neo-antigen[s] that can call forth a pathological adaptive immune response in susceptible hosts. In others it may act chiefly to slow the clearance and to increase the levels of amoxicillin, thus helping to give rise to a neo-antigen derived from amoxicillin.
Strengths of our study include its prospective nature with good follow-up; most surviving subjects agreed to return at 6 months and beyond if there was evidence of chronic DILI. The assessment of both severity and causality were done systematically using a Delphic process. In addition, all liver biopsies were reviewed by a single expert hepatopathologist [DEK] without prior reference to the clinical or laboratory features. We also recognize some limitations. One was that as per the DILIN protocol, liver biopsies are not required (but are performed as part of routine clinical care), and thus we do not have liver histology on all subjects. Despite this potential limitation, we doubt that the absence of histology on all subjects would change our conclusions. While not a limitation, a potential criticism is that our study was not a population-based study. Thus, subjects were identified at or referred to only a limited number of clinical sites (variably 5–8 in number over the years of the study). This may have led to recall or reporting bias since it is likely that not all patients with AC-DILI were referred to a DILIN site. Notwithstanding, given the large sample size, again it is unlikely that such a bias would change our conclusions.
In summary, AC is the most frequent cause of idiosyncratic DILI in the USA, and, indeed, probably throughout the world. Unlike most DILI, AC causes DILI more often in older men, with Caucasian men seeming to be at greatest risk. The clinical presentation is that of immuno-allergic type injury with prominent cholestatic features and chemistries. The initial levels of serum ALT and the R values tend to be higher both in women and men <50 years old versus those ≥50 years old, although these differences are not statistically significant. The prognosis is generally good. Nevertheless, a small proportion of patients develop acute liver failure and die or require liver transplantation. Ten percent of subjects continue to have ongoing evidence of liver injury 6 months after onset. The higher risk especially in older Caucasian men is worthwhile noting as is the finding that only three subjects, all of whom were women, required liver transplantation.
Supplementary Material
Acknowledgments
We thank Thomas Phillips from the Duke Clinical Research Institute for help with statistical analysis.
Grant Support The DILIN Network is structured as a U01 cooperative agreement with Funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants: 2U01-DK065176-06 (Duke), 2U01-DK065201-06 (UNC), 2U01-DK065184-06 (Michigan), 2U01-DK065211-06 (Indiana), 5U01DK065193-04 (UConn), 5U01-DK065238-08 (UCSF/CPMC), 1U01-DK083023-01 (UTSW), 1U01-DK083027-01 (TJH/UPenn), 1U01-DK082992-01 (Mayo), 1U01-DK083020-01 (USC). Additional funding is provided by CTSA grants: UL1 RR025761 (Indiana), UL1 RR025747 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and in part by the Intramural Research Program of The NIH, National Cancer Institute.
Footnotes
For the Drug-Induced Liver Injury Network (DILIN)
Electronic supplementary material The online version of this article (doi:10.1007/s10620-016-4121-6) contains supplementary material, which is available to authorized users.
Author Contributions Andrew S. deLemos was involved in study concept and design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for intellectual content; statistical analysis. Marwan Ghabril contributed to study concept and design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for intellectual content. Don C. Rockey was associated with study concept and design; analysis and interpretation of data; critical revision of the manuscript for intellectual content. Jiezhun Gu was involved in data collection and statistical analysis; critical revision of the manuscript for important intellectual content. Huiman X. Barnhart was involved in data collection and statistical analysis. Robert J. Fontana contributed to data collection; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. David E. Kleiner was associated with data collection; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Herbert L. Bonkovsky contributed to study concept and design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for intellectual content; statistical analysis.
Conflict of interest The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product.
References
- 1.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. 1934 e1921–1924. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Ice-land. Gastroenterology. 2013;144:1419–1425. 1425 e1411–1413. doi: 10.1053/j.gastro.2013.02.006. quiz e1419–e1420. [DOI] [PubMed] [Google Scholar]
- 4.Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146:914–928. doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JH, Zimmerman HJ. Drug- and chemical-induced cholestasis. Clin Liver Dis. 1999;3:433–464, vii. doi: 10.1016/s1089-3261(05)70079-9. [DOI] [PubMed] [Google Scholar]
- 6.Mohi-ud-din R, Lewis JH. Drug- and chemical-induced cholestasis. Clin Liver Dis. 2004;8:95–132, vii. doi: 10.1016/S1089-3261(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 7.Jakab SS, West AB, Meighan DM, Brown RS, Jr, Hale WB. Mycophenolate mofetil for drug-induced vanishing bile duct syndrome. World J Gastroenterol. 2007;13:6087–6089. doi: 10.3748/wjg.v13.45.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleiner DE. The pathology of drug-induced liver injury. Semin Liver Dis. 2009;29:364–372. doi: 10.1055/s-0029-1240005. [DOI] [PubMed] [Google Scholar]
- 9.Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci. 2005;50:1785–1790. doi: 10.1007/s10620-005-2938-5. [DOI] [PubMed] [Google Scholar]
- 10.Limauro DL, Chan-Tompkins NH, Carter RW, Brodmerkel GJ, Jr, Agrawal RM. Amoxicillin/clavulanate-associated hepatic failure with progression to Stevens–Johnson syndrome. Ann Pharmacother. 1999;33:560–564. doi: 10.1345/aph.18104. [DOI] [PubMed] [Google Scholar]
- 11.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110:1450–1459. doi: 10.1038/ajg.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alqahtani SA, Kleiner DE, Ghabril M, et al. Identification and characterization of cefazolin-induced liver injury. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucena MI, Andrade RJ, Fernandez MC, et al. Determinants of the clinical expression of amoxicillin–clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44:850–856. doi: 10.1002/hep.21324. [DOI] [PubMed] [Google Scholar]
- 16.Reddy KR, Brillant P, Schiff ER. Amoxicillin–clavulanate potassium-associated cholestasis. Gastroenterology. 1989;96:1135–1141. doi: 10.1016/0016-5085(89)91633-8. [DOI] [PubMed] [Google Scholar]
- 17.Hautekeete ML, Horsmans Y, Van Waeyenberge C, et al. HLA association of amoxicillin–clavulanate–induced hepatitis. Gastroenterology. 1999;117:1181–1186. doi: 10.1016/s0016-5085(99)70404-x. [DOI] [PubMed] [Google Scholar]
- 18.Larrey D, Vial T, Micaleff A, et al. Hepatitis associated with amoxycillin-clavulanic acid combination report of 15 cases. Gut. 1992;33:368–371. doi: 10.1136/gut.33.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foureau DM, Walling TL, Maddukuri V, et al. Comparative analysis of portal hepatic infiltrating leukocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin Exp Immunol. 2014 doi: 10.1111/cei.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucena MI, Molokhia M, Shen Y, et al. Susceptibility to amoxicillin–clavulanate–induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens C, Lopez-Nevot MA, Ruiz-Cabello F, et al. HLA alleles influence the clinical signature of amoxicillin–clavulanate hepatotoxicity. PLoS One. 2013;8:e68111. doi: 10.1371/journal.pone.0068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 23.Urban TJ, Shen Y, Stolz A, et al. Limited contribution of common genetic variants to risk for liver injury due to a variety of drugs. Pharmacogenet Genomics. 2012;22:784–795. doi: 10.1097/FPC.0b013e3283589a76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Haan F, Stricker BH. Liver damage associated with the combination drug amoxicillin-clavulanic acid (Augmentin) Ned Tijdschr Geneeskd. 1997;141:1298–1301. [PubMed] [Google Scholar]
- 25.Thomson JA, Fairley CK, Ugoni AM, et al. Risk factors for the development of amoxycillin-clavulanic acid associated jaundice. Med J Aust. 1995;162:638–640. doi: 10.5694/j.1326-5377.1995.tb126049.x. [DOI] [PubMed] [Google Scholar]
- 26.O’Donohue J, Oien KA, Donaldson P, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717–720. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia Rodriguez LA, Stricker BH, Zimmerman HJ. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch Intern Med. 1996;156:1327–1332. doi: 10.1001/archinte.1996.00440110099013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


