Summary
The sexually dimorphic distribution of adipose tissue influences the development of obesity-associated pathologies. The accumulation of visceral white adipose tissue (VWAT) that occurs in males is detrimental to metabolic health, while accumulation of subcutaneous adipose tissue (SWAT) seen in females may be protective. Here, we show that adipocyte hyperplasia contributes directly to the differential fat distribution between the sexes. In male mice, high-fat diet (HFD) induces adipogenesis specifically in VWAT, while in females HFD induces adipogenesis in both VWAT and SWAT in a sex hormone-dependent manner. We also show that the activation of adipocyte precursors (APs), which drives adipocyte hyperplasia in obesity, is regulated by the adipose depot microenvironment and not by cell-intrinsic mechanisms. These findings indicate that APs are plastic cells, which respond to both local and systemic signals that influence their differentiation potential independent of depot origin. Therefore, depot-specific AP microenvironment niches coordinate adipose tissue growth and distribution.
Graphical abstract
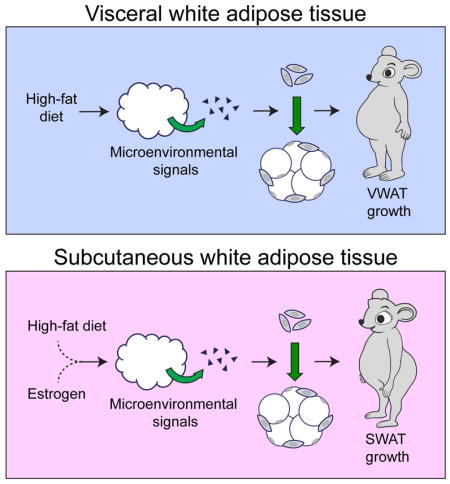
eTOC blurb
Jeffrey et al look at differences in fat depot expansion in male and female mice in response to a high fat diet. Subcutaneous white adipose tissue (SWAT) expansion only occurs in females. Furthermore depot-specific activation and differentiation of white adipocyte precursors is determined by the microenvironment rather than cell-intrinsic differences.
Introduction
The increasing rates of obesity and obesity-associated pathologies around the world highlight the need for an understanding of the mechanisms that underlie white adipose tissue (WAT) growth in vivo. The distribution of WAT into subcutaneous (SWAT) and visceral (VWAT) depots in obesity has strong links to metabolic disease. Increased VWAT mass is strongly associated with cardiometabolic risk and mortality across ethnicities (Coutinho et al., 2011; Lee et al., 2014; Nazare et al., 2012; Phillips and Prins, 2008; Pischon et al., 2008; Wormser et al., 2011). In contrast, elevated SWAT mass has been associated with improvements in plasma lipid profile, insulin sensitivity, blood pressure, and atherosclerosis (Appleton et al., 2013; Heitmann and Lissner, 2011; Manolopoulos et al., 2010; Snijder et al., 2003). These data suggest that the mechanisms underlying depot-specific WAT growth are directly relevant to metabolic health and disease, yet the regulation of this process in vivo is not well understood.
Subcutaneous and visceral adipose depots display an array of differences including gene expression (Cohen et al., 2014; Grove et al., 2010; Karastergiou et al., 2013), developmental lineage (Chau et al., 2014; Krueger et al., 2014; Sanchez-Gurmaches and Guertin, 2014), metabolic characteristics (Cohen et al., 2014; Karastergiou and Fried, 2013; Tran et al., 2008), and adipokine secretion profiles (Shi et al., 2009). Interestingly, males preferentially accumulate visceral adipose tissue, while premenopausal females accumulate more subcutaneous adipose tissue (Palmer and Clegg, 2015). In humans, these patterns of adipose distribution result in characteristically female “pear-shaped” obesity and male “apple-shaped” obesity (Gesta et al., 2007; Karastergiou et al., 2012). In postmenopausal women, adipose distribution shifts toward a male-like pattern, implicating sex hormones in maintaining a balance between subcutaneous and visceral depot mass (Palmer and Clegg, 2015). Importantly, however, the cellular and molecular mechanisms underlying differential adipose distribution in males and females are unclear.
Adipose tissue growth can occur through two distinct mechanisms: the increase in size of existing adipocytes (hypertrophy), or the increase in number of adipocytes (hyperplasia). Since mature adipocytes are post-mitotic, new adipocytes are derived from adipocyte precursor (AP) cells within the stroma of all white adipose depots (Berry et al., 2014). It was recently shown that the expansion of visceral adipose tissue in males occurs through adipocyte hyperplasia in both mice (Jeffery et al., 2015; Wang et al., 2013) and humans (Arner et al., 2013), while male subcutaneous adipose tissue in mice does not exhibit significant hyperplasia in response to obesogenic stimuli (Jeffery et al., 2015; Wang et al., 2013). Furthermore, studies of the dynamics of human adipose tissue indicate that weight loss is mediated primarily by a reduction in adipocyte size with no significant reduction in adipocyte number, even after bariatric surgery (Spalding et al., 2008). These data suggest that an increase in adipocyte number in obesity may have lasting effects on energy homeostasis and weight maintenance. Taken together, these findings indicate that depot-specific mechanisms are important in the control of WAT growth in obesity; however, the mechanisms driving these processes are not known.
We recently found that in male mice, obesogenic signals such as high-fat feeding or hyperphagia lead to the proliferation and differentiation of APs in VWAT, but not SWAT (Jeffery et al., 2015). The preferential growth of VWAT in male mice can thus be partially attributed to increased hyperplasia; however, the mechanism(s) underlying depot-specific AP activation remain unclear. One potential explanation for this phenomenon is that APs in different depots have distinct intrinsic abilities to respond to obesogenic signals. Indeed, previous reports have suggested that AP-intrinsic mechanisms are responsible for depot-specific differences in AP gene expression, in vitro differentiation potential, and contribution to metabolic disease (Baglioni et al., 2012; Fried et al., 2015; Loh et al., 2015; Macotela et al., 2012). However, the mechanisms that control depot-specific AP activation in vivo have not been investigated. Here, we show that obesogenic adipogenesis in SWAT is differentially regulated in males and females, explaining the differences in WAT distribution between the sexes. Furthermore, through AP transplant studies, we find that depot-specific proliferation and differentiation of APs is determined by the depot microenvironment, and not by cell-intrinsic differences in APs.
Results
Adipocyte hyperplasia contributes to depot- and sex-specific WAT growth in obesity
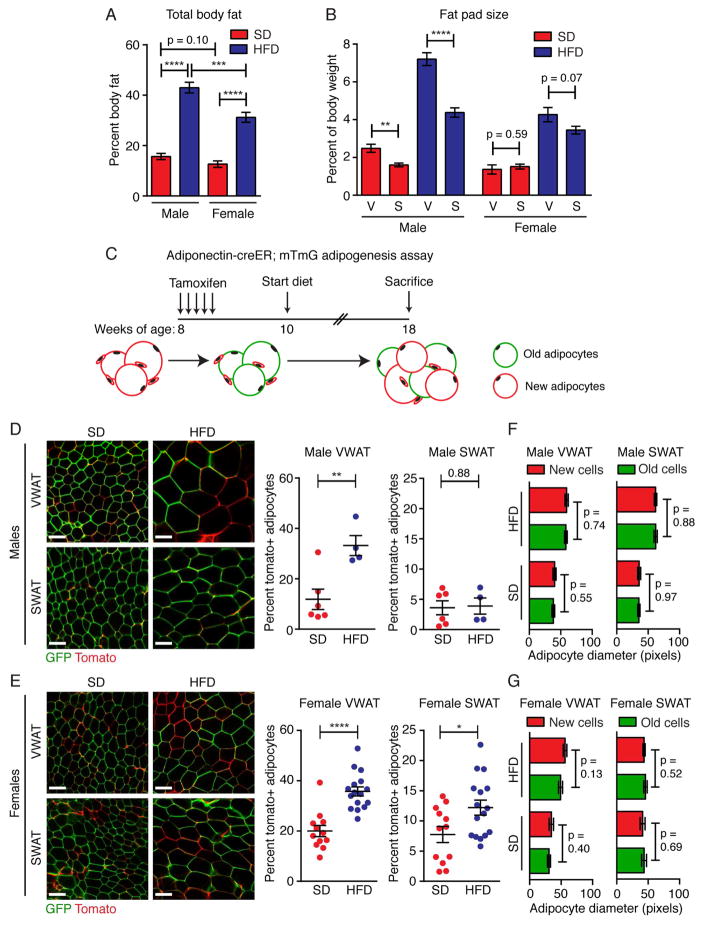
Sex-specific patterns of adipose distribution have been well documented in humans (Gesta et al., 2007; Karastergiou et al., 2012; Palmer and Clegg, 2015). To determine if C57Bl6/J mice display similar phenotypes in the high-fat diet (HFD) model of obesity, we measured whole body fat mass (Figure 1A) and fat pad weight of the perigonadal VWAT and the inguinal SWAT depots (Figure 1B) after 8 weeks of HFD or standard diet (SD) feeding. Although the precise anatomic location of these depots in mice differ from humans, these mouse depots impact metabolism similarly to human visceral adipose tissues (Barzilai et al., 1999; Foster et al., 2010, 2011; Gabriely and Barzilai, 2003) and gluteofemoral adipose tissue (Foster et al., 2013; Tran et al., 2008), respectively. We find that upon HFD feeding, males accumulate more fat mass than females (Figure 1A), consistent with previous reports (Grove et al., 2010; Medrikova et al., 2012). Interestingly, in both SD- and HFD-fed conditions, male VWAT is significantly larger than male SWAT, whereas these two depots are maintained at similar sizes in females (Figure 1B). These data indicate that, similar to humans, mice are sexually dimorphic with regard to fat mass distribution in obesity.
Figure 1. Adipocyte hyperplasia contributes to depot- and sex-specific WAT growth in obesity.
(A) Body fat as a percentage of total body weight after 8 weeks of SD or HFD feeding in male and female C57Bl/6J Adiponectin-creER; mTmG mice as measured by magnetic resonance imaging. (B) Weight of individual fat pads from male or female C57Bl/6J Adiponectin-creER; mTmG mice after 8 weeks of SD or HFD feeding. (In A and B, n = 9–15) (C) Experimental design for Adiponectin-creER; mTmG adipogenesis experiments. (D–E) Representative confocal images (left) and quantification (right) of adipocyte labeling in the indicated depots of Adiponectin-creER; mTmG male (D) or female (E) mice. (Males: n = 4–6. Females: n = 12–15) (F–G) Average adipocyte diameter of tdTomato+ (new) and eGFP+ (old) adipocytes from the indicated groups. (n = 4–6) Note: all data from A-E are from tamoxifen-treated animals. Significance was determined by comparing the indicated groups using an unpaired two-tailed student’s t-test. Scale bar is 100μM. Error bars represent mean ± S.E.M. See also Figure S1.
We recently showed that HFD-feeding induces adipocyte hyperplasia in male VWAT, but not male SWAT after 8 weeks of HFD feeding (Jeffery et al., 2015), contributing to the preferential growth of VWAT in males. To determine whether hyperplastic mechanisms influence sex-specific WAT growth, we used the Adiponectin-creER; mTmG mouse model to track the formation of new mature adipocytes in vivo in response to SD or HFD feeding. In this model, new adipocytes that form after tamoxifen injection are mTomato+ (mT), while pre-existing adipocytes will remain mGFP+ (mG) (Figure 1C, S1A–B and (Jeffery et al., 2014; Jeffery et al., 2015)). Of note, our data indicate that the low dose of 50mg/kg tamoxifen does not significantly affect body weight (Figure S1C). Consistent with previous findings, we observe VWAT-specific adipogenesis in males after 8 weeks of HFD feeding (Figure 1D). Interestingly, in females we find that HFD significantly increases adipogenesis in both VWAT and SWAT depots (Figure 1E), mirroring the similar expansion of these depots in response to HFD (Figure 1B). We also compared the average size of the newly formed mTomato+ adipocytes to the existing mGFP+ adipocytes within each depot after 8 weeks of diet, and found no significant differences (Figure 1F–G), which suggests the newly formed cells contribute significantly to fat mass.
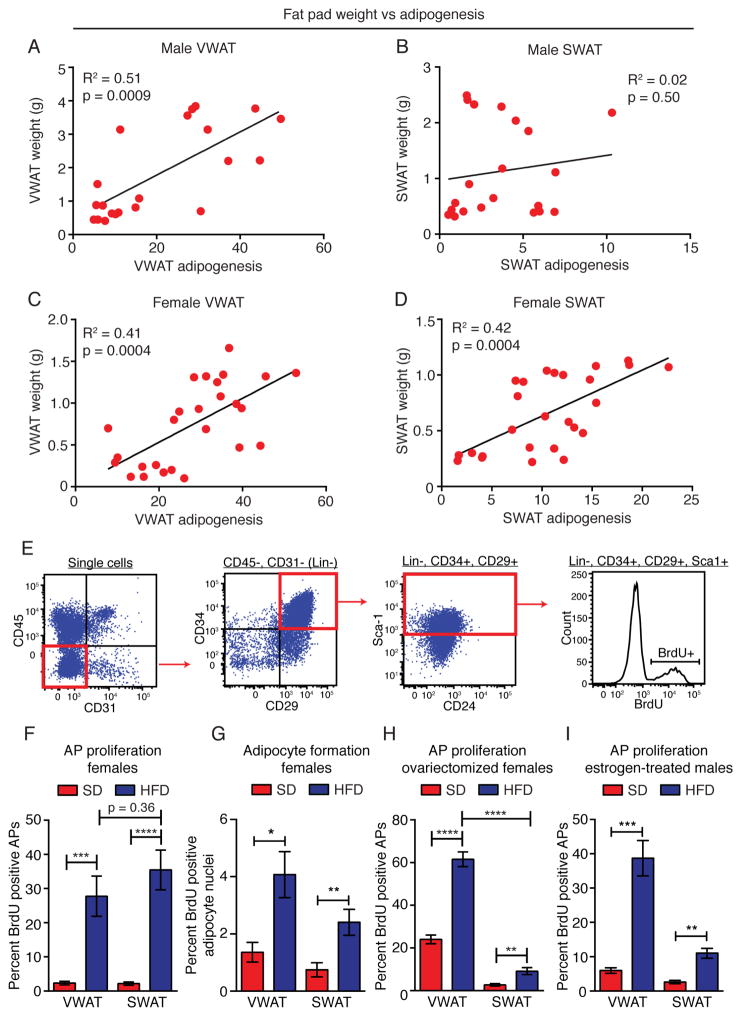
We next directly assessed the relationship between adipocyte formation and WAT depot mass. Indeed, comparison of depot size to adipocyte hyperplasia in individual animals shows that in male VWAT, female VWAT, and female SWAT depots, adipocyte formation is significantly correlated with fat pad size after 8 weeks of diet (Figure 2A–D). Furthermore, whole body fat mass is significantly correlated with adipogenesis within these depots (Figure S2). Conversely, there is no correlation between adipocyte formation and either fat pad mass or body fat mass in the non-adipogenic male SWAT depot (Figure 2B and S2B). These data indicate that adipocyte hyperplasia significantly contributes to depot- and sex-specific WAT growth in obesity.
Figure 2. Fat pad weight and adipocyte precursor activation mirror depot-specific patterns of adipogenesis.
(A–D) Correlation between adipogenesis and fat pad weight in the indicated depots of male (AB) or female (C–D) mice. Each point represents one mouse. (n = 21–26) (E) Representative flow cytometry plots to measure BrdU incorporation into adipocyte precursors. (F) BrdU incorporation into APs from the indicated depot of female mice following one week of SD or HFD and BrdU treatment. (n = 5 per group) (G) BrdU incorporation into adipocyte nuclei as measured via immunofluorescence in paraffin sections following one week of BrdU treatment (pulse) and 7 more weeks of the indicated diet (chase). (n = 8–10) (H–I) BrdU incorporation into APs from the indicated depot of ovariectomized female (H) or estrogen-treated male (I) mice following one week of SD or HFD and BrdU treatment. (n = 5–10) Significance in A–D was determined using Spearman’s non-parametric two-tailed correlation analysis. Significance in F–I was determined by comparing the indicated groups using an unpaired two-tailed student’s t-test. Error bars represent mean ± S.E.M. See also Figure S2.
The production of new adipocytes in vivo occurs through the proliferation and differentiation of APs within the adipose tissue stroma (Jeffery et al., 2015; Rodeheffer et al., 2008). In males, HFD feeding induces the transient proliferation of APs specifically in VWAT during the first week of HFD feeding, followed by adipogenesis within this depot (Jeffery et al., 2015). To determine whether the same mechanism of transient AP activation also occurs in female mice upon HFD feeding, we assessed the incorporation of bromodeoxyuridine (BrdU) into APs via flow cytometry (Figure 2E). We find that in both the female SWAT and VWAT depots, HFD feeding induces rapid and transient proliferation of APs (Figure 2F and S2E–F), similar to male VWAT (Jeffery et al., 2015). Furthermore, analysis of mature adipocyte nuclei after a 1-week BrdU pulse and 7-week chase indicates that HFD induces significant adipocyte formation from proliferative precursors in both the SWAT and VWAT depots of female mice (Figure 2G), whereas males on HFD display increased BrdU-positive adipocyte formation in VWAT (Jeffery et al., 2015), but not SWAT (Figure S2G).
Interestingly, after ovariectomy females display a more male-like pattern of AP activation in response to HFD, with significantly reduced AP proliferation in SWAT (Figure 2H), which suggests that sex hormones play a role in the activation of SWAT APs. To test whether estrogen influences depot-specific AP activation, we administered estrogen to male mice via mini osmotic pumps and assessed their AP proliferation response to HFD feeding. We find that estrogen treatment of males results in significantly increased AP proliferation in SWAT upon HFD feeding (Figure 2I), suggesting that estrogen influences the cellular response of the SWAT depot to diet. Taken together, these data show that adipocyte hyperplasia, mediated by the proliferation and differentiation of APs, is an important component of depot- and sex-specific WAT growth in obesity.
The adipose microenvironment determines adipocyte precursor activation
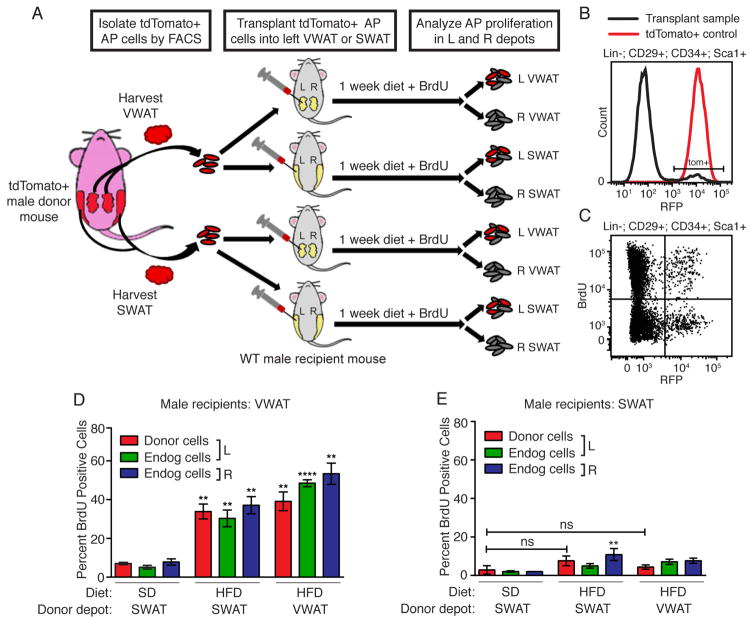
We next investigated the mechanisms regulating depot-specific AP activation. Previous in vitro studies suggest that APs from SWAT and VWAT exhibit cell-intrinsic differences that control their response to environmental cues and differentiation potential (Baglioni et al., 2012; Loh et al., 2015; Macotela et al., 2012). To determine whether cell-intrinsic phenomena explain depot-specific AP activation in vivo, we performed a series of AP transplant experiments (Figure 3A). In these experiments, APs were isolated from the VWAT or SWAT of tdTomato-expressing mice by fluorescence-activated cell sorting (FACS), and then transplanted into either the VWAT or SWAT of congenic wild type recipient mice. After recovery, the recipient mice were placed on either HFD or SD for 1 week, and the proliferation of tdTomato+ donor APs and tdTomato− endogenous APs was determined by BrdU incorporation. Flow cytometry analysis of APs demonstrates engraftment of tdTomato+ donor APs in recipient depots and permits the clear distinction between donor and recipient AP populations (Figure 3B–C and S3A–B). In recipient mice fed SD, neither donor nor endogenous APs are activated in either depot, indicating that transplantation alone does not induce AP proliferation (Figure 3D–E). In recipient mice fed HFD, APs derived from VWAT depots display high levels of proliferation when injected back into the VWAT depot, similar to endogenous cells and APs in the uninjected contralateral depot, supporting the ability of the transplanted cells to be activated by obesogenic stimuli (Figure 3D). Surprisingly, SWAT-derived donor AP cells are also induced to proliferate after HFD feeding when cells are placed in the VWAT microenvironment (Figure 3D), but neither VWAT nor SWAT-derived APs proliferate significantly when injected back into SWAT (Figure 3E). Furthermore, when male SWAT APs are injected into female SWAT, their proliferation is induced by HFD (Figure S3C), despite lower levels of engraftment (35% – 11/31) and increased background proliferation, which may be caused by sex mismatched transplants (Volk et al., 2015; Weiss et al., 2009). These data indicate that despite their separate developmental lineages (Chau et al., 2014; Krueger et al., 2014), the depot-specific response of APs to HFD feeding is determined by the WAT depot microenvironment, not by cell-intrinsic properties of APs within distinct depots.
Figure 3. Adipocyte precursor activation at the onset of obesity is determined by the adipose depot microenvironment.
(A) Experimental design for AP transplant experiment. (B–C) Representative flow cytometry histogram (B) and dot plot (C) demonstrating engraftment of donor-derived tdTomato+ APs in the recipient depot, and BrdU incorporation into both tdTomato− and tdTomato+ populations. (D–E) BrdU incorporation into APs in donor, endogenous, or contralateral populations after 1 week of the indicated diet and BrdU treatment. (n = 3–8) Astrisks indicate significance over SD controls. Significance was determined using one-way ANOVA. Error bars represent mean ± S.E.M. See also Figure S3.
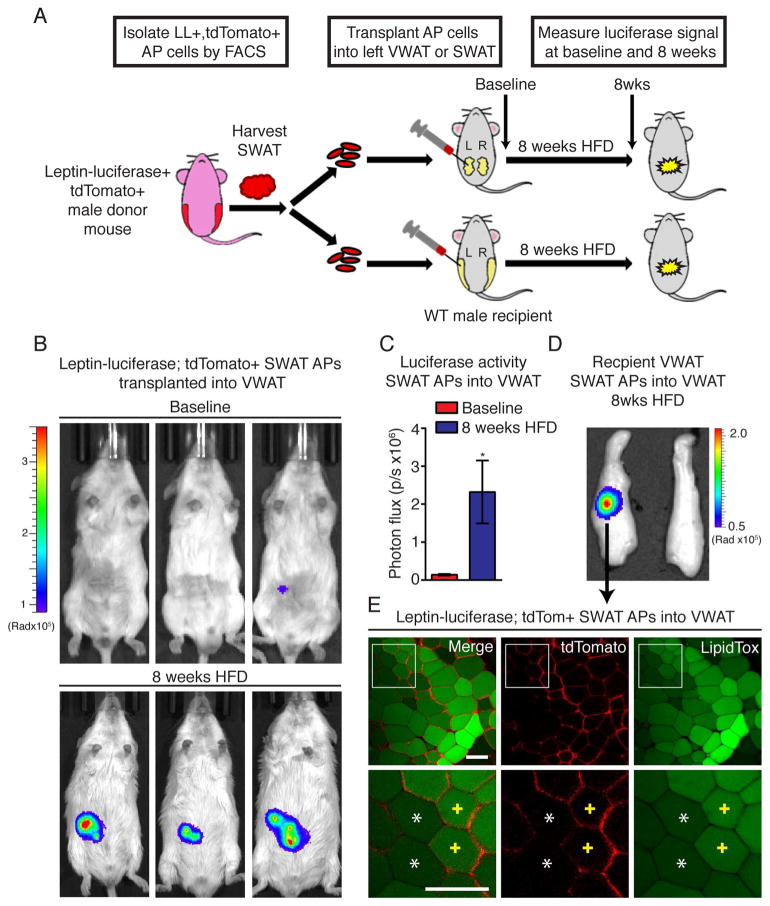
The depot-specific regulation of both AP proliferation (Figure 2–3 and (Jeffery et al., 2015)) and adipogenesis (Figure 1) suggests that AP differentiation is also controlled by the adipose depot microenvironment. To test this possibility, we performed transplant experiments with APs derived from the SWAT of male mice expressing luciferase under the control of the mature adipocyte-specific leptin promoter (Figure S3A and (Rodeheffer et al., 2008)), as well as the mT/mG cassette (leptin-luciferase; mT/mG mice). These mice allow both non-invasive assessment of adipocyte formation via luciferase activity and direct observation of transplanted cells via tdTomato fluorescence. Importantly, male mice with this genetic background (FVB/NJ) still display a VWAT-specific AP proliferation pattern in response to HFD feeding (Figure S4B). Leptin-luciferase; mT/mG SWAT APs were transplanted into the VWAT or SWAT of congenic wild type male recipient mice, and after recovery, mice were placed on HFD for 8 weeks. Luciferase signal was monitored after transplant of APs (baseline) and after 8 weeks of HFD feeding (Figure 4A). Excitingly, we find that upon HFD feeding, SWAT-derived APs display significantly increased adipogenesis when transplanted into the VWAT microenvironment (Figure 4B–C), but not when transplanted back into SWAT, despite clear engraftment of injected cells (Figure S4C–E). Furthermore, confocal analysis of adipose tissue from the luminescent region of the injected VWAT fat pad shows the presence of lipid-filled, tdTomato+ adipocytes, (Figure 4D), further confirming that HFD induces the differentiation of SWAT-derived APs in the VWAT microenvironment.
Figure 4. High-fat diet induced adipogenesis of adipocyte precursors is determined by the adipose depot microenvironment.
(A) Experimental design for AP transplant experiment. (B) Representative images of mice with luminescent signal overlay following luciferase injection at the indicated time following transplant of APs from the SWAT of leptin-luciferase; mTmG mice. (C) Quantification of luminescent signal at the indicated experimental time points following transplant of APs from the SWAT of leptin-luciferase; mTmG mice. (n = 12) (D) Representative image of VWAT depot with luminescent overlay showing the region with luciferase activity in the left pad where the transplant was performed. (E) Representative confocal images of the luminescent region in (D) showing tdTomato+ mature adipocytes with LipidTOX staining (yellow crosses) adjacent to endogenous tdTomato− adipocytes (white asterisks). Exposure time for each luminescent image was 1 minute. Scale bar is 100μM. Significance was determined using an unpaired two-tailed student’s t-test. Error bars represent mean ± S.E.M. See also Figure S4.
Discussion
Taken together, these data show that AP proliferation in response to HFD is regulated by cell-extrinsic factors in the depot microenvironment. In the inguinal SWAT depot, systemic levels of estrogen influence this response. The finding that APs from different WAT depots are functionally interchangeable in vivo suggests that developmental lineage does not irreversibly determine depot-specific AP behavior. Rather, APs are functionally plastic cells that are influenced by their physiologic context.
We employed the Adiponectin-creER model to study WAT dynamics, which utilizes tamoxifen treatment for cre activation and cell tracing. While higher doses of tamoxifen have detrimental effects on WAT (Ye et al., 2015), reports are conflicting about how the lower tamoxifen dose used here affects WAT (Hesselbarth et al., 2015; Liu et al., 2015). To control for the potential effects of tamoxifen treatment in the Adiponectin-creER experiments, both SD and HFD groups were treated with tamoxifen. Furthermore, if the lower doses of tamoxifen used here had long-term effects on creER activation, as has been shown for higher doses (Ye et al., 2015), we would not detect any adipocyte formation in our lineage-tracing experiments. We also show similar patterns of depot-specific adipocyte formation using the tamoxifen-independent BrdU tracing assay, demonstrating that the trends in adipogenesis that we report are not an artifact of tamoxifen treatment.
Similar to our previous studies of male mice (Jeffery et al., 2015), the BrdU and cre-based techniques detect similar depot-specific trends in adipogenesis, but result in different quantitative measurements of adipocyte formation, with the BrdU method consistently displaying a much lower percentage of new cells formed. For example, in the VWAT of HFD-fed females, the Adiponectin-creER method identifies 35.8 ± 1.8% of adipocytes as newly formed, while the Brdu method only labels 4 ± 0.81% of mature adipocytes. This discrepancy is explained by WAT biology, as the cre-based lineage tracing method assesses the contribution of both post-mitotic preadipocytes and proliferative adipocyte progenitors, while the BrdU technique only assesses the contribution of proliferative cells. We have previously shown that within the WAT stromal vascular fraction, the abundant CD24− preadipocytes are post-mitotic, while the comparatively rare CD24+ adipocyte progenitors proliferate in response to obesogenic stimuli, but rapidly lose CD24 expression upon activation to generate and replenish the CD24− preadipocytes (Berry and Rodeheffer, 2013; Jeffery et al., 2015). This large pool of post-mitotic preadipocytes can thus contribute to adipocyte formation without re-entering the cell cycle and incorporating BrdU. As a result, the cre-based method assesses the adipogenic capacity of both CD24+ and CD24− adipocyte precursor populations combined, while the BrdU assay only quantifies the contribution of the CD24+ adipocyte progenitors within the limited time window of the experiment. Therefore, the elevated assessment of adipogenesis in the cre model compared to the BrdU assay is accounted for by the differentiation of the large pool of committed, post-mitotic preadipocytes that were formed from the proliferative CD24+ adipocyte progenitors prior to the initiation of the experiment.
The clear links between depot- and sex-specific adipogenesis, AP activation, and fat pad weight demonstrate the important role that AP regulation and adipogenesis play in obesogenic WAT growth. Future studies aimed to define these regulatory signals and compare their function in adipogenic and non-adipogenic adipose tissue microenvironments may lead to novel approaches to control adipose tissue distribution in a manner that favors metabolically beneficial SWAT growth over detrimental VWAT growth.
Experimental Procedures
Animals
All animal studies followed guidelines issued by Yale University’s Institutional Animal Care and Use Committee (IACUC). See supplemental methods for details on mouse strains. VWAT refers to the perigonadal WAT and SWAT refers to inguinal WAT in mice. For BrdU experiments, mice were given 0.4–0.8 mg/mL BrdU in drinking water, refreshed every 2 days. High fat diet is from Research Diets (D12492). Standard diet is from Harlan Laboratories (2018S). Analysis of body composition was performed by Echo MRI (Echo Medical System, Houston, TX). For estrogen treatment, 8-week old male mice were implanted with mini osmotic pumps (Alzet 1004) delivering cyclodextran-coated estrogen (Sigma E4389) at a dose of 2μg/kg/day in PBS. Mice were allowed to recover for two weeks after pump implantation prior to experiment initiation.
Transplant assay
0.5–1 million APs were isolated from the indicated depot of male mice and injected into the indicated pad of 3–6 week old congenic wild type mice. After recovery, mice were placed on HFD or SD and treated with BrdU for 1 week then sacrificed or maintained on diet for 8 weeks prior to analysis. Left and right adipose tissue depots were excised and analyzed separately via flow cytometry, microscopy or luminescence (see supplemental methods).
Flow cytometry
Flow cytometry preparations were performed as described in (Jeffery et al., 2015) for BrdU analysis and (Berry and Rodeheffer, 2013) for cell sorting.
Confocal microscopy
The mT/mG quantification experiments were performed as described in (Jeffery et al., 2015), starting the 50mg/kg tamoxifen treatments at 8 weeks of age. See the supplemental methods for further details. For analysis of leptin-luciferase; tdTomato+ adipocytes following transplantation, tissue from the luminescent region of the VWAT was dissected and stained with HCS LipidTOX Green Neutral Lipid Stain (Invitrogen, H34475, used at 1–100) for at least 30 minutes before being washed in PBS and mounted onto slides in Fluoromount-G (Southern Biotech; 0100-01).
Statistical analysis
Statistical tests used are indicated in the figure legends. All statistical tests were performed using GraphPad Prism version 6.02 for Windows (GraphPad Software). Data are expressed as mean ± SEM, and P < 0.05 was considered statistically significant. Outliers were identified with the ROUT method test using GraphPad Prism software, setting Q to 10%. Sample sizes for main figures are listed in Table S1, and indicate individual animals (biological replicates).
Supplementary Material
Highlights.
High-fat diet feeding induces sex-specific patterns of adipogenesis.
The amount of obesogenic adipogenesis positively correlates with fat pad weight.
Estrogen potentiates obesogenic adipogenesis in the inguinal subcutaneous depot.
Depot-specific adipogenesis is regulated by the adipose tissue microenvironment.
Acknowledgments
We thank Ewa Menet in the Yale Flow Cytometry facility for her technical assistance. This work was supported by Women’s Health Research at Yale, NIDDK grant DK090489, ADA Award 7-12-JF-46, and Yale DERC DK045735 to MSR, and Lo Fellowships for Excellence in Stem Cell Research to RB and EJ.
Footnotes
Author Contributions
EJ and MSR designed experiments. RB generated the FVB/NJ; Leptin-luciferase: mT/mG mice. EJ, AW, LC and CDC performed experiments. EJ, AW, BH, JK, RS and ZS analyzed data. EJ, AW and MSR interpreted data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Andersson DP, Thorne A, Wiren M, Hoffstedt J, Naslund E, Thorell A, Ryden M. Variations in the size of the major omentum are primarily determined by fat cell number. J Clin Endocrinol Metab. 2013;98:E897–901. doi: 10.1210/jc.2012-4106. [DOI] [PubMed] [Google Scholar]
- Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F, et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012;7:e36569. doi: 10.1371/journal.pone.0036569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, Rossetti L. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19:8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho T, Goel K, Correa de Sa D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57:1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Foster MT, Shi H, Seeley RJ, Woods SC. Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physiol Behav. 2010;101:282–288. doi: 10.1016/j.physbeh.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Shi H, Seeley RJ, Woods SC. Removal of intra-abdominal visceral adipose tissue improves glucose tolerance in rats: role of hepatic triglyceride storage. Physiol Behav. 2011;104:845–854. doi: 10.1016/j.physbeh.2011.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Softic S, Caldwell J, Kohli R, de Kloet AD, Seeley RJ. Subcutaneous Adipose Tissue Transplantation in Diet-Induced Obese Mice Attenuates Metabolic Dysregulation While Removal Exacerbates It. Physiol Rep. 2013;1 doi: 10.1002/phy2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015;23:1345–1352. doi: 10.1002/oby.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely I, Barzilai N. Surgical removal of visceral adipose tissue: effects on insulin action. Curr Diab Rep. 2003;3:201–206. doi: 10.1007/s11892-003-0064-3. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 2010;34:989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmann BL, Lissner L. Hip Hip Hurrah! Hip size inversely related to heart disease and total mortality. Obes Rev. 2011;12:478–481. doi: 10.1111/j.1467-789X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- Hesselbarth N, Pettinelli C, Gericke M, Berger C, Kunath A, Stumvoll M, Blüher M, Klöting N. Tamoxifen affects glucose and lipid metabolism parameters, causes browning of subcutaneous adipose tissue and transient body composition changes in C57BL/6NTac mice. Biochem Biophys Res Commun. 2015;464:724–729. doi: 10.1016/j.bbrc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Jeffery E, Berry R, Church CD, Yu S, Shook BA, Horsley V, Rosen ED, Rodeheffer MS. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte. 2014;3:206–211. doi: 10.4161/adip.29674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou K, Fried SK. Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr Atheroscler Rep. 2013;15:361. doi: 10.1007/s11883-013-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou K, Fried SK, Xie H, Lee MJ, Divoux A, Rosencrantz MA, Chang RJ, Smith SR. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab. 2013;98:362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KC, Costa MJ, Du H, Feldman BJ. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports. 2014;3:1147–1158. doi: 10.1016/j.stemcr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Beretvas SN, Freeland-Graves JH. Abdominal adiposity distribution in diabetic/prediabetic and nondiabetic populations: a meta-analysis. J Obes. 2014;2014:697264. doi: 10.1155/2014/697264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zou P, Zheng L, Linarelli LE, Amarell S, Passaro A, Liu D, Cheng Z. Tamoxifen reduces fat mass by boosting reactive oxygen species. Cell Death Dis. 2015;6:e1586. doi: 10.1038/cddis.2014.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh NY, Neville MJ, Marinou K, Hardcastle SA, Fielding BA, Duncan EL, McCarthy MI, Tobias JH, Gregson CL, Karpe F, et al. LRP5 regulates human body fat distribution by modulating adipose progenitor biology in a dose- and depot-specific fashion. Cell Metab. 2015;21:262–272. doi: 10.1016/j.cmet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond) 2012;36:262–272. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Almeras N, Despres JP. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LK, Prins JB. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. 2008;10:156–164. doi: 10.1007/s11906-008-0029-7. [DOI] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res. 2003;11:104–111. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk V, Schneider A, Spineli LM, Grosshennig A, Stripecke R. The gender gap: discrepant human T-cell reconstitution after cord blood stem cell transplantation in humanized female and male mice. Bone Marrow Transplant. 2015 doi: 10.1038/bmt.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ES, Allen JG, Patel ND, Russell SD, Baumgartner WA, Shah AS, Conte JV. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation: analysis of 18 000 transplants in the modern era. Circ Heart Fail. 2009;2:401–408. doi: 10.1161/CIRCHEARTFAILURE.108.844183. [DOI] [PubMed] [Google Scholar]
- Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Wang QA, Tao C, Vishvanath L, Shao M, McDonald JG, Gupta RK, Scherer PE. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab. 2015;4:771–778. doi: 10.1016/j.molmet.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.