SUMMARY
The molecular mechanisms underlying the regulation of pluripotency by cellular metabolism in human embryonic stem cells (hESCs) are not fully understood. We found that high levels of glutamine metabolism are essential to prevent degradation of OCT4, a key transcription factor regulating hESC pluripotency. Glutamine withdrawal depletes the endogenous anti-oxidant glutathione, which results in the oxidation of OCT4 cysteine residues required for its DNA binding and enhanced OCT4 degradation. The emergence of the OCT4lo cell population following glutamine withdrawal did not result in greater propensity for cell death. Instead, glutamine withdrawal during vascular differentiation of hESCs generated cells with greater angiogenic capacity, thus indicating that modulating glutamine metabolism enhances the differentiation and functional maturation of cells. These findings demonstrate that the pluripotency transcription factor OCT4 can serve as a metabolic-redox sensor in hESCs and that metabolic cues can act in concert with growth factor signaling to orchestrate stem cell differentiation.
eTOC BLURB
Previous studies have shown a connection between energy metabolism and pluripotency in human embryonic stem cells. Marsboom et al. provide a molecular explanation for this observation and argue that the pluripotency regulator OCT4 is a redox-based metabolic sensor that undergoes oxidation and degradation during glutamine withdrawal.
INTRODUCTION
Human embryonic stem cells (hESCs) derived from the inner cell mass of the blastocyst give rise to cells from three germ layers when exposed to specific differentiation cues (Thomson et al., 1998). Energy metabolism in hESCs depends largely on cytosolic glycolysis (Turner et al., 2014), resembling the Warburg effect observed in cancer cells (Hsu and Sabatini, 2008). Evidence for the intertwined nature of pluripotency and metabolism comes from the observation that dedifferentiation of somatic cells into induced pluripotent stem cells (iPSCs) is accompanied by changes in energy metabolism, that are both crucial and precede the induction of pluripotency (Folmes et al., 2011). Human ESCs also have low levels of reactive oxygen species (ROS), which is important to maintain pluripotency (Cho et al., 2006; Ji et al., 2010; Song et al., 2014). Therefore, stem cell function is correlated both with energy metabolism and reactive oxygen species levels, however the molecular mechanisms behind this interaction are not clear (Perales-Clemente et al., 2014; Teslaa and Teitell, 2015).
At the core of maintaining pluripotency is the octamer-binding transcription factor 4 (OCT4), which is essential for the formation of the inner cell mass of the blastocyst (Nichols et al., 1998) and the generation of iPSCs (Yu et al., 2007). OCT4 is encoded by the POU5F1 gene, a member of the POU family of transcription factors that consists of 2 DNA binding domains capable of independently recognizing half-sites of an octameric DNA sequence motif. OCT4 binds to more than 600 promoters usually together with SOX2 and NANOG and can both induce gene expression related to pluripotency as well as silence genes involved in differentiation (Boyer et al., 2005). Depletion of OCT4 leads to spontaneous differentiation (Hay et al., 2004; Matin et al., 2004; Niwa et al., 2000) and regulation of OCT4 by growth factor signaling has been well described (Rizzino, 2013; Xu et al., 2005).
We demonstrate here that glutamine metabolism is a crucial regulator of pluripotency in hESCs because it directly regulates OCT4 oxidation and degradation. Glutamine is taken up avidly in hESCs (as shown by stable isotope-assisted metabolomic analysis) and glutamine metabolism is downregulated upon differentiation. Glutamine is required for maintaining high levels of the intracellular anti-oxidant glutathione and glutamine depletion leads to increased ROS levels, resulting in OCT4 cysteine oxidation. Oxidation of OCT4 leads to its rapid degradation and cysteines 185, 198, 221, and 252 were shown by site-directed mutagenesis to be essential for DNA binding of OCT4. Lowering OCT4 levels significantly enhanced endothelial differentiation and increased endothelial cell sprouting and neovascularization in vivo. Our findings therefore demonstrate that the metabolic pathways that are modulated during differentiation directly regulate OCT4, reinforcing and stabilizing the process of differentiation via a metabolism-differentiation feedback loop.
RESULTS
OCT4 Protein Levels are Regulated by Glutamine Metabolism
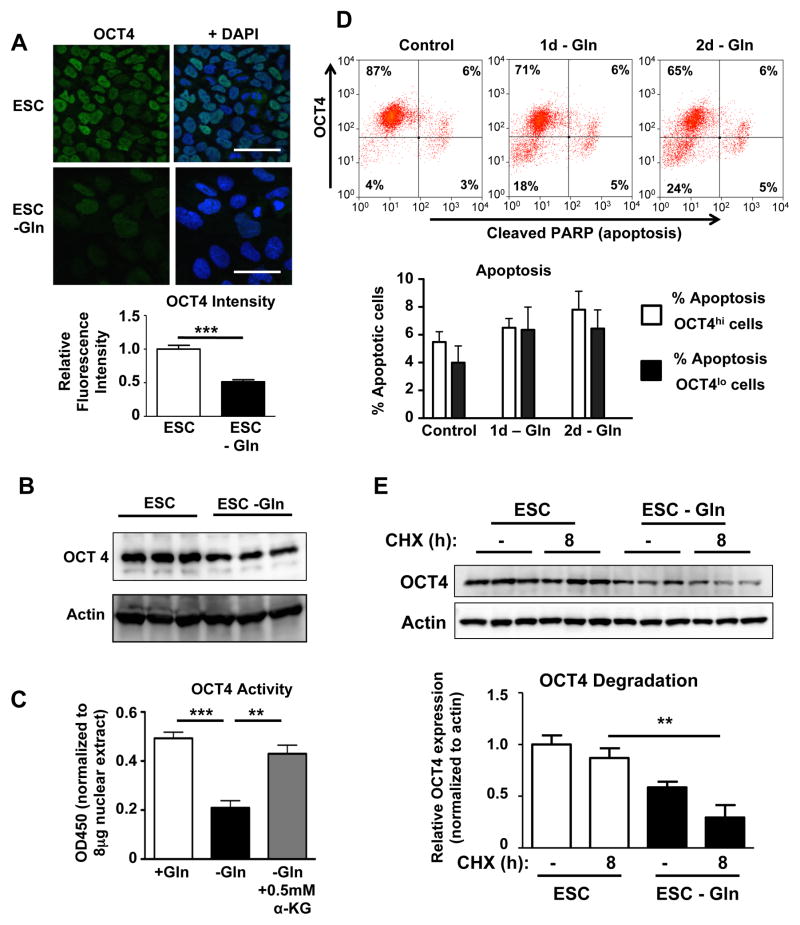
As cytosolic glycolysis leads to lactate production and secretion, other carbon sources are necessary to synthesize lipids and amino acids in a process referred to as anaplerosis (DeBerardinis et al., 2008). Glutamine is the highest consumed amino acid in hESCs (Christensen et al., 2014) and it improves the generation of embryos after in vitro fertilization (Devreker et al., 1998). Moreover, the expression of SLC1A5 and GLS2, respectively the major glutamine uptake transporter and mitochondrial glutaminase involved in converting glutamine into glutamate (Hensley et al., 2013), decreases during spontaneous differentiation (Figure S1A). Therefore, we decided to investigate the relationship between glutamine metabolism and pluripotency. Interestingly, glutamine withdrawal by itself led to a marked downregulation of OCT4 protein expression in hESCs (Figure 1A–B). In contrast to OCT4, the protein levels of the pluripotency regulator NANOG were not influenced by glutamine withdrawal (Figure S1B). We confirmed the functional significance of decreased OCT4 protein expression by showing lower OCT4 DNA binding activity (Figure 1C). Supplying alpha-ketoglutarate (α-KG), an intracellular metabolite of glutamine, rescued OCT4 DNA binding activity in glutamine-depleted hESCs. This suggested that the anaplerotic uptake of glutamine-derived carbon atoms into the TCA cycle or the generation of other alpha-ketoglutarate-derived metabolites was crucial for maintaining OCT4 levels. The downregulation of OCT4 could be either due to the decreased expression of OCT4 in hESC subsets or OCT4hi cells may selectively undergo cell death. We therefore performed intracellular flow cytometric analysis of OCT4 and cleaved PARP, a reliable marker of apoptosis (Figure S1C). We observed the appearance of an OCT4lo population as soon as 1 day after glutamine withdrawal, but importantly there was no significant increase in apoptosis with glutamine withdrawal (Figure 1D) and we also did not observe any significant difference in cell death between OCT4lo and OCT4hi cells. This indicates that the glutamine withdrawal-induced decrease of OCT4 is driven by the appearance of an OCT4lo cell population and not due to selective cell death. The reduction in OCT4 protein was also not due to changes in OCT4 mRNA levels (Figure S1D) but rather a consequence of accelerated OCT4 protein degradation (Figure 1E and Figure S1E). In contrast, NANOG half-life was not altered by glutamine withdrawal (Figure S1F). During spontaneous differentiation, OCT4 levels decreased only slightly after 4 days whereas glutamine withdrawal significantly accelerated OCT4 degradation during spontaneous differentiation and the magnitude of the glutamine effect on OCT4 downregulation was more profound than that of spontaneous differentiation alone (Figure S2A). We also determined whether the observed OCT4 loss was due to an overall decrease in the energy state of the cells. We found that in the absence of glutamine, hESCs were able to use glucose to maintain their ATP levels, thus indicating that the glutamine regulation of OCT4 levels was not secondary to a drop in ATP levels (Figure S2B).
Figure 1. Glutamine regulates OCT4 expression in hESCs.
(A) Immunofluorescent staining for OCT4 in hESCs cultured with or without glutamine for 4d showed marked decreases in the protein level of OCT4 in response to glutamine withdrawal. Images were obtained at the same magnification. Scale bar, 20μm. n=48–50 cells/group. (B) Immunoblot showing downregulation of OCT4 in hESCs cultured in the absence of glutamine for 4d. (C) OCT4 DNA binding activity to its canonical DNA sequence is decreased after 2d of glutamine withdrawal, indicating reduced OCT4 activity. Alpha-ketoglutarate (α-KG) restored normal OCT4 DNA binding, showing that the reduction in OCT4 activity is not a non-specific response to glutamine withdrawal. n=4/group. (D) Flow cytometry for OCT4 and cleaved PARP, a marker of apoptosis. Results show that a group of OCT4lo cells is present after just 1 day of glutamine withdrawal. At the same time, OCT4hi cells are not apoptotic, indicating that selective death of OCT4 high cells is not an explanation for the observed overall OCT4 downregulation. n=8/group. (E) Protein degradation was assessed using the protein synthesis inhibitor cycloheximide (CHX). In medium with glutamine, OCT4 protein levels were stable after 8h of CHX treatment, indicating a protein half-life of greater than 8h. Glutamine withdrawal for 2d lowered the baseline OCT4 protein level and enhanced its degradation leading to a protein half-life of approximately 8h. For a time course of OCT4 protein levels after CHX treatment, please see Figure S1E. *P<0.05, **P<0.01, ***P<0.001; Unpaired Student’s t-test for comparison between 2 groups, ANOVA with post hoc Tukey’s test for multiple group comparisons. Error bars show s.e.m.
Glutamine-Derived Glutathione is Required to Keep ROS Levels Low in Human ESCs while Increased ROS Leads to a Rapid Downregulation of OCT4
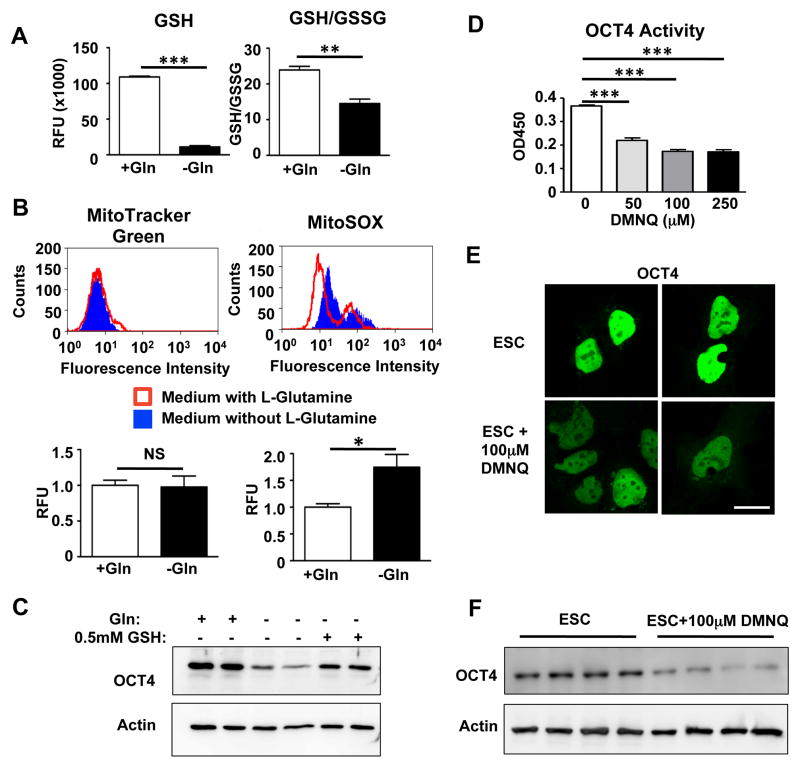
Because glutamine is a precursor of glutamate, a building block of the cellular antioxidant glutathione (GSH) (Sies, 1999), we next investigated whether OCT4 is oxidized after glutamine withdrawal. Glutathione levels were markedly lower after glutamine withdrawal (Figure 2A) and resulted in the oxidation of GSH to glutathione disulfide (GSSG, Figure 2A). We also used the mitochondrial superoxide-specific probe MitoSOX and found increased ROS levels in the absence of glutamine, confirming a more oxidized cellular environment (Figure 2B). To prove that a shift towards an oxidized redox state in the ESCs mediated the observed loss of OCT4, we cultured ESCs in presence of cell-permeable glutathione. Addition of glutathione rescued OCT4 degradation induced by glutamine withdrawal (Figure 2C), confirming the importance of GSH in maintaining cellular OCT4 levels. Finally, to demonstrate that increased ROS by itself can lead to reduced OCT4 activity, we incubated hESCs with DMNQ, a cell-permeable redox cycling quinone that leads to intracellular superoxide formation. We observed a dose-dependent decrease in OCT4 DNA binding within 5 hours (Figure 2D and Figure S2C) and a reduction of OCT4 protein levels (Figure 2E–F) clearly demonstrating that oxidation can lead to a rapid degradation of OCT4.
Figure 2. Glutamine withdrawal decreases anti-oxidant defenses and leads to OCT4 oxidation and inactivation.
(A) Glutamine withdrawal for 2d decreased intracellular glutathione (GSH) levels and decreased the ratio of reduced glutathione versus oxidized glutathione disulfide (GSSG). n=4/group. (B) Glutamine withdrawal for 2d increased mitochondrial superoxide levels as measured by MitoSOX fluorescence. MitoTracker Green signal intensity is not different between both groups, indicating equal total mitochondrial content. n=3/group. (C) Supplying cell-permeable glutathione prevents degradation of OCT4 induced by glutamine withdrawal. (D) OCT4 activity decreases in response to reactive oxygen species in cells treated for 5h with the intracellular superoxide generator DMNQ. n=4/group. (E) Treatment for 5h with 100μM DMNQ led to decreased OCT4 intensity in hESCs. Scale bar, 20μm. (F) Total OCT4 protein levels decrease after incubation with 100μM DMNQ for 5h indicating that oxidation leads to the rapid degradation of OCT4. n=4/group. *P<0.05, **P<0.01, ***P<0.001; Unpaired Student’s t-test for comparison between 2 groups and ANOVA with post hoc Dunnett’s test for multiple group comparisons. Error bars show s.e.m.
Cysteines Play a Crucial Role in DNA Binding of OCT4 and are Oxidized After Glutamine Withdrawal
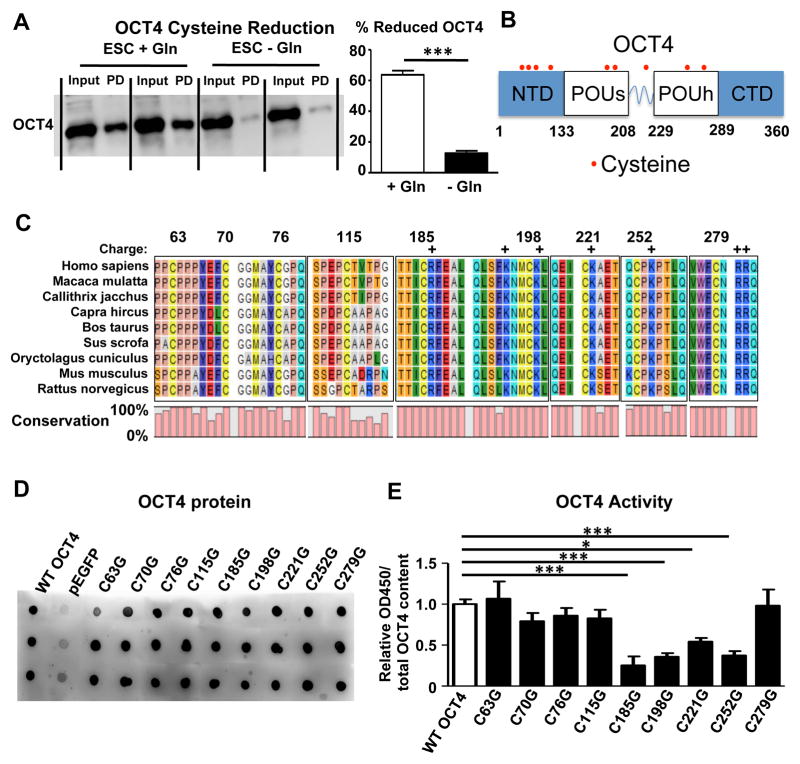
Cysteines are especially vulnerable to oxidative modifications (Cremers and Jakob, 2013) and regulation of transcription factor DNA binding activity by cysteine oxidation has been described for several transcription factors including p53, AP-1, and NF-κB (Abate et al., 1990; Hainaut and Milner, 1993; Matthews et al., 1992; Rainwater et al., 1995; Sun and Oberley, 1996). To investigate whether cysteine oxidation of OCT4 is a regulatory target of glutamine metabolism, we incubated protein lysates with maleimide-biotin to label reduced cysteine groups. Pulldown of biotin-labeled proteins followed by an immunoblot for OCT4 shows that OCT4 cysteines were oxidized after 2 days of glutamine withdrawal (Figure 3A). We observed a similar pattern of OCT4 oxidation during spontaneous differentiation in the absence of glutamine (Figure S2D). Of the 9 cysteines in OCT4, 4 are found in the N-terminal domain, while the DNA binding POUs and POUh domains each contain 2 cysteines. One final cysteine is present in the linker region between both DNA binding domains (Figure 3B). Since it is unknown which cysteines regulate OCT4 transcriptional activity, we first performed an alignment of OCT4 proteins from different mammal species. Importantly, all 9 cysteines are conserved across species further underscoring the importance of redox-sensitive cysteines as a conserved regulatory mechanism (Figure 3C). The reactivity of cysteines is enhanced by positively charged amino acids in their vicinity (Britto et al., 2002) and several cysteines contain either arginine or lysine immediately adjacent to them (Figure 3C). We then performed separate site-directed mutagenesis to convert each of the 9 cysteines present in OCT4 to glycine (Figure S2E). Transfection led to a uniform overexpression of OCT4 (Figure 3D). However, in vitro DNA binding activity was decreased when cysteines 185, 198 (in the POUs domain), 221 (in the linker region), and 252 (in the POUh domain) were mutated (Figure 3E) and all of these cysteines contain a positively charged amino acid in their vicinity (Figure 3C).
Figure 3. Cysteines 185, 198, 221, and 252 are required for DNA binding of OCT4.
(A) Protein lysates of hESCs were incubated with maleimide-biotin to label reduced cysteine groups. Pulldown of biotin-labeled proteins is followed by an immunoblot for OCT4. For each sample, total nuclear extract (Input) and pulled down (PD) or reduced OCT4 are shown side by side. The percentage of reduced OCT4 is calculated after measuring the relative band intensities. Glutamine withdrawal for 2d leads to a marked reduction in reduced cysteines as significantly less OCT4 is pulled down. n=5/group. (B) Schematic representation of OCT4 with cysteine amino acids indicated. In total, there are 9 cysteines in OCT4, 4 in the N-terminal domain (NTD), 2 in the POUs DNA binding domain, 1 in the linker and 2 in the POUh DNA binding domain. There are no cysteines in the C-terminal domain (CTD). (C) Sequence alignment of OCT4 from different mammals reveals that all 9 cysteines of OCT4 are conserved across species. Since the reactivity of cysteines is enhanced by positively charged amino acids like arginine (R) and lysine (K), we indicated those amino acids with a + sign. (D) Human OCT4 cDNA was cloned into the pEGFP vector and then site-directed mutagenesis was used to separately mutate each of the cysteines into glycine. Immunoblotting confirmed a robust overexpression of OCT4 for each of the mutations in HEK293T cells. (E) OCT4 activity in nuclear isolates was assessed after transfection. Mutation of Cysteines 185, 198, 221, and 252 each led to a significant reduction in DNA binding. The average empty vector measurement was subtracted from all sample measurements and the resulting value was normalized to the OCT4 expression level of each sample. n=4–6/group. *P<0.05, **P<0.01, ***P<0.001; Unpaired Student’s t-test for comparison between 2 groups and ANOVA with post hoc Dunnett’s test for multiple group comparisons. Error bars show s.e.m.
Glutamine Withdrawal and OCT4 Degradation Promote Endothelial Differentiation and Angiogenesis
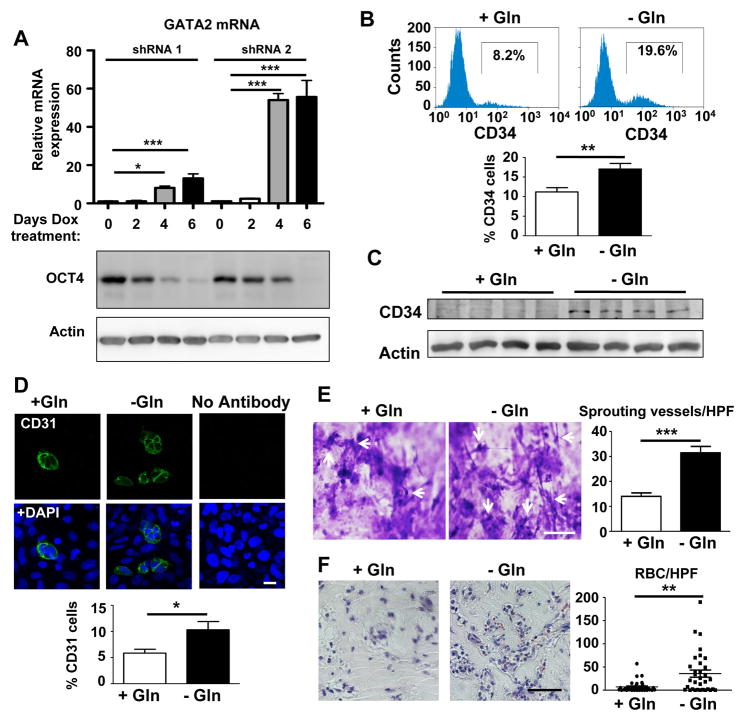
Downregulation of the pluripotency factor OCT4 secondary to a shift away from glutamine metabolism could serve as metabolic cue towards a differentiated state. To specifically evaluate the influence of OCT4 downregulation, we generated stable hESC cell lines expressing doxycycline-inducible shRNA against OCT4 (for validation see Figure S3A). We then cultured cells for different days in the presence of doxycycline and assessed expression of the transcription factors OTX2 (early ectodermal marker) (Zakin et al., 2000) and SOX17 (early mesendodermal marker) (Choi et al., 2012) as early indicators of differentiation. While ectodermal differentiation was not enhanced by the induced downregulation OCT4, we observed a clear increase in SOX17 expression (Figure S3B), thus suggesting that loss of OCT4 guides ESCs towards a mesendodermal lineage. To further evaluate mesendodermal differentiation, we investigated the expression of GATA2, a zinc finger transcription factor that is expressed during early differentiation of mesendodermal progenitors towards an endothelial lineage (Lugus et al., 2007). OCT4 downregulation resulted in marked increase of GATA2 (Figure 4A). Importantly, the order of magnitude of OCT4 reduction induced by individual shRNA clones after 4 days of induction (Figure 4A) was similar to that seen by glutamine withdrawal (Figure 1B), thus demonstrating that glutamine withdrawal results in functional decreases of OCT4 that can induce GATA2. Based on these findings, we hypothesized that endothelial differentiation of hESCs would be markedly enhanced following downregulation of glutamine metabolism and the resultant decrease in OCT4 activity.
Figure 4. Enhanced endothelial differentiation and angiogenesis in response to glutamine withdrawal.
(A) Stable hESC cell lines that express doxycycline-inducible shRNA against OCT4 were cultured in the presence of doxycycline for different days. Knockdown of OCT4 increases the zinc finger transcription factor GATA2, which is involved in early hemangioblast differentiation from mesodermal precursors, suggesting a mechanism by which OCT4 downregulation could lead to enhanced endothelial cell formation. Matching immunoblots are shown to indicate the residual level of OCT4 protein over time. Triplicate mRNA measurements were done for each clone at each time point. (B) Glutamine withdrawal during the final 2 days of a 4d endothelial differentiation protocol increases the percentage of CD34+ cells as shown by flow cytometry. n=6/group. (C) Immunoblot confirming the upregulation of CD34, indicative of a shift towards the endothelial cell lineage. n=4/group. (D) Quantification of CD31+ cells after endothelial differentiation of hESCs. Cells either received glutamine for the entire 4 days of differentiation or were differentiated in the absence of glutamine for the final 2 days. After differentiation, cells were subcultured onto collagen-coated coverslips and stained the next day for CD31. Quantification reveals a significant increase in the percentage of CD31+ cells. n=10 high power fields/condition. Scale bar, 20μm. (E) In vitro sprouting angiogenesis assay on collagen gels. Human ESCs were first differentiated for 4d and then 50,000 cells were plated on top of the collagen gel. After 72h, cells were fixed with 3% glutaraldehyde in PBS and invading sprouts were counted after staining with 0.1% toluidine blue in 30% methanol. Images were focused below the surface monolayer to visualize invading sprouts (white arrows). Sprouts were quantified by counting the number of sprouts per high power field (n=9 high power fields belonging to 3 different gels/group). Scale bar, 100μm. (F) Human ESCs were either differentiated towards endothelial cells for 4d (+Gln group) or exposed to glutamine withdrawal protocol during the final 2d of differentiation (-Gln group). Then equal numbers of cells were mixed with Matrigel and implanted subcutaneously for 7d. We observed increased formation of functional perfused blood vessels, measured by the number of red blood cells/high power field (RBC/HPF), in plugs with cells differentiated in the absence of glutamine (3 plugs/group with 10 high power fields/plug). Scale bar, 50μm. *P<0.05, **P<0.01, ***P<0.001; Unpaired Student’s t-test for comparison between 2 groups in panels B, D, E and ANOVA with post hoc Dunnett’s test for multiple group comparisons in panel A. Because data in panel F was not normally distributed (Kolmogorov-Smirnov test), a Mann Whitney test was used to compare RBCs/high power field. Error bars show s.e.m.
We developed an endothelial differentiation protocol consisting of 4 days of differentiation in the presence of VEGF and BMP4, which generates functional vascular endothelial cells with a typical cobblestone morphology and the ability to form vascular networks on Matrigel (Figure S3C). Moreover, cells uniformly expressed the endothelial markers VEGFR2, CD31 and VE-Cadherin (Figure S3D).
We first assessed the potential regulatory role of glutamine metabolism in endothelial differentiation by performing a stable isotopic study using [13C5]-labeled glutamine coupled with a metabolomic analysis. We found that ESC-derived endothelial cells demonstrate significantly lower entry of glutamine-derived metabolites into the TCA cycle, consistent with the idea that glutamine metabolism is downregulated during endothelial differentiation (Figure S4). This suggested that glutamine withdrawal could be used to enhance endothelial lineage commitment. Complete glutamine deprivation prevented successful endothelial formation, likely due to the fact that OCT4 activity is initially required to upregulate Brachyury, a transcription factor required for mesodermal differentiation (Karwacki-Neisius et al., 2013; Radzisheuskaya et al., 2013; Wang et al., 2012). However, targeted glutamine withdrawal during the second half of the 4 day differentiation protocol, once differentiating cells are committed to the mesodermal lineage, markedly increased the hematopoietic and endothelial marker CD34 (Figure 4B–C). The number of cells expressing the endothelial cell adhesion molecule CD31, a marker of endothelial maturation, also increased after targeted glutamine withdrawal (Figure 4D). To investigate the functional capacity of ESC-derived endothelial cells obtained under glutamine withdrawal, we used a collagen-based in vitro three-dimensional sprouting assay. We observed a significant enhancement of vessel sprouting in ESC-derived cells obtained under glutamine withdrawal (Figure 4E). This indicated that suppressing glutamine metabolism during ESC differentiation not only increased the number of endothelial cells but that they also exhibited increased capacity to form functional blood vessels. Importantly, we implanted hESC-derived endothelial cells into Matrigel plugs in immune-deficient NOD-SCID mice for 7 days to engineer human blood vessels in vivo. We found that hESCs differentiated with selective glutamine withdrawal demonstrated a 4-fold higher number of red blood cells in the newly formed blood vessels. This indicated that glutamine withdrawal during ESC differentiation increased the function of the engineered human blood vessels (Figure 4F).
DISCUSSION
We describe here an important mechanism by which glutamine metabolism regulates hESC pluripotency via redox-dependent oxidation and degradation of the transcription factor OCT4. We observed that genes involved in glutamine uptake and metabolism are highly expressed in undifferentiated hESCs compared to spontaneously differentiated cells. We also observed that glutamine is converted more efficiently into glutamate and leads to greater labeling of citrate when compared to differentiated endothelial cells. Other than fueling the TCA cycle, glutamine is also essential for maintaining cellular GSH levels. We observed that transient glutamine withdrawal increased ROS formation, cysteine oxidation of OCT4 and its degradation. Moreover, oxidation-dependent degradation of OCT4 induced by glutamine withdrawal is shown to enhance endothelial differentiation and in vivo blood vessel formation.
A major finding in our study is that glutamine, by maintaining a reduced redox state, regulates OCT4 levels. OCT4 (POU5F1) is a member of the POU family of transcription factors that consists of 2 DNA binding domains capable of independently recognizing half-sites of an octameric DNA sequence motif. OCT4 is essential both for the formation of the inner cell mass of the blastocyst (Nichols et al., 1998) and generation of iPSCs (Yu et al., 2007). The POU5F1 gene encodes for 3 different splice variants, but pluripotency depends only on the OCT4A isoform (Lee et al., 2006). OCT4 binds to more than 600 promoters, usually together with SOX2 and NANOG, and can both induce gene expression related to pluripotency and silence genes involved in differentiation (Boyer et al., 2005). Work in mouse ESCs and iPSCs has shown that pluripotency can be maintained with low levels of OCT4 and cells expressing low levels of OCT4 prior to differentiation are in a state of enhanced stability and less likely to undergo subsequent differentiation (Karwacki-Neisius et al., 2013; Radzisheuskaya et al., 2013). In contrast, OCT4 depletion in human ESCs leads to spontaneous endodermal (Hay et al., 2004) and trophectodermal differentiation (Matin et al., 2004). We observed that glutamine removal generates an OCT4lo population (Figure 1D) and it has been previously reported that OCT4lo cells fail to upregulate the mesodermal marker Brachyury in response to BMP4 (Wang et al., 2012), which is a prerequisite for endothelial differentiation. This emphasizes the importance of timing a metabolic intervention during the differentiation process. Indeed, glutamine withdrawal from the onset of differentiation inhibited endothelial formation whereas lowering OCT4 after differentiation has been initiated may enhance differentiation.
ESCs maintain low levels of ROS (Cho et al., 2006) and have a high expression of anti-oxidant proteins (Saretzki et al., 2008). Moreover ROS enhance and in some cases are essential for the differentiation of ESCs into different lineages (Crespo et al., 2010; Ji et al., 2010; Lin et al., 2012). This suggests that ROS levels and stem cell pluripotency are correlated, yet the mechanisms underlying the metabolic regulation of pluripotency and its functional importance for enhancing differentiation have not yet been understood (Perales-Clemente et al., 2014; Teslaa and Teitell, 2015). Our findings directly demonstrate that glutamine metabolism maintains the reduced redox state and that glutamine withdrawal leads to a rapid degradation of OCT4, thus establishing a direct relationship between glutamine metabolism and pluripotency regulation.
Chemical oxidation of OCT4 in a cell-free system has been shown to lower its in vitro DNA binding capacity (Guo et al., 2004) but our data demonstrate that OCT4 acts as a metabolic sensor for glutamine metabolism in hESCs and can respond to metabolic cues as potent drivers of stem cell differentiation. We observed an almost complete oxidation of OCT4 cysteines after glutamine withdrawal. Oxidation or modification of cysteines can similarly inactivate other transcription factors such as p53, AP-1, and NF-κB. For example, the DNA binding domain of p53 contains several cysteines that were shown by X-ray crystallography to electrostatically interact with a Zinc atom (Cho et al., 1994). Oxidation of thiols with diamide reduced DNA binding of p53 (Hainaut and Milner, 1993) and site-directed mutagenesis of cysteines in the DNA binding domain of p53 led to a complete loss of DNA binding activity (Rainwater et al., 1995). Similarly, cFos-cJun heterodimers were unable to bind to DNA once free thiols were modified with N-ethylmaleimide and mutagenesis identified a single cysteine residue that was necessary for DNA binding (Abate et al., 1990). Also, NF-κB activity is inhibited by sulfhydryl modifying agents and a single cysteine was shown to be necessary for DNA binding (Matthews et al., 1992). In addition, several other transcription factors have been shown to be impaired by cysteine oxidation (Sun and Oberley, 1996).
Our findings also complement a recent study that identified the role of glutamine metabolism as a regulator of epigenetic DNA methylation in murine ESCs (Carey et al., 2015), thus suggesting that glutamine can modulate gene expression via transcriptional as well as epigenetic mechanisms in embryonic stem cells. Our findings point to a rapid inactivation of OCT4 DNA binding in response to ROS generated by glutamine withdrawal. We also observed that during differentiation, ESCs downregulate glutamine metabolism which thus suggests an important feedback mechanism between the metabolic state of an ESC and its pluripotency. Differentiation downregulates glutamine metabolism which in turn enhances differentiation by oxidizing the transcription factor OCT4. This feedback mechanism can allow pluripotent stem cells to rapidly achieve a stable differentiated fate. It is also conceivable that the rapid direct inactivation of OCT4 and more permanent epigenetic modification of gene expression reinforce each other, highlighting the versatile roles of glutamine metabolism in regulating pluripotency and differentiation. Importantly, transient glutamine withdrawal during differentiation enables hESCs to significantly increase the generation of endothelial cells that can form functional blood vessels thus underscoring the role of metabolic shifts for enhancing the functional capacity of generated cells.
The importance of energy metabolism is further highlighted by a recent publication showing that glucose deprivation can be used to select for differentiated cardiomyocytes as these cells can typically use lactate to fuel the TCA cycle and can survive in the absence of glucose (Tohyama et al., 2013). Differentiation protocols often rely on growth factors or extracellular matrices to induce differentiation but our findings demonstrate that the metabolic milieu may be a similarly important factor in differentiating stem cells.
Our finding that the engineering of functional blood vessels from human ESCs is improved by metabolic modulation is especially important for translational applications to enhance angiogenesis with hESC-derived endothelial cells in situ and generate de novo blood vessels for tissue engineering. Our study focused on the role of glutamine metabolism in the generation of endothelial cells, but since OCT4 is a key regulator of pluripotency, it is likely that the regulatory role of glutamine metabolism also applies to the generation of other mature cell types and tissues during development and for regenerative tissue engineering. Future studies on the role of targeted metabolic interventions during the differentiation of pluripotent stem cells will help us understand and harness the complex interactions between the metabolic milieu and stem cell fate.
EXPERIMENTAL PROCEDURES
Additional experimental methods can be found in the Supplemental Experimental Procedures.
Human ESC culture and differentiation
H1 human ESCs (WiCell) were cultured with conditioned medium containing fresh 10ng/ml basic fibroblast growth factor (bFGF, R&D Systems) and 100ng/ml heparin (Sigma Aldrich). For spontaneous differentiation, medium was not conditioned and bFGF/heparin were left out. Endothelial differentiation was started by adding 20ng/ml bFGF, 25ng/ml bone morphogenetic protein 4 (BMP4), and 50ng/ml vascular endothelial growth factor (VEGF) (all from R&D Systems). To measure the effect of glutamine withdrawal on endothelial differentiation, glutamine was removed from the medium for the final 2 days of a 4 day differentiation.
OCT4 transcription factor assay and oxidation of OCT4
Nuclear extracts were incubated overnight in wells coated with double-stranded DNA containing the OCT4 response element and OCT4 binding was quantified according to the manufacturer’s instructions (Cayman Chemical). To induce intracellular superoxide formation, cells were treated for 5h with different concentrations of 2,3-Dimethoxy-1,4-naphthoquinone (DMNQ, Sigma Aldrich). OCT4 oxidation status was determined by incubating cells on ice for 4h with EZ-Link Maleimide-PEG2-Biotin (Thermo Fisher Scientific) to label reduced thiols.
GSH/GSSG measurements
Cells were first cultured in the presence or absence of glutamine for 2 days and then oxidized and reduced glutathione levels were measured using the GSH/GSSG-Glo Assay kit (Promega). Cell permeable glutathione (Glutathione reduced ethyl ester) was obtained from Sigma Aldrich and used at 0.5mM.
Endothelial sprouting assay
Invasion into 3-dimensional collagen gels was quantified using previously published methods (Bayless et al., 2009; Kang et al., 2015) using rat tail collagen type I containing 200ng/ml VEGF (R&D Systems). Human ESCs were differentiated toward endothelial cells for a total of 4 days and the glutamine withdrawal group did not receive glutamine during the final 2 days of differentiation. 50,000 cells were plated on top of the collagen gels in fresh endothelial basal medium (Lonza). After 72h endothelial sprouting was quantified as the number of structures invading beneath the monolayer per high power field.
Mouse experiments
Animal experiments were performed according to the NIH guidelines for the care and use of live animals and were approved by our IACUC committee. Ten week-old male NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (The Jackson laboratory, strain 005557) were injected with 4 million cells in 250μl growth factor reduced Matrigel (Corning). After 7 days, the number of red blood cells per high power field was determined.
Statistics
All values are given as mean±SEM. Inter-group differences were assessed by an unpaired Student’s t-test or an ANOVA with post hoc analysis using Tukey’s test when multiple groups were analyzed. Wherever applicable, normality was confirmed with the Kolmogorov-Smirnov test and for data that was not normally distributed (RBCs/high power field in Figure 4F), a Mann Whitney test was used. P<0.05 was considered statistically significant.
Supplementary Material
HIGHLIGHTS.
Glutamine metabolism regulates the pluripotency of human embryonic stem cells (ESCs)
OCT4 undergoes cysteine oxidation and degradation during glutamine withdrawal
Metabolic cues act in concert with growth factors to regulate stem cell differentiation
Glutamine withdrawal enhances the angiogenic capacity of ESC-derived endothelial cells
Acknowledgments
We would like to thank Li (Lucas) Zhong and Alexander Vladimirovich from the Roy J. Carver Biotechnology Center, University of Illinois at Urbana-Champaign for performing GC/MS analysis of metabolites. This work was supported in part by a Parker B. Francis fellowship and an American Heart Scientist Development grant 15SDG23250002 (GM), R01-GM094220 (JR), R01-HL118068 (JR and ABM), Roadmap Grant R33-DK070291 (HB), and R01-HL090152 (ABM).
Footnotes
AUTHOR CONTRIBUTIONS
J.R., A.B.M., and G.M. conceived the experiments and wrote the manuscript. J.R. directed the project. G.M. performed experiments and analyzed the data. G.F.Z. and H.B. performed isotope-assisted metabolic analysis. H.K., N.P.A., Y.Z., Y.Y., and B.H. performed experiments.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Kwak HI, Su SC. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nature protocols. 2009;4:1888–1898. doi: 10.1038/nprot.2009.221. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto PJ, Knipling L, Wolff J. The local electrostatic environment determines cysteine reactivity of tubulin. J Biol Chem. 2002;277:29018–29027. doi: 10.1074/jbc.M204263200. [DOI] [PubMed] [Google Scholar]
- Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park do J, Park KS, Lee HK. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472–1478. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Choi E, Kraus MR, Lemaire LA, Yoshimoto M, Vemula S, Potter LA, Manduchi E, Stoeckert CJ, Jr, Grapin-Botton A, Magnuson MA. Dual lineage-specific expression of Sox17 during mouse embryogenesis. Stem Cells. 2012;30:2297–2308. doi: 10.1002/stem.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DR, Calder PC, Houghton FD. Effect of oxygen tension on the amino acid utilisation of human embryonic stem cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;33:237–246. doi: 10.1159/000356665. [DOI] [PubMed] [Google Scholar]
- Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013;288:26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo FL, Sobrado VR, Gomez L, Cervera AM, McCreath KJ. Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells. 2010;28:1132–1142. doi: 10.1002/stem.441. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell metabolism. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Devreker F, Winston RM, Hardy K. Glutamine improves human preimplantation development in vitro. Fertil Steril. 1998;69:293–299. doi: 10.1016/s0015-0282(97)00463-9. [DOI] [PubMed] [Google Scholar]
- Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell metabolism. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Einhorn L, Kelley M, Hirota K, Yodoi J, Reinbold R, Scholer H, Ramsey H, Hromas R. Redox regulation of the embryonic stem cell transcription factor oct-4 by thioredoxin. Stem Cells. 2004;22:259–264. doi: 10.1634/stemcells.22-3-259. [DOI] [PubMed] [Google Scholar]
- Hainaut P, Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer research. 1993;53:4469–4473. [PubMed] [Google Scholar]
- Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. The Journal of clinical investigation. 2013;123:3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Ji AR, Ku SY, Cho MS, Kim YY, Kim YJ, Oh SK, Kim SH, Moon SY, Choi YM. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp Mol Med. 2010;42:175–186. doi: 10.3858/emm.2010.42.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Duran CL, Abbey CA, Kaunas RR, Bayless KJ. Fluid shear stress promotes proprotein convertase-dependent activation of MT1-MMP. Biochem Biophys Res Commun. 2015;460:596–602. doi: 10.1016/j.bbrc.2015.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacki-Neisius V, Goke J, Osorno R, Halbritter F, Ng JH, Weisse AY, Wong FC, Gagliardi A, Mullin NP, Festuccia N, et al. Reduced Oct4 expression directs a robust pluripotent state with distinct signaling activity and increased enhancer occupancy by Oct4 and Nanog. Cell stem cell. 2013;12:531–545. doi: 10.1016/j.stem.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006;281:33554–33565. doi: 10.1074/jbc.M603937200. [DOI] [PubMed] [Google Scholar]
- Lin CY, Peng CY, Huang TT, Wu ML, Lai YL, Peng DH, Chen PF, Chen HF, Yen BL, Wu KK, et al. Exacerbation of oxidative stress-induced cell death and differentiation in induced pluripotent stem cells lacking heme oxygenase-1. Stem Cells Dev. 2012;21:1675–1687. doi: 10.1089/scd.2011.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugus JJ, Chung YS, Mills JC, Kim SI, Grass J, Kyba M, Doherty JM, Bresnick EH, Choi K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, Moore HD, Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–668. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
- Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature genetics. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Perales-Clemente E, Folmes CD, Terzic A. Metabolic regulation of redox status in stem cells. Antioxid Redox Signal. 2014;21:1648–1659. doi: 10.1089/ars.2014.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzisheuskaya A, Chia Gle B, dos Santos RL, Theunissen TW, Castro LF, Nichols J, Silva JC. A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nature cell biology. 2013;15:579–590. doi: 10.1038/ncb2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainwater R, Parks D, Anderson ME, Tegtmeyer P, Mann K. Role of cysteine residues in regulation of p53 function. Molecular and cellular biology. 1995;15:3892–3903. doi: 10.1128/mcb.15.7.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A. Concise review: The Sox2-Oct4 connection: critical players in a much larger interdependent network integrated at multiple levels. Stem Cells. 2013;31:1033–1039. doi: 10.1002/stem.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L, et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free radical biology & medicine. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Song SH, Kim K, Park JJ, Min KH, Suh W. Reactive oxygen species regulate the quiescence of CD34-positive cells derived from human embryonic stem cells. Cardiovascular research. 2014;103:147–155. doi: 10.1093/cvr/cvu106. [DOI] [PubMed] [Google Scholar]
- Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free radical biology & medicine. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- Teslaa T, Teitell MA. Pluripotent stem cell energy metabolism: an update. The EMBO journal. 2015;34:138–153. doi: 10.15252/embj.201490446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell stem cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Turner J, Quek LE, Titmarsh D, Kromer JO, Kao LP, Nielsen L, Wolvetang E, Cooper-White J. Metabolic Profiling and Flux Analysis of MEL-2 Human Embryonic Stem Cells during Exponential Growth at Physiological and Atmospheric Oxygen Concentrations. PLoS One. 2014;9:e112757. doi: 10.1371/journal.pone.0112757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell stem cell. 2012;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nature methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zakin L, Reversade B, Virlon B, Rusniok C, Glaser P, Elalouf JM, Brulet P. Gene expression profiles in normal and Otx2−/− early gastrulating mouse embryos. Proc Natl Acad Sci U S A. 2000;97:14388–14393. doi: 10.1073/pnas.011513398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.