Zika virus (ZIKV) infection during pregnancy has been linked to birth defects,1 yet the magnitude of risk remains uncertain. A study of the 2013–2014 Zika outbreak in French Polynesia estimated that the risk of microcephaly due to ZIKV infection in the first trimester of pregnancy was 0.95% (95% confidence interval, 0.34–1.91%), on the basis of eight microcephaly cases identified retrospectively in a population of approximately 270,000 people with an estimated rate of ZIKV infection of 66%.2
In the current outbreak, thousands of suspected cases of infants with microcephaly or other developmental anomalies of the central nervous system that may be associated with ZIKV infection have been reported in Brazil. To estimate the magnitude of the risk of microcephaly in Brazil, we analyzed data from Bahia (see graph in Panel A). Serosurvey data from Yap Island, Federated States of Micronesia (where there was an outbreak in 2007), and French Polynesia indicate that reported Zika cases represent only a small fraction of the number of ZIKV infections that actually occur. The infection rate in Bahia cannot be reliably inferred from currently available data, so we assumed that it could range from 10% to 80% on the basis of estimates from Yap and French Polynesia (66 to 73%) and reports from non-outbreak ZIKV serosurveys (6 to 40%, see Supplementary Appendix, available with the full text of this article at NEJM.org). We apportioned this risk across 2015 according to the temporal distribution of reported cases (Panel B), assumed that all pregnant women were equally susceptible to infection (regardless of gestational age), and assessed the association of infection risk with microcephaly cases reported in the Brazilian Live Births Information System between July 2015 and February 2016 (as of March 21, 2016, accounting for a reporting delay and assuming that all reported births occurred at full term, Panel C).
Considering different infection-rate scenarios (from 10% to 80%), possible overreporting (0% or 100%), and an uncertain baseline microcephaly rate (2 to 12 cases per 10,000 births), we found a strong association between the risk of microcephaly and infection risk in the first trimester and a negligible association in the second and third trimesters, in keeping with the associations found in population-level estimates for French Polynesia (see Supplementary Appendix). The estimated baseline risk of microcephaly was low, approximately 2 per 10,000 births (Panels D–F; see also Supplementary Appendix), but the estimated risk due to infection in the first trimester ranged from 0.88% (95% credible interval, 0.80–0.97%), when we assumed an 80% overall ZIKV infection rate and 100% overreporting of microcephaly cases, to 13.2% (95% credible interval, 12.0–14.4%), when we assumed a 10% ZIKV infection rate and no overreporting.
The lower end of this range is similar to the approximately 1% risk estimated for French Polynesia, especially if infection rates in Bahia were high (40% or more with overreporting, 70% or more without overreporting). It is also possible that the French Polynesia estimate is an underestimate; it is from a single outbreak, and microcephaly cases were identified retrospectively. Furthermore, higher risks of microcephaly have been documented for some other viruses.2 Both estimates are consistent with the lack of reported microcephaly cases in Yap; if microcephaly risk due to ZIKV infection during the first trimester was 0.88 to 13.2%, then between zero and four microcephaly cases would have been expected.
There are uncertainties and limitations to all current estimates of microcephaly risk associated with ZIKV infection. First, available data are very limited, especially in recently affected areas such as Bahia where infection rates are unknown and microcephaly cases are still being reported and evaluated. The limited information on ZIKV infection rates is compounded by difficulty in the clinical confirmation of microcephaly, as evidenced by low confirmation rates in the independent, temporary microcephaly reporting system established by Brazil in late 2015. Carefully designed serosurveys and data from other locations can help in refining these estimates.
Recent studies have revealed associations between symptomatic ZIKV infection during all trimesters and adverse pregnancy outcomes3 and potential peak risk during gestational weeks 14 to 17.4 It is unclear how these outcomes relate to the clear association between first-trimester risk and microcephaly at the population level in French Polynesia and Bahia. On the population level, the temporal relationship is confounded by variation in infection risk, gestational age, and fetal outcome assessment. Here we assumed that all births were full term. While fetal loss and early termination have been documented, the delay between the Zika outbreak and microcephaly cases in French Polynesia and Bahia indicates that the majority of cases were associated with first trimester infection risk in full-term or near-full-term pregnancies. Meanwhile, our understanding of the biology of ZIKV infection in pregnancy is based on clinically described cases in pregnant women with symptomatic infection. We therefore have little knowledge of the effects of mild or asymptomatic ZIKV infections and infections in early pregnancy when pregnancy status may not be known. Risk of adverse events may be higher in symptomatic infections, but mild infections are probably more common and thus may also contribute substantially to the overall burden. Furthermore, microcephaly is only one possible adverse outcome among a spectrum of conditions that may be part of congenital Zika syndrome. A population-level increase in central nervous system anomalies has been observed in both French Polynesia and Brazil. More data are needed to refine gestational age–specific risk estimates for microcephaly and these other outcomes related to ZIKV infection, especially assessing population-level infection rates and the multiple effects of congenital Zika syndrome at all gestational ages related to both symptomatic and asymptomatic infection.
Although much remains unknown about the effects of ZIKV infection during pregnancy, population-level data from French Polynesia and Bahia reveal a clear association between first-trimester ZIKV infection and microcephaly risk. This pattern was likely similar in other parts of northeastern Brazil, where Zika outbreaks in early 2015 were followed by microcephaly outbreaks in late 2015. If the risk of infection and adverse outcomes is similar in the other geographic areas where ZIKV has since spread, many more cases of microcephaly and other adverse outcomes are likely to occur. In light of the growing evidence, it is prudent to take precautions to avoid ZIKV infection during pregnancy5 and for health care systems to prepare for an increased burden of adverse pregnancy outcomes in the coming years.
Supplementary Material
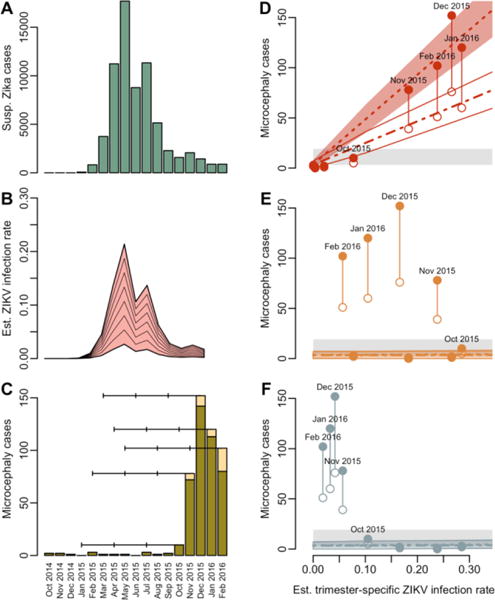
Relationship between Trimester-Specific ZIKV Infection Risk and Microcephaly in Bahia, Brazil.
Panel A shows the approximate number of suspected Zika cases reported in Bahia by month. Panel B shows the estimated ZIKV infection rate, assuming an overall infection rate of 10 to 80%. Panel C shows the numbers of microcephaly cases in Bahia including reported cases (green) and estimated additional cases (yellow), accounting for reporting delays (see Supplementary Appendix). Horizontal lines indicate the approximate gestational period by trimester for births in October 2015 through February 2016 assuming the pregnancies reached full term. In the remaining panels, the solid points represent the total number of microcephaly cases for each birth cohort (July 2015–February 2016) in Bahia (adjusted for reporting delays) relative to the estimated infection rate for the first (Panel D), second (Panel E), and third (Panel F) trimesters if the overall 2015 infection rate was 50%. The open points represent 50% of this value (reflecting potential overreporting), and the grey area represents expected baseline microcephaly rates of 2 to 12 cases per 10,000 births. Model-fitted estimates and 95% credible intervals for microcephaly cases are shown for data with (dot-dashed) and without overreporting (dashed).
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention. All data are publicly available, and code is available from Dr. Johansson upon request.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects — reviewing the evidence for causality. N Engl J Med. 2016 doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 2.Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016 doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasil P, Pereira JP, Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro — preliminary report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faria NR, Azevedo RD, Kraemer MU, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016 doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:30–3. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


