Abstract
Importance
Prostate-specific antigen (PSA) screening for prostate cancer is controversial. Experts have suggested more personalized or more conservative strategies to improve benefit-risk tradeoffs, but the value of these strategies—particularly when combined with increased conservative management for low-risk cases—is uncertain.
Objective
To evaluate the potential cost-effectiveness of plausible PSA screening strategies, and to assess the value added by increased use of conservative management among low-risk screen-detected cases.
Design
Micro-simulation model of prostate cancer incidence and mortality under alternative PSA screening strategies and either (1) “contemporary” treatment practices based on age, stage, and grade observed in the Surveillance, Epidemiology, and End Results program in 2010 or (2) “selective” treatment practices where cases with Gleason sum <7 and clinical T-stage ≤T2a are treated only after clinical progression and all others are treated according to “contemporary” treatment practices.
Setting
National and trial data on PSA growth, screening and biopsy patterns, incidence, treatment distributions, treatment efficacy, mortality, health-related quality of life, and direct medical expenditure.
Participants
A simulated contemporary cohort of U.S. men beginning at 40 years of age.
Interventions
18 screening strategies that vary by start and stop age, screening interval, and criteria for biopsy referral; “contemporary” or “selective” treatment practices.
Main Outcome Measures
Life years (LYs), quality-adjusted life years (QALYs), direct medical expenditure, and cost per LY and QALY gained.
Results
All screening strategies increased LYs (range 0.03–0.06) and costs ($300–$1,400) vs. no screening with cost per LY ranging from $7,300 to $21,600. With “contemporary” treatment, only strategies with biopsy referral when PSA >10.0 µg/L or age-dependent thresholds increased QALYs (0.002–0.004), and only quadrennial screening of ages 55–69 was potentially cost-effective in terms of cost per QALY (ICER=$92,400). With “selective” treatment, all strategies increased QALYs (0.002–0.004) and several strategies were potentially cost-effective in terms of cost per QALY (ICER=$70,800–$136,300).
Conclusions
For PSA screening to be cost effective it needs to be used conservatively and ideally in combination with a conservative management approach for low-risk disease.
Keywords: active surveillance, conservative management, cost-effectiveness, prostate cancer screening, prostatic neoplasms
INTRODUCTION
With the U.S. Preventive Services Task Force (USPSTF) recommendation against routine prostate-specific antigen (PSA) screening1, and conservative guidance from other national panels2–4, the future of PSA screening is uncertain. The recently updated guidelines relied heavily on results from two large trials conducted in the U.S. and Europe5–7. These results have been interpreted by some as demonstrating that PSA screening provides at most modest benefit, with unacceptable costs in terms of overdiagnosis and overtreatment8,9. However, over a long-term horizon, the lives saved by screening are likely to be considerably higher, and the fraction overdiagnosed considerably lower compared with the trials10–13. Rather than rejecting screening, we have recommended seeking more personalized (or “smarter”) screening strategies that preserve benefit while reducing harms13,14. Unfortunately, these strategies are unlikely to be evaluated in randomized trials due to resource and logistical constraints. Therefore, we have used modeling to conduct simulated comparisons of candidate screening approaches.
In a recent study15, we projected outcomes for a contemporary cohort of U.S. men using 35 screening strategies that varied by screening ages, inter-screening intervals, and criteria for biopsy referral. We identified several strategies that reduced screening harms by more than half yet retained the majority of lives saved relative to a “reference” annual screening strategy for men aged 50–74 years. These “smarter” strategies used longer inter-screening intervals and more conservative criteria for biopsy referral in older men. Other investigators have also proposed screening policies with similar objectives, including: stopping screening at age 60 if PSA <1.0 µg/L16, using baseline PSA at age 45–50 to identify men appropriate for less frequent screening17, and referring to biopsy only when PSA >10.0 µg/L9. However, no studies to date have evaluated how these strategies alter the benefit-risk balance of PSA screening, or if they represent high-value alternatives to no screening18.
Beyond “smarter” screening strategies, there is growing support for more selective treatment strategies. Active surveillance, which manages newly diagnosed patients conservatively with serial biopsies, is an increasingly common approach19–21 for treating low-risk cases—which constitute the majority of newly diagnosed prostate cancers. However, few studies have projected screening outcomes under alternative treatment practices.
The primary objective of this modeling study is to investigate whether “smarter” PSA prostate cancer screening strategies have the potential to be effective and cost-effective relative to no screening. Additionally, we investigate the potential added value of combining screening and treatment strategies by also projecting outcomes under “selective” treatment practices with increased use of conservative management among men with screen-detected low-risk disease.
METHODS
Overview
The Fred Hutchinson Cancer Research Center (FHCRC) micro-simulation model of prostate cancer (summarized in the Supplement) was developed as part of the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) prostate cancer working group22. The model is unique among prostate cancer models because it explicitly links cancer progression with individual PSA growth. This link is critical for evaluating screening strategies with PSA-dependent criteria for biopsy referral, inter-screening intervals, and/or early cessation. The model has been fit to U.S. incidence data (eFigure 1 in the Supplement), and has been used to study population incidence and mortality trends23 and evaluate the comparative effectiveness of alternative PSA screening policies15.
We expanded the FHCRC model to estimate quality-adjusted survival and costs for coordinated screening and treatment strategies from a U.S. healthcare payer perspective. For each strategy, the model simulated a cohort of men beginning at age 40 and projected prostate cancer outcomes over a lifetime horizon. We calculated outcomes using health state utility and cost weights applied to the person-years tallied in the healthy state and in the post-diagnosis states (eFigure 2 in the Supplement). Costs and survival outcomes were discounted at 3% per year in the base case, and cost outcomes are presented in 2014 USD. This modeling study was exempt from human subjects review.
Screening strategies
The strategies in our analysis (Figure 1) reflect promising strategies from our prior comparative effectiveness evaluation15 and approximations to the National Comprehensive Cancer Network recommendations (Strategy 2)24, the American Urologic Association guidelines statement (Strategy 14)2, and the commonest protocol used in the European Randomized Study of Screening for Prostate Cancer (Strategy 15)25. We also consider strategies that use a high PSA threshold (i.e., 10.0 µg/L) for referral to biopsy (Strategies 3–4, 9–12, and 16–18)—a value that would mandate a biopsy recommendation. Supplementing this selection, we also evaluated the cost-effectiveness of the superset of screening strategies comprising all 150 combinations of starting ages 45, 50, and 55; cessation ages 69 and 74; inter-screening intervals 1, 2, and 4 years and two PSA-dependent intervals (explained in the Supplement); and PSA threshold 3.0, 4.0, and 10.0 µg/L and two age-dependent PSA thresholds (explained in the Supplement).
Figure 1. Candidate PSA screening strategies.
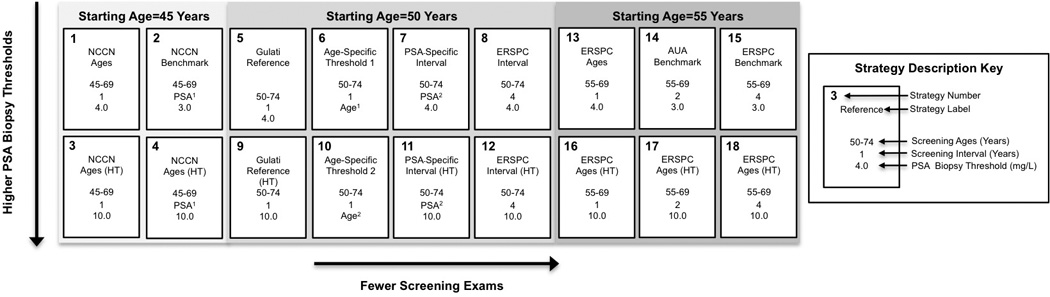
Strategies were suggested by published screening studies, approximation to a trial protocol, approximation to a clinical recommendation statement from a national organization, or a combination of sources. All strategies are compared to no screening.
NCCN=National Comprehensive Cancer Network
ERSPC=European Randomized Study of Screening for Prostate Cancer
AUA=American Urological Association
PSA1-dependent screening interval is every 1 year if PSA >3.0 µg/L and every 2 years otherwise.
PSA2-dependent screening interval is every 2 years if PSA >1.0 µg/L and every 4 years otherwise.
Age1-dependent PSA thresholds for biopsy referral are 3.5, 4.5, and 6.5 µg/L for ages 50–59, 60–69, and 70–74 y.
Age2-dependent PSA thresholds for biopsy referral are 4.5, 5.5, and 8.5 µg/L for ages 50–59, 60–69, and 70–74 y.
HT=high threshold
Survival model
In the absence of screening and curative treatment, prostate cancer survival is based on observed survival for untreated cases diagnosed in SEER in 1983–1986, just before the PSA era. Frequencies of curative surgery and/or radiation are based on SEER trends by age, stage, and grade at diagnosis, and frequencies of adjuvant hormone use are based on patterns observed in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database26. Effects of curative treatment are based on the Scandinavian randomized trial of prostatectomy vs. watchful waiting (HR=0.62)27 and assuming similar efficacy for contemporary radiation therapy28,29.
The model represents the effect of early detection on prostate cancer survival by assuming that would-be metastatic cases screen-detected at a local-regional stage have their survival changed to that associated with detection at the earlier stage. We previously showed that this effect is consistent with the published 21% mortality reduction reported in ERSPC14,15. Although the results of the U.S. Prostate, Lung, Colorectal, and Ovarian cancer screening trial14,30,31 did not show a reduction in the screen arm, we showed that the extensive control arm contamination suggests that a mortality benefit of this magnitude cannot be ruled out30.
In this study, the model was extended to track time spent in pre- and post-diagnosis states, including short- and long-term disease management states after receipt of curative treatment, a “no curative treatment” state for individuals not receiving curative treatment, and a two-year end-of-life state for men who die of prostate cancer. Cases with low-risk disease detected by screening may defer therapy until they progress to a point at which their disease would have become clinically apparent in the absence of screening. The Supplement summarizes health state definitions and durations.
“Contemporary” and “selective” treatment practices
We consider two initial treatment scenarios. Under “contemporary” treatment practices, all cases receive curative treatment (prostatectomy or radiation therapy, with or without androgen deprivation therapy) based on the frequencies of treatment observed in the SEER program in the year 2010 by age, stage, and grade. We do not model the small proportion of localized cases who receive androgen deprivation therapy alone. In contrast, under “selective” treatment practices, screen-detected cases with Gleason score <7 and clinical T-stage ≤T2a disease initially receive conservative management and all other cases receive the same treatments as under “contemporary” treatment practices. The Supplement describes extensions to the FHCRC model to identify cases eligible for conservative management and frequencies of immediate primary treatments (eTable 1).
We model a conservative management program in which curative treatment is offered once cases progress to the point of would-be clinical diagnosis in the absence of screening. Consequently, only non-overdiagnosed cases receive delayed curative treatment. This conservative version of active surveillance (AS) is modeled because there is no consensus around the appropriate conduct of AS, and the timing of progression to treatment under AS is therefore unclear. Further, the endpoint of would-be clinical diagnosis in the absence of screening is generated by the FHCRC model. We believe this represents a useful benchmark for comparison but acknowledge that under most contemporary AS approaches, curative therapy would likely be offered at an earlier time point.
Health-related quality of life and costs
Few studies have produced estimates of health state utilities for prostate cancer and its treatment. The health state utility for men without prostate cancer diagnosis was assumed to be 1.0 to represent full health. All other health state utilities were extracted from a prior U.S. study of 162 men aged ≥60 years or older that used standard gamble to elicit preferences for 19 prostate cancer health states (Table 1)32. Note that the short-term treatment health state utility decrement was applied for one year to localized cases receiving prostatectomy or radiation therapy, and reflects a weighted average of patients with and without major treatment side effects.
Table 1. Health state utility and direct medical expenditure model inputs.
The expenditure values below are presented as reported in the original publications. All costs were analyzed in 2014 USD.
| Parameter | Point Estimate |
Low Value |
High Value |
Distribution | Reference (Reference Number) |
|---|---|---|---|---|---|
| Direct Medical Expenditure Inputs | |||||
| PSA Test Cost (Per Procedure) | $27 | $22 | $32 | Normal | CMS Reimbursement Schedule (33) |
| Biopsy Cost (Per Procedure) | $688 | $550 | $826 | Normal | Hayes, Ann Int Med, 2013 (35) |
| Conservative Management Cost (Per Year)* | $476 | $381 | $571 | Normal | CMS Reimbursement Schedule (33) |
| Mean Prostatectomy Cost (Per Procedure) | $10,600 | $6,410 | $10,684 | Normal | Wang, Med Care, 2014 (34) |
| Mean Radiation Therapy Cost (Full Course of Treatment) |
$22,515 | $18,012 | $27,018 | Normal | Wang, Med Care, 2014 (34) |
| Androgen Deprivation Therapy Cost (Per Year)# | $2,267 | $1,814 | $2,720 | Normal | Cooperberg, BJU Int, 2013 (28) |
| Distant Stage Initial Treatment Cost (Full Course of Treatment)& |
$15,773 | $12,618 | $18,927 | Normal | Cooperberg, BJU Int, 2013 (28) |
| Distant Stage Management Cost (Per Year)& | $2,212 | $1,106 | $4,424 | Normal | Cooperberg, BJU Int, 2013 (28) |
| End-of-Life Cost (Last Year), Prostate Cancer Death |
$40,807 | $20,404 | $81,614 | Normal | Cooperberg, BJU Int, 2013 (28) |
| End-of-Life Cost (Last Year), Other Cause Death | $5,000 | $4,000 | $6,000 | Normal | Mobley, MDM, 2006 (37) |
| Surgical Complication Cost (Per Event) | $709 | $567 | $851 | Normal | Cooperberg, BJU Int, 2013 (28) |
| Radiation Therapy Complication Cost (Per Event) | $230 | $184 | $276 | Normal | Cooperberg, BJU Int, 2013 (28) |
| Office Visit Cost^ | $80 | $64 | $96 | Normal | CMS Reimbursement Schedule (33) |
| Health State Utility Value Inputs | |||||
| Healthy, Utility | 1.00 | 0.90 | 1.00 | Beta | Assumption |
| Symptomatic, Utility Decrement | 0.11 | 0.05 | 0.17 | Beta | Stewart, Med Care, 2005 (32) |
| Surveillance, Utility Decrement | 0.08 | 0.02 | 0.14 | Beta | Stewart, Med Care, 2005 (32) |
| Short-Term Treatment, Utility Decrement | 0.25 | 0.19 | 0.31 | Beta | Stewart, Med Care, 2005 (32) |
| Long-Term Treatment, Utility Decrement | 0.08 | 0.02 | 0.14 | Beta | Stewart, Med Care, 2005 (32) |
| Distant Stage, Utility Decrement | 0.25 | 0.22 | 0.28 | Beta | Stewart, Med Care, 2005 (32) |
| End-of-Life, Utility Decrement | 0.67 | 0.57 | 0.77 | Beta | Stewart, Med Care, 2005 (32) |
Conservative management was assumed to consist of an annual office visit, annual PSA test, and a biennial biopsy.
Men receiving initial curative treatment involving androgen deprivation therapy with prostatectomy or radiation therapy were assumed to receive one year of treatment.
The “distant stage initial treatment cost” was a one-time cost of initial treatment applied to men diagnosed with distant stage disease. The “distant stage management cost” reflects the ongoing cost of care in the distant stage state and was applied to all years in that state.
The cost of office visits was applied once annually in men without prostate cancer diagnosis.
We obtained cost estimates related to PSA testing, office visits, and conservative management by micro-costing resource use with the Centers for Medicare and Medicaid Services 2014 reimbursement schedule33. Costs for surgical treatment and radiation therapy episodes were derived from a prior SEER-Medicare analysis that calculated the mean procedure-attributable cost for patients receiving either type of treatment34. Biopsy, distant stage initial treatment (one-time), long-term management, end-of-life, and treatment complication costs were derived from prior economic analyses in prostate cancer (Table 1)28,35–37. Treatment complication costs were applied to 12.5% and 4.2% of men receiving prostatectomy and radiation therapy, respectively, based on the rates of Grade 3/4 complications in a prior analysis.28 Cost inputs were adjusted using the medical care component of the consumer price index to 2014 USD38.
Model outcomes
We used the model to calculate prostate cancer diagnosis, treatment, death, unadjusted life years, quality-adjusted life years (QALYs), and cost for each screening strategy. The incremental cost-effectiveness ratio (ICER) was calculated as the ratio of the difference in costs between strategies to the difference in effects (e.g., QALYs) between strategies38.
We calculated probabilistic outcomes using Monte Carlo simulation and conducted one-way sensitivity analyses to determine the inputs with the greatest influence on incremental QALY and cost outcomes39.
Cost-effectiveness was evaluated at willingness-to-pay thresholds ranging from $50,000 to $150,000 per QALY40–44. This range reflects the implied willingness-to-pay for cancer treatments in the U.S. and is consistent with values used in prior analyses40,44–46.
RESULTS
“Contemporary” treatment practices
Table 2 displays the results under “contemporary” treatment practices. Among the 18 screening strategies evaluated, all increased life years (range=0.03–0.06) compared with no screening, but only strategies with biopsy threshold at PSA >10.0 µg/L increased QALYs (range=0.002–0.004). Among this subset of strategies, cost per life year ranged from $12,000 to $21,000. Only quadrennial screening of ages 55–69 with a biopsy threshold at PSA >10.0 µg/L (Strategy 18) was potentially cost-effective in terms of cost per QALY ($92,446/QALY).
Table 2. Results in the “contemporary” and “selective” treatment scenarios. The PSA screening strategy results are listed in descending order of quality-adjusted life years in the “contemporary treatment” scenario.
We do not report results for strategies with PSA threshold for biopsy 10.0 µg/L in the “selective” treatment scenario because cases detected by screening are unlikely candidates for conservative management with delayed curative treatment.
| PSA Screening Strategy Number |
Screening Ages (Years) |
Inter- Screening Interval (Years) |
PSA Threshold for Biopsy Referral (µg/L) |
"Contemporary Treatment" Scenario | "Selective Treatment" Scenario | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Life Years |
Total QALYs |
Total Cost |
Cost Per Life Year Gained |
Cost Per QALY Gained |
Total Life Years |
Total QALYs |
Total Cost |
Cost Per Life Year Gained |
Cost Per QALY Gained |
||||
| No Screening |
- | - | - | 36.302 | 21.504 | $4,708 | Reference | Reference | 36.302 | 21.504 | $4,708 | Reference | Reference |
| 4 | 45–69 | PSA1 | 10 | 36.347 | 21.508 | $5,391 | $15,344 | $184,074 | - | - | - | - | - |
| 18 | 55–69 | 4 | 10 | 36.329 | 21.508 | $5,022 | $11,977 | $92,446 | - | - | - | - | - |
| 12 | 50–74 | 4 | 10 | 36.338 | 21.507 | $5,246 | $15,123 | $170,195 | - | - | - | - | - |
| 11 | 50–74 | PSA2 | 10 | 36.348 | 21.507 | $5,357 | $14,209 | $209,338 | - | - | - | - | - |
| 9 | 50–74 | 1 | 10 | 36.357 | 21.507 | $5,698 | $18,160 | $330,065 | - | - | - | - | - |
| 17 | 55–69 | 2 | 10 | 36.338 | 21.507 | $5,197 | $13,734 | $170,981 | - | - | - | - | - |
| 3 | 45–69 | 1 | 10 | 36.345 | 21.507 | $5,590 | $20,761 | $326,292 | - | - | - | - | - |
| 16 | 55–69 | 1 | 10 | 36.343 | 21.506 | $5,371 | $16,347 | $300,884 | - | - | - | - | - |
| 15 | 55–69 | 4 | 3 | 36.343 | 21.502 | $5,315 | $14,977 | Dominated | 36.338 | 21.508 | $4,971 | $7,335 | $70,831 |
| 8 | 50–74 | 4 | 4 | 36.348 | 21.502 | $5,513 | $17,466 | Dominated | 36.343 | 21.508 | $5,062 | $8,622 | $89,333 |
| 10 | 50–74 | 1 | Age2 | 36.361 | 21.502 | $5,818 | $19,006 | Dominated | 36.355 | 21.509 | $5,329 | $11,838 | $124,564 |
| 1 | 45–69 | 1 | 4 | 36.361 | 21.499 | $5,919 | $20,751 | Dominated | 36.354 | 21.509 | $5,404 | $13,409 | $163,214 |
| 7 | 50–74 | PSA2 | 4 | 36.359 | 21.499 | $5,730 | $17,983 | Dominated | 36.352 | 21.508 | $5,160 | $9,098 | $136,332 |
| 6 | 50–74 | 1 | Age1 | 36.363 | 21.498 | $5,928 | $19,972 | Dominated | 36.357 | 21.508 | $5,364 | $11,906 | $166,784 |
| 13 | 55–69 | 1 | 4 | 36.355 | 21.498 | $5,688 | $18,645 | Dominated | 36.350 | 21.508 | $5,187 | $9,985 | $128,680 |
| 14 | 55–69 | 2 | 3 | 36.353 | 21.498 | $5,597 | $17,390 | Dominated | 36.349 | 21.508 | $5,105 | $8,600 | $120,952 |
| 5 | 50–74 | 1 | 4 | 36.366 | 21.494 | $6,079 | $21,649 | Dominated | 36.360 | 21.507 | $5,411 | $12,293 | $243,768 |
| 2 | 45–69 | PSA1 | 3 | 36.360 | 21.494 | $5,835 | $19,622 | Dominated | 36.353 | 21.506 | $5,269 | $11,028 | $313,214 |
PSA1-dependent screening interval is every 1 year if PSA >3.0 µg/L and every 2 years otherwise.
PSA2-dependent screening interval is every 2 years if PSA >1.0 µg/L and every 4 years otherwise.
Age1-dependent PSA thresholds for biopsy referral are 3.5, 4.5, and 6.5 µg/L for ages 50–59, 60–69, and 70–74 y.
Age2-dependent PSA thresholds for biopsy referral are 4.5, 5.5, and 8.5 µg/L for ages 50–59, 60–69, and 70–74 y.
Corresponding results for the superset of screening strategies show that our selection of promising and policy-relevant strategies is representative of the range of cost-effectiveness outcomes (eFigure 3 in the Supplement). In general, only a small number of conservative screening strategies (4% of the superset) similar to those presented in Table 2 were potentially cost-effective at a willingness-to-pay of $150,000 or less per QALY.
“Selective” treatment practices
“Selective” treatment practices were implemented only for strategies with PSA thresholds below 10.0 µg/L because prostate cancer cases diagnosed with PSA >10.0 µg/L would not typically qualify as “low-risk” or candidates for delayed curative treatment. Among the 10 screening strategies evaluated (Table 2), Strategies 8, 14, and 15 compared most favorably with no screening, resulting in 0.041, 0.046, and 0.036 more life years, 0.004, 0.003, and 0.004 more QALYs, and $353, $397, and $262 greater cost, respectively. All of these strategies have an inter-screening interval of 2–4 years with PSA biopsy thresholds of 4.0, 3.0, and 3.0 µg/L; the ICERs for these strategies were $8,622, $7,335, and $8,600 per life year gained and $89,333, $70,831, and $120,952 per QALY gained, respectively.
Results for the superset of screening strategies with “selective” treatment practices, including those with biopsy threshold at PSA >10.0 µg/L, show that a large proportion of the strategies are potentially cost-effective at willingness-to-pay levels of $100,000 (43% of the superset) and $150,000 (70% of the superset) per QALY (eFigure 3 in the Supplement). The most cost-effective strategies in the superset are similar to the most cost-effective strategies in Table 2.
Sensitivity analysis
One-way sensitivity analyses focused on QALYs demonstrated that results were by far most sensitive to the health state utility in the conservative management state. One-way sensitivity analyses evaluating cost differences were most sensitive to the costs of prostate cancer death, radiation therapy, and PSA testing. All analyses were conditional on the assumed efficacy of curative treatment.
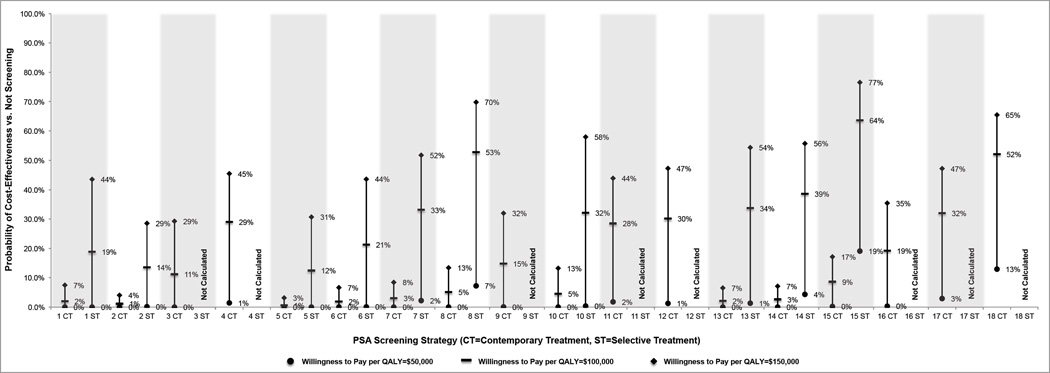
Under “contemporary” treatment practices, the probabilistic sensitivity analysis demonstrated a low probability of PSA screening cost-effectiveness at willingness-to-pay levels at or below $100,000 per QALY (Figure 2A). Only quadrennial screening of men age 55–69 with a PSA biopsy threshold of 10.0 µg/L had greater than a 50% probability of being potentially cost-effective at willingness-to-pay of $100,000 and $150,000 per QALY (Figure 2).
Figure 2.
Cost-effectiveness acceptability results for the “contemporary” and “selective” treatment scenarios at willingness to pay levels of $50,000–$150,000 per quality-adjusted life year gained. The strategy numbers relate to the strategies in Table 2. The percentages noted in the figure relate to the proportion of simulation runs in which the cost per quality-adjusted life year was less than or equal to the given willingness to pay. We do not report results for strategies with PSA threshold for biopsy of 10.0 µg/L in the “selective” treatment scenario because cases detected by screening are unlikely candidates for conservative management with delayed curative treatment.
Under “selective” treatment practices, the probabilistic sensitivity analysis demonstrated that no strategies had a greater than 50% probability of being cost-effective at a willingness-to-pay of $50,000 per QALY, and only quadrennial screening of men age 55–69 with a PSA biopsy threshold of 3.0 µg/L (Strategy 15) and quadrennial screening of men age 50–74 with a PSA biopsy threshold of 4.0 µg/L (Strategy 8) had greater than a 50% probability of being potentially cost-effective at a willingness-to-pay of $100,000 per QALY (Figure 2). Several other relatively conservative strategies (7, 10, 13, and 14) were potentially cost-effective at a willingness-to-pay of $150,000 per QALY (Figure 2).
DISCUSSION
The value of PSA screening for prostate cancer is uncertain, as reflected by variable clinical guidelines. This study provides the first quantitative framework to evaluate the comparative effectiveness of PSA-based screening strategies and selective treatment approaches, and it addresses an urgent need for direction concerning the future of PSA screening in the U.S. Our work indicates strategies with conservative screening frequency (e.g., quadrennial) and/or a higher PSA biopsy threshold (e.g., 4.0 µg/L) are potentially cost-effective when combined with increased use of conservative management for low-risk cases, but are unlikely to be cost-effective under contemporary treatment practices.
Our findings have clear implications for the future of PSA screening in the U.S. Rather than stopping PSA screening, as recommended by the USPSTF, implementation of strategies that extend the inter-screening interval and/or utilize higher PSA biopsy thresholds have the potential to preserve substantial benefit while controlling harm and costs. Though higher-threshold policies (e.g., 10.0 µg/L) are unlikely to be clinically appealing, they reinforce the general conclusion that conservative patterns of screening and biopsy referral are important directions to consider if PSA screening is to be both clinically effective and cost-effective.
All strategies evaluated were potentially cost-effective in terms of cost per life year (range=$7,300–$21,600). However, that metric ignores the important health-related quality of life impacts of cancer diagnosis, treatment, and associated complications. For this reason, our primary analysis evaluated the impacts of PSA screening in terms of cost per QALY. In analyses with “contemporary” treatment practices, we demonstrated that only strategies with highly conservative PSA biopsy thresholds (i.e., 10.0 µg/L) are expected to increase QALYs relative to no screening, and among those strategies only the most conservative (quadrennial screening of ages 55–69) was potentially cost-effective.
The contrasting cost-effectiveness results of the “contemporary” vs. “selective” treatment practices demonstrates the importance of conservative management of low-risk prostate cancer and the potential for increased use of active surveillance to make the benefit-risk tradeoffs and cost-effectiveness of screening acceptable. For example, quadrennial screening of men age 55–69 with biopsy threshold at 3.0 µg/L (Strategy 15) and quadrennial screening of men age 50–74 with biopsy threshold at 4.0 µg/L (Strategy 8) were both dominated under “contemporary” treatment practices but had ICERs of $89,300 and $70,800 per QALY under “selective” treatment practices, respectively. These favorable results in the “selective” treatment scenario are due to low-risk men on conservative management having better health-related quality of life, lower cost, and similar survival compared with low-risk men who receive immediate curative treatment. Additionally, we observed in the supplemental analysis of the superset of 150 screening strategies that the high PSA biopsy threshold (e.g., 10.0 µg/L) found to be favorable under “contemporary” treatments has similar value under “selective” treatments because men diagnosed with high PSA are more promising candidates for immediate treatment47 and are generally ineligible for surveillance programs.
There has been substantial discussion of the need for cost-effectiveness analyses exploring emerging PSA screening strategies, but few such studies have been reported in the literature48,49. A recent study used another CISNET micro-simulation model to evaluate the cost-effectiveness of a range of screening strategies in a European setting50. Their most cost-effective strategy screened men ages 55–59 at 2-year intervals, which is consistent with our conclusions that conservative use of the test is imperative. The authors concluded that shorter inter-screening intervals are more cost-effective than longer intervals when they examined strategies with cessation around age 60. In contrast, when they examined strategies with higher cessation ages, they found that longer screening intervals were more cost-effective. For example, quadrennial screening to age 69 or 74 achieved much lower additional costs but similar QALYs gained compared to biennial or annual screening (Figure 2B in that study). It should be noted that their model reflects a European setting with very different costs for many services, and with a lower frequency of curative treatments relative to the U.S. Additionally, several post-diagnosis utility values (e.g., active surveillance=0.97 and 1 year after initial primary treatment=0.95) were more favorable than ours (0.92 for both states). Nevertheless, despite these differences, and differences in how the two models represent and estimate prostate cancer natural history51,52, there is broad agreement between their study and ours that only a highly conservative PSA screening strategy will be cost-effective.
This analysis has several limitations that should be noted. First, this is a micro-simulation study that uses the best available evidence to project the comparative effectiveness of PSA screening strategies vs. no screening. Ideally, the comparative effectiveness of the PSA screening strategies would be evaluated head-to-head in “real world” settings prior to implementation. However, this is unlikely given the resource demands and complexity of designing studies to evaluate dozens of screening strategies. As a result, rigorously developed and validated disease models play an important role in projecting the comparative effectiveness of alternative PSA screening strategies. Nonetheless, our model evaluates a long-term time horizon, and there like to be is increasing uncertainty around model-projected results over time.
Few studies have elicited health state utilities for the PSA screening, making cost-effectiveness analyses challenging in this setting. As a result, we assume equivalence between several health states and those noted in prior studies (e.g., our conservative management utility was assumed to be equivalent to that of prostate cancer patients with a 20% chance of cancer spread not currently receiving treatment)53. However, we do allow a fraction of those cases to later receive curative treatment, and their utilities are modified accordingly at that time. We do not model the health-related quality of life impacts of biopsies. Neither do we model the impact of an elevated PSA (say 4.0 µg/L) that is still below the threshold for biopsy referral (say 10.0 µg/L) owing to a lack of data in this setting. Further, our analysis does not reflect the substantial costs of several recently approved systemic treatments for advanced prostate cancer. To the extent that screening reduces metastasis and castrate resistance, inclusion of these new treatments could improve screening cost-effectiveness outcomes relative to those projected in this study. Our previous studies have discussed other technical limitations of the FHCRC model15.
We recognize that the modeled conservative management program in the “selective” treatment scenario reflects a highly conservative approach to active surveillance. There is not a standard protocol for active surveillance, but most contemporary programs would likely identify and treat progressive cases before they progressed to clinically detected disease (when cases are treated in the model). Thus, the “selective” treatment scenario results might underestimate survival and costs compared with contemporary active surveillance protocols.
In conclusion, our work adds to a growing consensus50,54,55 that highly conservative use of the PSA test and biopsy referral is necessary if PSA screening is to be cost-effective. Among the strategies considered, less frequent screening and more restrictive criteria for biopsy resulted in greater chances of PSA screening being cost-effective—particularly when combined with “selective” treatment strategies that do not immediately treat low-risk screen-detected cases.
Supplementary Material
Acknowledgments
The authors thank Sigrid Carlsson, MD, PhD, of Memorial Sloan Kettering Cancer Center and Scott D. Ramsey, MD, PhD, of the Fred Hutchinson Cancer Research Center for helpful comments, and Annika C. Hanson of the Fred Hutchinson Cancer Research Center for technical assistance. Dr. Carlsson, Dr. Ramsey, and Ms. Hanson did not receive compensation for their contributions and provided written confirmation that they wanted to be acknowledged herein. Joshua Roth, PhD, MHA, Roman Gulati, MS, and Ruth Etzioni, PhD, were responsible for data analysis of this project. As Principal Investigator, Joshua Roth, PhD, MHA, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by Award Numbers R01CA131874 from the National Cancer Institute and U01CA157224 from the National Cancer Institute and the Centers for Disease Control and Prevention as part of the Cancer Intervention and Surveillance Modeling Network (CISNET). Roth JA is supported by grant number 1K12HS022982 from the Agency for Healthcare Research and Quality. The National Cancer Institute, Centers for Disease Control and Prevention, and Agency for Healthcare Research and Quality had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, the Centers for Disease Control and Prevention, or the Agency for Healthcare Research and Quality.
The authors have no conflicts of interest to declare.
Reproducible Research Statement: Study Protocol: Screening and treatment strategies available from Mr. Gulati (e-mail, rgulati@fredhutch.org). Statistical Code: Cost-effectiveness analysis code available from Dr. Roth (e-mail, jroth@fredhutch.org). Data/Model: Model source code (languages: C and R) available from Mr. Gulati (e-mail, rgulati@fredhutch.org). A detailed model description is available at http://cisnet.cancer.gov/prostate/profiles.html and a high-level overview of the model is available at https://resources.cisnet.cancer.gov/registry/packages/psapc-fhcrc.
REFERENCES
- 1.Moyer VA on behalf of the USPSTF. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 2.Carter HB, Albertsen PC, Barry MJ, et al. Early Detection of Prostate Cancer: AUA Guideline. The Journal of urology. 2013 doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P for the Clinical Guidelines Committee of the American College of P. Screening for Prostate Cancer: A Guidance Statement From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013 doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Oliver TK, Vickers A, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion. Journal of Clinical Oncology. 2012;30(24):3020–3025. doi: 10.1200/JCO.2012.43.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriole GL. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J. Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. New England Journal of Medicine. 2012;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou R, LeFevre ML. Prostate cancer screening--the evidence, the recommendations, and the clinical implications. Jama. 2011;306(24):2721–2722. doi: 10.1001/jama.2011.1891. [DOI] [PubMed] [Google Scholar]
- 9.Welch HG. A piece of my mind. Making the call. JAMA : the journal of the American Medical Association. 2011;306(24):2649–2650. doi: 10.1001/jama.2011.1898. [DOI] [PubMed] [Google Scholar]
- 10.Gulati R, Mariotto AB, Chen S, Gore JL, Etzioni R. Long-term projections of the harm-benefit trade-off in prostate cancer screening are more favorable than previous short-term estimates. Journal of clinical epidemiology. 2011;64(12):1412–1417. doi: 10.1016/j.jclinepi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeb S. What is the true number needed to screen and treat to save a life with prostate-specific antigen testing? J. Clin. Oncol. 2011;29:464–467. doi: 10.1200/JCO.2010.30.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijnsdijk EA. Quality-of-life effects of prostate-specific antigen screening. N. Engl. J. Med. 2012;367:595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etzioni R, Gulati R, Cooperberg MR, Penson DM, Weiss NS, Thompson IM. Limitations of basing screening policies on screening trials: The US Preventive Services Task Force and Prostate Cancer Screening. Medical Care. 2013;51(4):295–300. doi: 10.1097/MLR.0b013e31827da979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati R, Tsodikov A, Etzioni R, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer. 2014;120(22):3519–3526. doi: 10.1002/cncr.28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: Model estimates of potential benefits and harms. Annals of Internal Medicine. 2013;158(3):145–153. doi: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlsson S, Vickers AJ, Roobol M, et al. Prostate cancer screening: facts, statistics, and interpretation in response to the US Preventive Services Task Force Review. Journal of Clinical Oncology. 2012;30(21):2581–2584. doi: 10.1200/JCO.2011.40.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control study. Bmj. 2013;346:f2023. doi: 10.1136/bmj.f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilt TJ, Harris RP, Qaseem A. Screening for cancer: advice for high-value care from the american college of physicians. Ann Intern Med. 2015;162(10):718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 19.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156(8):591–595. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. European urology. 2015;67(1):44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. Jama. 2015;314(1):80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 22.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11(4):707–719. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer causes & control : CCC. 2008;19(2):175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll PR, Parsons JK, Andriole G, et al. Prostate cancer early detection, version 1.2014. Featured updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network : JNCCN. 2014;12(9):1211–1219. doi: 10.6004/jnccn.2014.0120. quiz 1219. [DOI] [PubMed] [Google Scholar]
- 25.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. The New England journal of medicine. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 26.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J. Clin. Oncol. 2010;28(7):1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. The New England journal of medicine. 2011;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 28.Cooperberg MR, Ramakrishna NR, Duff SB, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU international. 2013;111(3):437–450. doi: 10.1111/j.1464-410X.2012.11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boorjian SA, Karnes RJ, Viterbo R, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117(13):2883–2891. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gulati R, Tsodikov A, Wever EM, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer causes & control : CCC. 2012;23(6):827–835. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. New England Journal of Medicine. 2012;367(7):595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart ST, Lenert L, Bhatnagar V, Kaplan RM. Utilities for prostate cancer health states in men aged 60 and older. Medical care. 2005;43(4):347–355. doi: 10.1097/01.mlr.0000156862.33341.45. [DOI] [PubMed] [Google Scholar]
- 33.information. CfMMSBCfMMSOH-G. [Accessed 08/23, 2012];2012 http://www.cms.hhs.gov/MedHCPCSGenInfo/
- 34.Wang SY, Wang R, Yu JB, et al. Understanding regional variation in Medicare expenditures for initial episodes of prostate cancer care. Medical care. 2014;52(8):680–687. doi: 10.1097/MLR.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes JH, Ollendorf DA, Pearson SD, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158(12):853–860. doi: 10.7326/0003-4819-158-12-201306180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. Jama. 2010;304(21):2373–2380. doi: 10.1001/jama.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mobley LR, Hoerger TJ, Wittenborn JS, Galuska DA, Rao JK. Cost-effectiveness of osteoporosis screening and treatment with hormone replacement therapy, raloxifene, or alendronate. Medical decision making : an international journal of the Society for Medical Decision Making. 2006;26(2):194–206. doi: 10.1177/0272989X06286478. [DOI] [PubMed] [Google Scholar]
- 38.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 39.O'Hagan A, McCabe C, Akehurst R, et al. Incorporation of uncertainty in health economic modelling studies. PharmacoEconomics. 2005;23(6):529–536. doi: 10.2165/00019053-200523060-00001. [DOI] [PubMed] [Google Scholar]
- 40.Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? The oncologist. 2006;11(2):90–95. doi: 10.1634/theoncologist.11-2-90. [DOI] [PubMed] [Google Scholar]
- 41.Berry SR, Bell CM, Ubel PA, et al. Continental Divide? The attitudes of US and Canadian oncologists on the costs, cost-effectiveness, and health policies associated with new cancer drugs. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(27):4149–4153. doi: 10.1200/JCO.2010.29.1625. [DOI] [PubMed] [Google Scholar]
- 42.Neumann PJ, Palmer JA, Nadler E, Fang C, Ubel P. Cancer therapy costs influence treatment: a national survey of oncologists. Health Aff (Millwood) 2010;29(1):196–202. doi: 10.1377/hlthaff.2009.0077. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg D, Earle C, Fang CH, Eldar-Lissai A, Neumann PJ. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. Journal of the National Cancer Institute. 2010;102(2):82–88. doi: 10.1093/jnci/djp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. The New England journal of medicine. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 45.Carlson JJ, Garrison LP, Ramsey SD, Veenstra DL. The potential clinical and economic outcomes of pharmacogenomic approaches to EGFR-tyrosine kinase inhibitor therapy in non-small-cell lung cancer. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2009;12(1):20–27. doi: 10.1111/j.1524-4733.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 46.Myers EMA, Shen Lan, Posey RE, Gray R, Sanders GD. Value-of-Information Analysis for Patient-Centered Outcomes Research Prioritization. Patient-Centered Outcomes Research Institute; 2012. [Google Scholar]
- 47.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. The New England journal of medicine. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garg V, Gu NY, Borrego ME, Raisch DW. A literature review of cost-effectiveness analyses of prostate-specific antigen test in prostate cancer screening. Expert review of pharmacoeconomics & outcomes research. 2013;13(3):327–342. doi: 10.1586/erp.13.26. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Denton BT, Balasubramanian H, Shah ND, Inman BA. Optimization of PSA screening policies: a comparison of the patient and societal perspectives. Medical decision making : an international journal of the Society for Medical Decision Making. 2012;32(2):337–349. doi: 10.1177/0272989X11416513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heijnsdijk EA, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. Journal of the National Cancer Institute. 2015;107(1):366. doi: 10.1093/jnci/dju366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulati R, Wever EM, Tsodikov A, et al. What if I don't treat my PSA-detected prostate cancer? Answers from three natural history models. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(5):740–750. doi: 10.1158/1055-9965.EPI-10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wever EM, Draisma G, Heijnsdijk EA, et al. Prostate-specific antigen screening in the United States vs in the European Randomized Study of Screening for Prostate Cancer-Rotterdam. Journal of the National Cancer Institute. 2010;102(5):352–355. doi: 10.1093/jnci/djp533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sox HC. Quality of life and guidelines for PSA screening. New England Journal of Medicine. 2012;367(7):669–671. doi: 10.1056/NEJMe1207165. [DOI] [PubMed] [Google Scholar]
- 54.Vickers A, Carlsson S, Laudone V, Lilja H. It ain't what you do it's the way you do it: five golden rules for transforming prostate-specific antigen screening. European urology. 2014;66(2):188–190. doi: 10.1016/j.eururo.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 55.Murphy DG, Loeb S. Prostate cancer: Growth of AS in the USA signals reduction in overtreatment. Nature reviews. Urology. 2015 doi: 10.1038/nrurol.2015.236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.