Abstract
We previously found that aspirin decreases the risk of cerebral aneurysm rupture in humans. We aim to assess whether a sex differential exists in the response of human cerebral aneurysms to aspirin and confirm these observations in a mouse model of cerebral aneurysm. A nested case-control analysis from the International Study of Unruptured Intracranial Aneurysms was performed to assess whether a sex differential exists in the response of human cerebral aneurysms to aspirin. A series of experiments were subsequently performed in a mouse model of cerebral aneurysms. Aneurysms were induced with hypertension and elastase injection into mice basal cisterns. We found that aspirin decreased the risk of aneurysm rupture in men significantly more than women in the International Study of Unruptured Intracranial Aneurysms. In mice, aspirin and cyclooxygenase-2 inhibitor did not affect cerebral aneurysm formation but significantly decreased the incidence of rupture. The incidence of rupture was significantly lower in male vs. female mice on aspirin. Gene expression analysis from cerebral arteries showed higher 15-hydroxyprostaglandin dehydrogenase levels in male mice. The rate of cerebral aneurysm rupture was similar in male mice receiving aspirin and 15-hydroxyprostaglandin dehydrogenase inhibitor compared to females receiving aspirin and 15-hydroxyprostaglandin dehydrogenase agonist signaling a reversal of the sex-differential response to aspirin. Aspirin decreases aneurysm rupture in human and mice, in part through cyclooxygenase-2 pathways. Evidence from animal and human studies suggests a consistent differential effect by sex. 15-hydroxyprostaglandin dehydrogenase activation in females reduces the incidence of rupture and eliminates the sex-differential response to aspirin.
Keywords: aneurysm, aspirin, inflammation, sex, subarachnoid hemorrhage
Introduction
Inflammation in response to hemodynamic stress plays a critical role in cerebral aneurysm (CA) formation and rupture.1 The data derive from studies in both humans and animal models of CA and implicate several inflammatory cells and mediators in the cascade of events leading to CA rupture.2–8
Given the central role of inflammation in the pathogenesis of CAs, several anti-inflammatory therapeutic strategies have been tested. Aspirin is a promising agent that may decrease aneurysm rupture.9,10 In a nested case-control study from a large epidemiologic cohort study, the International Study of Unruptured Intracranial Aneurysms (ISUIA, published in Lancet in 2003),11 our group found that patients taking aspirin (325 mg) at least three times weekly had a significantly lower risk of subarachnoid hemorrhage (SAH) compared with those who never took aspirin (figure S1).12 In the present study, we further analyze the data from the ISUIA to evaluate whether a sex differential exists in the response of human CAs to aspirin.
Additionally, we aim to confirm these findings in a mouse model. Specifically, we assess whether aspirin decreases the incidence of CA rupture in mice and investigate whether the underlying mechanism is through inhibition of the Cyclooxygenase-2 (Cox-2) pathway. We also hypothesize that aspirin decreases the incidence of CA rupture more so in male than female mice due to a differential expression of 15-hydroxyprostaglandin dehydrogenase (15-PGDH).
Methods
Analysis of the sex differential response to aspirin in the ISUIA
ISUIA is an epidemiological cohort study that involved along-term follow-up of 2 prospective cohorts: untreated; and treated. Prospective case ascertainment was from 1991 to 1998 (phases I and II) and prospective follow-up of the prospective cohort (Phase III) was conducted from 2004 to 2007. A total of 1691 patients were managed conservatively, 1917 patients underwent surgery, and 451 patients underwent endovascular intervention. The patients analyzed in the present study are selected from the 1691 patients who were initially untreated. Of patients enrolled in the conservative management group, 545 ultimately underwent a surgical or endovascular procedure to secure an aneurysm during the overall follow-up period. Patients were enrolled in 61 centers in the United States, Canada, and Europe. Details of aneurysm and patient characteristics were previously published in the original ISUIA article. 11
To be eligible for the ISUIA, patients had to satisfy the following clinical and radiological inclusion and exclusion criteria: patients must have at least 1 unruptured intracranial aneurysm (symptomatic or asymptomatic). Patients who have had a ruptured aneurysm at another location that was treated must be able to care for themselves as determined at a follow-up evaluation at 30 days post-treatment (Rankin Grade 1 or 2). Patients with saccular aneurysms 2 mm maximum diameter or with fusiform, traumatic, or mycotic aneurysms were not eligible for the study. Hemorrhagic events were classified by diagnostic certainty and location of aneurysmal rupture. Frequency of aspirin use (325 mg) in female and male patients was grouped as “never,” “ once a month”, ” once a month to 2 times a week,” and “3 times weekly to daily”.
Subjects were selected from the prospective untreated cohort (n=1691) for a nested case-control study to determine whether there was a differential response by sex in the protective effect of aspirin against aneurysm rupture. Cases are subjects who had a primary SAH over a 5-year period after CA diagnosis. The cases and controls were matched based on aneurysm size and location. Hemorrhage cases were adjudicated based on diagnostic criteria with no knowledge of risk factors. Analysis was done by stratified analysis by sex of the association of aspirin and case-control classification.
Mouse model of CA
A series of 5 experiments were performed in mice. Care of the mice used in the experiments fully met the standards set forth by the National Institutes of Health (NIH) guidelines for the care and use of experimental animals. All procedures were approved by the University of Iowa Institutional Animal Care and Use Committee. CAs were induced using previously published methods as described in detail.2,13,14 Briefly, mice were anesthetized with ketamine (87.5 mg/kg) or xylazine (12.5 mg/kg) and a longitudinal incision was made in the scalp. A 1-mm hole was drilled in the skull, and elastase (20 mU in 2.5 μL) was stereotactically injected using the following coordinates: 2.7 mm posterior to the bregma, 1 mm to the right of the midline, and depth of 6.3 mm from the skull. Immediately after injection of elastase, an osmotic mini-pump that delivered a pressor dose of Angiotensin II (1000 ng/kg per minute) for 3 weeks was implanted subcutaneously. Blinded daily neurological examination was performed. Neurological symptoms were graded: 0, normal; 1, decreased drinking or eating with associated weight loss >2 g of body weight (approximately 10%) over 24 hours; 2, flexion of the torso and forelimbs on lifting of the animal by the tail; 3, circling to one side with a normal posture at rest; 4, leaning or falling to one side at rest; 5, no spontaneous activity. Mice were euthanized when they developed neurological symptoms (score 1 to 5). Mice without neurological signs were sacrificed 17–19 days following CA induction.
Immediately after euthanasia, the chest and abdomen of each mouse were exposed and examined for major bleeding or aneurysms of the aorta. Mice were perfused transcardially at physiological pressures with 10 to 15 mL of ice-cold physiological saline solution containing papaverine (100 μmol/L) to produce systemic vasodilation, followed by infusion of 2 mg/mL of bromophenol blue dye and 8% in gelatin saline to facilitate visualization of arteries and small vessels. The brain was then dissected and inspected for the presence of CAs and SAH. Aneurysms were defined as a localized outpouching arising from any cerebral artery with a diameter ≥1.5× the parent artery diameter as assessed by 2 independent observers blinded to the animal cohort.2,13,14 Animal cohorts were not revealed until all experimental groups had been euthanized.
Gene expression in mice cerebral arteries
Cerebral arteries (anterior and posterior cerebral arteries, anterior and posterior communicating arteries, middle cerebral arteries, and basilar arteries) were isolated from the above mice when possible and dissolved in TriZol (Life Technologies). qRT-PCR was used to quantify mRNA levels as described previously,13,14 with both primers for the gene of interest (FAM fluoror, Life Technologies or IDT) and the house-keeping β-actin (VIC fluoror, Life Technologies, for normalization) in the same reaction.
Statistical Analysis
Analysis was performed using Sigma Plot 12.5 (Systat Software, Inc) and Prism 6 (Graphpad, La Jolla, CA) and SAS Version 9.2. Categorical data (incidence of aneurysms and SAH) was compared with 2-tailed Fisher Exact Test. Kaplan–Meier survival analysis was performed with comparison between cohorts using the log-rank (Mantel-Cox) test. Aspirin effect by sex was evaluated by Mantel-Haenszel Chi-Square and conditional logistic regression analysis. A p value <0.05 was considered significant.
Results
1 Analysis of sex-differential effect of aspirin on risk of SAH in humans – results from the ISUIA
Twenty-eight of 52 men reported using aspirin, with 18 using aspirin ≥3x per week – daily. The proportion of SAH cases in males on aspirin ≥3x per week – daily (1/18, 6%) was significantly lower than in males who used aspirin less frequently (16/34, 47%, p<0.05, table 1).
Table 1.
Results from the International Study of Unruptured Intracranial Aneurysms; OR: Odds ratio
| Males
|
N | Hemorrhages
|
|||
|---|---|---|---|---|---|
| Aspirin use | 1 year | 5 year | >5 year | Proportion | |
| Never | 24 | 6 | 4 | 3 | 54.2% |
| <1 per month- 2 per week | 10 | 0 | 2–1 | 1 | 30.0% |
| 3 times per week - daily | 18 | 1 | 0 | 0 | 5.6% |
| P=0.0024 | OR(<2 week)=0.363 | OR(3+ week)=0.050 | |||
|
| |||||
|
Females
|
N |
Hemorrhages
|
|||
| Aspirin use | 1 year | 5 year | >5 year | Proportion | |
|
| |||||
| Never | 85 | 14 | 10 | 1 | 29.4% |
| <1 per month- 2 per week | 27 | 4 | 1 | 2 | 25.9% |
| 3 times per week - daily | 49 | 1 | 4 | 3 | 16.3% |
| P=0.2369 | OR(<2 week) =0.84 | OR(3+ week)= 0.468 | |||
Seventy-six of 161 women reported using aspirin, with 49 using aspirin ≥3x per week – daily. In contrast to men, the proportion of SAH cases in women on aspirin ≥3x per week – daily (8/49, 16%) did not differ from the proportion of SAH cases in those who used less aspirin (32/112, 29%, p≥0.05).
There was a significant decreasing OR for hemorrhage with the increasing use of aspirin in men (OR, 0.05; p=0.0024) but not in women (OR, 0.46; p=0.23).
2 Aspirin effect in mice
We have recently determined in a nested case-control study from the ISUIA that aspirin confers a protective effect against aneurysm rupture in humans.12 CAs were induced in C57BL/6J mice in the following four groups to confirm this finding and to investigate the underlying mechanism: 1) 14 mice receiving vehicle only (DMSO), 2) 14 mice receiving aspirin (25 mg/kg/day IP), 3) 14 mice receiving Cox-1 inhibitor (Sc-560 10mg/kg/day IP), and 4) 14 mice receiving Cox-2 inhibitor (NS-398 20 mg/kg/day IP, dose based on the literature15,16). Mice needed to be excluded due to death within 48 hours of surgery including 3 in the control group, none in the aspirin group, 3 in the COX-1 group, and 2 in the COX-2 group.
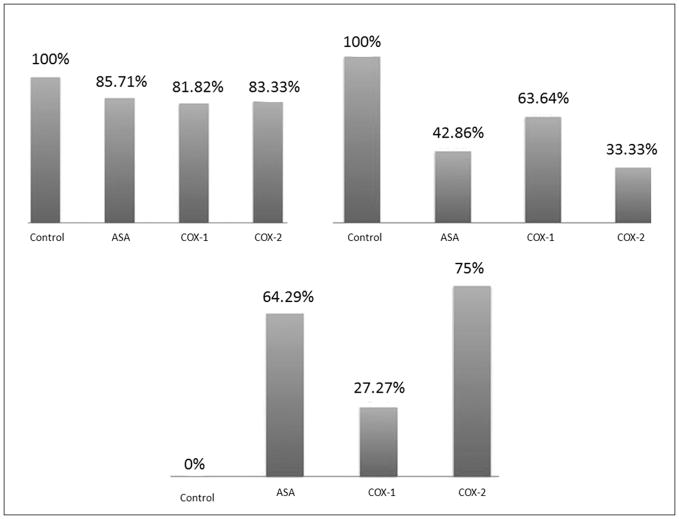
The incidence of CA formation was not different among the four groups (figure 1A). However, the incidence of CA rupture was significantly lower in mice receiving aspirin (p<0.05) or Cox-2 inhibitor (p<0.05) (figure 1B). Survival was also significantly higher in mice receiving aspirin or Cox-2 inhibitor (p<0.05) (figure 1C).
Figure 1.
CAs were induced in 56 C57BL/6J mice divided equally into mice receiving vehicle only (DMSO), aspirin (25 mg/kg/day IP), Cox-1 inhibitor (Sc-560 10mg/kg/day IP), and Cox-2 inhibitor (NS-398 20 mg/kg/day IP). (1A, upper left graph) Aspirin and Cox-2 inhibitor did not alter the rate of CA formation in mice. (1B, upper right graph) Aspirin and Cox-2 inhibitor significantly reduced the risk of SAH in mice. (1C, lower graph) Aspirin and Cox-2 inhibitor significantly prolonged asymptomatic survival in mice.
* indicates statistically significant difference.
To further assess the mechanisms behind alteration in aneurysm rupture, mRNA expression was assessed in cerebral arteries using qRT-PCR. 15-PGDH was significantly higher in aspirin (p<0.05), Cox-1 inhibitor (p<0.05), and Cox-2 inhibitor (p<0.05) groups compared with the control group. We also examined the expression of matrix metalloproteinases-9 (MMP-9), an extracellular enzyme that plays a key role in degradation of extracellular matrix in the wall of CAs.1,6 MMP-9 was significantly decreased in the aspirin (p<0.05) and Cox-2 inhibitor (p<0.05) groups. There was a trend towards decreased Cox-2 (p≥0.05) and monocyte chemoattractant protein-1 (MCP-1) expression (p≥0.05) in the aspirin group, and decreased expression of CD68 (p≥0.05), Cox-2 (p≥0.05) and MCP-1 (p≥0.05) in the Cox-2 inhibitor group but this was not statistically significant.
3 Cox-1 and ASA
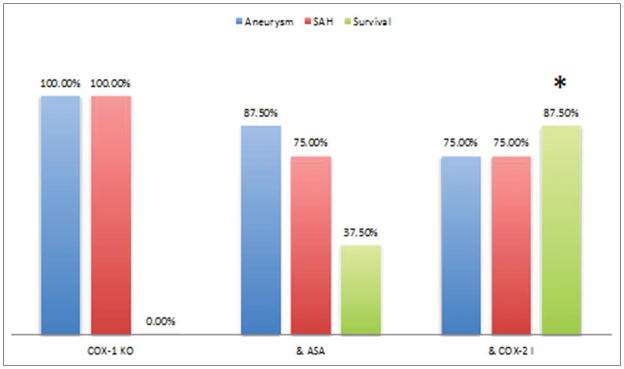
To examine the effect of Cox-1 on CA formation and rupture, aneurysms were induced in 36 Cox-1 knockout mice (C57Bl/6J genetic background –Taconic laboratories) and divided into three equal groups: 1) Cox-1 KO mice receiving vehicle (DMSO) and serving as a control 2) Cox-1 KO mice receiving aspirin (25 mg/kg/day IP), and 3) Cox-1 KO mice receiving Cox-2 inhibitor. Three mice were excluded (death within 48 hours) in the control group; 4 mice in the aspirin group; and 4 mice in the cox-2 inhibitor group. The incidences of CA formation and rupture were not statistically different among the three groups (figure 2). However, the asymptomatic survival rate was significantly higher in Cox-1 KO mice receiving Cox-2 inhibitor (p<0.05).
Figure 2.
Aneurysms were induced in 36 Cox-1 knockout mice and divided into three equal groups: 1) Cox-1 KO mice receiving vehicle (DMSO) and serving as a control 2) Cox-1 KO mice receiving aspirin (25 mg/kg/day IP), and 3) Cox-1 KO mice receiving Cox-2 inhibitor. Aspirin and Cox-2 inhibitor did not affect the incidences of CA formation and rupture in Cox-1 KO mice. Cox-2 inhibitor increased asymptomatic survival rate.
* indicates statistically significant difference.
4 Inhibition of Cox-2 in mPGES-1 KO mice
In a murine model of CA, we have previously shown that deficiency of microsomal prostaglandin E2 (PGE2) synthase type 1 (mPGES-1) is associated with a paradoxical increase in rupture of CAs, which was attenuated by low dose aspirin.14 Aspirin also attenuated mortality in mPGES-1 KO mice.14 A potential explanation for this phenomena is that genetic deficiency of mPGES-1 in mice leads to increased production of prostaglandin D2 (PGD2), prostaglandin F2α (PGF2α), and other mediators that induce a proinflammatory state and promote aneurysm rupture. We had postulated that aspirin may provide a protective effect by blocking this diversion upstream through inhibition of Cox-2.
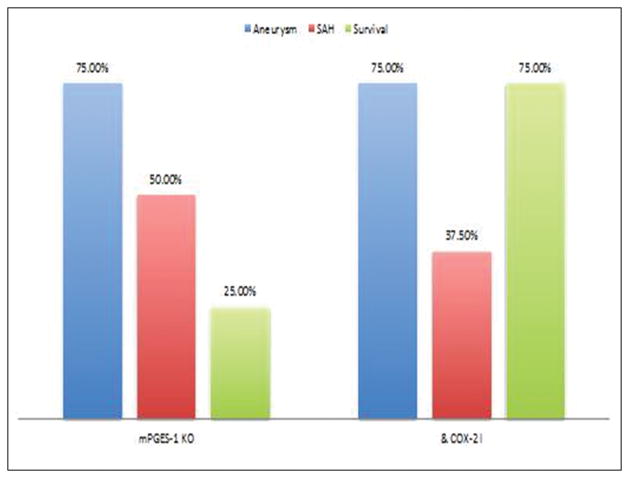
In this experiment, we examined whether a Cox-2 inhibitor has a similar effect to aspirin in mPGES-1 KO mice. CAs were induced in 16 mPGES-1 KO mice and divided into 2 groups: 1) 8 mice receiving vehicle, and 2) 8 mPGES-1 KO mice receiving a Cox-2 inhibitor (NS-398 20 mg/kg/day IP, dose based on the literature15,16). No mice were excluded. Both the incidence of CA formation and rupture did not differ between the 2 groups (figure 3). However, similarly to aspirin, there was a trend towards increased asymptomatic survival in mPGES-1 KO mice receiving a Cox-2 inhibitor (p≥0.05).
Figure 3.
CAs were induced in 16 mPGES-1 KO mice and divided into 2 groups: 1) 8 mice receiving vehicle, and 2) 8 mPGES-1 KO mice receiving a Cox-2 inhibitor (NS-398 20 mg/kg/day IP). Cox-2 inhibitor did not alter the incidences of CA formation and rupture in PGES-1 KO mice. There was a trend towards increased asymptomatic survival in PGES-1 KO mice receiving a Cox-2 inhibitor.
* indicates statistically significant difference.
5 Sex differential response to aspirin in mice
To assess whether there is a sex differential response to aspirin, CAs were induced in 2 groups of C57BL/6J mice: 1) 12 male mice receiving aspirin (25 mg/kg/day IP), and 2) 12 female mice receiving aspirin (25 mg/kg/day IP). Two male mice were excluded due to death within 48 hours. The incidence of CA formation was not statistically different between the 2 groups but the incidence of CA rupture/SAH was significantly lower in male mice (p<0.05).
Gene expression analysis from cerebral arteries using qRT-PCR showed that the expression of 15-PGDH was significantly higher in male mice (p<0.05). Cox-2 (p<0.05), CD-68 (p<0.05), MMP-9 (p<0.05), MCP-1 (p<0.05), and nuclear factor-kB (NF-kB) (p<0.05) were significantly higher in females (Figure S2).
6 15-PGDH activation in female mice on aspirin and 15-PGDH inhibition in male mice on aspirin
15-PGDH is a critical enzyme that catabolizes PGE2 and converts it to 15-keto-PGE2, an endogenous peroxisome proliferator-activated receptor γ (PPARγ) agonist. We have previously found that PPARy decreases aneurysm formation and rupture in mice.13 In this experiment, we assessed whether the differential expression of 15-PGDH in male vs. female mice affected the response to aspirin.
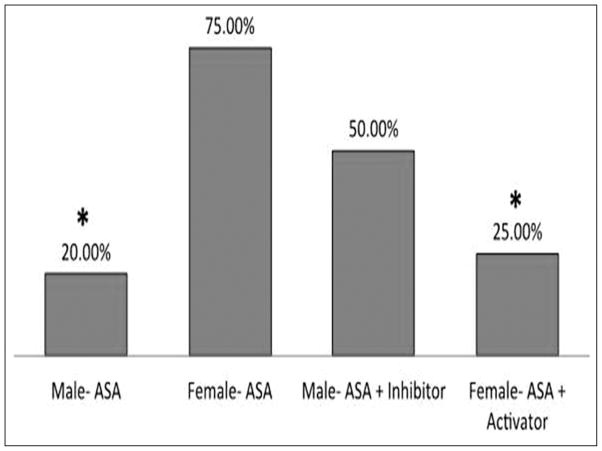
Cerebral aneurysms were induced in 2 groups of C57BL/6J mice: 1) 12 male mice receiving aspirin and 15-PGDH inhibitor (Cay10638; 0.25 mg/day IP, dose based on the literature17), and 2) 12 female mice receiving aspirin and 15-PGDH agonist (CDDO-Me, 250 microgram per day IP – dose based on literature18). Two male mice were excluded sue to death within 48 hours. Aneurysm formation did not differ between the 2 groups. However, the rate of CA rupture/SAH was similar between the 2 groups, signaling a reversal of the sex differential response to aspirin with modulation of the expression of 15-PGDH. When comparing female mice receiving aspirin alone and female mice receiving aspirin and 15-PGDH activator, the latter group had a significantly lower risk of CA rupture/SAH (75% vs. 25%, respectively, p<0.05) (figure 4). Female mice receiving aspirin and 15-PGDH activator had a similar risk of CA rupture/SAH compared with male mice receiving aspirin alone (25% vs. 20%, respectively, p≥0.05).
Figure 4.
CAs were induced in 2 groups of C57BL/6J mice: 1) 12 male mice receiving aspirin (25 mg/kg/day IP), and 2) 12 female mice receiving aspirin (25 mg/kg/day IP). Male mice treated with aspirin had lower rates of aneurysm rupture compared with female mice.
CAs were then induced in 2 groups of C57BL/6J mice: 1) 12 male mice receiving aspirin and 15-PGDH inhibitor (Cay10638; 1mg/kg/day IP), and 2) 12 female mice receiving aspirin and 15-PGDH agonist (CDDO-Me, 250 ng per day IP). The rate of CA rupture/SAH was similar between the 2 groups, indicating a reversal of the sex differential response to aspirin.
* indicates statistically significant difference.
Gene expression analysis from cerebral arteries of the 2 groups described above showed that 15-PGDH was still higher (p≥0.05) in male mice (but no longer significantly). Expressions of Cox-2 (p≥0.05), CD-68 (p≥0.05), MMP-9 (p≥0.05) and NF-kB (p≥0.05) were no longer statistically different between male and female mice (Figure S3). Levels of Cullin-3 (p<0.05) and Keap-1 (p<0.05) were significantly higher, and Nrf2 significantly lower in female mice (p<0.05).
Discussion
The major findings of this study are that: 1) In the IUSIA, frequent aspirin use decreased the risk of aneurysm rupture in men more than women; 2) aspirin decreases the risk of aneurysm rupture (not formation) in mice and attenuates the expression of inflammatory mediators in cerebral arteries; 3) the protective effects of aspirin are likely mediated by Cox-2 (not Cox-1); 4) the risk of aneurysm rupture is lower in male mice compared to female mice receiving aspirin. Female mice on aspirin have lower levels of 15-PGDH and higher levels of inflammatory molecules than male mice on aspirin. This sex differential response to aspirin is reversed by adding a 15-PGDH agonist to aspirin in females.
Growing evidence suggests that inflammation is a central factor in the pathogenesis of CAs. The inflammatory process is initiated by a hemodynamic insult, involves several inflammatory cells/mediators, and leads to degradation of the extracellular matrix by MMPs and apoptosis of smooth muscle cells. 1,6 These processes act in concert to weaken the arterial wall, resulting in dilatation, aneurysm formation, and ultimately rupture.1,6 Emerging data suggest that aspirin, through its antinflammatory action, may decrease the risk of CA rupture. 12,19 The protective effect of aspirin against aneurysm rupture initially noted in a nested case-control study from ISUIA 12 was later confirmed in a study from Europe that included 1340 cases of SAH and 10,000 controls.19 Among the findings of this study, the authors found that long-term low-dose aspirin therapy protects against SAH and does not increase the risk of intracerebral hemorrhage. Furthermore, the trend toward decreased risk of SAH was particularly pronounced in those on long-term aspirin therapy (>3 years). 19 Along similar lines, in 747 patients with CAs, Gross et al20 found the rate of hemorrhagic presentation to be significantly lower in patients taking aspirin (28% vs. 40%). Taken together these studies provided the background for further research into the role of aspirin in the prevention of SAH.
This study provides evidence that, in mice like in humans, aspirin decreases the risk of CA rupture (not formation) and improves asymptomatic survival. There was also decreased expression of inflammatory molecules in cerebral arteries of mice treated with aspirin, namely MMP-9, MCP-1, and Cox-2 all of which have been shown to be involved in CA pathogenesis.1,6 The mechanism through which aspirin may exert its protective effects is likely the Cox-2 pathway as evidenced by the similarity between the effects of aspirin and a Cox-2 inhibitor on aneurysm formation (no change), rupture (decreased), asymptomatic survival (increased) as well as the comparable anti-inflammatory profile induced in cerebral arteries. Moreover, both aspirin and Cox-2 inhibitor improved asymptomatic survival in mPGES-1 KO mice. These results are in agreement with the findings of a previously published study where we investigated the effects of aspirin on the expression of inflammatory molecules in human CAs.21 Decreased expression of cyclooxygenase-2 (Cox-2) (not Cox-1) and macrophages among other mediators were found in patients treated with aspirin compared with controls. This suggested that aspirin may exert its protective effects through the Cox-2 pathway. Figure S4 highlights some mechanisms through which aspirin may interact with cytokines and enzymes to protect against aneurysm rupture.
In this study, Cox-1 KO mice had a 100% rate of CA rupture and aspirin did not affect the risk of hemorrhage or mortality in this group, suggesting that Cox-1 may not be involved in the pathogenesis of CA rupture or the pathway through which aspirin exerts its protective effects.
This report brings evidence from both human and animal studies suggesting that the protective effects of aspirin against CA rupture vary by sex. In our matched case-control analysis of ISUIA,12 aspirin dramatically decreased the risk of SAH in men but only minimally in women. Similarly, in mice, aspirin decreased the risk of CA rupture in males more than in females. This novel observation reminds us of reports from the cardiovascular literature that aspirin decreases the risk of myocardial infarction in men but not in women.22 A meta-analysis by Berger et al22 that included 6 trials with a total of 95 456 patients showed that aspirin had no effect on myocardial infarction (MI) in women but was associated with a 32% reduction in the rate of MI in men.
Female mice receiving aspirin in this study had lower levels of 15-PGDH in cerebral arteries and higher levels of proinflammatory mediators namely Cox-2, CD-68, MMP-9, MCP-1, and NF-kB compared with male mice. With the addition of 15-PGDH activator, the levels of proinflammatory mediators in cerebral arteries as well as the risk of aneurysm rupture decreased significantly in female mice equaling those in male mice. These findings suggest that females may have lower levels of 15-PGDH, an enzyme that appears to mediate the protective effects of aspirin against SAH. Restoring 15-PGDH activity in females eliminates this sex difference. Other factors may contribute to the sex variation in aspirin effect including a known difference in aspirin metabolism with a reduced pharmacological effect of aspirin among women compared with men.23,24 Also, aspirin resistance tends to be more prevalent among women than men.25
There are currently no medical therapies recommended for patients with CAs. Both endovascular and microsurgical interventions may be associated with a risk of morbidity/mortality. A noninvasive medical treatment that lessens the risk of aneurysm rupture by targeting the underlying biological mechanism would be a beneficial therapy for a large number of patients, especially those who do not meet criteria for invasive treatment. Aspirin is also an inexpensive, widely available, and safe drug that has several other health benefits, including the prevention of MI, ischemic stroke, and colorectal cancer. Clinicians are also very familiar with aspirin, which facilitates its use for the prevention of SAH. Addition of 15-PGDH in women may be necessary to optimize the effects of aspirin.
Limitations
The rapidity of aneurysm formation and the use of exogenous elastase to induce CAs in mice may activate mechanisms that are distinct from those responsible for the natural progression of CAs in humans. An attempt to use Cox2 KO mice was not successful in these experiments due to the rapid death of these mice (within first 1–10 days) secondary to cardiac effusions and other unknown conditions. The dose of aspirin that we used in mice was higher than the dose used in the ISUIA. We chose to use this high dose of aspirin to obtain the anti-inflammatory rather than the anti-platelet effect. It would have been interesting to study whether different doses of aspirin would have different effects on aneurysm formation and rupture. We did not have enough tissue from cerebral arteries to perform Western blotting or immunohistochemistry to corroborate the PCR findings. Lastly, although the number of patients evaluated from ISUIA was rather small, the data still reached statistical significance.
Perspectives
Aspirin appears to decrease the risk of SAH in men more than women. Furthermore, 15-PGDH activation in females may reduce the incidence of aneurysm rupture and eliminate the sex differential response to aspirin. The present study also confirms in a mouse model the observation from ISUIA that aspirin decreases the risk of aneurysm rupture. Future clinical trials are indicated to determine the overall effect of aspirin on CA rupture and whether aspirin is indicated for prevention of subarachnoid hemorrhage.
Supplementary Material
Novelty and significance.
What is new?
The study provides novel data from both mice and humans showing that aspirin decreases the risk of cerebral aneurysm rupture, with a consistent sex differential that can be reversed with 15-PGDH modulation.
What is relevant?
Aspirin may be a potential medical therapy for prevention of cerebral aneurysm rupture
Summary
Aspirin decreases the risk of cerebral aneurysm rupture in men more than women
Acknowledgments
Funding Sources: This work was supported by the NIH (D. Hasan [R03NS079227, K08NS082363]
Footnotes
Disclosures: None
References
- 1.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–3622. doi: 10.1161/STROKEAHA.113.002390. [DOI] [PubMed] [Google Scholar]
- 2.Pena-Silva RA, Chalouhi N, Wegman-Points L, Ali M, Mitchell I, Pierce GL, Chu Y, Ballas ZK, Heistad D, Hasan D. Novel role for endogenous hepatocyte growth factor in the pathogenesis of intracranial aneurysms. Hypertension. 2015;65:587–593. doi: 10.1161/HYPERTENSIONAHA.114.04681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starke RM, Chalouhi N, Ding D, Raper DM, McKisic MS, Owens GK, Hasan DM, Medel R, Dumont AS. Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Translational Stroke Research. 2014;5:338–346. doi: 10.1007/s12975-013-0290-1. [DOI] [PubMed] [Google Scholar]
- 4.Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013;44:2594–2597. doi: 10.1161/STROKEAHA.113.002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starke RM, Raper DM, Ding D, Chalouhi N, Owens GK, Hasan DM, Medel R, Dumont AS. Tumor necrosis factor-alpha modulates cerebral aneurysm formation and rupture. Translational Stroke Research. 2014;5:269–277. doi: 10.1007/s12975-013-0287-9. [DOI] [PubMed] [Google Scholar]
- 6.Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. Biology of intracranial aneurysms: role of inflammation. Journal of Cerebral Blood Flow and Metabolism. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalouhi N, Jabbour P, Hasan D. Inflammation, macrophages, and targeted imaging in intracranial aneurysms. World Neurosurgery. 2014;81:206–208. doi: 10.1016/j.wneu.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K, Hasan DM, Greig NH, Owens GK, Dumont AS. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. Journal of Neuroinflammation. 2014;11:77. doi: 10.1186/1742-2094-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starke RM, Chalouhi N, Ding D, Hasan DM. Potential role of aspirin in the prevention of aneurysmal subarachnoid hemorrhage. Cerebrovascular Diseases. 2015;39:332–342. doi: 10.1159/000381137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalouhi N, Jabbour P, Starke RM, Hasan DM. Aspirin for prophylaxis against cerebral aneurysm rupture. World Neurosurgery. 2014;81:e2–3. doi: 10.1016/j.wneu.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 12.Hasan DM, Mahaney KB, Brown RD, Jr, Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC International Study of Unruptured Intracranial Aneurysms I. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasan DM, Starke RM, Gu H, Wilson K, Chu Y, Chalouhi N, Heistad DD, Faraci FM, Sigmund CD. Smooth Muscle Peroxisome Proliferator-Activated Receptor gamma Plays a Critical Role in Formation and Rupture of Cerebral Aneurysms in Mice In Vivo. Hypertension. 2015;66:211–220. doi: 10.1161/HYPERTENSIONAHA.115.05332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pena Silva RA, Mitchell IJ, Kung DK, Pewe LL, Granja MF, Harty JT, Faraci FM, Heistad DD, Hasan DM. Paradoxical Increase in Mortality and Rupture of Intracranial Aneurysms in Microsomal Prostaglandin E2 Synthase Type 1-Deficient Mice: Attenuation by Aspirin. Neurosurgery. 2015;77:613–620. doi: 10.1227/NEU.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke. 2002;33:2704–2710. doi: 10.1161/01.str.0000033132.85123.6a. [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Tai HH, Cho H. Synthesis and SAR of thiazolidinedione derivatives as 15-PGDH inhibitors. Bioorganic & Medicinal Chemistry. 2010;18:1428–1433. doi: 10.1016/j.bmc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Ames E, Harouna S, Meyer C, Welniak LA, Murphy WJ. The triterpenoid CDDO-Me promotes hematopoietic progenitor expansion and myelopoiesis in mice. Biology of Blood and Marrow Transplantation. 2012;18:396–405. doi: 10.1016/j.bbmt.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Rodriguez LA, Gaist D, Morton J, Cookson C, Gonzalez-Perez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology. 2013;81:566–574. doi: 10.1212/WNL.0b013e31829e6ffa. [DOI] [PubMed] [Google Scholar]
- 20.Gross BA, Rosalind Lai PM, Frerichs KU, Du R. Aspirin and aneurysmal subarachnoid hemorrhage. World Neurosurgery. 2014;82:1127–1130. doi: 10.1016/j.wneu.2013.03.072. [DOI] [PubMed] [Google Scholar]
- 21.Chalouhi N, Teufack S, Chandela S, Dalyai R, Tjoumakaris S, Hasan DM, Dumont AS, Gonzalez LF, Rosenwasser RH, Jabbour PM. Aneurysmal subarachnoid hemorrhage in patients under 35-years-old: a single-center experience. Clin Neurol Neurosurg. 2013;115:665–668. doi: 10.1016/j.clineuro.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 23.Spranger M, Aspey BS, Harrison MJ. Sex difference in antithrombotic effect of aspirin. Stroke. 1989;20:34–37. doi: 10.1161/01.str.20.1.34. [DOI] [PubMed] [Google Scholar]
- 24.Escolar G, Bastida E, Garrido M, Rodriguez-Gomez J, Castillo R, Ordinas A. Sex-related differences in the effects of aspirin on the interaction of platelets with subendothelium. Thrombosis Research. 1986;44:837–847. doi: 10.1016/0049-3848(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 25.Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, Sapp SK, Topol EJ. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. The American Journal of Cardiology. 2001;88:230–235. doi: 10.1016/s0002-9149(01)01631-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.