Abstract
BACKGROUND
Sexually transmitted infections (STIs) in pregnancy such as Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) may lead to adverse infant outcomes.
METHODOLOGY
Individual urine specimens from HIV-infected pregnant women diagnosed with HIV during labor were collected at the time of infant birth and tested by polymerase chain reaction for CT and NG. Infant HIV infection was determined at 3 months with morbidity/mortality assessed through 6 months.
RESULTS
Of 1373 maternal urines, 277 (20.2%) were positive for CT and/or NG; 249 (18.1%) for CT, 63 (4.6%) for NG, and 35 (2.5%) for both CT and NG. HIV infection was diagnosed in 117 (8.5%) infants. Highest rates of adverse outcomes (sepsis, pneumonia, congenital syphilis, septic arthritis, conjunctivitis, low birth weight, preterm delivery, death) were noted in infants of women with CT and NG (23/35, 65.7%) compared to NG (16/28, 57.1%), CT (84/214, 39.3%), and no STI (405/1096, 37%, p=0.001). Death (11.4% vs. 3%, p=0.02), low birth weight (42.9% vs. 16.9%, p=0.001), and preterm delivery (28.6% vs. 10.2%, p=0.008) were higher among infants of CT and NG co-infected women. Infants who had any adverse outcome and were born to women with CT and/or NG were 3.5 times more likely to be HIV-infected after controlling for maternal syphilis (OR 3.5, 95% CI 1.4-8.3). By adjusted multivariate logistic regression, infants born to mothers with any CT and/or NG were 1.35 times more likely to have an adverse outcome (OR 1.35, 95% CI 1.03-1.76).
CONCLUSION
STIs in HIV-infected pregnant women are associated with adverse outcomes in HIV-exposed infected and uninfected infants.
Keywords: HIV, pregnancy, chlamydia, gonorrhea, sexually transmitted infections, adverse infant outcomes
INTRODUCTION
In 2008, the World Health Organization estimated that 105.7 million new Chlamydia trachomatis (CT) and 106.1 million Neisseria gonorrhoeae (NG) infections occurred worldwide, with highest rates in low and middle-income countries.(1) Sexually transmitted infections (STIs) including CT and NG pose additional health risks for HIV-infected pregnant women. Untreated chlamydial and gonococcal infections in pregnancy can lead to fetal loss, premature rupture of membranes, and preterm labor and delivery.(2-6) Maternal chlamydial infections may lead to neonatal conjunctivitis and pneumonia, (4) whereas gonococcal infections may also predispose infants to conjunctivitis and in rare cases disseminated infections such as sepsis and septic arthritis.(3)
In response to limited published research from low and middle-income countries of HIV-infected pregnant women with CT and/or NG infections, the present sub-study aimed to assess the health of HIV-exposed infants in the first six months of life, particularly in association with these maternal STIs. We, therefore, evaluated adverse infant outcomes associated with these STIs during pregnancy including sepsis, pneumonia, congenital syphilis, septic arthritis, conjunctivitis, death, low birth weight, and premature delivery. In a separate report we described the prevalence of STIs in the NICHD HPTN 040 cohort and evaluated potential associations between maternal STIs and mother-to-child HIV transmission.(7)
METHODS
Study Design
This study was a sub-study of the National Institute of Child Health and Human Development (NICHD) HIV Prevention Trials Network (HPTN) 040 trial, also known as International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT P1043) NICHD/HPTN 040 (or P1043), a phase 3, triple-arm, randomized, open-label, multi-center study that evaluated the efficacy, safety, and tolerance of three different infant antiretroviral prophylaxis regimens for the prevention of intrapartum HIV transmission to infants born to HIV-infected pregnant women, who had not received antiretroviral drugs during pregnancy.(8) Study enrollment consisted of 1684 HIV-infected pregnant women diagnosed with HIV infection at the time of labor and delivery. All women provided written informed consent. Enrollment occurred at multiple sites in Brazil, South Africa, Argentina, and the United States. Infants <32 weeks of gestational age were excluded from the study.
Maternal plasma HIV RNA levels and CD4+ T-lymphocyte subsets were obtained at the time of labor and delivery. Syphilis testing was performed at the time of labor and delivery using Venereal Disease Research Laboratory (VDRL) test titers with confirmatory treponemal syphilis antibody tests, per standard of care. The primary endpoint of the parent study was HIV infection status at 3 months of age. However, infants were followed until 6 months of age for safety and toxicity monitoring in the parent study. Adverse infant outcome data through age 6 months were collected, which included the variables of interest in this sub-study: sepsis, pneumonia, congenital syphilis, septic arthritis, conjunctivitis, death, low birth weight (<2500 g), and premature delivery (<37 weeks and ≥32 weeks).
HIV Diagnosis
HIV DNA polymerase chain reaction (PCR) was performed on infants within 48 hours of birth and at 10-14 days, 4-6 weeks, 3 months, and 6 months of age. Repeat HIV DNA PCR testing was performed to confirm a positive result. Diagnosis of infant HIV infection required two positive HIV DNA PCR test (Roche Molecular Systems Inc., Basel, Switzerland) results collected on different days. During the primary study, infants with a positive HIV DNA PCR test result at birth and positive results on repeat testing were classified as having in utero HIV infection. Infants with a negative HIV DNA PCR result at birth and a positive HIV DNA PCR result on subsequent testing were classified as having intrapartum HIV infection. All HIV-exposed infants enrolled in the study were exclusively formula fed.
Specimen Collection and Chlamydia and Gonorrhea Testing
Stored maternal urine samples, one per patient, collected at the time of labor and delivery or within 48 hours of birth were frozen at − 80 °C and stored at study sites. Stored urine was thawed and aliquots (7 mL each) were shipped on dry ice for testing at Cepheid, Sunnyvale, CA. Urines were tested for the presence of CT and NG using the Xpert® CT/NG assay. Results were reported as positive, negative or indeterminate. Indeterminate test results were repeated up to two times, and those that remained indeterminate were excluded from data analysis.
Statistical Analysis
Chi-square (or Fisher’s exact) test was used to assess the difference in proportions of infants with adverse outcomes, including sepsis, pneumonia, congenital syphilis, septic arthritis, conjunctivitis, death, low birth weight (<2500g), and premature delivery (<37 weeks) according to maternal STI status (only CT-infected, only NG-infected, CT and NG co-infected, or CT and/or NG uninfected) and infant HIV status (HIV-infected (in utero and intrapartum) or HIV-uninfected), respectively. Univariate and multivariate logistic regression modeling (or exact logistic regression as necessary) was used to examine the relationship between adverse clinical outcome and infants born to women with CT and/or NG infection. All computations were done using SAS software v9.3 (Cary, NC, USA).
Human Subjects
Both the parent trial and the present analysis were approved by the institutional review boards and national ethics committees at each study site.
RESULTS
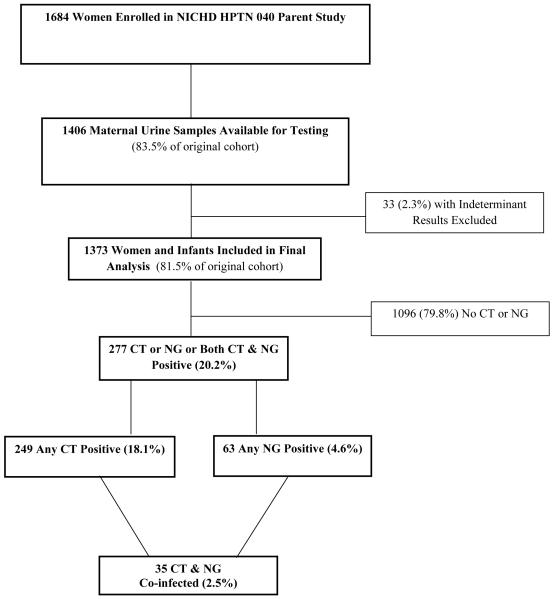
Urine samples from 1406 HIV-1 infected women were tested for CT and NG infections. After excluding 33 indeterminate results (2.3% of samples), 1373 maternal urine test results with linked infant outcomes (81.5% of the 1684 women enrolled in the original study) were included in the analysis. Women were from study sites in Brazil (68.3%), South Africa (29.8%), Argentina (1.4%), and the United States (0.5%). Further detail on the sociodemographics of our cohort was previously described in our earlier manuscript.(7) For the 1373 HIV-infected pregnant women included, 249 (18.1%) had any CT, 63 (4.6%) any NG, and 35 (2.5%) had both CT and NG; 277 women (20.2%) were positive overall for CT and/or NG. One hundred seventeen (8.5%) infants were HIV-infected including 75 (64.1%) infants infected in utero and 42 (35.9%) infants infected intrapartum. (Figure 1)
Figure 1.
Flow chart of subjects enrolled in the present analysis.
Adverse Infant Outcomes and Maternal Chlamydia and Gonorrhea
Of 1373 infants, 528 (38.5%) had at least one of the following adverse outcomes (sepsis, pneumonia, congenital syphilis, septic arthritis, conjunctivitis, death, low birth weight, or prematurity), and significant differences were noted among infants born to CT and/or NG infected as compared to uninfected women (p=0.001). The highest rates of any of those adverse outcomes were noted among infants born to women with both CT and NG (65.7%) as compared to those with NG only (57.1%), CT only (39.3%), and no STI (37%) (p=0.001). (Table 1)
Table 1.
Adverse Infant Outcomes by Sexually Transmitted Infection Status among Infants Born to HIV-infected Pregnant Women.
| Total | CT & NG | CT Only | NG Only | No STI | ||
|---|---|---|---|---|---|---|
| (N = 1373) | (N = 35) | (N = 214) | (N = 28) | (N = 1096) | p value* |
|
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
|
Any of the
following Adverse Infant Outcomes |
||||||
| No | 845 (61.5) | 12 (34.3) | 130 (60.8) | 12 (42.9) | 691 (63.0) | 0.001 |
| Yes | 528 (38.5) | 23 (65.7) | 84 (39.3) | 16 (57.1) | 405 (37.0) | |
| Sepsis | ||||||
| No | 1318 (96.0 | 33 (94.3) | 206 (96.3) | 25 (89.3) | 1054 (96.2) | 0.30 |
| Yes | 55 ( 4.0) | 2 ( 5.7) | 8 ( 3.7) | 3 (10.7) | 42 ( 3.8) | |
| Pneumonia | ||||||
| No | 1305 (95.0) | 33 (94.3) | 204 (95.3) | 24 (85.7) | 1044 (95.3) | 0.15 |
| Yes | 68 ( 5.0) | 2 ( 5.7) | 10 ( 4.7) | 4 (14.3) | 52 ( 4.7) | |
|
Congenital
Syphilis |
||||||
| No | 1289 (93.9) | 32 (91.4) | 203 (94.9) | 26 (92.9) | 1028 (93.8) | 0.85 |
| Yes | 84 ( 6.1) | 3 ( 8.6) | 11 ( 5.1) | 2 ( 7.1) | 68 ( 6.2) | |
| Septic Arthritis | ||||||
| No | 1372 (99.9) | 35 (100.0) | 214 (100.0) | 28 (100.0) | 1095 (99.9) | 0.97 |
| Yes | 1 ( 0.1) | 0 ( 0.00) | 0 ( 0.00) | 0 ( 0.00) | 1 ( 0.1) | |
| Conjunctivitis | ||||||
| No | 1371 (99.9) | 35 (100.0) | 212 (99.1) | 28 (10 0.0) | 1096 (100.0) |
0.01 |
| Yes | 2 ( 0.1) | 0 ( 0.00) | 2 ( 0.9) | 0 ( 0.00) | 0 ( 0.00) | |
| Death | ||||||
| No | 1332 (97.0) | 31 (88.6) | 210 (98.1) | 28 (100.0) | 1063 (97.0) | 0.02 |
| Yes | 41 ( 3.0) | 4 (11.4) | 4 ( 1.9) | 0 ( 0.00) | 33 ( 3.0) | |
|
Low Birth
Weight |
||||||
| ≥2500g | 1129 (82.2) | 20 (57.1) | 176 (82.2) | 22 (78.6) | 911 (83.1) | 0.001 |
| <2500g | 244 (17.8) | 15 (42.9) | 38 (17.8) | 6 (21.4) | 185 (16.9) | |
| Gestational Age | ||||||
| ≥ 37 weeks | 1225 (89.2) | 25 (71.4) | 191 (89.2) | 25 (89.3) | 984 (89.8) | 0.008 |
| <37 weeks | 148 (10.8) | 10 (28.6) | 23 (10.8) | 3 (10.7) | 112 (10.2) |
CT= Chlamydia trachomatis. NG= Neisseria gonorrhoeae.
P-value calculated using Chi-square (or Fisher’s exact) test between groups.
In the cohort, 41 (3.0%) infant deaths occurred, and differences in infant death rates were noted among those born to STI-infected and uninfected women (p=0.02). Death rates were highest among infants born to women with CT and NG (11.4%) compared to those born to women with NG only (0%), CT only (1.9%), and CT or NG uninfected (3%) women.
Two hundred forty-four (17.8%) of the births resulted in low birth weight infants, whereas 148 (10.8%) infants were born preterm. Significant differences in birth weight (p=0.001) and preterm birth (p=0.008) were observed among infants born to women with and without these STIs. Low birth weight rates were highest among infants born to women with CT and NG (42.9%) compared to those born to women with NG only (21.4%), CT only (17.8%), and neither CT or NG (16.9%). Similar differences in death and low birth weight rates among these STI groups were noted when infants with congenital syphilis were excluded from the analysis.
Infants born to women with CT and NG had the highest rates of preterm delivery: 28.6% of infants born to women with CT and NG were born preterm in comparison to 10.2% of those born to women without CT or NG (p=0.008). Significant differences in infant adverse events were not noted among individual maternal STI groups (CT only, NG only, CT and NG, and no CT/NG) for infants with sepsis, pneumonia, congenital syphilis, or septic arthritis. The two cases of infant conjunctivitis occurred in women with CT.
Among 1373 infants, 117 (8.5%) were HIV-infected. Not surprisingly, differences in rates of any of the adverse events were noted when comparing HIV-infected (58.1%) versus HIV-uninfected infants (36.6%), (p<0.0001). Rates of sepsis (12% vs 3.3%, p<0.0001), pneumonia (18.8% vs 3.7%, p< 0.0001), congenital syphilis (10.3% vs 5.7%, p=0.05), death (13.7% vs 2%, p<0.0001), and low birth weight (25.6% vs 17%, p=0.02) were higher in HIV-infected versus HIV-uninfected infants. Infants who had adverse outcomes (Table 2) and were born to women with CT and/or NG were 3.5 times more likely to be HIV-infected (OR 3.5, 95% CI 1.4-8.3) after controlling for maternal syphilis infection. Similar adjusted associations were noted for individual adverse outcomes in infants exposed to maternal CT and/or NG and the likelihood of HIV infection: pneumonia (OR 4.7, 95% CI 1.5-14.9), and death (OR 6.3, 95% CI 1.4-28.1).
Table 2.
Relationship of Infant HIV Infection Status with Adverse Infant Outcomes among Infants Born to HIV-infected Women with Any Sexually Transmitted Infection (CT/NG Including CT & NG Co-infection)
| Total |
HIV-
infected |
HIV-
uninfected |
Unadjusted
|
Adjusted
†
|
|||
|---|---|---|---|---|---|---|---|
| (N=277) | (N=28) | (N=249) | |||||
| n (col %) | n (row %) | n (row %) | OR (95% CI) | p- value |
OR (95% CI) | p-value | |
|
Any of the
following Adverse Infant Outcomes |
|||||||
| No | 154 (55.6) | 8 (5.2) | 146 (94.8) | 1.00 | 1.00 | ||
| Yes | 123 (44.4) | 20 (16.3) | 103 (83.7) | 3.54 (1.50 - 8.36) | 0.004 | 3.45 (1.43 - 8.31) |
0.006 |
| Sepsis | |||||||
| No | 264 (95.3) | 26 (9.8) | 238 (90.2) | 1.00 | 1.00 | ||
| Yes | 13 (4.7) | 2 (15.4) | 11 (84.6) | 1.66 (0.35 - 7.92) | 0.52 | 1.52 (0.31 - 7.40) |
0.60 |
| Pneumonia | |||||||
| No | 261 (94.2) | 23 (8.8) | 238 (91.2) | 1.00 | 1.00 | ||
| Yes | 16 (5.8) | 5 (31.3) | 11 (68.8) | 4.70 (1.50 - 14.72) | 0.008 | 4.74 (1.51 - 14.90) |
0.008 |
|
Congenital
Syphilis |
|||||||
| No | 261 (94.2) | 25 (9.6) | 236 (90.4) | 1.00 | -- | ||
| Yes | 16 (5.8) | 3 (18.8) | 13 (81.3) | 2.18 (0.58 - 8.17) | 0.25 | -- | |
|
Septic
Arthritis |
|||||||
| No | 277 (100) | 28 (10.1) | 249 (89.9) | -- | -- | ||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | -- | -- | ||
| Conjunctivitis | |||||||
| No | 275 (99.3) | 28 (10.2) | 247 (89.8) | 1.00 | 1.00 | ||
| Yes | 2 (0.7) | 0 (0.0) | 2 (100) | 3.69 (0 – 31.24) | 1.0 | 3.92 (0 – 33.21) | 1.0 |
| Death | |||||||
| No | 269 (97.1) | 25 (9.3) | 244 (90.7) | 1.00 | 1.00 | ||
| Yes | 8 (2.9) | 3 (37.5) | 5 (62.5) | 5.86 (1.32 - 25.97) | 0.02 | 6.30 (1.41 – 28.14) | 0.016 |
|
Low Birth
Weight |
|||||||
| ≥ 2500g | 218 (78.7) | 22 (10.1) | 196 (89.9) | 1.00 | 1.00 | ||
| <2500g | 59 (21.3) | 6 (10.2) | 53 (89.8) | 1.01 (0.39 - 2.61) | 0.99 | 0.95 (0.36 - 2.50) |
0.92 |
|
Gestational
Age |
|||||||
| ≥ 37 weeks | 241 (87.0) | 22 (9.1) | 219 (90.9) | 1.00 | 1.00 | ||
| <37 weeks | 36 (13.0) | 6 (16.7) | 30 (83.3) | 1.99 (0.75 - 5.30) | 0.17 | 2.08 (0.77 - 5.57) |
0.15 |
CT= Chlamydia trachomatis. NG= Neisseria gonorrhoeae.
OR: odds ratio. CI: confidence interval. OR calculated by univariate or exact logistic regression as necessary.
Each adverse infant outcome was adjusted for maternal syphilis status using multivariable logistic regression.
In an adjusted multivariate logistic regression controlling for infant HIV-infection status, the risk of any adverse infant outcome (e.g. including sepsis, pneumonia, low birth weight, prematurity and/or death) in infants born to mothers with CT, NG, or both was 1.35 (OR 1.35, 95% CI 1.03 – 1.76) times more than infants born to mothers without any STI. However, no associations were noted for individual infant adverse outcomes and those maternal STIs. (Table 3)
Table 3.
Relationship of CT/NG with Adverse Infant Outcomes with/without Adjusting for Infant HIV Infection Status
| Unadjusted (Predictor = Any CT/NG) |
After Adjusting for HIV
status (Predictor – Any CT/NG + HIV Status) |
|||
|---|---|---|---|---|
| Adverse Infant Outcomes | OR (95% CI) | p-value | OR (95% CI) | p-value |
|
Any Adverse
Infant Outcomes (yes) |
1.36 (1.04 - 1.78) | 0.02 | 1.35 (1.03 - 1.76) | 0.03 |
| Sepsis (yes) | 1.24 (0.65 - 2.34) | 0.51 | 1.19 (0.62 - 2.26) | 0.60 |
| Pneumonia (yes) | 1.23 (0.69 - 2.19) | 0.48 | 1.16 (0.65 - 2.10) | 0.61 |
|
Low birth weight
<2500g |
1.33 (0.96 - 1.85) | 0.086 | 1.32 (0.95 - 1.83) | 0.099 |
|
Gestational age
<37 weeks |
1.31 (0.88 - 1.96) | 0.18 | 1.31 (0.87 - 1.95) | 0.19 |
| Death (yes) | 0.96 (0.44 - 2.10) | 0.91 | 0.88 (0.40 - 1.96) | 0.76 |
CT= Chlamydia trachomatis. NG= Neisseria gonorrhoeae. OR: odds ratio. CI: confidence interval.
DISCUSSION
We evaluated the association of CT and NG infections with adverse infant outcomes. We found that infants born to HIV-infected women with CT and/or NG infection were more likely to have an adverse event through age 6 months as compared to infants born to HIV-infected women without these STIs. In our stratified analysis, the differences for death, low birth weight, and preterm delivery were most pronounced for those infants born to women with dual CT and NG infection.
Death in the first 6 months of life occurred in 11.4% of HIV-exposed infants born to women with CT and NG dual infection, but there was no mortality difference for those with only CT or only NG infections when compared to infants of mothers without either infection. Congenital syphilis and HIV-infection are known risk factors for infant death; however, these differences for CT and NG co-infection persisted even after controlling for these confounders.(9-11) Although limited, the few other existing published studies have also suggested that STIs such as CT or NG during pregnancy may be associated with increased rates of neonatal and infant death.(6, 12-15) One prior study found that stillbirth or neonatal death occurred 10 times more often among CT-infected women than uninfected matched controls.(13) Another study, which found reductions in neonatal death with presumptive STI treatment for Trichomonas vaginalis, bacterial vaginosis, Chlamydia trachomatis, and Neisseria gonorrhoeae in pregnancy, also provides some support for the causal association of maternal STIs in pregnancy and increased neonatal death.(16)
Preterm birth and low birth weight have been attributed to many different risk factors.(17, 18) It is likely that the women in our cohort had some degree of baseline risk for these types of outcomes, particularly given self-reports of high rates of prior preterm birth, usage of alcohol, tobacco, and illegal substances, and lack of prenatal care as discussed in our prior analysis.(7) Nevertheless, our study provides additional support for the deleterious role of STIs and adverse birth outcomes such as preterm birth and low birth weight. These findings were particularly striking among the women infected with both CT and NG, where 42.9% delivered low birth weight infants and 28.6% had premature infants. These co-infected women also delivered infants with average birth weights that were 358.2g less than women uninfected with either of these STIs. Several studies apart from ours have previously suggested that STIs in pregnancy such as CT and/or NG may also be linked with delivery of low birth weight and/or premature infants. (6, 16, 19-24) However, studies that have focused on treatment of genital infections and/or chorioamnionitis have reported conflicting results with respect to improvement in birth outcomes with these interventions.(6, 16, 19, 24-29)
HIV infection during pregnancy, particularly in women with lower CD4 T-cell counts, higher serum HIV viral loads, and higher placental HIV viral loads, have also all been associated with an increased prevalence of low birth weight and/or premature infants.(30-33) Yet, our stratified analysis findings demonstrating that women with STIs had higher rates of low birth weight and premature infants remained, even after controlling for infant HIV status. Those findings were also not explained by high rates of untreated maternal syphilis (10%), as they persisted in a second analysis controlling for this variable. (34)
In fact, our findings may actually underestimate the extent of this problem since very low birth weight and early premature infants were unable to be included; gestational age ≥ 32 weeks was required for enrollment in the parent study.
In our evaluation of the effect of chlamydial and gonococcal infections during pregnancy and adverse events among a cohort of HIV-exposed infants, it may not be surprising that we found high overall rates of adverse outcomes and also significant differences in adverse outcome rates when comparing HIV-infected and HIV-uninfected infants. (31, 32, 35-37) (38-42) Existing research on HIV-exposed infants has suggested that HIV exposure alone may be a risk factor for other infections, particularly in infants born to women with advanced HIV infection, due to genital colonization of pathogens, subclinical chorioamnionitis, and lower protective antibody titers resulting in decreased transfer of passive immunity across the placenta.(43) However, while our infant cohort appeared to be at risk for adverse outcomes from HIV-exposure at baseline, we still found that infants born to CT and/or NG-infected mothers were more likely to have an adverse outcome, irrespective of infant HIV-infection. In our multivariate logistic regression analysis, although risk could not be associated with individual adverse outcomes (likely due to sample size), the risk of any adverse outcome was 1.36 times more likely in infants of women with CT and/or NG. When the analysis was adjusted for HIV-infection status, the risk of any adverse outcome remained essentially unchanged (adjusted odds ratio = 1.35).
One limitation of our study was that the sample size to evaluate the impact of STIs on infant outcomes in the first six months of life was based on convenience. The ability to detect differences in adverse infant outcomes by maternal STI group may have been limited by the modest sample size, particularly when STI exposures were combined with analysis of the infant HIV status. Furthermore, although data were collected on all types of serious adverse events and clinical outcomes that occurred in the parent study, our analysis was limited to adverse infant outcomes that were more frequently reported with CT and/or NG infections as opposed to other nonspecific adverse outcomes such as respiratory distress, hypoglycemia, neutropenia, thrombocytopenia, gastroenteritis that were not included. These other types of adverse outcomes have been reported in a separate analysis of a cohort of 1000 HIV-exposed uninfected infants in NICHD HPTN 040.(44)
CONCLUSION
This study provides important information about the potential deleterious effects of untreated maternal STIs such as Chlamydia trachomatis and Neisseria gonorrhoeae on the well-being of infants born to high-risk groups of HIV-infected women in low and middle income countries. The combination of untreated CT and/or NG infection in pregnancy and HIV-exposure appears to increase the likelihood of adverse outcomes in these infants beyond the risk of HIV acquisition. This additional sub-study again highlights the potential benefits of prenatal laboratory-based STI screening and treatment programs, particularly for high risk groups such as HIV-infected pregnant women, which may aid in preventing these types of adverse infant outcomes.
Acknowledgments
The NICHD HPTN 040 study was supported by NICHD Contract # HHSN267200800001C (NICHD Control # N01-HD-8-0001) and U01 AI047986 (Brazilian AIDS Prevention Trials International Network), National Institute of Allergy and Infectious Diseases (NIAID)/ NIH. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers NIAIDU01 AI068632, UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH (AI068632). In addition, the parent study was supported in part by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI), and GlaxoSmithKline on behalf of ViiV Healthcare. We thank the patients and their families who enrolled in this trial. We also thank Marita McDonough and Lauren Petrella from Boehringer Ingelheim Pharmaceuticals and Helen Watson from GlaxoSmithKline (on behalf of ViiV Healthcare) for assistance with the donation of study drugs from their respective companies for the conduct of the parent study. This particular sub-study was supported by Cepheid, Sunnyvale, CA, where CT and NG testing of specimens was performed. Support for Kristina Adachi's work on this sub-study was also provided in part by the UCLA Children's Discovery and Innovation Institute (CDI). Support was also provided by the UCLA Center for AIDS Research (CFAR) NIH/ NIAID AI028697. We would also like to acknowledge two laboratory personnel who conducted all of the urine specimen preparation and shipment, Mary Ann Hausner and Jessica Liu. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all aspects of manuscript development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, affiliated universities, programs or companies of the authors.
The authors thank the patients and their families who enrolled in this trial. In addition to the authors, members of the NICHD/HPTN 040/PACTG 1043 protocol team include the following: Argentina, Buenos Aires-Foundation for Maternal and Infant Health (FUNDASAMIN): Edgardo Szyld, Silvia Marzo. Brazil, Belo Horizonte-Federal University of Minas Gerais: Flavia Faleiro Ferreira, Fabiana Kakehasi. Porto Alegre-Hospital Nossa Senhora da Conceicao: Rita Lira. Porto Alegre-Hospital Femina: Carla Franceschini de Fraga Rita Lira. Porto Alegre-Irmandade da Santa Casa de Misericordia de Porto Alegre: Debora Fernandes Coelho, Alberto Sanseverino, Luis Carlos Ribeiro. Rio de Janeiro-Hospital dos Servidores do Estado: M. Leticia Santos Cruz, Ezequias Martins, Jacqueline Anita de Menezes, Luisa Andrea Torres Salgado. Rio de Janeiro-Hospital Geral de Nova Iguaçu: Ana Valeria Cordovil, Andréa Gouveia, Priscila Mazzucanti, Jorge Eurico Ribeiro. Ribeirao Preto -Universidade de Sao Paulo: Geraldo Duarte, Adriana Aparecida Tiraboschi Barbaro, Carolina Sales Vieira. Sao Paulo-Universidade Federal de Sao Paulo: Regina Succi. South Africa, Capetown-Stellenbosch University and Tygerberg Hospital: Mark Cotton, Jeanne Louw, Elke Maritz. Johannesburg-Perinatal HIV Research Unit, University of Witwatersrand and Chris Hani Baragwanath Hospital: Sarita Lalsab, Shini Legoete, James Alasdair McIntyre, Mandisa Nyati. United States, Baltimore-Johns Hopkins University: Allison Agwu, Jean Anderson, Joan Bess, Jonathan Ellen, Todd Noletto, Nancy Hutton. Gainesville-Shands Hospital: Carol Delany, Robert M. Lawrence. Jacksonville-University of Florida: Chas Griggs, Mobeen Rathore, Kathleen Thoma, Michelle Tucker. Long Beach-Miller Childrens Hospital: Audra Deveikis, Susan Marks. Newark-University Medical and Dental School of NJ: Linda Bettica, James M. Oleske. San Juan City-San Juan City Hospital: Midnela Acevedo Flores, Elvia Pérez. Oswaldo Cruz Foundation, Rio de Janeiro (FIOCRUZ): Ronaldo I. Moreira, Marilia Santini de Oliveira, Monica Derrico, Valéria Ribeiro, Thiago Torres e FIOTEC (Fundação para o Desenvolvimento Científico e Tecnológico). University of California-Davis: Ruth Dickover. Boston University: Mark Mirochnick. Westat, Inc.: Margaret Camarca, James Bethel, Emmanuel Aluko, Yolanda Bertucci, Jennifer Bryant, Patty Chen, Barbara Driver, Ruby Duston, Adriana Ferreira, Priya Guyadeen, Sarah Howell, Marsha Johnson, Linda Kaufman, Naomi Leshabane, Lilya Meyerson, Rita Patel, Lubima Petrova, Georgine Price, Susan Raitt, Scott Watson, Yiling Xu, Eunice Yu. Other protocol team members included, Jennifer Read and Jack Moye from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Elizabeth Smith and Sheryl Zwerski from the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Clinical Trials Registration Number: NCT00099359. The content, conclusions and opinions expressed in this article are those of the authors and do not necessarily represent those of the National Institutes of Health, the U.S. Department of Health and Human Services, or the U.S. Department of State. Contributors Statement Page: Kristina Adachi drafted the initial sub-study design and data analysis, drafted the initial manuscript, revised, and approved the final manuscript as submitted. Jeffrey D. Klausner provided oversight for the current sub-study design, data analysis, reviewed, revised, and approved final manuscript as submitted. Jiahong Xu designed the data collection instruments, organized data entry for the initial study, provided methods for data analysis, and performed primary data analysis for the study results presented in this paper. Bonnie Ank assisted with preparation and coordination of urine samples from study sites to Cepheid, Inc. and provided laboratory support in the U.S. Claire C. Bristow performed some of the preliminary data analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. Fred Weir provided direct oversight of specimen analysis at Cepheid with the Xpert® CT/NG assay. David Persing facilitated specimen analysis at Cepheid. Mariza Morgado provided laboratory support in Brazil for study conduct, specimen storage, transfer of specimens to the US, and participated in data analysis. Esau Joao, Jose Henrique Pilotto, Glenda Gray, Gerhard Theron, Breno Santos, Rosana Fonseca, Regis Kreitchmann, Jorge Pinto, Marisa M. Mussi-Pinhata, Mariana Ceriotto, Daisy Machado, Beatriz Grinsztejn, and Francisco I. Bastos were responsible for initial study design, patient recruitment and patient care enrolled in this study at sites in Brazil and in South Africa. They also reviewed and revised the manuscript, and approved the final manuscript as submitted. D. Heather Watts, Valdilea Veloso, Lynne Mofenson, Yvonne Bryson, George Siberry, and Karin Nielsen-Saines supervised the design of the data collection instruments, supervised data collection at all sites, critically reviewed the manuscript, and approved the final manuscript as submitted. Dr. Nielsen-Saines was the principal investigator of the parent study as well as this current sub-study.
Disclosures and Conflict of Interest: None of the authors have any financial relationships or conflicts of interest to disclose except for the following: Fred Weir PhD is the Director of Research and Development at Cepheid. David Persing MD, PhD is the Executive Vice President, Chief Medical&Technology Officer of Cepheid.
Prior Presentations: Preliminary data were presented at the Pediatric Academic Societies Conference and Asian Society for Pediatric Research Joint Meeting in Vancouver, Canada on May 5, 2014. Abstract number 754433. Preliminary data restricted to the Americas cohort were presented at the Centers for Disease Control and Prevention STD Prevention Conference in collaboration with the 15th International Union against Sexually Transmitted Infections (IUSTI) World Congress and 2nd Latin American International Union against Sexually Transmitted Infections (IUSTI-ALACITS) Congress in Atlanta, Georgia, U.S. on June 12, 2014. Abstract number 34402.
References
- 1.World Health Organization . Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections--2008. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- 2.Silveira MF, Ghanem KG, Erbelding EJ, Burke AE, Johnson HL, Singh RH, et al. Chlamydia trachomatis infection during pregnancy and the risk of preterm birth: a case-control study. International journal of STD&AIDS. 2009;20(7):465–9. doi: 10.1258/ijsa.2008.008388. Epub 2009/06/23. PubMed PMID: 19541887. [DOI] [PubMed] [Google Scholar]
- 3.Woods CR. Gonococcal infections in neonates and young children. Seminars in pediatric infectious diseases. 2005;16(4):258–70. doi: 10.1053/j.spid.2005.06.006. Epub 2005/10/08. PubMed PMID: 16210106. [DOI] [PubMed] [Google Scholar]
- 4.Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(Suppl 3):S99–102. doi: 10.1093/cid/cir699. Epub 2011/12/07. PubMed PMID: 22080275. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Global Strategy for Prevention and Control of Sexually Transmitted Infections: 2006-2105. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 6.Silva MJ, Florencio GL, Gabiatti JR, Amaral RL, Eleuterio Junior J, Goncalves AK. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2011;15(6):533–9. doi: 10.1590/s1413-86702011000600006. Epub 2012/01/06. PubMed PMID: 22218511. [DOI] [PubMed] [Google Scholar]
- 7.Adachi K, Klausner JD, Bristow CC, Xu J, Ank B, Morgado MG, et al. Chlamydia and Gonorrhea in HIV-Infected Pregnant Women and Infant HIV Transmission. Sexually transmitted diseases. 2015;42(10):554–65. doi: 10.1097/OLQ.0000000000000340. Epub 2015/09/16. PubMed PMID: 26372927; PubMed Central PMCID: PMC4571193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen-Saines K, Watts DH, Veloso VG, Bryson YJ, Joao EC, Pilotto JH, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. The New England journal of medicine. 2012;366(25):2368–79. doi: 10.1056/NEJMoa1108275. Epub 2012/06/22. PubMed PMID: 22716975; PubMed Central PMCID: PMC3590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2013;91(3):217–26. doi: 10.2471/BLT.12.107623. Epub 2013/03/12. PubMed PMID: 23476094; PubMed Central PMCID: PMC3590617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuznik A, Habib AG, Manabe YC, Lamorde M. Estimating the Public Health Burden Associated With Adverse Pregnancy Outcomes Resulting From Syphilis Infection Across 43 Countries in Sub-Saharan Africa. Sexually transmitted diseases. 2015;42(7):369–75. doi: 10.1097/OLQ.0000000000000291. Epub 2015/07/30. PubMed PMID: 26222749; PubMed Central PMCID: PMC4520246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapogiannis BG, Soe MM, Nesheim SR, Abrams EJ, Carter RJ, Farley J, et al. Mortality trends in the US Perinatal AIDS Collaborative Transmission Study (1986-2004) Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(10):1024–34. doi: 10.1093/cid/cir641. Epub 2011/10/18. PubMed PMID: 22002982; PubMed Central PMCID: PMC3202314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gencay M, Koskiniemi M, Saikku P, Puolakkainen M, Raivio K, Koskela P, et al. Chlamydia trachomatis seropositivity during pregnancy is associated with perinatal complications. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1995;21(2):424–6. doi: 10.1093/clinids/21.2.424. Epub 1995/08/01. PubMed PMID: 8562754. [DOI] [PubMed] [Google Scholar]
- 13.Martin DH, Koutsky L, Eschenbach DA, Daling JR, Alexander ER, Benedetti JK, et al. Prematurity and perinatal mortality in pregnancies complicated by maternal Chlamydia trachomatis infections. JAMA : the journal of the American Medical Association. 1982;247(11):1585–8. Epub 1982/03/19. PubMed PMID: 7062461. [PubMed] [Google Scholar]
- 14.Panaretto KS, Lee HM, Mitchell MR, Larkins SL, Manessis V, Buettner PG, et al. Prevalence of sexually transmitted infections in pregnant urban Aboriginal and Torres Strait Islander women in northern Australia. The Australian&New Zealand journal of obstetrics&gynaecology. 2006;46(3):217–24. doi: 10.1111/j.1479-828X.2006.00577.x. Epub 2006/05/18. PubMed PMID: 16704476. [DOI] [PubMed] [Google Scholar]
- 15.Bouwhuis SA, Davis MD. Contribution of sexually transmitted diseases and socioeconomic factors to perinatal mortality in rural Ghana. International journal of dermatology. 2004;43(1):27–30. doi: 10.1111/j.1365-4632.2004.01841.x. Epub 2003/12/25. PubMed PMID: 14693017. [DOI] [PubMed] [Google Scholar]
- 16.Gray RH, Wabwire-Mangen F, Kigozi G, Sewankambo NK, Serwadda D, Moulton LH, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. American journal of obstetrics and gynecology. 2001;185(5):1209–17. doi: 10.1067/mob.2001.118158. Epub 2001/11/22. PubMed PMID: 11717659. [DOI] [PubMed] [Google Scholar]
- 17.March of Dimes P. Save the Children, WHO . Born Too Soon: The Global Action Report on Preterm Birth. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- 18.Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC pregnancy and childbirth. 2010;10(Suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1. Epub 2010/03/27. PubMed PMID: 20233382; PubMed Central PMCID: PMC2841772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Mudenda V, Read JS, Brown ER, Sinkala M, Kamiza S, et al. HPTN 024 study: histologic chorioamnionitis, antibiotics and adverse infant outcomes in a predominantly HIV-1-infected African population. American journal of obstetrics and gynecology. 2006;195(4):1065–74. doi: 10.1016/j.ajog.2006.05.046. Epub 2006/08/01. PubMed PMID: 16875654. [DOI] [PubMed] [Google Scholar]
- 20.Donders GG, Desmyter J, De Wet DH, Van Assche FA. The association of gonorrhoea and syphilis with premature birth and low birthweight. Genitourinary medicine. 1993;69(2):98–101. doi: 10.1136/sti.69.2.98. Epub 1993/04/01. PubMed PMID: 8509101; PubMed Central PMCID: PMC1195038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews WW, Goldenberg RL, Mercer B, Iams J, Meis P, Moawad A, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. American journal of obstetrics and gynecology. 2000;183(3):662–8. doi: 10.1067/mob.2000.106556. Epub 2000/09/19. PubMed PMID: 10992190. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs L, Nagy E, Berbik I, Meszaros G, Deak J, Nyari T. The frequency and the role of Chlamydia trachomatis infection in premature labor. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1998;62(1):47–54. doi: 10.1016/s0020-7292(98)00075-7. Epub 1998/08/29. PubMed PMID: 9722125. [DOI] [PubMed] [Google Scholar]
- 23.Blas MM, Canchihuaman FA, Alva IE, Hawes SE. Pregnancy outcomes in women infected with Chlamydia trachomatis: a population-based cohort study in Washington State. Sexually transmitted infections. 2007;83(4):314–8. doi: 10.1136/sti.2006.022665. Epub 2007/03/09. PubMed PMID: 17344249; PubMed Central PMCID: PMC2598687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg RL, Mwatha A, Read JS, Adeniyi-Jones S, Sinkala M, Msmanga G, et al. The HPTN 024 Study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. American journal of obstetrics and gynecology. 2006;194(3):650–61. doi: 10.1016/j.ajog.2006.01.004. Epub 2006/03/09. PubMed PMID: 16522393. [DOI] [PubMed] [Google Scholar]
- 25.Martin DH, Eschenbach DA, Cotch MF, Nugent RP, Rao AV, Klebanoff MA, et al. Double-Blind Placebo-Controlled Treatment Trial of Chlamydia trachomatis Endocervical Infections in Pregnant Women. Infectious diseases in obstetrics and gynecology. 1997;5(1):10–7. doi: 10.1155/S1064744997000057. Epub 1997/01/01. PubMed PMID: 18476128; PubMed Central PMCID: PMC2364533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen I, Veille JC, Calkins BM. Improved pregnancy outcome following successful treatment of chlamydial infection. JAMA : the journal of the American Medical Association. 1990;263(23):3160–3. Epub 1990/06/20. PubMed PMID: 2348525. [PubMed] [Google Scholar]
- 27.Ryan GM, Jr., Abdella TN, McNeeley SG, Baselski VS, Drummond DE. Chlamydia trachomatis infection in pregnancy and effect of treatment on outcome. American journal of obstetrics and gynecology. 1990;162(1):34–9. doi: 10.1016/0002-9378(90)90815-o. Epub 1990/01/01. PubMed PMID: 2301514. [DOI] [PubMed] [Google Scholar]
- 28.McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double-blind, placebo-controlled trial of erythromycin treatment. American journal of obstetrics and gynecology. 1990;163(5 Pt 1):1580–91. doi: 10.1016/0002-9378(90)90632-h. Epub 1990/11/01. PubMed PMID: 2240110. [DOI] [PubMed] [Google Scholar]
- 29.Andrews WW, Klebanoff MA, Thom EA, Hauth JC, Carey JC, Meis PJ, et al. Midpregnancy genitourinary tract infection with Chlamydia trachomatis: association with subsequent preterm delivery in women with bacterial vaginosis and Trichomonas vaginalis. American journal of obstetrics and gynecology. 2006;194(2):493–500. doi: 10.1016/j.ajog.2005.08.054. Epub 2006/02/07. PubMed PMID: 16458652. [DOI] [PubMed] [Google Scholar]
- 30.Turner AN, Tabbah S, Mwapasa V, Rogerson SJ, Meshnick SR, Ackerman WEt, et al. Severity of maternal HIV-1 disease is associated with adverse birth outcomes in Malawian women: a cohort study. J Acquir Immune Defic Syndr. 2013;64(4):392–9. doi: 10.1097/QAI.0b013e3182a2d13c. Epub 2013/07/13. PubMed PMID: 23846560; PubMed Central PMCID: PMC3940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. British journal of obstetrics and gynaecology. 1998;105(8):836–48. doi: 10.1111/j.1471-0528.1998.tb10227.x. Epub 1998/09/24. PubMed PMID: 9746375. [DOI] [PubMed] [Google Scholar]
- 32.Kim HY, Kasonde P, Mwiya M, Thea DM, Kankasa C, Sinkala M, et al. Pregnancy loss and role of infant HIV status on perinatal mortality among HIV-infected women. BMC pediatrics. 2012;12:138. doi: 10.1186/1471-2431-12-138. Epub 2012/09/04. PubMed PMID: 22937874; PubMed Central PMCID: PMC3480840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marazzi MC, Palombi L, Nielsen-Saines K, Haswell J, Zimba I, Magid NA, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS. 2011;25(13):1611–8. doi: 10.1097/QAD.0b013e3283493ed0. Epub 2011/06/16. PubMed PMID: 21673553. [DOI] [PubMed] [Google Scholar]
- 34.Yeganeh N, Watts HD, Camarca M, Soares G, Joao E, Pilotto JH, et al. Syphilis in HIV-infected mothers and infants: results from the NICHD/HPTN 040 study. The Pediatric infectious disease journal. 2015;34(3):e52–7. doi: 10.1097/INF.0000000000000578. Epub 2015/03/06. PubMed PMID: 25742089; PubMed Central PMCID: PMC4352722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen-Saines K, Komarow L, Cu-Uvin S, Jourdain G, Klingman KL, Shapiro DE, et al. Infant outcomes after maternal antiretroviral exposure in resource-limited settings. Pediatrics. 2012;129(6):e1525–32. doi: 10.1542/peds.2011-2340. Epub 2012/05/16. PubMed PMID: 22585772; PubMed Central PMCID: PMC3362906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owor M, Mwatha A, Donnell D, Musoke P, Mmiro F, Allen M, et al. Long-term follow-up of children in the HIVNET 012 perinatal HIV prevention trial: five-year growth and survival. J Acquir Immune Defic Syndr. 2013;64(5):464–71. doi: 10.1097/QAI.0000000000000015. Epub 2013/10/15. PubMed PMID: 24121753; PubMed Central PMCID: PMC4172334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newell ML, Borja MC, Peckham C. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111(1):e52–60. doi: 10.1542/peds.111.1.e52. Epub 2003/01/02. PubMed PMID: 12509595. [DOI] [PubMed] [Google Scholar]
- 38.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. The Journal of infectious diseases. 2012;206(11):1695–705. doi: 10.1093/infdis/jis553. Epub 2012/10/16. PubMed PMID: 23066160; PubMed Central PMCID: PMC3488194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41(4):504–8. doi: 10.1097/01.qai.0000188122.15493.0a. Epub 2006/05/03. PubMed PMID: 16652060. [DOI] [PubMed] [Google Scholar]
- 40.Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. The Pediatric infectious disease journal. 2011;30(1):45–51. doi: 10.1097/INF.0b013e3181ecbf7e. Epub 2010/12/22. PubMed PMID: 21173675. [DOI] [PubMed] [Google Scholar]
- 41.Kourtis AP, Wiener J, Kayira D, Chasela C, Ellington SR, Hyde L, et al. Health outcomes of HIV-exposed uninfected African infants. AIDS. 2013;27(5):749–59. doi: 10.1097/QAD.0b013e32835ca29f. Epub 2013/05/31. PubMed PMID: 23719347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollins NC, Coovadia HM, Bland RM, Coutsoudis A, Bennish ML, Patel D, et al. Pregnancy outcomes in HIV-infected and uninfected women in rural and urban South Africa. J Acquir Immune Defic Syndr. 2007;44(3):321–8. doi: 10.1097/QAI.0b013e31802ea4b0. Epub 2007/01/02. PubMed PMID: 17195768. [DOI] [PubMed] [Google Scholar]
- 43.Mussi-Pinhata MM, Freimanis L, Yamamoto AY, Korelitz J, Pinto JA, Cruz ML, et al. Infectious disease morbidity among young HIV-1-exposed but uninfected infants in Latin American and Caribbean countries: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Pediatrics. 2007;119(3):e694–704. doi: 10.1542/peds.2006-1856. Epub 2007/02/14. PubMed PMID: 17296782. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen-Saines K, Watts DH, Joao EC, Pilotto JH, Gray G, Theron G, Santos B, Siberry G, Xu J, Camarca M, Bethel J, Veloso VG, Bryson Y, Mofenson L, for the NICHD HPTN 040/PACTG 1043 Protocol Team To formula feed or not: Infectious morbidity, mortality and growth of HIV-exposed, uninfected, formula-fed infants in Brazil and South Africa enrolled in NICHD/ HPTN 040/ PACTG 1043. 3rd Pediatrics HIV Conference; Rome, Italy. 2011. [Google Scholar]