Introduction
As a whole, the possible adverse effects of breast cancer and its treatment on cognitive function are now widely acknowledged, but the risks of specific chemotherapies are still undetermined. A recent retrospective cross-sectional study found lower memory scores on average two years post-treatment among breast cancer survivors who underwent anthracycline (ANTHRA) treatment versus those who underwent other chemotherapies or no chemotherapy, accompanied by lower functional connectivity in Default Mode Network regions1. However, in an earlier report examining a large sample of breast cancer patients immediately post primary treatment and adjuvant chemotherapy (the Mind Body Study [MBS]),2 we found no association between ANTHRA exposure and cognitive complaints. To determine risk of lasting cognitive decline with ANTHRA treatment, we performed a secondary analysis of cognitive function across multiple domains from the MBS prospective longitudinal study of breast cancer survivors, with evaluations up to four time points.
Methods
We analyzed data from the MBS,2,3,4 in which breast cancer survivors underwent baseline neuropsychological evaluations within three months following primary treatments (T1, n=190), at 6-months (T2, n=173), at 1 year (T3, n=173), and on average 4.8 (±0.7, range 3.5–6.9) years post-treatment (TF, n=102). To examine the effects of ANTHRA, we categorized women by receipt of chemotherapy or not: no chemotherapy (No Chemo); chemotherapy but not ANTHRA (Chemo/no ANTHRA); chemotherapy with ANTHRA (Chemo/ANTHRA). The neuropsychological tests selected for these analyses measure memory, processing speed, and executive functions. The full study design and the neuropsychological battery have been detailed elsewhere2. Raw neuropsychological test scores were compared among treatment groups across all four assessment points using mixed models controlling for age, IQ, and history of treatment with endocrine therapy. The MBS was approved by the UCLA IRB and all participants provided written informed consent.
Results
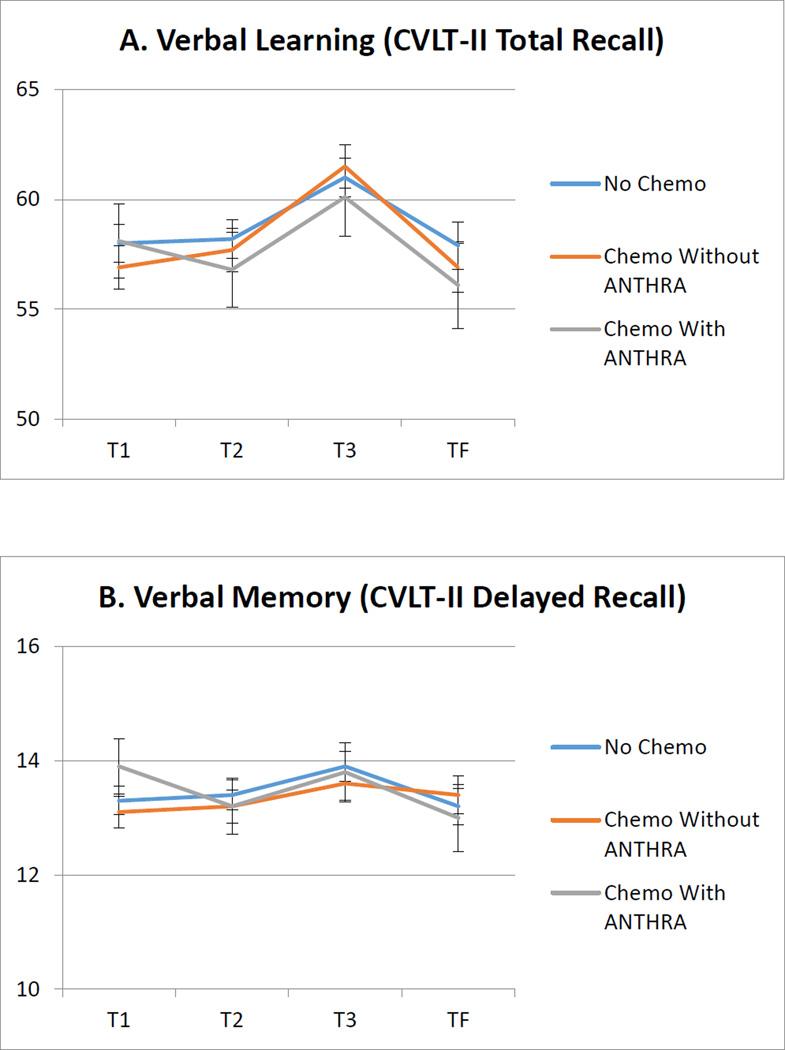
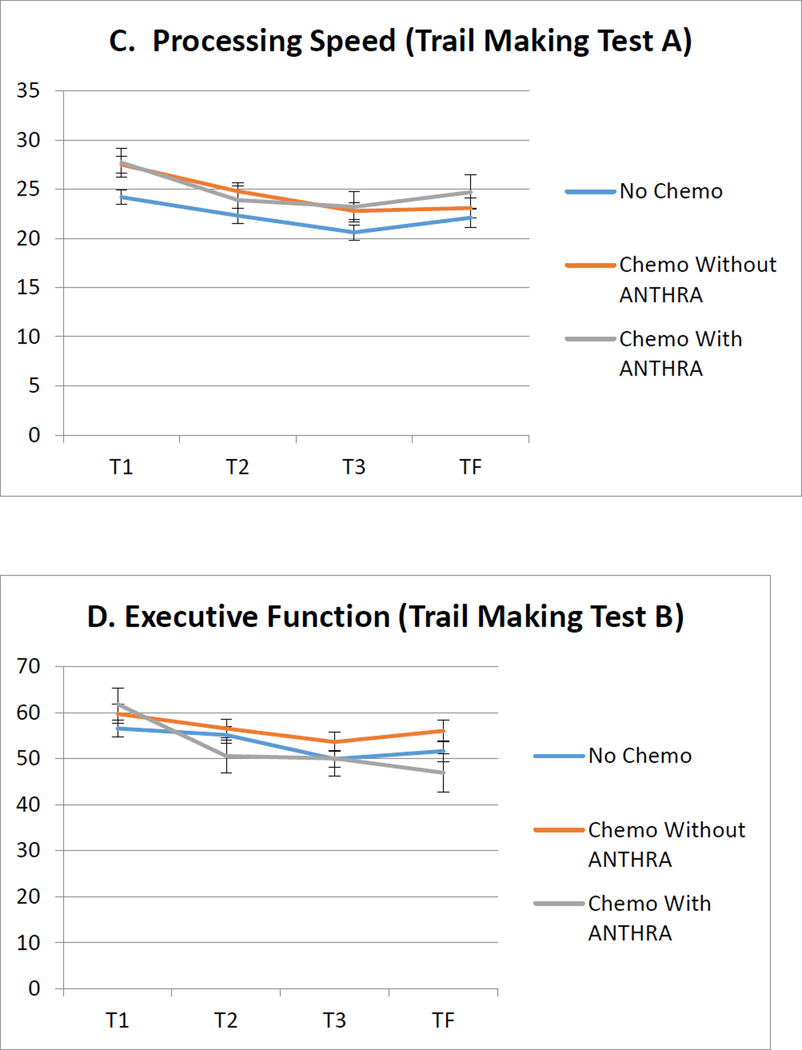
See Table 1 for group demographic and clinical characteristics at T1. Of the seven neuropsychological tests examined in mixed models, there were no significant group effects. There were also no significant group × time interactions in any of the seven mixed models (p’s > .05). In addition to the four neuropsychological test results presented in Figure 1, no group effects or interactions were found on tests of executive functioning/verbal fluency (FAS) or visual memory (Brief Visual Memory Test-Revised, total and delayed recall scores).
Table 1.
Sample Characteristics by Treatment Group
| No Chemo (n=92) | Chemo/No ANTHR (n=74) |
Chemo/ANTHR (n=24) |
Total (n=190) | |
|---|---|---|---|---|
| Age*, years mean (SD), min-max | 53.7 (7.4) 34–66 | 50.1 (7.9), 32–66 | 49.9 (11.0), 31–65 | 51.8 (8.3), 31–66 |
| White, n (%) | 76 (83%) | 56 (76%) | 19 (79%) | 151 (79%) |
| Education (College or more), n (%) | 76 (83%) | 59 (80%) | 21 (88%) | 156 (82%) |
| WTAR IQ mean (SD), min-max | 115.1 (8.6), 89–126 | 112.6 (10.1), 81–125 | 116.4 (6.3), 105–126 | 114.3 (9.1), 81–126 |
| Disease Stage at Diagnosis* n 0, I, II, III (%) | 25 (27%) 57 (62%) 10 (11%) 0 (0%) |
0 (0%) 28 (38%) 37 (50%) 9 (12%) |
0 (0%) 3 (13%) 12 (50%) 9 (38%) |
25 (13%) 88 (46%) 59 (31%) 18 (9%) |
| Chemo Only*, n (%) | 0 (0%) | 17 (23%) | 3 (13%) | 20 (11%) |
| Radiation Only*, n (%) | 64 (70%) | 0 (0%) | 0 (0%) | 64 (34%) |
| fBoth Chemo and Radiation*, n (%) | 0 (0%) | 57 (77%) | 21 (88%) | 78 (41%) |
| Received Endocrine Therapy Ever (%) | 59 (66%) | 58 (79%) | 15 (63%) | 132 (71%) |
| Post-menopausal at BL, n (%) | 55 (60%) | 35 (47%) | 11 (46%) | 101 (53%) |
p<.05 group differences;
WTAR = Wechsler Test of Adult Reading
Figure 1.
Longitudinal adjusted means of raw neuropsychological test scores by treatment group. Error bars show the standard error of the adjusted means.
All means adjusted for age, IQ, and history of treatment with endocrine therapy. CVLT-II = California Verbal Learning Test 2nd Edition.
Discussion
We examined the association of treatment type (chemotherapy with and without ANTHRA, and no chemotherapy) with neuropsychological performance among breast cancer survivors immediately after primary treatment through at least three years post treatment. We found no differences among groups across time points and no group × time interaction on any measure. Our findings indicate that cognitive functioning following cancer treatment in the areas of memory, processing speed, and executive functioning is comparable among those who receive chemo with or without anthracycline and those who did not receive chemotherapy. Further, cognitive functioning over time (i.e., during and after recovery) is also comparable between groups up to four years post-treatment. We did not find an association between anthracycline exposure and neuropsychological performance on any measure examined.
Our findings based on data from a longitudinal prospective study are in contrast to the Kesler et al. cross-sectional study1. We found no differential effect of anthracycline treatment on neuropsychological performance. Further, our neuropsychological battery employed more challenging memory measures than those in Kesler’s study, so the discrepancy is not due to differential sensitivity. In conclusion, in this study we could not find evidence to support the claim that anthracycline treatment confers greater risk of cognitive decline for breast cancer survivors.
Acknowledgments
This research was supported by funding from the National Cancer Institute R01 CA 109650 and the Breast Cancer Research Foundation. Drs. Ganz and Van Dyk had full access to all the data in the study and Dr. Ganz takes responsibility for the integrity of the data and the accuracy of the data analysis. Laura Petersen conducted the data analysis.
Role of the Funder: Neither of the funding organizations played any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: Dr. Ganz would like to disclose that she is a member of the Scientific Advisory Board of the Breast Cancer Research Foundation. There are otherwise no disclosures for any of the authors.
References
- 1.Kesler SR, Blayney DW. Neurotoxic Effects of Anthracycline- vs Nonanthracycline-Based Chemotherapy on Cognition in Breast Cancer Survivors. JAMA Oncol. 2015:1–8. doi: 10.1001/jamaoncol.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz PA, Petersen L, Castellon SA, et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J Clin Oncol. 2014;32(31):3559–3567. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganz PA, Petersen L, Bower JE, et al. Impact of Adjuvant Endocrine Therapy on Quality of Life and Symptoms: Observational Data Over 12 Months From the Mind-Body Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.64.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]