SUMMARY
Evolution of hematophagy in blood-sucking parasites likely involves communication with their hosts. We find that Ixodes ticks are responsive to IFNγ acquired in a blood meal from mice infected with the Lyme disease-causing bacteria Borrelia burgdorferi, leading to induction of antimicrobial responses. Ixodes ticks parasitizing B. burgdorferi-infected mice up-regulated an I. scapularis Rho-like GTPase (IGTPase). IGTPase knockdown enhanced B. burgdorferi levels in post-fed ticks, suggesting this protein controls spirochete survival. Notably, IGTPase was only induced during pathogen acquisition from mice and not upon transmission to naïve hosts. Microinjection of ticks with IFNγ induced IGTPase, and ticks parasitizing IFNγ knockout mice failed to upregulate IGTPase. Additionally, ticks lacking the transcription factor STAT, which signals downstream of IFNγ, did not induce IGTPase. IGTPase expression induced antimicrobial peptides, including Dae2, previously shown to inhibit B. burgdorferi. These results identify an interspecies signaling cascade allowing ticks to detect invading bacteria and mount microbicidal responses.
INTRODUCTION
Like all metazoans, arthropods must control the proliferation of invading pathogens. This is especially challenging for hematophagous vectors like ticks that ingest relatively large volumes of blood meal that can be infested with microbes. Arthropods defend themselves from infection through innate immune responses that include the Toll, immune deficiency (Imd), and Janus kinase (JAK)-signaling transducer activator of transcription (STAT) pathways (De Gregorio et al., 2002; Dostert et al., 2005; Gillespie et al., 1997). The JAKSTAT pathway, originally identified as a cytokine-signaling pathway in mammals (Shuai et al., 1993), is also operational in arthropods, including Ixodes scapularis ticks, and triggers antimicrobial peptide (AMP) responses against Anaplasma phagocytophilum infection (Liu et al., 2012), by an unknown mechanism. Notwithstanding the presence of many orthologs of immunity-related genes in the I. scapularis genome (Smith and Pal, 2014), tick responses to invading pathogens remain enigmatic. As ticks rank second only to mosquitoes in their role as vectors for many human pathogens, additional information is necessary to develop new interventions against many prevalent infections.
AMPs constitute key components of the innate immune system against pathogens (Zasloff, 2002). Ixodes ticks produce several AMPs (Smith and Pal, 2014); in I. ricinus, for example, a defensin-like gene is upregulated in the gut after infection with B. burgdorferi (Hynes et al., 2005) while a 5.3-kDa AMP controls A. phagocytophilum proliferation in the tick salivary glands (Liu et al., 2012). An AMP, Dae2, limits B. burgdorferi proliferation within I. scapularis (Chou et al., 2015). However, the molecular mechanisms that regulate AMP synthesis have not been elucidated.
During blood feeding, arthropods acquire not only pathogen and pathogen-derived molecules from infected hosts but also a vast array of immune effector molecules that may impact their physiology. Thus, some arthropods evolved to use mammalian factors to activate their immune system and control bacterial proliferation. For example, mammalian TGFβ (Luckhart et al., 2003) ingested with the blood meal influences the development of malarial parasites within the mosquito gut by unknown immune pathways. Ticks acquire B. burgdorferi when feeding on infected mammals, for example wild rodents, which are regarded as the primary reservoirs for Lyme disease spirochetes (Radolf et al., 2012). Upon infection, mice produce several local and systemic cytokines that circulate in the blood, such as TNF, IFNγ, IL-1β, IL-6, or IL-12 (Hedrick et al., 2006; Isogai et al., 1996). Whether one or more of these cytokines influence innate immune signaling in ticks, and thus bacterial burdens within the vector, is currently unknown.
A large family of small GTPases, including Rho GTPases, regulates critical functions in virtually all living organisms. They control fundamental processes common to eukaryotes, such as morphogenesis, cell migration and division, and immunity (Jaffe and Hall, 2005). In arthropods, Rho GTPases mediate various cellular functions, including immune responses (Shandala and Brooks, 2012). In addition, Rho GTPases can regulate signaling pathways leading in gene expression changes (Jaffe and Hall, 2005). In this study, we identified an I. scapularis small GTPase, termed I. scapularis Rho-like GTPase, (IGTPase) that is induced by a host-derived cytokine, IFNγ, and functions as a signaling intermediary that cues incoming B. burgdorferi infection within ticks. IFNγ induces, in a STAT-dependent fashion, the expression of the borreliacidal peptide, Dae2, limiting spirochete persistence within ticks. These data stress the close vector-host relationships and identify mammalian IFNγ and a tick small GTPase as critical B. burgdorferi-induced factors that bridge protective responses across species.
RESULTS
A tick Rho GTPase gene is highly induced during B. burgdorferi acquisition from infected hosts
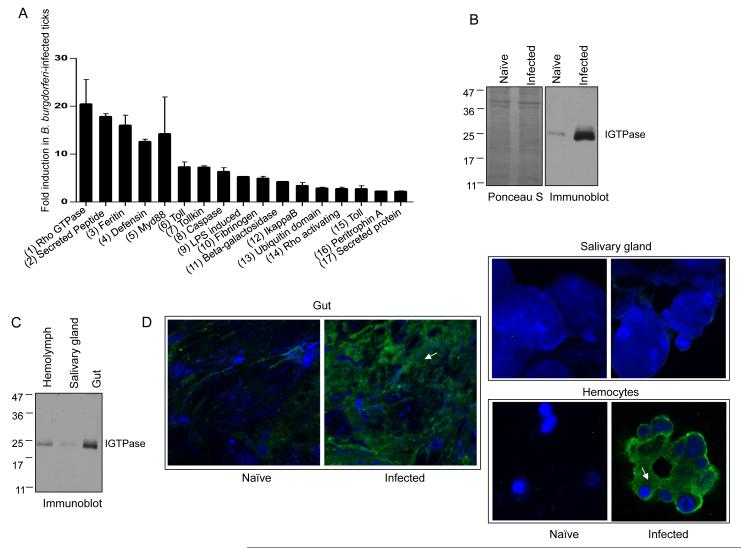
To identify tick genes regulated during acquisition of B. burgdorferi, we analyzed the expression of 270 I. scapularis genes categorized to be part of nine major innate immunity-related pathways (Smith and Pal, 2014). Fifty nymphs per group were allowed to parasitize uninfected or two-week B. burgdorferi-infected C3H mice, collected after 48 hours of feeding and analyzed for the relative gene expression. While 17 genes were upregulated in ticks that had fed on infected-mice (Figure 1A), 56 were downregulated (Figure S1A), and most (198) remained unaltered (data not shown). Notably, a putative Rho GTPase gene (ISCW004348) encoding a protein that is 50% identical to the mammalian RhoA GTPase displayed the highest level of upregulation (up to 18-fold) during tick acquisition of B. burgdorferi (Figure 1A). Among the at least 14 Rho and Rho-related genes present in the I. scapularis genome, ISCW004348 (named hereafter as IGTPase) was the most upregulated in ticks fed on infected mice (Figure S1B), suggesting a specific role of this gene in Ixodes immunity against invading spirochetes. IGTPase remained highly expressed throughout the blood meal on infected hosts, and in repleted ticks (Figure S1C).
Figure 1. An I. scapularis Rho-like GTPase, termed IGTPase, is dramatically induced during spirochete acquisition in ticks.
(A) B. burgdorferi infection-induced transcript levels of selected I. scapularis genes. Bars represent two-fold or higher increase in transcript levels in 48 hour fed ticks that parasitized on B. burgdorferi-infected mice relative to uninfected hosts. Accession numbers: (1) ISCW004348, (2) AAV63544, (3) ISCW015079, (4) ISCW022102, (5) ISCW008802, (6) ISCW007724, (7) ISCW022120, (8) ISCW003039, (9) ISCW020307, (10) ISCW009412, (11) ISCW000651, (12) ISCW007030, (13) ISCW023764, (14) ISCW003559, (15) ISCW017724, (16) ISCW006076, (17) ISCW024521. The data represent the mean values ± SEM of 20 ticks.
(B) Dramatic induction of IGTPase protein levels in B. burgdorferi-infected ticks. Equal amount of lysates from ticks that fed on naïve or B. burgdorferi-infected mice for 24 hours were resolved on an SDS-PAGE gel, transferred onto nitrocellulose, stained with Ponceau S, and immunoblotted with IGTPase antisera. Molecular mass markers (kDa) are indicated on the left.
(C) IGTPase is predominantly detectable in the tick gut. Equal amount of hemolymph, salivary glands and gut lysates from B. burgdorferi-infected ticks (Figure 1B) were immunoblotted using IGTPase antisera.
(D) Localization of native IGTPase in the I. scapularis. Tick tissues (Figure 1C) were probed with IGTPase antisera and a FITC-labeled anti-mouse IgG (green) and DAPI (blue). IGTPase was detectable in the gut and hemocytes (arrows).
To further study IGTPase production and function in ticks, we generated recombinant protein in Escherichia coli and produced high-titer antibodies by immunizing a group of mice. Western blotting analysis using whole tick lysates demonstrated a native protein of the expected size (25 kDa), dramatically upregulated during B. burgdorferi acquisition from infected hosts (Figure 1B). Immunoblot and immunofluorescence analysis further revealed that IGTPase is predominantly expressed in the gut but also localized in the cellular component of the hemolymph of ticks that engorged on B. burgdorferi-infected hosts (Figure 1C and D). Thus, the acquisition of B. burgdorferi by ticks results in the regulation of a limited set of Ixodes immune genes, and most noticeably a gene encoding a putative Rho GTPase-like protein.
IGTPase controls B. burgdorferi colonization within the vector
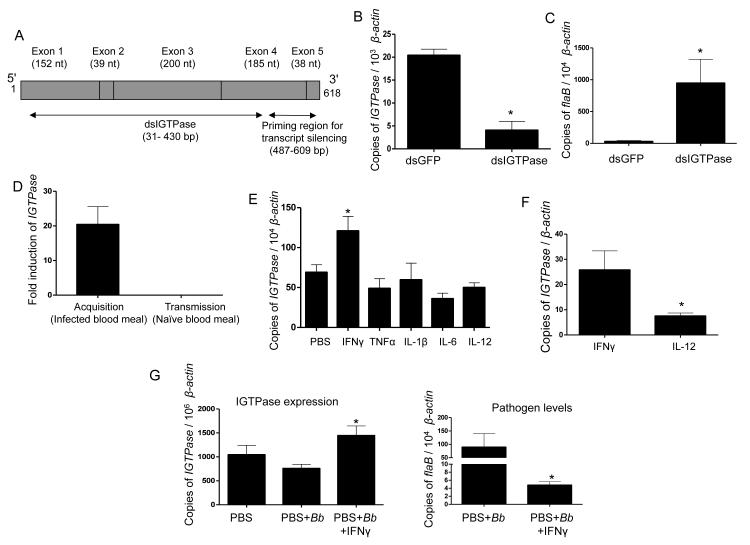
The IGTPase gene encompasses 6643 nucleotides, encoding a 618-base pair mRNA spanning five exons (Figure 2A). I. ricinus, a predominant disease vector in Eurasia, also encodes an IGTPase ortholog (accession number: V5HT37) with more than 99% identity at the amino acid level (data not shown). We assessed the potential role of IGTPase in B. burgdorferi colonization by repressing its expression via RNA interference. The silencing of IGTPase (Figure 2B), which did not affect expression of other tick Rho and Rho-related genes (Figure S1B), resulted in a significant increase in spirochete levels in post-fed ticks that had engorged on infected mice (Figure 2C). We also examined the effects of Clostridium difficile toxin B, which targets and artificially glycosylates RhoA GTPase in a threonine at position 37 within the switch I domain, conserved in IGTPase, resulting in the functional inactivation of the protein (Genth et al., 1999). Naïve nymphal ticks microinjected with toxin B in the gut and allowed to engorge on infected hosts for 48 hours exhibited higher levels of B. burgdorferi, compared to controls (Figure S2A). Confocal microscopic analysis suggested negligible toxic effects of toxin B, particularly within 48 hours of treatment, while more spirochetes were evident in the treated ticks (Figure S2B). These results demonstrate that IGTPase is involved in the control of pathogen burdens within the vector.
Figure 2. IGTPase influences persistence of B. burgdorferi in feeding ticks and is induced by a mammalian cytokine.
(A) Schematic representation of IGTPase open reading frame showing multiple exons and regions targeted for RNA interference. Exons (gray boxes), regions for dsRNA, and primers for assessment of transcript silencing are shown.
(B) Knockdown of IGTPase transcripts via RNA interference. Nymphal ticks (25/group) were injected with IGTPase dsRNA or control GFP dsRNA, allowed to feed on infected mice and collected after feeding. IGTPase transcript levels in gut tissues were measured by quantitative RT-PCR and normalized for tick β-actin. The data represent the mean ± SEM values from five individual ticks. * p < 0.0001.
(C) Silencing of IGTPase expression results in higher levels of B. burgdorferi in ticks. Nymphs were microinjected with IGTPase dsRNA or GFP dsRNA, allowed to parasitize B. burgdorferi-infected mice and spirochete burden in ticks was detected by measuring flaB transcripts normalized with tick β-actin. The mean ± SEM values from five individual ticks are presented. * p < 0.05.
(D) Selective induction of IGTPase during B. burgdorferi acquisition from infected mice. Naïve nymphal ticks that engorged on B. burgdorferi-infected mice for 48 hours show an average of 20-fold higher IGTPase transcript levels compared to naïve ticks or to B. burgdorferi-infected ticks that parasitized uninfected hosts during spirochete transmission. The data represent the mean ± SEM values from pools of 20 ticks per group.
(E) Expression of IGTPase is induced by a mammalian cytokine. Groups of nymphal ticks were microinjected with PBS (control) or a panel of mammalian cytokines induced during B. burgdorferi infection and allowed to parasitize naïve mice for 48 hours. The amounts of IGTPase transcripts normalized against tick β-actin were then analyzed by qRT-PCR. The data presented correspond to at least three ticks from each group. * p < 0.02.
(F) Expression of IGTPase is significantly induced in the presence of IFNγ. Groups of nymphal ticks (25/group) were injected with IFNγ or a control cytokine (IL-12) and examined for upregulation of IGTPase transcripts, as described for Figure 2E. The data represent the mean ± SEM values from 10 individual ticks. * p < 0.05.
(G) IGTPase induction and its spirochete control require the presence of IFNγ. Groups of nymphal ticks (25/group) were injected with buffer (PBS), B. burgdorferi, or B. burgdorferi plus IFNγ, and examined for upregulation of IGTPase transcripts (left panels) and spirochete levels (right panels), as described for Figure 2E and 2C, respectively. The data represent the mean ± SEM values from 10 individual ticks. * p < 0.05.
A mammalian factor present in infected blood meal induces IGTPase expression during spirochete acquisition
To determine whether IGTPase is also upregulated during B. burgdorferi transmission from the infected tick gut to naïve hosts, we analyzed IGTPase transcript levels in ticks that had acquired B. burgdorferi by feeding on infected mice and those already infected with spirochetes and fed on naïve mice. IGTPase expression levels in infected ticks that received a naïve blood meal did not change compared to the dramatic upregulation observed in ticks that received an infected blood meal (Figure 2D). Therefore, the physical presence of B. burgdorferi in the tick gut does not induce the expression of IGTPase in the tick.
We also assessed whether introduction of infected sera into ticks, in the absence of pathogens, induces IGTPase levels. Ticks were microinjected in the gut with two-week infected C3H mouse serum free of spirochetes and allowed to feed on naïve mice for 48 hours. The injection of infected serum induced a noticeable increase in IGTPase expression compared to ticks injected with normal serum (Figure S3A), indicating that a murine serum factor induced during infection with B. burgdorferi regulates the expression levels of IGTPase.
Numerous blood-derived factors, including cytokines, which are transferred into feeding arthropods (Luckhart et al., 2003; Pakpour et al., 2013), remain immunologically active. We microinjected nymphs with biologically active cytokines and allowed to feed on uninfected mice for 48 hours. IGTPase expression was specifically induced in IFNγ-injected ticks, while none of the other cytokines had an effect on IGTPase expression levels (Figure 2E and F). Except for IGTPase, IFNγ injection did not alter the expression of any other Rho or related proteins (as described in Figure S1B), confirming the specificity of IGTPase induction by the cytokine (data not shown). In addition, IGTPase was not induced when B. burgdorferi was microinjected into nymphal ticks (Figure 2G), but a significant upregulation was observed when the injection inoculum included IFNγ, leading to decreased spirochete levels in post-fed ticks (Figure 2G). Together, these studies provide a direct evidence of linking mouse IFNγ to IGTPase induction and B. burgdorferi control within the tick.
IFNγ from B. burgdorferi-infected blood increases IGTPase expression during acquisition
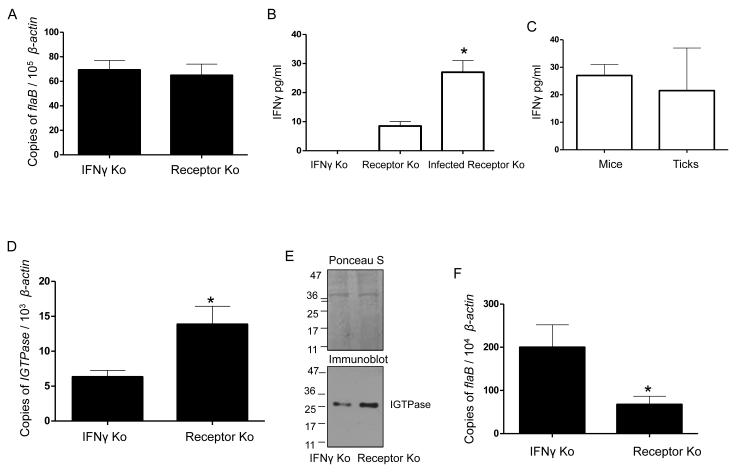
To confirm that mouse IFNγ acquired with the blood meal induces the upregulation of IGTPase, ticks were fed on IFNγ-knockout (KO) mice. As controls, we used mice that lack the capacity to respond to IFNγ (IFNγ receptor-deficient), which harbor equivalent bacterial loads in the skin upon infection (Figure 3A). Infection of IFNγ receptor-deficient mice resulted in increased IFNγ levels (Figure 3B) that were comparable to those present in fed ticks (Figure 3C), confirming that the cytokine is acquired during the blood meal. Compared to IFNγ receptor-deficient mice, IGTPase expression levels were significantly reduced in ticks that had fed on IFNγ-KO mice (Figure 3D). The absence of IFNγ also resulted in lower IGTPase protein levels in feeding ticks (Figure 3E). More importantly, ticks that had fed on IFNγ-KO mice showed significantly increased spirochete burdens (Figure 3F). Overall, these data suggest that the acquisition of B. burgdorferi from murine hosts triggers the upregulation of IGTPase by IFNγ resulting a microbicidal response.
Figure 3. Mammalian IFNγ is responsible for IGTPase induction.
(A) B. burgdorferi burden in IFNγ knockout or IFNγ receptor knockout mice. Mice were infected with spirochetes (105 cells/mouse) and their levels in skin were assessed at two weeks post-infection via qRT-PCR. Bars represent the mean ± SEM of six mice in three independent experiments.
(B) Systemic IFNγ levels in mice. Serum samples from infected animals were measured for IFNγ by ELISA. The data represent the mean ± SEM of pooled mouse serum analyzed in triplicate experiments. *p < 0.01.
C) IFNγ levels in ticks that engorged on infected IFNγ receptor knockout mice. The IFNγ receptor mice infected with B. burgdorferi of Figure 3B were fed by nymphs and IFNγ levels in the tick blood meals were measured by ELISA. The data correspond to the mean ± SEM values from ten ticks. Differences in the levels of IFNγ between mice and ticks were non-significant (p > 0.05).
(D) Murine IFNγ induces expression of IGTPase. Nymphal ticks (25/group) were allowed to parasitize on B. burgdorferi-infected mice. At 48 hours of feeding, ticks were collected, and IGTPase transcripts were measured in 25 nymphs in each group and normalized against tick β-actin. The data represent the mean ± SEM values. *p < 0.001.
(E) IGTPase protein levels are enhanced by murine IFNγ. Twenty four-hour fed ticks were processed for IGTPase levels using immunoblot analysis. Equal protein loading was evidenced by Ponceau S staining.
(F) B. burgdorferi persistence in feeding ticks is decreased in the presence of IFNγ. Nymphal ticks (25/group) were allowed to parasitize B. burgdorferi-infected mice and spirochete levels in 48-hour fed ticks were individually measured using qRT-PCR. The data represent the mean ± SEM values. * p < 0.02.
IGTPase regulates the expression of antimicrobial peptides and is regulated by the JAK-STAT pathway
Since AMP synthesis participates in innate immune responses in the tick (Smith and Pal, 2014), we investigated whether IGTPase-mediated immune responses involve AMP function. Based on the Ixodes genome annotation and homology sequences (Smith and Pal, 2014), and a list of putative AMPs (http://aps.unmc.edu/AP/main.php), we performed a screening of all AMPs and orthologs upregulated during tick acquisition of spirochetes. The expression of only three of 14 tested AMP genes (SP10, defensin, and Dae2) was significantly upregulated during spirochete acquisition (Figure 4A). Dae2, almost absent in naïve ticks, was dramatically induced in B. burgdorferi-infected ticks (Figure S3B). Importantly, Dae2 expression levels were highly downregulated in IGTPase-silenced ticks compared to controls (Figure 4B), while non-significant or small differences in the expression of Defensin or SP10 were observed (Figure 4B). These data demonstrate that IGTPase regulates expression of antimicrobial peptide Dae2.
Figure 4. Ixodes JAK-STAT signaling cascade controls expression of IGTPase and regulates the expression of the borreliacidal peptide, Dae2.
(A) B. burgdorferi-infection induced transcript levels of potential I. scapularis genes encoding potential antimicrobial peptides. Bars represent fold changes ± SEM (20 ticks) of the target transcript levels in 48-hour fed ticks that parasitized on B. burgdorferi-infected mice relative to naive hosts. Accession numbers: (1) AAV63544, (2) ISCW006596, (3) ISCW022102, (4) ISCW005928, (5) ISCW016466, (6) ISCW016747, (7) ISCW018425, (8) ISCW002331, (9) ISCW002695, (10) ISCW018541, (11) ISCW001310, (12) ISCW017494, (13) ISCW014204, (14) ISCW009364.
(B) IGTPase deficiency results in the dramatic reduction of Dae2 transcripts. Ticks were microinjected with IGTPase dsRNA or GFP dsRNA and allowed to parasitize B. burgdorferi-infected mice. After feeding, ticks transcript levels of SP10, Dae2, and defensin were individually measured in 10 nymphs in each group by qRT-PCR normalized against tick β-actin. The bars represent the mean ± SEM values from 10 individual ticks. **p < 0.01; * p < 0.05.
(C) Reduced phosphorylated I. scapularis STAT in ticks engorged on IFNγ knockout mice. Fed ticks were analyzed for phosphorylated I. scapularis STAT levels by immunoblotting. Equal protein loading was evidenced by Ponceau S staining.
(D) Silencing of STAT transcripts via RNA interference. Nymphal ticks (25/group) were injected with STAT dsRNA or control GFP dsRNA, fed on mice, and processed for transcript silencing. The bars represent the mean ± SEM values. *, p < 0.001.
(E) STAT knockdown affects expression of IGTPase transcripts. Groups of 25 control and STAT-silencing ticks were examined for expression of IGTPase transcripts via qRT-PCR. *p < 0.01.
(F) Expression of antimicrobial peptide Dae2 is severely impaired in STAT knockdown ticks. Groups of 25 control and STAT-silencing ticks were examined for Dae2 transcript levels via qRT-PCR. *p < 0.01.
The I. scapularis genome encodes for many orthologs of three signaling pathways relevant to innate immune responses: Toll, Imd, and JAK-STAT (Smith and Pal, 2014). Although we detected the expression of two key representative members of the Toll and Imd pathways in feeding ticks, PGRP (ISCW022212) and Myd88 (ISCW008802), their silencing via RNAi did not alter IGTPase expression (data not shown). Ticks also contain a functional JAK-STAT pathway with a single annotated STAT (Liu et al., 2012), which was less phosphorylated when ticks engorged on IFNγ-deficient mice compared to control mice producing IFNγ (Figure 4C). IGTPase and JAK (ISCW016158), expressed by an I. scapularis cell line, IDE12 (Mattila et al., 2007), also underwent significant upregulation when the cells were treated with IFNγ (Figure S4A). We also silenced I. scapularis STAT via RNA interference and allowed ticks to feed for 48 hours on B. burgdorferi-infected C3H mice. Decreased STAT expression in dsSTAT-injected nymphs (Figure 4D) resulted in significantly reduced IGTPase expression (Figure 4E). Importantly, STAT-deficient nymphal ticks fed on B. burgdorferi-infected C3H mice showed significantly decreased expression of the borreliacidal peptide Dae2 (Figure 4F). Except for Dae2, expression of other AMPs was not altered in STAT-silenced ticks (data not shown). These data show that Ixodes STAT-mediated signaling events induce the upregulation of IGTPase and the borreliacidal peptide Dae2.
DISCUSSION
Disease-transmitting arthropods evolved to control the proliferation of pathogens that persist within them. For example, the malarial parasites encounter a remarkable bottleneck within the mosquito gut, where the majority of invading pathogens are killed by potent Anopheles immune responses (Smith et al., 2014). Many arthropods combat microbial infections through processes mediated by immune cells, including hemocytes (Smith et al., 2014). Arthropods can also rapidly synthesize a suite of AMPs that may persist for weeks (Rolff and Schmid-Hempel, 2016). Here, we unveil how the I. scapularis tick, the vector for the Lyme disease pathogen, B. burgdorferi, takes advantage of host-derived IFNγ to restrict spirochete proliferation within the gut. IFNγ induces the activation of the evolutionarily conserved JAK-STAT signaling pathway ultimately leading to the synthesis of the recently described anti-borrelial peptide, Dae2, in the tick gut. Induction of Dae2 expression by IFNγ is mediated by a tick small GTPase, IGTPase, which is highly induced during B. burgdorferi acquisition.
Rho proteins are a family of highly conserved small GTPases found in all eukaryotic cells. The I. scapularis genome encodes for at least 14 putative Rho and Rho-related genes (Smith and Pal, 2014). Of those, only IGTPase is dramatically induced during B. burgdorferi infection, and its repressed expression results in increased tick susceptibility to B. burgdorferi infection, supporting its immunological relevance. Very limited information is available on the transcriptional regulation of Rho GTPases in ticks and the extent to which they play a role in immunity or physiology. We demonstrate that IGTPase is regulated by the specific action of host-derived IFNγ induced during infection with B. burgdorferi. While cytokines induced during mammalian infection with spirochetes are known to exert critical modulatory functions in the host, herein we describe a trans-species effect exerted by B. burgdorferi-induced factors in the tick vector. Crosstalk between mammals and arthropods has been previously described for mosquito vectors (Luckhart et al., 2003; Pakpour et al., 2013). The conservation of critical signaling pathways in arthropods and mammals raises the possibility that host-derived factors can impact the physiology of blood-feeding arthropods and their response to ingested pathogens. This may occur because selectively retaining and preserving mammalian cytokine function is beneficial as they augment arthropod defenses against invading microbes.
It is currently unknown whether or how tick proteins can activate the JAK-STAT signaling pathway, which also mediates innate immune defenses in flies and mosquitos (Dostert et al., 2005). The binding of the ligand Upd to the transmembrane receptor Domeless triggers the activation of the JAK-STAT pathway in Drosophila (Brown et al., 2001), but no Upd ortholog is evidently present in the Ixodes genome. Activation of this signaling pathway is not initiated either by the direct interaction of B. burgdorferi ligands with a putative receptor, since the presence of spirochetes alone did not induce the expression of IGTPase. However, our data demonstrate that mammalian IFNγ from infected hosts activates the tick JAK-STAT pathway, possibly via binding to a noncanonical receptor, the identity of which is currently unknown. Although decreased STAT expression in ticks slightly reduces B. burgdorferi levels (Narasimhan et al., 2014), and we show that STAT-knockdown reduces transcript levels of the antimicrobial peptide Dae2, spirochete levels in these ticks remained unaffected (Figure S4B), suggesting that a single recognizable I. scapularis STAT could have opposite effects on B. burgdorferi persistence. In fact, multiple signaling pathways converge on the STAT protein (Herrington et al., 2000), which can direct seemingly contradictory responses (Levy and Lee, 2002). Nevertheless, in agreement with the pronounced borreliacidal effects of Dae2 reported in the post-fed ticks (Chou et al., 2015), we observed the most dramatic reduction in spirochete levels in repleted IGTPase knockdown nymphal ticks.
Overall, we describe an anti-B. burgdorferi immune response that is activated in I. scapularis during acquisition of the spirochete from infected mammalian hosts. Our results highlight the presence of conserved interspecies communication pathways that bridge immune responses between ticks and mammals mediated by the cytokine IFNγ and that involve the small GTPase, IGTPase. These data unveil the existence of an unprecedented co-evolutionary mechanism that permits the arthropod vector to both identify B. burgdorferi-infected hosts and initiate defense mechanisms that limit the proliferation of ingested pathogens within the vector environment.
EXPERIMENTAL PROCEDURES
Bacteria, mice, and ticks
The B. burgdorferi B31 isolate A3 was grown in Barbour-Stoenner-Kelly H (BSK-H) media (Yang et al., 2014). Four- to-six-week-old female C3H/HeN or B6 mice deficient for IFN-γ or IFN-γ receptor were purchased from the National Institutes of Health, Charles River Laboratories, or the Jackson Laboratories. Mixed offspring from multiple adult I. scapularis females were reared (Yang et al., 2014) and monitored for tick-borne pathogens using PCR. All animal experiments were performed under approval of the Institutional Animal Care and Biosafety Committee.
Nucleic acid isolation and PCR
Isolation of nucleic acid and PCR were performed as detailed (Yang et al., 2009; Yang et al., 2014) and further described in the Supplemental Experimental Procedures.
Recombinant proteins and antibodies, and immunoblotting
Recombinant IGTPase was produced in E. coli using the pET-28a vector (Invitrogen) with primers detailed in Table S1. Protein purification, generation of polyclonal antisera, and Western blotting was performed as detailed (Kariu et al., 2013). Immunodetection of phospho-STAT in ticks was performed using a commercial antibody (Life Technologies) raised against a tyrosine phosphorylation residue Y699 that is conserved in Ixodes STAT.
Confocal immunofluorescence microscopy
Nymphal ticks engorged on mice for 24-48 hours were processed for confocal microscopy (Yang et al., 2014). Isolated tick tissues or organs were fixed, incubated with a 1:100 dilution of IGTPase antisera and Alexa 488-labeled secondary antibodies and DAPI (Life Technologies), and imaged by a LSM 510 laser confocal microscope (Zeiss).
RNA interference
Silencing of target tick genes was accomplished via RNA interference using published procedures (Yang et al., 2014) and further described in the Supplemental Experimental Procedures.
Microinjection of cytokines in ticks
Nymphs (20-30) were injected with biologically active cytokines obtained from BD Biosciences: TNF (0.3 pg/μl), IFNγ (5 pg/μl), IL-6 (6ug/μl), IL-1β (0.3 pg/μl), and IL-12 (5 pg/μl). Ticks were allowed to recover for 3 hours before being fed on naïve mice and collected 48 hours into feeding and assessed for induction of target transcripts using qRT-PCR.
Murine infection studies
Wild-type C3H, IFNγ-deficient, or IFNγ receptor-deficient mice were infected with 105spirochetes, as detailed (Yang et al., 2014). After 14 days, naïve or microinjected ticks were placed on the infected mice (25 ticks/mouse) and detached after 48 hours or allowed to feed to repletion. The flaB transcripts or target tick transcripts were detected using qRT-PCR. At least three independent animal experiments were performed; from each mouse, 5-10 ticks were recovered and analyzed individually. Levels of IFNγ in mouse serum or in engorged ticks were detected using a commercially available ELISA kit (Bio-Techne Inc.).
Bioinformatics and statistical analysis
Protein annotations are based on the VectorBase database (www.vectorbase.org). Results are expressed as the mean ± standard error (SEM). Statistical significance of differences were analyzed using GraphPad Prism v5 (La Jolla, CA). One- or two-tailed Student's t-tests were used to compare the mean values, and p < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank John Anderson, Toru Kariu, Faith Kung, Kavita Sharma, Brian Backstedt, Juraj Koci, and Meghna Thakur for their assistance with this study. We would like to thank Ulrike Munderloh, University of Minnesota, for providing the IDE12 cell line. This work was supported by funding from University of Maryland, College Park and the National Institute of Allergy and Infectious Diseases Award Number AI080615 and AI116620 to U.P. O.B. is supported by funding from Ondokuz Mayis University and Turkish Higher Education Council. A.M. is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Disease. J.A. is funded by the European Union (Grant Agreement number 602272).
Footnotes
AUTHOR CONTRIBUTIONS
A.S., X.Y., C.W. and O.B. conducted the experiments. A.S., N.N., X.Y., J.A. and U.P. designed the experiments. A.S., N.N., J.A. and U.P. wrote the paper. A.S., N.N., O.B., A.M., J.A. and U.P. discussed the manuscript and provided important feedback. U.P. supervised the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–1705. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- Chou S, Daugherty MD, Peterson SB, Biboy J, Yang Y, Jutras BL, Fritz-Laylin LK, Ferrin MA, Harding BN, Jacobs-Wagner C, et al. Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature. 2015;518:98–101. doi: 10.1038/nature13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Genth H, Aktories K, Just I. Monoglucosylation of RhoA at threonine 37 blocks cytosol-membrane cycling. J Biol Chem. 1999;274:29050–29056. doi: 10.1074/jbc.274.41.29050. [DOI] [PubMed] [Google Scholar]
- Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- Hedrick MN, Olson CM, Jr., Conze DB, Bates TC, Rincon M, Anguita J. Control of Borrelia burgdorferi-specific CD4+-T-cell effector function by interleukin-12- and T-cell receptor-induced p38 mitogen-activated protein kinase activity. Infect Immun. 2006;74:5713–5717. doi: 10.1128/IAI.00623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19:2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- Hynes WL, Ceraul SM, Todd SM, Seguin KC, Sonenshine DE. A defensin-like gene expressed in the black-legged tick, Ixodes scapularis. Med Vet Entomol. 2005;19:339–344. doi: 10.1111/j.1365-2915.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Isogai E, Isogai H, Kimura K, Hayashi S, Kubota T, Nishikawa T, Nakane A, Fujii N. Cytokines in the serum and brain in mice infected with distinct species of Lyme disease Borrelia. Microb Pathog. 1996;21:413–419. doi: 10.1006/mpat.1996.0072. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annual review of cell and developmental biology. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kariu T, Yang X, Marks CB, Zhang X, Pal U. Proteolysis of BB0323 results in two polypeptides that impact physiologic and infectious phenotypes in Borrelia burgdorferi. Mol Microbiol. 2013;88:510–522. doi: 10.1111/mmi.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Dai J, Zhao YO, Narasimhan S, Yang Y, Zhang L, Fikrig E. Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J Infect Dis. 2012;206:1233–1241. doi: 10.1093/infdis/jis484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart S, Crampton AL, Zamora R, Lieber MJ, Dos Santos PC, Peterson TM, Emmith N, Lim J, Wink DA, Vodovotz Y. Mammalian transforming growth factor beta1 activated after ingestion by Anopheles stephensi modulates mosquito immunity. Infect Immun. 2003;71:3000–3009. doi: 10.1128/IAI.71.6.3000-3009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila JT, Munderloh UG, Kurtti TJ. Phagocytosis of the Lyme disease spirochete, Borrelia burgdorferi, by cells from the ticks, Ixodes scapularis and Dermacentor andersoni, infected with an endosymbiont, Rickettsia peacockii. J Insect Sci. 2007;7:58. doi: 10.1673/031.007.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan J, Eppler-Epstein R, Deponte K, Fish D, Fikrig E. Gut Microbiota of the Tick Vector Ixodes scapularis Modulate Colonization of the Lyme Disease Spirochete. Cell Host Microbe. 2014;15:58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakpour N, Akman-Anderson L, Vodovotz Y, Luckhart S. The effects of ingested mammalian blood factors on vector arthropod immunity and physiology. Microbes Infect. 2013;15:243–254. doi: 10.1016/j.micinf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff J, Schmid-Hempel P. Perspectives on the evolutionary ecology of arthropod antimicrobial peptides. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2016;371 doi: 10.1098/rstb.2015.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandala T, Brooks DA. Innate immunity and exocytosis of antimicrobial peptides. Communicative & integrative biology. 2012;5:214–216. doi: 10.4161/cib.19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Ziemiecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993;366:580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Smith AA, Pal U. Immunity-related genes in Ixodes scapularis--perspectives from genome information. Frontiers in cellular and infection microbiology. 2014;4:116. doi: 10.3389/fcimb.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Vega-Rodriguez J, Jacobs-Lorena M. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Memorias do Instituto Oswaldo Cruz. 2014;109:644–661. doi: 10.1590/0074-0276130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Coleman AS, Anguita J, Pal U. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog. 2009;5:e1000326. doi: 10.1371/journal.ppat.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Smith AA, Williams MS, Pal U. A Dityrosine Network Mediated by Dual Oxidase and Peroxidase Influences the Persistence of Lyme Disease Pathogens within the Vector. J Biol Chem. 2014;289:12813–12822. doi: 10.1074/jbc.M113.538272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.