Abstract
Much of the work in cognitive neuroscience is shifting from a focus on single brain regions to a focus on the connectivity between multiple brain regions. These inter‐regional connectivity patterns contribute to a wide range of behaviors and are studied with models of functional integration. The rapid expansion of the literature on functional integration offers an opportunity to scrutinize the consistency and specificity of one of the most popular approaches for quantifying connectivity: psychophysiological interaction (PPI) analysis. We performed coordinate‐based meta‐analyses on 284 PPI studies, which allowed us to test (a) whether those studies consistently converge on similar target regions and (b) whether the identified target regions are specific to the chosen seed region and psychological context. Our analyses revealed two key results. First, we found that different types of PPI studies—e.g., those using seeds such as amygdala and dorsolateral prefrontal cortex (DLPFC) and contexts such as emotion and cognitive control, respectively—each consistently converge on similar target regions, thus supporting the reliability of PPI as a tool for studying functional integration. Second, we also found target regions that were specific to the chosen seed region and psychological context, indicating distinct patterns of brain connectivity. For example, the DLPFC seed reliably contributed to a posterior cingulate cortex target during cognitive control but contributed to an amygdala target in other contexts. Our results point to the robustness of PPI while highlighting common and distinct patterns of functional integration, potentially advancing models of brain connectivity. Hum Brain Mapp 37:2904–2917, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: coordinate‐based meta‐analysis, CBMA, coupling, effective connectivity, brain connectivity, fMRI
INTRODUCTION
Neuroscience seeks to understand how the brain enables behavior by characterizing structure‐function relationships [Fink et al., 2003; Raichle, 2003]. Much of this interest was sparked by lesion studies examining the debilitating behavioral deficits that follow from brain damage [Adolphs et al., 1994; Calder et al., 2000; Goodale and Milner, 1992; Scoville and Milner, 1957]. Although lesion studies will remain an important tool for inferring structure‐function relationships [Rorden and Karnath, 2004], functional neuroimaging has become the primary method in characterizing how individual brain regions respond to a given task [Fellows et al., 2005]. This approach has been applied widely and led to a sizable literature of functional neuroimaging studies on a range of topics spanning the breadth of cognitive neuroscience [Huettel, 2012; Poldrack, 2008, 2010]. The growth in fMRI studies further permits quantitative syntheses to formally assess the consistency and specificity of previously characterized structure‐function relationships [Wager et al., 2009; Yarkoni et al., 2010]. Such approaches have been successful across several areas, including language [Binder et al., 2009], memory [Murty et al., 2010], emotion [Buhle et al., 2014], and decision making and valuation [Bartra et al., 2013; Clithero and Rangel, 2014]. These studies have honed our understanding of structure‐function relationships by characterizing how individual brain regions respond to a task.
The responses of individual brain regions, however, may be inadequate for representing the complex links between structure and function. Indeed, given the various anatomical inputs to single regions, there can be several functions associated with such regions, making it challenging to understand how the brain enables behavior [Friston, 2005; Park and Friston, 2013]. Solving this challenge rests with our ability to understand brain connectivity because the function of a region depends on its interactions with other brain regions [Fox and Friston, 2012]. Brain connectivity is now being explored more frequently using various techniques that range in their ability to quantify neuronal coupling between brain regions [Friston, 2009; Smith et al., 2011; Sporns, 2014]. For example, researchers are collecting resting‐state functional magnetic resonance imaging data (fMRI) and quantifying functional connectivity (i.e., statistical dependencies or correlations) between brain regions [Biswal et al., 2010; Shehzad et al., 2009]. These approaches are becoming increasingly popular because of their application to individual differences and classifying distinct groups of individuals [Hariri, 2009; Kelley et al., 2015], such as depressed [Berman et al., 2011] and schizophrenic [Manoliu et al., 2014] patients. Functional connectivity can also be measured during tasks and contrasted with resting states, which has revealed key similarities [Smith et al., 2009] and differences [Utevsky et al., 2014] in context‐dependent brain organization. In addition, meta‐analytic connectivity approaches that quantify how distal brain regions are reliably coactivated [Robinson et al., 2010] have used functional connectivity to reveal novel functional parcellations within various brain regions, including the orbitofrontal cortex [Zald et al., 2014], parietal operculum [Eickhoff et al., 2010], cerebellum [Riedel et al., 2015], and insula [Chang et al., 2013]. Taken together, these observations highlight how the responses in individual brain regions can be combined through functional connectivity.
Yet, researchers have long recognized that functional connectivity suffers from important pitfalls that limit its insight into neuronal coupling [Gerstein and Perkel, 1969]. For example, changes in functional connectivity could reflect changes in another connection, observational noise, or neuronal fluctuations [Friston, 2011]. To address these confounds, many groups have employed computational approaches that estimate effective connectivity [Valdes‐Sosa et al., 2011]. Unlike functional connectivity, effective connectivity quantifies directed relationships between brain regions and controls for confounds that limit functional connectivity—features that facilitate insight into functional integration [Park and Friston, 2013]. Effective connectivity can be measured with different approaches. One such approach, reserved for very simple models of effective connectivity (e.g., those with two regions), is psychophysiological interactions (PPI) [Friston et al., 1997]. This approach measures whether a psychological context alters how one brain region (a “seed region”) contributes to another brain region (a “target region”) by explicitly testing whether a significant interaction between psychological context and the seed region is expressed in the target region (Fig. 1A). Although PPI studies have become increasingly popular over the years (Fig. 1B), it remains unclear whether the target regions identified by PPI results are consistent across similar studies (i.e., studies using the same seed region and psychological context) and specific to a given seed region and psychological context. These issues may undermine the applicability of PPI analyses to structure‐function relationships and functional integration.
Figure 1.
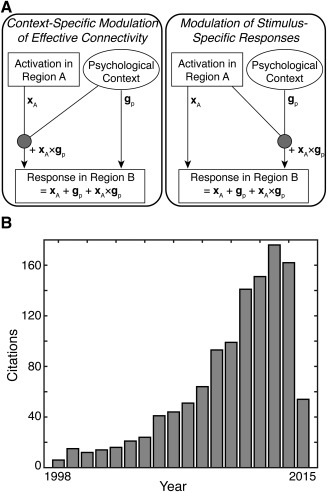
Overview of Psychophysiological Interactions. (A) The goal of a psychophysiological interaction (PPI) analysis is to explain the responses of one brain region (i.e., a target region) in terms of an interaction (gp × xA) between the influence of another brain region (i.e., a seed region; xA) and a particular psychological context (gp). There are two perspectives on PPIs. On the left, a PPI reflects a context‐specific change in effective connectivity between the two regions. In this case, the psychological context (e.g., attention) modulates the contribution of the seed region (region A) to the target region (region B). On the right, a PPI reflects a modulation of stimulus‐specific responses. In this case, the seed region (region A) modulates the response of the target region (region B) to the psychological context. [cf. Friston et al., 1997] (B) The usage of PPI in neuroimaging has increased rapidly since its inception. This popularity and widespread usage has led to a large corpus of PPI studies that can be probed using meta‐analytic techniques.
We investigated whether a quantitative synthesis of PPI studies would reveal common and distinct patterns of connectivity—findings that would contribute toward a cumulative science of functional integration. We formed a corpus of studies comprising all published PPI experiments. Of course, synthesizing the results of PPI experiments (or any approach examining context‐specific changes in effective connectivity) presents a challenge for appropriately grouping studies. We therefore grouped studies according to their chosen seed region and psychological context (Fig. 2), allowing us to formally evaluate whether the associated target regions were common or distinct. We used coordinate‐based‐meta‐analysis and activation likelihood estimation [Eickhoff et al., 2012, 2009] to quantify consistency and specificity across distinct groups of PPI studies. Our analyses focused on two key questions. First, do PPI studies consistently converge on similar target regions? Second, are these target regions dependent on the chosen seed region and psychological context?
Figure 2.
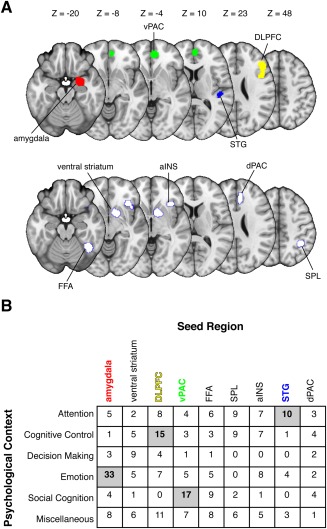
Aggregating studies of psychophysiological interactions. Aggregating across PPI studies requires explicit consideration of the variables that contribute to the psychophysiological interactions: the seed region and the psychological context. (A) We identified common seed regions within our corpus of PPI studies using a coordinate‐based meta‐analytic approach. This analysis revealed several regions that were commonly used as seed regions: the amygdala, ventral paracingulate cortex (vPAC), superior temporal gyrus (STG), and dorsolateral prefrontal cortex (DLPFC), fusiform face area (FFA), ventral striatum, anterior insula (aINS), dorsal paracingulate cortex (dPAC), and superior parietal lobe (SPL). (B) We then parsed studies according to the general psychological context used in the PPI analysis (see Materials and Methods for article coding scheme). The matrix depicts the number of studies using a particular combination of seed region and psychological context (i.e., a specific PPI). Our primary analyses focused on bidirectional contrasts of cells containing 10 or more studies (gray), excluding those in the miscellaneous category. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
MATERIALS AND METHODS
Interpreting Psychophysiological Interactions
The main limitation of a typical functional connectivity analysis is the reliance on correlations, which could reflect changes in another connection, observational noise, or neuronal fluctuations [Friston, 2011]. Psychophysiological interaction (PPI) analysis overcomes these limitations because, in a very strict sense, it is a test for effective connectivity. PPI analyses should be interpreted as a (simple) test for effective connectivity because they are based on an explicit (and often linear) model of coupling between one or more brain regions. This model allows researchers to test for directed changes in connectivity by establishing a significant interaction between the seed region and the psychological context (Fig. 1A). Although the direction of the change is explicitly specified in the model, we note that the post hoc interpretation of the results can be ambiguous. For example, a significant increase in coupling from one region to another region is likely to be significant when testing for a PPI in the opposite direction; e.g., a significant PPI effect could be observed by reversing the seed and target regions. This ambiguity can be resolved by submitting the regions comprising the putative functional circuit to dynamic causal modeling and then conducting Bayesian model comparisons [Friston, 2009; Friston et al., 2003]. Furthermore, we note that a PPI model better approximates effective connectivity as more regions are added to the model [Friston et al., 1997], but this approach is rarely done in practice due to multicollinearity and the relative paucity of observations compared to potential regions (i.e., degrees of freedom). In short, when we discuss changes in effective connectivity, we do so in light of these qualifications.
Study Identification
We created a corpus of PPI studies using forward reference searches on PubMed and Web of Science. Our searches identified published articles (by February 2014) citing the original PPI article [Friston et al., 1997]. We excluded articles that did not employ a PPI model or contain empirical PPI results (i.e., coordinates of significant target regions). This procedure eliminated review papers, commentaries, and other articles that did not contain PPI results. We also excluded articles that focused strictly on group differences (or other types of individual differences) in PPI effects. Although group differences in PPI results are important, such effects are necessarily driven by a third factor (i.e., group), thus making it impossible to combine with simpler, within‐subject PPI results that are only driven by seed location and psychological construct (Fig. 1A). Taken together, these exclusions left us with a total of 396 studies for initial analysis.
Coordinate‐Based Meta‐Analysis
Within any scientific discipline, it is imperative to synthesize the results of independent experiments [Stanley and Spence, 2014]. Although quantitative meta‐analyses provide a gold standard for synthesizing results from different experiments, such approaches have unique challenges within the context of neuroimaging data. Results from neuroimaging experiments are typically reported in the form of coordinates within a stereotaxic system—namely, Talairach and Montreal Neurological Institute (MNI) coordinate spaces [Lancaster et al., 2007]. Thus, the key data recorded across independent neuroimaging experiments is the spatial location of an effect (in terms of a coordinate); this feature has motivated the development of tools for performing coordinate‐based meta‐analysis (CBMA). Rather than testing whether an effect magnitude is consistent across studies, CBMA tests whether an effect location is consistent across studies [Fox et al., 1998].
We computed all CBMA results using GingerALE (v2.3.3; http://www.brainmap.org/ale/), which relies on the activation likelihood estimation (ALE) metric [Eickhoff et al., 2012] and incorporates recent improvements that allow for random‐effects inference [Eickhoff et al., 2009]. Prior to calculating the ALE metric, all coordinates were converted to MNI space using the “icbm2tal” transformation [Lancaster et al., 2007] to facilitate aggregation across studies. The ALE metric is quantified in two primary steps. First, each study is converted to a modeled activation (MA) map in which each voxel represents the probability that a true result lies in that location. These probabilities are obtained by convolving each of the reported coordinates (or foci) using 3D Gaussian probability distribution function (PDF). Crucially, the full width at half maximum (FWHM) value of the PDF depends on the number of participants in the study—fewer participants equates to greater spatial uncertainty and hence a larger FWHM. This procedure accounts for heterogeneity in spatial uncertainty across studies and improves generalization beyond the corpus of studies under investigation [Eickhoff et al., 2009]. We also note that the number of coordinates reported in a study does not influence the resulting MA map: when multiple coordinates contribute to a voxel's MA value, the maximum probability is used [Turkeltaub et al., 2012]. Second, the MA maps are combined (via a probabilistic union) to create an image containing the ALE statistic at each voxel. The ALE statistic represents the probability that at least one true result lies in that voxel across the population of all possible studies.
Statistical inference on ALE images requires distinguishing random convergence (i.e., noise) from locations of true convergence between experiments. Previous work has addressed this issue by permuting voxel locations to collect the empirical null distributions [Turkeltaub et al., 2002; Wager et al., 2007]. Yet, recent developments have abandoned this approach in favor of a nonlinear histogram integration approach [Eickhoff et al., 2012]. This revised approach considers distinct MA values instead of distinct voxels, thus greatly reducing number of required permutations. We therefore used 1,000 permutations to analytically compute the null distribution and assess significance in each ALE map [Eickhoff et al., 2012]. We also implemented another recent development that allows for cluster‐level inference to be implemented within the CBMA framework [Eickhoff et al., 2012]. We first applied an uncorrected voxelwise threshold of P < 0.001, and the resulting clusters were held to a family‐wise‐error‐rate (FWER) of P = 0.00625 to account for bidirectional comparisons on four distinct cells in our matrix of final studies (see below).
Matrix of Final Studies
A CBMA on PPI data must consider how the results (i.e., target coordinates) in each experiment are based on a specific seed location and psychological context. Although equating for seed location and psychological context necessarily winnows down our list of candidate studies, this procedure is necessary for synthesizing PPI results. Unfortunately, seed location varies widely across PPI studies, making it difficult to identify commonly used seed regions and group studies appropriately. We addressed this problem empirically by performing a CBMA of the seed locations extracted from our 396 studies. We note that some studies performed multiple PPI analyses, with some using distinct seed regions (e.g., amygdala and DLPFC) and others using similar seed regions (e.g., different parts of the DLPFC). Thus, our total number of seed region coordinates was 602. These seed region coordinates were submitted to a CBMA to identify regions that were reliably used as seeds. To facilitate aggregation across studies, we assumed no differences in laterality and forced all seed regions into the right hemisphere prior to analysis. Although collapsing seed coordinates into the right hemisphere ignores hemispheric asymmetries, such as those in prefrontal cortex [Binder et al., 2009], testing for lateralization and potentially doubling our pool of seed regions would necessitate a much larger corpus of PPI studies. (We did not make analogous lateralization assumptions for target regions.) We thresholded the CBMA map at P < 0.00001 (uncorrected), which yielded nine meta‐seed regions (Fig. 2A; Table 1). We then grouped our seed region coordinates (and hence our PPI experiments) according to their proximity to the nine meta‐seed regions. To maximize the number of included studies and to account for spatial uncertainty across studies [Brett et al., 2002; Devlin and Poldrack, 2007; Eickhoff et al., 2012], we excluded studies with seeds that were greater than 10 mm from any of the 9 meta‐seed regions. We chose 10 mm to avoid cases where a study could be counted as part of multiple seeds (e.g., amygdala and ventral striatum). After excluding studies that could not be unambiguously grouped with a given seed region—a key consideration for conducting accurate PPI meta‐analyses—we were left with a total of 284 independent studies containing data from 5,997 participants (see Supporting Information Tables I–IX). In cases where studies used two or more overlapping seed regions (e.g., different parts of the DLPFC, or left and right amygdala), we combined the associated target coordinates to prevent experiments from being counted more than once in our analyses.
Table 1.
Seed coordinates
| Name | x | y | z | Size |
|---|---|---|---|---|
| Amygdala | 25 | −2 | −21 | 326 |
| Ventral striatum | 13 | 9 | −6 | 218 |
| DLPFC | 46 | 19 | 26 | 470 |
| vPAC | 4 | 49 | 3 | 315 |
| FFA | 41 | −53 | −21 | 274 |
| SPL | 38 | −46 | 49 | 150 |
| aINS | 37 | 23 | −8 | 136 |
| STG | 48 | −23 | 7 | 103 |
| dPAC | 7 | 35 | 25 | 95 |
Shown are the MNI coordinates for the center of gravity associated with each of our meta‐seed regions. We report size in terms of the number of 2 mm3 voxels.
vPAC, ventral paracingulate cortex; STG, superior temporal gyrus; DLPFC, dorsolateral prefrontal cortex; FFA, fusiform face area; aINS, anterior insula; dPAC, dorsal paracingulate cortex; SPL, superior parietal lobe.
Next, we coded the remaining studies into one of five broad psychological contexts: Attention, Cognitive Control, Decision Making, Emotion, and Social Cognition. Our coding schemes for each category are presented in Table 2; these categories were defined a priori to prevent bias and to capture the largest array of psychological contexts used in PPI studies. Most PPI studies—like most studies employing cognitive subtractions [Friston et al., 1996]—aim to isolate a psychological context using a contrast. For example, “cognitive control” might reflect high load > low load. This feature of PPI raises two important points about our analyses. First, our coding scheme does not explicitly consider whether the PPI resulted from a contrast of two conditions (e.g., high load > low load) or the main effect of a single condition relative to baseline (e.g., high load > fixation). Second, recent work has demonstrated PPIs with more than two conditions should use a generalized PPI (gPPI) approach that models the psychophysiological interactions for each condition separately [McLaren et al., 2012]. The gPPI approach results in greater sensitivity and specificity [McLaren et al., 2012], but its relatively recent development precludes focusing on gPPI studies within our analyses, which include a mix of standard and gPPI studies. Accordingly, whether a study optimally modeled differences in psychological context with gPPI may introduce noise to our analyses, but it is very unlikely to systematically bias our effects.
Table 2.
Psychological contexts
| Psychological context | Description |
|---|---|
| Emotion | The purpose of the task is to elicit or regulate an emotional response (positive, neutral, or negative). |
| Attention | The task requires attention to stimuli, which can be visual, auditory, gustatory, olfactory, nociceptive, or multimodal. |
| Cognitive control (incl. working memory) | The task involves cognitive flexibility such as working memory, problem solving, or goal‐directed behavior. |
| Social cognition | The task involves making judgments or thinking about, interacting with, or inferring the cognitive state of another individual. |
| Decision making | The purpose of the task is to make choices between different options (free or forced choice). |
To form a psychophysiological interaction, a study must have activity from a seed region and a specific psychological context. We therefore grouped our PPI studies according to the seed and the psychological context used to form the psychophysiological interaction. We used relatively broad definitions of psychological context, which are shown in the table.
We evaluated reproducibility of our coding scheme for psychological contexts using a subsample of studies (N = 50) coded by two independent raters (authors MG and MES). Both raters produced very similar results (inter‐rater agreement: κ = 0.71, P < 0.001), thus supporting the reproducibility of our coding scheme for the psychological context categories. Studies that did not fit in any of the five broad categories were assigned to a miscellaneous category. Although these categories are broad and somewhat imprecise, we emphasize that further divisions would render our meta‐analysis ineffective due to a paucity of observations for most analyses. Indeed, many of the cells in our final matrix of studies already contain too few experiments to perform robust CBMAs (Fig. 2B). We therefore focused on cells containing 10 or more experiments (N = 4; denoted with gray shading in Fig. 2B), excluding the miscellaneous category. We note that our threshold of 10 experiments is arbitrary, but it reflects a principled, a priori cutoff because CBMAs within the ALE framework are unlikely to produce robust results with fewer than 10 experiments [Eickhoff et al., 2012].
Contrasts and Conjunctions of ALE Images
Our matrix of studies created a unique opportunity to investigate common and distinct patterns of connectivity. We quantified common patterns of connectivity using conjunction analyses. In this case, two thresholded ALE images are combined using the minimum statistic [Nichols et al., 2005] and then re‐thresholded to test whether any clusters are significant in both ALE images. This approach has been used effectively within the literature; for example, other meta‐analyses have identified brain regions that compute value for different types of reward [Bartra et al., 2013; Clithero and Rangel, 2014].
In addition to conjunction analyses, multiple ALE images can be contrasted to reveal distinct patterns of connectivity. Like conjunction analyses, the contrast analyses also utilize thresholded images as inputs, thus limiting the need for additional corrections for multiple comparisons [Eickhoff et al., 2011]. Contrasts in CBMA are carried out in a series of steps and inference is based on permutation analyses. First, subtracting one input image from the other forms ALE contrast images. These contrast images do not account for differences in study sizes (i.e., whether one ALE image was created using more experiments). Next, to account for differences in study sizes, the algorithm creates simulated data by pooling coordinate datasets and then randomly splitting them into two new groupings of the same size original datasets. Finally, an ALE image is created for each new simulated dataset, subtracted from the other, and then compared to the true data. This process is repeated 10,000 times to form null distributions that allow for statistical inference. The resulting statistical images were subjected to an additional voxelwise threshold of P < 0.01.
RESULTS
Assessing Reliable Patterns of Brain Connectivity Across Multiple Seeds and Contexts
Given the underpowered nature of many neuroscience studies, assessing the reliability of the results is becoming increasingly important [Button et al., 2013]. Yet very little is known about the reliability of brain connectivity findings, particularly those relying on psychophysiological interactions (PPI). Thus, one open and important question regarding PPI studies is whether the obtained results—i.e., the target regions—are consistently found across multiple studies.
We addressed this question for four combinations of seed regions and psychological context containing a sufficient number of experiments for CBMA (Fig. 2B). First, we examined studies using the superior temporal gyrus (STG) as a seed region under the psychological context of attention. Our results indicated that these studies reliably report targets in the fusiform face area (FFA; MNIx , y , z = 32, −65, −11) and the primary somatosensory area (S1; MNIx , y , z = −42, −20, 57), suggesting these regions are influenced by the STG across tasks classified under the construct of attention (Fig. 3A). Second, we examined studies using the dorsolateral prefrontal cortex (DLPFC) as a seed region under the psychological context of cognitive control. Our results indicated that these studies reliably report targets in the posterior cingulate cortex (PCC; MNIx , y , z = −10, −58, 45) and proximal portions of DLPFC (MNIx , y , z = 48, 25, 31), suggesting these regions are influenced by the DLPFC in tasks involving cognitive control (Fig. 3B). Third, we examined studies using the amygdala as a seed region under the psychological context of emotion. Our results indicated that these studies reliably report targets in the inferior lateral occipital cortex (iLOC; MNIx , y , z = −46, −66, −14), the ventral paracingulate cortex (vPAC; MNIx , y , z = 9, 47, 0), and the dorsal anterior cingulate cortex (dACC; MNIx , y , z = 9, 29, 20), suggesting these regions are influenced by the amygdala in tasks involving emotion (Fig. 3C). Fourth, we examined studies using the vPAC as a seed region under the psychological context of social cognition. [Note that vPAC is anatomically similar to what is typically labeled as medial prefrontal cortex in the literature [Amodio and Frith, 2006].] Our results indicated these studies reliably report targets in the posterior superior temporal sulcus (pSTS; MNIx , y , z = 57, −43, 1), suggesting that this region is influenced by the vPAC in tasks involving social cognition (Fig. 3D). Taken together, these results highlight the reliability of PPI results for multiple seed regions and contexts.
Figure 3.
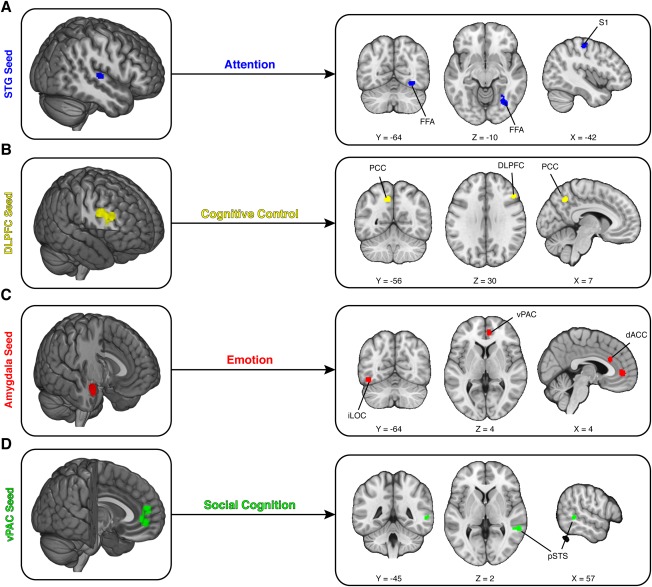
Meta‐analytic evidence of psychophysiological interactions. We used a coordinate‐based meta‐analytic approach to quantify whether PPI studies converged on reliable target regions. (A) Studies using superior temporal gyrus (STG) as the seed region and attention as the psychological context reliably found sensory targets, including the right fusiform face area (FFA) and left primary somatosensory cortex (S1). (B) Studies using dorsolateral prefrontal cortex (DLPFC) as the seed region and cognitive control as the psychological context reliably found targets in the DLPFC and the posterior cingulate cortex (PCC). (C) Studies using amygdala as the seed region and emotion as the psychological context reliably found targets in dorsal anterior cingulate cortex (dACC), inferior lateral occipital cortex (iLOC), and ventral paracingulate cortex (vPAC). (D) Studies using the vPAC as the seed region and social cognition as the psychological context reliably found targets in posterior superior temporal sulcus (pSTS). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Identifying Common and Distinct Patterns of Brain Connectivity
Our findings demonstrate that multiple seed regions and psychological contexts reliably influence specific target regions. Yet, these observations—and hence our understanding of task dependent brain connectivity—could be confounded by multiple factors. For example, a network of regions could work together to influence a single region, thus complicating models of brain connectivity with common inputs. In addition, although the observed patterns of connectivity with a seed region should depend on psychological context, it could be the case that the observed connectivity is merely a general feature of the chosen seed region. For instance, a seed region may contribute to a particular target region irrespective of psychological context, thus casting doubt on the importance of psychological context when using that seed region. These issues raise serious concerns about the interpretation of PPI results and, potentially, models of task‐dependent brain connectivity more generally.
Our meta‐analytic approach can be extended to assess these possibilities and clarify models of brain connectivity. We first attempted to rule out the influence of other seed regions (i.e., common inputs) by performing, for each psychological context, bidirectional comparisons between the seed region of interest and all other seed regions. If the observed influences on the target regions are specific to the seed region, then subtracting out the effect of other seed regions should leave similar target regions. We tested this possibility for each of the results described in Figure 3. Our analysis revealed two key results. First, in tasks involving attention, the FFA (MNIx , y , z = 34, −66, −10) was reliably modulated by the STG more so than any other seed region in our corpus of studies (Fig. 4A). Second, in tasks involving cognitive control, the PCC (MNIx , y , z = −11, −60, 45) was reliably modulated by the DLPFC more so than any other seed region in our corpus of studies (Fig. 4B). Interestingly, we also found that tasks involving emotion tend to reliably modulate the amygdala (MNIx , y , z = −28, 0, −16) and the STG (MNIx , y , z = −65, −25, 10) (Fig. 4C). This result implies that the psychological context of emotion might generally influence responses in the left amygdala and the left STG (e.g., right‐hand panel of Fig. 1A).
Figure 4.
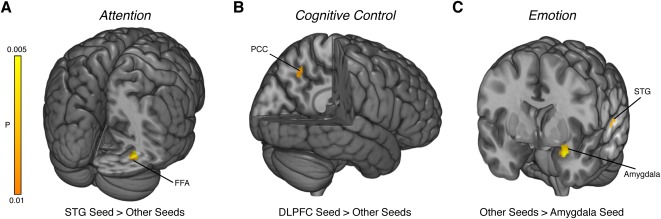
Psychophysiological interactions depend on seed region. Models of brain connectivity assessed through PPI have difficulty ruling out whether common inputs contribute to the observed effects. We therefore tested whether our meta‐analytic PPI target regions were dependent on the seed region (i.e., the input region) by contrasting studies employing the seed region of interest against studies using other seed regions. (A) For studies utilizing attention as the psychological context, we found that the superior temporal gyrus (STG) seed (relative to other seeds) selectively contributed to the fusiform face area (FFA). (B) Similarly, for studies utilizing cognitive control, we found that the dorsolateral prefrontal cortex (DLPFC) seed (relative to other seeds) selectively contributed to the posterior cingulate cortex (PCC). (C) In contrast, studies using emotion as the psychological context and the amygdala as the seed region were not uniquely tied to any target regions. Instead, emotion studies that did not use the amygdala as seed region were uniquely associated with amygdala and STG targets. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Our next set of analyses focused on the specificity of psychological context. A given psychological context could modulate similar target regions, irrespective of the chosen seed region. Alternatively, a given seed region could modulate distinct target regions, depending on the psychological context. We evaluated these possibilities by calculating, for each seed region, bidirectional contrasts between the psychological context of interest and all other contexts. We found one result: DLPFC modulates the PCC (MNIx , y , z = −9, −59, 45) during cognitive control and modulates the amygdala (MNIx , y , z = 20, −6, −16) during other contexts (Fig. 5). These observations point to the flexibility of DLPFC and demonstrate how its interactions with other brain regions are fundamentally dependent on psychological context.
Figure 5.
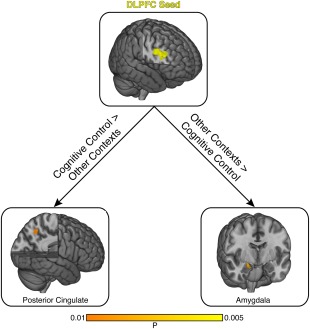
Psychophysiological interactions depend on psychological context. We investigated the influence of psychological context on PPI results by contrasting studies using the same seed region with different psychological contexts. We found that only one seed region, the DLPFC, contributed to distinct target regions depending on psychological context. Specifically, with psychological contexts involving cognitive control, DLPFC contributed to the posterior cingulate. In contrast, with other psychological contexts, DLPFC contributed to the amygdala. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For completeness, we also investigated, in a series of post hoc analyses, common and distinct patterns of connectivity with each seed region (ignoring psychological context). These post hoc analyses maintained a relatively conservative threshold for the ALE images (i.e., clusters were held to FWER of P = 0.01); and resulting contrast and conjunction analyses were subjected to a voxelwise threshold of P < 0.005. We first performed bidirectional comparisons between the amygdala and each alternative seed region. Compared to the FFA seed and the DLPFC seed, the amygdala seed was more likely to contribute to target region in medial prefrontal cortex (Supporting Information Fig. 1). Next, we examined bidirectional comparisons between the ventral striatum seed and each alternative seed region. Compared to the anterior insula seed, the DLPFC seed, and the ventral paracingulate cortex seed, the ventral striatum seed was more likely to influence a target region in ventral lateral prefrontal cortex (Supporting Information Fig. 2). Notably, recent work has suggested that this particular corticostriatal pathway may be important for distinguishing the hedonic and reinforcing properties of an experienced reward [Smith et al., 2016]. Further pairwise contrasts of each seed region revealed no other differences. We also examined pairwise conjunctions to test whether any seed regions influence a common target region. Our analyses indicated that the FFA seed and the DLPFC seed influence a target region in the left amygdala (Supporting Information Fig. 3), suggesting the FFA and DLPFC serve as common inputs to the amygdala.
DISCUSSION
A key goal of neuroscience is to understand structure‐function relationships in the brain. However, examining single brain regions in isolation without examining connections between them severely limits our ability to accurately characterize the consistency and specificity of structure‐function relationships. Thus, we sought to investigate this outstanding yet critical question—that is, whether similar seed regions yield similar target regions across multiple studies—by performing a coordinate‐based meta‐analysis on 284 studies that utilized PPI (5,997 participants). We identified the most common seed regions spanning diverse psychological contexts (e.g., attention, cognition control, emotion, and social cognition), which included the amygdala, fusiform face area (FFA), superior temporal gyrus (STG), dorsolateral prefrontal cortex (DLPFC), and ventral paracingulate cortex (vPAC). This approach allowed us to demonstrate that: (1) PPI studies provide consistent patterns of effective connectivity; (2) such patterns are largely dependent on the reference region, thus mitigating concerns over common inputs; and (3) a single region may participate in multiple psychological functions depending on its connectivity with other regions. Taken together, these results support the robustness of PPI as a tool to examine functional integration, while broadening our understanding of models of brain connectivity to emphasize a shift from functional to effective connectivity.
The results are particularly significant when considering the recent emphasis on reproducibility of findings [Nosek et al., 2015; Open Science, 2015]. While replications are indeed necessary [Ioannidis, 2005], the meta‐analytic approach allows us to draw broader conclusions across a body of work to provide new insight that often goes beyond the scope of a few studies [Stanley and Spence, 2014]. That is, our work serves to confirm results and support the reliability of PPI findings—for instance, by highlighting that studies on social cognition using the vPAC as the seed region show functional connectivity with pSTS, a result consistent with the larger literature on the processing of social cues, agency of actions, and mentalizing [Amodio and Frith, 2006; Tankersley et al., 2007]. However, our analysis also achieves the exciting goal of characterizing general and distinct patterns of effective connectivity traversing various psychological contexts. For example, the FFA and DLPFC had a general contribution to the amygdala, regardless of psychological context, whereas DLPFC had a selective contribution to PCC in studies on cognitive control, but contributed to the amygdala in other psychological contexts.
To illustrate this further, we focus discussion on some of the specific results we observed. Studies on emotion that used the amygdala as a seed region, for example, yielded targets in regions previously implicated in the cognitive regulation of emotion, such as the dACC and vPAC [Ochsner and Gross, 2005], as well as the iLOC, which is broadly linked to visual perception of shape from motion [Ferber et al., 2003]. The dACC is thought to be active during conflict or performance monitoring, emotion regulation [Kanske and Kotz, 2011], and reward‐based decision making and learning [Bush et al., 2002], whereas the vPAC is most closely linked to social cognition [Amodio and Frith, 2006], and may reflect the social nature of stimuli used in these studies (e.g., faces or images of people experiencing emotion).
The amygdala's contribution to both cognitive (dACC) and affective (vPAC) aspects of the cingulate is in line with models of the cognitive control of emotion [Ochsner and Gross, 2005; Ochsner et al., 2012]. However, such regions are predicted to modulate emotional responses via projections to affective regions, such as the amygdala, rather than in the reverse direction. There is some evidence of coupling between the amygdala and either the prefrontal cortex or cingulate during emotion regulation [Blair et al., 2007; Delgado et al., 2008; Kim and Hamann, 2007; Ochsner et al., 2004; Phan et al., 2005], but functional connectivity studies cannot speak to directionality. Only a handful of studies have actually tested a direct relationship between the prefrontal cortex and amygdala, for instance, demonstrating that the DMPFC or VLPFC reduce amygdala activity through modulation of the VMPFC [Johnstone et al., 2007; Urry et al., 2006], with one study also showing a subsequent reduction in negative affect [Wager et al., 2008]. Our meta‐analytic PPI results indicated a similar relationship, in that the DLPFC contributed to the amygdala in studies with psychological contexts outside of cognitive control. This finding may suggest a more general role of DLPFC‐amygdala connectivity in affective, attentional, and social processing, which may be due to the fact that such processes are often emotionally embedded. Importantly, there could be other interpretations of the potential relationship between DLPFC and amygdala that include distinct task demands and stimuli used across all studies, from motor demands of a particular paradigm to context such as task difficulty which could be commonly elicited across all contexts. Our results demonstrating the amygdala's role in emotion as both an input region and recipient from prefrontal and cingulate regions highlight the significance of examining inter‐regional connectivity patterns as a function of behavior.
Similar inferences can be made from our observed results on neuroimaging studies of cognitive control and attention that seek to understand how the brain allocates resources for information processing and attends to relevant stimuli in the environment. We observed that studies on cognitive control that used DLPFC as the seed region found targets in the DLPFC and PCC. The DLPFC as a seed and target region for studies on cognitive control is fitting given its known association with working memory, planning, decision‐making and cognitive flexibility [Cieslik et al., 2013; Qin et al., 2009]. There is evidence to suggest that the PCC dampens in response to attentional control or working memory demands, given its role in the default mode network [Buckner et al., 2008; Hayden et al., 2009; Whitfield‐Gabrieli et al., 2011], but has increased activity during self‐relevant thinking such as memory retrieval or planning [Brewer et al., 2011; Lemogne et al., 2011]. Single unit studies linking PCC activity to cognitive control are supportive of these observations [Hayden et al., 2010], and together with the discussed neuroimaging results suggest that DLPFC contributions to PCC reflect changes in attentional or cognitive demands during task performance. Interestingly, we also found that studies on attention using the STG as the seed region contributed to targets in sensory regions such as the FFA and S1, potentially consistent with the involvement of STG in attentional disorders [Karnath et al., 2004; Smith et al., 2013a]. Although our analysis of attention‐related PPI studies collapsed across sensory modalities (e.g., visual, auditory) because previous work has suggested that some aspects of attentional control operate similarly across sensory modalities [Smith et al., 2010; Wu et al., 2007], we note that future connectivity work could explore how different sensory modalities are integrated and influenced by attention [Busse et al., 2005; Donohue et al., 2015, 2011; Laing et al., 2015; Mayer and Vuong, 2014]. In addition, it would also be important to disentangle the effects of stimulus content (e.g., faces) from psychological context (e.g., selective attention).
Beyond the observed general and specific connectivity patterns, our work also provides a distinct perspective on brain connectivity. The current zeitgeist in brain connectivity has been largely fueled by functional connectivity approaches measuring correlations between brain regions [Smith et al., 2013b; Tomasi and Volkow, 2011]. This approach is also endemic within the CBMA framework and is commonly called meta‐analytic connectivity modeling [Eickhoff et al., 2011; Robinson et al., 2012]. Although functional connectivity metrics have tremendous utility in classifying individual differences [Hariri, 2009; Kelley et al., 2015], these approaches provide limited insight into the mechanisms of functional integration [Friston, 2011]. Indeed, a change in correlation between two brain regions could arise due to factors that are unrelated to neuronal coupling: changes in another connection; changes in observational noise; or changes in neuronal fluctuations [Friston, 2011]. Our approach—which focuses exclusively on psychophysiological interactions—eschews these confounds and provides mechanistic insight into functional integration. We provide a new perspective by providing meta‐analytic insights into simple models of effective connectivity. By identifying common and distinct patterns of psychophysiological interactions across multiple seed regions and psychological contexts, our study may catalyze new research efforts and shape future questions about brain connectivity. Future studies could build on our findings by formalizing how effective connectivity is constrained by structural connections [e.g., Mayer and Vuong, 2014].
Yet, these advances should be interpreted within the context of two primary limitations. First, we stress that PPI results are models of contribution (Fig. 1A) and do not imply causation (i.e., responses in the seed region do not cause responses in target region). Inferring causation from neuroimaging data is challenging [Ramsey et al., 2010] and requires alternative approaches, such as dynamic causal modeling (DCM), which evaluates multiple models of context‐specific changes in effective connectivity between regions [Friston, 2009; Friston et al., 2003]. We chose not to focus on DCM studies in our meta‐analysis due to the difficulty of aggregating findings across DCM experiments. These experiments vary on multiple factors: number of ROIs, locations of ROIs, connections between those ROIs, and psychological contexts. Although these factors make it challenging to perform meta‐analyses on DCM experiments, we note that recent work has also highlighted the test‐retest reliability of the DCM approach [Frassle et al., 2015].
Second, idiosyncratic features of our corpus of PPI studies could influence our results. For example, we synthesized findings from specific sets of PPI experiments. Although we sampled broadly from the literature, it is possible that selection bias could influence our findings [Ahmed et al., 2012; Egger et al., 1997]. For example, the seed region used in an experiment may depend on the psychological context under investigation. This dependency was evident in our matrix of studies: each seed region was disproportionately represented by a psychological context (e.g., emotion and the amygdala). Nevertheless, we emphasize that our contrasts partially mitigate this concern by controlling for the influence of other factors, including alternative seed regions and psychological contexts. In addition, our efforts to group PPI studies into broad categories and relatively coarse seed regions that ignore lateralization may have added noise to our analyses, thus limiting our ability to detect true effects. For example, collapsing across studies using left and right DLPFC as the seed region neglects hemispheric differences in DLPFC, potentially missing effects that are specific to a hemisphere [Binder et al., 2009]. With continued growth of PPI studies, future work could quantify hemispheric differences in connectivity by contrasting studies using left‐lateralized seed regions against studies using right‐lateralized seed regions.
There are other considerations that could add noise to the analysis. For instance, some patterns of connectivity could also be tied to subtle distinctions in psychological contexts that evoke different responses in different individuals [Smith et al., 2014a; van den Bos et al., 201]). Likewise, some patterns of connectivity could be tied to very small differences in seed location. Indeed, recent work has pointed to the importance of seed region location [Cole et al., 2010], which has helped motivate the use of alternative tools that quantify connectivity with large‐scale neural networks [Leech et al., 2011; Smith et al., 2015, 2014b]. As these factors add noise to our analyses and thus reduce the likelihood of detecting true convergence across studies, we caution against drawing any conclusions regarding the absence of meta‐analytic effects. In other words, the absence of meta‐analytic effects should not be interpreted as evidence against the reliability or consistency of PPI. Taken together, these questions are important for advancing models of functional integration [Park and Friston, 2013], but we stress that the current corpus of PPI studies does not permit robust CBMAs on such questions [Eickhoff et al., 2012]. We hope that the continued growth of PPI creates an opportunity to investigate these issues and refine our study with targeted contrasts and new analyses.
CONCLUSIONS
Despite these caveats, our study validates the utility of the psychophysiological interactions and provides a first step toward a cumulative science of functional integration. Our core results lead to two broad conclusions. First, different types of PPI studies each consistently converge on similar target regions, indicating PPI can be a reliable tool for studying functional integration. Second, target regions identified by PPI can be specific to the chosen seed region and psychological context, suggesting PPI can reveal distinct patterns of brain connectivity and functional integration. The complex nature of functional integration—like many issues within cognitive neuroscience [Yarkoni et al., 2010]—requires synthesizing data from multiple experiments. Our work reveals how distinct brain systems interact and modulate other regions across multiple psychological contexts, which may help elucidate the mechanisms that contribute to disorders hypothesized to reflect disconnections, such as autism [Just et al., 2007] and schizophrenia [Friston, 2002].
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Authors thank John Clithero and Brynne DiMenichi for helpful comments on previous drafts of this manuscript. Authors also thank Karl Friston for advice on interpreting psychophysiological interactions.
See related article http://onlinelibrary.wiley.com/doi/10.1002/hbm.23354/full
Contributor Information
David V. Smith, Email: smith@psychology.rutgers.edu.
Mauricio R. Delgado, Email: delgado@psychology.rutgers.edu.
REFERENCES
- Adolphs R, Tranel D, Damasio H, Damasio A (1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669–672. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Sutton AJ, Riley RD (2012): Assessment of publication bias, selection bias, and unavailable data in meta‐analyses using individual participant data: A database survey. BMJ 344:d7762. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD (2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7:268–277. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013): The valuation system: A coordinate‐based meta‐analysis of bold FMRI experiments examining neural correlates of subjective value. Neuroimage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J (2011): Depression, rumination and the default network. Soc Cognit Affect Neurosci 6:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kutter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ (2007): Modulation of emotion by cognition and cognition by emotion. Neuroimage 35:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM (2002): The problem of functional localization in the human brain. Nat Rev Neurosci 3:243–249. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H (2011): Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA 108:20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2014): Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cereb Cortex 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2002): Dorsal anterior cingulate cortex: A role in reward‐based decision making. Proc Natl Acad Sci USA 99:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse L, Roberts KC, Crist RE, Weissman DH, Woldorff MG (2005): The spread of attention across modalities and space in a multisensory object. Proc Natl Acad Sci USA 102:18751–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR (2013): Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev 14:365–376. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW (2000): Impaired recognition and experience of disgust following brain injury. Nat Neurosci 3:1077–1078. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2013): Decoding the role of the insula in human cognition: Functional parcellation and large‐scale reverse inference. Cereb Cortex 23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2013): Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation‐based parcellation. Cereb Cortex 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A (2014): Informatic parcellation of the network involved in the computation of subjective value. Soc Cognit Affect Neurosci 9:1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF (2010): Advances and pitfalls in the analysis and interpretation of resting‐state Fmri data. Front Syst Neurosci 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA (2008): Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Poldrack RA (2007): In praise of tedious anatomy. Neuroimage 37:1033–1041. discussion 1050‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue SE, Green JJ, Woldorff MG (2015): the effects of attention on the temporal integration of multisensory stimuli. Front Integr Neurosci 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue SE, Roberts KC, Grent T, Äòt‐Jong T Woldorff MG (2011): The cross‐modal spread of attention reveals differential constraints for the temporal and spatial linking of visual and auditory stimulus events. J Neurosci 31:7982–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997): Bias in meta‐analysis detected by a simple, graphical test. BMJ 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta‐analysis revisited. Neuroimage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011): Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TEJ (2010): Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci 30:6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH (2005): Method matters: An empirical study of impact in cognitive neuroscience. J Cognit Neurosci 17:850–858. [DOI] [PubMed] [Google Scholar]
- Ferber S, Humphrey GK, Vilis T (2003): The lateral occipital complex subserves the perceptual persistence of motion‐defined groupings. Cereb Cortex 13:716–721. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Noth J, Zilles K (2003): Remarks on this special issue. Neuroimage 20:S1. [DOI] [PubMed] [Google Scholar]
- Fox PT, Friston KJ (2012): Distributed processing; distributed functions? Neuroimage 61:407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Parsons LM, Lancaster JL (1998): Beyond the single study: Function/location metanalysis in cognitive neuroimaging. Curr Opin Neurobiol 8:178–187. [DOI] [PubMed] [Google Scholar]
- Frassle S, Stephan KE, Friston KJ, Steup M, Krach S, Paulus FM, Jansen A (2015): Test‐retest reliability of dynamic causal modeling for FMRI. Neuroimage 117:56–66. [DOI] [PubMed] [Google Scholar]
- Friston K (2009): Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol 7:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (2002): Dysfunctional connectivity in Schizophrenia. World Psychiatry 1:66–71. [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (2005): Models of brain function in neuroimaging. Annu Rev Psychol 56:57–87. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: A review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ (1996): The trouble with cognitive subtraction. Neuroimage 4:97–104. [DOI] [PubMed] [Google Scholar]
- Gerstein GL, Perkel DH (1969): Simultaneously recorded trains of action potentials: Analysis and functional interpretation. Science 164:828–830. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD (1992): Separate visual pathways for perception and action. Trends Neurosci 15:20–25. [DOI] [PubMed] [Google Scholar]
- Hariri AR (2009): The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci 32:225–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML (2009): Electrophysiological correlates of default‐mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA 106:5948–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML (2010): Cognitive control signals in posterior cingulate cortex. Front Hum Neurosci 4:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA (2012): Event‐related FMRI in cognition. Neuroimage 62:1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP (2005): Why most published research findings are false. PLoS Med 2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ (2007): Failure to regulate: Counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. J Neurosci 27:8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ (2007): Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex 17:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Kotz SA (2011): Emotion triggers executive attention: Anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Hum Brain Mapp 32:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Fruhmann Berger M, Küker W, Rorden C (2004): The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cereb Cortex 14:1164–1172. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Wagner DD, Heatherton TF (2015): In search of a human self‐regulation system. Annu Rev Neurosci 38:389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S (2007): Neural correlates of positive and negative emotion regulation. J Cognit Neurosci 19:776–798. [DOI] [PubMed] [Google Scholar]
- Laing M, Rees A, Vuong QC (2015): Amplitude‐modulated stimuli reveal auditory‐visual interactions in brain activity and brain connectivity. Front Psychol 6:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the Icbm‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ (2011): Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 31:3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Gorwood P, Bergouignan L, Pelissolo A, Lehericy S, Fossati P (2011): Negative affectivity, self‐referential processing and the cortical midline structures. Soc Cognit Affect Neurosci 6:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, Peters H, Zimmer C, Förstl H, Bäuml J, Wohlschläger AM, Sorg C (2014): Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in Schizophrenia. Schizophrenia Bull 40:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KM, Vuong QC (2014): TBSS and probabilistic tractography reveal white matter connections for attention to object features. Brain Struct Funct 219:2159–2171. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (Gppi): A comparison to standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS (2010): Fmri studies of successful emotional memory encoding: A quantitative meta‐analysis. Neuropsychologia 48:3459–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25:653–660. [DOI] [PubMed] [Google Scholar]
- Nosek BA, Alter G, Banks GC, Borsboom D, Bowman SD, Breckler SJ, Buck S, Chambers CD, Chin G, Christensen G, Contestabile M, Dafoe A, Eich E, Freese J, Glennerster R, Goroff D, Green DP, Hesse B, Humphreys M, Ishiyama J, Karlan D, Kraut A, Lupia A, Mabry P, Madon TA, Malhotra N, Mayo‐Wilson E, McNutt M, Miguel E, Paluck EL, Simonsohn U, Soderberg C, Spellman BA, Turitto J, VandenBos G, Vazire S, Wagenmakers EJ, Wilson R, Yarkoni T (2015): Promoting an open research culture. Science 348:1422–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci 9:242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ (2004): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23:483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT (2012): Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann NY Acad Sci 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Open Science, C. (2015): Estimating the reproducibility of psychological science. Science 349:aac4716. [DOI] [PubMed] [Google Scholar]
- Park HJ, Friston K (2013): Structural and functional brain networks: From connections to cognition. Science 342:1238411. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME (2005): Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry 57:210–219. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2008): The role of FMRI in cognitive neuroscience: Where do we stand? Curr Opin Neurobiol 18:223–227. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2010): Mapping mental function to brain structure: How can cognitive neuroimaging succeed? Perspect Psychol Sci 5:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G (2009): Acute psychological stress reduces working memory‐related activity in the dorsolateral prefrontal cortex. Biol Psychiatry 66:25–32. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2003): Functional brain imaging and human brain function. J Neurosci 23:3959–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C (2010): Six problems for causal inference from FMRI. Neuroimage 49:1545–1558. [DOI] [PubMed] [Google Scholar]
- Riedel MC, Ray KL, Dick AS, Sutherland MT, Hernandez Z, Fox PM, Eickhoff SB, Fox PT, Laird AR (2015): Meta‐analytic connectivity and behavioral parcellation of the human cerebellum. Neuroimage 117:327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, Fox PT (2012): The functional connectivity of the human caudate: An application of meta‐analytic connectivity modeling with behavioral filtering. Neuroimage 60:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT (2010): Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum Brain Mapp 31:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO (2004): Using human brain lesions to infer function: A relic from a past era in the FMRI age? Nat Rev 5:812–819. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957): Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP (2009): The resting brain: Unconstrained yet reliable. Cereb Cortex 19:2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Clithero JA, Boltuck SE, Huettel SA (2014a): Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Soc Cognit Affect Neurosci 9:2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Clithero JA, Rorden C, Karnath HO (2013a): Decoding the anatomical network of spatial attention. Proc Natl Acad Sci USA 110:1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Davis B, Niu K, Healy EW, Bonilha L, Fridriksson J, Morgan PS, Rorden C (2010): Spatial attention evokes similar activation patterns for visual and auditory stimuli. J Cogn Neurosci 22:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Rigney AE, Delgado MR (2016): Distinct reward properties are encoded via corticostriatal interactions. Sci Rep 6:20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Sip KE, Delgado MR (2015): Functional connectivity with distinct neural networks tracks fluctuations in gain/loss framing susceptibility. Hum Brain Mapp 36:2743–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Utevsky AV, Bland AR, Clement N, Clithero JA, Harsch AE, Carter RM, Huettel SA (2014b): Characterizing individual differences in functional connectivity using dual‐regression and seed‐based approaches. Neuroimage 95:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, Kelly M, Laumann T, Miller KL, Moeller S, Petersen S, Power J, Salimi‐Khorshidi G, Snyder AZ, Vu AT, Woolrich MW, Xu J, Yacoub E, Ugurbil K, Van Essen DC, Glasser MF, Consortium WUMH (2013b): Resting‐state FMRI in the human connectome project. Neuroimage 80:144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi‐Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW (2011): Network modelling methods for FMRI. Neuroimage 54:875–891. [DOI] [PubMed] [Google Scholar]
- Sporns O (2014): Contributions and challenges for network models in cognitive neuroscience. Nat Neurosci 17:652–660. [DOI] [PubMed] [Google Scholar]
- Stanley DJ, Spence JR (2014): Expectations for replications: Are yours realistic? Perspect Psychol Sci 9:305–318. [DOI] [PubMed] [Google Scholar]
- Tankersley D, Stowe CJ, Huettel SA (2007): Altruism is associated with an increased neural response to agency. Nat Neurosci 10:150–151. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2011): Functional connectivity hubs in the human brain. Neuroimage 57:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ (2006): Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26:4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA (2014): Precuneus is a functional core of the default‐mode network. J Neurosci 34:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes‐Sosa PA, Roebroeck A, Daunizeau J, Friston K (2011): Effective connectivity: Influence, causality and biophysical modeling. Neuroimage 58:339–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM (2014): Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. J Neurosci 34:10298–10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L (2007): Meta‐analysis of functional neuroimaging data: Current and future directions. Soc Cognit Affect Neurosci 2:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX (2009): Evaluating the consistency and specificity of neuroimaging data using meta‐analysis. Neuroimage 45:S210–S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Moran JM, Nieto‐CastaÒÛn A, Triantafyllou C, Saxe R, Gabrieli JDE (2011): Associations and dissociations between default and self‐reference networks in the human brain. Neuroimage 55:225–232. [DOI] [PubMed] [Google Scholar]
- Wu CT, Weissman DH, Roberts KC, Woldorff MG (2007): The neural circuitry underlying the executive control of auditory spatial attention. Brain Res 1134:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Van Essen DC, Wager TD (2010): Cognitive neuroscience 2.0: Building a cumulative science of human brain function. Trends Cogn Sci 14:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR (2014): Meta‐analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex 24:232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


