SUMMARY
Based on studies in mouse tumor models, granulocytes appear to play a tumor-promoting role. However, there are limited data about the phenotype and function of tumor-associated neutrophils (TANs) in humans. Here, we identify a subset of TANs that exhibited characteristics of both neutrophils and antigen-presenting cells (APC) in early-stage human lung cancer. These APC-like “hybrid neutrophils”, which originate from CD11b+CD15hiCD10−CD16low immature progenitors, are able to cross-present antigens, as well as trigger and augment anti-tumor T cell responses. IFN-γ and GM-CSF are requisite factors in the tumor that working through the Ikaros transcription factor, synergistically exert their APC-promoting effects on the progenitors. Overall, these data demonstrate the existence of a specialized TAN subset with anti-tumor capabilities in human cancer.
INTRODUCTION
Tumor-associated inflammation contributes to cancer development and progression and is often associated with a high degree of inflammatory cell infiltration (Grivennikov, et al, 2010). Tumor-associated neutrophils (TANs) represent a significant portion of tumor-infiltrating cells and accumulate in many types of cancers, including lung cancer (Carus, et al, 2013; Ilie, et al, 2012). Although the role of TANs in tumor development is beginning to be investigated in murine models, it remains largely unexplored in humans.
In murine studies, it appears that TANs can exert both pro-tumor and anti-tumor effects (Brandau, 2013; Fridlender, et al, 2009). Numerous studies have shown that neutrophils can promote tumor progression by degrading matrix, immunosculpting, stimulating tumor cell proliferation, increasing metastasis, and enhancing angiogenesis (Houghton, 2010; Piccard, et al, 2012). However, they can also exert anti-tumor functions such as inducing tumor cell death via their powerful antimicrobial killing machinery (Dallegri and Ottonello, 1992; van Egmond and Bakema, 2013) and producing factors to recruit and activate cells of the innate and adaptive immune system (Mantovani, et al, 2011). Given these varying effects of mouse TANs on tumor growth, the paradigm of anti-tumor “N1 neutrophils” versus pro-tumor “N2 neutrophils” was proposed (Fridlender, et al, 2009). However, most of these data were derived from mouse models that use tumor cell lines adapted to grow rapidly in vivo and have thus already undergone cancer immunoediting (Schreiber, et al, 2011). These models are also characterized by high tumor burden, minimal matrix, and rapid tumor growth. Because these features are dissimilar to human cancers that evolve slowly over time, the role of tumor-infiltrating myeloid cells in human cancers may not be the same and the function of human TANs, particularly in the early stages of tumor development, remains largely unexplored.
Understanding the role of TANs in the regulation of the T cell response in cancer patients is important because the cytotoxic T lymphocytes are the major effector cells mediating antigen-driven anti-tumor immunity. We recently demonstrated that early stage lung cancers are highly infiltrated with activated neutrophils and that these TANs exhibit heterogeneous expression of T cell co-stimulatory molecules (Eruslanov, et al, 2014). In contrast to the data from murine studies, TANs isolated from vast majority of small early-stage tumors were not immunosuppressive, but in fact, they stimulated T cell responses (Eruslanov, et al, 2014). Interestingly, the T cell activation property of TANs became less prominent with disease progression, consistent with the emerging concept of an immunogenic “switch” from anti-tumor to pro-tumor phenotype (Granot and Fridlender, 2015).
As part of our phenotypic analysis of early stage lung cancer TANs (Eruslanov, et al, 2014), we identified a subset of cells exhibiting the hybrid phenotype of both neutrophils and antigen-presenting cells (APCs). We hypothesized that early stage tumors, where the immunosuppressive environment might not be fully developed, can drive recruited granulocytes to further differentiate into a specialized cell subset with strong T cell stimulatory activity. The purpose of this study was to characterize the phenotype, function, and origin of these hybrid cells in lung cancer patients.
RESULTS
Early-stage human lung cancers accumulate a neutrophil subset with a composite phenotype of granulocytes and antigen-presenting cells
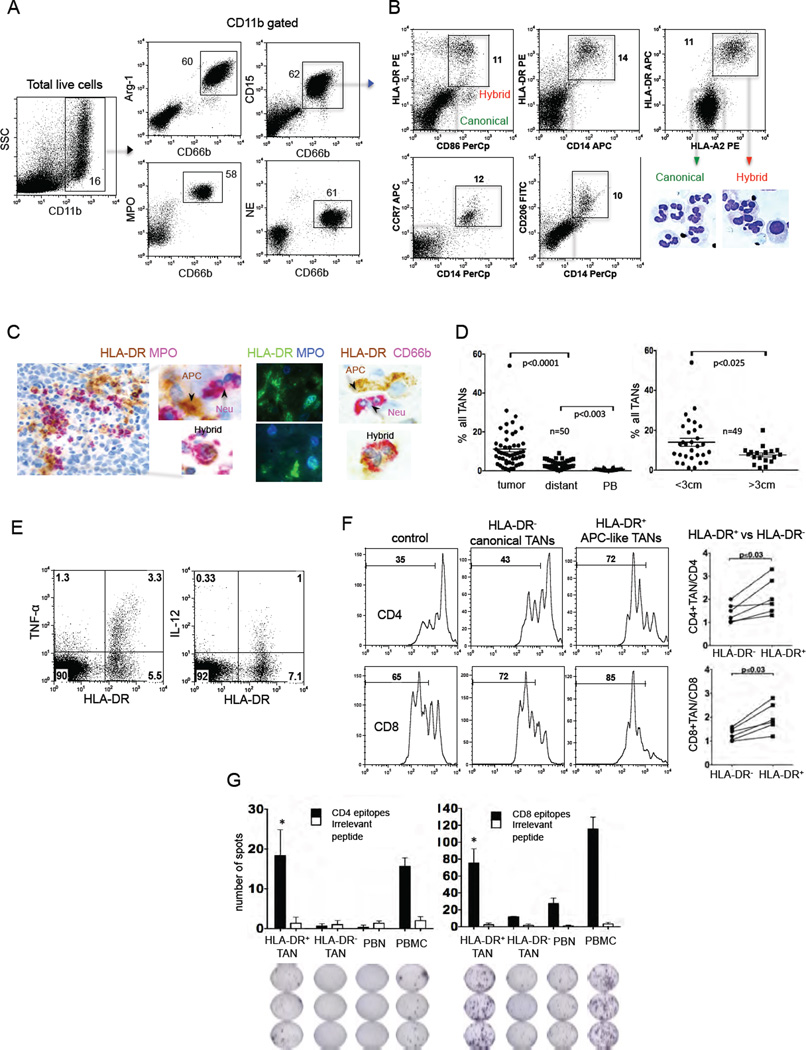
Since TANs in patients with early stage lung cancer have the ability to heterogeneously express some T cell co-stimulatory molecules (Eruslanov, et al, 2014), we postulated that there might be a subset of TANs with characteristics of antigen–presenting cells (APC). We thus analyzed the expression of APC surface markers on neutrophils from three locations: lung cancer tissue, adjacent (within the same lobe) lung parenchyma (termed “distant tissue”), and peripheral blood (Figure S1A). We performed phenotypic analysis of 50 random patients with Stage I–II non-small cell lung cancer (NSCLC). Detailed characteristics of all patients involved in this study are shown in Table S1. Fresh tissue was digested using defined conditions that minimize enzyme-induced ex-vivo effects on the viability, premature activation, phenotype, and function of neutrophils (Quatromoni, et al, 2015). Previously, we performed extensive phenotypic analysis of neutrophils in NSCLC and characterized TANs as CD11b+CD15hiCD66b+MPO+Arg1+CD16intIL-5Rα− cells (Eruslanov, et al, 2014). Importantly, all CD66b+CD11b+ cells also expressed the other neutrophil/myeloid cell markers CD15, MPO (myeloperoxidase), Arg-1 (Arginase-1) and NE (neutrophil elastase) at very high levels (Figure 1A- blue boxes) and thus could be segregated from other CD15loMPOloNEloArg-1− non-granulocytic CD11b+ myeloid cells. Since there was a high concordance amongst the selected neutrophil markers, for our studies, we defined TANs as CD15hiCD66b+CD11b+ cells. Our analysis revealed that the majority of neutrophils from lung tumors, which we term “canonical TANs”, expressed only these classic neutrophil markers (Figure 1A and S1A). However, we also identified TANs with surface expression of additional markers normally expressed on APC, specifically HLA-DR, CD14, CD206, CD86, and CCR7 (Figure S1B–F). These receptors were completely absent in peripheral blood neutrophils (PBNs). The “distant tissue” neutrophils also expressed these APC markers, however, at much lower levels in comparison to TANs.
Figure 1. A subset of TANs with hybrid characteristics of neutrophils and APCs.
(A) A single-cell suspension was obtained from fresh tumor and the expression of the indicated granulocytic markers was analyzed by flow cytometry on gated live CD11b cells. Total TANs are shown in blue boxes.
(B) Flow cytometric analysis of the expression of APC markers on gated CD11b+CD15hiCD66b+ TANs. The representative cytomorphology of canonical (green boxes) and APC-like hybrid TANs (red boxes) in NSCLC. Scale bar, 10 µm.
(C) The presence of APC-like hybrid TANs in tumor detected by IHC and IF double-staining. Scale bar: 50 µm (left image) and 10 µm (other images).
(D) The frequency of APC-like hybrid neutrophils in tumors, distant lung tissue, peripheral blood (PB) (right graph) and in tumors of different sizes (left graph) (line represents mean ± SEM, n = 50, one-way ANOVA test and unpaired t test). APC-like hybrid TANs were defined as a live HLA-DR+CD11b+CD15hiCD66b+ cells.
(E) Intracellular TNF-α and IL-12 production by HLA-DR+ hybrid or HLA-DR− canonical TANs after stimulation with LPS. TANs were gated on CD11b+CD15hiCD66b+ cells. Representative results from 1 of 5 experiments are shown.
(F) The proliferation of autologous CFSE-labeled PBMC stimulated with plate-bound anti-CD3 Abs in the presence of hybrid HLA-DR+ or canonical HLA-DR− TANs. T cell stimulatory activity was defined as the ratio CFSElo (T cells+TANs) / CFSElo (T cells) (n=6, Wilcoxon matched-pairs rank test).
(G) Autologous virus-specific memory T cell responses in the presence of APC-like hybrid HLA-DR+ or canonical HLA-DR− TANs. IFN-γ-ELISPOT assay (mean ± SEM, n=3, *p ≤ 0.01 canonical vs. hybrid, Mann-Whitney test).
See also Figure S1.
Further analysis revealed that the APC markers (CD14+HLA-DR+HLA-ABChiCCR7+ CD86+CD206+) were co-expressed on a unique subset of CD11b+CD66b+CD15hi TANs (Figure 1B), exhibiting a composite phenotype of canonical neutrophils and APC. We termed this subset “APC-like hybrid TANs”. This population of hybrid TANs expressed some markers of the APC phenotype (e.g. CD14, HLA-DR, CCR7, CD86, and CD206) but lacked other defining markers of “professional APC” such as CD209, CD204, CD83, CD40, CD163, CD1c and CCR6 (data not shown). Of note, the expression of CD206, CCR7 and CD86 varied, whereas there was a consistent co-expression of HLA-DR and CD14 on hybrid TANs. Cytospins prepared from flow-sorted HLA-DR− canonical and HLA-DR+ hybrid TANs revealed that some of the hybrid TANs had round and oval nuclear shapes in comparison to the classic nuclear segmentation of canonical TANs (Figure 1B). Histological review of lung tumors also revealed “double-positive” MPO+HLA-DR+ and CD66b+HLA-DR+TANs that were scattered throughout lung tumors (Figure 1C). Additionally, we detected a small, but clearly distinguishable population of HLA-DR+CD15hiCD66b+CD11b+ cells in the draining lymph nodes of several lung cancer patients (Figure S1G).
The frequency of this identified subset of TANs varied from 0.5% to 25% among all TANs (Figure 1D) and from 0.1% to 4.3% among all cells in tumor digests (Figure S1H). The hybrid population was significantly higher in patients with adenocarcinoma (AC) compared to patients with squamous cell carcinoma (SCC) (Figure S1I). There were no significant associations between the frequency of APC-like TANs and tumor stage or smoking history (Figure S1J and S1K). Interestingly, we found a significantly smaller percentage of HLA-DR+ hybrid neutrophils amongst TANs in large tumors (diameter > 3 cm) versus the small tumors (diameter < 3 cm) (Figure 1D and S1L). Thus, the hybrid population appears to decline as tumors enlarge, and it is completely absent in tumors greater than 5 to 7 cm in diameter. Together, these data demonstrate that neutrophils in some early-stage lung tumors undergo unique phenotypic changes, yielding a subset of TANs with composite characteristics of neutrophils and APC.
APC-like hybrid TANs stimulate and support T cell responses
Previously, we showed that TANs isolated from early-stage small lung cancers were able to stimulate antigen non-specific T cell responses (Eruslanov, et al, 2014). Having identified these APC-like TANs, we hypothesized that this subset may be primarily responsible for stimulating T cell responses in these early stage lung tumors.
We first evaluated the functional activity of APC-like TANs to ensure that these activated cells were not “exhausted” or hypofunctional. TANs were thus isolated from tumors and stimulated with LPS. After LPS stimulation, HLA-DR+ hybrid TANs produced much more TNF-α and IL-12 when compared to HLA-DR− canonical TANs (Figure 1E). Furthermore, HLA-DR+ hybrid TANs phagocytosed E. coli bioparticles more efficiently than HLA-DR− canonical TANs (Figure S1M). These data demonstrate that APC-like hybrid TANs are fully functional and, in fact, perform major functions such as cytokine production and phagocytosis superior to canonical TANs.
To determine the effect of APC-like hybrid TANs on T cell responses, we isolated TAN subsets by flow cytometry cell sorting (Figure S1N and S1O). Each sorted TAN subset was co-cultured with autologous CFSE-labeled PBMCs that had been stimulated with plate-bound anti-CD3 antibodies (Abs) (Figure 1F). We observed that the proliferation of CD4 and CD8 cells after 4 days of stimulation was markedly augmented after exposure to HLA-DR+ hybrid TANs versus the HLA-DR− canonical TANs (Figure 1F).
We next determined whether APC-like hybrid TANs could trigger and sustain antigen-specific T-cell responses. Therefore, we co-cultured autologous T cells with TAN subsets that had been pulsed with peptides from commercially available peptide pools that contained mixtures of overlapping peptides. Each peptide pool corresponded to defined HLA class I or II restricted T-cell epitopes from Cytomegalovirus, Epstein-Barr virus, Influenza virus or Clostridium tetani designed to stimulate T cells with a broad array of HLA types. As shown in Figure 1G, the HLA-DR+ hybrid TANs efficiently triggered memory CD8 and CD4 T cell responses to HLA class I and II restricted T cell epitopes, respectively. Canonical TANs and PBNs induced only weak CD8 T cell responses and did not trigger CD4 T cell responses. Together, these data demonstrate that HLA-DR+ hybrid TANs are able to function as efficient APCs.
Long-lived immature neutrophils recapitulate the phenotype of APC-like hybrid TANs in the presence of tumor-derived factors
Given the potential anti-tumor activity of APC-like TANs due to their strong stimulatory effect on T cell responses, we investigated the mechanisms by which these cells could originate and expand in the human tumor microenvironment.
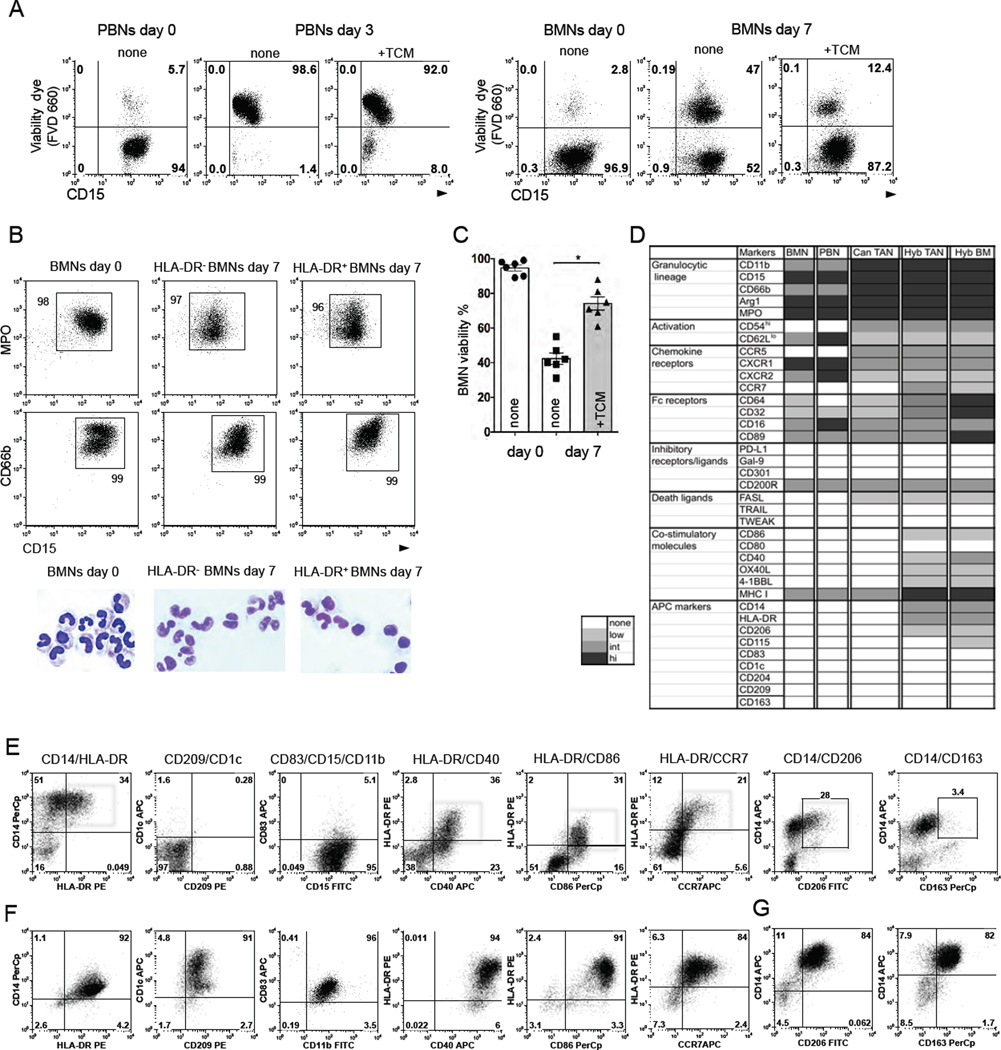
We collected tumor-conditioned media (TCM) from digested lung cancers that contained ≥ 15% of hybrid TANs among all TANs (termed hybrid-inducing TCM). We exposed purified PBNs to hybrid-inducing TCM and found that PBNs did not differentiate into the HLA-DR+CD14+ neutrophils (data not shown) and died within three days (Figure 2A). To determine whether more immature neutrophils with a higher degree of plasticity differentiate into APC-like hybrid neutrophils, we obtained a highly enriched population of immature human bone marrow neutrophils (BMNs). Isolated BMNs expressed the myeloid/granulocytic specific markers CD11b, CD66b, CD15, Arg-1, NE, and MPO and were mostly “band”-like immature neutrophils in appearance (Figure 2B and S2A). Of note, the purified BMNs did not express HLA-DR and CD14 and were not contaminated with macrophages and monocytes (Figure S2A). Unlike blood neutrophils, about 40% of these BMNs could survive in cell culture for up to one week and dramatically increased their viability in the presence of TCM (Figure 2A, 2C, and S2B). Thus, BMNs with a prolonged lifespan in vitro provided us with large quantities of cells that could be used to model the origins and differentiation process of neutrophils in the tumor microenvironment.
Figure 2. Tumor-derived factors differentiate long-lived immature BMNs into a hybrid subset with a partial phenotype of dendritic cells and macrophages.
(A) Fixable Viability Dye eFluor® 660 (FVD 660) was used to discriminate viable neutrophils in cell culture. Representative dot plots from 1 of 6 experiments are shown.
(B) Flow cytometric analysis of the expression of MPO, CD66b, and CD15 markers on freshly isolated BMNs (day 0) and BMNs cultured with (HLA-DR+ BMNs) or without hybrid-inducing TCM (HLA-DR− BMNs) for 7 days. Cytospins show the cytomorphology of these BMNs. Scale bar, 10 µm.
(C) Survival of BMNs in the cell culture in the presence or absence of TCM. Viability dye FVD 660 was used to discriminate viable BMNs in cell culture (mean ± SEM, n = 6, *p ≤ 0.01,Wilcoxon matched-pairs rank test).
(D) Heat map comparing the phenotypes of BMNs, PBNs, canonical TANs (Can TAN), hybrid TANs (Hyb TAN) and BM-derived hybrid neutrophils (Hyb BM).
(E–G) Flow cytometric analysis of the expression of indicated APC markers on BM-derived hybrid neutrophils (E) (red boxes), dendritic cells (F) and macrophages (G). Expression of APC markers was analyzed by flow cytometry on gated CD11b+CD15hiCD66b+ BMNs.
See also Figure S2.
After 7 days of incubation of BMNs with hybrid-inducing TCMs, we observed the formation of a cell subset that retained all its granulocytic markers (Figure2B and 2D) and acquired the same phenotype as the tumor-derived hybrid TANs (HLA-DR+ CD14+CD86+CD206+CCR7+) (Figure 2E). Similar to hybrid TANs, most of the BMNs also changed their nuclear shape from band-like to oval when they converted into hybrid BMNs (Figure 2B). A detailed phenotypic comparison of PBNs, BMNs, BM- and tumor-derived hybrid neutrophils is summarized in Figure 2D. The differentiation of BMNs into HLA-DR+CD14+ APC-like hybrid BMNs after exposure to hybrid-inducing TCM was donor-dependent and varied from 20% to 80% of the initial BMN population (Figure S2C). BMNs began to upregulate CD14 within 24 hours of co-culturing with hybrid-inducing TCM, while the expression of HLA-DR, CD86, CCR7, and CD206 markers did not appear until day 4 (Figure S2D). This suggests that these late APC markers are synthesized de novo.
Similar to hybrid TANs, differentiated hybrid BMNs acquired only the partial phenotype of DC and macrophages (Mph) (HLA-DR+CD14+CD86+CD206+) (Figure 2E–G). The hybrid subset of BMNs and TANs differed from BM-derived DC and Mph by absence of CD1c, CD83, CD163, CD209, markers and low expression of CD40, CD86, CD115 and CCR7 (Figure 2D–G). The level of the transcription factor IRF8, which regulates monocyte/DC lineage commitment (Yanez, et al, 2015), was not dramatically changed in hybrid BMNs and was much lower than the amount detected in BM-derived Mph and DC (Figure S2E).
We next asked whether the differentiated APC-like hybrid BMNs could proliferate in the presence of hybrid-inducing TCM and thus represent a self-maintained population of neutrophils. A BrdU incorporation assay revealed that within 24 hours of treatment with hybrid-inducing TCM, 10–15% of BMNs begin to synthesize DNA in vitro (Figure S2F). As the differentiation process progressed, a small proportion of HLA-DR− BMNs continued to incorporate BrdU up to day 8, whereas the differentiated HLA-DR+ neutrophils lost proliferative potential (Figure S2F).
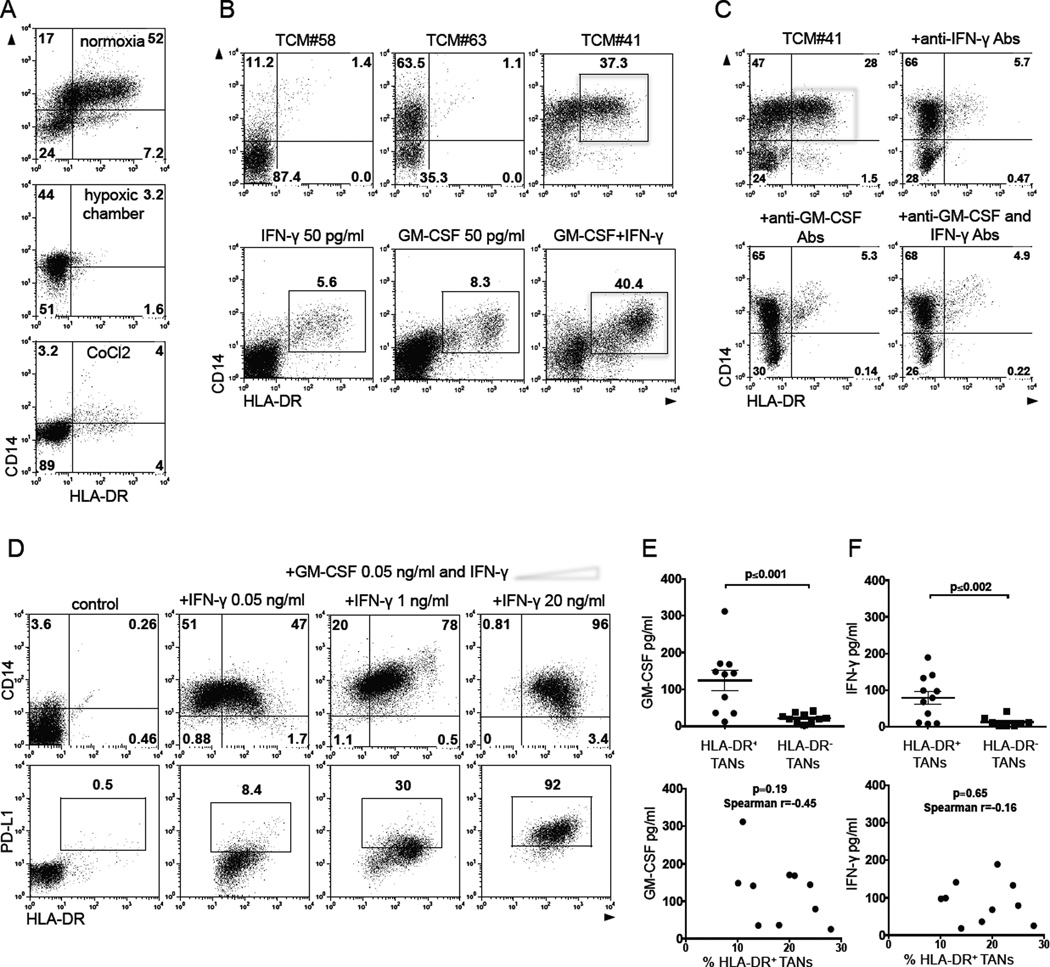
Given that the frequency of hybrid TANs was reduced in large tumors (Figure 1D), we hypothesized that hypoxia, which is strongly associated with the tumor progression, may negatively regulate the formation of hybrid neutrophils. Thus, BMNs were cultured in the presence of hybrid-inducing TCM for 6 days under normoxic (5% CO2 and 21% O2) and hypoxic (5% CO2 and 5% O2) cell culture conditions. We also cultured BMNs in the presence of hybrid-inducing TCM and cobalt chloride, an agent that induces hypoxia-inducible factor-1α, the main transcriptional factor activated in hypoxic conditions (Dai, et al, 2012). We found that the development of hybrid CD14+HLA-DR+ neutrophils was profoundly inhibited under these hypoxic and hypoxia-simulating conditions (Figure 3A).
Figure 3. Tumor-derived IFN-γ and GM-SCF synergistically differentiate immature neutrophils into a subset of APC-like hybrid neutrophils.
(A) Flow cytometric analysis of CD14 and HLA-DR expression on gated live CD11b+CD15hiCD66b+ BMNs cultured in the presence of hybrid-inducing TCM under normoxic and hypoxic cell culture conditions.
(B) Flow cytometric analysis of CD14 and HLA-DR expression on gated live CD11b+CD15hiCD66b+ BMNs cultured in the presence of different TCMs (top panel) or with IFN-γ and/or GM-CSF (low panel).
(C) The effect of IFN-γ and GM-CSF blocking Abs (5 µg/ml) in blunting the formation of HLA-DR+CD14+ hybrid neutrophils in vitro (red box).
(D) The expression of CD14 and HLA-DR markers on live CD11b+CD15hiCD66b+ BMNs (top panel) and PD-L1 on gated HLA-DR+CD14+ hybrid neutrophils (low panel) differentiated with GM-CSF (50 pg/ml) and the increasing doses of IFN-γ in vitro. (E and F) Levels of IFN-γ and GM-CSF in supernatants collected from the cell culture of small size tumor digests where APC-like hybrid TANs were or were not previously detected (set-off was >10% among all TANs) (line represents mean ± SEM, n = 10, Mann-Whitney test for unpaired data). Lower panels represent the correlation between the absolute levels of IFN-γ and GM-CSF in the TCM and the frequency of hybrid neutrophils in each tumor showed in top graphs. Non-parametric Spearman test was used to determine the degree of correlation.
Representative dot plots from 1 of 5 experiments are shown in (A–D).
See also Figure S3.
IFN-γ and GM-CSF are requisite factors in the tumor microenvironment for the development of hybrid neutrophils
To determine the particular tumor-specific factors that promote the formation of hybrid TANs, we screened primary TCMs collected from 20 consecutive lung cancer patients, and categorized the TCMs based on their ability to induce: (i) the full phenotype of hybrid cells (CD14+HLA-DR+ CD11b+CD66b+CD15hi) (Figure 3B, example-TCM#41), (ii) the partial phenotype of hybrid cells (CD14+HLA-DR− CD11b+ CD66b+CD15hi) (Figure 3B, example-TCM#63), or (iii) no phenotypic changes (Figure 3B, example-TCM#58). We evaluated each TCM using a multiplex cytokine/chemokine bead assay and found that those TCMs that induced CD14+HLA-DR+ hybrid cells had increased amounts of G-CSF, IL-6, IL-15, GM-CSF, IFN-γ, MIP-1α, TNF-α, MCP-1, and MIG compared to TCMs that did not induce hybrid cells. When we tested the ability of each of these factors (at the low concentrations found in the TCMs) to induce the CD14+HLA-DR+ hybrid phenotype in BMNs, we found that only IFN-γ and GM-CSF were able to induce the phenotype, although in a relatively low percentage of cells (Figure 3B and S3A). However, we observed that these factors worked in a synergistic manner: when combined at very low concentrations of 50 pg/ml of each factor, they induced expression of APC markers in a large proportion (>40%) of the cells in a donor-dependent fashion (Figure 3B and S3A). The addition of neutralizing monoclonal antibodies for either IFN-γ or GM-CSF completely inhibited the formation of BM hybrid cells in the presence of hybrid-inducing TCM (Figure 3C), thereby confirming that both IFN-γ and GM-CSF play a key role in the induction process. Interestingly, incubation of BMNs with a low dose of GM-CSF (50 pg/ml) and increasing concentrations of IFN-γ (from 50 pg/ml to 20 ng/ml) resulted in the expansion of CD14+HLA-DR+ BMNs from 40 to 96% among all BMNs (Figure 3D, top panel). However, the treatment of BMNs with IFN-γ at concentration more than 1 ng/ml gradually induced the expression of PD-L1 on the HLA-DR+ BMNs (Figure 3D, bottom panel) resulting in the formation of hybrid neutrophils with T cell suppressive activity (described in detail below).
We next analyzed the frequency of APC-like TANs in the tumor digests, and, in parallel, measured the concentration of IFN-γ and GM-CSF in the supernatants collected from digested autologous tumor cell cultures. Figure 3E demonstrates that the levels of IFN-γ and GM-CSF were statistically higher in tumors where there was a high proportion of hybrid TANs (>10% of all TANs). However, the generation of hybrid neutrophils in vivo is most likely more complex and not solely due to IFN-γ and GM-CSF levels, because the absolute levels of IFN-γ and GM-CSF in the TCM did not necessarily correlate with the frequency of hybrid neutrophils (>10% of all TANs) in each tumor as shown in Figure3E and 3F. Also, when we exposed BMNs from the same donor to different hybrid-inducing TCMs containing variable concentrations of IFN-γ and GM-CSF, we were also unable to observe a clear relationship between absolute levels of GM-CSF and IFN-γ and the degree of hybrid neutrophil formation (Figure S3B). These data suggest that there is a requisite threshold level of GM-CSF and IFN-γ and additional tumor-derived factors may contribute to the process of hybrid neutrophil differentiation.
CD11b+CD15hiCD10−CD16int/low progenitors give rise to APC-like hybrid neutrophils
The low frequency of APC-like hybrid TANs along with the high heterogeneity in the accumulation in cancer patients suggested that there might be precursor cells that could differentiate into this unique subset of neutrophils under specific favorable conditions in some tumors. Therefore, we sought to determine if the ability of long-lived immature BMNs to develop hybrid neutrophils is either shared by all immature subsets or limited to a specific differentiation stage.
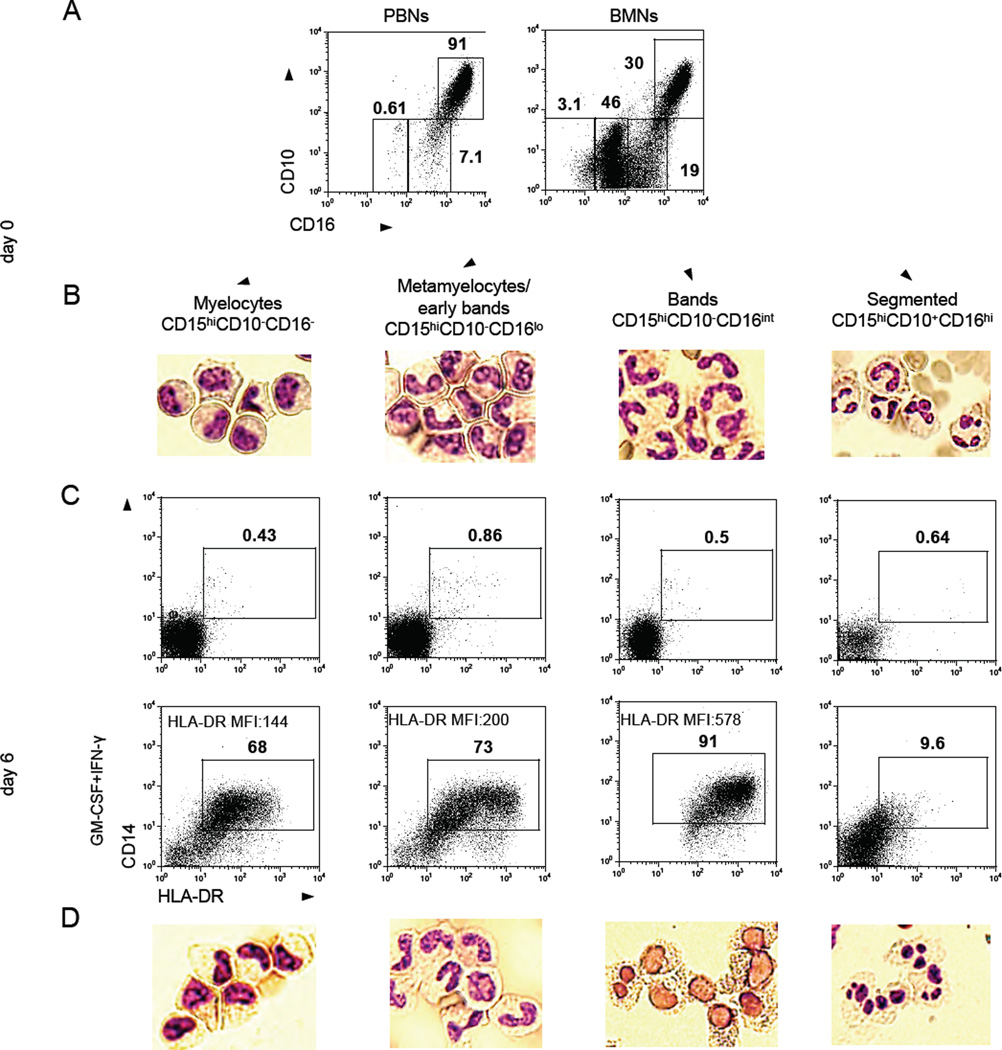
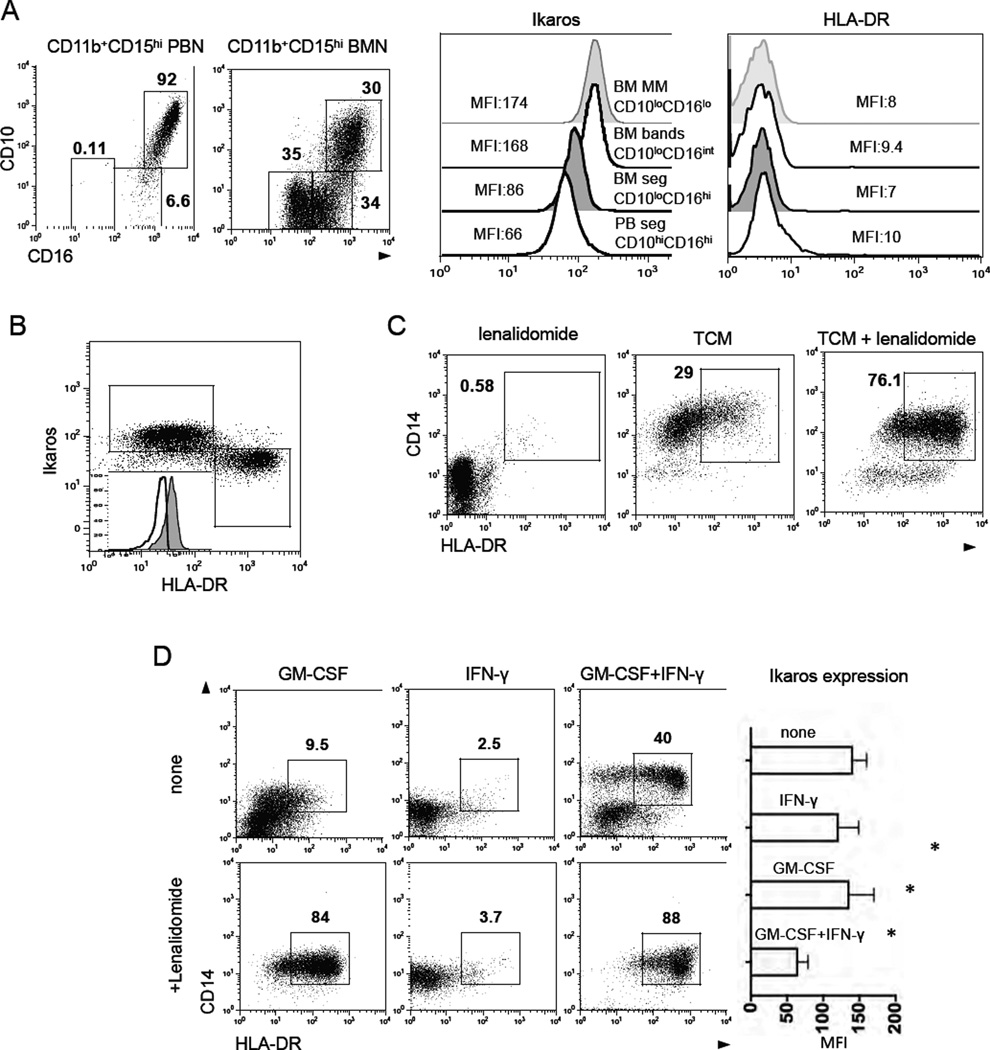
To address this question, we used the combined expression of the CD11b, CD15, CD10, CD49d and CD16 to distinguish the different maturational states of BMNs (Elghetany, 2002). As expected, we found that CD11b+CD15hi BMNs consist of a heterogeneous combination of mature CD16hiCD10+CD49d− cells, immature CD16intCD10−CD49d− band cells, and CD16low/−CD10−CD49d+ metamyelocytes/myelocytes (Figure 4A, expression of CD49d is not shown). Of note, all mature and immature BMNs express CD66b but at slightly different levels (Figure S4A). The detailed phenotype of neutrophils at different maturation stages is summarized in Figure S4B. We isolated BMNs at different stages of maturation by flow cytometry sorting based on these phenotypes. Cytomorphology confirmed that each population was associated with distinct maturation stages (Figure 4B). These sorted subsets of BMNs were cultured in the presence of low concentration of IFN-γ (50 pg/ml) and GM-CSF (50 pg/ml) for six days, and then the resulting CD11b+CD15hiCD66b+ neutrophil populations were analyzed for surface expression of CD14 and HLA-DR (Figure 4C). Our data revealed that CD14+HLA-DR+ hybrid neutrophils could be generated from all immature stages of neutrophils, except the terminally differentiated, mature, segmented neutrophils. However, the level of HLA-DR expression on these hybrid neutrophils was affected by the degree of immaturity of the neutrophils prior to exposure to IFN-γ and GM-CSF: the more mature CD15hiCD10−CD16int band cells gave rise to hybrid neutrophils with the highest expression of HLA-DR on the surface when compared to hybrid neutrophils differentiated from CD15hiCD10−CD16−/lo myelocytes and metamyelocytes/early bands (Figure 4C). Interestingly, the majority of the neutrophils differentiated from CD15hiCD10−CD16int band cells were able to change their nuclear contour from band-like to oval when compared to neutrophils differentiated from CD15hiCD10−CD16−/lo myelocytes, and metamyelocytes/early bands (Figure 4D).
Figure 4. APC-like hybrid neutrophils originate from CD11b+CD15hiCD66b+CD10−CD16lo/int progenitors.
(A) Flow cytometric analysis of the expression of CD10 and CD16 on gated live CD11b+CD15hiCD66b+ neutrophils isolated from peripheral blood (PBNs) and bone marrow (BMNs) of cancer patients.
(B) Cytospins were made from sorted BMNs at different stages of maturation and stained with the Hema3 Stat Pack Kit (Wright-Giemsa-like stain).
(C) Sorted BMNs at different stages of maturation were differentiated in the presence of IFN-γ (50 pg/ml) and GM-CSF (50 pg/ml) in vitro. Expression of HLA-DR and CD14 markers was analyzed by flow cytometry on CD11b+CD15hiCD66b+ BMNs.
(D) Cytomorphology of APC-like HLA-DR+ hybrid neutrophils differentiated from the sorted populations of BMNs at different stages of maturation.
Representative results from 1 of 4 experiments are shown in (A–D). Scale bar, 10 µm. See also Figure S4.
Importantly, the circulating blood CD16int/loCD10− immature neutrophils that could potentially traffic into tumors were also able to differentiate into hybrid neutrophils in the presence of hybrid-inducing TCM or IFN-γ and GM-CSF (Figure S4C).
Ikaros negatively regulates the development of APC-like hybrid neutrophils
Murine models have shown that the transcription factor Ikaros is involved in control of neutrophil differentiation by silencing specific pathways in common precursors that allow for macrophage-monocyte development (Dumortier, et al, 2003; Papathanasiou, et al, 2003). Given that hybrid neutrophils exhibit some characteristics of monocytic lineage cells, but can be differentiated from granulocyte-committed precursors, we hypothesized that the hybrid-inducing ability of TCM may be due to two possible synergistic effects on granulocyte progenitor cells: 1) premature downregulation of Ikaros, thus allowing some degree of monocyte differentiation to occur, and 2) the provision of the appropriate macrophage stimulating factors (i.e. GM-CSF) to activate the monocyte differentiation pathways.
We measured the level of Ikaros expression in BMNs at different stages of maturation and found that Ikaros was upregulated in all immature neutrophils (bands and metamyelocytes) with lower levels in mature BMNs and PBNs (Figure 5A). The analysis of BMNs treated with hybrid-inducing TCM revealed that Ikaros level was lower in HLA-DR+ hybrid BMNs compared to HLA-DR− canonical BMNs (Figure 5B). Thus hybrid-inducing TCM induced premature downregulation of Ikaros in HLA-DR+ hybrid BMNs. We next cultured BMNs with hybrid-inducing TCM in the presence or absence of the drug lenalidomide, which causes proteasomal degradation of the human Ikaros proteins (Kronke, et al, 2014). The addition of lenalidomide to TCM-treated BM neutrophils dramatically facilitated the development of HLA-DR+CD14+ hybrid neutrophils (Figure 5C). Together, these data suggest that Ikaros negatively regulates this process in the presence of tumor-derived factors.
Figure 5. Transcription factor Ikaros negatively regulates the differentiation of hybrid neutrophils.
(A) Flow cytometric analysis of the level of Ikaros and HLA-DR expression in PBNs and BMNs at different stages of maturation Results are shown as Mean Fluorescence Intensity (MFI).
(B) Flow cytometric analysis of the level of Ikaros expression in the HLA-DR+ hybrid and HLA-DR− canonical CD11b+CD15hiCD66b+ BMNs.
(C) Flow cytometric analysis of CD14 and HLA-DR expression on gated live CD11b+CD15hiCD66b+ BMNs cultured in the presence of lenalidomide (10 µM) and hybrid-inducing TCM (30% v/v) for 6 days.
(D) The effect of IFN-γ (50 pg/ml) and GM-CSF (50 pg/ml) on the formation of HLA-DR+ CD14+ hybrid neutrophils in the absence (top panel) or presence (bottom panel) of lenalidomide (10 µM) in vitro. The level of Ikaros expression (MFI) in BMNs treated with IFN-γ (50 pg/ml) and GM-CSF (50 pg/ml) for 5 days (mean ± SEM, n = 3, *p ≤ 0.01, Wilcoxon matched-pairs rank test).
Representative dot plots from 1 of 6 experiments are shown in (A–D).
We then measured the level of Ikaros in BMN progenitors incubated with or without low dose IFN-γ and/or GM-CSF at days 1, 3, and 5. Downregulation of Ikaros was only observed when both IFN-γ and GM-CSF were present for at least 5 days confirming their synergistic effect in this process (Figure 5D). Next, we downregulated Ikaros in BMNs by adding lenalidomide and cultured these cells with either IFN-γ or GM-CSF. The incubation of BMNs with the combination of GM-CSF and lenalidomide, but not IFN-γ and lenalidomide, resulted in efficient development of HLA-DR+CD14+ hybrid cells (80%–90% among all BMNs) (Figure 5D). These data confirm the hypothesis that both the premature downregulation of Ikaros in concert with the macrophage stimulatory factor GM-CSF are requisite for the development of hybrid neutrophils from neutrophil progenitors.
BM-derived hybrid neutrophils recapitulate the function of APC-like hybrid TANs
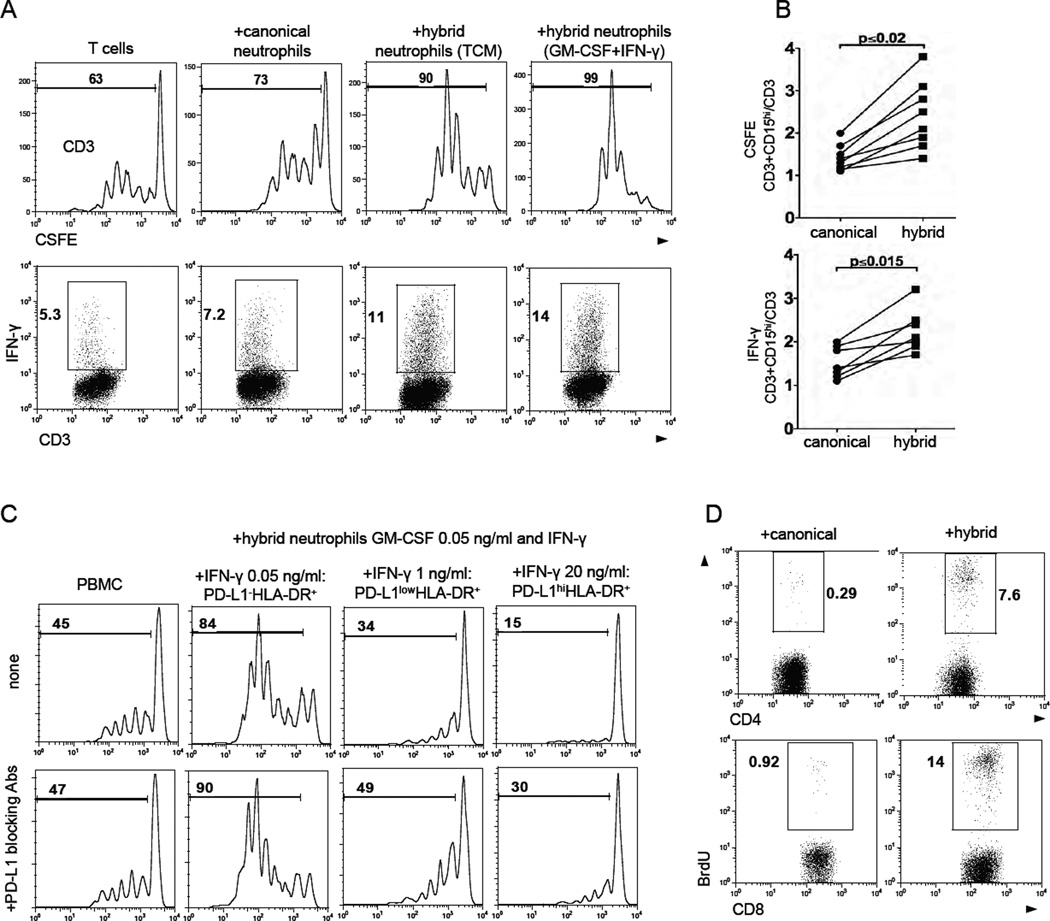
Next we investigated whether the BM-derived hybrid neutrophils also functionally resemble hybrid TANs in their ability to stimulate T cell responses. For this purpose, we differentiated immature BMNs into activated canonical and hybrid neutrophils (Figure S5A) and co-cultured them with autologous PBMCs stimulated with plate-bound anti-CD3 Abs. We found that both subsets of neutrophils augmented the expression of activation markers CD25 and CD69 on stimulated T cells to the same degree (Figure S5B). However, HLA-DR+ hybrid neutrophils exerted a significantly stronger stimulatory effect on T cell proliferation and IFN-γ production than the canonical neutrophils (Figure6A and 6B). The BM-derived hybrid neutrophils differentiated with low doses of IFN-γ and GM-CSF also recapitulated the T cell stimulatory activity of hybrid TANs (Figure 6A). However, as described above, the treatment of BMNs with a low dose of GM-CSF and IFN-γ at concentrations more than 1 ng/ml gradually induced the expression of PD-L1 on the HLA-DR+ BMNs (Figure 3D, low panel). When we co-cultured these PD-L1+ HLA-DR+ BMNs with autologous PBMC stimulated with anti-CD3 Abs, we found marked suppression of T cell proliferation (Figure 6C, top panel), which was substantially inhibited by PD-L1 blocking Abs (Figure 6C, low panel). Thus, high doses of IFN-γ can convert the T cell stimulatory HLA-DR+ BMNs into a suppressive population via upregulation of PD-L1. These results demonstrate some functional plasticity in the APC-like neutrophils.
Figure 6. APC-like hybrid neutrophils stimulate antigen non-specific T cell responses.
(A) The proliferation and IFN-γ production of anti-CD3 Abs stimulated autologous T cells in the presence of BM-derived canonical and hybrid neutrophils differentiated with hybrid-inducing TCM or IFN-γ (50 pg/ml) and GM-CSF (50 pg/ml).
(B) Summary results of autologous T cell proliferation (top graph) and IFN-γ production (bottom graph) in the presence of canonical and hybrid neutrophils. Data are presented as a ratio (CD3 cells+CD15hi)/(CD3) (n = 8,Wilcoxon matched-pairs rank test).
(C) The proliferation of CFSE-labeled autologous PBMCs cultured with hybrid BMNs with different level of PD-L1 expression in the presence (bottom panel) or absence PD-L1 blocking Abs (5 µg/ml) (top panel). PD-L1−/lo/hiHLA-DR+ hybrid neutrophils were differentiated with GM-CSF (50 pg/ml) and the increasing doses of IFN-γ.
(D) The proliferation of allogeneic T cells from healthy donors in the presence of APC-like hybrid neutrophils in mixed lymphocyte reaction (MLR).
Representative results from 1of 6 experiments are shown in (C) and (D).
See also Figure S5.
To determine whether the hybrid neutrophils are able to induce the proliferation of allogeneic T cells in a mixed-lymphocyte reaction (MLR), we co-cultured BM-derived hybrid and canonical neutrophils with allogeneic T cells purified from the peripheral blood of healthy donors. BrdU incorporation assay revealed that hybrid neutrophils, but not canonical neutrophils, were able to initiate the allogeneic proliferation of both CD4 and CD8 cells (Figure 6D). Additionally, similar to hybrid TANs, BM-derived hybrid neutrophils pulsed with a peptide pool of viral antigens were able to initiate the autologous memory CD8 and CD4 cell response more efficiently than canonical neutrophils (Figure S5C). These data demonstrate the functional resemblance between BM-derived and tumor-derived hybrid neutrophils, and they justify the use of this model to investigate additional functions of this rare subset of TANs.
APC-like hybrid neutrophils stimulate and augment anti-tumor effector T cell responses
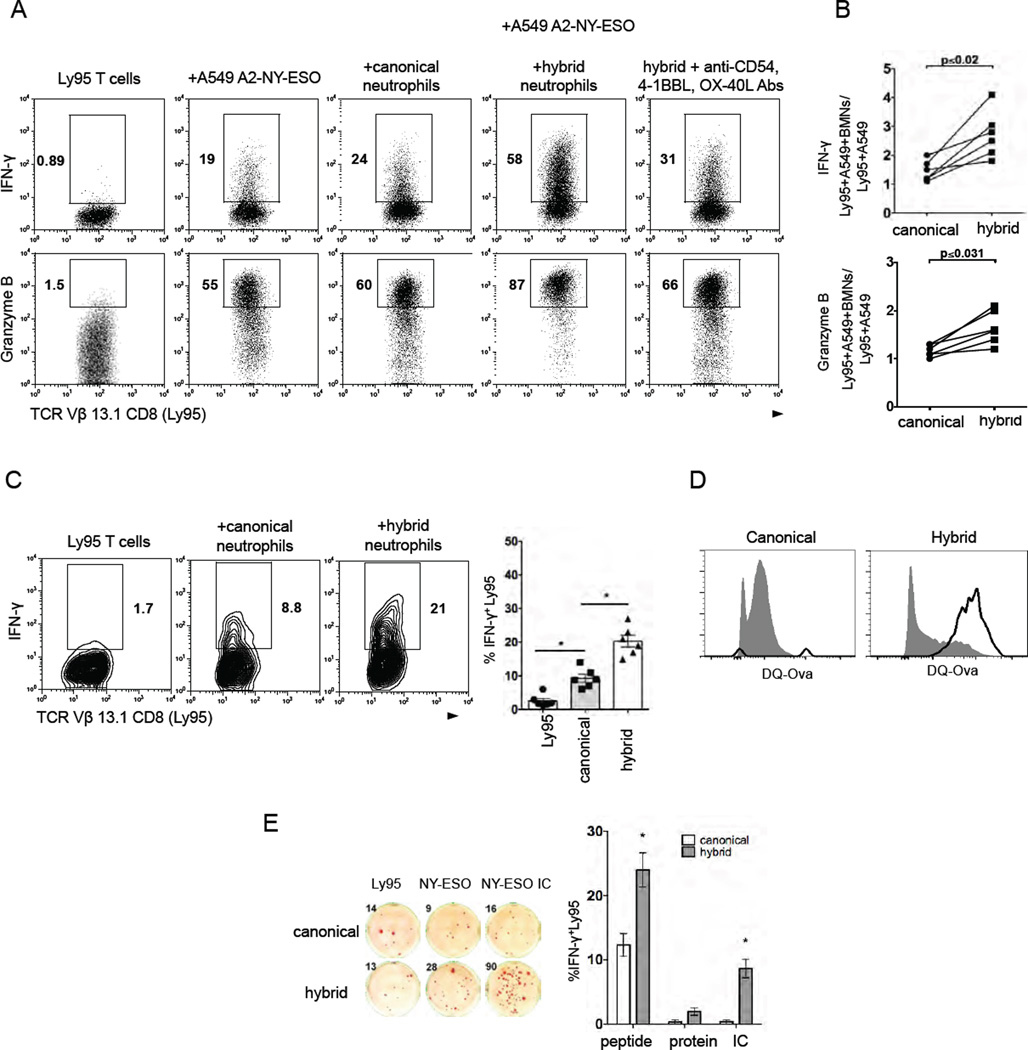
Next, we evaluated the effect of canonical and hybrid neutrophils on anti-tumor effector T cells using a newly developed in vitro model. We transduced human T cells with a high-affinity transgenic T cell receptor (TCR) called Ly95 that recognizes an HLA-A*0201-restricted peptide sequence in the human cancer testis antigen, NY-ESO-1. (Moon, et al, 2015). As target cells, we used a genetically modified A549 human lung adenocarcinoma cell line expressing the NY-ESO-1 protein in the context of HLA-A* 0201 (A549A2-NY-ESO-1 cells) (Moon, et al, 2015). Co-culturing of Ly95 T cells with A549A2-NY-ESO-1 tumor cells resulted in robust production of IFN-γ and Granzyme B in Ly95 T cells (Figure 7A). When we added BM-derived hybrid neutrophils into this system, the production of IFN-γ and Granzyme B in Ly95 T cells was markedly elevated (Figure7A and 7B) and increased compared to canonical neutrophils. Of note, the addition of the hybrid neutrophils into Ly95 T cells co-cultured with control A549 cells did not induce the production of these factors, indicating that hybrid neutrophil-mediated stimulation of Ly95 cells was NY-ESO-1-specific and not the result of allostimulation (data not shown).
Figure 7. APC-like hybrid neutrophils are able to trigger and stimulate NY-ESO specific effector T cell responses.
(A) NY-ESO-specific Ly95 cells (TCR Vβ13.1+CD8+) were stimulated with A549 tumor cell line expressing NY-ESO-1 in the context of HLA-A*02 (A2/NY-ESO-1 A549) in the presence of BM-derived canonical and hybrid neutrophils. Intracellular IFN-γ and Granzyme B production was measured by flow cytometry.
(B) Cumulative results showing the Ly95 cell stimulatory activity of canonical and hybrid neutrophils. Stimulatory activity was defined as a ratio (Ly95 cells+A549-NY-ESO+BMN)/(Ly95cells+A549-NY-ESO) (n=6,Wilcoxon matched-pairs rank test).
(C) HLA-A02+ canonical or hybrid neutrophils were pulsed with synthetic NY-ESO-1 peptide and co-cultured with Ly95 cells for 24 hr. Intracellular IFN-γ was assessed by flow cytometry, (mean ± SEM, n = 6, *p ≤ 0.01,Wilcoxon matched-pairs rank test).
(D) DQ-OVA uptake and processing by BM-derived canonical or hybrid neutrophils (open histograms). Cells incubated at 4°C served as controls (shaded histograms).
(E) Cross-presentation of NY-ESO-1 epitopes to Ly95 cells by HLA-A02+ canonical or hybrid neutrophils preloaded with NY-ESO-1 protein, NY-ESO-1 peptide or NY-ESO-immune complex (IC). IFN-γ ELISpot (mean ± SEM, n = 6, *p ≤ 0.01 canonical vs. hybrid, Wilcoxon matched-pairs rank test).
Using a transwell assay system, we found that HLA-DR+ hybrid BMNs induced the stimulation of IFN-γ production by Ly95 T cells only when the cells were in direct contact (Figure S5D). Since hybrid BMNs are characterized by increased expression of co-stimulatory molecules OX40L, 4-1BBL CD86, CD54 (Figure 2D, 2E and Figure S2D), we co-cultured Ly95 T cells activated with A549 A2-NY-ESO-1 tumor cells and with hybrid BMNs in the presence of blocking Abs to these up-regulated co-stimulatory molecules. Figure 7A shows a representative experiment where the stimulatory effect of hybrid neutrophils was partially abrogated in the presence of anti-CD54, 4-1BBL, OX-40L, and CD86 blocking Abs (Figure 7A).
Next we asked whether APC-like hybrid neutrophils could directly trigger NY-ESO-1 specific response of Ly95 cells. Given that Ly95 cells specifically recognize HLA-A*02-restricted peptide of NY-ESO-1, we pulsed HLA-A*02+ BM-derived canonical and hybrid neutrophils with the NY-ESO-1 (157-165, SLLMWITQV) peptide and then co-cultured the exposed neutrophils with Ly95 T cells for 24 hours. We found that hybrid HLA-A*02+HLA-DR+ hybrid neutrophils preloaded with the peptide triggered IFN-γ production in Ly95 T cells more effectively than peptide-loaded canonical neutrophils (Figure 7C). These data demonstrate that hybrid neutrophils can trigger and significantly augment the activation of antigen-specific effector T cells.
APC-like hybrid neutrophils are able to cross-present tumor antigens
The assays described above evaluated the ability of the TANs to present antigenic peptides, but they did not address the ability of hybrid neutrophils to process antigens, as the high affinity MHC class I binding peptides could bind directly to the surface MHC class I and do not require uptake and processing. We therefore performed experiments with DQ™ ovalbumin (DQ-OVA) and demonstrated that hybrid neutrophils were able to uptake and process ovalbumin to a higher degree than canonical neutrophils (Figure 7D). To evaluate whether hybrid neutrophils are able to present extracellular protein to effector CD8 cells (cross-presentation), we preloaded HLA-A*02 positive BM-derived hybrid and canonical neutrophils with full-length NY-ESO-1 protein and mixed them with Ly95 cells for 24 hr (Figure 7E). We found that these canonical and hybrid neutrophils were not sufficient to trigger Ly95 T cell response. Ly95 T cells mixed with control, unloaded neutrophils generated a low background of IFN-γ positive spots due to endogeneous activity of Ly95 cells due to prior CD3 stimulation required for expansion of these cells after TCR transduction (Figure 7E).
Next, we sought to employ the Fc receptors (FcγR) that are highly expressed on hybrid neutrophils (Figure 2D) and deliver the NY-ESO protein as an IgG-immune complex to trigger the more efficient FcγR-mediated Ag uptake and presentation. For this purpose, we pre-exposed the neutrophil subsets to NY-ESO-1 immune complexes (IC) formed by incubating the NY-ESO-1 protein with anti-NY-ESO-1 monoclonal Abs and mixed them with Ly95 cells for 24 hr. Under these conditions, we observed that hybrid neutrophils, but not canonical neutrophils, were able to cross-present NY-ESO epitopes and induce low-level, but NY-ESO specific production of IFN-γ by Ly95 T cells (Figure 7E). These data demonstrate that hybrid neutrophils have the ability to uptake and cross-presenting exogenous tumor antigens (Ags), at least under the conditions used here.
DISCUSSION
We identified a subset of TANs that exhibited the hybrid phenotypic and functional characteristics of neutrophils and antigen-presenting cells. These APC-like hybrid TANs were superior to canonical TANs in their ability to induce and stimulate anti-tumor T cell responses. We identified the progenitors of APC-like hybrid TANs, along with the tumor-derived and transcriptional factors responsible for the development of these cells. Thus, our findings demonstrate that the early stage lung tumor microenvironment can drive neutrophils to differentiate into a cell subset with enhanced anti-tumor capabilities.
It has been previously recognized that neutrophils can acquire characteristics of “professional APCs” under certain non-cancerous physiological and pathological conditions (Ashtekar and Saha, 2003). Specifically, neutrophils have been reported to upregulate MHC class II molecules and co-stimulatory molecules, such as CD80 and CD86, in response to inflammatory cytokines and during autoimmune pathology or inflammatory diseases (Iking-Konert, et al, 2005; Wagner, et al, 2006). There have also been several reports demonstrating the ability of neutrophils to present viral and bacterial antigens to T cells, provide accessory signals for T cell activation, and prime antigen-specific Th1 and Th17 cells (Abi Abdallah, et al, 2011; Radsak, et al, 2000; Tvinnereim, et al, 2004). Little is known, however, about factors that trigger the formation of these cells, the origin of these cells, and the precise functional capacities they possess, especially in humans. Moreover, the detailed phenotype and function of these APC-like neutrophils have not yet been reported and studied in cancer patients. The most similar cells to hybrid TANs appear to be those recently identified by Matsushima et al. and Geng et al. who described the ability of a subset of murine neutrophils to acquire markers of dendritic cells (CD11c, MHC II, CD80, and CD86) and termed these cells “neutrophil-DC hybrids” (Geng, et al, 2013; Matsushima, et al, 2013). However, the phenotype of these neutrophil-DC hybrids is somewhat different from APC-like hybrid TANs that exhibit a partial phenotype of DC (MHC class II, CD86, CCR7) along with a partial phenotype of monocyte/macrophages (CD14, CD206, CD64hi, CD32hi) and lacked other defining markers of dendritic cells and macrophages such as CD209, CD204, CD83, CD80, CD40, CD1c, CD163, and CCR6.
Over the last decade, there has been an increasing focus on the interactions between inflammatory myeloid cells and T cells within the tumor microenvironment. In this study, we identified a specialized neutrophil subpopulation enabling to augment both antigen non-specific and tumor-specific T cell responses by providing a co-stimulatory signals through the OX40L, 4-1BBL CD86, CD54 molecules. Our data are concordant with previous studies showing that activated granulocytes can provide accessory signals for T cell activation (Radsak, et al, 2000). We also found evidence for functional plasticity in the generation of APC-like neutrophils in terms of regulation of T cell response. Differentiation of immature neutrophils with low doses of IFN-γ resulted in highly immunostimulatory cells; however, high doses of IFN-γ resulted in formation of PD-L1hi hybrid neutrophils that profoundly suppressed T cell responses. This dichotomous in vitro effect of neutrophils on T cell response may suggest an important role of hybrid neutrophils in the regulation of the normal physiological inflammatory processes where T cell stimulation needs to be followed by suppression in order to resolve an inflammatory process.
One of the key functional findings of our study were that APC-like hybrid TANs acquired new functions compared to canonical TANs and were able to uptake, degrade and cross-present tumor antigens. Cross-presentation was triggered in hybrid neutrophils when NY-ESO protein was delivered as an IgG-immune complex, however this cross-presentation occurred at a relatively low level. This data suggest that the hybrid neutrophils can uptake and process Ags by means of the high-affinity IgG receptors FcγRI and FcγRII, which are highly expressed on hybrid neutrophils compared to canonical neutrophils. These hybrid neutrophils may “regurgitate” processed peptide outside of the cell (Potter and Harding, 2001) and thus facilitate the Ag uptake and processing by other professional APC. Our data are consistent with previous studies showing the critical role of FcγR in enhancing the cross-presentation of NY-ESO-1 by professional APC (Nagata, et al, 2002).
The ability of human neutrophils to express some APC markers in vitro after stimulation with inflammatory factors such as M-CSF, IFN-γ, GM-CSF, TNF-α, IL-3, IL-1β and IL-4 was discovered over decade ago (Gosselin, et al, 1993; Reinisch, et al, 1996; Oehler, et al, 1998). For example, the combination of GM-CSF, IL-4, and TNFα induced the expression of MHC II, CD40, CD86, CD1a, CD1b, and CD1c, whereas the combination of GM-CSF, TNFα and IFN-γ triggered MHC II, CD80, CD83, and CD86 expression by human neutrophils (Iking-Konert, et al, 2001; Oehler, et al, 1998). Moreover, it has been reported that neutrophils could even be reprogrammed and trans-differentiated into macrophages and DC after co-culture with multiple cytokines in vitro (Araki, et al, 2004; Iking-Konert, et al, 2005; Koffel, et al, 2014). It should be noted, however, that high (generally non-physiologic) concentrations of inflammatory factors (ranging from 1 to 100 ng/ml) were used in all these previous studies to induce the upregulation of APC markers, and differentiation could even be observed in mature end-stage neutrophils. It seems unlikely that neutrophils would encounter these artificial conditions in vivo and it has been unknown whether these factors are required and sufficient for the generation of APC-like neutrophils in vivo. We found that the tumors containing increased number of hybrid TANs secreted many of the inflammatory factors described above at very low concentrations when compared to tumors without hybrid TANs. When tested, however, only IFN-γ and GM-CSF at the low concentrations found in TCM were able to synergistically induce hybrid neutrophils. The development of APC-like neutrophils occurred over a five day time period and required the synthesis of APC receptors de novo in the long-lived CD10−CD16/low/int immature neutrophils, suggesting that hybrid neutrophils are a result of a differentiation process and not simply an acute activation event.
Mechanistically, we also found IFN-γ and GM-CSF exert their APC-promoting effects on immature neutrophils partially via the downregulation of the transcription factor Ikaros. Although Ikaros is well known as a key regulator of lymphocyte differentiation (O'Brien, et al, 2014), our data suggest that Ikaros has a much broader role in hematolymphoid differentiation. Our results are consistent with previous studies in murine cells demonstrating that Ikaros controls neutrophil differentiation by silencing of genes that are necessary for macrophage-monocyte development (Papathanasiou, et al, 2003).
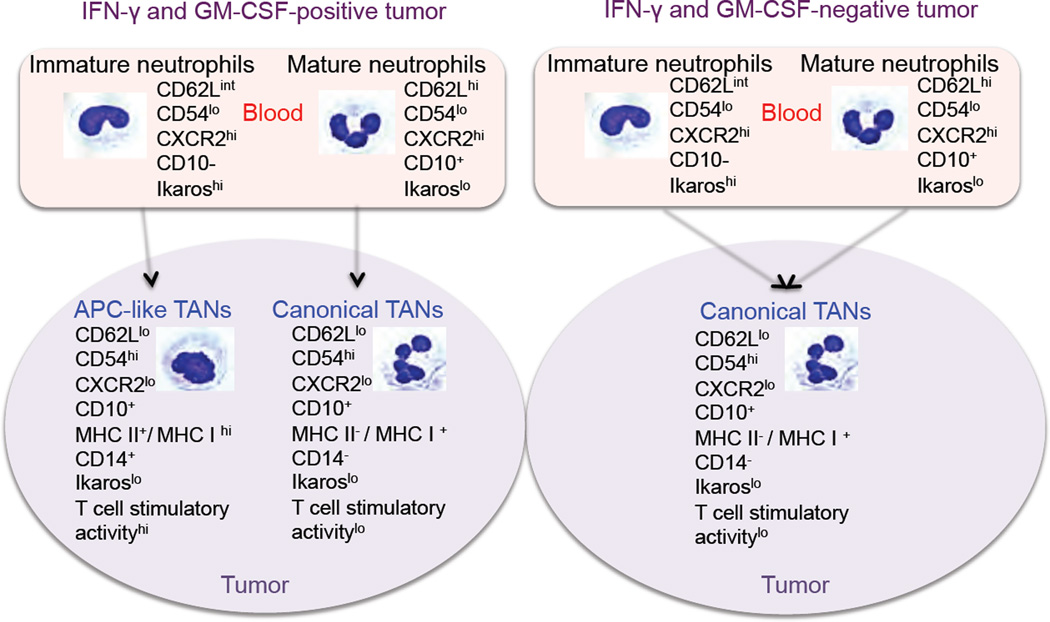
Although the in vitro conditions used in our experiments may not necessarily reflect what actually transpires in vivo, we propose a model of human neutrophil differentiation in different lung tumor microenvironments at early stages (Figure 8). According to this model, neutrophils adapt differently to the tumor microenvironment. Mature neutrophils recruited into tumors acquire the phenotype of highly activated neutrophils resulting in the formation of canonical TANs. However, the immature neutrophils, which circulate to varying degrees in cancer patients, are more plastic. When immature neutrophils are recruited into tumors which produce appropriate levels of IFN-γ and GM-CSF, they change their differentiation program and give rise to a subset of APC-like hybrid TANs exhibiting composite characteristics of neutrophils and APC. However, if the level of IFN-γ and GM-CSF is not significant to initiate the differentiation of immature neutrophils into the hybrid neutrophils, or other inhibitory factors or conditions are present in the tumor (i.e. hypoxia), the immature neutrophils use a default pathway of differentiation and become activated canonical TANs.
Figure 8. Schematic model of neutrophil differentiation in early-stage human lung cancer.
The concept of neutrophil diversity and plasticity has begun to emerge in a variety of inflammatory disorders and in murine tumor models, however, to date, there has been no convincing evidence showing that specialized neutrophil subpopulations with different functions exist in human cancers. Our data suggest that the tumor microenvironment in some early stage lung tumors can induce the formation of an immunostimulatory subset of neutrophils that have the ability to activate T cells and, in a general way, resemble the N1 neutrophil phenotype previously described in mice (Fridlender, et al, 2009). By gaining a better understanding of the cellular and molecular processes in early stage cancers that control tumor growth, it may be possible to enhance or mimic these factors and conditions in order to potentially boost natural or vaccine induced anti-tumor immunity.
EXPERIMENTAL PROCEDURES
Study Design
A total of 109 patients with Stage I–II lung cancer, who were scheduled for surgical resection, were consented for tissue collection of a portion of their tumor and/or blood for research purposes at the Hospital of the University of Pennsylvania and The Philadelphia Veterans Affairs Medical Center after obtaining consents that had been approved by their respective Institutional Review Boards. Detailed characteristics of the patients can be found in the Supplemental Experimental Procedures and Table S1.
Preparation of a single-cell suspension from lung tumor tissue
We used our optimized disaggregation method for fresh human lung tumors that preserves the phenotype and function of the immune cells as previously described in details (Quatromoni, et al, 2015).
Neutrophil isolation
TANs were isolated from tumor single cell suspensions using positive selection of CD15+ or CD66b+ cells with microbeads as previously described (Eruslanov, et al, 2014). TAN subsets were flow sorted based on the phenotype of canonical (CD11b+CD66b+CD15hiHLA-DR−) and hybrid (CD11b+CD66b+CD15hiHLA-DR+) TANs. PBNs and BMNs were isolated from EDTA anticoagulated peripheral blood and BM single cell suspension cell suspension respectively, using positive selection of CD15+ or CD66b+ cells with microbeads. See Supplemental Procedures for details
Generation of BM-derived macrophages, dendritic cells, hybrid and canonical neutrophils
To differentiate BMNs into the cells that resemble canonical and hybrid TANs, we culture the purified BMNs with a different types of TCM for 7 days. Alternatively, hybrid-neutrophils were differentiated from BMNs with low doses IFN-γ and GM-CSF. BM-derived macrophages and dendritic cells were generated by culturing CD15−CD11b+ BM cells with M-CSF or IL-4 and GM-CSF respectively.
Flow Cytometry
Flow cytometric analysis was performed according to standard protocols. For phenotypic and functional analysis PBNs, BMNs and TANs were gated on live CD11b+CD15hiCD66b+cells. For more details see the Supplementary Procedures.
Antigen non-specific T cell response
To induce antigen non-specific T cell response, PBMC or purified T cells were stimulated with plate-bound anti-human CD3 and/or anti-CD28 antibodies. For more details see the Supplementary Procedures.
Virus-specific memory T cell response
Peripheral blood autologous T cells were used as responders and co-cultured with neutrophil subsets that had been pulsed with mixture of viral peptides with a broad array of HLA types. The T cell response was quantified by IFN-γ ELISPOT.
NY-ESO-specific T cell response
To study the regulation of antigen-specific effector T cell responses by neutrophil subsets, we used Ly95 TCR transduced T cells recognizing the HLA-A2 restricted NY-ESO-1:157-165 peptide antigen (Moon, et al, 2015). Generation of NY-ESO specific Ly95 TCR T cells and details of the experimental procedures are provided in the Supplemental Procedures.
Allogeneic Mixed Lymphocyte Reaction
Purified allogeneic T cells from healthy donor PBMCs were used as responders and reacted with canonical or hybrid neutrophils (inducers) from lung cancer patients at a ratio of 1:1. Five days later, the proliferation of CD4 and CD8 T cells was measured using BrdU incorporation assay.
Statistics
Comparisons between two groups were assessed with a 2-tailed Student’s t-test for paired and unpaired data if data were normally distributed. Non-parametric Wilcoxon matched-pairs test and Mann-Whitney unpaired test were used when the populations were not normally distributed. Likewise, multiple groups were analyzed by one-way analysis of variance (ANOVA) with corresponding Tukey's multiple comparison test if normally distributed, or by the Kruskal–Wallis with Dunn's multiple comparison test if not normally distributed. All statistical analyses were performed with GraphPad Prism 6. A p value <0.05 was considered statistically significant.
Supplementary Material
SIGNIFICANCE.
Tumor-associated neutrophils (TANs) represent a significant fraction of the inflammatory cells in the tumor microenvironment, however the contribution of these cells in inhibiting or promoting tumor expansion in humans remains unclear. Although the concept of neutrophil phenotypic and functional diversity has emerged in murine tumor models, it is unknown whether TAN subsets with different functions exist in humans. Here, we provide evidence that early stage lung tumors can induce the formation of a unique subset of TANs that can trigger and support anti-tumor T cell responses. These findings demonstrate the potential anti-tumor role of TANs in early stage cancer and may provide opportunities to boost the anti-tumor efficacy of cytotoxic T lymphocytes.
HIGHLIGHTS.
Lung tumors accumulate a subset of TANs with a granulocyte and APC hybrid phenotype
APC-like hybrid neutrophils are able to stimulate the anti-tumor T cell responses
IFN-γ and GM-CSF are requisite factors for the development of hybrid neutrophils
Ikaros negatively regulates the development of hybrid neutrophils from progenitors
In brief.
Singhal et al. identify a subset of tumor-associated neutrophils (TANs) that can cross present tumor antigens and activate anti-tumor T cells in stage I/II human lung cancer. The induction of these hybrid TANs from progenitors requires GM-CSF and IFNγ and reduction of Ikaros.
Acknowledgments
This work was supported by the Department of Defense (LC140199 to E.E.), NIH (RO1 CA187392-01A1 to E.E.), NIH (R01 CA193556 to S. S), NIH (K12CA076931 to A.G) and the Lung Cancer Translation Center of Excellence of the Abramson Cancer Center at the University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
No conflict of interest exists.
AUTHOR CONTRIBUTIONS
S.S., S.A. and E.E. conceived of the study. S.S. P.B., A.R., T.S., S.B., E.M., J.Q., C.D., M.F., and E.E. performed experiments and data analysis. Reagents were contributed by E.M. S.A., S.S., A.G., W.H., and J.C-G., Manuscript preparation was performed by S.S. and E.E. Manuscript revisions were performed by S.A., W.H., J.C-G. All authors read and approved the final manuscript.
REFRENCES
- Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 2011;5:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki H, Katayama N, Yamashita Y, Mano H, Fujieda A, Usui E, Mitani H, Ohishi K, Nishii K, Masuya M, et al. Reprogramming of human postmitotic neutrophils into macrophages by growth factors. Blood. 2004;8:2973–2980. doi: 10.1182/blood-2003-08-2742. [DOI] [PubMed] [Google Scholar]
- Ashtekar AR, Saha B. Poly's plea: membership to the club of APCs. Trends Immunol. 2003;9:485–490. doi: 10.1016/s1471-4906(03)00235-7. [DOI] [PubMed] [Google Scholar]
- Brandau S. The dichotomy of neutrophil granulocytes in cancer. Semin. Cancer Biol. 2013;3:139–140. doi: 10.1016/j.semcancer.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;1:130–137. doi: 10.1016/j.lungcan.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Dai ZJ, Gao J, Ma XB, Yan K, Liu XX, Kang HF, Ji ZZ, Guan HT, Wang XJ. Up-regulation of hypoxia inducible factor-1alpha by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells. J. Exp. Clin. Cancer Res. 2012 doi: 10.1186/1756-9966-31-28. 28-9966-31-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallegri F, Ottonello L. Neutrophil--mediated cytotoxicity against tumour cells: state of art. Arch. Immunol. Ther. Exp. (Warsz) 1992;1:39–42. [PubMed] [Google Scholar]
- Dumortier A, Kirstetter P, Kastner P, Chan S. Ikaros regulates neutrophil differentiation. Blood. 2003;6:2219–2226. doi: 10.1182/blood-2002-05-1336. [DOI] [PubMed] [Google Scholar]
- Elghetany MT. Surface antigen changes during normal neutrophilic development: a critical review. Blood Cells Mol. Dis. 2002;2:260–274. doi: 10.1006/bcmd.2002.0513. [DOI] [PubMed] [Google Scholar]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Invest. 2014;12:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer. Cell. 2009;3:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Page K, Blumenthal RM, Ward NL, Miyazaki T, Takashima A. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;10:1690–1700. doi: 10.1182/blood-2012-07-445197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J. Immunol. 1993;3:1482–1490. [PubMed] [Google Scholar]
- Granot Z, Fridlender ZG. Plasticity beyond Cancer Cells and the "Immunosuppressive Switch". Cancer Res. 2015;21:4441–4445. doi: 10.1158/0008-5472.CAN-15-1502. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;6:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell. Cycle. 2010;9:1732–1737. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- Iking-Konert C, Cseko C, Wagner C, Stegmaier S, Andrassy K, Hansch GM. Transdifferentiation of polymorphonuclear neutrophils: acquisition of CD83 and other functional characteristics of dendritic cells. J. Mol. Med. (Berl) 2001;8:464–474. doi: 10.1007/s001090100237. [DOI] [PubMed] [Google Scholar]
- Iking-Konert C, Ostendorf B, Sander O, Jost M, Wagner C, Joosten L, Schneider M, Hansch GM. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Ann. Rheum. Dis. 2005;10:1436–1442. doi: 10.1136/ard.2004.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coelle C, Mouroux J, Hofman P. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;6:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- Koffel R, Meshcheryakova A, Warszawska J, Hennig A, Wagner K, Jorgl A, Gubi D, Moser D, Hladik A, Hoffmann U, et al. Monocytic cell differentiation from band-stage neutrophils under inflammatory conditions via MKK6 activation. Blood. 2014;17:2713–2724. doi: 10.1182/blood-2014-07-588178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronke J, Hurst SN, Ebert BL. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology. 2014;7:e941742. doi: 10.4161/21624011.2014.941742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;8:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N, Kotol PF, Chojnacki BJ, Miyazaki T, Gallo RL, Takashima A. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;10:1677–1689. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EK, Ranganathan R, Eruslanov E, Kim S, Newick K, O'Brien S, Lo A, Liu X, Zhao Y, Albelda SM. Blockade of Programmed Death 1 Augments the Ability of Human T cells Engineered to Target NY-ESO-1 to Control Tumor Growth after Adoptive Transfer. Clin. Cancer Res. 2016;22:436–447. doi: 10.1158/1078-0432.CCR-15-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Ono S, Matsuo M, Gnjatic S, Valmori D, Ritter G, Garrett W, Old LJ, Mellman I. Differential presentation of a soluble exogenous tumor antigen, NY-ESO-1, by distinct human dendritic cell populations. Proc. Natl. Acad. Sci. U. S. A. 2002;16:10629–10634. doi: 10.1073/pnas.112331099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S, Thomas RM, Wertheim GB, Zhang F, Shen H, Wells AD. Ikaros imposes a barrier to CD8+ T cell differentiation by restricting autocrine IL-2 production. J. Immunol. 2014;11:5118–5129. doi: 10.4049/jimmunol.1301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler L, Majdic O, Pickl WF, Stockl J, Riedl E, Drach J, Rappersberger K, Geissler K, Knapp W. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J. Exp. Med. 1998;7:1019–1028. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanasiou P, Perkins AC, Cobb BS, Ferrini R, Sridharan R, Hoyne GF, Nelms KA, Smale ST, Goodnow CC. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity. 2003;1:131–144. doi: 10.1016/s1074-7613(03)00168-7. [DOI] [PubMed] [Google Scholar]
- Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. Hematol. 2012;3:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J. Immunol. 2001;5:2538–2546. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- Quatromoni JG, Singhal S, Bhojnagarwala P, Hancock WW, Albelda SM, Eruslanov E. An optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells. J. Leukoc. Biol. 2015;1:201–209. doi: 10.1189/jlb.5TA0814-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hansch GM. Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;4:521–530. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch W, Tillinger W, Lichtenberger C, Gangl A, Willheim M, Scheiner O, Steger G. In vivo induction of HLA-DR on human neutrophils in patients treated with interferon-gamma. Blood. 1996;7:3068. [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;6024:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J. Immunol. 2004;3:1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin. Cancer Biol. 2013;3:190–199. doi: 10.1016/j.semcancer.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Wagner C, Iking-Konert C, Hug F, Stegmaier S, Heppert V, Wentzensen A, Hansch GM. Cellular inflammatory response to persistent localized Staphylococcus aureus infection: phenotypical and functional characterization of polymorphonuclear neutrophils (PMN) Clin. Exp. Immunol. 2006;1:70–77. doi: 10.1111/j.1365-2249.2005.02963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez A, Ng MY, Hassanzadeh-Kiabi N, Goodridge HS. IRF8 acts in lineage-committed rather than oligopotent progenitors to control neutrophil vs monocyte production. Blood. 2015;9:1452–1459. doi: 10.1182/blood-2014-09-600833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.