Abstract
Excess weight and physical inactivity are modifiable risk factors for breast cancer. Behavioral intervention is particularly important among women with an elevated risk profile. This trial tested an intervention that trained women to use a self-monitoring website to increase activity and lose weight. Women with BMI≥27.5 kg/m2 at elevated breast cancer risk were randomized to the intervention (N=71) or usual care (N=34). The intervention group received telephone-based coaching and used web-based self-monitoring tools. At 6 months, significant weight loss was observed in the intervention group (4.7% loss from starting weight; SD=4.7%) relative to usual care (0.4% gain; SD=3.0%) (p<.0001). By 12 months, the intervention group had lost 3.7% of weight (SD=5.4%), compared to 1.3% (SD=4.2) for usual care (p=.003). At 12 months, accelerometer-measured moderate-to-vigorous physical activity increased by 12 min/day (SD=24) compared to no change in usual care (p=.04. In summary, this web- and phone-based approach produced modest but significant improvements in weight and physical activity for women at elevated breast cancer risk.
Keywords: Weight loss, breast cancer, internet, technology, self-monitoring, physical activity
With 232,000 incident cases per year, breast cancer is the most common cancer among US women (American Cancer Society, 2013). Excess weight is an established risk factor for post-menopausal breast cancer, with a body mass index (BMI) ≥28 kg/m2 associated with 34% higher risk among women who have never taken hormone replacement therapy (Heo et al., 2015). Similarly, 10–20% of breast cancer cases are attributable to insufficient physical activity (Brenner, 2014; Lee et al., 2012). Interventions for weight management and physical activity promotion therefore present a valuable opportunity to reduce breast cancer risk. This may be particularly true for women who have already been identified as being at elevated risk due to family history, reproductive factors, and or a previous biopsy. An observational study among women at elevated risk (those for whom a first-degree relative had recently been diagnosed with breast cancer) found that 41% of women improved one or more health behaviors (diet, physical activity, alcohol intake and/or smoking) and that those with a greater perception of risk were more likely to make these changes (Lemon et al., 2004). While many weight loss and lifestyle trials have been conducted in breast cancer survivors (Demark-Wahnefried et al., 2012; Greenlee et al., 2013; Patterson et al., 2015; Rock et al., 2015) and in the general population of healthy women (Heshka et al., 2003; Jakicic et al., 2003; Silva et al., 2010), little work has been conducted specifically with women at elevated breast cancer risk.
Although caloric restriction is arguably the single most important component of successful weight loss (Johns et al., 2014), promotion of increased moderate-intensity physical activity has been shown to contribute to weight loss among post-menopausal women (Befort et al., 2011; Friedenreich et al., 2011; Jakicic et al., 2003) and plays a key role in weight loss maintenance (Dombrowski et al., 2014; Swift et al., 2014). The theory-based behavior change technique most strongly associated with successful dietary and physical activity change is the use of self-monitoring, particularly when augmented with one or more additional self-regulatory techniques (such as goal-setting, frequent behavioral feedback, or review of goals) (Abraham & Michie, 2008; Michie et al., 2009; Michie et al., 2011). The use of self-regulatory techniques for physical activity has also been shown to improve maintenance of weight loss, as compared to a standard behavioral intervention (Nicklas et al., 2014). Although detailed logging of food intake and physical activity can be burdensome, a variety of consumer-based technologies (e.g., websites, apps) are now available which can markedly reduce the time and effort required. By entering information online, users can receive automatically calculated estimates of caloric intake and energy expenditure to assist them in their weight loss efforts. A recent systematic review and meta-analysis reported that integrating eHealth components with traditional intervention approaches can enhance intervention efficacy (Hutchesson et al., 2015). One such approach is the use of telephone-based coaching, which has been shown to be an effective method of supporting dietary change (Pierce et al., 2004; Tucker et al., 2008) to support and enhance the impact of web-based weight loss tools.
The randomized controlled trial reported here examined the effects of a phone- and web-based intervention for weight loss and physical activity among women at elevated risk for future breast cancer. The intervention strategy was first tested in a 12-week pilot trial among 51 women, and was found to be associated with significant improvements in weight and accelerometer-measured physical activity as compared to a usual care group (Cadmus-Bertram et al., 2013). The current study was then conducted to test the intervention in a larger sample across a longer time period of one year. The goal of the study was to determine the effect of the intervention on weight change (primary outcome) and objectively-measured physical activity (secondary outcome).
Methods
This randomized controlled trial tested a phone- and web-based lifestyle intervention, versus a usual care group, for weight and physical activity among middle-aged and older women at elevated risk for developing breast cancer. Data were collected from 2011–2014 and all procedures were approved by the University of California, San Diego (UCSD) Human Research Protections Program. Sample size was determined based on pilot research (Cadmus-Bertram et al., 2013).
Participants
Participants were 105 women aged 40–75 years with BMI ≥27.5 kg/m2. Women were eligible if they had a previous history of ductal or lobular carcinoma in situ (DCIS/LCIS) or a Gail model score of ≥1.7. The Gail model is a standard risk assessment tool that incorporates age, family history, age at menarche, age at first live birth, and previous biopsy to estimate the 5-year risk of incident breast cancer. A score of 1.7 refers to a 1.7% risk of developing breast cancer within the next 5 years and is used as a standard cutoff for elevated risk (Gail et al., 1989; Gail et al., 2007). Women were excluded if they were performing >150 min/week of moderate-to-vigorous intensity physical activity (MVPA), were currently enrolled in another dietary or physical activity trial, did not have access to high speed internet, or were not fluent in English. Participants were excluded if they reported that they had any medical or psychological condition or other problem that would interfere with participation (e.g., advanced osteoarthritis, cardiac problems, severe depression).
Participants were recruited through the mammography clinic at the Moores Cancer Center at the University of California, San Diego. Identification of potential participants was facilitated by the Athena UCSD Breast Imaging Registry, a project of the University of California Athena Breast Health Network. Those patients from the registry who met the criteria for age, BMI, and breast cancer risk (or history of DCIS/LCIS) were contacted by telephone to solicit their interest and eligibility. Those who were both eligible and interested were then scheduled for a baseline visit at the Moores UCSD Cancer Center.
Baseline Visit
At the baseline visit, the study coordinator obtained written informed consent from the participant, measured her height and weight, and provided materials for the completion of the web-based Baseline Questionnaire. The participant was taught how to wear the ActiGraph accelerometer and provided with a return mailer.
Randomization
A computerized randomization scheme was used to randomly assign each participant with 2:1 probability to either the phone- and web-based intervention group or to a usual care group. Randomization was blocked on age and BMI. Randomization was conducted by the UCSD Biostatistics and Bioinformatics Shared Resource and codes were provided to the study coordinator. Participants were informed of group assignment via telephone and sent intervention materials via postal mail.
Intervention Group
Participants assigned to the intervention group received a 12-month weight loss intervention that focused on the development and practice of self-monitoring and self-regulatory skills. Participants were asked to perform 150 minutes/week of moderate-to-vigorous intensity physical activity and to restrict calories at a level sufficient to induce initial weight loss of 1–2 lbs/week (approximate deficit of 500 kcal/day), although it was understood that not all participants would attain this rate of weight loss. Dietary goals emphasized increased intake of fruits, vegetables, and fiber, and decreased intake of unhealthy fats and refined grains. The first 3–6 months of the intervention were focused on weight loss (goal: 10% loss from baseline weight), with the remaining 6–9 months focused on maintenance.
The intervention was delivered via 18, 30-minute phone-based health coaching sessions delivered by trained lay coaches following a protocol previously shown to be effective in achieving major dietary change (Madlensky et al., 2008; Newman et al., 2005; Pierce et al., 2007), physical activity promotion (Cadmus-Bertram et al., 2013), and short-term weight loss (Cadmus-Bertram et al., 2013). Each participant was matched with a single coach to provide continuity throughout the intervention. The schedule of these sessions was designed to provide maximum support and training during the early phase of behavior change, followed by a gradual transition to greater self-reliance. The initial call was scheduled in Week 1; participants then received twice weekly calls in Weeks 2–3, weekly calls in Weeks 5–8, biweekly call in Weeks 10–12, monthly calls in Weeks 16–24, and quarterly calls in weeks 28–52. The intervention was based on Social Cognitive Theory (Bandura, 1986) and followed a phased, step-wise approach focused on (a) helping the person to establish a series of short-term goals and (b) assisting the participant to evaluate performance in a manner that would maintain or improve self-efficacy. Each call included a specific behavioral focus. Example topics include meal planning, increasing vegetable intake, reducing refined carbohydrates, dining out, increasing daily steps, increasing moderate-intensity physical activity, managing stress, and proper sleep. Each participant received a manual that included detailed information on these topics.
Participants were taught to self-monitor their diet and physical activity using the free website Sparkpeople.com, which offers online food and physical activity logs. To speed the process of logging dietary intake, Sparkpeople’s dietary tracker offers a large database of nutritional data for various food items and the ability to save frequently consumed meals or combinations of foods. As foods are entered, the user is provided with daily totals for calories and macronutrients. The website also has forums where users can obtain social support and motivation as well as share recipes and tips. Participants were taught how to set up an account, use basic features (e.g., entering daily steps), log food intake, and interpret caloric and macronutrient feedback. This feedback could be viewed simultaneously by the counselor and participant to facilitate discussion during coaching calls. The coaching sessions allowed for sufficient flexibility to provide website training at a pace appropriate to the individual participant. A basic step-counting pedometer was provided as an intervention tool to assist with physical activity monitoring.
Usual Care Group
Participants assigned to the usual care group received a copy of the US Dietary Guidelines for Americans. To maintain engagement with the study and reduce loss to follow-up, they also received a brief 15-minute telephone call every three months. These calls did not include in-depth coaching or recommendations for diet or physical activity change. The coach would simply re-establish rapport with the participant, ask whether the participant had reviewed the materials and which sections had been most helpful. If a participant mentioned a personal weight loss goal, this was acknowledged but not followed with specific recommendations or coaching.
Measures
Anthropometrics
A standard stadiometer was used to measure height to the nearest 0.1 cm. The measurement was taken twice and the mean of both readings was used. Weight was measured on a digital scale to the nearest 0.1 kg. Height was measured at the baseline visit only; weight was taken at the baseline, 6-month, and 12-month study visits.
Questionnaires
Prior to randomization, participants completed an online questionnaire, which assessed demographics, technology use, and medical history.
Physical activity assessment
Prior to randomization and again at 12 months, each participant wore an ActiGraph GT3X+ accelerometer (ActiGraph, Pensecola, FL) on the hip during their waking hours for 7 consecutive days. At both time points, data were promptly downloaded after the 7-day wear period and screened for completeness and irregularities. Participants were asked to re-wear the accelerometer if it was not worn for at least 10 hours per day for 5 days. Ninety consecutive zeroes were designated as non-wear time and standard calibration thresholds were used to aggregate data into minutes spent in light, moderate, and vigorous activity using the Freedson cutpoints (Freedson et al., 1998). The accelerometer also provided data for the amount of time spent in bouts (≥10 continuous minutes) of moderate-to-vigorous intensity physical activity. It was worn at baseline and 12 months but was not used at 6 months in order to reduce participant burden.
Statistical Analysis
All statistical analyses were conducted using SAS version 9.4 (SAS; Cary, NC). Baseline characteristics between the two groups were assessed using t-tests and chi-squared tests. Changes in anthropometric measures between groups were analyzed using a longitudinal model controlling for baseline level of the anthropometric variable. Changes in physical activity measures between groups were analyzed using a longitudinal model using the subject’s day level activity and controlling for ActiGraph wear time. Three physical activity variables were used: minutes/day of total physical activity (includes light-, moderate-, and vigorous-intensity activity), minutes/day of total MVPA (includes moderate- and vigorous-intensity activity), and minutes/day of MVPA performed in bouts of 10 minutes or more, which corresponds to the type of activity prescribed by national physical activity guidelines (Office of Disease Prevention and Health Promotion, 2008). All longitudinal analyses were carried out with a subject-level random intercept using an unstructured covariance model, as determined by model AIC comparisons. All outcome variables were modeled on a linear, continuous scale with one exception. Physical activity in bouts was analyzed using a negative binomial regression model to account for the heavy right skew and over-dispersed nature of the variable. While analysis of physical activity was carried out using day level data (minutes per day), descriptive statistics (mean, SD, and range) are presented in average minutes per day, calculated by taking the mean value of each subject’s day level activity.
In the analysis of physical activity and percent weight loss, we used total accelerometer-measured activity (or total MVPA) at the 12-month follow-up as our outcome variable and percent weight loss as the independent variable due to the repeated nature of the day-level activity measurements. This model was used to assess the association between the two variables and model estimates were obtained for activity at varying levels of weight loss. Percent weight loss was calculated as the percent change in weight at 12 months compared to the baseline and both activity models controlled for baseline activity (total activity or total MVPA).
Demographic characteristics were not included as covariates because baseline analysis revealed that the randomization had been successful in eliminating demographic differences by treatment group. This choice was confirmed by sensitivity analysis. Missing data were accounted for in the longitudinal random effects models by using a likelihood-based estimation method, which uses all available data and does not ignore subjects with missing data.
Results
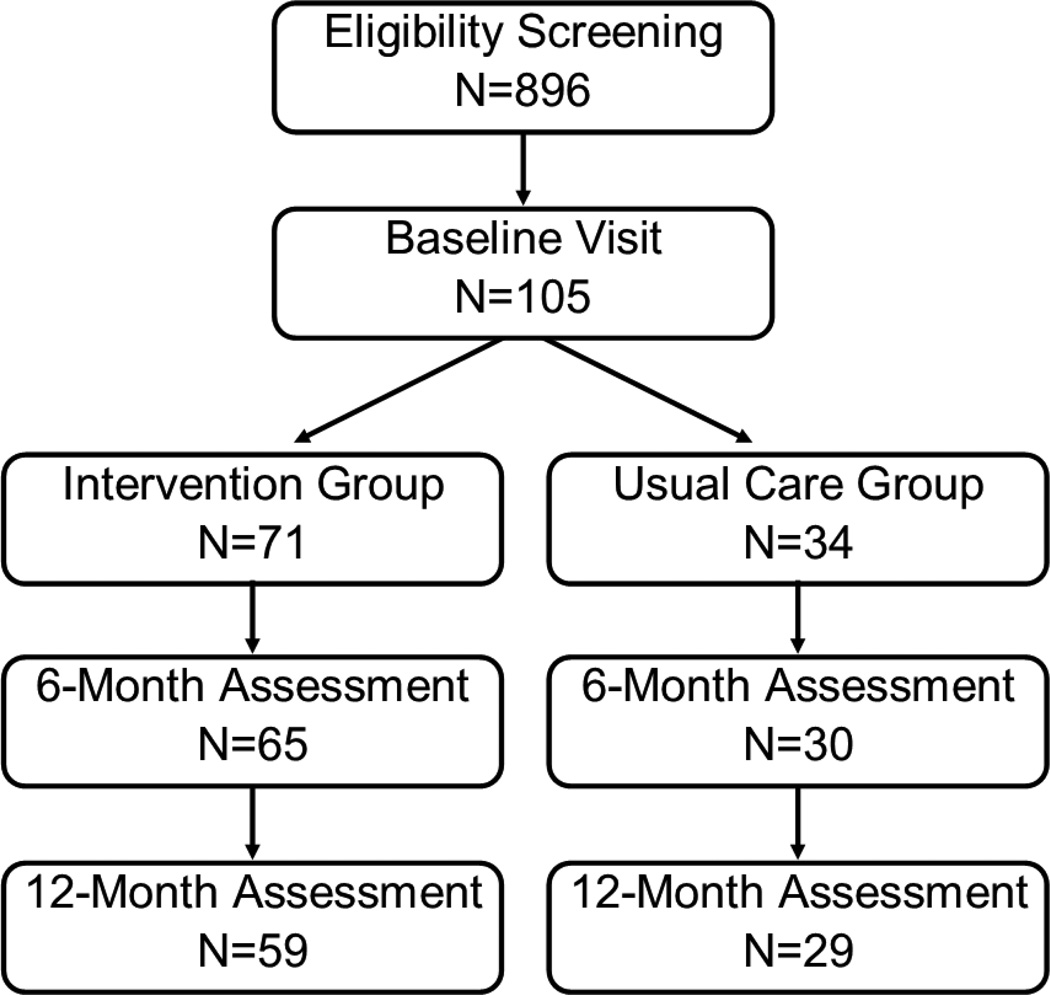
A total of 105 women were enrolled in this randomized trial (intervention: N=71; usual care: N=34) (Figure 1). Full six-month data were available for 65 intervention group participants (92%) and 30 usual care group participants (88%). Complete twelve-month data were available for 59 intervention group participants (81%) and 29 usual care group participants (85%).
Figure 1.
Flow of participants through the randomized controlled trial.
Baseline characteristics
Participants were, on average, 60.3 years of age (SD=6.2) (Table 1). The mean Gail Model score was 2.6 (SD=1.2), indicating an average 2.6% likelihood of developing invasive breast cancer within 5 years. Fifty-three percent of participants had undergone at least one biopsy and 78% had at least one first-degree relative with breast cancer. Mean BMI was 32.1 kg/m2 (SD=4.0) and 86% were daily internet users. At baseline, participants were performing 10 min/day (SD=13) of accumulated MVPA and only 39% of participants had at least one 10-minute bout/week of MVPA. The study groups did not differ with respect to baseline characteristics, including accelerometer wear time. Among the intervention group, 87% of participants completed at least 11 of the 12 calls in the first six months and 64% completed at least 15 of the 18 calls across the entire 12-month intervention.
Table 1.
Baseline characteristics of participants randomized to a weight loss trial (N=105).
| Intervention Group | Usual Care Group | p-value | |
|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | ||
| N | 71 | 34 | |
| Demographics | |||
| Age | 60.0 (6.3) | 60.8 (6.2) | .55 |
| College degree or higher | 67% | 82% | .11 |
| Non-Hispanic White | 83% | 94% | .22 |
| Married or living as married | 66% | 76% | .29 |
| Adiposity | |||
| Weight | 84.9 (12.1) | 85.3 (13.4) | .94 |
| BMIa (kg/m2) | 32.0 (3.6) | 32.2 (4.9) | .91 |
| Physical activity | |||
| ActiGraph wear time (min/day) | 865 (154) | 824 (128) | .20 |
| Total physical activityb (min/day) | 337 (46) | 330 (109) | .54 |
| Accumulated MVPAc (min/day) | 11 (13) | 9 (13) | .22 |
| MVPA within 10-min bouts (min/day) | 4 (10) | 3 (10) | .50 |
| Technology use | |||
| Daily internet user | 86% | 85% | .57 |
| Enjoys computer use | 79% | 77% | .78 |
Body mass index
Includes light, moderate, and vigorous-intensity activity
Moderate-to-vigorous intensity physical activity
Weight
At 6 months, the intervention group had lost an average of 3.9 kg (SD=3.8), compared to a gain of 0.3 kg (SD=2.6) in the usual care group (between-group p<.001) (Table 2). At 12 months, weight loss from baseline was 2.9 kg (SD=4.3) in the intervention group, compared to 1.2 kg (SD=3.8) in the usual care group (between-groups p=.06). As a proportion of starting body weight, the intervention group lost 4.7% of baseline weight by 6 months compared to a 0.4% increase in the usual care group (p<.001); this narrowed to 3.7% and 1.3% loss for the two groups at 12 months (p=.003). The apparent slight re-gain of weight during months 6–12 in the intervention group was not statistically significant (p=.67), however the small 1.2 kg loss in the usual care group was significant (p=.01).
Table 2.
Changes in weight and physical activity by study arm in a randomized weight loss trial (N=105).
| Intervention Group | Usual Care Group | p-value | |||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | ||
| Weight (kg)a | |||||
| Baseline | 84.9 (12.1) | 66.6 – 121.2 | 85.3 (13.4) | 60.4 – 110.6 | 0.94 |
| 6 months | 81.3 (13.8) | 57.3 – 122.8 | 85.8 (14.2) | 61.1 – 113.4 | <.0001 |
| 12 months | 81.6 (14.2) | 57.1 – 122.9 | 84.9 (13.9) | 61.6 – 114.7 | 0.01 |
| 6 months - Baseline |
−3.9 (3.8) | −17.0 – 3.6 | 0.3 (2.6) | −5.7 – 6.4 | <.0001 |
| 12 months - Baseline |
−2.9 (4.3) | −13.4 – 7.2 | −1.2 (3.8) | −7.7 – 8.3 | 0.059 |
| Percent weight loss from baseline (%)a | |||||
| 6 months | 4.7 (4.7) | −4.3 – 18.1 | −0.4 (3.0) | −8.3 – 5.6 | <.0001 |
| 12 months | 3.7 (5.4) | −8.9 – 19.0 | 1.3 (4.2) | −7.8 – 8.3 | 0.003 |
| Total physical activityb (average min/day) (accelerometer-measured)d | |||||
| Baseline | 334 (105) | 94 – 530 | 328 (85) | 174 – 551 | 0.74 |
| 12 months | 326 (124) | 51 – 627 | 267 (96) | 114 – 486 | 0.39 |
| 12 months - Baseline |
−13 (106) | −267 – 195 | −61 (101) | −258 – 101 | 0.03 |
| Accumulated MVPAc (average min/day) (accelerometer-measured)d | |||||
| Baseline | 11 (10) | 1 – 46 | 9 (9) | 1 – 39 | 0.53 |
| 12 months | 22 (25) | 0 – 109 | 12 (14) | 0 – 41 | 0.03 |
| 12 months - Baseline |
12 (24) | −17 – 99 | 0 (11) | −24 – 30 | 0.04 |
| MVPA in 10-min bouts (average min/day) (accelerometer-measured)d | |||||
| Baseline | 4 (6) | 0 – 32 | 3 (6) | 0 – 19 | 0.5 |
| 12 months | 13 (24) | 0 – 89 | 1 (5) | 0 – 23 | 0.97 |
| 12 months - Baseline |
9 (24) | −21 – 83 | −2 (7) | −19 – 16 | 0.77 |
Due to missing follow-up data, the difference of the means does not equal the mean of differences between each set of values. Calculations for percent weight loss are similarly affected.
Includes light, moderate, and vigorous-intensity activity
Moderate-to-vigorous intensity physical activity
Model adjusted for accelerometer wear time
Physical activity
The ActiGraph was worn at baseline and 12 months. The intervention group increased total accelerometer-measured MVPA by 12 min/day (SD=24) versus no change in the usual care group (mean=0, SD=11; p=.04) (Table 2). For MVPA accumulated in bouts of at least 10 minutes, the intervention group increased by 9 min/day (SD=24), versus a decrease of 2 min/day (SD=7) in the usual care group (p=.77). Total physical activity (which includes light-intensity activity), decreased in both groups from baseline to 12 months. The observed decrease was 13 min/day (SD=106) in the intervention group and 61 min/day (SD=101) in the usual care group (the decrease was significantly less in the intervention group (p=.03)). Wear time did not change significantly within either group between the two time points.
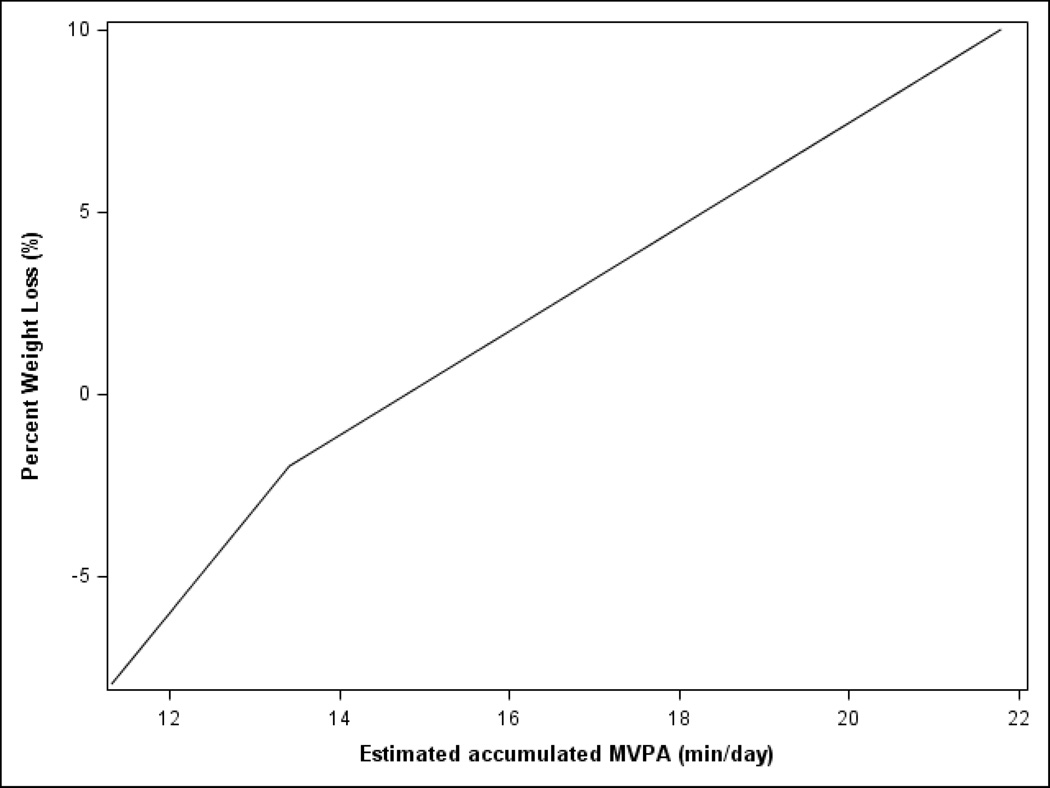
Weight loss by physical activity
After controlling for mean-centered baseline physical activity, mean-centered baseline weight, and intervention group, the percent weight loss at 12 months was significantly associated with min/day at 12 months of total physical activity (p=.048), min/day of accumulated MVPA (p=.041), and min/day of MVPA in bouts (p<.001) (curves for all three outcomes were comparable, therefore only data for accumulated 12-month MVPA is shown in Figure 2). Data are presented for both groups together because a similar, significant relationship between physical activity and weight loss was observed in each group. As illustrated in the figure, weight loss was associated with completing ≥16 min/day (112 min/week) of MVPA, while MVPA levels <16 min/day were associated with weight maintenance or gain.
Figure 2.
Percent weight loss at 12-months by estimated 12-month moderate-vigorous intensity physical activity (MVPA); controlling for intervention group, baseline physical activity, and baseline weight and adjusted for accelerometer wear time. (N=105).
Discussion
Relative to a usual care group, a phone- and web-based self-monitoring intervention was associated with significant weight loss among women at increased risk for developing breast cancer. The 6-month weight loss of 3.9 kg was slightly higher than we observed in our 12-week pilot study, which reported a mean loss of 3.3 kg (SD=4.0) (Cadmus-Bertram et al., 2013). In this study, the between-group difference in weight change was highest at 6 months. This decreased somewhat by 12 months, driven by a non-significant re-gain in the intervention group coupled with a small weight loss in the usual care group. The finding that participants in the intervention group maintained their weight loss but did not continue to lose weight may be related to the reduction in the frequency of telephone coaching calls during the second half of the year. The 1.2 kg loss observed in the usual care group, while not expected, may simply be due to the small size of that group (N=34), which renders it more sensitive to individual observations. Together, our findings are consistent with previous research with respect to the difficulty in achieving and maintaining substantial weight loss over time (Peirson et al., 2015).
The intervention effect on MVPA was statistically significant but modest. The intervention’s heavy focus on dietary change may have resulted in weight loss achieved primarily through caloric reduction, without large changes in activity level. An alternative explanation is that participants may have increased their physical activity early in the intervention, then experienced a degree of regression towards the later months of the study, resulting in a small 12-month change in activity. It should also be noted that because accelerometry removes the potential for self-report error and bias in physical activity measurement, it typically results in physical activity estimates that are lower but more accurate than those observed by self-report questionnaires (Jakicic et al., 2015; Prince et al., 2008). However, this would not necessarily impact the magnitude of baseline-to-12-month changes. While the usual care group experienced a substantial decrease in min/day of total physical activity (including all light, moderate, and vigorous-intensity activity), the intervention was successful in averting this decline. The lack of an observed increase in total physical activity in the intervention group (despite no significant change in accelerometer wear time) suggests that participants may have increased MVPA by displacing light-intensity activity. This is a positive finding given that the health benefits of MVPA typically exceed those of light-intensity activity (Kokkinos et al., 2010; Peters et al., 2009). Additional focus on sedentary time is needed and being addressed in a number of ongoing trials.
This study included a large proportion of women with salient breast cancer risk factors (e.g., previous biopsy, first-degree relative with breast cancer), therefore many women may have been aware of or concerned about the possibility of increased risk prior to being contacted by the study. This may result in a more motivated or adherent sample than the general population of middle-aged and older women. As noted earlier, those with higher breast cancer risk scores on the Gail model were more likely to complete the study. However perceived threat of negative health outcomes has been shown to be only a weak predictor of health behavior (Carpenter, 2010) and receiving information about familial risk of chronic disease does not necessarily increase motivation to engage in risk-reducing behaviors (Prichard et al., 2015).
Beyond this study and our own pilot study, only one previous trial has tested a weight loss intervention specifically among women known to be at elevated breast cancer risk (Harvie et al., 2010). Premenopausal women (N=78; mean age = 40) with a family history of breast cancer (estimated lifetime breast cancer risk 16–40%) were assigned to a 12-month intervention including group visits and monthly in-person visits with the study dietician. A modest weight loss (4 kg) was observed at 6 months and maintained at 12 months, with no changes in the control group. This study is consistent with our findings, suggesting that while modest weight loss can be achieved, women at elevated risk for breast cancer are no more likely than other groups to achieve marked weight loss. However, given that obesity and weight gain are consistently associated with breast cancer risk, over the long term, the potential benefit of lifestyle intervention may be driven by prevention of weight gain rather than substantial weight loss (Ahn et al., 2007; Neuhouser et al., 2015).
Strengths of this study include (a) a randomized design, (b) use of an elevated risk population, (c) objective assessment of physical activity, and (d) an intervention strategy that leveraged free, web-based self-monitoring resources to enhance a phone-based coaching program. Limitations include (a) a sample that was primarily well-educated and non-Hispanic White, (b) the absence of accelerometer data at the 6-month time point, which would have provided additional useful information about the trajectory of change in physical activity during the yearlong intervention, and (c) lack of website usage data that could provide insight on how participants used the Sparkpeople self-monitoring tools (e.g., frequency of logging physical activity or dietary intake).
Since the initiation of this trial in 2012, the consumer market for web-based self-monitoring has exploded, including many new options including accelerometer-based smartphone apps, physical activity trackers/ wristbands, and wireless scales (Linde et al., 2015). These tools offer the opportunity for more accurate and detailed self-monitoring of physical activity and diet, which may assist with development of self-regulation skills and successful weight loss. Much more research is needed to determine the best ways to leverage consumer-based self-monitoring technologies (Levine et al., 2015) and combine them successfully with proven traditional approaches (Hutchesson et al., 2015), especially in populations at increased risk of developing cancer or with a history of cancer. This yearlong study affirms and extends the findings of our 12-week pilot trial, demonstrating that integrating traditional intervention approaches (e.g., telephone-based coaching) with technology-based self-monitoring tools is a feasible and effective method for achieving modest weight loss and increased physical activity in this population.
Acknowledgments
FUNDING: This study was funded by (a) the University of California (UC) Athena Breast Health Network, via the Safeway Foundation and University of California Office of the President, and (b) by a gift from Carol Vassiliadis and family. Dr. Cadmus-Bertram is supported by grant K07CA178870 and Dr. Hartman by grant K07CA181323, both from the National Cancer Institute.
Footnotes
DISCLOSURE: The authors declare no conflict of interest
References
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychology. 2008;27(3):379–387. doi: 10.1037/0278-6133.27.3.379. [pii]10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- Ahn J, Schatzkin A, Lacey JV, Jr, Albanes D, Ballard-Barbash R, Adams KF, Leitzmann MF. Adiposity, adult weight change, and postmenopausal breast cancer risk. Archives of Internal Medicine. 2007;167(19):2091–2102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Breast Cancer Facts and Figures 2013–2014. Atlanta: American Cancer Society, Inc.; 2013. [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, Fabian CJ. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Research and Treatment. 2011;132(2):631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: Implications for prevention. Preventive Medicine. 2014;66:131–139. doi: 10.1016/j.ypmed.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Cadmus-Bertram L, Wang JB, Patterson RE, Newman VA, Parker BA, Pierce JP. Web-based self-monitoring for weight loss among overweight/obese women at increased risk for breast cancer: the HELP pilot study. Psycho-Oncology. 2013;22(8):1821–1828. doi: 10.1002/pon.3219. [DOI] [PubMed] [Google Scholar]
- Carpenter CJ. A meta-analysis of the effectiveness of health belief model variables in predicting behavior. Health Communication. 2010;25(8):661–669. doi: 10.1080/10410236.2010.521906. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, Cohen HJ. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. Journal of Clinical Oncology. 2012;30(19):2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. British Medical Journal. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine and Science in Sports and Exercise. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Woolcott CG, McTiernan A, Terry T, Brant R, Ballard-Barbash R, Courneya KS. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: A randomized controlled trial. International Journal of Obesity (2005) 2011;35(3):427–435. doi: 10.1038/ijo.2010.147. [pii]10.1038/ijo.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. Journal of the National Cancer Institute. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- Gail MH, Costantino JP, Pee D, Bondy M, Newman L, Selvan M, Bernstein L. Projecting individualized absolute invasive breast cancer risk in African American women. Journal of the National Cancer Institute. 2007;99(23):1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- Greenlee HA, Crew KD, Mata JM, McKinley PS, Rundle AG, Zhang W, Hershman DL. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (Silver Spring) 2013;21(1):65–76. doi: 10.1002/oby.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Cohen H, Mason C, Mercer T, Malik R, Adams J, Howell A. Adherence to a diet and exercise weight loss intervention amongst women at increased risk of breast cancer. The Open Obesity Journal. 2010;2:71–80. [Google Scholar]
- Heo M, Kabat GC, Strickler HD, Lin J, Hou L, Stefanick ML, Rohan TE. Optimal cutoffs of obesity measures in relation to cancer risk in postmenopausal women in the Women's Health Initiative Study. Journal of Womens Health. 2015;24(3):218–227. doi: 10.1089/jwh.2014.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, Pi-Sunyer FX. Weight loss with self-help compared with a structured commercial program: a randomized trial. Journal of the American Medical Association. 2003;289(14):1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- Hutchesson MJ, Rollo ME, Krukowski R, Ells L, Harvey J, Morgan PJ, Collins CE. eHealth interventions for the prevention and treatment of overweight and obesity in adults: A systematic review with meta-analysis. Obesity Reviews. 2015;16(5):376–392. doi: 10.1111/obr.12268. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, King WC, Gibbs BB, Rogers RJ, Rickman AD, Davis KK, Belle SH. Objective versus self-reported physical activity in overweight and obese young adults. J Phys Act Health. 2015 doi: 10.1123/jpah.2014-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. Journal of the American Medical Association. 2003;290(10):1323–1330. doi: 10.1001/jama.290.10.1323. 290/10/1323 [pii] [DOI] [PubMed] [Google Scholar]
- Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P Behavioural Weight Management Review G. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114(10):1557–1568. doi: 10.1016/j.jand.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos P, Myers J. Exercise and physical activity: Clinical outcomes and applications. Circulation. 2010;122(16):1637–1648. doi: 10.1161/CIRCULATIONAHA.110.948349. [DOI] [PubMed] [Google Scholar]
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT Lancet Physical Activity Series Working G. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon SC, Zapka JG, Clemow L. Health behavior change among women with recent familial diagnosis of breast cancer. Preventive Medicine. 2004;39(2):253–262. doi: 10.1016/j.ypmed.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Levine DM, Savarimuthu S, Squires A, Nicholson J, Jay M. Technology-assisted weight loss interventions in primary care: a systematic review. Journal of General Internal Medicine. 2015;30(1):107–117. doi: 10.1007/s11606-014-2987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde JA, Jeffery RW, Crow SJ, Brelje KL, Pacanowski CR, Gavin KL, Smolenski DJ. The Tracking Study: description of a randomized controlled trial of variations on weight tracking frequency in a behavioral weight loss program. Contemporary Clinical Trials. 2015;40:199–211. doi: 10.1016/j.cct.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlensky L, Natarajan L, Flatt SW, Faerber S, Newman VA, Pierce JP. Timing of dietary change in response to a telephone counseling intervention: Evidence from the WHEL study. Health Psychology. 2008;27(5):539–547. doi: 10.1037/0278-6133.27.5.539. [pii] 10.1037/0278-6133.27.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychology. 2009;28(6):690–701. doi: 10.1037/a0016136. [pii] 10.1037/a0016136. [DOI] [PubMed] [Google Scholar]
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychology & Health. 2011;26(11):1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Anderson GL. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women's Health Initiative randomized clinical trials. JAMA Oncology. 2015;1(5):611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman VA, Thomson CA, Rock CL, Flatt SW, Kealey S, Bardwell WA, Pierce JP. Achieving substantial changes in eating behavior among women previously treated for breast cancer--an overview of the intervention. Journal of the American Dietetic Association. 2005;105(3):382–391. doi: 10.1016/j.jada.2004.12.008. quiz 488. doi: S0002822304018504 [pii] 10.1016/j.jada.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Gaukstern JE, Beavers KM, Newman JC, Leng X, Rejeski WJ. Self-monitoring of spontaneous physical activity and sedentary behavior to prevent weight regain in older adults. Obesity (Silver Spring) 2014;22(6):1406–1412. doi: 10.1002/oby.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RE, Marinac CR, Natarajan L, Hartman SJ, Cadmus-Bertram L, Flatt SW, Kerr J. Recruitment strategies, design, and participant characteristics in a trial of weight-loss and metformin in breast cancer survivors. Contemporary Clinical Trials. 2015 doi: 10.1016/j.cct.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Usman Ali M, Raina P, Sherifali D. Strategies for weight maintenance in adult populations treated for overweight and obesity: A systematic review and meta-analysis. CMAJ Open. 2015;3(1):E47–E54. doi: 10.9778/cmajo.20140050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TM, Moore SC, Gierach GL, Wareham NJ, Ekelund U, Hollenbeck AR, Schatzkin A, Leitzmann MF. Intensity and timing of physical activity in relation to postmenopausal breast cancer risk: The prospective NIH-AARP Diet and Health Study. BMC Cancer. 2009;9:349. doi: 10.1186/1471-2407-9-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Newman VA, Flatt SW, Faerber S, Rock CL, Natarajan L, Kealey S. Telephone counseling intervention increases intakes of micronutrient- and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. Journal of Nutrition. 2004;134(2):452–458. doi: 10.1093/jn/134.2.452. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Newman VA, Natarajan L, Flatt SW, Al-Delaimy WK, Caan BJ, Parker BA. Telephone counseling helps maintain long-term adherence to a high-vegetable dietary pattern. Journal of Nutrition. 2007;137(10):2291–2296. doi: 10.1093/jn/137.10.2291. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard I, Lee A, Hutchinson AD, Wilson C. Familial risk for lifestyle-related chronic diseases: Can family health history be used as a motivational tool to promote health behaviour in young adults? Health Promotion Journal of Australia. 2015;26(2):122–128. doi: 10.1071/HE14104. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, Wyatt H. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A behavioral weight loss intervention in overweight or obese breast cancer survivors. Journal of Clinical Oncology. 2015;33(28):3169–3176. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MN, Vieira PN, Coutinho SR, Minderico CS, Matos MG, Sardinha LB, Teixeira PJ. Using self-determination theory to promote physical activity and weight control: A randomized controlled trial in women. Journal of Behavioral Medicine. 2010;33(2):110–122. doi: 10.1007/s10865-009-9239-y. [DOI] [PubMed] [Google Scholar]
- Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Progress in Cardiovascular Diseases. 2014;56(4):441–447. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LA, Cook AJ, Nokes NR, Adams TB. Telephone-based diet and exercise coaching and a weight-loss supplement result in weight and fat loss in 120 men and women. American Journal of Health Promotion. 2008;23(2):121–129. doi: 10.4278/ajhp.07051646. [DOI] [PubMed] [Google Scholar]