Abstract
Background
Though commonly noted in clinical practice, it is unknown if decongestion in acute heart failure (AHF) results in increased serum bicarbonate.
Methods and Results
For 678 AHF patients in the DOSE-AHF, CARRESS-HF, and ROSE-AHF trials, we assessed change in bicarbonate (baseline to 72-96 hours) by decongestion strategy, and the relationship between bicarbonate change and protocol-defined decongestion. Median baseline bicarbonate was 28 mEq/L. Patients with baseline bicarbonate ≥28 mEq/L had lower EF, worse renal function and higher NT-proBNP than those with baseline bicarbonate <28 mEq/L There were no differences in bicarbonate change between treatment groups in DOSE-AHF or ROSE-AHF (all p>0.1). In CARRESS-HF, bicarbonate increased with pharmacologic care but decreased with ultrafiltration (median +3.3 vs. -0.9 mEq/L respectively; p<0.001). Bicarbonate change was not associated with successful decongestion (p>0.2 for all trials).
Conclusions
In AHF, serum bicarbonate is most commonly elevated in patients with more severe heart failure. Despite being used in clinical practice as an indicator for decongestion, change in serum bicarbonate was not associated with significant decongestion.
Keywords: heart failure, edema, diuretics
Introduction
Acute heart failure (AHF) is common and treatment decisions are often based on an assessment of a combination of clinical conditions and laboratory measures.1 Many clinicians view increasing serum bicarbonate levels as a sign of volume contraction and use it as a marker of decongestion.3 However, empirical evidence to support this practice is lacking. Using data from three AHF trials, we sought to describe the characteristics of patients hospitalized for AHF by serum bicarbonate levels at baseline and follow up for different treatment strategies, and describe the association between serum bicarbonate and decongestion.
Methods
Data Source and Study Population
This analysis was performed using data from three National Heart, Lung, and Blood Institute (NHLBI)-sponsored Heart Failure Network trials, Diuretic Optimization Strategy Evaluation in Acute Heart Failure (DOSE-AHF), Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF), and Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF). The design and primary results of these trials have been published previously.4-9
All study participants provided written informed consent. The studies were approved by protocol review and data safety monitoring committees as well as each participating site's institutional review board.
Patients enrolled in the DOSE-AHF, CARRESS-HF, and ROSE-AHF trials were included in this study population if they had had a serum bicarbonate level measured at baseline and follow up at 72 hours or 96 hours.
Statistical Analyses
Baseline characteristics were compared between patients with a baseline serum bicarbonate level above and below the median using the Wilcoxon rank-sum test for continuous variables and the Pearson chi-square test for categorical variables. Baseline characteristics were described using medians and 25th and 75th percentiles for continuous variables and frequencies and proportions for categorical variables.
Linear regression was used to estimate the serum bicarbonate change differences across decongestion strategies within each trial. The models were adjusted for baseline serum bicarbonate. For analyses pooling all trials, an indicator variable for trial was also included. Complete decongestion was defined per study protocol as jugular venous distention < 8cm, trace or no peripheral edema, and no orthopnea.5,7
Spearman correlations were used to assess the association between the change in serum bicarbonate and the following: change in weight, change in renal function, and change in NT-proBNP.
Results
Of 835 unique patients in the DOSE-AHF, CARRESS-HF, and ROSE-AHF trials, 678 patients had a serum bicarbonate level measured at baseline and at follow-up (72 hours or 96 hours)—225 in DOSE-AHF, 309 in ROSE-AHF, and 144 in CARRESS-HF. Patients with baseline serum bicarbonate above the median (≥ 28 mEq/L) were significantly more likely to have a reduced EF, and at baseline had a lower serum sodium, and higher blood urea nitrogen, creatinine, and NT-proBNP (Table 1).
Table 1. Baseline Characteristics by Baseline Median Serum Bicarbonate Level*.
| Variable | Serum bicarbonate (mEq/L) < 28 N=362 | Serum bicarbonate (mEq/L) ≥ 28 N=316 | p-value† |
|---|---|---|---|
| Characteristics | |||
| Age, years | 68.5 (61.0, 78.0) | 68.5 (58.0, 78.0) | 0.46 |
| Gender, Male | 279 (77.1%) | 223 (70.6%) | 0.05 |
| Race, White | 291 (80.4%) | 208 (65.8%) | < 0.001 |
| Ejection fraction, %, | 33.6 (28.2, 40.0) | 30.4 (25.7, 36.0) | < 0.001 |
| Preserved ejection fraction | 121 (33.7%) | 80(25.6%) | 0.03 |
| Heart failure hospitalization in last year | 262 (73.6%) | 217 (69.1%) | 0.20 |
| Past Medical History | |||
| Ischemia as cause of heart failure | 222 (61.3%) | 174 (55.1%) | 0.10 |
| Atrial fibrillation/flutter | 221 (61.0%) | 155 (49.1%) | 0.002 |
| Diabetes | 218 (60.2%) | 172 (54.4%) | 0.13 |
| Chronic Obstructive Pulmonary Disease | 103 (28.5%) | 77 (24.4%) | 0.23 |
| Medications before Hospitalization | |||
| Beta Blockers | 292 (80.7%) | 266 (84.2%) | 0.23 |
| Aldosterone antagonist | 902 (24.9%) | 83 (26.3%) | 0.68 |
| Furosemide equivalent dose, mg/day | 120.0 (80.0, 160.0) | 80.0 (80.0, 160.0) | 0.002 |
| Baseline Evaluation | |||
| Weight, lbs | 216.9 (185.4, 267.4) | 196.2 (168.0, 241.8) | < 0.001 |
| Systolic blood pressure, mmHg | 114.0 (103.0, 126.0) | 116.0 (104.0, 127.0) | 0.36 |
| Heart rate, beats/min | 75.0 (67.0, 84.0) | 76.5 (66.5, 87.5) | 0.18 |
| Jugular Venous Pressure ≥ 8 cm | 330 (95.7%) | 284 (93.4%) | 0.21 |
| Orthopnea | 317 (91.1%) | 276 (92.3%) | 0.58 |
| New York Heart Association Class | 0.77 | ||
| I | 1 (0.3%) | 0 (0.0%) | |
| II | 8 (2.4%) | 9 (3.0%) | |
| III | 217 (64.8%) | 191 (64.1%) | |
| IV | 109 (32.5%) | 98 (32.9%) | |
| Sodium, mg/L | 139.0 (136.0, 141.0) | 138.0 (135.0, 140.0) | 0.001 |
| Blood urea nitrogen, mg/dl | 36.5 (26.0, 51.0) | 40.0 (27.0, 59.0) | 0.05 |
| Creatinine, mg/dl | 1.6 (1.2, 2.0) | 1.7 (1.3, 2.3) | < 0.001 |
| NT-pro BNP‡, pg/ml | 3929 (1999, 8493) | 6230 (2886, 12200) | < 0.001 |
| eGFR‡ | 43.2 (34.1, 55.9) | 38.7 (28.2, 53.6) | 0.001 |
Presented as Presented as N (%) or median (25th, 75th percentile)
p-values obtained using Wilcoxon rank-sum test for continuous variables and Pearson chi-square test for categorical variables
Abbreviations: NT-proBNP: N-terminal brain natriuretic peptide, eGFR: indexed glomerular filtration rate
No difference could be detected in the change in serum bicarbonate between bolus versus infusion (p = 0.40) or low-dose versus high-dose diuretics (p = 0.10) in DOSE-AHF, or between dopamine versus nesiritide versus placebo (p = 0.37) in ROSE-AHF (Table 2). In CARRESS-HF, subjects randomized to stepped pharmacologic care showed an increase in serum bicarbonate from baseline to 96 hours compared to those on ultrafiltration (change +3.3 mEq/L vs -0.9 mEq/L, p <0.001).
Table 2. Change in Serum Bicarbonate and Frequency of Significant Rise in Serum Bicarbonate by Decongestion Strategy.
| Trial/Decongestion Strategy | Baseline Bicarbonate (mEq/L) Mean (SD) | 72 or 96 hours Bicarbonate (mEq/L) Mean (SD) | Change in Bicarbonate (mEq/L) Mean (SD) | p-value* |
|---|---|---|---|---|
| DOSE-AHF | ||||
| Bolus (N=117) | 28.2 (4.2) | 29.3 (3.8) | 1.1 (3.5) | 0.40 |
| Infusion (N=108) | 28.0 (4.2) | 29.5 (4.0) | 1.5 (3.8) | |
| Low Dose (N=110) | 28.0 (4.1) | 29.0 (3.9) | 1.0 (3.6) | 0.10 |
| High Dose (N-115) | 28.2 (4.2) | 29.8 (3.9) | 1.6 (3.7) | |
| ROSE-AHF | ||||
| Dopamine (N=105) | 27.4 (4.5) | 30.0 (4.6) | 2.5 (3.6) | 0.37 |
| Nesiritide (N=102) | 27.0 (4.0) | 29.0 (4.0) | 2.1 (3.7) | |
| Placebo (N=102) | 27.4 (3.4) | 29.7 (3.8) | 2.2 (3.3) | |
| CARRESS-HF | ||||
| Stepped pharmacologic care (N=75) | 27.9 (4.4) | 31.2 (4.1) | 3.3 (3.9) | <0.001 |
| Ultrafiltration (N=69) | 28.1 (4.6) | 27.2 (4.8) | -0.9 (3.9) |
p values obtained from linear regression model. Models were adjusting for baseline serum bicarbonate.
There was no association between successful decongestion and change in serum bicarbonate from baseline to 72 or 96 hours (Table 3). Across all trials, the mean change in serum bicarbonate was 2.3 mEq/L for those who achieved successful decongestion by 72 or 96 hours and 1.6 mEq/L for those who did not (p = 0.85).
Table 3. Association of Success of Decongestion and Change in Serum Bicarbonate by Trial.
| Trial/Decongestion Status | Baseline | 72 or 96 hours | Change | p-value* |
|---|---|---|---|---|
| All Trials | 0.85 | |||
| Successful Decongestion† (N=83) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 26.4 (4.3) | 28.7 (3.6) | 2.3 (4.0) | |
| Unsuccessful Decongestion (N=580) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 27.9 (4.1) | 29.6 (4.2) | 1.6 (3.7) | |
| DOSE-AHF | 0.26 | |||
| Successful Decongestion (N=33) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 26.1 (4.9) | 28.9 (3.7) | 2.8 (4.3) | |
| Unsuccessful Decongestion (N=187) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 28.4 (4.0) | 29.5 (3.9) | 1.1 (3.3) | |
| ROSE-AHF | 0.79 | |||
| Successful Decongestion (N=39) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 26.4 (4.1) | 28.8 (3.7) | 2.4 (3.3) | |
| Unsuccessful Decongestion (N=263) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 27.4 (4.0) | 29.6 (4.2) | 2.2 (3.5) | |
| CARRESS-HF | 0.48 | |||
| Successful Decongestion (N=11) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 26.9 (2.6) | 27.8 (3.7) | 0.9 (5.2) | |
| Unsuccessful Decongestion (N=130) | ||||
| Bicarbonate (mEq/L), Mean (SD) | 28.2 (4.5) | 29.4 (4.9) | 1.2 (4.3) |
p values obtained from linear regression model. Models were adjusting for baseline serum bicarbonate (and trial when all trials were combined)
Successful decongestion is defined as JVP < 8cm, no orthopnea, and trace or no peripheral edema
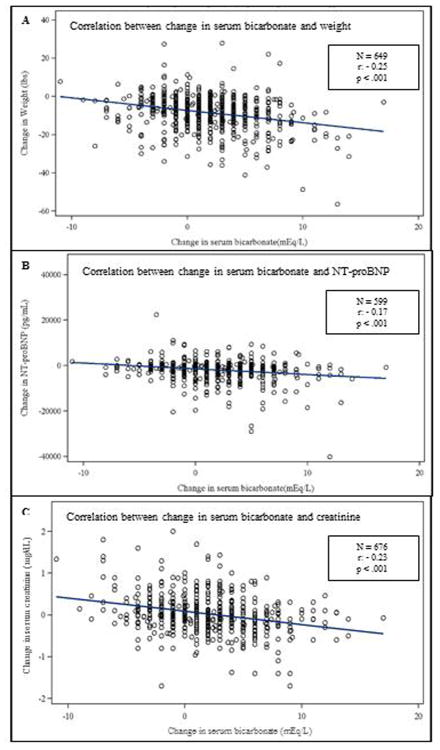
Figure 1 demonstrates the relationship between change in serum bicarbonate and other measures of congestion. While correlations were modest, across all trials combined as serum bicarbonate increased from baseline, weight decreased (Panel A), NT-proBNP decreased (Panel B), and serum creatinine decreased (Panel C).
Figure 1.
Scatterplots of change in serum bicarbonate and other measures of congestion. Panel A: Relationship between change in bicarbonate and change in weight. N=649, r: -0.25, p < 0.001. Panel B: Relationship between change in bicarbonate and NT-proBNP. N=599, r: -0.17, p < 0.001. Panel C: Relationship between change in bicarbonate and change in creatinine. N=676, r: -0.23, p < 0.001.
Discussion
Current treatment strategies in AHF rely predominantly on the use of loop and thiazide diuretics. The mechanisms by which the use of these diuretics results in metabolic alkalosis have been well described.10 “Contraction alkalosis,” due to decreased extracellular fluid volume resulting in increased bicarbonate concentration has only a small effect on serum bicarbonate levels. Diuretic induced acidification of the distal nephron stimulates increased production of bicarbonate, and decreased effective blood volume results in a decrease in the glomerular filtration rate, hindering the amount of bicarbonate filtered.10,11 Moreover, heart failure represents a state of contracted effective blood volume, setting up a substrate for development of metabolic alkalosis.
In our study, patients with worse heart failure had higher serum bicarbonate at baseline, and all decongestion strategies except ultrafiltration showed an increase in serum bicarbonate, consistent with the known mechanisms by which heart failure and diuretics result in metabolic alkalosis. However, no difference in serum bicarbonate change was detected between low-dose and high-dose diuretic regimens. Furthermore, while patients treated with ultrafiltration had considerable volume loss there was not an increase in bicarbonate. These results highlight that “contraction” is not the only source of increased serum bicarbonate and metabolic alkalosis during decongestion therapy, and further underscore the complexity of acid-base regulation in the kidney.
Health care providers rely on various subjective and objective features to determine when adequate decongestion has been achieved. In this study, an increase in serum bicarbonate with treatment was associated with other surrogate markers of decongestion, including weight loss and decrease in NT-proBNP. However, change in serum bicarbonate was not associated with worsening renal function or clinical decongestion as determined by history and physical exam findings. The majority of hospitalizations for heart failure are due to congestion, thus adequate decongestion is a primary goal; furthermore inadequate decongestion during hospitalization is associated with poor outcomes.12-15 Therefore, the finding of an elevated serum bicarbonate level in isolation may not be sufficient evidence for healthcare providers to stop or slow down decongestion efforts in patients with AHF.
Our study has several limitations. First, there is a selection bias inherent to all clinical trials. Second, while both ROSE-AHF and DOSE-AHF enrolled patients within 24 hours of hospital admission and collected serum bicarbonate at baseline and 72 hours after treatment, CARRESS-HF enrolled patients within 10 days of hospital admission—though 78.2% were enrolled within the first 72 hours— and collected serum bicarbonate at the start of the intervention and then after 96 hours. Our study did not account for treatment prior to baseline measurement of serum bicarbonate. Finally, we were limited by the data available. We evaluated lab values and clinical status at 72 or 96 hours after initiation of treatment when only a minority of patients had achieved successful decongestion. We were unable to examine values at other time points, such as later in the hospitalization, at the point of successful clinical decongestion, or at hospital discharge.
In conclusion, an association between change in serum bicarbonate and degree of decongestion for different treatment strategies in AHF could not be detected, though effective decongestion achieved with ultrafiltration was less likely to be associated with an increase in serum bicarbonate compared with a strategy based on pharmacological decongestion. Adequate decongestion is a key goal in the treatment of patients with AHF; thus isolated modest increases in serum bicarbonate should not prompt a decrease or cessation of diuresis.
Highlights.
Elevated serum bicarbonate is a common observation in acute heart failure patients
In AHF, bicarbonate increased with diuretics but decreased with ultrafiltration
Bicarbonate change was not associated with clinical signs of decongestion
Adequate decongestion is a key goal in the treatment of patients with AHF
Modest increases in bicarbonate should not prompt decrease or cessation of diuresis
Acknowledgments
The authors would like to thank Dr. Javed Butler and Dr. Horng Chen for their contributions to this manuscript, including assistance with study design, data analysis, and manuscript review.
Funding Sources: DOSE-AHF, CARRESS-HF, and ROSE-AHF were funded by the National Heart, Lung, and Blood Institute. Dr. Cooper is supported by grant T32HL069749-11A1 from the National Institute of Health.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr. Hernandez reports having served as a consultant for Amgen and Novartis. Dr. Cooper, Dr. Mentz, Ms. Gallup, Dr. Lala, Dr. DeVore, Dr. Vader, Dr. AbbouEzzeddine, Dr. Bart, Dr. Anstrom, and Dr. Felker report no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Peixoto AJ, Alpern RJ. Treatment of severe metabolic alkalosis in a patient with congestive heart failure. Am J Kidney Dis. 2013;61:822–7. doi: 10.1053/j.ajkd.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C. Fluid Overload: Diagnosis and Management. Karger; 2010. [Google Scholar]
- 4.Felker GM, O'Connor CM, Braunwald E. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bart BA, Goldsmith SR, Lee KL, Redfield MM, Felker GM, O'Connor CM, et al. Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS-HF, for the Heart Failure Clinical Research Network. J Card Fail. 2012;18:176–82. doi: 10.1016/j.cardfail.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HH, AbouEzzeddine OF, Anstrom KJ, Givertz MM, Bart BA, Felker GM, et al. Targeting the kidney in acute heart failure: can old drugs provide new benefit? Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) trial. Circ Heart Fail. 2013;6:1087–94. doi: 10.1161/CIRCHEARTFAILURE.113.000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. J Am Med Assoc. 2013;310:2533–43. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol. 2011;31:542–52. doi: 10.1016/j.semnephrol.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Palmer BF, Alpern RJ. Metabolic alkalosis. J Am Soc Nephrol. 1997;8:1462–9. doi: 10.1681/ASN.V891462. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119:S3–s10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 14.Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140:840–7. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 15.Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF) Circ Heart Fail. 2015;8:741–8. doi: 10.1161/CIRCHEARTFAILURE.114.001957. [DOI] [PMC free article] [PubMed] [Google Scholar]