Abstract
In recent years miscellaneous smart micro/nanosystems that respond to various exogenous/endogenous stimuli including temperature, magnetic/electric field, mechanical force, ultrasound/light irradiation, redox potentials, and biomolecule concentration have been developed for targeted delivery and release of encapsulated therapeutic agents such as drugs, genes, proteins, and metal ions specifically at their required site of action. Owing to physiological differences between malignant and normal cells, or between tumors and normal tissues, pH-sensitive nanosystems represent promising smart delivery vehicles for transport and delivery of anticancer agents. Furthermore, pH-sensitive systems possess applications in delivery of metal ions and biomolecules such as proteins, insulin, etc., as well as co-delivery of cargos, dual pH-sensitive nanocarriers, dual/multi stimuli-responsive nanosystems, and even in the search for new solutions for therapy of diseases such as Alzheimer’s. In order to design an optimized system, it is necessary to understand the various pH-responsive micro/nanoparticles and the different mechanisms of pH-sensitive drug release. This should be accompanied by an assessment of the theoretical and practical challenges in the design and use of these carriers.
INTRODUCTION
Over the last two decades, stimuli-responsive smart nanomaterials have increasingly been considered to be attractive vehicles for the release of drugs and genes. Smart nanoparticles (NPs) are capable of reacting to cues in the form of changes in several environmental parameters including temperature,1 pH,2,3 light,4 electric and magnetic fields,5,6 ultrasound,7,8 mechanical stress, and biochemical stimuli.9 Therefore, owing to their nonlinear response to variations in these external signals, they have been called ‘smart or intelligent materials’.10 Thanks to these properties, they have been utilized for applications in drug delivery,11 catalysts,12 biosensors,13 membranes,14 etc.
Among this whole range of different stimuli that have been tried, materials which are pH-sensitive, have attracted widespread applications due to their relevance in biology.15 In fact, there are pH differences between many tissues and cellular compartments of the human body.16,17 For instance, there is a big range in pH values throughout the digestive tract ranging from pH 2 in the stomach to pH 7 in the colon. Moreover, tumor tissue is 0.5–1 pH-units lower than the pH value in surrounding normal tissue due to metabolic glycolysis and lactic acid production. At the cellular level, there are pH differences among cellular compartments such as lysosomes (pH 4.5–5), endosomes (pH 5.5–6), and the cytosol (pH 7.4). Furthermore, microorganisms directly or by the release of enzymes, and also wounds themselves can be either acidic or alkaline depending on the biological environment. Hence, nanocarriers designed to be responsive to specific defined pH values, can target a specific area in the body to release their encapsulated drugs with maximum therapeutic impact and minimum side-effects.18–20 This realization has made pH-responsive carriers very interesting to be studied as drug delivery systems (DDSs).
Biopolymers have been frequently used for the design of drug delivery vehicles. Because of the ability of polymers to be precisely tailored according to their specific application, together with their biocompatibility, biodegradability, and biological functionality,21 synthetic functional polymers as well as organic polymers can act as carriers ranging from the macroscale (e.g., gels and hydrogels) to the nano-scale (e.g., micelles, nanogels, NPs, etc.). Generally speaking, the response of pH-sensitive polymers to variations in their surrounding environmental pH stems from changes in their physical properties. These properties include volume, solubility, conformation such as hydrophilic/hydrophobic balance and configuration such as crystalline/amorphous transition.22 These alterations can be reversible such as transformation in chain conformations of soluble polymers, or could be irreversible with collapse or degradation of the carrier caused by dissolution/swelling of polymers with pH variation.23
This review aims to comprehensively cover pH-sensitive nanocarriers, and highlight their importance and extensive applications in medicine and pharmaceutical science. As mentioned above, the more acidic environment of tumors, compared to the normal surrounding tissues, provides a specific opportunity which has been applied for targeted treatment of cancer. In addition, we address various methods for preparation of these materials and discuss two distinct mechanisms and a combined route for drug release. Last but not least, the challenges and difficulties which researchers have dealt with in the synthesis and application of these vehicles are summarized.
FEATURES OF MALIGNANT CELLS AND TUMORS AND DESIGN OF DIFFERENT NANOCARRIERS
Smart nanocarriers are advanced drug delivery vehicles that can overcome the biological barriers faced by conventional drug dosage formulations.24 The last decade has seen great strides made in developing efficient nanocarriers for selective targeting and delivery of cytotoxic agents against cancer cells.25,26 Efficient nanocarriers with controlled drug release can be designed by employing knowledge and a sound understanding of the physiology and microenvironment of cancer cells. Various nanocarriers like core-shell structures, polymers, liposomes, dendrimers, and metallic NP-based nanocarriers have been studied extensively. To date, more than 250 nanocarrier-based drug delivery strategies have been reported to be in the preclinical and clinical development pipelines.27
Despite the advances made in this regard, a few challenges like poorly-controlled drug release and limited availability of the drug at the tumor site still have remained unsolved. To address these challenges, several stimuli-responsive NP-based carriers have been devised.28,29 The activating stimulus can either be external or internal in nature, but both lead to alteration of the physiochemical properties of the carrier and aid in drug release or targeting. Differences in pH, temperature, redox potential,30 enzyme activation,31 ligand–target molecular interactions,32 variation in electrical and magnetic fields33 can all be used as stimuli for drug release. Nevertheless to make the use of smart nanocarriers more rational, one should have a better understanding of the features of the tumor microenvironment and how it differs from normal healthy tissue. For example, cancerous sites and tumor tissues have many peculiar properties including aberrant tumor vascularization and heterogeneous vascular density (a potential route for passive targeting of nanocarriers),34 a preponderance of veins/venules and a paucity of arteries/arterioles35 leading to both irregular blood flow within tumor (i.e., higher around the periphery than in the central parts).36 There is a positive net pressure difference in tumors (i.e., elevated interstitial fluid pressure (IFP) of about 60 mm Hg) (especially in the central parts compared to the periphery) in contrast to a negative pressure difference between blood vessels and interstitial space in normal tissues. A positive pressure difference deters convective transvascular transport of large molecules to the deeper areas of tumor tissues, but a negative pressure difference facilitates biomolecular transport to the interstitium.37–40 Tumors have a reduced number of pericytes around the blood vessels inducing leaky vasculature.41 Other factors like interstitial fibrosis, the compact and dense nature of the tumor cell populations, and poorly formed or absent lymphatic drainage further elevates the IFP.42,43
Poor lymphatic drainage and accumulation of macromolecules in tumors (due to poor clearance) is termed the ‘enhanced permeability and retention’ (EPR) effect in tumors.44–46 Here, the large gaps in the tumor vasculature allow selective extravasation of macromolecules (and even NPs) that will accumulate within tumor tissues. This property can be successfully used for targeting NPs, which accumulate in the tumor interstitium and are able to release therapeutic or cytotoxic agents.
Rapid tumor growth crucially depends on oxygen and nutrient supplies, and when these are consumed and cannot be resupplied due to a lack of blood vessels in the tumor core, this rapid growth induces hypoxia (i.e., lack of oxygen).47 Hypoxia leads to angiogenesis42 and also increases the malignant properties and proliferation rate of tumors, and their invasiveness into other areas.48
The reticuloendothelial system (RES) and renal clearance constitute the natural processes for elimination of NPs which are considered as foreign bodies in the bloodstream.49 The process of opsonization (i.e., the coverage of the surface of the NPs by a protein layer or a protein corona which facilitates this elimination process) must be considered in the design of NPs.50,51 For example, 100–200 nm sized NPs show avoidance of RES clearance, a size below 100 nm (10–100 nm) shows reduced clearance, and a size of 20–150 nm is used for passive targeting of tumors via the EPR effect.52
Tumor Extracellular pH Targeting
The tumor mass at both the early stage of growth and at later growth stages and when it is present as metastases has a significantly lower extracellular pH (pHe) than the surrounding normal tissue (pH about 7.4). This lower level of pH varies for different cancers (e.g., breast cancer, lung cancer, and gastrointestinal cancer) depending upon cancer type, size and its anatomical location.53 However, occasionally the environmental pH in a tumor can increase rather than decrease due to necrosis occurring in a large tumor mass.54
Hypoxia induced by the high proliferation rate in the tumor mass and lower blood flow in tumor vessels activates a range of genes, which are responsible for changing metabolism in the cells.55 For example, hypoxia up-regulates hypoxia inducible factor (HIF 1) which triggers the expression of glycolytic enzymes and glucose transporters (GLUT1 and GLUT3).56 Anaerobic glycolysis that occurs at low oxygen concentrations converts glucose molecules to pyruvate, which are then used as the source of energy in cancer cells. In this condition, pyruvate instead of going through tricarboxylic acid (TCA) cycle, is converted into lactic acid directly, which consequently lowers the pH.57 Pyruvate molecules are then eventually transported outside of the cell membrane.
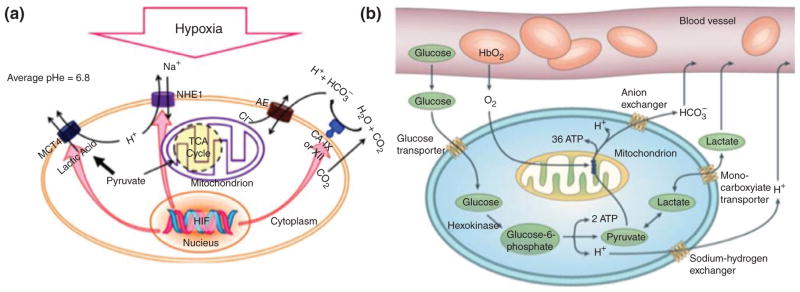
Another fundamental peculiarity of cancer cells is based on the Warburg effect, i.e., the preference for glucose fermentation remains even in the presence of adequate supplies of oxygen (i.e., aerobic glycolysis).58 This is despite the fact that the aerobic glycolysis pathway produces less adenosine triphosphate (ATP) molecules than the TCA cycle.59 Large amounts of H+ ions which are produced by glycolysis and lactic acid must be washed out. The overproduced H+ ions tend to accumulate in the tumor interstitium due to the poor perfusion rate in tumor vessels and the effects of hypoxia on the function of some transporters.60 Carbonic anhydrase (CA), which is present in the cell membrane and is induced by HIF, is another factor in the overproduction of H+ ions. CA converts carbon dioxide into carbonic acid, which eventually diffuses out of the membrane.61 Carbonic acid decomposes into HCO3− and H+. The H+ remains in the extracellular fluid and the HCO3− is taken up by the cell. Hence, lactic acid (in the form of lactate in physiological conditions) and carbonic acid are the two main acids giving rise to low pH in the tumor mass.62 Figure 1 illustrates the hypoxia in tumor cells (Figure 1(a)) as well as the glucose metabolism involving aerobic glycolysis (Warburg effect) (Figure 1(b)).
FIGURE 1.
(a) Hypoxia and the resultant decreased pHe induced by different routes including production and export of H+ and lactate (through up-regulation of NHE1, MCT4), conversion of CO2 to carbonic acid, influx of the dissociated weak base HCO3− while H+ is left outside, etc., (b); glucose metabolism in mammalian cells involving aerobic glycolysis metabolism (i.e., Warburg effect). ((a) Reprinted with permission from Ref.62 Copyright 2012 Elsevier. (b) Reprinted with permission from Ref.63 Copyright 2004 Nature)
It is noteworthy that the low pH in tumors can reduce the activity of some important anticancer drugs, such as doxorubicin (DOX).64 Finding a way to overcome these problems and while at the same time using the low tumor-pH for better drug delivery is a major challenge. Recent studies highlight the development of some promising carriers with pH-sensitivity and therefore the capability for tumor-selective delivery.65
Tumor Intracellular pH Targeting
Some intracellular organelles naturally have mildly to moderately acidic pH (pH 4.5 to 6.8). Drugs and other macromolecules are engulfed by invaginations in the plasma membrane, which are then internalized into the cell inside early endosomes by the endocytic uptake pathway.66 An ATP-driven H+ pump, which is located in the endosomal membrane pulls H+ ions into lumen from the cytosol and further reduces the pH. When the endosomes are internalized into the cell, the internal pH is lowered.
Finally, the late endosome fuses with another intracellular organelle containing enzymes released from the Golgi apparatus to form lysosomes. When the pH reaches ~5 (by the action of the ATP-driven H+ pump) lysosomal enzymes are activated and are able to digest all foreign biomolecules. If the endosome carries a drug, to avoid degradation in the lysosomes, the drug must be released from the endosomes (endosomolysis) before fusion to obtain the maximum pharmacological effect.67,68
These extracellular and intracellular pH gradients can be efficiently exploited to design nanocarriers for a selective drug delivery approach.
pH-SENSITIVE NANOCARRIERS
pH-Sensitive nanocarriers for drug/gene delivery systems can be constructed from organic and inorganic materials including polymers, lipids (liposomes, nanoemulsions, and solid-lipid NPs), metal, and ceramic NPs.69 A summary of the various types of pH-responsive nanocarriers is presented in Table 1.
TABLE 1.
Examples of Different Kinds of pH-Responsive Nanocarriers
| Nanocarrier Type | Material – Polymer, Lipid, etc. | Drug | Properties and Therapeutic Outcome | Drug Locating Strategy in Nanocarrier | Ref. |
|---|---|---|---|---|---|
| Micelles | poly(ethylene glycol)–polyaspartate copolymer with 45 nm particle size | proteasome inhibitor MG132 | Low in vivo toxicity of micelles than free MG132, prolonged circulation of drug-loaded micelles in the bloodstream, high stability in physiological pH, hydrolytic degradation in late-endosomes and lysosomes, release after EPR-mediated accumulation of drugs in the tumor, retained cytotoxicity by proteasome inhibition of released MG132-conjugated carriers | covalent binding of MG132 bound to the block copolymer | 70 |
| Liposomes | multifunctional envelope-type nanodevice encompassing pH-5 lipid YSK05 (YSK05-MEND) | anti-miR-122 | Enhance in target genes in the liver followed by reduction in plasma cholesterol, | miR-122 (AMO122) encapsulated in YSK05-MEND | 71 |
| nanogels | Interpenetrating polymeric network (IPN) spherical nanogels composed of poly(acrylamidoglycolic acid) and natural gelatin biological protein with 100 nm size | Curcumin | Good bioavailability and dispersion in aqueous solution, encapsulation efficiency of 42–48%, uniform distribution of curcumin in nanogel network, high anticancer activity than pristine curcumin, promising carriers for colorectal cancer delivery | Hydrophobic curcumin was encapsulated into the nanogels | 72 |
| Polymer-drug conjugates | Poly(ethylene oxide)-block-Polyphosphoester-graft-Paclitaxel conjugates with acid-sensitive linker | Paclitaxel | Enhanced drug release in acidic conditions with pH-trigger, increased cytotoxicity against cancer cells, capability to conjugate imaging labels, stability during blood circulation and uptake by tumor by EPR effect | Paclitaxel conjugated to polymer backbone via Acid-labile β-thiopropionate linkage | 73 |
| Core-shell NPs | Chitosan-alginate NPs with 100–200 nm size and spherical/sub-spherical shape | Insulin | Enhanced insulin bio-efficiency, Significant hypoglycemic effects with improved insulin-relative bioavailability, high retention of encapsulated insulin in simulated gastric buffer, sustained release in simulated intestinal milieu, no systemic toxicity, promising oral insulin delivery | Insulin entrapped in alginate core | 74 |
| Inorganic NPs | Mesoporous silica NPs (MSNs) with acetal-modified dextran as gating valves | DOX | Valves opened by acetal hydrolysis to retrieve hydrophilic state of acetal-modified dextran in model of endosomal/lysosomal compartments inducing rapid drug release after endocytosis, restricted release in pH 7.4, comparable cytotoxicity with free DOX, biocompatible carriers | DOX entrapped inside the MSN pores | 75 |
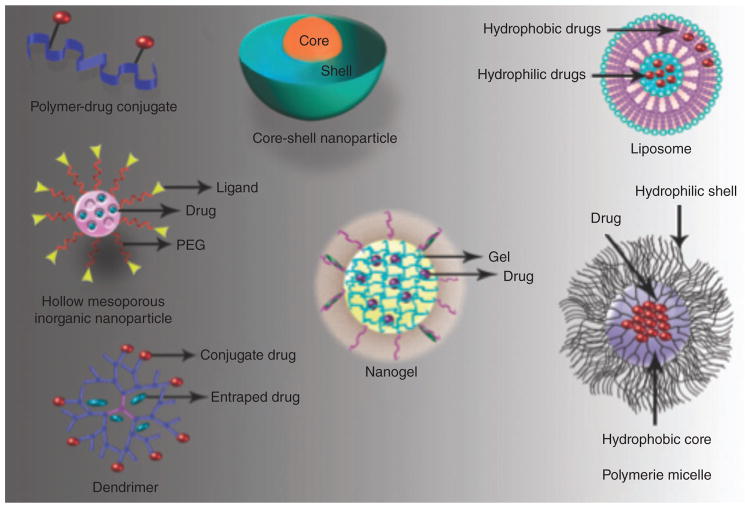
Various nanostructures that have been evaluated as pH-sensitive nanocarriers are illustrated in Figure 2. pH-Sensitive nanocarriers can be categorized into three main groups; polymeric nanocarriers, (which are sub-divided into four types including nanogels, micelles, polymer-drug conjugates, and core-shell polymeric NPs), liposomes, and inorganic NPs.
FIGURE 2.
Several examples of pH-sensitive nanocarrier platforms.
Polymeric Nanocarriers
Polymeric Nanogels
Cross-linked hydrophilic polymer chains can form a highly porous three-dimensional network either by self-assembly or by formation of covalent bonds. Drugs can be encapsulated into the inner gel structure and released by swelling caused by environmental pH changes.76,77 Nanogels possess higher capacity than micelles and liposomes for drug loading, but they cannot completely isolate hydrophobic drugs in their cores. They can be adapted for targeted delivery by appropriate surface modifications.78 Nanogels can be simply prepared by proper combination of amphiphilic block copolymers leading to binding of oppositely charged polymeric chains.79 One method for preparation of large pore-size nanogels is chemical crosslinking. Crosslinkers prevent rapid dissolution of the hydrophilic polymer chains in aqueous environments.80
Abandansari et al.81 synthesized a new pH-sensitive nanogel based on H40 by using a click reaction via mini-emulsion polymerization. Boltorn® H40 (H40) is a hyper-branched aliphatic polyester with attractive properties such as biodegradability, biocompatibility, globular architecture, and possibility for functionalized chains. This research group used H40-poly(ξ-caprolactone) (H40-PCL) as a core for improving the hydrophobicity of the core nanogel to load hydrophobic drugs, together with poly(vinylpyridine) (PVP) as a pH-sensitive crosslinker, and poly(ethylene glycol) (PEG) for enhancing the water solubility of the nanogels and increasing biocompatibility in the body environment.
Rigogliuso et al.82 synthesized nanogels by pulsed electron irradiation. The results showed non-toxicity of the nanogels toward cells.
Polymeric Micelles
Micelles are spherical supramolecular aggregates of amphiphilic molecules forming a liquid colloid. Nanosized polymeric micelles are formed by amphiphilic block copolymers which can self-assemble via hydrophobic and ionic interactions between the polymer blocks in an aqueous environment.83 They have been investigated as drug nanocarriers and have several attractive features such as their ability to solubilize water-insoluble drugs in the hydrophobic interior, high solubility, low toxicity, and the ability to take advantage of the EPR effect for passive tumor-targeting.84,85 Different functional groups including targeting ligands such as monoclonal antibodies, and cell-penetrating peptides to improve intracellular uptake can be attached to the hydrophilic exterior of micelles. pH-sensitive polymeric micelles take advantage of the lower tumor-pH to selectively release their cargo.84
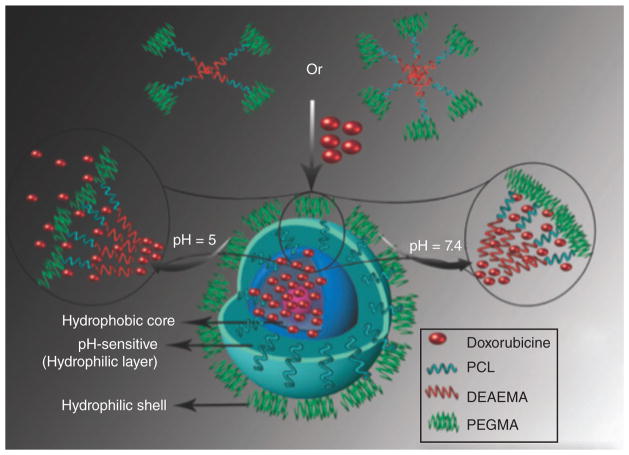
Yang et al.86 synthesized a series of amphiphilic 4- and 6-armed star triblock co-polymers-poly(e-caprolactone)-b-poly(2-(diethylamino)ethyl methacrylate)-b-poly(poly(ethylene glycol) methyl ether methacrylate) (4/6AS-PCL-b-PDEAEMA-b-PPEGMA) by using two polymerization methods including ring opening polymerization and a continuous activated radical polymerization process regenerated by electron transfer. The three-layered self-assembled micelles were pH-sensitive for the release of the hydrophobic anticancer drug DOX. Figure 3 schematically shows the loading and release of drugs from pH-sensitive micelles. The micelles swelled at acidic pH due to the ionization of tertiary amine groups in DEAEMA in the middle layer of the micelles, and the rate of DOX release increased when pH decreased from 7.4 to 5.0. The in vitro cytotoxicity of DOX-encapsulated micelles to HepG2 (hepatocellular carcinoma) cells showed higher anticancer activity and bioavailability than free DOX, so a lower amount of drug could be used for therapeutic applications.
FIGURE 3.
Schematic illustration of drug loading and release of drug in a pH-sensitive micelle.
Liu et al.87 prepared a pH-sensitive amphiphilic copolymer nanocarrier. Here, the amide groups in the core of micelles underwent pH-dependent hydrolysis that changed the charge of the PEI from negative to positive in the acidic tumor environment, thus the size of the micelles significantly reduced from 90 to 25 nm. This shrinkage disassembled the micelles and released the drug.
Kamimura et al.88 synthesized polyionic complex micelles from poly(ethylene glycol)-block-poly(4-vinylbenzylphosphonate) (PEG-b-PVBP) loaded with DOX as pH-sensitive nanocarriers (DOX@PNP). These self-assembled NPs (45–55 nm) overcame the premature drug release problem by hydrophobic and electrostatic interactions between phosphonate groups of PEG-b-PVBP as hydrophobic segments and cationic DOX. These DOX@PNPs showed high stability when diluted. After cellular endocytosis, DOX was delivered into endosomal and/or lysosomal compartments and spreaded into cytosol stepwise and transported into the cell nuclei.
Polymer-drug Conjugates (Prodrugs)
Conjugation of drugs to pH-sensitive polymers can be used as a carrier in DDSs. The drug molecules can be covalently bound to the polymer chains, or alternatively they can be encapsulated via electrostatic or hydrophobic interactions. Polymer-drug conjugates can possess long blood circulation time and stability against environmental destruction.89 Moreover poorly water-soluble drugs can be conjugated to water-soluble polymer.82,90 However it is still necessary to improve the control of drug release at the target sites. For this purpose Shixian et al.89 prepared 3,3′-dithiodipropionic acid functionalized poly(ethylene glycol)-b-poly(L-lysine) (mPEG-b-P(LL-DTPA)) with paclitaxel (PTX) directly conjugated via ester bonds. She et al.91 prepared DOX conjugated to a dendronized heparin block via an acid-labile hydrazine linkage and self-assembly, that showed safe and efficient pH-sensitive drug release. Du et al.92 prepared a folate-bovine serum albumin (BSA)-cis-aconitic anhydride-DOX prodrug. Folic acid was linked to BSA to improve tumor targeting ability of prodrugs. BSA enhanced the water solubility of drugs and cis-aconitic anhydride acted as pH-sensitive linker between the BSA and DOX.
Core-Shell Polymeric NPs
Polymeric NPs with colloidal, spherical, and branched properties can have core-shell architectures. They can be synthesized form biodegradable synthetic or natural polymers by methods such as salting out, spontaneous emulsion, nanoprecipitation, emulsion evaporation, supercritical CO2 polymerization, etc.93
Liu et al.94 prepared pH-sensitive poly(ethylene glycol)-poly(L-histidine)-poly(L-lactide) (PEG-PH-PLLA) core-shell NPs as antitumor drug carriers encapsulated DOX. The results showed that the size of blank NPs and drug-loaded NPs at pH 7.4 were smaller than at pH 5.0 and the release of DOX at pH 5.0 was faster than pH 7.4. In vitro experiments showed the prepared NPs were nontoxic to both NIH 3T3 fibroblasts and HepG2 cells. Xu et al.95 prepared pH-sensitive NPs with potential as a protein delivery system. The core contained glassy NPs formed from cross-linked dextran that was pH-sensitive and the protein was encapsulated inside.
Liposomes
Liposomes are self-assembled spherical vesicles made up of a single (unilamellar) or several (multilamellar) concentric lipid bilayers with various sizes from 50 nm to several micrometers. A liposome surrounds an aqueous solution inside a hydrophobic membrane. The encapsulated hydrophilic interior solution cannot easily pass through the membrane so liposomes can be used delivery vehicles for hydrophobic molecules (contained within the bilayer) or hydrophilic molecules (contained in the aqueous interior). Moreover by the addition of agents to the membrane surface, properties such as size, surface charge, and targeting to diseased cells or tissue can be tailored.96–98 In comparison with micellar systems, liposomes possess a better biocompatibility profile, making them good candidate for drug/gene delivery systems.99 However, they have some limitations such as low encapsulation efficiency, too rapid a release rate of the drug, low storage stability and lack of tunable triggers for drug release.100 As a result, several investigations have focused on enhancing liposome stability and circulation half-life, for example by surface modification, and assembly of layer-by-layer liposomal NPs (LBL-LNPs)88 to improve targeting and drug release. pH-Sensitive liposomes including various pH-sensitive components have been designed to release the active contents from the inside the endosomes into the cytoplasm.98
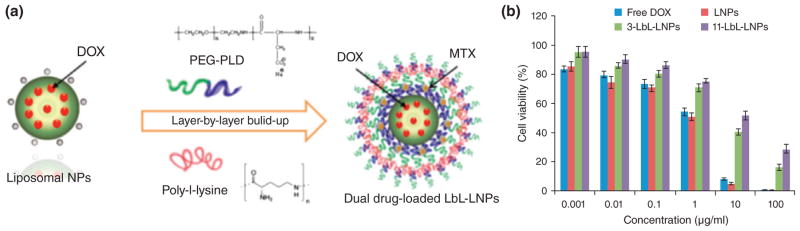
Ramasamy et al.96 prepared LBL-LNP nanocarriers loaded with DOX and mitoxantrone (MTX). These LBL were assembled by consecutive deposition of poly-L-lysine (PLL) and poly(ethylene glycol)-block-poly(L-aspartic acid) (PEG-b-PLD) (Figure 4 (a)). The results showed effectively-controlled burst-release kinetics, increased drug half-life, and improved biodistribution. Furthermore, free DOX (as well as LNPs), showed higher cytotoxicity than LBL-LNPs due to accelerated drug diffusion into the cell nucleus (Figure 4(b)).
FIGURE 4.
(a) Schematic illustration of the LBL-coated LNPs fabrication process, and (b) cell viability of LNPs, LBL-LNPs, and free DOX. (Reprinted with permission from Ref.96 Copyright 2014 Elsevier)
Yoshizaki et al.101 synthesized cationic lipid-incorporated liposomes modified with an acid-labile polymer hyper-branched poly(glycidol) (HPG) that could be used as pH-sensitive nanocarriers for efficient delivery of antigen molecules to the cytosol and endosomes/lysosomes for cancer immunotherapy. They reported that the modified liposomes were taken up by murine dendritic cells more than unmodified liposomes, and the modified ovalbumin (OVA)-loaded liposomes decreased the burden of an OVA-expressing tumor in a mouse model. Cationic lipid incorporation noticeably decreased tumor volume although OVA-loaded unmodified liposomes slightly reduced tumor growth. In another work pH-sensitive fusogenic polymer-(SucPG-) modified liposomes were prepared as vaccine carriers.102
In another study,103 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-28[methoxy(polyethylene glycol)] (mPEG-DSPE) and stearoyl-poly(ethylene glycol)-poly(methacryloyl sul-29 fadimethoxine) copolymer (stearoyl-PEG-polySDM) were used to form pH-sensitive liposomes for anticancer drug delivery. The ionization of polySDM in the acidic tumor environment led to aggregation of the liposomes. The relatively small amount of stearoyl-PEG-polySDM led to rapid rearrangement in the tumor environment.
Inorganic NPs
Inorganic NPs formed from mesoporous silica, gold, or CaCO3 have been used as nanocarriers for drug/gene delivery systems. They display good encapsulation capability and their rigid surfaces allow controlled functionalization.95 Some inorganic NPs can be used as pH-sensitive functional materials when combined with organic components such as chitosan,104 polydopamine,105 poly(acrylic acid),106 etc.
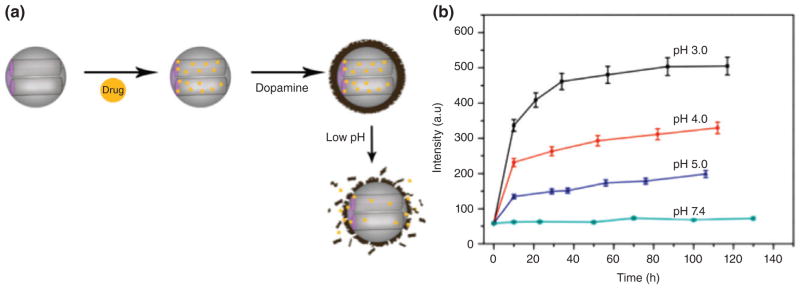
Mesoporous silica NPs (MSNs) have favorable properties such as structural stability, large area for functionalization, tunable pore size, and good bio-compatibility.107 Zheng et al.105 prepared pH-sensitive nanocarriers from polydopamine (PDA) coated MSNs with a large pore size obtained by using 1,3,5-trimethylbenzene as a pore expanding agent (Figure 5(a)). They were coated with PDA by a self-polymerization method that were stable at neutral pH but released DOX in acidic media (Figure 5 (b)). PDA showed good biocompatibility and low cytotoxicity.108
FIGURE 5.
(a) Schematic of fabrication of PDA coated MSNs and pH-dependent drug release, (b) DOX release profile from MSN-DOX@PDA at various pH values. (Reprinted with permission from Ref.105 Copyright 2014 Elsevier)
Chitosan-coated MSN have been used as pH-sensitive nanocarriers. Deng et al.104 used TNF-α as an anticancer drug, and antibodies that recognized ErbB2 attached to the surface of the chitosan-MSN in order to release the drug at the target sites. Here, mono-disperse cationic polystyrene (PS) nanospheres were synthesized as templates for preparation of hollow silica NPs (HNPs).
Yang et al.109 prepared multilayered, multifunctional nanocarriers as DDSs. They used superparamagnetic iron oxide NPs (SPIONs) sandwiched between the hydrophobic head of a pH-sensitive amphiphilic polymer (HAMAFA-b-DBAM) and MSN, which were surface-modified by the hydrocarbon octadecyltrimethoxysilane (C18). High drug loading was achieved and drug release was delayed because of the surface modification. They could be tracked by MRI due to the inclusion of SPIONs.
THE MECHANISMS OF DRUG RELEASE FROM pH-SENSITIVE NPs
Different pH-sensitive nanocarriers that swell or dissolve in response to a pH stimulus have been used to release drugs or genes at the target.11,110 The main mechanisms of pH-induced instability are discussed below.
Drug Release due to Dissolution of the Nanocarriers at Specific pH Values
pH-sensitive nanocarriers generally display a burst-release kinetic profile when the NPs dissolve or destabilize. Nanocarriers made from polycarboxylic acids are solid matrices at low pH that encapsulate the drug, but as soon as the pH changes from acidic to neutral, the carboxylic acid groups de-protonate, the linear polymers dissolve and drug release takes place.111
Calcium phosphate (CaP), due to its excellent biocompatibility, is a promising candidate for DDSs. As the pH decreases, the aqueous solubility of CaP increased. At a physiological pH, CaP has a fixed structure in contrast to the acidic endosomal environment when it dissolves. Unmodified carboxylate groups in carboxymethyl-chitosan have been used to bind Ca2+ ions.83 Poly (β-amino ester) (PbAE) belongs to a group of biodegradable cationic polymers used in pH-sensitive nanocarriers. At low levels of pH (pH ≤ 6.5), PbAE dissolves rapidly and releases the drug.112 Dan et al.113 prepared acid-labile micelles based on a β-thiopropionate linker, to encapsulate hydrophobic dye. The micelles were stable at neutral pH, but dissolved in an acidic environment due to hydrolysis of the ester functionality of the micelles, thus selectively releasing the dye which is illustrated in Figure 6.
FIGURE 6.
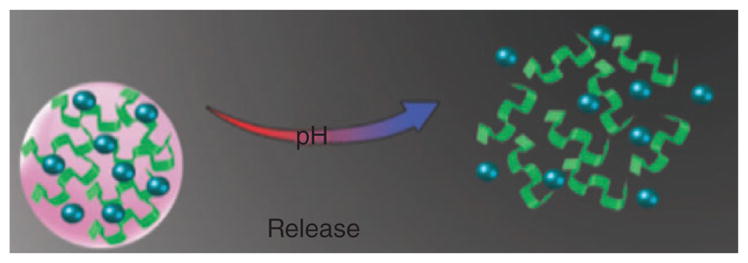
Dissolution of micelles based on pH changes.
Ulbrich et al.114 synthesized an antibody-targeted pH-sensitive polymer-drug conjugate. They selected a water-soluble polymer as the carrier with a hydrolytic-labile linker including hydrazine bonds. These conjugates were stable in the blood circulation, but with changes in pH inside the target cells, released DOX. Water-soluble, biodegradable, hydrolytically-labile, amine-functionalized polyacetals (APEGs) were synthesized by tri-polymerization of PEG, divinyl ethers, and serinol. Polyacetals quickly underwent hydrolysis, as the pH decreased.115 Different methods have been developed to release the liposomal contents into the cytoplasm by using pH-responsive functionalities. Inside the endosomes, the low pH destabilizes the liposomal membrane, allowing interaction with the endosomal membrane and leading to drug release into the cytoplasm. pH-Sensitive liposomes often use fusogenic lipids incorporated in the liposome composition such as unsaturated dioleoyl-phosphatidyl-ethanolamine (DOPE).116 Mildly acidic amphiphiles can be added to DOPE, to act as stabilizers at neutral pH, such as cholesteryl hemisuccinate (CHEMS), oleic acid (OA), and palmitoyl-homocysteine (PHC). In the acidic environment their carboxyl groups protonate, leading to shrinkage of the hydrophilic part causing membrane destabilization and drug-release.117
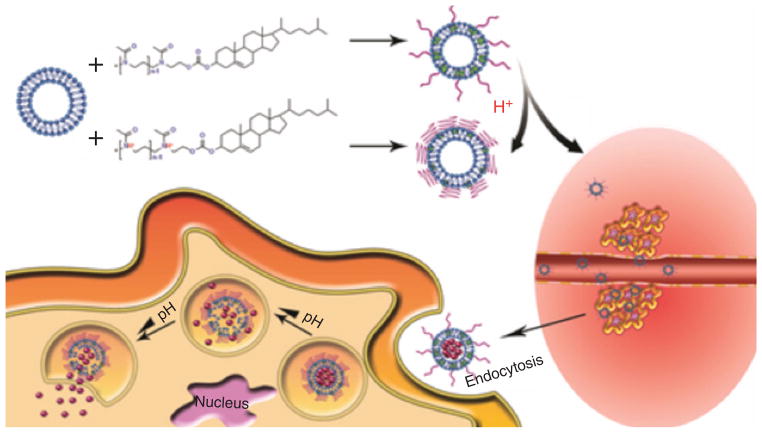
pH-Responsive liposomes may undergo transformation from a conventional lipid-bilayer to an inverted hexagonal phase, when the carboxylic groups become protonated. In addition to drug release due to liposome destabilization, the endosomal membrane can also be destabilized, and the drugs can be released directly into the cytoplasm.118 Micelles can release the loaded drug by two different mechanisms: the first one is to protonate the hydrophobic inner part of the micelle at low pH, which disassembles the micelle and releases the encapsulated drug. Poly(L-histidine)-b-PEG(PHisb-PEG) and poly(L-lactic acid)-b-PEG-b-polyHis-ligand (PLLA-b-PEG-b-Phis-ligand) exhibit this behavior. The second way is to utilize acid-degradable linker units to attach the drug to the hydrophobic block of the amphiphilic polymer. At low pH (around 6 or lower), drug release is enhanced in both mechanisms.119 Figure 7 illustrates drug release from liposomes associated with dissolution of the nanocarrier with pH variation.
FIGURE 7.
Schematic representation of drug delivery and release by using liposomes.
Drug Release as a Result of Polymer Swelling at Specific pH Values
Another mechanism for drug release from the nanocarriers is the pH-induced swelling of the materials. Figure 8 shows swelling of various nanocarriers at certain pH values. The most commonly used functional groups for synthesis of pH-sensitive NPs are carboxyl and pyridine groups.
FIGURE 8.
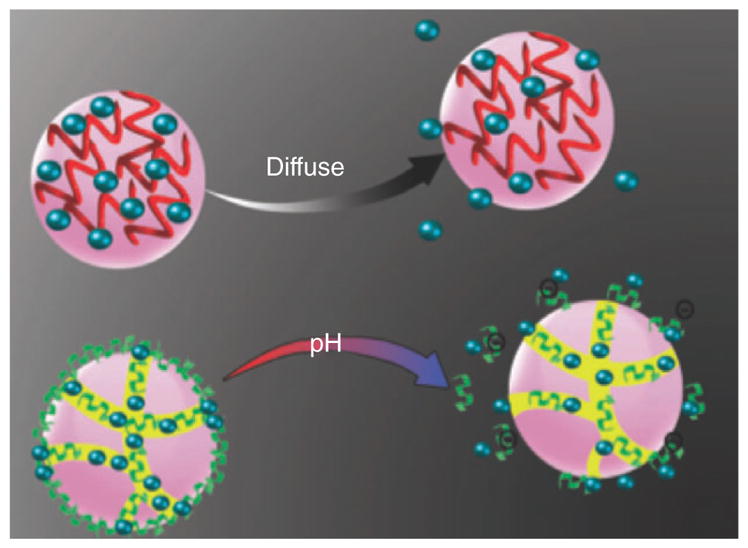
The swelling mechanism for different kinds of nanocarriers.
Alkali-swellable Carboxyl Groups
At acidic pH values, cross-linked polymers with carboxyl groups maintain a dense conformation, which results in a reduction of the porosity of the matrix and resistance against diffusion of the drug. In fact, at low pH levels, carboxyl groups undergo protonation and hydrophobic interactions dominate, which causes the volume shrinkage of the polymer. In contrast, at basic pH, the NPs swell and reach the highest porosity, thus diffusion resistance is reduced, and the release of the drug become much easier. At high levels of pH, the polymer swells because of the dissociation of carboxyl groups into carboxylate anions, which results in increased charge density in the polymer.
The hydrolysis of polycarbonate acetals within polymersomes and micelles, which were synthesized based on PEG and an acid-labile polycarbonate, poly (2,4,6-trimethoxybenzylidenepentaerythritol carbonate) (PTMBPEC) was evaluated. The results showed that the hydrolysis rate from PEG(1.9K)-PTMBPEC (6K) was affected by pH changes. According to dynamic light scattering (DLS) measurements, there was a rapid size change in response to acetal hydrolysis at low pH, indicating swelling.120
The release of erythromycin (EM) at low pH was quite low, while at pH values above 7 the amount of drug release increased. Therefore EM release would be minimal in the stomach (pH < 3), due to absence of swelling of the NPs, which would protect EM against destruction by gastric acid. As the NPs pass along the intestinal tract, the increased pH, would cause ionization of the carboxylic groups which increased the swelling of the NPs.121
Acid-swellable Pyridine Groups
In contrast to alkali-swellable carboxyl group, at acidic pH, pyridine groups become protonated leading to internal charge repulsion between the pyridinium groups. An enlargement in the overall dimensions of the NP occurs, as a result of the charge repulsion. At higher pH, the polymer groups become less ionized which causes a decrease in the charge repulsion and an increase in the polymer–polymer interaction, leading to a reduction of the overall hydrodynamic diameter of the polymer. Poly(vinyl-pyridine), formed from monomers, like 4-vinylpyridine (4VP) and 2-vinylpyridine (2VP) is one of the most widely used polymers in this class.122
Drug Release as a Result of Both Polymer Dissolution and Swelling
It is difficult to separate these two mechanisms, namely dissolution and swelling, from each other. Some DDSs might employ both mechanisms to release the encapsulated drug. In the DDSs loaded with EM, the NPs possessed partial degradability in the intestine at the presence of dextranase enzymes, so release occurred as a result of both swelling and enzyme-mediated dissolution.121 Li et al.123 reported the release of hydrophilic insulin from Eudragit L100-55 polymer-coated chitosan NPs. The results showed that the NP size remained unchanged because of the low water permeation into the particle at low pH, while when the pH reached 5.8, Eudragit L100-55 dissolved and water penetrated into the core of the particles and insulin was released due to swelling and higher porosity of chitosan.
CHALLENGES AND ISSUES IN THE DESIGN AND USE OF pH-SENSITIVE NANOCARRIERS
In the preparation process of pH-sensitive nanocarriers, researchers have recognized several challenges in terms of selection of materials, choosing appropriate methods of synthesis, selection, and measurement of properties, etc. Moreover there are several possible routes and applications to utilize these materials for drug release. Some of these challenges will be discussed below.
Materials and Preparation Methods
The first step in selection of materials for pH-sensitive nanocarriers is choosing ionizable polymers which possess pKa values between 3 and 10. These polymers tend to be polyelectrolytes and typically, they are weak acids and bases with pendant groups which can be ionized with variation in environmental pH.23 In addition, the pH-sensitivity of different polymers varies markedly. Carboxylic groups, for example, owing to their inherent pKa do not respond to small variations around physiological pH.124
One of the most important problems with pH-sensitive polymers is the rapid cleavage of micelles and polymers containing acetal, hydrazone, orthoester, and ketal functionalities in an acidic environment. This makes it difficult to achieve true controlled release. In the study by Zhang et al.,125 pyrene-containing surface-cross-linked micelles (SCMs) were prepared with 8–10 nm diameter. They reported that the cross-linkages within these SCMs were cleaved rapidly (<1 min) and as a consequence the loaded drugs were released during a few minutes. As a result, researchers have proposed β-thiopropionate as a crosslinker which possesses a relatively slow hydrolysis rate for better control. The effect of introducing sulfur atoms leads to development of a partial positive charge on the ester carbonyl carbon and this in turn, controls the hydrolysis rate.113
Although polymers with high molecular weights provide several benefits such as stability and better targeting, they may lack biodegradability. Since biopolymers should be removed after use, their nonbiodegradability is assumed to be one of the most critical limitations. Although biodegradability is not strictly necessary for some local and topical application routes such as oral drug delivery, its desirability for systemic applications such as intravenous injection have been widely discussed. Therefore, with regard to biodegradability, pH-sensitive biopolymers consisting of polypeptides, proteins, and polysaccharides have been proposed as good choices.126 Sun et al.127 studied hemi-cellulose (HC) based hydrogels synthesized from HC extracted from wheat straw together with acrylic acid (AAc) as monomers. According to swelling evaluation with pH variation, they proposed bulk erosion as the main process involved in degradation of the hydrogels. In this process, water diffusion into samples occurs at a faster rate than the rate of the hydrolysis reaction. Moreover, increase in the HC content led to a greater weight loss of samples whereas a higher density of crosslinking had the opposite effect. Moreover, in the presence of proteases, degradation was increased, and degradation occurred more rapidly in simulated gastric fluid. Wang et al.128 introduced hydrogels based on poly(e-caprolactone); PCL, methacrylic acid (MAA), and Pluronic (L35) as a potential biodegradable polymer for drug delivery use. The hydrolytic degradation behavior was higher with an increase in proportion of PCL due to the acid cleavage of ester bonds. Furthermore, a higher proportion of the crosslinker, i.e., N,N0-methylene-bis-acrylamide (BIS), resulted in decreased water diffusion into the hydrogel network and consequently a lower degradation rate.
Furthermore, pH-sensitive polymers often suffer from low mechanical strength. According to Kim et al.,129 the compressive strength of hydrogel formed from poly(acrylamide-co-acrylic acid)/polyethylenimine (P(AM-co-AA)/PEI) decreased with an increase in polyacrylic acid (a classical polyelectrolyte). The reduction of mechanical strength was even accompanied by cracking at a high concentration of AA. This phenomenon stemmed from excessive swelling stress during water uptake. If water diffusion takes place faster than relaxation of the polymer molecules, stress accumulates in the polymer structure and cracking can easily occur. With this in mind, various studies have attempted to improve the mechanical properties of the hydrogels through addition of a secondary component in the nanocomposites. For instance, it was observed that addition of 0.3 wt. % of graphene oxide nanosheet (GONS) to a poly acrylic acid/gelatin (PAA/gel) resulted in significant improvements in tensile strength and elongation at breaking point by 71% and 26%, respectively.130
Applications and Delivery Routes
pH-Sensitive hydrogels have been utilized for controlled release of drugs in two main applications. Firstly, they can be placed into capsules. Gutowska et al.131 examined drug delivery of hydrogels prepared by a mechanical squeezing process. Drug-loaded hydrogels placed in capsules were examined in various release media. After immersion, the gel swelled immediately to its equilibrium swelling state, resulting in closing of the pores in the capsule. Under certain circumstances such change in pH, the gel shrank, squeezing the capsule to open the holes. Consequently, drug could be released through the open holes with a controlled rate. In the field of cross-linked nanosphere carriers, disintegration at the correct pH depending on the desired stage of the digestive tract is required. Sonaje et al.132 reported that drug release from chitosan-poly(L-glutamic acid); γ-PGA occurred at pH 7–7.4 which simulates the intestinal environment. However the carboxyl groups of γ-PGA were protonated at the low pH of stomach which led to instability of the nanospheres owing to reduced electrostatic interaction. To rectify this problem, the nanospheres were freeze-dried and filled in an enteric-coated capsule.
Secondly, pH-sensitive nanocarriers can be applied embedded in silicone matrices. In a study by Carelli et al.,133 the hydrogels were semi-interpenetrating polymer networks [semi-IPN(s)] containing various amounts of poly(methacrylic acid-co-methylmethacrylate) (Eudragit (EUD) L100) and polyethylene glycol 8000 (P8000C) as a crosslinker. 35 wt. % Hydrogel particles with a diameter ranging from 89 to 123 μm, loaded with 15 wt.% prednisolone (PDN), were placed into silicone microspheres with a 500–1000 μm size range with acceptable morphology, by a modified emulsion vulcanization method.
There are other challenges that may be faced in employing these materials. For instance, the stability in serum is considered to be a major point to be considered in drug delivery carriers. According to Ashley et al.,134 liposomes composed of dioleoylphosphati-dylethanolamine (DOPE) exhibit poor stability in serum. Accordingly, Wu et al.135 designed liposomes composed of copolymer soy phosphatidylcholine (SPC) for solving the problem. They observed that in comparison to DOPE-liposomes, the SPC copolymer –liposomes released lower amounts of calcein in the PBS buffer solution during 20 h, which indicated higher stability in serum.
Importantly, elimination of undesirable premature drug release is a critical issue. For example, premature degradation of therapeutic agents that can occur in lysosomes can be a problem. The process of endosomolysis (the destabilization of the endosomal membrane at low pH) by designing nanocarriers that are taken up via endocytosis can be a solution.136
Dual/multi responsive DDSs that are pH-responsive in addition to another stimulus-responsive feature are new concepts in efficient targeting and release of therapeutic agents from NPs or membranes. Thus various dual/triple stimuli sensitive systems such as pH/magnetic, pH/redox, pH/temperature, pH/biomolecules, pH/thermo/redox, pH/temperature/magnetic, and pH/redox/magnetic have been developed with properties such as unique control of drug delivery and release, and significant anticancer efficacies.17,29 A pH/biomolecule responsive MSN based nanocarrier with immobilized polycaprolactone (esterase degradable) in the MSN core and pH-labile polyacrylic acid (PAA) showed a payload (DOX) release only in presence of both the acidic milieu and an esterase found in tumors (i.e., AND logic gate).137 A pH/redox nanohydrogels showed negligible premature drug release in bloodstream but rapid release in low pH milieu and glutathione (GSH) presence.138
Theronostic DDSs with both therapeutic and imaging capability have also been tested as pH-responsive nanosystems. A CdTe quantum dot (with pH-dependent fluorescence intensity) loaded into nanogels (QDs-NGs) showed promising drug delivery and tracking capabilities, and was suggested for cellular imaging of methotrexate (MTX) intracellular delivery in clinical therapy.139 In another attempt, a graphene oxide (GO)-based platform with a pH-labile fluorescence trace (rhodamine dye) used for concurrent pH-sensing and targeted release of RNAi in acidic tumors by a rhodamine-triggered competition reaction.140
Various disorders and diseases such as Wilson’s disease (i.e., copper (Cu) ion deficiency) and Alzheimer’s disease (due to dysregulation of Cu ions followed by aggregation of myloid-beta (Aβ) peptides in the brain) have been reported to have involvement of pH-alteration. Thus efficient pH-sensitive cargo (e.g., Cu)-delivery systems can be designed for treatment of such diseases.141 Recently, pH-responsive nanocarriers for intracellular delivery of antioxidants [natural scavengers of reactive oxygen species (ROSs)] have been designed with high stability, low cytotoxicity, and antioxidant activity. Such systems can be used for therapy of cardiovascular diseases (e.g., atherosclerosis) and neurological disorders (e.g., Alzheimer’s).142
Parameters involved in delivery of drugs can be enhanced by using pH-sensitive nanosystems. Docetaxel (DTX)-loaded micelles in a pH-responsive hydrogel showed enhanced oral bioavailability and small intestine targeted release that produced inhibition of subcutaneous breast tumor growth.143 In some cases due to limited drug bioavailability, a single high dosage of oral administration is required. This applies to delivery of therapeutic protein with high isoelectric point-exhibiting.144 Also, in some conditions such as long-term administration of therapeutic agents, nanosystems like carbon dot coated alginate beads (CA-CD) can be applied for gastrointestinal tract administration.145
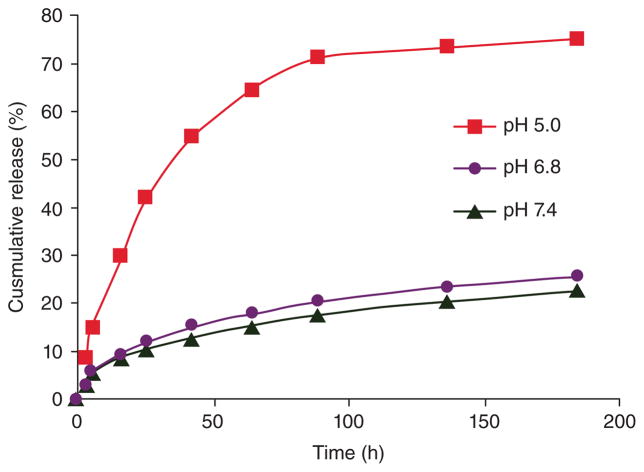
Dual pH-triggered release of drugs has been developed. A polymer-DOX conjugate nanocarrier was designed to be sensitive to both the extracellular pH and the intracellular pH of tumor tissues. At extracellular pH (e.g., 6.8), cellular uptake was facilitated due to positive to negative change in the surface charge. According to Figure 9 which shows cumulative release of DOX versus time for different pH values, in the intracellular endosomal compartment at pH 5.0, faster drug release was induced. This could significantly enhance cytotoxicity.146
FIGURE 9.
Cumulative release of DOX from polymer-drug conjugate NPs through cleavage of acid-labile hydrazonelinker. (Reprinted with permission from Ref.146 Copyright 2011 American Chemical Society)
Co-delivery systems have been considered as an innovative approach in design of pH-sensitive nanocarriers. For example, a dual-cargo nanocarrier was developed for delivery of small interfering RNA (siRNA) and anticancer PTX. Hence, simultaneous expression of gene silencing nucleic acids and cytotoxic chemotherapy was demonstrated in carcinoma cell lines. Such approaches could be used to overcome cancer multi-drug resistance (MDR).147 pH-responsive MSNs were fabricated for which one cargo was released at pH 7.0 and the second cargo was released at pH 2.0.148
The development of novel pH-sensitive NPs and novel applications has been a challenging goal. Some specific examples are metal-phenolic network (MPN) capsules with low fouling, fast assembly and pH-degradability,149 and amphiphilic ‘Janus’ NPs (i.e., emulsion droplets) with a pH-dependent dynamically-tunable structure and aggregation/dispersion behavior150 have been reported. Such novel particles could provide new concepts in the design of pH-responsive DDSs.
CONCLUSION AND FUTURE DIRECTION
In the field of stimuli-responsive nanocarriers, those nanovehicles that respond to changes in pH have possibly attracted the most attention compared to other stimuli. The reasons for this predominance are threefold and depend on the versatility of the approach. Firstly, the ability to have drugs released in a controlled manner in the acidic environment of endosomes and lysosomes (pH 4.5–5.5), and moreover to also possess the ability to carry out endosomolysis (destruction of the intracellular organelle) so the active ingredients can gain access to the cytoplasmic milieu where their effects are optimal is highly attractive. Secondly, the well-described low pH environment characteristic of tumors caused by the switch to the glycolytic metabolism (Warburg effect) typical of malignancies, has encouraged the development of drug-release vehicles tailored to respond to the relatively limited reduction in tumor pH (pH 6.8). Thirdly the ability to tailor drug-release either at the very low pH environment in the stomach (pH < 3), or alternatively at the higher pH environment in the intestines (pH > 7) is very important for oral drug delivery. It should be noted that there is an increasing trend for oral delivery of medicines previously routinely administered by injection (e.g., insulin). Furthermore, the impressive novel applications of pH-dependent DDSs in treatment of Alzheimer’s disease, dual-pH-sensitive delivery systems, dual/multi stimuli-responsive DDSs, theranostics, co-delivery systems, etc., is likely to increase in the future.
Looking forward to the future, highly sensitive nanocarriers leading to on-demand and controlled drug delivery and release, can be produced by combining pH-responsive elements with other stimuli-responsive elements. These other elements could be responsive to either externally applied forces or fields (heat, light, ultrasound, magnetic, or electric fields) or to other internal triggers (enzymes, redox, and specific biomolecules). Furthermore, by fabrication of nanosystems that are sequentially-responsive to different pH values that are typical of different biological environments, new breakthroughs can be obtained for orchestrated delivery of several different therapeutic cargos, in such a way that each cargo is released at a specific milieu/pH value. Thus strategy may be particularly applicable for gene/drug co-delivery systems which have recently been introduced for efficient cancer therapy and related MDR inhibition.
The capability of pH-responsive DDSs may pave the way for finding new therapies for other common but serious affiliations such as Alzheimer’s disease. Delivery of various biomolecules such as proteins, peptides, vaccines, and antigens while maintaining their biological activity is a challenging issue for which smart DDSs (especially pH-sensitive nanocarriers) have been suggested. In recent years, advent of multifunctional nanocarriers particularly the ones including pH-responsiveness (like multifunctional micellar NPs), suggests novel approaches for combining active targeting and smart stimuli-responsive DDSs, for theranostic applications and more efficient cancer therapy.
Many of the individual properties of DDSs such as bioavailability, biodegradability, and biodistribution, as well as efficient loading of cargo, controlled delivery, and release without premature drug leakage can be improved by taking advantage of the innovations in pH-sensitive nanosystems.
When looking toward the future, the effect that pH variations within different biological environments such as the gastrointestinal tract and the tumor extracellular/intracellular milieus have on the fate of the NPs, their nanotoxicity/biocompatibility (reduction of toxicity toward normal cells/tissues, and increased toxicity toward diseased sites and cancerous cells), targeting and cell uptake ability, drug release rate as well as protein corona formation around the NPs should be more comprehensively taken into account. Overcoming the unfortunate side-effects of potent anticancer chemotherapy drugs such as DOX, motivates investigations in which protecting the drug and eliminating its premature release and mitigating its harmful activity en route to its target, are indispensable.
It is expected that pH-responsive drug delivery vehicles will continue to be an active area of research involving polymer chemists, nanotechnologists, cell biologists, physicians and eventually biotechnology and pharmaceutical companies. Unceasing progress in pH-sensitive nanocarrier technology and related new advances in delivery of triggered therapeutic agents will continue for the foreseeable future.
Acknowledgments
Michael R. Hamblin was supported by US NIH grant R01AI050875.
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1.Ding Z, Liu Z, Hu J, Swaddiwudhipong S, Yang Z. Inhomogeneous large deformation study of temperature-sensitive hydrogel. Int J Solids Struct. 2013;50:2610–2619. [Google Scholar]
- 2.Tomar LK, Tyagi C, Choonara YE, Kumar P, Pillay V. Rheological and swelling behavior of pH sensitive hydrogel particles. APCBEE Procedia. 2014;9:192–196. [Google Scholar]
- 3.Dong K, Dong Y, You C, Xu W, Huang X, Yan Y, Zhang L, Wang K, Xing J. Assessment of the safety, targeting, and distribution characteristics of a novel pH-sensitive hydrogel. Colloids Surf B Biointerfaces. 2014;123:965–973. doi: 10.1016/j.colsurfb.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 4.Chiang C-Y, Chu C-C. Synthesis of photoresponsive hybrid alginate hydrogel with photo-controlled release behavior. Carbohydr Polym. 2014;119:18–25. doi: 10.1016/j.carbpol.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 5.Indermun S, Choonara YE, Kumar P, du Toit LC, Modi G, Luttge R, Pillay V. An interfacially plasticized electro-responsive hydrogel for transdermal electro-activated and modulated (TEAM) drug delivery. Int J Pharm. 2014;462:52–65. doi: 10.1016/j.ijpharm.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Karthik S, Puvvada N, Kumar BN, Rajput S, Pathak A, Mandal M, Singh ND. Photoresponsive coumarin-tethered multifunctional magnetic nanoparticles for release of anticancer drug. ACS Appl Mater Interfaces. 2013;5:5232–5238. doi: 10.1021/am401059k. [DOI] [PubMed] [Google Scholar]
- 7.Cavalieri F, Micheli L, Kaliappan S, Teo BM, Zhou M, Palleschi G. Antimicrobial and biosensing ultrasound responsive lysozyme-shelled microbubbles. ACS Appl Mater Interfaces. 2013;5:464–471. doi: 10.1021/am302660j. [DOI] [PubMed] [Google Scholar]
- 8.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai F, Li H, Luo R. Chemo-electro-mechanical modeling of ionic-strength-sensitive hydrogel: Influence of Young’s modulus. Int J Solids Struct. 2010;47:3141–3149. [Google Scholar]
- 10.Ballauff M, Lu Y. ‘Smart’ nanoparticles: preparation, characterization and applications. Polymer (Guildf ) 2007;48:1815–1823. [Google Scholar]
- 11.Liua J, Huanga Y, Kumara A, Tanc A, Jina S, Mozhia A, Lianga X. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Bergbreiter D, Brenda C, Yun-Shan L, Caraway WJ. Poly(N-isopropylacrylamide) soluble polymer supports in catalysis and synthesis. Macromolecules. 1998;31:6053–6062. [Google Scholar]
- 13.Kim JH, Yoon LS, Nam DH, Ryu J, Ku SH, Park CB. Biosensors and bioelectronics self-assembled, photoluminescent peptide hydrogel as a versatile platform for enzyme-based optical biosensors. Biosens Bioelectron. 2011;26:1860–1865. doi: 10.1016/j.bios.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Kim D-Y, Kim HJ. pH-sensitive optode membrane covalently incorporated with an ICT dye for low pH values. Sens Actuators B Chem. 2015;206:508–515. [Google Scholar]
- 15.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 16.Iemma F, Spizzirri UG, Puoci F, Muzzalupo R, Trombino S, Cassano R, Leta S, Picci N. pH-sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int J Pharm. 2006;312:151–157. doi: 10.1016/j.ijpharm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh RP, Pillay V, Choonara YE, du Toit LC, Ndesendo VM, Bawa P, Cooppan S. A review of multi-responsive membranous systems for rate-modulated drug delivery. AAPS PharmSciTech. 2010;11:441–459. doi: 10.1208/s12249-010-9403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez-Lorenzo C, Concheiro A. Smart drug delivery systems: from fundamentals to the clinic. Chem Commun. 2014;50:7743–7765. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Lorenzo C, Concheiro A. Chapter 1: From drug dosage forms to intelligent drug delivery systems: a change of paradigm. Smart Mater Drug Deliv. 2013;1:1. doi: 10.1039/9781849736800-00001. [DOI] [Google Scholar]
- 20.Binauld S, Stenzel MH. Acid-degradable polymers for drug delivery: a decade of innovation. Chem Commun. 2013;49:2082–102. doi: 10.1039/c2cc36589h. [DOI] [PubMed] [Google Scholar]
- 21.Prabaharan M, Mano JF. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermo-responsive polymers as novel biomaterials. Macromol Biosci. 2006;6:991–1008. doi: 10.1002/mabi.200600164. [DOI] [PubMed] [Google Scholar]
- 22.De Las Heras Alarcon C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 23.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 24.d’Angelo I, Conte C, Miro A, Quaglia F, Ungaro F. Core-shell nanocarriers for cancer therapy. Part I: biologically oriented design rules. Expert Opin Drug Deliv. 2014;11:283–297. doi: 10.1517/17425247.2014.868881. [DOI] [PubMed] [Google Scholar]
- 25.Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- 26.Bickerton S, Jiwpanich S, Thayumanavan S. Interconnected roles of scaffold hydrophobicity, drug loading, and encapsulation stability in polymeric nanocarriers. Mol Pharm. 2012;9:3569–3578. doi: 10.1021/mp3004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 29.Cheng R, Meng F, Deng C, Klok H-A, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Bio-materials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 30.Reisner E, Arion VB, Guedes da Silva MF, Lichtenecker R, Eichinger A, Keppler BK, Kukushkin VY, Pombeiro AJ. Tuning of redox potentials for the design of ruthenium anticancer drugs – an electrochemical study of [ trans-RuCl 4 L ( DMSO )] - and indazole. Inorg Chem. 2004;43:7083–7093. doi: 10.1021/ic049479c. [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Zhang G, Liu S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem Soc Rev. 2012;41:5933–5949. doi: 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- 32.Vyas SP, Singh A, Sihorkar V. Ligand-receptor-mediated drug delivery: an emerging paradigm in cellular drug targeting. Crit Rev Ther Drug Carrier Syst. 2001;18(1):1–76. [PubMed] [Google Scholar]
- 33.Hayashi K, Ono K, Suzuki H, Sawada M, Moriya M, Sakamoto W, Yogo T. High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl Mater Interfaces. 2010;2:1903–1911. doi: 10.1021/am100237p. [DOI] [PubMed] [Google Scholar]
- 34.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Wang J, Wientjes MG, Au JL-S. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev. 2012;64:29–39. doi: 10.1016/j.addr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Less JR, Skalak TC, Sevick EM, Jain RK. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. 1991 [PubMed] [Google Scholar]
- 37.Heldin C-H, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 38.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;500:3039–3052. [PubMed] [Google Scholar]
- 39.Rofstad EK, Galappathi K, Mathiesen BS. Tumor interstitial fluid pressure-a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia. 2014;16:586–594. doi: 10.1016/j.neo.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 41.Warren BA. The vascular morphology of tumors. Tumor Blood Circ. 1979;645:1–48. [Google Scholar]
- 42.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 43.Curti BD, Urba WJ, Alvord WG, Janik JE, Smith JW, 2nd, Madara K, Longo DL. Interstitial pressure of subcutaneous nodules in melanoma and lymphoma patients: changes during treatment. Cancer Res. 1993;53(10):2204–2207. [PubMed] [Google Scholar]
- 44.Matsumura Y, Maeda H. A new concept for macro-molecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 part 1):6387–6392. [PubMed] [Google Scholar]
- 45.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 46.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- 47.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 48.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 49.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Protein à nanoparticle interactions: opportunities and challenges. 2011;111(9):5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 51.Monopoli MP, Aberg C, Salvati A, Dawson KA. Bio-molecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 52.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 53.Volk T, Jaihdel E, Fortmeyer HP, Glisenkampl K, Rajewskyl MF. pH in human tumour xenografts: effect of intravenous administration of glucose. Br J Cancer. 1993;68(3):492–500. doi: 10.1038/bjc.1993.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Criscione JM, Le BL, Stern E, Brennan M, Rahner C, Papademetris X, Fahmy TM. Self-assembly of pH-responsive fluorinated dendrimer-based particulates for drug delivery and noninvasive imaging. Biomaterials. 2009;30:3946–3955. doi: 10.1016/j.biomaterials.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9:10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 56.Naha PC, Bhattacharya K, Tenuta T, Dawson KA, Lynch I, Gracia A, Lyng FM, Byrne HJ. Intracellular localisation, geno- and cytotoxic response of polyN-isopropylacrylamide (PNIPAM) nanoparticles to human keratinocyte (HaCaT) and colon cells (SW 480) Toxicol Lett. 2010;198:134–143. doi: 10.1016/j.toxlet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 57.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Hsu PP, Sabatini DM. Cancer cell metabolism: War-burg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 59.Kim J-W, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 60.Kojima C. A collagen-mimic dendrimer capable of controlled release. J Am Chem Soc. 2009;131:6052–6053. doi: 10.1021/ja809639c. [DOI] [PubMed] [Google Scholar]
- 61.Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61(21):7992–7998. [PubMed] [Google Scholar]
- 62.Tian L, Bae YH. Cancer nanomedicines targeting tumor extracellular pH. Colloids Surf B Biointerfaces. 2012;99:116–126. doi: 10.1016/j.colsurfb.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 64.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bio-conjug Chem. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 65.Manchun S, Dass CR, Sriamornsak P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012;90:381–387. doi: 10.1016/j.lfs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1985;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 67.Cerletti a, Drewe J, Fricker G, Eberle aN, Huwyler J. Endocytosis and transcytosis of an immunoliposome-based brain drug delivery system. J Drug Target. 2000;8:435–446. doi: 10.3109/10611860008997919. [DOI] [PubMed] [Google Scholar]
- 68.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 70.Quader S, Cabral H, Mochida Y, Ishii T, Liu X, Toh K, Kinoh H, Miura Y, Nishiyama N, Kataoka K. Selective intracellular delivery of proteasome inhibitors through pH-sensitive polymeric micelles directed to efficient antitumor therapy. J Control Release. 2014;188:67–77. doi: 10.1016/j.jconrel.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 71.Hatakeyama H, Murata M, Sato Y, Takahashi M, Minakawa N, Matsuda A, Harashima H. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J Control Release. 2013;173:43–50. [PubMed] [Google Scholar]
- 72.Madhusudana Rao K, Krishna Rao KSV, Ramanjaneyulu G, Ha C-S. Curcumin encapsulated pH sensitive gelatin based interpenetrating polymeric network nanogels for anti cancer drug delivery. Int J Pharm. 2015;478:788–795. doi: 10.1016/j.ijpharm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 73.Zou J, Zhang F, Zhang S, Pollack SF, Elsabahy M, Fan J, Wooley KL. Poly(ethylene oxide)-block-poly-phosphoester-graft-paclitaxel conjugates with acid-labile linkages as a pH-sensitive and functional nanoscopic platform for paclitaxel delivery. Adv Healthc Mater. 2014;3:441–448. doi: 10.1002/adhm.201300235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mukhopadhyay P, Chakraborty S, Bhattacharya S, Mishra R, Kundu PP. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int J Biol Macromol. 2015;72:640–648. doi: 10.1016/j.ijbiomac.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 75.Lin Z, Li J, He H, Kuang H, Chen X, Xie X, Jing X, Huang Y. Acetalated-dextran as valves of mesoporous silica particles for pH responsive intracellular drug delivery. RSC Adv. 2015;5:9546–9555. [Google Scholar]
- 76.Chacko RT, Ventura J, Zhuang JM, Thayumanavan S. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev. 2012;64:836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raemdonck K, Demeester J, Smedt SD. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–715. [Google Scholar]
- 78.Soni G, Yadav KS. Nanogels as potential nanomedicine carrier for treatment of cancer: a mini review of the state of the art. Saudi Pharm J. 2014 doi: 10.1016/j.jsps.2014.04.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yallapu MM, Jaggi M, Chauhan SC. Design and engineering of nanogels for cancer treatment. Drug Discov Today. 2001;16:457–463. doi: 10.1016/j.drudis.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinogradov SV. Colloidal microgels in drug delivery applications. Curr Pharm Des. 2006;12:4703–4712. doi: 10.2174/138161206779026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abandansari HS, Nabid MR, Rezaei SJT, Niknejad H. pH-sensitive nanogels based on Boltorn® H40 and poly(vinylpyridine) using mini-emulsion polymerization for delivery of hydrophobic anticancer drugs. Polymer (Guildf ) 2014;55:3579–3590. [Google Scholar]
- 82.Rigogliuso S, Sabatino MA, Adamo G. Polymeric nanogels: nanocarriers for drug delivery application. Chem Eng Transact. 2012;27:247–252. [Google Scholar]
- 83.Lv Y, Huang H, Yang B, Liu H, Li Y, Wang J. A robust pH-sensitive drug carrier: aqueous micelles mineralized by calcium phosphate based on chitosan. Carbohydr Polym. 2014;111:101–107. doi: 10.1016/j.carbpol.2014.04.082. [DOI] [PubMed] [Google Scholar]
- 84.Wu H, Zhu L, Torchilin VP. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials. 2013;34:1213–1222. doi: 10.1016/j.biomaterials.2012.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 86.Yang YQ, Zhao B, Li ZD, Lin WJ, Zhang CY, Guo XD, Wang JF, Zhang LJ. pH-sensitive micelles self-assembled from multi-arm star triblock copolymers poly(ε-caprolactone)-b-poly(2-(diethylamino)ethyl methacrylate)-b-poly(poly(ethylene glycol) methyl ether methacrylate) for controlled anticancer drug delivery. Acta Biomater. 2013;9:7679–7690. doi: 10.1016/j.actbio.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 87.Liu GY, Li M, Zhu CS, Jin Q, Zhang ZC, Ji J. Charge-conversional and pH-sensitive PEGylated polymeric micelles as efficient nanocarriers for drug delivery. Macromol Biosci. 2014;14:1280–1290. doi: 10.1002/mabi.201400162. [DOI] [PubMed] [Google Scholar]
- 88.Kamimura M, Nagasaki Y. pH-Sensitive polymeric micelles for enhanced intracellular anti-cancer drug delivery. J Photopolym Sci Technol. 2013;26:161–164. [Google Scholar]
- 89.Lv S, Tang Z, Zhang D, Song W, Li M, Lin J, Liu H, Chen X. Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. J Control Release. 2014;194:220–227. doi: 10.1016/j.jconrel.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Zou J, Jafr G, Themistou E, Yap Y, Wintrob ZA, Alexandridis P, Ceacareanu AC, Cheng C. pH-Sensitive brush polymer-drug conjugates by ring-opening metathesis copolymerization. Chem Commun (Camb) 2011;47(15):4493–4495. doi: 10.1039/c0cc05531j. [DOI] [PubMed] [Google Scholar]
- 91.She W, Li N, Luo K, Guo C, Wang G, Geng Y, Gu Z. Dendronized heparin-doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials. 2013;34:2252–2264. doi: 10.1016/j.biomaterials.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 92.Du C, Deng D, Shan L, Wan S, Cao J, Tian J, Achilefu S, Gu Y. A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials. 2013;34:3087–3097. doi: 10.1016/j.biomaterials.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 93.Soppimath K, Aminabhavi T, Kulkarni A, Rudzinski W. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 94.Liu R, Li D, He B, Xu X, Sheng M, Lai Y, Wang G, Gu Z. Anti-tumor drug delivery of pH-sensitive poly (ethylene glycol)-poly (L-histidine-)-poly (L-lactide) nanoparticles. J Control Release. 2011;152:49–56. doi: 10.1016/j.jconrel.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 95.Dan X, Wu F, Chen Y, Wei L, Yuan W. pH-sensitive degradable nanoparticles for highly efficient intracellular delivery of exogenous protein. Int J Nanomedicine. 2013;8:3405. doi: 10.2147/IJN.S47701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramasamy T, Haidar ZS, Tran TH, Choi JY, Jeong JH, Shin BS, Choi HG, Yong CS, Kim JO. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014;10:5116–5127. doi: 10.1016/j.actbio.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 97.Blume G, Cevc G. Liposomes for the sustained drug release in vivo. Biochim Biophys Acta Biomembr. 1990;1029:91–97. doi: 10.1016/0005-2736(90)90440-y. [DOI] [PubMed] [Google Scholar]
- 98.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 99.VPT Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 100.Sihorkaer V, Vyas SP. Potential of polysaccharide anchored liposomes in drugs delivery, targeting and immunization. J Pharm Pharm Sci. 2001;4:138–158. [PubMed] [Google Scholar]
- 101.Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials. 2014;35:8186–8196. doi: 10.1016/j.biomaterials.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 102.Watarai S, Iwase T, Tajima T, Yuba E, Kono K. Efficiency of pH-sensitive fusogenic polymer-modified liposomes as a vaccine carrier. Sci World J. 2013;2013:Article ID 903234, 7. doi: 10.1155/2013/903234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bersani S, Vila-Caballer M, Brazzale C, Barattin M, Salmaso S. pH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine) decorated liposomes for the delivery of gemcitabine to cancer cells. Eur J Pharm Biopharm. 2014;88:670–682. doi: 10.1016/j.ejpb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 104.Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK. Hollow chitosan-silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials. 2011;32:4976–4986. doi: 10.1016/j.biomaterials.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 105.Zheng Q, Lin T, Wu H, Guo L, Ye P, Hao Y, Guo Q, Jiang J, Fu F, Chen G. Mussel-inspired polydopamine coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Int J Pharm. 2014;463:22–26. doi: 10.1016/j.ijpharm.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 106.Yuan L, Tang Q, Yang D, Zhang JZ, Zhang F, Hu J. Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery. J Phys Chem C. 2011;115:9926–9932. [Google Scholar]
- 107.Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocom-patibility and drug delivery. Adv Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 108.Kim S, Park C. Mussel-inspired transformation of CaCO3 to bone minerals. Biomaterials. 2010;31:6628–6634. doi: 10.1016/j.biomaterials.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 109.Yang S, Chen D, Li N, Mei X, Qi X, Li H, Xua Q, Lu J. A facile preparation of targetable pH-sensitive polymeric nanocarriers with encapsulated magnetic nanoparticles for controlled drug release. J Mater Chem. 2012;22:25354. [Google Scholar]
- 110.Nowag S, Haag R. pH-responsive micro- and nanocarrier systems. Angew Chem Int Ed. 2014;53:49–51. doi: 10.1002/anie.201308619. [DOI] [PubMed] [Google Scholar]
- 111.Sonaje K, Lin K, Wang JJ, Mi FL, Chen CT, Juang JH, Sung HW. Self-assembled pH-sensitive nanoparticles: a platform for oral delivery of protein drugs. Adv Funct Mater. 2010;20:3695–3700. [Google Scholar]
- 112.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. in vivo distribution and tumor localization studies. Pharm Res. 2005;22:2107–2114. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dan K, Pan R, Ghosh S. Aggregation and pH responsive disassembly of a new acid-labile surfactant synthesized by thiol - acrylate michael addition reaction. Langmuir. 2011;27:612–617. doi: 10.1021/la104445h. [DOI] [PubMed] [Google Scholar]
- 114.Ulbrich K, Subr V. Polymeric anticancer drugs with pH-controlled activation. Adv Drug Deliv Rev. 2004;56:1023–1050. doi: 10.1016/j.addr.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 115.Tomlinson R, Heller J, Brocchini S, Duncan R. Polyacetal-doxorubicin conjugates designed for pH-dependent degradation. Bioconjug Chem. 2003;14:1096–1106. doi: 10.1021/bc030028a. [DOI] [PubMed] [Google Scholar]
- 116.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2012;64:302–315. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 117.Legendre J, Szoka FJ. Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res. 1992;9:1235–1242. doi: 10.1023/a:1015836829670. [DOI] [PubMed] [Google Scholar]
- 118.Varkouhi A, Scholte M, Storm G, Haisma H. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 119.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 120.Chen W, Meng F, Cheng R, Zhong Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: a comparative study with micelles. J Control Release. 2010;142:40–46. doi: 10.1016/j.jconrel.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H, Wu H, Fan L, Li F, Gu C, Jia M. Preparation and characteristics of pH-sensitive derivated dextran hydrogel nanoparticles. Polym Compos. 2009;30:1243–1250. [Google Scholar]
- 122.Wang X-Q, Zhang Q. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur J Pharm Biopharm. 2012;82:219–229. doi: 10.1016/j.ejpb.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 123.Li MG, Lu WL, Wang JC, Zhang X, Zhang H, Wang XQ, Wu CS, Zhang Q. Preparation and characterization of insulin nanoparticles employing chitosan and poly(methylmethacrylate/methylmethacrylic acid) copolymer. J Nanosci Nanotechnol. 2006;6:2874–2886. doi: 10.1166/jnn.2006.411. [DOI] [PubMed] [Google Scholar]
- 124.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 125.Zhang S, Zhao Y. Rapid release of entrapped contents from multi-functionalizable, surface cross-linked micelles upon different stimulation. J Am Chem Soc. 2010;132:10642–10644. doi: 10.1021/ja103391k. [DOI] [PubMed] [Google Scholar]
- 126.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 127.Sun X, Wang H, Jing Z, Mohanathas R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr Polym. 2013;92:1357–1366. doi: 10.1016/j.carbpol.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 128.Wang K, Xu X, Liu T, Fu S, Guo G, Gu Y, Luo F, Zhao X, Wei Y, Qian Z. Synthesis and characterization of biodegradable pH-sensitive hydrogel based on poly ( e -caprolactone ), methacrylic acid, and Pluronic ( L35) q OH. Carbohydr Polym. 2010;79:755–761. [Google Scholar]
- 129.Kim D, Park K. Swelling and mechanical properties of superporous hydrogels of poly(acrylamide-co-acrylic acid)/polyethylenimine interpenetrating polymer networks. Polymer (Guildf ) 2004;45:189–196. [Google Scholar]
- 130.Faghihi S, Karimi A, Jamadi M, Imani R, Salarian R. Graphene oxide/poly(acrylic acid)/gelatin nanocomposite hydrogel: experimental and numerical validation of hyperelastic model. Mater Sci Eng C Mater Biol Appl. 2014;38:299–305. doi: 10.1016/j.msec.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 131.Gutowska A, Barka JS, Kwona IC, Baea YH, Chab Y, Kima SW. Squeezing hydrogels for controlled oral drug delivery. J Control Release. 1997;48:141–148. [Google Scholar]
- 132.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ, Sung HW. Enteric-coated capsules filled with freeze-dried chitosan/poly (γ-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31:3384–3394. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 133.Carelli V, Coltelli S, Di Colo G, Nannipieri E, Serafini MF. Silicone microspheres for pH-controlled gastrointestinal drug delivery. Int J Pharm. 1999;179:73–83. doi: 10.1016/s0378-5173(98)00387-1. [DOI] [PubMed] [Google Scholar]
- 134.Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Hanna TN, Liu J, Phillips B, Carter MB, Carroll NJ, Jiang X, Dunphy DR, Willman CL, Petsev DN, Evans DG, Parikh AN, Chackerian B, Wharton W, Peabody DS, Brinker CJ. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu X, Wang Z, Zhu D, Zong S, Yang L, Zhong Y, Cui Y. pH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer-lipid bilayer. ACS Appl Mater Interfaces. 2013;5:10895–10903. doi: 10.1021/am403092m. [DOI] [PubMed] [Google Scholar]
- 136.Torchilin VP. Multifunctional, stimuli-sensitive nano-particulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen X, Soeriyadi AH, Li X, Sagnella SM, Kavallaris M, Gooding JJ. Dual bioresponsive mesoporous silica nanocarrier as an ‘AND’ logic gate for targeted drug delivery cancer cells. Adv Funct Mater. 2014;24:6999–7006. [Google Scholar]
- 138.Zhou T, Zhao X, Liu L, Liu P. Preparation of biodegradable PEGylated pH/reduction dual-stimuli responsive nanohydrogels for controlled release of an anti-cancer drug. Nanoscale. 2015;7:12051–12060. doi: 10.1039/c5nr00758e. [DOI] [PubMed] [Google Scholar]
- 139.Li Z, Xu W, Wang Y, Shah BR, Zhang C, Chen Y, Li Y, Li B. Quantum dots loaded nanogels for low cytotoxicity, pH-sensitive fluorescence, cell imaging and drug delivery. Carbohydr Polym. 2015;121:477–485. doi: 10.1016/j.carbpol.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 140.Hsieh CJ, Chen YC, Hsieh PY, Liu SR, Wu SP, Hsieh YZ, Hsu HY. Graphene oxide based nanocarrier combined with a pH-sensitive tracer: a vehicle for concurrent pH sensing and pH-responsive oligonucleotide delivery. ACS Appl Mater Interfaces. 2015;7:11467–11475. doi: 10.1021/acsami.5b02397. [DOI] [PubMed] [Google Scholar]
- 141.Treiber C, Quadir MA, Voigt P, Radowski M, Xu S, Munter LM, Bayer TA, Schaefer M, Haag R, Multhaup G. Cellular copper import by nanocarrier systems, intracellular availability, and effects on amyloid β peptide secretion. Biochemistry. 2009;48:4273–4284. doi: 10.1021/bi900290c. [DOI] [PubMed] [Google Scholar]
- 142.Tang C, Amin D, Messersmith PB, Anthony JE, Prud’homme RK. Polymer directed self-assembly of pH-responsive antioxidant nanoparticles. Langmuir. 2015;31:3612–3620. doi: 10.1021/acs.langmuir.5b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y, Chen L, Tan L, Zhao Q, Luo F, Wei Y, Qian Z. PEG–PCL based micelle hydrogels as oral docetaxel delivery systems for breast cancer therapy. Biomaterials. 2014;35:6972–6985. doi: 10.1016/j.biomaterials.2014.04.099. [DOI] [PubMed] [Google Scholar]
- 144.Koetting MC, Peppas NA. pH-Responsive poly(itaconic acid-co-N-vinylpyrrolidone) hydrogels with reduced ionic strength loading solutions offer improved oral delivery potential for high isoelectric point-exhibiting therapeutic proteins. Int J Pharm. 2014;471:83–91. doi: 10.1016/j.ijpharm.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gogoi N, Chowdhury D. Novel carbon dot coated alginate beads with superior stability, swelling and pH responsive drug delivery. J Mater Chem B. 2014;2:4089–4099. doi: 10.1039/c3tb21835j. [DOI] [PubMed] [Google Scholar]
- 146.Du J-Z, Du X-J, Mao C-Q, Wang J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc. 2011;133:17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 147.Yu H, Xu Z, Chen X, Xu L, Yin Q, Zhang Z, Li Y. Reversal of lung cancer multidrug resistance by pH-responsive micelleplexes mediating co-delivery of siRNA and paclitaxel. Macromol Biosci. 2014;14:100–109. doi: 10.1002/mabi.201300282. [DOI] [PubMed] [Google Scholar]
- 148.Gui W, Wang W, Jiao X, Chen L, Wen Y, Zhang X. Dual-cargo selectively controlled release based on a pH-responsive mesoporous silica system. Chem-PhysChem. 2015;16:607–613. doi: 10.1002/cphc.201402732. [DOI] [PubMed] [Google Scholar]
- 149.Ju Y, Cui J, Müllner M, Suma T, Hu M, Caruso F. Engineering low-fouling and pH-degradable capsules through the assembly of metal-phenolic networks. Biomacromolecules. 2015;16:807–814. doi: 10.1021/bm5017139. [DOI] [PubMed] [Google Scholar]
- 150.Tu F, Lee D. Shape-changing and amphiphilicity-reversing Janus particles with pH-responsive surfactant properties. J Am Chem Soc. 2014;136:9999–10006. doi: 10.1021/ja503189r. [DOI] [PubMed] [Google Scholar]