SUMMARY
Hypersomnolence plays a sizeable role in the course and morbidity of psychiatric disorders. Current sleep medicine nosology is reliant on the multiple sleep latency test (MSLT) to segregate hypersomnolence associated with psychiatric disorders from other central nervous system causes. However, the evidence base regarding sleep propensity in psychiatric hypersomnolence as measured by the MSLT has not been systematically evaluated, which is vital to clarify the utility and validity of current nosological schema. In this review, the use of sleep propensity assessed by the MSLT in patients with psychiatric hypersomnolence is systematically evaluated, using both qualitative and quantitative assessment. Findings demonstrate high heterogeneity and potential for bias among studies, with a pooled estimate of sleep propensity among patients with psychiatric hypersomnolence similar to normative values. Additionally, approximately 25% of patients with psychiatric hypersomnolence demonstrate a mean sleep latency below 8 minutes, the current cutpoint to define pathologic sleepiness. These data underscore the limitations of the MSLT in segregating psychiatric hypersomnolence from other central nervous system hypersomnias. Further research is warranted to evaluate novel measures and biomarkers of excessive sleepiness to advance clinical practice, as well as dimensional approaches to classification of hypersomnolence disorders.
Keywords: hypersomnolence, sleepiness, psychiatric, multiple sleep latency test, depression
INTRODUCTION
Hypersomnolence, broadly defined as excessive daytime sleepiness (EDS) and/or excessive sleep duration, commonly occurs in patients with psychiatric disorders. Hypersomnolence plays a significant role in the course of psychiatric illness, particularly mood disorders, and is associated with treatment resistance, symptomatic relapse, increased risk of suicide, and functional impairment [1–6]. Despite its importance, there has generally been limited research on hypersomnolence in psychiatric disorders, particularly related to objective measures of sleepiness in these patients.
The multiple sleep latency test (MSLT) is widely considered the gold standard measure of daytime sleepiness, and as such, it is commonly used in the practice of sleep medicine for diagnostic purposes [7, 8]. It is generally accepted that there is no objective evidence that patients with mood disorders have abnormal mean sleep latency on the MSLT [9]. As a result, nosologies central to the practice of sleep medicine have emphasized that psychiatric hypersomnolence is characterized by sleep latencies that are often within normal limits, in contrast to other central nervous system (CNS) hypersomnias [8, 10].
However, despite the importance of hypersomnolence in psychiatric disorders and the reliance on the MSLT to guide diagnostic classification in sleep medicine, there have been no systematic reviews conducted to synthesize the literature and estimate objective sleep propensity in this patient population, a vital component of evidence-based medicine [11]. Thus, the primary aim of this review was to systematically analyze the available literature regarding sleep propensity in psychiatric hypersomnolence as measured by the MSLT, with the intention that such a synthesis would inform clinical practice and further refine the nosology of sleep disorders.
METHODS
Criteria for considering studies of this review
Types of participants
Studies that employed the MSLT to assess sleep propensity in patients or research subjects with psychiatric disorders were included. Since MSLT protocols utilized in research and clinical settings can vary [7], the operationalized definition of an MSLT utilized in this study required multiple repeated nap opportunities occurring within the same day, during which the participant tried to fall asleep, and latency to sleep was quantified. Studies were limited to those assessing adults, as there is limited normative data for the MSLT in pediatric populations [7].
Types of studies
All studies that utilized the MSLT to evaluate hypersomnolence in persons with psychiatric disorders were considered. Studies were included that reported MSLT findings in psychiatric disorders, even if such measures were not a primary aim of the study (e.g., a drug-study evaluating the efficacy of a pharmacologic treatment on sleep propensity would be included, as long as MSLT findings at baseline were reported).
Search Strategy
Searches were conducted using Pubmed and PsychINFO, as well as “waterfall” and “ancestral” searches of related materials. There were no limitations on year of publication or language of article. The following search parameters were utilized: (psychiatr* OR depress* OR mood OR anxiety OR "attention deficit" OR ADHD OR ADD OR schizophr* OR bipolar) AND (hypersom* OR mslt OR "multiple sleep latency test" OR "mean sleep latency"). Both peer reviewed publications and unpublished literature (meeting abstracts, dissertations/theses, etc.) were considered, since the likelihood of unpublished studies, and thus publication bias, is higher in studies of diagnostic tests [12]. The author conducted all searches. The last search was performed November 3, 2015.
Eligibility
The following criteria were required for inclusion: 1) use of MSLT (according to operational definition) to quantify sleep propensity and 2) evaluation of sleep propensity of patients/subjects with psychiatric diagnoses with clinical symptoms of excessive sleepiness and/or sleep duration. Exclusion criteria included: 1) individual nap opportunities occurring on different days, 2) use of non-EEG based method to derive sleep onset (e.g. self-report, actigraphy), 3) testing performed under experimental conditions that were not standard for the patient/subject (e.g. MSLT performed after sleep deprivation), and 4) insufficient data for qualitative analysis or quantitative meta-analysis.
Data Extraction
The author extracted all data (unblinded). Extracted data included: author/journal, year of publication, study design, number of MSLT naps, definition of sleep latency, number and demographics (ages and sex), psychiatric diagnoses, and mean sleep latency. Sleep onset REM periods (SOREMs) were also noted, if reported. Study quality was assessed (unblinded) by the author using the Methodological Index for Non-Randomized Studies (MINORS) rating scale [13].
Analysis
All studies that met inclusion/exclusion criteria were analyzed in the qualitative assessment of the literature on this topic. In addition, since normative data for the MSLT exists for 4–5 nap protocols, studies that utilized fewer naps (i.e. two nap protocols), were excluded from the quantitative meta-analysis. Attempts were made to obtain data from studies that did not report sufficient data for meta-analysis, but might otherwise qualify for inclusion. This included contacting corresponding authors of studies in which the MSLT was employed in psychiatric populations and an assessment of hypersomnolence was obtained in the study (e.g. Epworth sleepiness scale) even if it was not used to segregate groups in the primary study, since this subset of data might be applicable to these analyses.
Meta-analysis was performed using random-effects model (DeSimonian-Laird), utilizing OpenMetaAnalyst, an open-source, cross-platform software for advanced meta-analysis (http://www.cebm.brown.edu/open_meta/) [14]. The primary variable of interest was mean sleep latency on MSLT. Secondary variables of interest were proportions of patients with mean sleep latency (MSL) less than 8 and 5 minutes, as these cutpoints have been used to define pathologic sleepiness [8, 10, 15]. I2 was utilized to assess heterogeneity among studies, with cutoffs 0%, 25%, 50%, and 75% used to define no, low, moderate, and high heterogeneity [16, 17]. It was anticipated a priori that likely confounders that would affect meta-analysis could include age, sex, MSLT protocol variables (number of naps, definition of sleep latency), psychiatric diagnoses, and definition of hypersomnolence (i.e. excessive sleepiness vs. excessive sleep duration).
RESULTS
Study Inclusion and Assessment
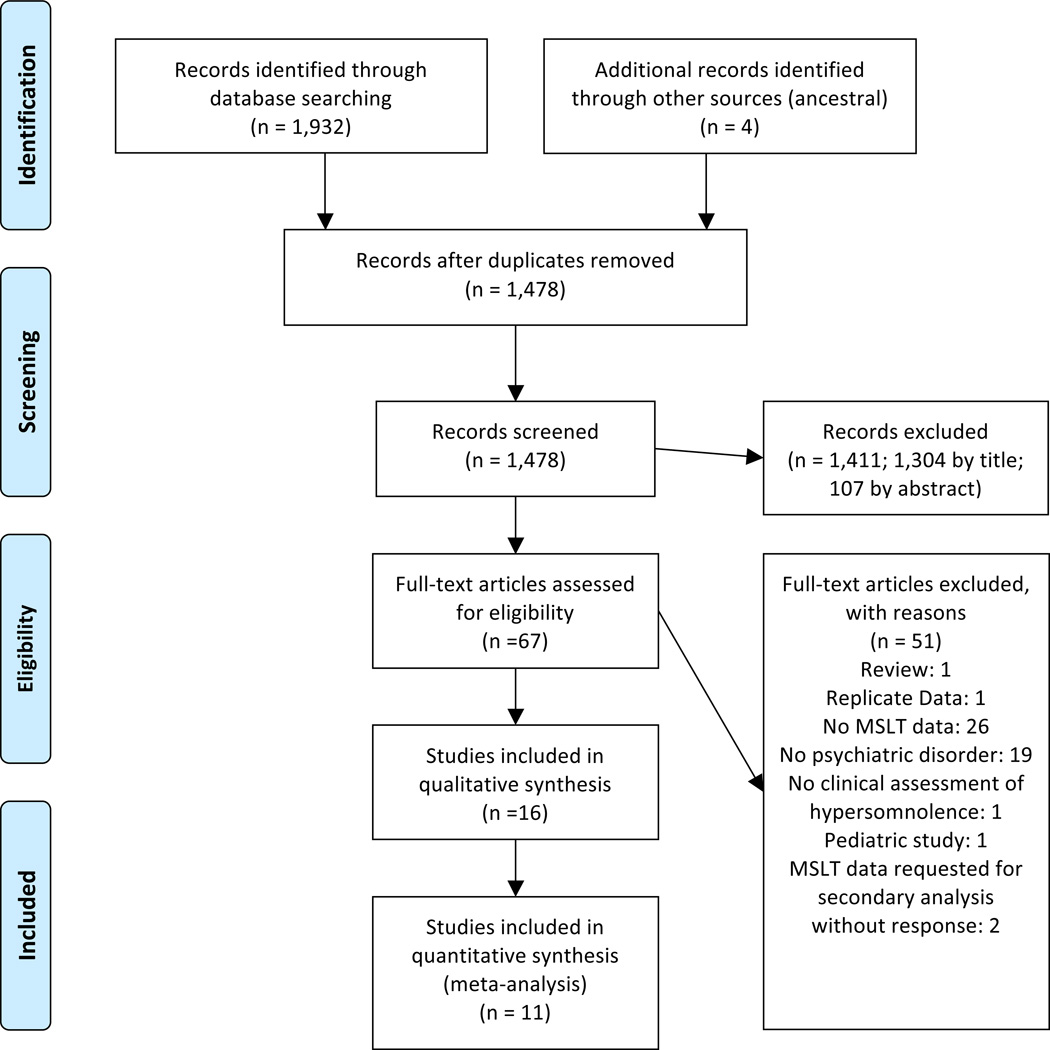
The Preferred Reporting Items for Systematic Reviews (PRISMA)[18] flow diagram is presented in Figure 1. After removal of duplicates, database and other searches identified 1,478 possible records, which were subsequently screened. Reasons full-text articles were excluded are enumerated in Figure 1. Ad hoc data from two studies in which psychiatric patients with ESS>10 were able to be obtained, were subsequently included in analyses (Schredl M, personal communication; Delesie L, personal communication) [19, 20]. Additionally, MSLT data exclusively for patients with psychiatric disorders were obtained from a study in which results were originally presented in the published manuscript combined with behaviorally induced insufficient sleep syndrome (Peter-Derex L, personal communication) [21]. One study [22] utilized a group of dysthymic hypersomnolent patients that were a subset of patients published in a prior report (Billiard M, personal communication) [23]. Because the study utilizing a subset of patients also included a healthy control group not included in the parent study, the smaller study was included in the qualitative synthesis, but excluded from meta-analysis to avoid biasing results. One included study was published in German [24] and the article was translated using Google Translate (http://translate.google.com); otherwise, all articles were published in English. In all, sixteen studies met inclusion/exclusion criteria for qualitative review [19–34], eleven of which met inclusion criteria for quantitative meta-analysis of the primary variable of interest, MSL among patients with psychiatric hypersomnolence (Figure 1). Notably, two studies did not have sufficient data to include in the pooled analysis of MSL, but did report sufficient data to be included in secondary analyses of the proportion of patients with MSL <5 and/or <8 minutes [24, 34].
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Qualitative synthesis
The definitions and criteria used to define hypersomnolence varied among studies (Table 1). The majority of studies considered patients with a complaint of excessive daytime sleepiness, however, precise definitions were often not described. In some cases, all patients had evidence of excessive sleepiness using a validated subjective instrument such as the ESS (e.g. [29, 31]), however the majority of studies did not employ such requirements as part of inclusion/exclusion criteria. One study focused primarily on excessive time in bed and nap duration, rather than difficulty with daytime vigilance [28].
Table 1.
Table of Evidence.
| Study | Study design (MINORS score) |
Sample Size/Age/G ender |
Nosology/ Diagnoses |
#Naps /SL definition |
MSL±SD (minutes) |
Notes |
|---|---|---|---|---|---|---|
| Bassetti et al. 2003 [29] | Case review of consecutive patients with hypersomnia (18) | 6/40±14.7/1M, 5F | ICSD criteria/3 depression, 3 affective d/o | 5/NR | 7.83±3.97 | All patients complained of EDS and had ESS>10; No SOREMs among mood d/o; 4/6 with MSL<8 min; 2/6 with MSL=5 min; 0/6 with MSL<5 min |
| Billiard et al. 1994 [23] | Database review of subjects given diagnosis of hypersomnia associated with a mood d/o (14) | 36/median 45 (range 23–68)/29M, 7F | 31 dysthymia, 4 bipolar d/o (3 depressed; 1 manic), 1 major recurrent depression | 5*/NR | 12.40±5.51 | All subjects were drug free for at least 15 days; 8/36 had MSL<7 ; 13/36 had MSL ≤10; MSLT came after 22:30–7:30 night of sleep and was followed by second night of uninterrupted sleep |
| Dolenc et al. 1996 [22] | Case-control, patients referred with a complaint of excessive night and daytime sleepiness (15) | 12/median 37, range 23–57/7M, 5F | Dysthymia; diagnosis based on clinical interview | 5/NR | 13±1 (unclear if ±SD or ±SEM) | Dysthymic subjects were a subset of patients reported in Billiard et al. 1994* |
| Kayumov et al. 2000 [33] | Consecutive depressed patients attending sleep and alertness clinic for assessment of sleep disturbance (12) | 22/42.6±13.5/11M, 11F | Clinical assessment based on DSM-IV criteria | 4–5/NR | NR | Only correlations between PSG parameters, MSLT, and MWT reported; patients were free of psychotropic medications |
| Kofmel et al. 2014 [31] | Retrospective comparison of patients previously studied at outpatient clinic (15) | 19/37.4±11.4/7M, 12F | ICD-10 criteria; depressive episode, recurrent depressive d/o, and dysthymia | NR/NR | 8.1±4.0 | Inclusion required ESS>10; 5/19 were on antidepressants; No SOREMs in psychiatric group; 12/19 had MSL<8 minutes |
| Mariman et al, 2013 [19] | Prospective evaluation of patients admitted to tertiary center for evaluation of unexplained chronic fatigue; subset with ESS>10* (11) | 12/34.5±10.6 years/1M,11F | Assessed with integrated diagnostic pathway | 4/NR | 14.2±4.4 | No patients had MSL<5 minutes; 1 had MSLT<8 minutes (mood d/o); SOREMs not reported; MSL12.8±4.8 in 6 patients with mood d/o vs. 15.5±3.9 in 6 patients with other Axis 1 d/o |
| Nofzinger et al. 1991 [28] | Prospective study; hypersomnia defined as spending 1 or more hours in bed each day than usual and napping more than 30 minutes most days (19) | 25/36.6±11.9/10M,15F | RDC; Bipolar outpatients | 5/NR | 13.7±3.82 | 2-week drug and alcohol washout prior to testing; only 20% of bipolar depressed group fell asleep during all 5 naps; No bipolar patients had MSL<5 minutes; 2 bipolar patients had 1 SOREM and 2 had 2 SOREMs |
| Peter-Derex et al. 2013 [21] | Prospective inclusion of patients admitted to sleep unit for EDS; subset of patients with psychiatric d/o* (18) | 27 (PC)/37.44±13.16/NS | ICSD-2; nearly all had depression and 2 had anxiety | NR/NR | 13.16±3.247 | 2 had MSL<8; 0 had MSL<5 min |
| Reynolds et al. 1982 [26] | Drug-free patients with a history of excessive daytime sleepiness (13) | 7/37.6±14.3/2M, 5F | RDC; Primary non-delusional depression | 4/≥3 epochs of stage 1 or 1 epoch of stage 2 sleep | 9.975±5.70 | Patients were psychotropic and alcohol free for at least 2 weeks prior to studies; only 1 depressed patient had 1 SOREM; If patient did not fall asleep, that nap was excluded from analysis |
| Sangal et al.1992 [34] | Series of consecutive patients whose clinical presentation required evaluation for EDS, evaluated with MSLT and MWT (17) | 28/NR/NR | Diagnosis of depression not defined | 4/1st epoch of any stage of sleep | NR | 6/28 depressed patients had MSL<7.3 min on MSLT; 10/28 had low (<29.38 min) MWT scores; 9/28 had low MWT but high MSLT scores; authors estimate >95% of all patients were free of CNS active meds prior to testing |
| Shen et al. 2011 [30] | Depressed patients participating in an open-label study of mirtazapine for sleepiness and fatigue (9) | 16/NR/NR | Depression with scores on RSD-17≥17, BDI-II≥15, CES-D≥16 | 4–5/NR | 7.8±5.0 | Subjects free of psychotropic meds for 2 weeks prior to study; Probably demographics 47.1±11.6 years (range 29–60), 2M, 14F based on prior report [55] |
| Sobanski et al, 2014 [20] | Adults with ADHD recruited from consecutive referrals versus healthy controls: subset with ESS>10* (19) | 6/34.33±6.86 years/5M,1F | ADHD diagnosed according to DSM-IV and according to expert consensus | “most” had 5/first epoch of sleep | 10.27±4.32 | Participants were off medications >4 weeks; Excluded if BDI was>10 or positive urine drug screen; 1 patient with MSL≤ 5 minutes; 3 with MSL≤ 8 minutes |
| van den Hoed et al. 1981 [25] | Consecutive series of 100 sleep apnea free patients with complaint of EDS (16) | 18/34.1±9.0/NR | Final diagnosis recorded in patient’s chart following ASDC Criteria; included depressive ad nonaffective d/o | 5/latency to 2 epochs of stage 1 or 1 epoch of any other stage | 10.6±5.2 | 2/18 had MSL<5 minutes; 11/18 had MSL<11 minutes; no psychiatric patients were on stimulants at the time of their study |
| Vgontzas et al. 2000 [32] | Consecutive patients with a chief complaint of EDS (18) | 23/39.9±11.5/NR | Semistructured psychiatric interview following DSM-IV; 12 mood, 6 somatoform, 4 anxiety, 1 personality d/o | 2/≥1 min of any stage, but if stage 1, had to be followed by at least 1 minute consecutively by any other sleep stage | 33.3±13.9 | Naps were 60 minutes; Patients with SOREMs on PSG or either nap were excluded; Patients on psychotropic medications were excluded; 36% (21/59) of primary hypersomnia comparison group met criteria for a secondary psychiatric d/o (primarily mood d/o) |
| Volk et al. 1992 [24] | Case series of patients with EDS (14) | 15/38.8±13.8/8M,7F | Endogenous depression or dysthymic d/o according to DSM-IIIR | 5/1st epoch NREM sleep | NR | No REM was observed during naps in psychiatric group; All psychiatric hypersomnia had MSL>10 minutes |
| Zorick et al. 1982 [27] | Database Review of 161 patients referred for EDS complaint (12) | 13/43.9±13.5/5M,8F | ASDC; specific psychiatric d/o not identified | 4/latency to stage 1 | 9.97±8.67 | ~11% of naps had SOREMs in psychiatric group; MSL and SD estimated from graphs |
ADHD=attention deficit hyperactivity disorder; ASDC=Association of Sleep Disorders Centers; BDI=Beck Depression Inventory; CES-D=Center for Epidemiologic Studies Depression scale; CNS=central nervous system; DSM=Diagnostic and Statistical Manual; EDS=excessive daytime sleepiness; ESS=Epworth Sleepiness Scale; F=female; HRSD=Hamilton Rating Scale for Depression; ICD=International classification of disease; ICSD=International Classification of Sleep Disorders; M-male; MSLT=multiple sleep latency test; MSL-mean sleep latency; MWT=maintenance of wakefulness test; NR=not reported; RDC=Research Diagnostic Criteria; PSG=polysomnography; SD=standard deviation; SEM=standard error of the mean; SOREM=sleep onset rapid eye movement;
=see text for details regarding personal communication.
Psychiatric diagnoses of participants were also variable among studies (Table 1). Mood disorders were the most common class of disorders considered [22–24, 26, 28–31, 33, 34]. Frequently, findings were reported for all psychiatric patients as a heterogeneous group comprised of individuals with and without mood disorders [19, 21, 25, 32], or with the composition undefined [27]. One sample consisted exclusively of adults with attention-deficit hyperactivity disorder (ADHD) without depressive symptoms [20].
Despite the variability regarding definitions of hypersomnolence and psychiatric disorders evaluated, all studies examined patients in a relatively narrow age-range (mean/median age 30s–40s). All studies examined groups consisting of both men and women. Among studies that reported gender distributions, the majority had more women than men [19, 27–31], with three studies reporting more men than women [22–24].
Among studies, only three employed the use of a concurrent healthy control group for comparison purposes [20, 22, 32]. One of these studies used a non-standard 2-nap MSLT protocol [32], demonstrating patients with psychiatric hypersomnia had significantly longer sleep latency than healthy controls during the second, but not first nap opportunity. Conversely the other two studies demonstrated patients with dysthymia and ADHD had slightly shorter mean sleep latencies than healthy controls, neither of which reached statistical significance [20, 22]. If a given study involved a comparison of MSLT results between psychiatric hypersomnolence and other patient groups, the most common comparison was made with patients with narcolepsy [21, 25–29, 31, 34].
Five studies reported the number or frequency of SOREMs in patients with psychiatric hypersomnia. Two studies demonstrated no SOREMs within their psychiatric sample [29, 31]. One study had 1 out of 7 patients demonstrate 1 SOREMP, with no patients with multiple SOREMs [26]. In a study of hypersomnolent bipolar patients, two patients had 2 SOREMs and 2 had 1 SOREM out of 25 total patients [28]. One study reported that approximately 11% of naps in the psychiatric group demonstrated a SOREM, but did not report proportions of patients with 1 or 2 SOREMs [27].
High potential for bias was a significant issue in the majority of published studies. No studies explicitly reported the use of blinding in study design. In many reports, it was not clear if the diagnosis was established before or after MSLT, or how MSLT findings were interpreted when segregating patients into diagnostic categories. Additionally, many studies were non-consecutive, increasing the potential for sampling bias.
Quantitative synthesis
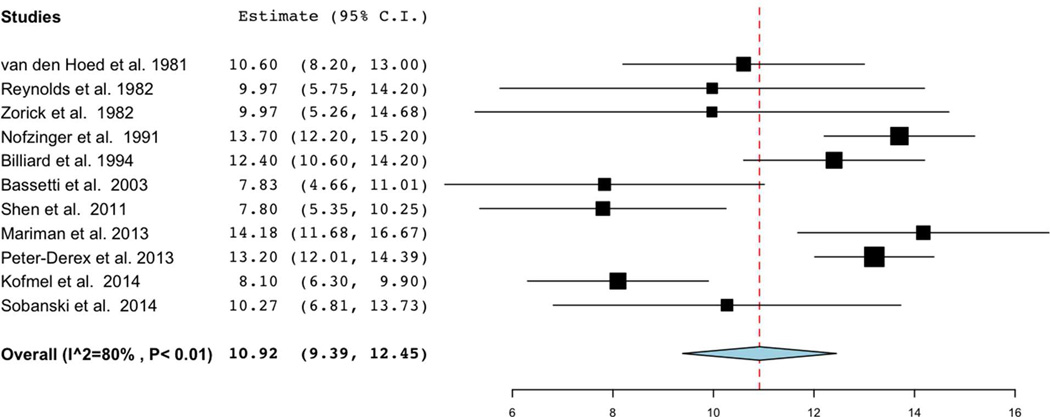
Eleven studies met criteria for inclusion in meta-analysis of mean sleep latency on MSLT [19–21, 23, 25–31], the primary variable of interest. Reasons studies were included in qualitative analysis but not primary meta-analysis were as follows: absence of MSL values [33, 34], absence of estimate of variance of MSL [24], non-standard MSLT protocol [32], and replicate data [22]. Meta-analysis demonstrated a pooled estimate of MSL of 10.92 minutes (95% CI: 9.39, 12.45), with notably high heterogeneity among studies (I2=80%) (Figure 2). Exploratory analyses to identify sources of heterogeneity, which included evaluating studies segregated by number of naps in MSLT protocol, definitions of sleep latency, criteria for hypersomnolence, and class of psychiatric disorder, did not appreciably affect heterogeneity. Notably, six studies had individual confidence intervals for which the lower bounds was below 8 minutes [20, 26, 27, 29–31], but only one small study had a lower bounds below 5 minutes [29].
Figure 2.
Forest plot of mean sleep latency (minutes) on MSLT in psychiatric hypersomnolence. Note high heterogeneity (I2=80%) among studies, and pooled estimate of 10.92 (95%CI: 9.39–12.45 minutes).
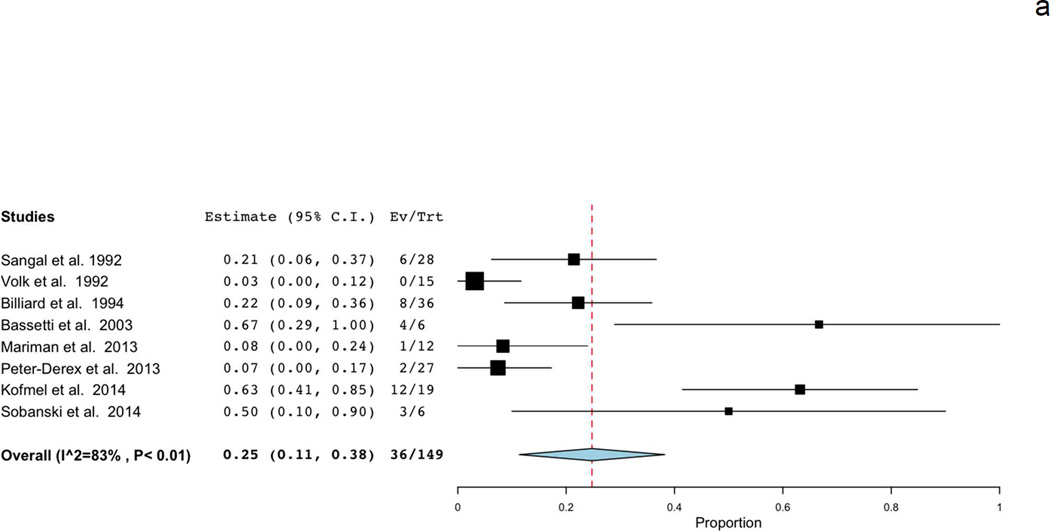
Secondary meta-analyses that examined proportions of patients with MSL below 8 and 5 minutes included different studies than the primary analysis due to availability of data. Eight studies had sufficient data to estimate the proportion of patients with MSL<8 minutes [19–21, 23, 24, 29, 31, 34]. The pooled estimate was 0.25 (95%CI: 0.11, 0.38), with high heterogeneity among studies (I2=83%) (Figure 3a). Similar to overall MSL analysis, a source of heterogeneity among studies (type of psychiatric disorder, number of naps, etc.) could not be identified. One included study reported the proportion of patients with MSL<7.3 minutes rather than 8 minutes [34], however exclusion of this study from the pooled estimate did not substantially alter findings (pooled estimate 0.26, 95%CI: 0.11, 0.41).
Figure 3.
Forest plots of proportions of patients with psychiatric hypersomnolence demonstrating mean sleep latency A) below 8 minutes and B) below 5 minutes on MSLT. Estimates based on available data.
Seven studies had sufficient data to estimate the proportion of patients with MSL<5 minutes [19–21, 24, 25, 28, 29]. The pooled estimated was 0.03 (95%CI: 0.00, 0.06), without heterogeneity among studies (I2=0%)(Figure 3b). In one study, 2 patients had MSL equal to 5 minutes, however, inclusion of these participants in the estimate did not alter findings [29].
DISCUSSION
This systematic review and meta-analysis demonstrates that patients with psychiatric hypersomnolence demonstrate mean sleep latency on the MSLT comparable to normative values [7]. In addition, similar to the general population [35], roughly one-quarter of patients with psychiatric hypersomnolence will have MSL below 8 minutes on the MSLT. Although these findings must be interpreted with caution in light of significant heterogeneity and potential for bias among studies, they have significant implications for both nosological classifications in Sleep Medicine, as well as clinical practice.
The MSLT, although the most widely utilized tool for objectively measuring sleep propensity, has significant limitations when applied to patients with complaints of hypersomnolence. In general, there is no perfect cutoff to define pathological sleep propensity on the MSLT—all will result in a significant number of normal persons defined as having pathological sleepiness (false positives), or miss patients with true hypersomnolence (false negatives) [36]. From a nosological standpoint, this limitation of the MSLT has been demonstrated by shifting cutoffs used to define disorders of hypersomnolence over time. The first edition of the ICSD required a MSL of <5 minutes for a diagnosis of narcolepsy, but <10 minutes for idiopathic hypersomnia (IH) [37]. Starting with the ICSD-2, the pathological cutoff of <8 minutes was applied to both narcolepsy and IH [10]. Although done for simplicity, this change altered the sensitivity and specificity of the MSLT differentially for these disorders, and was recognized as being highly debatable at the time [38]. A perhaps unintended consequence of simplifying the ICSD was to create a de facto cutpoint to delineate pathologic from subjective sleepiness, which may have limited utility in a number of disorders, particularly since a sizeable proportion of the general population will demonstrate MSL values below this cutpoint [39].
In the case of a healthy individual with no complaint of sleepiness who demonstrates such ‘high sleepability’ on the MSLT [40], the term ‘false positive’ is clearly applicable. However, in the case of a patient with psychiatric hypersomnolence who demonstrates pathologic sleep latency on the MSLT, who also has a clinical complaint of sleepiness, the use of the term ‘false positive’ is a misnomer. The results of this meta-analysis suggest that roughly 25% of patients with psychiatric hypersomnolence will demonstrate objective pathological sleep propensity using a MSL cutoff of 8 minutes, however, very few, if any, will have a MSL below 5 minutes. The use of the MSL cutpoint of 5 versus 8 minutes to define pathologic sleepiness marginally alters test performance in the diagnosis of narcolepsy [41], but substantially affects the ability to diagnose IH, which tends to have higher MSL than narcolepsy, with mean values of approximately 8 minutes reported in large cohorts [42, 43].
Delineating IH from psychiatric hypersomnolence can be one of the most difficult diagnostic challenges faced in the practice of sleep medicine. In patients who do not have a psychiatric disorder, segregating the two is not difficult, as psychiatric hypersomnolence by definition requires the presence of a psychiatric condition. However, because up to 20–35% of patients with IH have co-occurring psychiatric symptoms or meet criteria for a secondary psychiatric disorder [32, 43–45], most commonly depression, distinguishing the two can be particularly challenging in such instances. Roughly 60% of patients with IH will demonstrate a MSL<8 minutes [42, 43], which is certainly higher than the approximately 25% of patients with psychiatric hypersomnolence estimated by this meta-analysis. However, there is also clearly sizeable overlap between these two groups using a threshold for pathologic sleepiness of 8 minutes. Notably, when considering the differential diagnosis of IH, recent nosologies have noted that “[t]he MSLT in hypersomnia associated with a psychiatric disorder does not demonstrate a short mean sleep latency” [8]. As a result, many practitioners have subsequently adopted the strategy of depending on MSLT results to segregate IH from psychiatric hypersomnolence, with patients with MSL below 8 minutes categorized as the former and those above 8 minutes as the latter. The results of this meta-analysis, as well as the sizeable portion of patients with IH who do not demonstrate MSL below 8 minutes, clearly demonstrate the potential pitfalls of reliance on this diagnostic strategy.
In instances where MSL is less than 5 minutes, data from this meta-analysis suggest a lower probability of psychiatric hypersomnolence, however, in clinical practice many IH patients will not have MSL below 5 minutes. In fact, roughly 40% of IH patients may have MSL above 8 minutes, and among long sleepers, over 70% of IH patients have a MSL above 8 minutes and 50% above 10 minutes on the MSLT [43]. As a result, the recently updated ICSD-3 now includes criteria for excessive sleep duration, objectively measured by 24-hour EEG or actigraphy, as an alternative to pathological sleep propensity in the diagnosis of IH [8]. In this context, it is noteworthy that the only study included in this systematic review that universally defined hypersomnolence in psychiatric patients based on sleep duration rather than excessive sleepiness had a relatively higher MSL compared to many other studies [28]. Additionally, Billiard et al. reported a subset (n=5) of hypersomnolent mood-disordered patients in their study slept longer than 9 hours on ad libitum electroencephalographic recordings, none of whom demonstrated a MSL below 11 minutes [23]. Thus, systematic evaluation of sleep duration in psychiatric hypersomnolence as compared to IH may be a fruitful area of investigation to clarify how objective sleep duration segregates disorders, and what threshold (i.e. 9, 10, 11 hours) provides optimal diagnostic performance in discriminating between psychiatric hypersomnolence and IH.
However, a more constructive strategy may be to consider hypersomnolence as a comorbid phenomenon with a given psychiatric illness, rather than a problem secondary to or caused by a specific disorder. Such a perspective shift has already occurred for insomnia, and is reflected in both the ICSD-3 and the most recent Diagnostic and Statistical Manual (DSM-5), the primary nosology used in psychiatry [46]. This change has resulted in large part from the now widely appreciated bidirectional relationship between sleep disturbance and mental illness [47]. However, it is noteworthy that a conceptual schism still exists between the DSM-5 and ICSD-3 when considering hypersomnolence, with the former nosology adopting greater flexibility to consider hypersomnolence disorder as comorbid with other psychiatric illnesses, while the ICSD-3 emphasizes hypersomnolence associated with psychiatric illness as distinct from other CNS hypersomnias [8, 46]. Given high rates of psychiatric diagnoses in patients with primary hypersomnia disorders in prior investigations [32, 44], which were notably not included in this meta-analysis, coupled with the observation that for some of these patients, the severity of hypersomnolence may parallel the severity of mood symptoms [44], future studies that examine MSLT findings in patients meeting criteria for DSM-5 hypersomnolence disorder, with and without psychiatric comorbidities, may further elucidate the utility of the MSLT in these patients.
In a related vein, a significant limitation of the literature examining psychiatric hypersomnolence is the high potential for bias influencing results. Many studies were conducted as retrospective chart reviews in which the potential for bias, particularly in light of the aforementioned nosological considerations, are substantial. If MSLT results are considered as part of the diagnostic strategy, there is a high likelihood that patients with pathological sleep latencies will be classified as having CNS hypersomnias other than psychiatric hypersomnolence, which would lead to an underestimate of the true mean sleep latency. Evidence, though circumstantial, that bias is an important factor in studies that were considered in this systematic review is the high heterogeneity among studies, which could not be explained by obvious clinical or methodological diversity. The importance of continuous and systematic evaluation of the literature to minimize the influence of bias, particularly in the delineating nosology in sleep medicine, is evidenced by the fact that comprehensive reviews on the topic of hypersomnolence in mood disorders [3, 9] and all ICSD editions [8, 10, 37] in sum only referenced 4 out of 16 studies identified in this systematic review [22, 23, 28, 32], of which only 2 [23, 28] were ultimately included in the meta-analysis of MSL values, for reasons previously described.
In addition to systematic review of the evidence base, prospective research designs in which diagnostic categories are clearly determined prior to MSLT, thus minimizing bias, are required to more accurately estimate MSL in psychiatric hypersomnolence. Moreover, the use of healthy comparison groups would be particularly meaningful in light of the limited normative data for the MSLT [7]. Finally, supporting clinical and polysomnographic findings suggested as ancillary measures to segregate CNS hypersomnias [8], such as excessive sleep inertia and sleep efficiency, also require systematic and prospective evaluation to verify their clinical and diagnostic utility.
Ultimately, however, it may be more pragmatic to supplement current nosological categories that depend on the MSLT to segregate CNS hypersomnias not characterized by hypocretin/orexin deficiency with a dimensional approach to evaluation and treatment. This may be particularly salient given problems with repeatability of the MSLT in patients with hypersomnolence, as the test-retest reliability of the MSLT in patients with CNS hypersomnias (without cataplexy) has recently been demonstrated to be poor, with 40% of patients demonstrating a change in MSL crossing the conventional boundary of 8 minutes [48].
The MSLT quantifies the ability to fall asleep on multiple repeated nap opportunities, and as such measures sleep propensity under soporific conditions, but does not quantify facets of sleepiness such as excessive sleep inertia or the ability to maintain vigilance, highlighting the need move beyond the MSLT in sleep research and clinical care [49]. Hypersomnolence is a multi-faceted construct unlikely to be entirely quantified by a single measure, and as such there is a vital need to develop, validate, and apply additional measures of sleepiness [50]. It has long been appreciated that the MSLT and maintenance of wakefulness test (MWT), which measures the ability to stay awake under soporific conditions, can be discordant [34]. This is particularly pertinent for hypersomnolence in mood disorders, in which nearly half of hypersomnolent depressed patients demonstrate objective abnormalities on one of the two tests when administered as part of a combined protocol [34]. Infrared pupillometry, which measures pupillary oscillations in darkness, has also demonstrated potential utility to quantify drowsiness in mood-disordered patients [31]. Other measures of neurobehavioral alertness, such as the psychomotor vigilance task (PVT), which correlates poorly with the MSLT [51], may also be useful in the diagnosis of CNS hypersomnias and measuring response to treatment [52, 53]. Finally, the use of auditory evoked potentials as an objective measure of sleep inertia has also shown promise as a measure in the assessment of CNS hypersomnias [21].
Development and application of other objective measures of hypersomnolence has the potential benefits of improved diagnostic accuracy as well as measuring response to treatment in specific domains to document efficacy. However, like the MSLT, such measurements do not directly address the neurobiology underlying clinical symptomatology. Emerging hypotheses and evaluation strategies for CNS hypersomnias, such as assessment for γ-aminobutyric acid-related hypersomnolence, offer the promise of identifying specific pathological causes of symptoms in patients toward which treatment may be directed [54]. However, as CNS hypersomnias are likely to be a heterogeneous group of disorders, the use of diagnostic testing to measure domains of impairment are likely to remain a vital component of clinical care for the foreseeable future.
There are limitations of this meta-analysis that merit discussion. First, the identified studies examined a relatively narrow age-range of patients, and thus results should not be extended to adolescent or geriatric populations, particularly in light of age-related effects on the MSLT [7]. Second, although sizeable efforts were made to include all relevant studies, it is possible that the systematic search strategy and additional efforts to contact authors for additional data/clarification employed did not capture all relevant data. Third, the MSL values were measured in patients with presumed active psychiatric disorders, and it is unclear whether these values would change with the resolution of psychiatric symptoms (consistent with a state-related measure), or remain constant (reflecting a trait phenomenon).
CONCLUSIONS
In summary, this systematic review and meta-analysis demonstrates that patients with psychiatric hypersomnolence demonstrate sleep propensity on the MSLT on par with the general population, including approximately 25% of patients demonstrating pathological sleep latencies according to current diagnostic standards. These results highlight the limitations of the MSLT as an exclusive measure of sleepiness, and the need to improve objective measures for quantifying distinct facets of hypersomnolence in the evaluation and treatment of CNS hypersomnias.
Practice Points.
Roughly 1 in 4 patients with psychiatric hypersomnolence will demonstrate mean sleep latency below 8 minutes on multiple sleep latency testing
The MSLT has significant limitations as a tool to segregate psychiatric hypersomnolence from idiopathic hypersomnia with comorbid psychiatric symptoms, particularly in patients with long sleep duration
A mean sleep latency below 5 minutes on MSLT is less likely to be observed in hypersomnolence related to a psychiatric disorder
Research Agenda.
Prospective studies in which diagnostic categories are determined prior to MSLT and include healthy comparison groups will more accurately determine MSLT values in patients with psychiatric hypersomnolence
Research that examines other facets of hypersomnolence and/or novel biomarkers as a means to discriminate psychiatric hypersomnolence from other CNS hypersomnias is warranted
In lieu of the development of other diagnostic strategies, a dimensional approach to quantifying hypersomnolence may be a more pragmatic and accurate means of evaluating patients with hypersomnolence
Acknowledgments
Conflicts of Interest
Dr. Plante is supported by NIMH (K23MH099234), the Brain and Behavior Research Foundation, and the American Sleep Medicine Foundation. None of the funding sources had any further role in the study design, data collection, analysis and interpretation of the data, and the decision to submit the paper for publication. Dr. Plante has also received unrelated royalties from Cambridge University Press.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- ASDC
Association of Sleep Disorders Centers
- BDI
Beck Depression Inventory
- CES-D
Center for Epidemiologic Studies Depression scale
- CNS
central nervous system
- DSM
Diagnostic and Statistical Manual
- EDS
excessive daytime sleepiness
- ESS
Epworth sleepiness scale
- F
female
- HRSD
Hamilton rating scale for depression
- ICD
International Classification of Disease
- ICSD
International Classification of Sleep Disorders
- IH
Idiopathic hypersomnia
- M
male
- MSLT
multiple sleep latency test
- MSL
mean sleep latency
- MWT
maintenance of wakefulness test
- NR
not reported
- RDC
Research Diagnostic Criteria
- PSG
polysomnography
- SD
standard deviation
- SEM
standard error of the mean
- SOREM
sleep onset rapid eye movement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
* Indicates key references
- 1.Worthington J, Fava M, Davidson K, Alpert J, Nierenberg AA, Rosenbaum JF. Patterns of improvement in depressive symptoms with fluoxetine treatment. Psychopharm Bull. 1995;31:223–226. [PubMed] [Google Scholar]
- 2.Zimmerman M, McGlinchey JB, Posternak MA, Friedman M, Boerescu D, Attiullah N. Differences between minimally depressed patients who do and do not consider themselves to be in remission. J Clin Psychiatry. 2005;66:1134–1138. doi: 10.4088/jcp.v66n0908. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan KA, Harvey AG. Hypersomnia across mood disorders: a review and synthesis. Sleep Med Rev. 2009;13:275–285. doi: 10.1016/j.smrv.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan KA, Gruber J, Eidelman P, Talbot LS, Harvey AG. Hypersomnia in inter-episode bipolar disorder: does it have prognostic significance? J Affect Disord. 2011;132:438–444. doi: 10.1016/j.jad.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein TR, Bridge JA, Brent DA. Sleep disturbance preceding completed suicide in adolescents. J Consult Clin Psychol. 2008;76:84–91. doi: 10.1037/0022-006X.76.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald CT, Messias E, Buysse DJ. Teen sleep and suicidality: results from the youth risk behavior surveys of 2007 and 2009. J Clin Sleep Med. 2011;7:351–356. doi: 10.5664/JCSM.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MSLT and MWT. Sleep. 2005;28:123–144. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Sleep Medicine. International Classification of Sleep Disorders. Third. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 9.Dauvilliers Y, Lopez R, Ohayon M, Bayard S. Hypersomnia and depressive symptoms: methodological and clinical aspects. BMC Med. 2013;11:78. doi: 10.1186/1741-7015-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Sleep M. International Classification of Sleep Disorders: Diagnostic and coding manual. Second. Darien, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 11.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376–380. doi: 10.7326/0003-4819-126-5-199703010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol. 1995;48:119–130. doi: 10.1016/0895-4356(94)00099-c. [DOI] [PubMed] [Google Scholar]
- 13.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 14.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Statistical Software. 2012;49:5. [Google Scholar]
- 15.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariman A, Delesie L, Tobback E, Hanoulle I, Sermijn E, Vermeir P, et al. Undiagnosed and comorbid disorders in patients with presumed chronic fatigue syndrome. J Psychosom Res. 2013;75:491–496. doi: 10.1016/j.jpsychores.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Sobanski E, Alm B, Hennig O, Riemann D, Feige B, Schredl M. Daytime Sleepiness in Adults With ADHD: A Pilot Trial With a Multiple Sleep Latency Test. J Atten Disord. 2014 doi: 10.1177/1087054714529456. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Peter-Derex L, Perrin F, Petitjean T, Garcia-Larrea L, Bastuji H. Discriminating neurological from psychiatric hypersomnia using the forced awakening test. Neurophysiol Clin. 2013;43:171–179. doi: 10.1016/j.neucli.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Dolenc L, Besset A, Billiard M. Hypersomnia in association with dysthymia in comparison with idiopathic hypersomnia and normal controls. Pflugers Archiv. 1996;431:R303–R304. doi: 10.1007/BF02346389. [DOI] [PubMed] [Google Scholar]
- 23.Billiard M, Dolenc L, Aldaz C, Ondze B, Besset A. Hypersomnia associated with mood disorders: a new perspective. J Psychosom Res. 1994;38(Suppl 1):41–47. doi: 10.1016/0022-3999(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 24.Volk S, Dyroff J, Georgi K, Pflug B. Quality of day time sleep in the multiple sleep latency tests in patients with narcolepsy, obstructive sleep apnea and psychogenic hypersomnia. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb. 1992;23:210–214. [PubMed] [Google Scholar]
- 25.van den Hoed J, Kraemer H, Guilleminault C, Zarcone VP, Miles LE, Dement WC, et al. Disorders of excessive daytime somnolence: polygraphic and clinical data for 100 patients. Sleep. 1981;4:23–37. doi: 10.1093/sleep/4.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds CF, Coble PA, Kupfer DJ, Holzer BC. Application of the multiple sleep latency test in disorders of excessive sleepiness. Electroencephalogr Clin Neurophysiol. 1982;53:443–452. doi: 10.1016/0013-4694(82)90009-8. [DOI] [PubMed] [Google Scholar]
- 27.Zorick F, Roehrs T, Koshorek G, Sicklesteel J, Hartse K, Wittig R, et al. Patterns of sleepiness in various disorders of excessive daytime somnolence. Sleep. 1982;5(Suppl 2):S165–S174. doi: 10.1093/sleep/5.s2.s165. [DOI] [PubMed] [Google Scholar]
- 28.Nofzinger EA, Thase ME, Reynolds CF, 3rd, Himmelhoch JM, Mallinger A, Houck P, et al. Hypersomnia in bipolar depression: a comparison with narcolepsy using the multiple sleep latency test. Am J Psychiatry. 1991;148:1177–1181. doi: 10.1176/ajp.148.9.1177. [DOI] [PubMed] [Google Scholar]
- 29.Bassetti C, Gugger M, Bischof M, Mathis J, Sturzenegger C, Werth E, et al. The narcoleptic borderland: a multimodal diagnostic approach including cerebrospinal fluid levels of hypocretin-1 (orexin A) Sleep Med. 2003;4:7–12. doi: 10.1016/s1389-9457(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 30.Shen J, Hossain N, Streiner DL, Ravindran AV, Wang X, Deb P, et al. Excessive daytime sleepiness and fatigue in depressed patients and therapeutic response of a sedating antidepressant. J Affect Disord. 2011;134:421–426. doi: 10.1016/j.jad.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 31.Kofmel NC, Schmitt WJ, Hess CW, Gugger M, Mathis J. Sleepiness and performance is disproportionate in patients with non-organic hypersomnia in comparison to patients with narcolepsy and mild to moderate obstructive sleep apnoea. Neuropsychobiology. 2014;70:189–194. doi: 10.1159/000365486. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Bixler EO, Kales A, Criley C, Vela-Bueno A. Differences in nocturnal and daytime sleep between primary and psychiatric hypersomnia: diagnostic and treatment implications. Psychosom Med. 2000;62:220–226. doi: 10.1097/00006842-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Kayumov L, Rotenberg V, Buttoo K, Auch C, Pandi-Perumal SR, Shapiro CM. Interrelationships between nocturnal sleep, daytime alertness, and sleepiness: two types of alertness proposed. J Neuropsychiatry Clin Neurosci. 2000;12:86–90. doi: 10.1176/jnp.12.1.86. [DOI] [PubMed] [Google Scholar]
- 34.Sangal RB, Thomas L, Mitler MM. Maintenance of wakefulness test and multiple sleep latency test. Measurement of different abilities in patients with sleep disorders. Chest. 1992;101:898–902. doi: 10.1378/chest.101.4.898. [DOI] [PubMed] [Google Scholar]
- 35.Mignot E, Lin L, Finn L, Lopes C, Pluff K, Sundstrom ML, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129:1609–1623. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 36.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 37.American Sleep Disorders Association. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. Rochester, NY: American Academy of Sleep Medicine; 1990. [Google Scholar]
- 38.Billiard M. Diagnosis of narcolepsy and idiopathic hypersomnia. An update based on the International classification of sleep disorders, 2nd edition. Sleep Med Rev. 2007;11:377–388. doi: 10.1016/j.smrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Goldbart A, Peppard P, Finn L, Ruoff CM, Barnet J, Young T, et al. Narcolepsy and predictors of positive MSLTs in the Wisconsin Sleep Cohort. Sleep. 2014;37:1043–1051. doi: 10.5665/sleep.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison Y, Horne JA. "High sleepability without sleepiness". The ability to fall asleep rapidly without other signs of sleepiness. Neurophysiol Clin. 1996;26:15–20. doi: 10.1016/0987-7053(96)81530-9. [DOI] [PubMed] [Google Scholar]
- 41.Aldrich MS, Chervin RD, Malow BA. Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep. 1997;20:620–629. [PubMed] [Google Scholar]
- 42.Anderson KN, Pilsworth S, Sharples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30:1274–1281. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:753–759. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth B, Nevsimalova S. Depresssion in narcolepsy and hypersommia. Schweiz Arch Neurol Neurochir Psychiatr. 1975;116:291–300. [PubMed] [Google Scholar]
- 45.Vandeputte M, de Weerd A. Sleep disorders and depressive feelings: a global survey with the Beck depression scale. Sleep Med. 2003;4:343–345. doi: 10.1016/s1389-9457(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 47.Reynolds CF., 3rd Troubled Sleep, troubled minds, and DSM-5. Arch Gen Psychiatry. 2011;68:990–991. doi: 10.1001/archgenpsychiatry.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9:789–795. doi: 10.5664/jcsm.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullington JM, Czeisler CA, Goel N, Krueger JM, Balkin TJ, Johns M, et al. Panel discussion: current status of measuring sleepiness. J Clin Sleep Med. 2011;7:S22–S25. doi: 10.5664/JCSM.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer G, Lammers GJ. The MSLT: More objections than benefits as a diagnostic gold standard? Sleep. 2014;37:1027–1028. doi: 10.5665/sleep.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–1316. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomann J, Baumann CR, Landolt HP, Werth E. Psychomotor vigilance task demonstrates impaired vigilance in disorders with excessive daytime sleepiness. J Clin Sleep Med. 2014;10:1019–1024. doi: 10.5664/jcsm.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trotti LM, Saini P, Freeman AA, Bliwise DL, García PS, Jenkins A, et al. Improvement in daytime sleepiness with clarithromycin in patients with GABA-related hypersomnia: Clinical experience. J Psychopharmacol. 2013;28:697–702. doi: 10.1177/0269881113515062. [DOI] [PubMed] [Google Scholar]
- 54.Rye DB, Bliwise DL, Parker K, Trotti LM, Saini P, Fairley J, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med. 2012;4:161ra51. doi: 10.1126/scitranslmed.3004685. [DOI] [PubMed] [Google Scholar]
- 55.Shen J, Chung SA, Kayumov L, Moller H, Hossain N, Wang X, et al. Polysomnographic and symptomatological analyses of major depressive disorder patients treated with mirtazapine. Can J Psychiatry. 2006;51:27–34. doi: 10.1177/070674370605100106. [DOI] [PubMed] [Google Scholar]