Abstract
Background
The immediate effect of aortic valve replacement (AVR) for aortic stenosis on perioperative myocardial function is unclear. Left ventricular (LV) function may be impaired by cardioplegia-induced myocardial arrest and ischemia-reperfusion injury, especially in patients with LV hypertrophy. Alternatively, LV function may improve when afterload is reduced following AVR. The right ventricle (RV), however, experiences cardioplegic arrest without benefiting from improved loading conditions. Which of these effects on myocardial function dominate in patients undergoing AVR for aortic stenosis has not been thoroughly explored. Our primary objective thus to characterize the effect of intraoperative events on LV function during AVR using echocardiographic measures of myocardial deformation. Secondarily, we evaluated RV function.
Methods
In this supplementary analysis of 100 patients enrolled in a clinical trial (NCT01187329), 97 patients underwent AVR for aortic stenosis. Of these patients, 95 had a standardized intraoperative transesophageal echocardiographic examination of systolic and diastolic function performed before surgical incision and repeated after chest closure. Echocardiographic images were analyzed off-line for global longitudinal myocardial strain and strain rate using 2-dimensional speckle-tracking echocardiography. Myocardial deformation assessed at the beginning of surgery was compared with the end of surgery using paired t-tests corrected for multiple comparisons.
Results
LV volumes and arterial blood pressure decreased, and heart rate increased at the end of surgery. Echocardiographic images were acceptable for analysis in 72 patients for LV strain, 67 for LV strain rate, and 54 for RV strain and strain rate. In 72 patients with LV strain images, 9 patients required epinephrine, 22 required norepinephrine, and 2 required both at the end of surgery. LV strain did not change at the end of surgery compared with the beginning of surgery [difference: 0.7 (97.6%CI: 0.2, 1.5)%; P =0.07] while LV systolic strain rate improved (became more negative) [−0.3 (−0.4, −0.2) sec−1; P<0.001]. In contrast, RV systolic strain worsened (became less negative) at the end of surgery [difference: 4.6 (3.1, 6.0)%; P< 0.001] although RV systolic strain rate was unchanged [0.0 (97.6% CI: −0.1, 0.1); P = 0.83].
Conclusion
LV function improved after replacement of a stenotic aortic valve demonstrated by improved longitudinal strain rate. In contrast, RV function, assessed by longitudinal strain, was reduced.
Introduction
Patients with symptomatic aortic stenosis are at high risk of death or heart failure unless aortic valve replacement (AVR) is performed.1 Long-term survival and quality of life are improved with surgical AVR;2 however, when myocardial dysfunction occurs early after surgery, unadjusted 30-day mortality is increased nearly 5-fold.3 Further, the presence of perioperative right ventricular (RV) impairment increases the risk for in-hospital mortality or postoperative circulatory failure nearly 25-fold.4 A better understanding of intraoperative left ventricular (LV) and RV response to AVR may help guide anesthetic and hemodynamic management during and after surgery to reduce postoperative myocardial dysfunction and ultimately improve outcomes following AVR.
Myocardial function after surgery is affected by opposing factors that may worsen or improve ventricular function. For example, cardioplegia-induced myocardial arrest and an ischemia-reperfusion sequence adversely affect LV function,5–7 especially when LV hypertrophy is present.8,9 In contrast, removal of a stenotic aortic valve abruptly decreases LV afterload thus improving LV ejection.10,11 RV function, in contrast, may be more susceptible to injury from cardioplegic arrest than the LV,12 without benefiting from afterload reduction. The net effect of these events on intraoperative RV and LV function in patients undergoing AVR for aortic stenosis has not been fully characterized.
Though load-independent measures of ventricular contractility, such as the slope of the end-systolic pressure volume relationship13,14 or preload recruitable stroke work,15 are sensitive to changes in inotropy, they are invasive, cumbersome, and thus not suitable for the clinical setting. Ejection phase measures, including LV ejection fraction (LVEF), are dependent on loading conditions; however, they provide clinically meaningful information, because an increase in afterload induces physiologic compensatory changes in preload and contractility in an intact cardiovascular system to offset a pure increase in afterload.16 Furthermore, echocardiographically measured LVEF is noninvasive, easy to measure, and thus suitable for the clinical setting. LVEF, however, evaluates volumetric changes during systole and diastole, rather than the magnitude and speed of myocardial muscle contraction, which are important descriptors of myocardial function.17 LVEF also relies on geometric assumptions which are subject to measurement error. Therefore a noninvasive echocardiographic measure that provides a reproducible and quantitative assessment of the magnitude and rate of myocardial contraction would be useful.
Myocardial strain and strain rate assess myocardial deformation and provide quantitative measures of myocardial contractility. Strain and strain rate correlate with load-dependent measures including LVEF18 and the rate of rise of left ventricular pressure.19 Strain rate also correlates with a load-independent measure of LV function, the slope of the end-systolic pressure-volume relationship.20 Strain and strain rate assess the percent and rate of change in ventricular wall dimensions and are measured by tracking displacement of “speckles” from 2-dimensional (D) echocardiographic images.21 Longitudinal strain in healthy individuals measured by transthoracic echocardiography varies somewhat depending upon the analysis technique, but is typically between −18 and −21%,21–23 while longitudinal strain rate is −1.1 ± 0.2 sec−1.21,22 The effect of AVR on postoperative strain one week or longer after AVR has been described,24–26 but acute intraoperative changes in myocardial deformation with transesophageal echocardiography (TEE) have not yet been reported.
Using strain and strain rate measured by speckle-tracking echocardiography, our primary objective was to characterize the effect of intraoperative events on LV and RV function after surgical replacement of a stenotic aortic valve. Specifically, we tested the hypothesis that LV function measured by strain and strain rate was improved at the end of surgery. Secondarily, the change in RV function was evaluated during cardiac surgery. Patient characteristics and perioperative variables, which potentially contribute to the change in myocardial deformation, were also assessed.
Methods
With approval from the Cleveland Clinic IRB and written patient consent, we evaluated 100 patients scheduled for AVR who were enrolled in a randomized controlled investigation entitled, “The effect of the hyperinsulinemic normoglycemic clamp on myocardial function and utilization of glucose” (ClinicalTrials.gov #NCT01187329, Andra Duncan, Principal Investigator, registered on August 19, 2010).27 Briefly, patients who were considered to be at increased risk for myocardial injury induced by cardioplegic arrest (patients with aortic stenosis and LV hypertrophy)28,29 were randomized to intraoperative glucose control using standard glucose control (insulin treatment for blood glucose >150 mg/dL) versus hyperinsulinemic normoglycemia. Hyperinsulinemic normoglycemia involves a high-dose insulin infusion at a fixed rate (5 mU/kg/min) with a concomitant variable glucose (dextrose 20%) infusion supplemented with potassium (40 mEq/L) and phosphate (30 mmol/L), titrated to a target glucose concentration of 80 – 110 mg/dL.27 Because the primary results did not find a meaningful difference in myocardial function between groups (minimal change in LV strain rate which was statistically significant [−0.16 (−0.30, −0.03) sec−1, P = 0.007], but not clinically meaningful, and no difference in LV strain, RV strain or strain rate),27 the study groups were combined for this supplementary analysis to assess change in LV and RV myocardial deformation during AVR surgery.
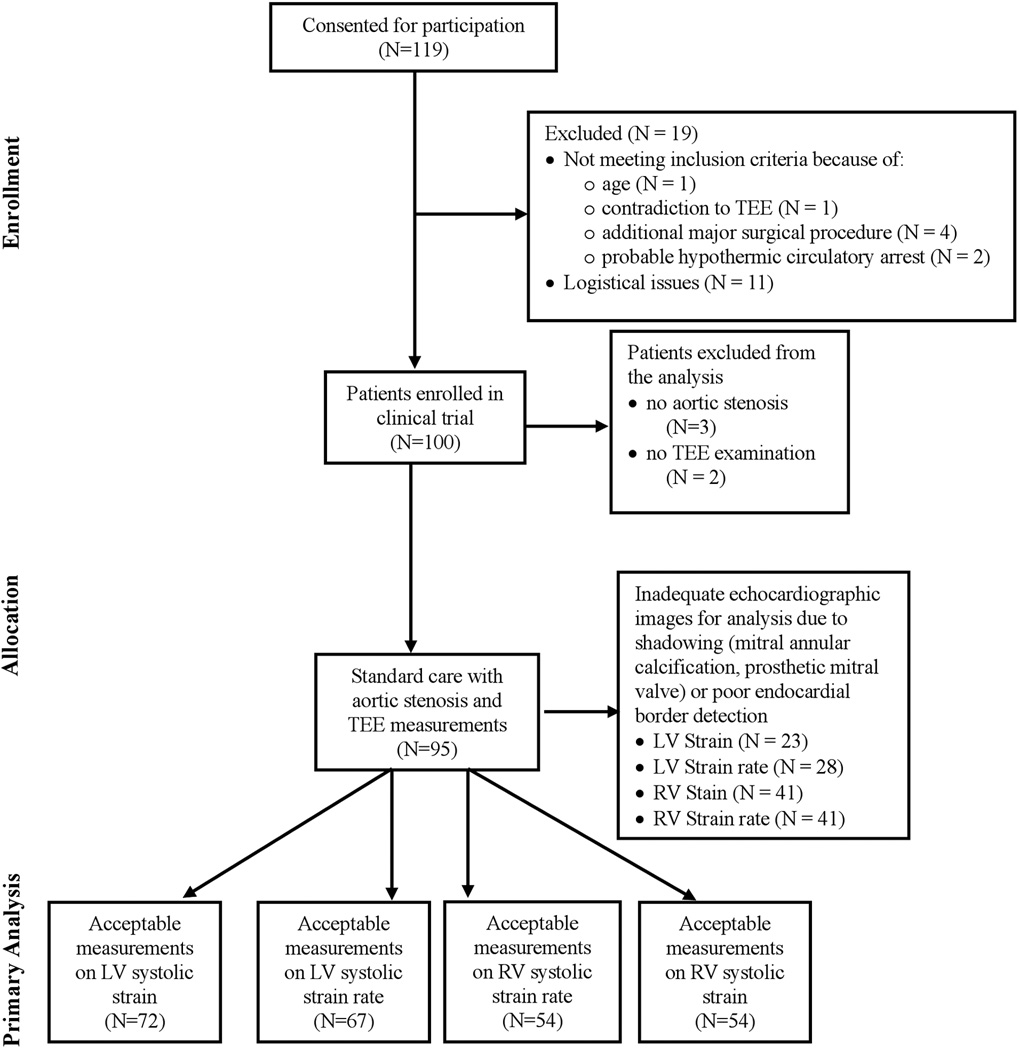
Exclusion criteria included the presence of aortic insufficiency without aortic stenosis, contraindication for TEE, poor quality echocardiographic images which were unsatisfactory for speckle-tracking strain analysis (>3 unacceptable myocardial segments as deemed by a blinded investigator), and requirement for intraoperative hypothermic circulatory arrest. Of 100 patients enrolled in the randomized controlled trial, 3 patients with aortic insufficiency as the predominant valvular pathophysiology and 2 patients with contraindications for TEE examinations were excluded. Twenty-three patients had echocardiographic images that were unacceptable for LV strain analysis, 28 were unacceptable for LV strain rate analysis and 41 for RV strain and strain rate analysis (Figure 1). Thus echocardiographic images were acceptable in 72 patients for LV strain analysis, and 67 for LV strain rate analysis. RV strain and strain rate analysis was adequate for 54 patients. Demographics and patient characteristics for 97 patients with aortic stenosis and a subgroup of 72 patients with LV strain data are shown in Table 1.
Figure 1.
Consolidated Statement of Reporting Trials flow diagram. LV = left ventricular; RV = right ventricular; TEE = transesophageal echocardiographic examination
Table 1.
Preoperative baseline characteristics of patients with aortic stenosis (N=97). A subgroup of patients with acceptable echocardiographic images for LV strain analysis (N = 72) have similar demographic data and perioperative characteristics. Data are presented as N (%), mean ± SD, or median [25th, 75th %].
| Variables | N | All patients with aortic stenosis (N = 97) |
N | Subgroup of patients with LV strain data (N = 72) |
|---|---|---|---|---|
| Demographics | ||||
| Age (year) | 97 | 70 ± 10 | 72 | 69 ± 9 |
| Gender, female, N (%) | 97 | 30 (31) | 72 | 24 (33) |
| Body Mass Index (kg/m2) | 97 | 31 ± 8 | 72 | 31 ± 8 |
| Medical history, N (%) | ||||
| Diabetes | 97 | 26 (27) | 72 | 22 (31) |
| Heart failure | 97 | 15 (15) | 72 | 11 (15) |
| Hypertension | 97 | 24 (25) | 72 | 16 (22) |
| Myocardial infarction | 97 | 8 (8) | 72 | 6 (8) |
| Chronic obstructive pulmonary disease |
97 | 9 (9) | 72 | 6 (8) |
| Pulmonary hypertension | 97 | 16 (16) | 72 | 13 (18) |
| Stroke | 97 | 5 (5) | 72 | 3 (4) |
| Peripheral vascular disease | 97 | 9 (9) | 72 | 7 (10) |
| Dialysis | 97 | 0 (0) | 72 | 0 (0) |
| Cardiogenic shock | 97 | 0 (0) | 72 | 0 (0) |
| Previous vascular surgery | 97 | 3 (3) | 72 | 7 (10) |
|
3D LV ejection fraction (LVEF) |
66 | 48 | ||
| LVEF ≥ 60% | 36 (55) | 27 (56) | ||
| LVEF 50 – 59% | 23 (35) | 17 (35) | ||
| LVEF < 50% | 7 (10) | 4 (8) | ||
| Preoperative laboratory values | ||||
| Hematocrit (%) | 96 | 40 ± 4 | 71 | 41 ± 4 |
| Creatinine (mg/dL) | 97 | 0.9 [0.8, 1.1] | 72 | 0.9 [0.8, 1.1] |
| NT-pro-BNP (pg/mL) | 83 | 320 [150, 807] |
61 | 287 [139, 627] |
| Aortic valve disease | ||||
| Peak transvalvular gradient (mmHg) |
96 | 83 ± 21 | 72 | 83 ± 22 |
| Mean transvalvular gradient (mmHg) |
96 | 49 ± 14 | 72 | 49 ± 15 |
| Dimensionless index | 97 | 0.23 ± 0.05 | 72 | 0.22 ± 0.05 |
| Aortic valve area (cm2) | 88 | 0.7 ± 0.2 | 72 | 0.73 ± 0.16 |
| Aortic insufficiency | 96 | |||
| 0 | 41 (43) | 72 | 28 (39) | |
| 1 – 2+ | 49 (51) | 72 | 40 (56) | |
| 3 – 4+ | 6 (6) | 72 | 4 (5) | |
| Intraoperative data | ||||
| Fentanyl dose (mg) | 96 | 1.0 ± 0.2 | 71 | 1.0 ± 0.2 |
| Mean end-tidal isoflurane concentration (%)† |
69 | 1.3 ± 0.5 | 53 | 1.4 ± 0.5 |
| HNC treatment | 97 | 49 (51) | 72 | 36 (50) |
| Surgical characteristics | ||||
| Duration of surgery (min) | 97 | 369 [316, 426] |
72 | 373 [316, 435] |
| Cardiopulmonary bypass (min) | 97 | 87 [64, 116] | 72 | 86 [67, 119] |
| Aortic cross-clamp (min) | 97 | 64 [49, 79] | 72 | 64 [50, 81] |
| Surgical procedure, N (%) | 97 | 72 | ||
| AVR | 50 (52) | 41 (57) | ||
| AVR + CABG | 29 (30) | 18 (25) | ||
| AVR ± CABG + other | 17 (18) | 13 (18) | ||
| Tricuspid valve repair | 1 (1) | 1 | ||
| Maze procedure | 2 (2) | 2 | ||
| Aortoplasty | 4 (4) | 2 | ||
| Ascending aorta replacement |
6 (6) | 6 | ||
| Mitral valve replacement |
1 (1) | 0 | ||
| Mitral valve repair | 2 (2) | 1 | ||
| Septal myectomy | 1 (1) | 1 | ||
| Previous cardiac surgery, N (%) | 97 | 23 (24) | 72 | 18 (25) |
| Cardioplegia, N (%) | 96 | 72 | ||
| Buckbergs | 82 (86) | 62 (86) | ||
| Del Nido | 13 (13) | 9 (13) | ||
| Microplegia | 1 (2) | 1 (1) |
LV= Left ventricular; HNC = Hyperinsulinemic normoglycemic clamp; AVR = Aortic valve replacement; CABG = Coronary artery bypass grafting
End-tidal isoflurane concentration was measured during ventilation before and after cardiopulmonary bypass. Patients routinely received isoflurane 1% during cardiopulmonary bypass, though end-tidal concentrations could not be measured.
Anesthetic and surgical management
Routine procedures for anesthesia, surgery, and conduct of cardiopulmonary bypass (CPB) were used as previously described.27 Epinephrine was administered for low cardiac index (< 2.0 L·min−1·m−2) and/or norepinephrine was given for low systemic vascular resistance (<700 dyn·sec·cm−5) following separation from CPB to maintain mean arterial blood pressures higher than 80 mmHg and cardiac index greater than 2.0 L·min−1·m−2.
Collection of echocardiographic data
TEE was performed as previously described.27 Briefly, Vivid S6 or Vivid E9 Ultrasound systems (GE Healthcare Vingmed Ultrasound AS, Horten, Norway) with a multiplane phased array GE 6Tc-RS 2.9–8.0 MHz transducer or an active matrix 4D volume phased array 3.0–8.0 MHz transducer were used to collect echocardiographic data for off-line analysis using dedicated analysis software (EchoPAC v.112, GE Healthcare Vingmed Ultrasound AS, Horten, Norway).
A standardized TEE examination was performed following anesthetic induction (prior to surgical incision) and repeated near end of surgery after sternal closure by one of three experienced staff cardiothoracic anesthesiologists who are certified in Perioperative Transesophageal Echocardiography by the National Board of Echocardiography. Standard echocardiographic parameters of LV systolic and diastolic function using 2D and Doppler echocardiography were performed as previously described.27 LV end-systolic meridional wall stress (LVESS) measured in dynes·cm−2 was calculated using the equation:
where ESD represents end-systolic dimension (cm) and h represents the end-systolic posterior wall thickness (cm).30,31 In order to apply this calculation to patients with aortic stenosis whose echocardiographic measurements were collected with TEE, the equation was modified as follows: LV peak pressure was estimated as the sum of systolic blood pressure and the peak intraoperative aortic transvalvular gradient; ESD was measured as the anterior-inferior end-systolic internal dimension measured from the transgastric mid-papillary LV short-axis echocardiographic view (cm); end-systolic inferior wall thickness measured from the transgastric mid-papillary LV short-axis was used as h rather than using the measurement of the thickness of the posterior myocardial wall, which is compromised by poor resolution by TEE.
RV systolic function was assessed in the 2D transesophageal 4-chamber view with focus on the RV at 0° by fractional area change (%).32 M-mode measurement of tricuspid annular plane systolic excursion (TAPSE) was not possible because of poor alignment of the tricuspid annular motion with the echocardiographic beam, thus TAPSE was measured off-line on 2D images by measuring the apical displacement of the lateral tricuspid annulus (cm) between systole and diastole.
Echocardiographic analysis of myocardial deformation using speckle-tracking echocardiography
Echocardiographic data were digitally collected and stored for off-line analysis of myocardial deformation with speckle-tracking analysis software (EchoPAC v. 112, GE Healthcare Vingmed Ultrasound AS, Horten, Norway). Two-dimensional strain analysis uses grayscale (B-mode) sector images and is based on frame-by-frame tracking of myocardial movement and deformation using a unique pattern of bright and dark pixels, or speckles, in echocardiographic images.21 These speckles, which are constructive and destructive interference patterns generated by reflected ultrasound from inhomogeneous myocardial tissue, are tracked from one frame to another throughout the cardiac cycle, and are used to assess myocardial deformation. Analysis of echocardiographic views for strain analysis involves tracing the endocardial contour on an end-systolic cavitary frame and defining the thickness of the myocardial region. The software automatically tracks the ventricular wall on subsequent frames and divides it into 6 segments. Manual adjustment of the endocardial contour and thickness of the region is performed when necessary. The software program deems tracking quality acceptable or unacceptable. However, the user can override this designation based on visual confirmation of proper tracking of myocardial motion.
Serial echocardiographic examinations were collected at equally spaced intervals of 60 degrees (i.e. 0, 60, 120°) of rotation of the transducer in attempts to reproduce images for each echocardiographic examination, while circumferentially describing global LV function. Frame rates between 40 and 90 Hz were used. For LV analysis, 6-segment LV strain and strain rate measurements from 3 views, including the midesophageal 4-chamber, mitral commissural, and long-axis view, were averaged (total of 18 segments). All measurements that included at least 15 “acceptable” segments were included in the LV analysis. Our previous report demonstrated accurate and consistent results with inclusion of assessments with a minimum of 15 acceptable segments.27
For RV analysis, strain and strain rate measurements from the 4-chamber view centered on the RV were used. At least 5 of 6 acceptable myocardial segments were required for analysis of the RV, though all segments from the RV free wall were required. LV and RV early diastolic strain rate were also assessed. All analyses of myocardial deformation were performed by the same investigator. We adhere to the convention of referring to the absolute value when comparing 2 strain measurements (e.g. a change in strain from −18% to −12% reflects a decrease in myocardial shortening and thus a “decrease” in strain).33
Hemodynamic data collection
Invasive arterial blood pressures were recorded on all patients using radial or brachial arterial catheters. Patients with normal biventricular function and scheduled for isolated AVR received central venous catheterization. Those with abnormal myocardial function or scheduled for complex cardiac surgery (combined AVR with coronary artery bypass grafting or additional valve procedure) received pulmonary artery catheterization. Data recorded from patients with pulmonary artery catheters included systolic and diastolic pulmonary artery pressures, thermodilution cardiac output, and cardiac index. Cardiac output/index data were only reported from patients with pulmonary artery catheters and calculated using thermodilution. Hemodynamic data were recorded during TEE examination which occurred after anesthesia induction before surgical incision and at the end of surgery after sternal closure.
Statistical Analysis
Patient demographics, clinical characteristics, and co-morbidities were summarized using standard descriptive statistics. The primary analysis was to assess the change in systolic LV myocardial function (strain and strain rate) between baseline measured after anesthesia induction and the end of surgery using paired t-tests. The change in systolic RV myocardial function (strain and strain rate) was assessed secondarily. Furthermore, the relationship between LV and RV strain and strain rate and 15 potential risk factors were assessed in a multivariable regression model. Due to small sample size and large number of risk factors, univariable analysis with P <0.05 were used to select initial candidates. A stepwise variable selection procedure with inclusion/exclusion criterion of P <0.05 was used to select the final variables.
Comparisons on additional prespecified intraoperative echocardiographic and hemodynamic parameters between baseline and the end of surgery were implemented using separate paired t-tests. The paired binary myocardial pacing (atrial and ventricular pacing) status between baseline and the end of surgery was compared by the McNemar’s test.
Intraobserver variability of the speckle-tracking analysis was examined using Lin's Concordance Correlation,34 Bland-Altmann Limits of Agreement and the binomial exact method. We conducted a preliminary analysis to assess the change in LV strain and strain rate in the first 45 patients. We used the O’Brien-Fleming alpha-spending method to adjust for this look (efficacy alone). As such, with an overall alpha of 0.05, the remaining alpha for the final analysis was 0.048. Using a Bonferroni correction, the significance criterion was 0.048/2 = 0.024 for each of the 2 primary and secondary analyses. SAS statistical software v. 9.4, Carey, NC, was used for all analyses.
Sample Size Consideration
With the attained sample size of 72 for change in LV strain and observed standard deviation of 3.1 and a correlation coefficient of 0.73 between pre and post-CPB measurements, we had 90% power at the overall 0.025 significance level (Bonferroni correction for 2 primary outcomes) to detect a change in mean strain change of 1% or larger. Similarly, with an observed total sample size of 67 and standard deviation of 0.30 and observed correlation coefficient of 0.52 between pre- and post-CPB measurements, we had 90% power to detect mean change of 0.13 sec−1 or more in strain rate.
Results
Clinical characteristics and events
Table 1 presents the demographics, clinical characteristics, co-morbidities, surgical and anesthesia variables for 97 patients with aortic stenosis. A subgroup of 72 patients who had acceptable echocardiographic images for LV strain are also shown in Table 1. In all patients with aortic stenosis, the mean (± SD) age was 70 ± 10 years, 30 (31%) were female, and cardiac reoperations were performed in 23 (24%) patients. Valve replacement was successful in all patients, as indicated by absence of significant residual regurgitation or transvalvular stenosis.
Primary and secondary outcomes of myocardial deformation
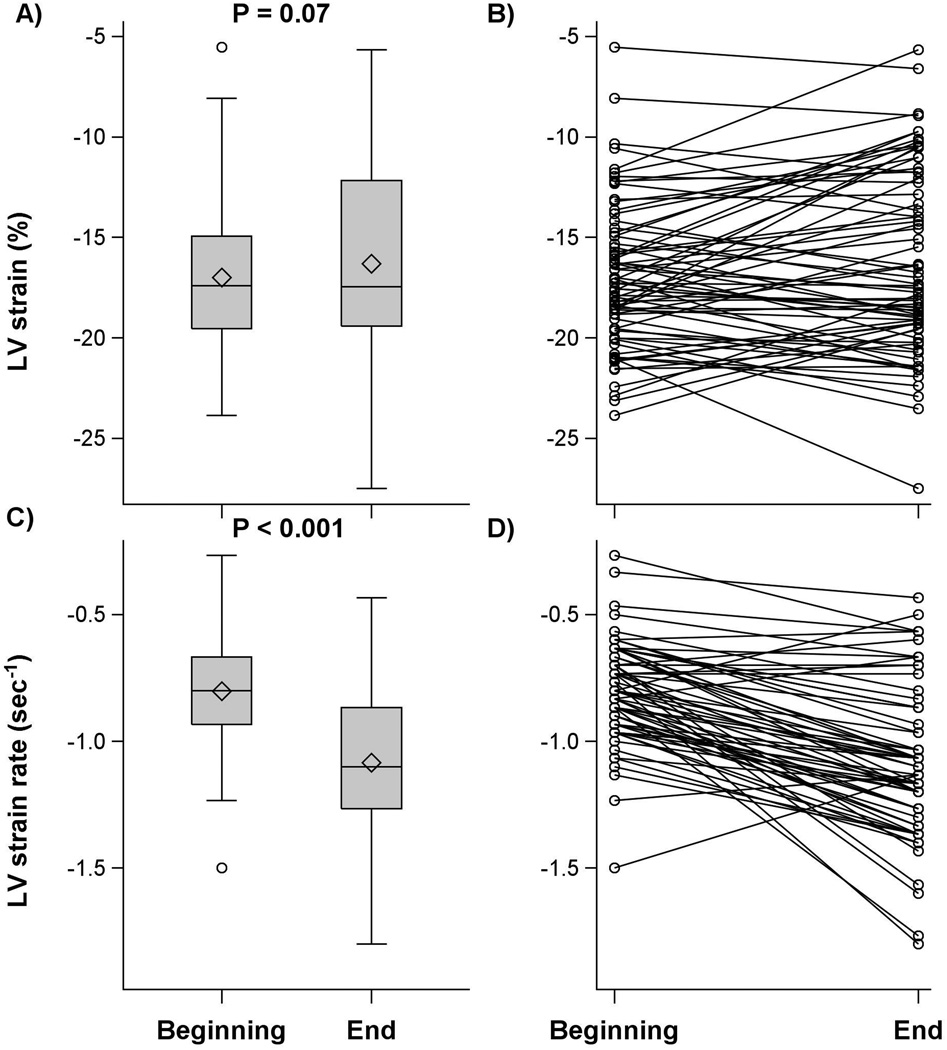
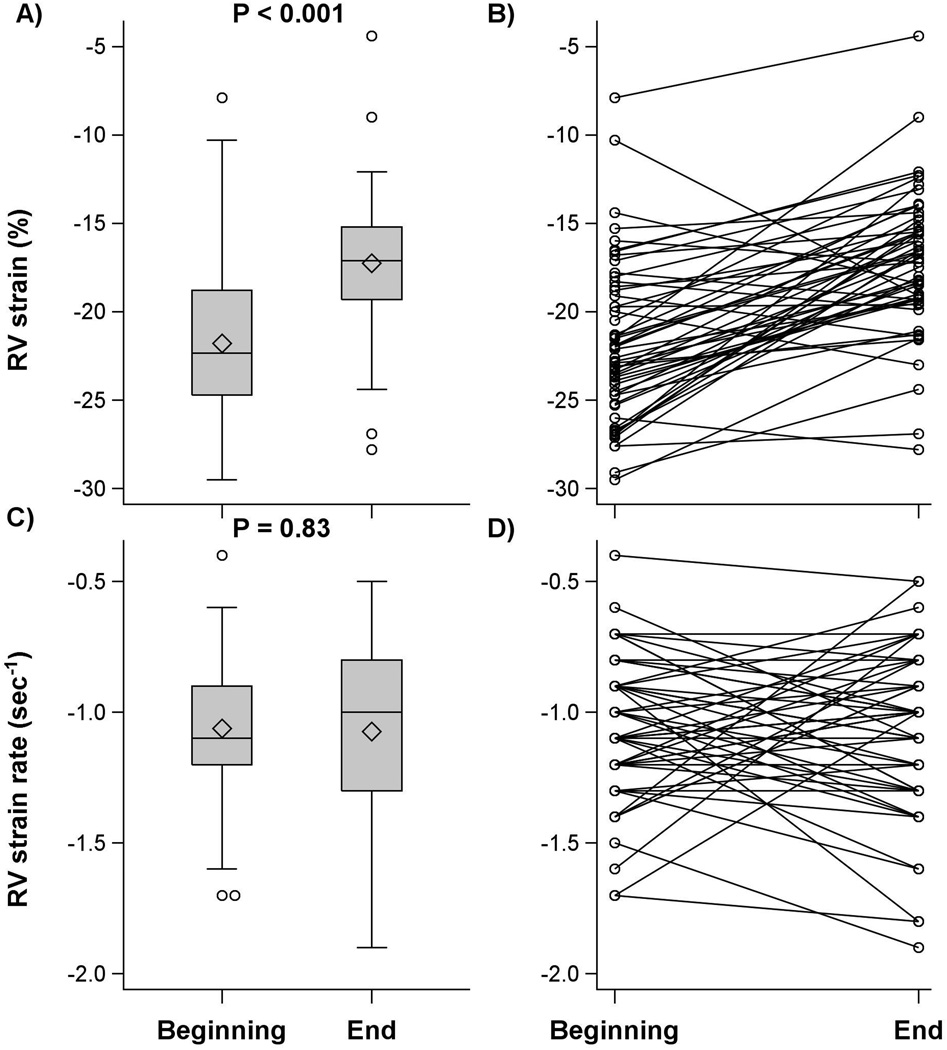
LV strain did not change at the end of surgery compared with baseline measurement [difference: 0.7 (97.6%CI: 0.2, 1.5)%; P =0.071] while LV systolic strain rate improved (became more negative) [−0.3 (−0.4, −0.2) sec−1; P<0.001]. In contrast, RV systolic strain worsened (became less negative) at the end of surgery [difference: 4.6 (3.1, 6.0) %; P< 0.001] although RV systolic strain rate was unchanged [0.0 (97.6% CI: −0.1, 0.1) sec−1; P = 0.83], (Table 2, Figures 2 and 3).
Table 2.
Echocardiographic and hemodynamic parameters during surgery (N = 97). Certain echocardiographic and perioperative variables were not available in all patients; thus for these variables, N < 97.
| Intraoperative Variables | N | Beginning of surgery (mean ± SD) |
End of surgery (mean ± SD) |
Change (97.6% CI) |
P* |
|---|---|---|---|---|---|
| Strain and strain rate outcomes | |||||
| Primary analysis | |||||
| Left systolic strain (%) | 72 | −17.0 ± 3.6 | −16.3 ± 4.6 | 0.7 (−0.2, 1.5) | 0.071 |
| Left systolic strain rate (sec−1) | 67 | −0.8 ± 0.2 | −1.1 ± 0.3 | −0.3 (−0.4, −0.2) | <0.001 |
| Secondary analysis | |||||
| Right systolic strain (%) | 54 | −21.8 ± 4.5 | −17.3 ± 4.0 | 4.6 (3.1, 6.0) | <0.001 |
| Right systolic strain rate (sec−1) | 54 | −1.1 ± 0.3 | −1.1 ± 0.3 | 0.0 (−0.1, 0.1) | 0.83 |
|
Additional LV echocardiographic parameters |
Change (95% CI) | ||||
| Ejection fraction (%) | 88 | 62 ± 13 | 66 ± 13 | 5 (2, 7) | <0.001 |
| Mitral lateral annular s' velocity (cm/sec) |
91 | 4.7 ± 1.6 | 6.5 ± 2.7 | 1.8 (1.3, 2.3) | <0.001 |
| Mitral lateral annular e' velocity (cm/sec) |
91 | 5.2 ± 1.8 | 5.3 ± 2.2 | 0.1 (−0.3, 0.5) | 0.64 |
| Mitral lateral annular a' velocity (cm/sec) |
90 | 4.9 ± 2.0 | 5.6 ± 2.5 | 0.7 (0.2, 1.3) | 0.007 |
| Early transmitral flow velocity (E- wave, m/sec) |
92 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.0 (0.0, 0.1) | 0.093 |
| Late transmitral flow velocity (A- wave, m/sec) |
87 | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.1 (0.0, 0.1) | 0.004 |
| E / A ratio | 87 | 1.4 ± 0.6 | 1.3 ± 0.6 | −0.1 (−0.2, 0.1) | 0.23 |
| E / e' ratio | 89 | 17.7 ± 12.1 | 20.1 ± 24.5 | 2.4 (−1.2, 6.0) | 0.19 |
| Deceleration time (msec) | 89 | 257 ± 79 | 243 ± 73 | −14 (−32, 3) | 0.11 |
| Velocity of propagation (cm/sec) | 91 | 46 ±18 | 46 ± 17 | −1 (6, 4) | 0.74 |
| LV diastolic strain rate (sec−1) | 52 | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.1 (0.0, 0.2) | 0.11 |
| LV end-systolic meridional wall stress (dynes/cm2) |
73 | 77 ± 52 | 48 ± 49 | −29 (−35, −22) | <0.001 |
| LV end-systolic dimension (cm) | 81 | 3.1 ± 1.1 | 2.8 ± 1.0 | −0.3 ± 0.6 | <0.001 |
|
Additional RV echocardiographic parameters |
|||||
| Tricuspid annular plane systolic excursion (cm) |
69 | 1.6 ± 0.4 | 0.9 ± 0.3 | −0.7 (−0.8, −0.6) | <0.001 |
| RV end-diastolic area (cm2) | 68 | 22.84 ± 6.00 | 20.78 ± 5.75 | −2.06 (−3.04, −1.07) | <0.001 |
| RV end-systolic area (cm2) | 68 | 13.18 ± 4.36 | 13.07 ± 4.46 | −0.12 (−0.83, −0.60) | 0.74 |
| RV fractional area change (%) | 68 | 43 ± 11 | 37 ± 12 | −5 (−8, −2) | 0.001 |
| RV early diastolic strain rate (sec−1) | 53 | 1.2 ± 0.3 | 1.1 ± 0.4 | −0.1 (−0.2, 0.1) | 0.15 |
| Aortic valve parameters | |||||
| Peak transvalvular gradient (mmHg) | 83 | 60 ± 19 | 18 ± 7 | −42 (−47, −38) | <0.001 |
| Mean transvalvular gradient (mmHg) |
83 | 36 ± 12 | 8 ± 3 | −28 (−30, −25) | <0.001 |
| Hemodynamic parameters | |||||
| Heart rate (bpm) | 87 | 56 ± 9 | 69 ± 11 | 14 (11, 16) | <0.001 |
| Mean arterial pressure (mmHg) | 96 | 84 ± 11 | 74 ± 10 | −10 (−13, −7) | <0.001 |
| Central venous pressure (mmHg) | 93 | 15 ± 5 | 15 ± 6 | 0 (−2, 1) | 0.56 |
| Pulmonary artery systolic pressure (mmHg) |
69 | 39 ± 9 | 36 ± 11 | −2 (−4, 0) | 0.028 |
| Pulmonary artery diastolic pressure (mmHg) |
68 | 29 ± 7 | 20 ± 7 | −9 (−11, −8) | <0.001 |
| Cardiac output (L/min) | 61 | 4.1 ± 1.0 | 5.0 ± 1.2 | 1.0 (1.0, 1.3) | <0.001 |
| Cardiac index (L/min/m2) | 61 | 2.0 ± 0.5 | 2.5 ± 0.5 | 0.5 (0.3, 0.6) | <0.001 |
| Pharmacologic hemodynamic support at the end of surgery |
|||||
| Epinephrine | 97 | 18 (19%) | |||
| Norepinephrine | 97 | 33 (34%) | |||
| Epinephrine + norepinephrine | 97 | 7 (7%) | |||
| Epinephrine, norepinephrine, +milrinone |
97 | 1 (1%) |
LV = Left ventricle; RV = Right ventricle
P values from paired t-test; the significance criterion of 0.024 for two primary analyses, and 0.024 for two secondary analyses after using the O’Brien-Fleming alpha-spending method to adjust for an interim analysis and Bonferroni correction.
Figure 2.
Boxplot and series plot for left ventricular (LV) systolic strain and strain rate at the beginning and end of surgery in patients with aortic stenosis. Interquartile range (IQR, box), median (horizontal line), high and low values within 1.5 IQR (whiskers), and mean (diamond) are shown.
Figure 3.
Boxplot and series plot for right ventricular (RV) systolic strain and strain rate at the beginning and end of surgery in patients with aortic stenosis. Interquartile range (IQR, box), median (horizontal line), high and low values within 1.5 IQR (whiskers), and mean (diamond) are shown.
Additional echocardiographic and hemodynamic outcomes
Pairwise comparisons (end of surgery minus beginning of surgery) in echocardiographic and hemodynamic parameters are shown in Table 2. Pairwise comparisons limited to the subgroup of patients with acceptable images for LV strain analysis are shown in the Supplemental Digital Content, Table A. Ten patients with RV strain data did not have acceptable images for LV strain analysis, thus only 44 patients with RV strain data are included in Table A. Considering that TEE measurement of LV chamber size may be foreshortened and result in volume measurements smaller than those measured by 3D echocardiography (although LVEF estimates remain accurate),35,36 end-diastolic LV volumes [82 ± 44 (beginning) vs. 69 ± 38 cc (end of surgery); change (95% CI) −13 (−20, −7) cc; P <0.001, or −9% (95%CI: −23, 6%)] and end-systolic volumes [39 ± 35 (beginning) vs. 27 ± 32 cc (end of surgery); change −10 (−13, −7) cc; P <0.001, or −25% (−34, −17%)] were lower at the end of surgery in all patients with aortic stenosis. Measures of LV systolic function, including LVEF and peak systolic myocardial velocity, improved at the end of surgery, but LV diastolic function was not different at the end of surgery. As expected, LV afterload measured by LVESS and aortic transvalvular gradient were lower at the end of surgery (Table 2). RV function, measured by conventional echocardiographic measures (TAPSE, fractional area change) was reduced at the end of surgery.
Assessment of intraobserver agreements between the first and secondary readings of LV strain and strain rate were excellent, with the Lin's Concordance Correlation (95% CI) of 0.94 (0.87, 0.98) for strain, and 0.93 (0.85, 0.97) for strain rate. Bland-Altmann Limits of Agreement and the binomial exact method demonstrated good to excellent intraobserver agreement, as previously described.27
Heart rate was more rapid and arterial blood pressure lower at the end of surgery (Table 2; all P<0.001). More patients had atrial pacing at the end of surgery [2 (2%) beginning vs. 11 (11%) at the end of surgery, McNemar’s test, P<0.001]; but ventricular pacing at the beginning [1(1%)] compared with end of surgery 6(6%) was not different; P=0.06]. Cardiac output and cardiac index increased at the end of surgery. Of all 97 patients with aortic stenosis, 11 (11%) required IV infusion of epinephrine only; 23 (24%) required IV infusion of norepinephrine only; 6 (6%) required both epinephrine and norepinephrine, and 1(1%) required epinephrine, norepinephrine, and milrinone. In the subgroup of 72 patients with images for LV strain analysis, 9 (13%) required epinephrine, 22 (31%) required norepinephrine, 2 (3%) required both, and 1 (1%) required epinephrine, norepinephrine, and milrinone.
The relationship between baseline and intraoperative factors and LV / RV strain and strain rate
Results of the univariable and multivariable relationship examining the change in LV and RV strain and strain rate with patient characteristics and intraoperative variables are listed in Table 3 and Table 4. In the multivariable model, aortic cross-clamp time and previous cardiac procedure were associated with change in LV strain. For every 10 min increase in aortic cross-clamp time, mean LV strain worsened by 0.6%, P = 0.001, and those who had previous cardiac surgery had a mean change in LV strain of 1.8% (worsening) compared with those having a primary surgery, P = 0.018. Use of the hyperinsulinemic normoglycemic clamp (P = 0.005) and epinephrine use (P=0.04) were significantly associated with change in LV strain rate. Patients who received the hyperinsulinemic normoglycemic clamp had a −0.2 sec−1 mean improvement in LV strain rate compared with those who did not receive this treatment. Patients who received epinephrine had a 0.2 sec−1 worse LV strain rate compared to patients who did not receive epinephrine, P = 0.04. Women had a worse RV strain (less negative) at the end of surgery (P=0.04), and patients with a history of hypertension had improved RV strain rate (more negative) at the end of surgery, P = 0.02.
Table 3.
Univariable relationships between the change in left (LV) and right (RV) ventricular strain and strain rate and risk factors. Of the 97 patients with aortic stenosis, only patients with adequate images for strain/strain rate analysis are included in this table.
| Variables | Change in LV strain (N=72) |
Change in LV strain rate (N= 67) |
Change in RV strain (N=54) |
Change in RV strain rate (N=54) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean(SE)^ | P- value* |
Mean(SE)^ | P- value* |
Mean(S E)^ |
P- value * |
Mean(SE) ^ |
P- value* |
|
| Age (unit = 10) | −0.3 (0.4) | 0.42 | −0.01(0.00) | 0.19 | −1.0 (0.6) | 0.12 | −0.01 (0.1) | 0.72 |
| Gender | 0.35 | 0.04** | 0.04** | 0.59 | ||||
| Female | 1.2 (0.6) | −0.2 (0.1) | 6.2 (1.0) | 0.03 (0.1) | ||||
| Male | 0.4 (0.5) | −0.3 (0.04) | 3.6 (0.8) | −0.03 (0.1) | ||||
| Diabetes | 0.30 | 0.72 | 0.48 | 0.90 | ||||
| Yes | 1.3 (0.4) | −0.3 (0.1) | 4.8 (0.7) | 0.01 (0.1) | ||||
| No | 0.4 (0.7) | −0.3 (0.04) | 3.8 (1.2) | 0.02 (0.1) | ||||
| Hypertension | 0.005** | 0.53 | 0.54 | 0.02** | ||||
| Yes | 2.6 (0.7) | −0.3(0.1) | 3.6 (1.6) | −0.3 (0.1) | ||||
| No | 0.1 (0.4) | −0.3 (0.04) | 4.7 (0.7) | 0.04 (0.1) | ||||
|
Hyperinsulinemic normoglycemic clamp |
0.10 | 0.004** | 0.19 | 0.10 | ||||
| Yes | 0.1 (0.5) | −0.4 (0.04) | 3.7 (0.9) | −0.1 (0.1) | ||||
| No | 1.3 (0.5) | −0.2 (0.04) | 5.3 (0.9) | 0.1 (0.1) | ||||
|
Aortic cross- clamp time (unit = 10 min) |
0.1 (0.01) | <0.001** | 0.02 (0.01) |
0.07 | 0.1 (0.3) | 0.64 | 0.003(0.02) | 0.92 |
|
Surgical procedure |
0.006** | 0.75 | 0.95 | 0.32 | ||||
| AVR | −0.3 (0.5) | −0.3 (0.04) | 4.4 (0.9) | 0.003 (0.1) |
||||
| AVR + CABG | 1.9 (0.7) | −0.3(0.1) | 4.9 (1.4) | −0.1 (0.1) | ||||
| AVR + CABG + other |
2.2 (0.8) | −0.2 (0.1) | 4.6 (1.4) | 0.1 (0.1) | ||||
|
Previous cardiac procedure |
0.01** | 0.24 | 0.10 | 0.33 | ||||
| Yes | 2.3 (0.7) | −0.2 (0.1) | 2.5 (1.4) | −0.1 (0.1) | ||||
| No | 0.1 (0.4) | −0.3 (0.04) | 5.1 (0.7) | 0.01 (0.1) | ||||
|
Aortic insufficiency grade |
0.17 | 0.27 | 0.84 | 0.82 | ||||
| 0 | 0.9 (0.6) | −0.2 (0.1) | 4.6 (1.0) | 0.03 (0.1) | ||||
| 1–2+ | 0.3 (0.5) | −0.3 (0.04) | 4.3 (0.9) | −0.04 (0.1) | ||||
| 3–4+ | 3.2 (1.5) | −0.2 (0.1) | 5.8 (2.7) | −0.03 (0.2) | ||||
| Surgical incision | 0.03** | 0.37 | 0.97 | 0.49 | ||||
| Full-stemotomy | 1.3 (0.4) | −0.3 (0.03) | 4.6 (0.8) | −0.04 (0.1) | ||||
| Mini- stemotomy |
−0.4 (0.6) | −0.2 (0.1) | 4.5 (1.0) | 0.04 (0.1) | ||||
| Cardioplegia | 0.0497** | 0.99 | 0.38 | 0.12 | ||||
| Buckbergs | 1.0 (0.4) | −0.3 (0.0) | 4.3 (0.7) | −0.05 (0.1) | ||||
| Others | −1.1 (1.0) | −0.3 (0.0) | 5.6 (1.4) | 0.1 (0.1) | ||||
|
Change in LV diastolic dimension |
−0.1 (0.2)a | 0.42 | 0.1 (0.2)c | 0.51 | 0.04 (3.7)e |
0.99 | 0.2 (0.3)e | 0.50 |
|
Change in LVESS (unit = 100) |
−0.8 (0.01)b |
0.57 | 0.001(0.01)d | 0.87 | 3.8 (2.8)f | 0.17 | 0.06 (0.02)f |
0.01** |
|
Post CPB Epinephrine |
0.007** | 0.03** | 0.97 | 0.35 | ||||
| Yes | 2.9 (0.9) | −0.1 (0.1) | 4.5 (1.6) | −0.1 (0.1) | ||||
| No | 0.2 (0.4) | −0.3 (0.03) | 4.6 (0.7) | 0.0 (0.1) | ||||
|
Post CPB Norepinephrine |
0.94 | 0.051 | 0.50 | 0.59 | ||||
| Yes | 0.6 (0.7) | −0.4 (0.1) | 5.2 (1.1) | −0.1 (0.1) | ||||
| No | 0.7 (0.5) | −0.2 (0.04) | 4.3 (0.8) | 0.0 (0.1) | ||||
AVR = Aortic valve replacement, CABG = Coronary artery bypass grafting; CPB = Cardiopulmonary bypass, LVESS= left ventricular end-systolic meridional wall stress
Univariable P values;
significant with P<0.05.
Slope (SE) for continuous variables (age, aortic cross-clamp time) is interpreted as the estimated mean change for each unit increase in predictor; estimated mean change (SE) for each level of categorical variables from the univariable regression.
a, b, c, d, e and f present 66, 62, 61, 58, 49 and 44 missing points, respectively
Table 4.
Multivariable Relationships between the change in left (LV) and right (RV) ventricular strain and strain rate and risk factors.*
| Outcome | Predictor | Slope (SE)** | P |
|---|---|---|---|
| Change in LV strain (N=72) | Aortic cross-clamp time (unit = 10 min) |
0.6 (0.14)a | <0.001 |
| Previous cardiac procedure | 1.8 (0.72) | 0.018 | |
|
Change in LV strain rate (N= 67) |
Hyperinsulinemic normoglycemic clamp |
−0.2 (0.06) | 0.005 |
| Post CPB Epinephrine | 0.2 (0.08) | 0.04 | |
| Change in RV strain (N=54) | Female | 2.6 (1.3)b | 0.04 |
|
Change in RV strain rate (N=54) |
Hypertension | −0.32 (0.14) | 0.02 |
SE= Standard error
The relationship between LV and RV strain and strain rate and 15 potential risk factors (listed in Table 3) were assessed in a multivariable regression model. Due to small sample size and large number of risk factors, univariable analysis with P <0.05 were used to select initial candidates (Table 3). A stepwise variable selection procedure with inclusion/exclusion criterion of P <0.05 was used to select the final variables.
For example, slopes for continuous predictors were interpreted as:
an estimated mean change in strain increased by 0.6%, with each 10 minute increase in aortic cross-clamp time; for binary predictor:
for female, the estimated mean change in RV strain was 2.6% higher than for males.
Discussion
Our investigation evaluated the percent and rate of myocardial longitudinal shortening with strain and strain rate and found that RV and LV function demonstrate acute and divergent changes immediately following AVR. Despite intraoperative cardioplegic arrest, an ischemia-reperfusion sequence, and possible myocardial stunning, LV function improved as documented by a nearly 40% increase in LV strain rate. However, LV strain, which measures the amount of LV longitudinal systolic shortening, was unchanged. In contrast, RV strain decreased while RV strain rate was unchanged, demonstrating a reduction in the amount of RV systolic shortening without affecting rate of contraction.
LV strain rate, a robust noninvasive measure of contractility representing the rate of myocardial shortening,18–20,37 was greatly increased after AVR, consistent with improved LV function. Strain rate correlates with the rate of LV pressure rise, a well-established measure of LV contractility which is based on evidence that the greater the contractile force exerted, the greater the rate of increase in LV pressure.38 LV strain, in contrast, did not improve. The lack of improvement in LV strain was unexpected because strain detects subtle changes in myocardial function that are not apparent with conventional echocardiography.39–41 Decreased LV preload, documented by smaller intraventricular volumes at the end of surgery, reduces strain by the Frank Starling mechanism42 and may have contributed to the lack of improvement in LV strain.
Other clinical settings have similarly documented improved contractility by an improvement in strain rate without corresponding changes in LV strain. Beta-adrenergic stimulation with dobutamine infusion in normally perfused myocardium increases strain rate with a minimal effect on strain,43 and, patients with low flow-low gradient aortic stenosis increase peak strain rate, but not strain, at peak stress during dobutamine stress echocardiography.44 Strain rate is less sensitive to changes in preload and heart rate than strain,18,45,46 and thus served as a more robust measure of LV function in our patients.
Whether the increase in LV strain rate was related to an improvement in intrinsic myocardial contractility, the myocardial response to an acute decrease in afterload, or a mild increase in heart rate cannot be determined from our results, because strain and strain rate are load-dependent measures.42,47 But there certainly was a substantial decrease in afterload as demonstrated by a nearly 5-fold reduction in the mean transvalvular gradient and 40% decrease in LVESS. Several techniques adjust myocardial deformation measures for changes in load, though each method has limitations. Some investigations “normalize” LV strain for changes in preload by adjusting for end-diastolic volume,48 though this modifies strain from a dimensionless quantity (it has the same value regardless of units) to a measure restricted to end-diastolic volume. In addition, normalizing strain for end-diastolic volume may result in a measurement that reflects LV size more than myocardial contraction. One investigation, for example, adjusted strain for end-diastolic volume in patients with aortic regurgitation48 and reported that absolute (actual) values of strain were not improved by corrective surgery, but normalized (strain/end-diastolic volume) strain values improved. However, the improvement in normalized strain was driven by a reduction in LV size, not a change in strain, and thus could be interpreted that LV volume decreases after AVR, rather than strain improves.48 Another important point is that loading conditions do not change in isolation and adjustment for a single factor ignores the effects of other important variables. Indeed, changes in preload also affect contractility and afterload and invoke baroreceptor responses and reflex changes that further alter the inotropic state.16,49 Another method of adjustment uses a multivariable model to adjust for loading conditions,50 but this method assumes a linear relationship between strain or strain rate and other variables, which may not be accurate.
Because of limitations associated with the above methods to adjust for loading changes, we chose to report the actual strain values without adjustment in our primary results. Our secondary analysis, however, explored the association between myocardial deformation and afterload using LVESS49 and preload assessed by LV end-diastolic dimension. Neither the change in preload or afterload were associated with the change in measures of myocardial deformation, suggesting that other factors may have contributed to an improvement in strain rate such as activation of autonomic reflexes or increases in endogenous and exogenous circulating catecholamines at the end of surgery. Heart rate is also increased by these factors which may further improve myocardial contractility. Harpole et al.51 reported that intrinsic myocardial contractility assessed by a load-independent measure, the stroke work-end-diastolic volume relationship was unchanged after replacement of a stenotic aortic valve. This difference from our results was likely due to the use of load-dependent versus load-independent measures.
Less is known about RV function during cardiac surgery, because its complex geometry and crescent-like shape complicate echocardiographic assessment.52 Using myocardial deformation analysis, our investigation demonstrated that, in distinct contrast to the beneficial effects of AVR on the LV, RV function did not improve after surgery. In fact, reduced longitudinal strain suggests worse RV function. Other echocardiographic measures of RV function including fractional area change and TAPSE similarly decreased at the end of surgery, consistent with worsening of RV function, though RV strain rate was unchanged. These results are consistent with the divergent effects of AVR on perioperative RV and LV function.
Why RV strain as well as other measures of RV function worsened at the end of surgery is unclear. One study similarly reports a decline in RV function after cardiac surgery and suggests that inadequate myocardial protection and subsequent interventricular septal dysfunction contribute to this finding.53 Another report suggests that pericardiotomy caused a decline in RV function.54 Others described a change in the pattern of RV contraction after surgery where longitudinal shortening was reduced while transverse shortening increased, thus maintaining low normal RV function.55 Hemodynamic variables may also affect RV function: the LV benefits from an acute reduction in afterload while RV afterload is maintained, demonstrated by preserved pulmonary artery pressures. RV preload, however, was reduced as indicated by a decrease in RV end-diastolic area. Other contributing factors may include interventricular dependence, where the size, shape, and compliance of one ventricle affects the other through direct mechanical interaction. Whether this change in RV function has clinical implications is unclear. Our study population was small with few adverse events and thus could not assess whether there was an association with adverse postoperative complications. However, because overall mortality after AVR is widely reported to be less than 2%,56,57 this reduction in RV strain does not appear to profoundly impact postoperative outcomes.
In addition to the expected hemodynamic effects of removal of a stenotic aortic valve, our secondary analysis examined other contributors to the change in myocardial deformation at the end of surgery. As reported previously, patients who received the hyperinsulinemic normoglycemic clamp demonstrated increased LV strain rate,27 but not other measures of myocardial deformation. Prolonged aortic cross-clamp time decreased LV strain, though LV strain rate and RV deformation parameters were unaffected. Interestingly, patients who did not receive epinephrine had a greater improvement in strain rate compared with those who received epinephrine, providing evidence that an increase in strain rate after surgery was not related to use of epinephrine. Alternatively, epinephrine use likely identified patients who experienced post-CPB myocardial dysfunction and thus required inotropic support. Similarly, epinephrine and norepinephrine use was not related to the change in other myocardial deformation measures.
Strain and strain rate assessments are uncommon in the operating room because of a lack of availability and experience with these techniques. Furthermore, strain rate measurements are characterized by significant noise and require substantial experience for interpretation. However, future improvements in image acquisition, analysis programs, real-time availability of strain and strain rate, and use of 3D assessment may increase use of strain and strain rate in the operating room. As demonstrated by our investigation, strain and strain rate may be used to detect subtle improvements or decrements in myocardial performance that cannot be appreciated by simple visual assessment of LV or RV motion by echocardiography. Importantly, there is currently no 2D-echocardiographic method available for assessment of rate of systolic contraction comparable to strain rate.
Although strain can be measured in longitudinal, radial, and circumferential dimensions, we focused on longitudinal strain because it provides a more reliable and reproducible measure of systolic function.58,59 Importantly, longitudinal function plays an important role in patients with aortic stenosis.39,60 Further, longitudinal strain predicts outcomes in patients with aortic stenosis,61 and other clinical scenarios including ischemic cardiomyopathy,62 heart failure,63 acute myocardial infarction,64 and mitral valve repair.65 In patients with heart failure, longitudinal strain best discriminates between patients who will require rehospitalization or die from cardiac causes.66 Importantly, longitudinal strain is easily calculated from routinely collected echocardiographic views that are acquired during the intraoperative period. Because the RV is more dependent upon longitudinal shortening during ejection than the LV,67 the use of longitudinal strain and strain rate measurements are especially well-suited for assessment of RV function.
This investigation has limitations. As discussed above, strain and strain rate are both affected by loading conditions. Strain and strain rate naturally vary among individuals. For example, strain ranged between −5.5 and −27.5% and strain rate was between −0.3 and −1.8 sec−1 in our study population, but by using a paired analysis, with patients serving as their own controls, we were able to isolate the specific effects of surgical events. Midesophageal echocardiographic images used for myocardial deformation analysis may have been subject to foreshortening; however, our analysis assessed the within-patient change from baseline thus reducing bias from foreshortened images. Although this investigation was limited to patients with aortic stenosis having AVR, there was variability in the surgical procedure, surgical approach, and myocardial protection strategy; however, our supplemental analysis found that the effect of these variables on myocardial deformation was not significant and this heterogeneity enhances the generalizability of our results. Strain and strain rate measurements may vary somewhat among operators; we thus restricted all echocardiographic measurements and analyses to a single experienced investigator who used a software analysis program from a single vendor. Intraobserver variability demonstrated excellent agreement between measurements, though the use of a single observer may result in a consistent bias. Because measurements of strain rate using speckle-tracking are limited by a lower frame rate (typically 50–90 frames per second) compared with tissue Doppler measurements (>100 frames per second), undersampling resulting in reduced peak strain rate may have occurred,68 though all patients would be similarly affected. Finally, we present a subanalysis of a larger study in which patients were randomized to a hyperinsulinemic normoglycemic clamp or routine glucose management. However, the hyperinsulinemic normoglycemic clamp had minimal clinical effect27 and the contribution of this treatment was evaluated in the secondary analysis.
In conclusion, surgical removal of a stenotic aortic valve improves LV function, as measured intraoperatively by myocardial strain rate. LV strain, in contrast, did not improve, possibly because loading conditions also changed considerably after valve replacement. RV strain, however, was reduced, though the clinical implications of this finding require further exploration.
Supplementary Material
Acknowledgments
Funding: This investigation was supported by NIH HL093065 (Dr. Duncan) and the Departments of Cardiothoracic Anesthesia, OUTCOMES RESEARCH, Quantitative Health Sciences and Cardiovascular Medicine, Cleveland Clinic, Cleveland, OH.
Footnotes
Disclosures
Name: Andra E. Duncan, MD, MS
Contribution: Andra Duncan was responsible for the study design, conduct of the study, data collection, data analysis, data interpretation, and manuscript preparation. Andra Duncan is the archival author.
Attestation: Andra Duncan attests to the integrity of the original data and analysis and approved the final manuscript.
Conflicts of Interest: None.
Name: Sheryar Sarwar, MD
Contribution: Sheryar Sarwar was responsible for data collection and manuscript preparation.
Attestation: Sheryar Sarwar approved the final manuscript.
Conflicts of Interest: None.
Name: Babak Kateby Kashy, MD
Contribution: Babak Kateby Kashy was responsible for data collection and manuscript preparation.
Attestation: Babak Kateby Kashy approved the final manuscript.
Conflicts of Interest: None.
Name: Abraham Sonny, MD
Contribution: Abraham Sonny was responsible for data collection and manuscript preparation.
Attestation: Abraham Sonny approved the final manuscript.
Conflicts of Interest: None.
Name: Shiva Sale, MD
Contribution: Shiva Sale was responsible for data collection and manuscript preparation.
Attestation: Shiva Sale approved the final manuscript.
Conflicts of Interest: None.
Name: Andrej Alfirevic, MD
Contribution: Andrej Alfirevic was responsible for data collection and manuscript preparation.
Attestation: Andrej Alfirevic approved the final manuscript.
Conflicts of Interest: None.
Name: Dongsheng Yang, MS
Contribution: Dongsheng Yang was responsible for data analysis and interpretation.
Attestation: Dongsheng Yang approved the final manuscript.
Conflicts of Interest: None.
Name: James D. Thomas, MD
Contribution: James Thomas was responsible for study design, conduct of study, data interpretation, and manuscript preparation.
Attestation: James Thomas approved the final manuscript.
Conflicts of Interest: None.
Name: Marc Gillinov, MD
Contribution: Marc Gillinov was responsible for conduct of study, data interpretation, and manuscript preparation.
Attestation: Marc Gillinov approved the final manuscript.
Conflicts of Interest: Marc Gillinov serves as a consultant for Edwards Lifesciences, Medtronic, Tendyne, Abbott, On-X, and PleuraFlow. Dr. Gillinov has served as a speaker and/or received honoraria from Edward Lifesciences, Medtronic, and Intuitive Surgical and receives research support from St. Jude Medical.
Name: Daniel I. Sessler, MD
Contribution: Daniel Sessler was responsible for study design, conduct of study, data interpretation, and manuscript preparation.
Attestation: Daniel Sessler attests to the integrity of the original data and analysis and approved the final manuscript.
Conflicts of Interest: None.
This manuscript was handled by: Martin J. London, MD
Partial results were presented at the American Society of Anesthesiologists Scientific meeting in San Francisco on October 12, 2013.
The complete results were presented at the American Society of Anesthesiologists Scientific meeting in New Orleans on October 14, 2014.
Contributor Information
Andra E. Duncan, Department of Cardiothoracic Anesthesia and Outcomes Research, Cleveland Clinic, Cleveland, Ohio.
Sheryar Sarwar, Department of Outcomes Research, Cleveland Clinic, Cleveland, Ohio.
Babak Kateby Kashy, Department of Outcomes Research, Cleveland Clinic, Cleveland, Ohio.
Abraham Sonny, Anesthesia Institute, Cleveland Clinic, Cleveland Ohio.
Shiva Sale, Department of Cardiothoracic Anesthesia, Cleveland Clinic, Cleveland, Ohio.
Andrej Alfirevic, Department of Cardiothoracic Anesthesia, Cleveland Clinic, Cleveland, Ohio.
Dongsheng Yang, Departments of Quantitative Health Sciences and OUTCOMES RESEARCH, Cleveland Clinic, Cleveland, Ohio.
James D. Thomas, Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland, Ohio.
Marc Gillinov, Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Cleveland, Ohio.
Daniel I. Sessler, Department of Outcomes Research, Cleveland Clinic, Cleveland, Ohio.
References
- 1.Carabello BA. Introduction to Aortic Stenosis. Circulation Research. 2013;113:179–185. doi: 10.1161/CIRCRESAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 2.Brennan JM, Edwards FH, Zhao Y, O'Brien SM, Douglas PS, Peterson ED Developing Evidence to Inform Decisions About Effectiveness-Aortic Valve Replacement Research T. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 1991 to 2007. Circulation. 2012;126:1621–1629. doi: 10.1161/CIRCULATIONAHA.112.091371. [DOI] [PubMed] [Google Scholar]
- 3.Vanky FB, Hakanson E, Svedjeholm R. Long-term consequences of postoperative heart failure after surgery for aortic stenosis compared with coronary surgery. Ann Thorac Surg. 2007;83:2036–2043. doi: 10.1016/j.athoracsur.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Haddad F, Denault AY, Couture P, Cartier R, Pellerin M, Levesque S, Lambert J, Tardif J-C. Right Ventricular Myocardial Performance Index Predicts Perioperative Mortality or Circulatory Failure in High-Risk Valvular Surgery. Journal of the American Society of Echocardiography. 2007;20:1065–1072. doi: 10.1016/j.echo.2007.02.017. x00E, ois. [DOI] [PubMed] [Google Scholar]
- 5.Kouchoukos NT, Blackstone EH, Hanley FL, Kirklin JK. Cardiac Surgery. Fourth. Philadelphia: Elsevier Saunders; 2013. pp. 133–162. [Google Scholar]
- 6.Buckberg GD. Myocardial protection: entering the new millennium. J Card Surg. 2002;17:447–450. doi: 10.1111/j.1540-8191.2001.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 7.Breisblatt WM, Stein KL, Wolfe CJ, Follansbee WP, Capozzi J, Armitage JM, Hardesty RL. Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol. 1990;15:1261–1269. doi: 10.1016/s0735-1097(10)80011-7. [DOI] [PubMed] [Google Scholar]
- 8.McAinsh AM, Turner MA, O'Hare D, Nithythyananthan R, Johnston DG, O'Gorman DJ, Sheridan DJ. Cardiac hypertrophy impairs recovery from ischaemia because there is a reduced reactive hyperaemic response. Cardiovasc Res. 1995;30:113–121. [PubMed] [Google Scholar]
- 9.Schonekess BO, Allard MF, Lopaschuk GD. Recovery of glycolysis and oxidative metabolism during postischemic reperfusion of hypertrophied rat hearts. Am J Physiol. 1996;271:H798–H805. doi: 10.1152/ajpheart.1996.271.2.H798. [DOI] [PubMed] [Google Scholar]
- 10.Ross J., Jr Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog Cardiovasc Dis. 1976;18:255–264. doi: 10.1016/0033-0620(76)90021-9. [DOI] [PubMed] [Google Scholar]
- 11.Harpole DH, Jr, Gall SA, Jr, Wolfe WG, Rankin JS, Jones RH. Effects of valve replacement on ventricular mechanics in mitral regurgitation and aortic stenosis. Ann Thorac Surg. 1996;62:756–761. doi: 10.1016/s0003-4975(96)00378-5. [DOI] [PubMed] [Google Scholar]
- 12.Allen BS, Winkelmann JW, Hanafy H, Hartz RS, Bolling KS, Ham J, Feinstein S. Retrograde cardioplegia does not adequately perfuse the right ventricle. J Thorac Cardiovasc Surg. 1995;109:1116–1124. doi: 10.1016/S0022-5223(95)70195-8. discussion 1124–6. [DOI] [PubMed] [Google Scholar]
- 13.Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res. 1973;32:314–322. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- 14.Sagawa K, Suga H, Shoukas AA, Bakalar KM. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am J Cardiol. 1977;40:748–753. doi: 10.1016/0002-9149(77)90192-8. [DOI] [PubMed] [Google Scholar]
- 15.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Jr, Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 16.Bugge-Asperheim B, Kiil F. Preload, contractility, and afterload as determinants of stroke volume during elevation of aortic blood pressure in dogs. Cardiovasc Res. 1973;7:328–341. [PubMed] [Google Scholar]
- 17.Janssen PM. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol. 2010;299:H1092–H1099. doi: 10.1152/ajpheart.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weidemann F, Jamal F, Kowalski M, Kukulski T, D'Hooge J, Bijnens B, Hatle L, De Scheerder I, Sutherland GR. Can strain rate and strain quantify changes in regional systolic function during dobutamine infusion, B-blockade, and atrial pacing--implications for quantitative stress echocardiography. J Am Soc Echocardiogr. 2002;15:416–424. doi: 10.1067/mje.2002.116535. [DOI] [PubMed] [Google Scholar]
- 19.Jamal F, Strotmann J, Weidemann F, Kukulski T, D'hooge J, Bijnens B, Van de Werf F, De Scheerder I, Sutherland GR. Noninvasive quantification of the contractile reserve of stunned myocardium by ultrasonic strain rate and strain. Circulation. 2001;104:1059–1065. doi: 10.1161/hc3501.093818. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ. Doppler-derived myocardial systolic strain rate Is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 21.Duncan AE, Alfirevic A, Sessler DI, Popovic ZB, Thomas JD. Perioperative assessment of myocardial deformation. Anesth Analg. 2014;118:525–544. doi: 10.1213/ANE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80–84. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Rost C, Korder S, Wasmeier G, Wu M, Klinghammer L, Flachskampf FA, Daniel WG, Voigt JU. Sequential changes in myocardial function after valve replacement for aortic stenosis by speckle tracking echocardiography. Eur J Echocardiogr. 2010;11:584–589. doi: 10.1093/ejechocard/jeq017. [DOI] [PubMed] [Google Scholar]
- 25.Iwahashi N, Nakatani S, Kanzaki H, Hasegawa T, Abe H, Kitakaze M. Acute improvement in myocardial function assessed by myocardial strain and strain rate after aortic valve replacement for aortic stenosis. Journal of the American Society of Echocardiography. 2006;19:1238–1244. doi: 10.1016/j.echo.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 26.Carasso S, Cohen O, Mutlak D, Adler Z, Lessick J, Reisner SA, Rakowski H, Bolotin G, Agmon Y. Differential effects of afterload on left ventricular long- and short-axis function: insights from a clinical model of patients with aortic valve stenosis undergoing aortic valve replacement. Am Heart J. 2009;158:540–545. doi: 10.1016/j.ahj.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Duncan AE, Kashy BK, Sarwar S, Stenina-Adognravi O, Christoffersen S, Alfirevic A, Sale S, Yang D, Thomas JD, Gillinov AM, Sessler DI. Hyperinsulinemic normoglycemic does not meaningfully improve myocardial performance during cardiac surgery: A randomized trial. Anesthesiology. 2015;123:272–287. doi: 10.1097/ALN.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan AI, Lowe BS, Garcia MJ, Xu M, Gillinov AM, Mihaljevic T, Koch CG. Influence of concentric left ventricular remodeling on early mortality after aortic valve replacement. Ann Thorac Surg. 2008;85:2030–2039. doi: 10.1016/j.athoracsur.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 29.Mehta RH, Bruckman D, Das S, Tsai T, Russman P, Karavite D, Monaghan H, Sonnad S, Shea MJ, Eagle KA, Deeb GM. Implications of increased left ventricular mass index on in-hospital outcomes in patients undergoing aortic valve surgery. J Thorac Cardiovasc Surg. 2001;122:919–928. doi: 10.1067/mtc.2001.116558. [DOI] [PubMed] [Google Scholar]
- 30.DePace NL, Ren JF, Iskandrian AS, Kotler MN, Hakki AH, Segal BL. Correlation of echocardiographic wall stress and left ventricular pressure and function in aortic stenosis. Circulation. 1983;67:854–859. doi: 10.1161/01.cir.67.4.854. [DOI] [PubMed] [Google Scholar]
- 31.Santo-Domingo NE, Orlov M, Wong ND, Kurosaki T, Reid CL, Hsieh AM, Gardin JM. Left ventricular end-systolic stress in young adults: distribution, risk factors, and relation to cardiovascular disease events. Echocardiography. 2009;26:1006–1011. doi: 10.1111/j.1540-8175.2009.00921.x. [DOI] [PubMed] [Google Scholar]
- 32.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 33.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 35.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Meris A, Santambrogio L, Casso G, Mauri R, Engeler A, Cassina T. Intraoperative three-dimensional versus two-dimensional echocardiography for left ventricular assessment. Anesth Analg. 2014;118:711–720. doi: 10.1213/ANE.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 37.Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002;283:H792–H799. doi: 10.1152/ajpheart.00025.2002. [DOI] [PubMed] [Google Scholar]
- 38.Wallace AG, Skinner NS, Jr, Mitchell JH. Hemodynamic determinants of the maximal rate of rise of left ventricular pressure. Am J Physiol. 1963;205:30–36. doi: 10.1152/ajplegacy.1963.205.1.30. [DOI] [PubMed] [Google Scholar]
- 39.Lafitte S, Perlant M, Reant P, Serri K, Douard H, DeMaria A, Roudaut R. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur J Echocardiogr. 2009;10:414–419. doi: 10.1093/ejechocard/jen299. [DOI] [PubMed] [Google Scholar]
- 40.Florescu M, Benea DC, Rimbas RC, Cerin G, Diena M, Lanzzillo G, Enescu OA, Cinteza M, Vinereanu D. Myocardial systolic velocities and deformation assessed by speckle tracking for early detection of left ventricular dysfunction in asymptomatic patients with severe primary mitral regurgitation. Echocardiography. 2012;29:326–333. doi: 10.1111/j.1540-8175.2011.01563.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim MS, Kim YJ, Kim HK, Han JY, Chun HG, Kim HC, Sohn DW, Oh BH, Park YB. Evaluation of left ventricular short- and long-axis function in severe mitral regurgitation using 2-dimensional strain echocardiography. Am Heart J. 2009;157:345–351. doi: 10.1016/j.ahj.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Rosner A, Bijnens B, Hansen M, How OJ, Aarsaether E, Muller S, Sutherland GR, Myrmel T. Left ventricular size determines tissue Doppler-derived longitudinal strain and strain rate. Eur J Echocardiogr. 2009;10:271–277. doi: 10.1093/ejechocard/jen230. [DOI] [PubMed] [Google Scholar]
- 43.Voigt J-U, Exner B, Schmiedehausen K, Huchzermeyer C, Reulbach U, Nixdorff U, Platsch G, Kuwert T, Daniel WG, Flachskampf FA. Strain-Rate Imaging During Dobutamine Stress Echocardiography Provides Objective Evidence of Inducible Ischemia. Circulation. 2003;107:2120–2126. doi: 10.1161/01.CIR.0000065249.69988.AA. [DOI] [PubMed] [Google Scholar]
- 44.Bartko PE, Heinze G, Graf S, Clavel MA, Khorsand A, Bergler-Klein J, Burwash IG, Dumesnil JG, Senechal M, Baumgartner H, Rosenhek R, Pibarot P, Mundigler G. Two-dimensional strain for the assessment of left ventricular function in low flow-low gradient aortic stenosis, relationship to hemodynamics, and outcome: a substudy of the multicenter TOPAS study. Circ Cardiovasc Imaging. 2013;6:268–276. doi: 10.1161/CIRCIMAGING.112.980201. [DOI] [PubMed] [Google Scholar]
- 45.Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, Veulemans P, Pellens M, La Gerche A, Rademakers F, Herijgers P, D'Hooge J. The relative value of strain and strain rate for defining intrinsic myocardial function. Am J Physiol Heart Circ Physiol. 2012;302:H188–H195. doi: 10.1152/ajpheart.00429.2011. [DOI] [PubMed] [Google Scholar]
- 46.Weytjens C, D'Hooge J, Droogmans S, Van den Bergh A, Cosyns B, Lahoutte T, Herijgers P, Van Camp G. Influence of heart rate reduction on Doppler myocardial imaging parameters in a small animal model. Ultrasound Med Biol. 2009;35:30–35. doi: 10.1016/j.ultrasmedbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Burns AT, La Gerche A, D'Hooge J, MacIsaac AI, Prior DL. Left ventricular strain and strain rate: Characterization of the effect of load in human subjects. Eur J Echocardiogr. 2010;11:283–289. doi: 10.1093/ejechocard/jep214. [DOI] [PubMed] [Google Scholar]
- 48.Smedsrud MK, Pettersen E, Gjesdal O, Svennevig JL, Andersen K, Ihlen H, Edvardsen T. Detection of Left Ventricular Dysfunction by Global Longitudinal Systolic Strain in Patients with Chronic Aortic Regurgitation. J Am Soc Echocardiogr. 2011;24:1253–1259. doi: 10.1016/j.echo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Reichek N, Wilson J, St John Sutton M, Plappert TA, Goldberg S, Hirshfeld JW. Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation. 1982;65:99–108. doi: 10.1161/01.cir.65.1.99. [DOI] [PubMed] [Google Scholar]
- 50.Saito M, Negishi K, Eskandari M, Huynh Q, Hawson J, Moore A, Koneru S, Foster S, Marwick TH. Association of left ventricular strain with 30-day mortality and readmission in patients with heart failure. J Am Soc Echocardiogr. 2015;28:652–666. doi: 10.1016/j.echo.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Harpole DH, Rankin JS, Wolfe WG, Smith LR, Young WG, Clements FM, Jones RH. Assessment of left ventricular functional preservation during isolated cardiac valve operations. Circulation. 1989;80:III1–III9. [PubMed] [Google Scholar]
- 52.Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg. 2009;108:407–421. doi: 10.1213/ane.0b013e31818f8623. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen T, Cao L, Movahed A. Altered Right Ventricular Contractile Pattern after Cardiac Surgery: Monitoring of Septal Function Is Essential. Echocardiography. 2014;31:1159–1165. doi: 10.1111/echo.12657. [DOI] [PubMed] [Google Scholar]
- 54.Unsworth B, Casula RP, Kyriacou AA, Yadav H, Chukwuemeka A, Cherian A, Stanbridge Rde L, Athanasiou T, Mayet J, Francis DP. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J. 2010;159:314–322. doi: 10.1016/j.ahj.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raina A, Vaidya A, Gertz ZM, Susan C, Forfia PR. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post-surgical assessment of right ventricular function. J Heart Lung Transplant. 2013;32:777–783. doi: 10.1016/j.healun.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Singh V, Badheka AO, Patel SV, Patel NJ, Thakkar B, Patel N, Arora S, Patel A, Savani C, Ghatak A, Panaich SS, Jhamnani S, Deshmukh A, Chothani A, Sonani R, Bhatt P, Dave A, Bhimani R, Mohamad T, Grines C, Cleman M, Forrest JK, Mangi A. Comparison of Inhospital Outcomes of Surgical Aortic Valve Replacement in Hospitals With and Without Availability of a Transcatheter Aortic Valve Implantation Program (from a Nationally Representative Database) Am J Cardiol. 2015 doi: 10.1016/j.amjcard.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 57.Parikh R, Goodman AL, Barr T, Sabik JF, Svensson LG, Rodriguez LL, Lytle BW, Grimm RA, Griffin BP, Desai MY. Outcomes of surgical aortic valve replacement for severe aortic stenosis: Incorporation of left ventricular systolic function and stroke volume index. J Thorac Cardiovasc Surg. 2015;149:1558 e1–1566 e1. doi: 10.1016/j.jtcvs.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, Friedberg MK. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr. 2011;24:37–44. doi: 10.1016/j.echo.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Risum N, Ali S, Olsen NT, Jons C, Khouri MG, Lauridsen TK, Samad Z, Velazquez EJ, Sogaard P, Kisslo J. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr. 2012;25:1195–1203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Donal E, Thebault C, O'Connor K, Veillard D, Rosca M, Pierard L, Lancellotti P. Impact of aortic stenosis on longitudinal myocardial deformation during exercise. Eur J Echocardiogr. 2011;12:235–241. doi: 10.1093/ejechocard/jeq187. [DOI] [PubMed] [Google Scholar]
- 61.Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, Grimm RA, Griffin BP, Desai MY. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7:938–945. doi: 10.1161/CIRCIMAGING.114.002041. [DOI] [PubMed] [Google Scholar]
- 62.Bertini M, Ng ACT, Antoni ML, Nucifora G, Ewe SH, Auger D, Marsan NA, Schalij MJ, Bax JJ, Delgado V. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circulation: Cardiovascular Imaging. 2012;5:383–391. doi: 10.1161/CIRCIMAGING.111.970434. [DOI] [PubMed] [Google Scholar]
- 63.Iacoviello M, Puzzovivo A, Guida P, Forleo C, Monitillo F, Catanzaro R, Lattarulo MS, Antoncecchi V, Favale S. Independent role of left ventricular global longitudinal strain in predicting prognosis of chronic heart failure patients. Echocardiography. 2013;30:803–811. doi: 10.1111/echo.12142. [DOI] [PubMed] [Google Scholar]
- 64.Mollema SA, Delgado V, Bertini M, Antoni ML, Boersma E, Holman ER, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ. Viability assessment with global left ventricular longitudinal strain predicts recovery of left ventricular function after acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:15–23. doi: 10.1161/CIRCIMAGING.108.802785. [DOI] [PubMed] [Google Scholar]
- 65.Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, Versteegh MI, Holman ER, Schalij MJ, Bax JJ, Klautz RJ, Marsan NA. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging. 2013;14:69–76. doi: 10.1093/ehjci/jes155. [DOI] [PubMed] [Google Scholar]
- 66.Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, Monzy S, Roudaut R, Habib G, Lafitte S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr. 2010;23:1019–1024. doi: 10.1016/j.echo.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Vitarelli A, Terzano C. Do we have two hearts? New insights in right ventricular function supported by myocardial imaging echocardiography. Heart Fail Rev. 2010;15:39–61. doi: 10.1007/s10741-009-9154-x. [DOI] [PubMed] [Google Scholar]
- 68.Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, La Gerche A, Rademakers F, Herijgers P, D'Hooge J. Assessment of strain and strain rate by two-dimensional speckle tracking in mice: comparison with tissue Doppler echocardiography and conductance catheter measurements. Eur Heart J Cardiovasc Imaging. 2013;14:765–773. doi: 10.1093/ehjci/jes274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.