Abstract
Adolescence is a unique period of development, marked by maturation of the prefrontal cortex (PFC), a region important for executive functioning. During this time, the human PFC decreases in overall volume and thickness. Likewise in adolescent rodents, losses of neurons, dendrites, dendritic spines and neurotransmitter receptors have been documented within the medial prefrontal cortex (mPFC), sometimes with sex and layer specificity. However, changes in the number of synapses during this time have not been examined. In the present study, we stereologically quantified the number of synaptophysin-immunoreactive boutons in the male and female rat mPFC across multiple time points from the juvenile period through adulthood (postnatal days (P) 25, 35, 45, 60 and 90). In females, there was a significant decrease in synaptophysin boutons between P35 and P45, coinciding with the onset of puberty. In males, there was no significant main effect of age on synaptophysin boutons; however, in both males and females, pubertal onset was associated with significant synaptic losses. These results suggest that puberty is a critical period for synaptic pruning within the rat mPFC, potentially contributing to maturation of adolescent executive function.
Keywords: synapse, pruning, puberty, stereology, synaptophysin
Graphical abstract
Stereological assessment of immunoreactive synaptophysin boutons in the mPFC between early adolescence and adulthood revealed significant pruning that was larger in females than males.. Additionally, pubertal onset in both males and females was associated with decreases in synapse number, suggesting a role for pubertal hormones in regulating synaptic pruning.
INTRODUCTION
Adolescence, the transition from childhood into adulthood, is characterized by unique behavioral and physiological phenotypes observed across several mammalian species. Notably, adolescents differ from children and adults on a variety of behaviors involving the prefrontal cortex (PFC), suggesting that the PFC undergoes developmental remodeling during this time. Anatomical MRI scans indicate throughout childhood PFC gray matter increases to a peak volume in early adolescence when it begins to gradually decline to a mature volume by early adulthood (Giedd, 2004; Lenroot and Giedd, 2006; Jernigan et al., 1991; Sowell et al., 1999). This anatomical pattern also occurs in both non-human primates and rats (Knickmeyer et al., 2010; Van Eden and Uylings, 1989).
There is evidence that gray matter losses in the PFC may be due to synaptic pruning. In humans, an increase in synaptic density occurs across early childhood, peaking around the time of puberty, followed by a decrease across adolescence into adulthood (Huttenlocher and Dabholkar, 1997; Glantz et al., 2007). Changes in spine density often are layer-specific and may not reach adult levels until the third decade of life (Petanjek et al., 2011). This synaptic pruning pattern has also been found in the cortex of nonhuman primates (Bourgeois and Rakic, 1993; Anderson et al., 1995; Bourgeois et al., 1994).
Currently, the existing literature only examines changes in synaptic density, as opposed to the total number of synapses, though there are volumetric changes across adolescence. While this is understandable given the difficulties in parcellating cortical subregions in primate brains, estimations of synapse number using only measurements of synaptic density in the PFC could be confounded by changes in PFC volume between adolescence and adulthood. Another limitation is that postmortem human brain tissue is difficult to obtain, leading to small sample sizes and significant age gaps between subjects. To circumvent these problems in the existing human literature, the rodent PFC can be used as a model for adolescent development, as rats have a PFC both neuroanatomically and functionally homologous to that of humans and nonhuman primates (Uylings et al., 2003). Thus using a rat model, two of the goals of the present study is to sample multiple ages to better expound upon the timing of pruning and to include volume changes in the calculation so that the total number of synapses can be ascertained.
Additionally, current literature on synaptic pruning during adolescence focuses primarily on male subjects, though work from our laboratory suggests differential trajectories between sexes. For example, while neurons themselves are pruned in the rat mPFC during adolescence, pruning is considerably more prominent in females than males (Markham et al., 2007; Willing and Juraska, 2015). Additionally, dendritic spine density increases in Layer V pyramidal neurons for both male and female rats during the prepubertal period (P20 to P35) and then decreases between adolescence and adulthood (P35 to P90); however, only females lose basilar dendrites in this region between adolescence and adulthood (Koss et al., 2014). The measure of dendritic spines at only three time points and only one type of neuron within a specific layer is indicative but leaves an incomplete picture of synaptic loss, which we hope to clarify by examining multiple age groups.
Furthermore, the rise in ovarian hormones at puberty may have a role in synaptic loss. Koss et al. (2015) found that prepubertal ovariectomy resulted in more neurons in the mPFC in adulthood, while prepubertal gonadectomy was without effect in males. The timing and extent of neuronal loss in Willing and Juraska (2015) further supports a role for ovarian hormones. Therefore as a start in exploring this, both sexes will be examined and pubertal status will be tracked in the present study. The number of synapses within the mPFC will be stereologically calculated through the use of synaptophysin immunocytochemistry across five ages in these rats.
MATERIALS AND METHODS
Subjects
Subjects were offspring of Long-Evans hooded rats obtained from Harlan Laboratories (Indianapolis, IN) and bred in the vivarium of the Psychology Department at the University of Illinois. Subjects were weaned at postnatal day (P) 24, and housed in same sex pairs or triplets until sacrifice at one of five time points. These ages include the juvenile period through adulthood: P25 is the juvenile period; P35 and P45 are the respective average onsets for female and male puberty; and P60 and P90 represent late adolescence and adulthood. Each age group contained 10–11 subjects per sex for a total of 105 animals. Within each age group, animals came from a minimum of five separate litters, and no age had more than two animals of the same sex from one litter. Subjects were given ad libitum access to water and standard rat chow while kept on a 12:12 hour light-dark cycle throughout their life. All procedures adhered to the National Institute of Health guidelines on the ethical use of animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
Pubertal status was assessed using identifiable anatomical indicators for all animals that reached puberty prior to sacrifice. In female subjects, pubertal onset was marked by vaginal opening, which coincides with maturation of the hypothalamic-pituitary-ovarian axis, increases plasma LH and FSH levels as well as the appearance of the estrous cycle (Halász et al., 1988; da Silva Faria et al., 2004). Pubertal onset was verified in male rats by preputial separation, or separation of the prepuce from the glans penis which is dependent upon the pubertal surge of endogenous androgen hormones (Korenbrot et al., 1977).
Tissue collection
Experimental subjects were deeply anesthetized with sodium pentobarbital. Subjects were then perfused intracardially with 0.1M phosphate buffered saline (PBS) with a pH of 7.4, followed by 4% paraformaldehyde fixative in PBS. Brains were removed, stored in the same fix solution for an additional 24 hours, and cryoprotected in a PBS solution with 30% sucrose for three days. All brains were coded at this time so that the experimenters were blind to the animal’s group. Once the brain had sunk in sucrose solution, it was sliced with a freezing microtome into 40µm coronal sections. Every fifth PFC section was then placed into 0.1M PBS and mounted on gelatin-coated slides. These slices were stained with methylene blue/azure II, a cell body stain previously used by our laboratory for volumetric estimations (Chisholm and Juraska, 2012). These sections were used to calculate mPFC volume and neuron and glia number (Willing and Juraska, 2015).
Volume Estimation
The area of the ventral mPFC (prelimbic (PL) and infralimbic (IL)) region on each mounted slice was parcellated and divided into layers (layer I, layer II/III, and layer V/VI) by using the same cytoarchitectural guides described by previous studies (Markham et al., 2007; Uylings et al., 2003) with StereoInvestigator software (MicroBrightField). This area was measured across each mounted PFC slice, with the anterior edge of the mPFC marked by the emergence of the underlying white matter and the most posterior border of the mPFC defined by the crossing of the genu of the corpus callosum.
The thickness of the mounted PFC sections was determined by measuring the depth of the focal length from the top to the bottom of the tissue. This analysis was done using a motorized stage (Prior Scientific), which measured the thickness of the z-plane across more than 50 sites for each subject. Average tissue thickness was then calculated. Total volume of the mPFC was calculated using StereoInvestigator software by multiplying the area of the mPFC on each mounted slice by the average thickness of the tissue and the distance between slices.
Immunocytochemistry
Every fifth section containing mPFC was stained for synaptophysin, a presynaptic vesicle protein used as a marker for synapses (Mouton et al., 1997). Sections were first rinsed three times (5 minutes each) in Tris Buffered Saline (TBS, pH 7.6). Sections were then incubated in a blocking solution (20% normal goat serum, 1% bovine serum albumin, 1% hydrogen peroxide in TBS) for 30 minutes. Tissue was then put in primary antibody (anti-synaptophysin, 1:5000, Sigma-Aldrich; mouse monoclonal) for 48 hours. Primary antibody dilution was made in tris-triton goat (TTG) solution (2% normal goat serum, 0.3% triton X-100 in TBS). After two days in primary antibody solution, PFC slices were rinsed three times with TTG (5 minutes each) and incubated in a biotinylated secondary antibody (Anti-Mouse IgG Antibody, 5ug/ml, Vector Laboratories) for 90 minutes at room temperature. Following rinses in TTG and TBS, sections were placed in avidin-biotin complex (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA) for one hour at room temperature. Lastly, tissue was stained with diaminobenzidine (DAB) (SigmaFast 3–3’ Diaminobenzidine Tablets) for two minutes. The avidin-biotin + DAB technique is an effective method of visualizing specific antibodies with high staining intensity (Hsu et al., 1981). After rinsing off DAB with TBS, stained sections were mounted on gelatin-coated slides and cover-slipped with permount.
To confirm the specificity of the primary synaptophysin antibody, a negative control staining protocol was conducted on mPFC tissue slices without the anti-synaptophysin primary antibody. This resulted in no detectable immunolabeling, thus confirming the synaptic localization of our primary antibody.
Stereology and Synapse Number Estimation
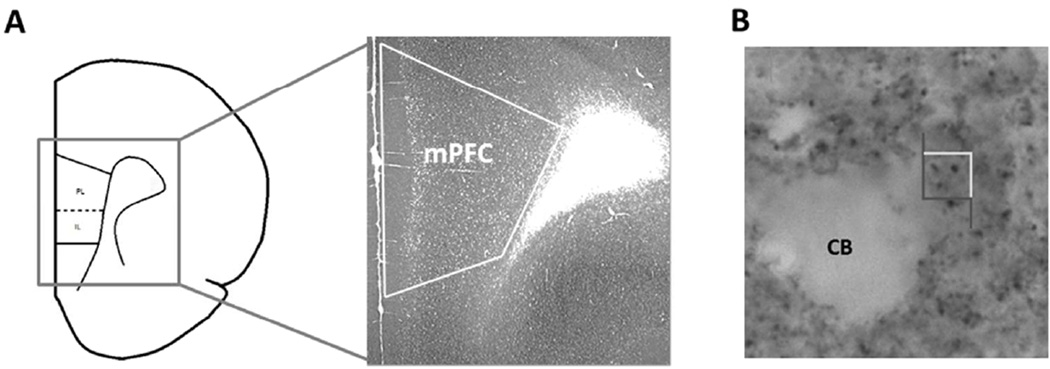
Stereological analysis of tissue stained for synaptophysin has been shown to be a reliable measure of synaptic numbers within the central nervous system (Calhoun et al., 1996). The overall density of the labeled synaptophysin boutons within the mPFC was determined using the optical disector in Stereoinvestigator. Prior to counting, the experimenter manually parcellated out layers I, II/III, and V/VI of the mPFC across the right and left hemispheres (Fig. 1A). The optical disector then imposes a grid (here 125 µm × 125 µm) across the tissue and places a counting frame (here 4µm × 4µm) in the corner of each grid square to ensure systematic uniform random sampling across the mPFC.
Fig. 1.
Coronal section of the mPFC that illustrates the immuno-labeled tissue used for counting synaptophysin across all cellular layers. PL = prelimbic, IL = infralimbic (A). Photograph within the mPFC showing immunoreactive synaptophysin boutons. The stereological counting frame (4µm × 4µm) is shown. CB = cell body (B).
The counting frame perimeter is made up of two “acceptance” edges and two “exclusion” edges. If a synaptophysin bouton falls entirely within the counting frame or it touches the “acceptance” edge, it is counted. All boutons that contact the “exclusion” end are omitted from analysis (Fig. 1B). The depth of the counting frame was kept to 6 µm with guard zones of 0.1 µm, and the Stereoinvestigator software tracks total bouton counts and the number of counting sites visited for each subject. The same technique has been reliably used by our laboratory to quantify the number of synapses in the aging mPFC (Chisholm and Juraska, 2012).
Synaptophysin boutons were quantified separately in layers I, II/III and V/VI of the rat ventral mPFC. For each animal, the recommended minimum of 200 boutons were counted within each layer (Gundersen and Jensen, 1987), resulting in an average of 251, 343, 594 synaptic boutons per animal counted in layer I, layer II/III, and layer V/VI, respectively. The number of counted boutons was divided by the total volume of the counting sites to obtain the synaptic density. Density was then multiplied by the previously calculated volume of the mPFC to determine the total number of synaptophysin immunoreactive boutons within each layer of the 9 mPFC. This was subsequently added across all layers resulting in an estimation of the total number of synapses in the mPFC.
Statistical Analysis
Due to the sex difference in pubertal onset, different trajectories of synaptic loss in males and females were hypothesized. Thus, male and female subjects were analyzed separately. For the overall number of synapses, a one-way analysis of variance (ANOVA) with age as the factor was run with litter as a cofactor, combining counts from all layers. Separate ANOVAs were also conducted for each cellular layer (layer I, layer II/III, and layer V/VI). When age was significant, four post hoc comparisons of Fisher’s least square difference (LSD) were run that compared adjacent ages to each other (P25 vs 35, 35 vs 45, 45 vs 60, 60 vs 90). In addition, the effect size was assessed using Cohen’s d for all findings (Cohen, 1992).
To specifically examine the effects of puberty on synapse number, a student’s t-test was used to compare the number of synapses at P35 and P45 for females and males, respectively that had or had not yet reached puberty. Subjects that had not yet reached puberty were also compared to their next older age counterparts. In females, a t-test was run comparing all pre-pubertal females at P35 to all post-pubertal females at P45. In males, the same test was used, comparing all pre-pubertal males at P45 to all post-pubertal males at P60.
RESULTS
Age of Pubertal Onset
As previously reported by Willing et al. (2015) for animals that were not sacrificed prior to pubertal onset, female subjects reached puberty earlier than male subjects. The average age for the onset of puberty in females was P34.9, with a range of 32–38. In males, the average age of pubertal onset was P44.9, with a range of 42–48.
Age and Synaptophysin Boutons
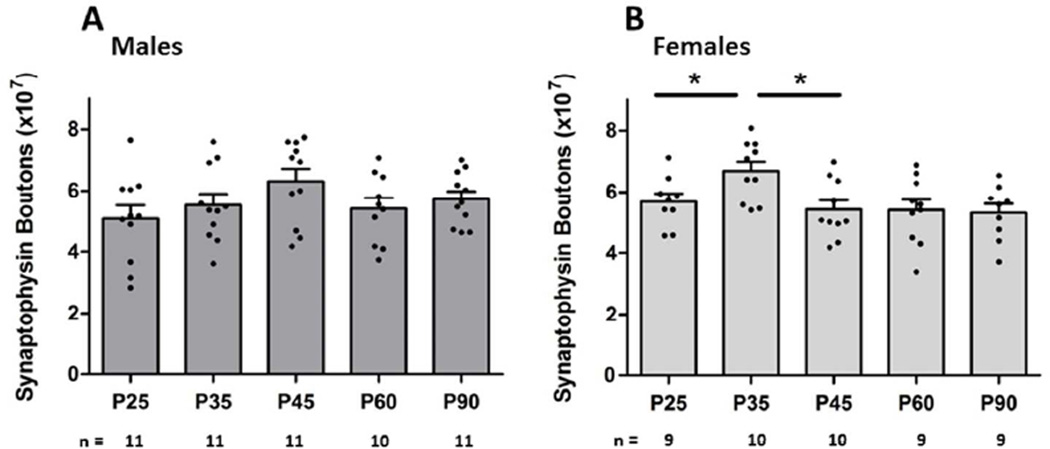
Males
A one-way ANOVA did not show a main effect of age on the total number of synapses in male subjects [F(4,36) = 1.49; p = 0.226] (Fig. 2A). Effect sizes of d = 0.61 and d = 0.73 (moderate effects) were calculated between P35–P45 and P45–P60, respectively. There was not a main effect of age in layers I or II/III, though there was a significant effect of age seen in layer V/VI [F(4,36) = 2.68; p = 0.047]. Post hoc tests revealed a significant increase in synaptic boutons from P35 to P45 [p < 0.05; d = 1.20] (above 1 is considered a large effect) (Table 1).
Fig. 2. Effects of age on synaptophysin boutons.
A main effect of age on the overall number of synapses in the male mPFC was not detected (A). There was a significantly higher number of synapses at P35 compared to both P25 and P45 [p = 0.01] (B).
Table 1.
Number of synaptophysin boutons (× 106) by cellular layer for males and females
| P25 | P35 | P45 | P60 | P90 | ||
|---|---|---|---|---|---|---|
| Males | Layer I | 3.03 ± .26 | 3.12 ± .19 | 3.64 ± .19 | 3.39 ± .23 | 3.52 ±.15 |
| Layer II/III | 17.1 ± 1.7 | 20.9 ± 1.9 | 22.1 ± 1.5 | 20.3 ± 1.8 | 19.8 ± 1.4 | |
| Layer V/VI | 29.6 ± 2.8 | 26.5 ± 2.2 | 36.6 ± 2.9*** | 29.8 ± 1.8 | 33.1 ± 2.2 | |
| Females | Layer I | 3.08 ± .14 | 3.95 ± .23*** | 3.15 ± .13*** | 3.01 ± .19 | 2.95 ± .22 |
| Layer II/III | 21.4 ± 1.4 | 23.9 ± 1.5 | 20.7 ± 1.1 | 21.0 ± 1.9 | 20.1 ± 1.4 | |
| Layer V/VI | 31.5 ± 1.85 | 35.8 ± 2.6 | 29.9 ± 2.5 | 29.5 ± 2.1 | 29.4 ± 2.2 | |
indicates P < 0.05 when compared to previous age group
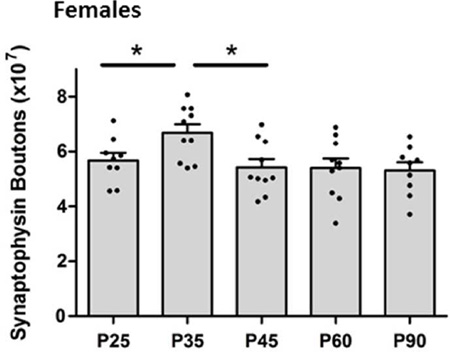
Females
In females, the one-way ANOVA revealed a main effect of age on total numbers of synapses [F(4,30) = 4.25; p = 0.01] (Fig. 2B). Post hoc analysis indicated a significant increase in synapses from P25 to P35 [p < 0.05; d = 1.11], as well as a decrease in synapse numbers between P35 and P45 [p < 0.05; d = 1.31]. There was no significant difference between any of the other adjacent ages (Fig. 2B).
Analyses of layers found a significant main effect of age on the number of synapses in layer I [F(4, 30) = 5.315; p < 0.01]. Post hoc tests show significantly higher numbers of synapses at P35 compared to both P25 [p < 0.05; d = 1.42] and P45 [p < 0.05; d = 1.33]. There was no main effect of age in layers II/III or V/VI (Table 1).
Puberty and Synaptophysin Boutons
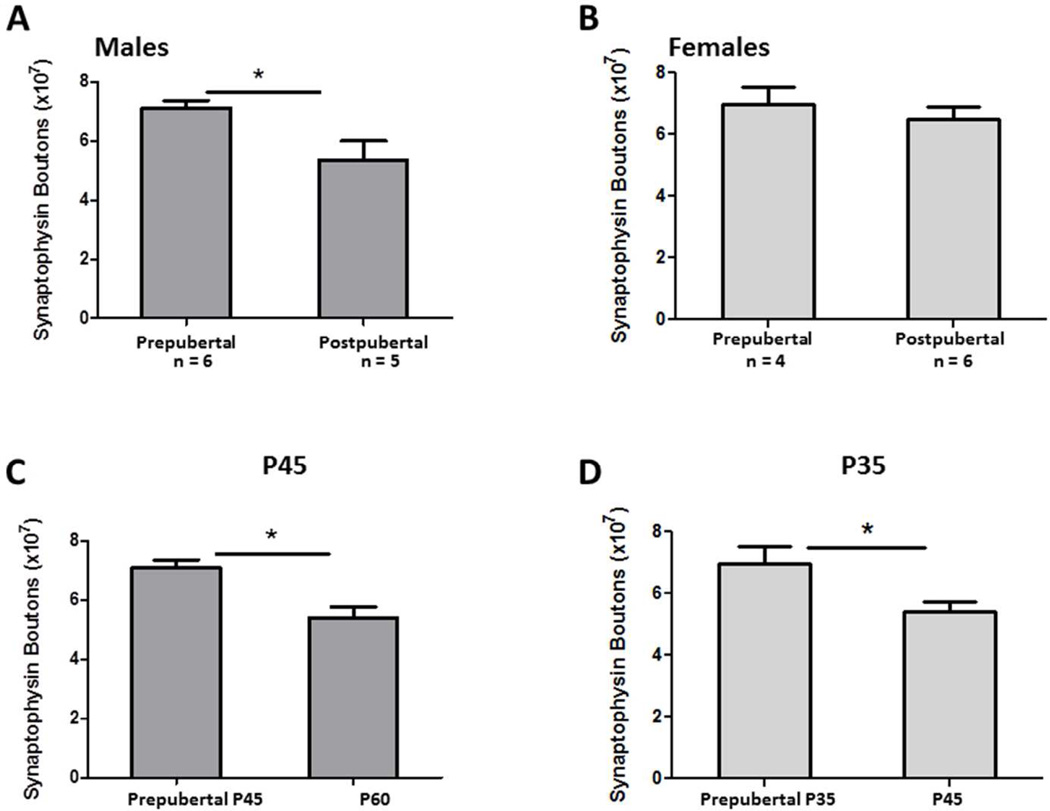
Males
To examine the effects of puberty on the total number of synapses, a t-test was run for males at P45 comparing those that had reached puberty to those who had not. Males that had reached puberty by P45 had significantly fewer synapses than males who had not [t(10) = 2.76; p = 0.02; d = 1.67] (Fig. 3A). Additionally, the pre-pubertal males at P45 had more synaptophysin boutons than all the post-pubertal males at P60 [t(14) = 3.36; p < 0.01; d = 1.73] (Fig. 3C).
Fig. 3. Effects of puberty on synaptophysin boutons.
Males that had reached puberty by P45 had significantly fewer synapses than those who had not [p = 0.02] (A). Prepubertal males at P45 had significantly more synapses than the post pubertal males at P60 [p < 0.01] (C). Female pubertal status at P35 did not significantly affect the number of synaptophysin bouton counts (B), though females at P35 that had not yet undergone puberty had significantly more synapses than post pubertal females at P45 [p = 0.02] (D).
Females
There were no significant differences in synapse number at P35 between females who had or had not gone through puberty [t(9) = 0.75; p = 0.47; d = 0.50] (Fig. 3B). However, the number of synapses in pre-pubertal females at P35 was significantly higher than the post-pubertal females at P45 [t(12) = 2.68; p = 0.02; d = 1.59] (Fig. 3D).
DISCUSSION
The present study quantified synaptic number changes within the adolescent mPFC from the juvenile period into adulthood, using synaptophysin as an immunoreactive marker for synapses. We found a peak in overall synapse numbers within the female mPFC at P35, and synaptic losses associated with pubertal onset in the male and female mPFC. Although previous literature has reported a decrease in synaptic density during adolescence (Bourgeois and Rakic, 1993; Anderson et al., 1995; Huttenlocher and Dabholkar, 1997; Huttenlocher 1979), the changes in prefrontal volume that occur during adolescence in rats (Van Eden and Uylings, 1989; Markham et al, 2007) and humans (Lenroot and Giedd, 2006) mean that changes in synaptic density do not accurately indicate the number of synaptic boutons. The present study is the first to document losses in the total number of synapses during adolescence. Moreover, this study is the first to find an association between pubertal status and number of synapses in the male and female mPFC, suggesting a role for gonadal hormones in synaptic pruning.
The results show a peak in synaptic numbers in the female cortex at P35, the average age of pubertal onset, followed by a drop at P45 that persisted into adulthood. The timing of these losses indicates that synaptic pruning in the female mPFC may be affected by ovarian hormones. This is consistent with findings from the rat visual cortex where ovarian hormones have been shown to influence neuronal pruning during adolescence (Nuñez et al., 2002). Within the rat mPFC, neuron losses during adolescence are more pronounced in females and occur between P35 and P45, coinciding with the onset of puberty (Willing and Juraska, 2015). Moreover, female mPFC neuronal pruning can be prevented by pre-pubertal removal of the ovaries, again suggesting a role for ovarian hormones in mPFC gray matter losses (Koss et al., 2015). If ovarian hormones are responsible for neuronal loss in the mPFC, it is reasonable that they would influence synapse numbers as well. Our laboratory has previously shown an inverted U-shaped trajectory of dendritic spines in layer V of the mPFC of both sexes across the juvenile period and into adulthood, and female subjects also had a marked loss of basilar dendrites between adolescence and adulthood (Koss et al., 2014). Thus the degree to which the loss of synapses is due to neuronal pruning cannot be determined in females, at least in the present data.
Though earlier work from our laboratory has previously noted small neuronal losses in the male mPFC during adolescence (Markham et al., 2007), we have subsequently found no significant relationship between neuronal pruning and male pubertal onset (Willing and Juraska, 2015). In spite of this, we report here that males that had undergone puberty had significantly fewer synapses at P45 than those that had not, implying a role for male pubertal hormones in synaptic pruning. Thus synaptic pruning can be independent of neuronal pruning. Additionally, the pre-pubertal males at P45 had significantly more synapses than the post-pubertal males at P60, further suggesting that synaptic pruning is more attributable to pubertal status than age. Other telencephalic neural areas in which prepubertal gonadectomy is known to affect spine density include the hippocampal CA1 (Meyer et al., 1978), and the medial amygdala (Cooke and Woolley, 2009), though it is not known how widespread synaptic pruning is during adolescence.
Layer-specific analysis revealed similar patterns of synaptic pruning between cortical layers in both sexes, but age was significant in only some of the layers. All layers of the female mPFC show a peak in synapses at puberty (P35), though it was only significant in layer I. In the male mPFC, again there appeared to be a peak in all layers at puberty (P45), but only layer V/VI was significant. It is difficult to know the meaning of this specificity, especially since it varies by sex and virtually nothing is known about sex differences in afferents to the rat mPFC. Layer-dependent maturation of amygdalo-cortical fibers have been demonstrated within the male mPFC during adolescence (Cunningham et al., 2002), suggesting that cortical layers may undergo differential rates of synaptic pruning patterns, though whether this is due to changes in the basolateral amygdala or the mPFC itself cannot be discerned from this study.
Selective pruning of synaptic connections could be a mechanism to “fine tune” the system to be more adaptable to changes in environment (Jacobson, 1973). The morphological changes within the mPFC might explain the unique transition in the behavioral/cognitive phenotype between adolescence and adulthood. Changes in synaptic numbers across the pubertal period may partly explain the deficits adolescents show on cognitive tasks that involve the mPFC. Notably, adolescent rats generally perform worse than adults on tasks that involve complex cognitive behaviors, such as working memory paradigms, attentional tasks, behavioral inhibition, and decision-making strategies (Sturman et al., 2010; Andrzejewski et al., 2011; Koss et al., 2011; Newman and McGaughy, 2011) (although greater cognitive flexibility was found in juveniles in Johnson and Wilbrecht (2011)). If pubertal hormones are responsible for synaptic pruning, then puberty may play an important role in cognitive development. Data from our laboratory supports this hypothesis, in that pre-pubertal male and female adolescents tend to show more cognitive rigidity and perseveration than recently post-pubertal and adult animals during a cognitive flexibility task (Willing et al., 2016).
The rapidly changing structure of the adolescent brain may confer a unique vulnerability, as previous research demonstrates that brain regions undergoing periods of rapid change may be more susceptible to negative external factors (Andersen, 2003; Andersen et al., 2008; Andersen and Teicher, 2004; reviewed by Eiland and Romeo, 2013). Leussis and Andersen (2008) support this idea, finding that the developing adolescent PFC is particularly vulnerable to social stressors. Both male and female rats that experienced social isolation during adolescence displayed increases in depressive- and anxiety-like behaviors. Furthermore, they found that socially isolated rats showed deficits in synaptic plasticity proteins, including decreases in frontal synaptophysin levels, suggesting a link between adolescent stressors and increased synaptic pruning. Mechanisms by which the environment might perturb pruning during puberty require further investigation.
Acknowledgments
We thank the Beckman Institute Imaging Technology Group for use of the microscopy suite. This work was supported by NIHMH099625 to JM Juraska and NIH Training Grant T32 ES007326 partially supported J Willing.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2004;31:83–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neurosci. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Feit EC, Harris R, McKee BL, Schochet TL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav Neurosci. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun M, Jucker M, Martin L, Thinakaran G, Price DL, Mouton P. Comparative evaluation of synaptophysin based methods for quantification of synapses. J Neurocytol. 1996;25:821–828. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- Chisholm N, Juraska J. Effects of long-term treatment with estrogen and medroxyprogesterone acetate on synapse number in the medial prefrontal cortex of aged female rats. Menopause. 2012;19:804–811. doi: 10.1097/gme.0b013e31824d1fc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev Neurobiol. 2009;69:141–152. doi: 10.1002/dneu.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- da Silva Faria T, da Fonte Ramos, Sampaio FJB. Puberty onset in the female offspring of rats submitted to protein or energy restricted diet during lactation. J Nutri Biochem. 2004;15:123–177. doi: 10.1016/j.jnutbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neurosci. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation to early adulthood. Neurosci. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász B, Köves K, Molnár J, Balika K, Stoll V. Hypothalamus and puberty. Brain Res Bull. 1988;20:709–712. doi: 10.1016/0361-9230(88)90081-0. [DOI] [PubMed] [Google Scholar]
- Hsu S, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P, Dabholkar A. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Genesis of neuronal specificity. In: Rockstein M, editor. Development and Aging in the Nervous System. New York: Academic Press; 1973. pp. 105–119. [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Johnson C, Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev Cog Neuro. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, Gilmore JH. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20:1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Koss WA, Franklin AD, Juraska JM. Delayed alternation in adolescent and adult male and female rats. Dev Psychobiol. 2011;53:724–731. doi: 10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LN, Juraska JM. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol. 2015;57:305–312. doi: 10.1002/dev.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Meyer G, Ferres-Torres R, Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice: a golgi study. Brain Res. 1978;155:108–112. doi: 10.1016/0006-8993(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Price DL, Walker LC. Empirical assessment of synapse numbers in primate neocortex. J Neurosci Meth. 1997;75:119–126. doi: 10.1016/s0165-0270(97)00058-7. [DOI] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;30:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Eden CG. Development of connections between the mediodorsal nucleus of the thalamus and the prefrontal cortex in the rat. J Comp Neurol. 1989;244:349–359. doi: 10.1002/cne.902440307. [DOI] [PubMed] [Google Scholar]
- Willing J, Drzewiecki CM, Cuenod BA, Cortes LR, Juraska JM. A role for puberty in water maze performance in male and female rats. Behav Neurosci. 2016 doi: 10.1037/bne0000145. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neurosci. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]