Abstract
Background/Aims
Ulcerative colitis (UC) patients are at greater risk for the development of colorectal neoplasia. Several individual studies have demonstrated associations between severity of histologic inflammation and colorectal neoplasia. However, a comprehensive systematic review has not been completed. We performed a systematic review and meta-analysis to explore the relationship between histologic inflammation and risk for neoplasia among available observational studies.
Methods
Three databases (EMBASE, MEDLINE and the Cochrane Library) were systematically searched. Studies were included if they included UC patients who underwent colonoscopic assessment and when histologic inflammation and colorectal neoplasia were both reported. Colorectal neoplasia rates were compared. Quantitative meta-analysis was attempted.
Results
Four of 1,422 records found were eligible. Results from 2 case-control studies reported a 3.5-fold increased risk for colorectal neoplasia associated with a single point increase in histologic inflammation. This result was further corroborated by one cohort study that demonstrated increased hazard ratios. The second cohort study reported outcomes for patients with normal gross endoscopy, but had increased histological inflammation when neoplasia was assessed. Finally, this study reported increased risk for neoplastic progression by histological inflammation among patients who were normal by gross endoscopic evaluation. Quantitative meta-analysis was unsuccessful due to heterogeneity between study measures.
Conclusions
There is strong evidence that histologic inflammation among patients with UC increases the risk of colorectal neoplasia. The depth and nature of assessment of additional clinical variables was varied and may have resulted in greater outcome discrepancy. Additional study related to mechanisms of inflammation-related neoplasia and therapeutic modification is needed.
Keywords: Colitis, ulcerative; Histologic inflammation; Colorectal neoplasms; Dysplasia
INTRODUCTION
It has been well-described that patients with IBD have an increased risk of developing colorectal neoplasia. Multiple studies have corroborated and confirmed this concern and demonstrated that IBD patients with dysplasia have a high risk of synchronous or metachronous colorectal cancer. In particular, there is consensus that patients with poorly-controlled disease and evidence of chronically active inflammation are most at risk for neoplasia and progressive neoplastic disease.
Rutter and colleagues from St. Mark's Hospital in England were the first to justify these concerns with empirical evidence. Using their large and long-standing registry, they demonstrated that the severity of colonic inflammation is an important risk determinant for colorectal neoplasia in UC.1 This case-control study confirmed that elevated risk was not only associated with endoscopic inflammation, but that by multi-variate analysis it was also particularly associated with long standing extensive histologic inflammation. The risk associated with these factors was substantial and was associated with an approximately five times increased risk of colorectal neoplasia.1
Over the last two decades, multiple studies from the United States and Europe have demonstrated similar patterns of elevated risk among patients who present with more severe histologic inflammation relative to their respective UC populations. 1,2,3,4 While this same trend has been observed in samples that have utilized different histologic scoring systems, significant methodological variation renders interpretation difficult and applicability limited. Given these limitations, we performed a systematic review and meta-analysis to assess the cumulative evidence for the relationship between inflammation severity and risk of colorectal neoplasia. In particular, we aimed to better understand the significance of histologic inflammation and its relationship with increased risk for neoplasia. In addition, we sought to assess the differences in relative risk between dysplasia, colorectal cancer, and subtypes (low-grade vs high-grade) of dysplasia.
METHODS
1. Search Strategy
Our systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA statement.5 Three databases – EMBASE, MEDLINE and the Cochrane library – were systematically searched from inception to October 2014. The systematic search was executed with specific terms (neoplasia, histology and inflammation and UC) and their associated synonyms. These terms were searched as both free text and Medical Subject Headings (MeSH) terms if available. These results were limited to publications with human subjects. No language exclusions were used. Two authors independently reviewed all articles.
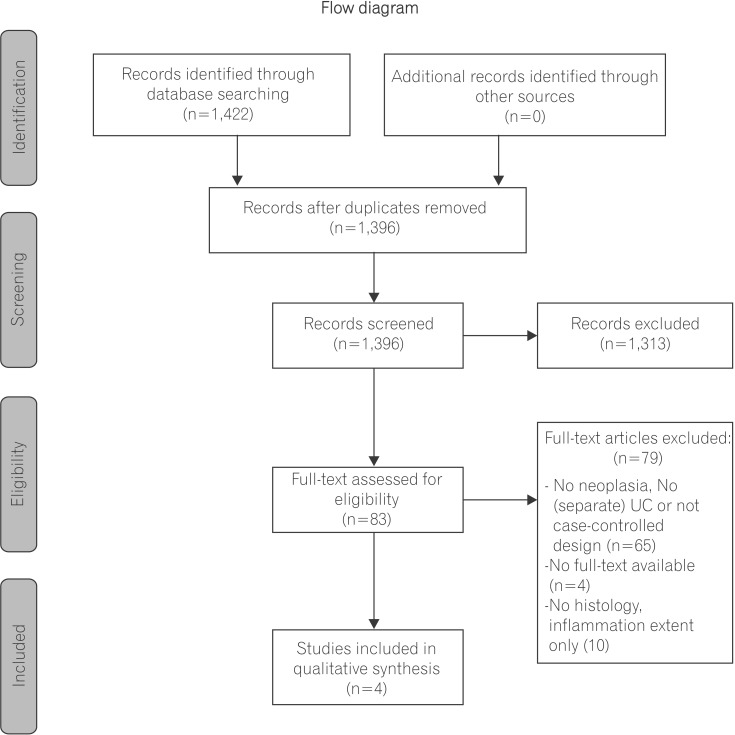
Observational studies were included based on the Populations, Exposure, Control, Outcomes, Study design (PECOS) question (Fig. 1). Study inclusion criteria required that patients have a prior UC diagnosis, previous colonoscopic as sessment in which inflammation scores and neoplastic outcomes were reported, and secondary colonoscopic follow-up assessment. In addition, articles were included if they examined and compared both patients with and without neoplasia. Studies were excluded if they did not report neoplastic outcomes or if they lacked histologic inflammation assessment. Studies were also excluded if they did not report separate clinical outcomes for patients with UC.
Fig. 1. Flow diagram of search strategy. Adapted from PRISMA Statement.5.
2. Quality of Studies
Two versions of the Newcastle-Ottawa Scale were used to assess for research quality and risk of bias among selected studies.6 The Newcastle-Ottawa Scale is a scale used for assessing of quality of non-randomized studies and has two versions one for cohort studies and one for case-control studies (Table 1A and 1B). Both versions rate quality of study design and analyses based on selection and comparability of cases-controls/cohorts, exposure, outcome and follow-up.
Table 1A. Newcastle-Ottawa Scale for Assessing Quality of Case-Control Studies.
| Quality assessment scale | Accepted criteria | Rutter et al. (2004)1 | Rubin et al. (2013)4 |
|---|---|---|---|
| Selection | |||
| 1. Is the case definition adequate? | Independent validation; Record linkage | - | - |
| 2. Representativeness of the cases | Representative of average UC Sex, age and disease severity |
- | * |
| 3. Selection of controls | Population from similar setting | * | * |
| 4. Definition of controls | No history of neoplasia | * | * |
| Comparability | |||
| Comparability of cases and controls on basis of the design and follow-up | Cases and controls were adequately matched | * | * |
| Exposure | |||
| Ascertainment of exposure | Secure histology records | - | - |
| Same method of ascertainment for cases and controls | Same histological scores between cases-controls | * | * |
| Total | (max=8) | 4 | 5 |
*Method accepted; - Method not accepted.
Table 1B. Newcastle-Ottawa Scale for Assessing Quality of Cohort Studies.
| Quality assessment scale | Accepted criteria | Gupta et al. (2007)2 | Korelitz et al. (2014)3 |
|---|---|---|---|
| Selection | |||
| Representativeness of the exposed cohort | Representative of average UC Sex, age and disease severity |
- | - |
| Representativeness of the non-exposed cohort | Drawn from the same community as exposed cohort | * | * |
| Ascertainment of exposure | Secure records | * | - |
| Demonstration that outcome of interest was not present at start of study | No neoplasia (or history of neoplasia) on first colonoscopy | * | - |
| Comparability | |||
| Comparability of cohorts on the basis | Match between design and confounders of cases-controls | - | - |
| Outcome | |||
| Follow-up long enough for outcome to occur? | Assessment of outcome | - | * |
| Adequacy of follow up of cohorts | Follow-up of complete cohort or unlikely to introduce bias? | * | * |
| Total | (max=8) | 4 | 3 |
*Method accepted; - Method not accepted.
3. Statistical Analysis
Data from the included studies were reviewed and outcomes were compared (Table 2). Neoplasia risk based on the histological severity of eligible studies were compared between studies and conceptualized as overall neoplasia risk. Additional analyses examined individual risk ratios for dysplasia (low-grade and high grade dysplasia), colorectal cancer and/or advanced neoplasia (high-grade dysplasia and colorectal cancer combined). Further statistical analyses with meta-analysis was attempted, to compare studies with homogenous methods, histology inflammation scales, regression models (e.g., conditional logistic regression, stratified logistic regression and cox regression), and observational study design.
Table 2. Study Characteristics.
| Study | Study type | Dx | Aims | Inclusion | Specific exclusion mentioned | No. of patients | Histology measures | Other factors |
|---|---|---|---|---|---|---|---|---|
| Rutter et al. (2004)1 | Case-control | UC | Examine risk factors for colorectal neoplasia | All Neoplasia cases Controls with intact colons and matched for a variety of factors Matched by age, sex, and age at symptom onset and UC duration and year of index scope (to match time eras) |
Neoplasia cases: 68 Matched-controls: 136 |
Simple histologic index (0-4): Mean scores per scope Mean scores overall |
PSC Smoking history Family history of CRC Mesalamine use Azathioprine use Folate supplements |
|
| Gupta et al. (2007)2 | Cohort | UC | To determine whether severity of microscopic inflammation over time is an independent risk factor for neoplastic progression in UC | Prior colonoscopy UC ≥7 years No prior dysplasia or CRC At least 1 F/U colonoscopy |
Prior colorectal surgery CD Indeterminate colitis |
Total cohort: 418 15 progressed to HGD/CRC 65 to any neoplasia |
Histologic activity index (0-3): IS-mean (average inflammation score over time) IS-bin (binary inflammation score) 1 if IS-mean ≥1 IS-max (maximum inflammation score over time) |
No. of colonoscopies Extent, duration of disease age at diagnosis PSC Medication exposure (5-ASA, thiopurines, steroids, cyclosporine, folate Male sex |
| Rubin et al. (2013)4 | Case-control | UC | To evaluate CRN in UC by degree of inflammation over time | Colon Matched by: extent, age at dx, and duration of dx (matched 1-4 individuals) |
Neoplasia proximal to colon Prior colorectal surgery |
Neoplasia cases: 59 Matched-controls: 141 |
Histological inflammatory activity ( scores 0-5): Mean score for all procedures combined Maximum score for single biopsy |
Smoking Family history of CRC PSC Use of 5-ASA, IMMs, steroid, folate, NSAIDs |
| Korelitz et al. (2014)3 | Cohort | UC | To evaluate if histologic inflammation in absence of endoscopic inflammation leads to an increased advanced CRN risk | UC ≥20 years ≥3 scopes+biopsies, 10 years after diagnosis |
Total cohort: 68 20 progressed to HGD/ CRC 48 did not develop advanced neoplasia |
No specific histologic activity index reported No mean or maximum scores calculated |
NA |
Dx, diagnosis; CRN, colorectal neoplasia; F/U, follow-up; HGD, high-grade dysplasia; PSC, primary sclerosing cholangitis; F/U, follow-up; 5-ASA, 5-aminosalicylate; IMM, immunomodulator; NA, not applicable.
RESULTS
1. Literature Search
The literature search yielded 1,422 records. Eighty-three records were eligible for full-text review after duplicate removal and titles and abstract screening. Of these 83 reviewed articles, 4 manuscripts were included in our final analysis. Articles were excluded due to methodological variation (no UC patients, no neoplasia, or no report of both patients with and without neoplasia n=65), no full-text availability (n=4) or no report of inflammation as a histological outcome (n=10) (Fig. 1). The 4 eligible manuscripts included 2 case-control studies and 2 cohort studies.1,2,3,4
2. Case-Control Studies
The 2 case-control studies included 127 cases and 277 patient controls.1,4 The first case-control study by Rutter et al. evaluated the impact of histological inflammation on the development of neoplasia. Rutter et al. conceptualized histological inflammation as an independent risk factor and found a 5-fold increase in risk for neoplasia for every 1-unit increase in endoscopical or histological mean score (Table 3).1 The 68 neoplastic patients included in this study were each matched with 2 neoplasia-naïve patients, resulting in 136 case-controls. The histological mean score was comprised of the mean of all histological scores for all combined surveillance colonoscopies. After accounting for other confounding factors, they found a similar increase in risk when histological scores (OR, 4.69; 95% CI, 2.1–10.48) were analyzed by multivariate analysis. the risk for endoscopic scores disappeared.
Table 3. Neoplasia Risk Ratios (If Reported).
| Study | Neoplasia categories | Analysis type | Cases, mean (SD) | Controls, mean (SD) | OR or HRa (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Rutter et al. (2004)1 | Any neoplasia | Univariate | 2.38 (0.56) (n=68) | 2.05 (0.41) (n=136) | 5.13 (2.36-11.14)b | <0.001 |
| Multivariate | - | - | 4.69 (2.10-10.48)b | <0.001 | ||
| CRC only | Univariate and multivariatec | 2.09 (0.44) | 2.54 (0.60) | 6.33 (1.24-32.33) | 0.030 | |
| Gupta et al. (2007)2 | Any neoplasia | Univariate | (n=65) | (n=353) | 1.4 (0.90-2.30)a | NS |
| Advanced neoplasiad | Univariate | (n=15) | (n=403) | IS-meana: 3.0 (1.40-6.30) |
<0.050 | |
| IS-bine: 3.4 (1.10-10.40) | ||||||
| IS-maxf: 2.2 (1.20-4.20) | ||||||
| Multivariate | (n=15) | (n=403) | IS-meana: 3.8 (1.70-8.60) 5.4 (1.70-17.00)g |
<0.050 | ||
| Rubin et al. (2013)4 | Any neoplasia | Univariate | 2.00 (0.89) (n=32) | 1.55 (0.68) (n=139) | Mean-score: 2.56 (1.45-4.54) |
0.001 |
| Any neoplasia | Maximum-score: 1.41 (1.03-1.91) |
0.030 | ||||
| Multivariate | - | - | Mean-score: 3.68 (1.69-7.98) |
0.001 | ||
| Dysplasia | Univariate | 2.07 (0.97) (n=44) | 1.52 (0.71) (n=104) | 2.54 (1.35-4.78) | 0.004 | |
| Cancer | Univariate | 1.79 (0.51) (n=15) | 1.62 (0.60) (n=37) | 2.64 (0.69-10.2) | NS |
Outcomes only reported for significant values, unless no outcome was significant for a specific neoplasia outcome.
aHazard ratio of IS-mean (For 1-unit increase the cumulative mean histologic inflammation score, there was a X fold increase for neoplasia).
bOdds ratio of colorectal neoplasia if 1-unit increase in histological score.
cSame effect size and significance, for both univariate and multivariate model, because histologic inflammation was the only variable in model.
dAdvanced neoplasia: high-grade dysplasia and cancer.
eBinary inflammation score: 1 if IS-mean ≥1.
fMaximum inflammation score over time.
gControlled for 1 or more colonoscopies per year.
HR, hazard ratio; CRC, colorectal cancer.
When patients with limited neoplasia (dysplasia only) were excluded from analyses, the odds of cancer increased 6-fold for every 1-point increase in the histological mean score. Of interest, this study did not identify any additional factors that contributed to neoplasia (such as family history, primary sclerosing cholangitis, smoking status, or medication usage). That said, it is worth noting that all patients in this single academic center study had at least macroscopic disease beyond the splenic flexure, indicating a UC population with extensive disease. In addition, the total number of colonoscopies per patient was varied and ranged between 1–17 among cases and between 1–13 in controls.
A more recent case-control study by Rubin et al. from the University of Chicago explored the relationship between colorectal neoplasia risk and a single episode of severe inflammation compared to that which exists in patients with multiple distinct relapses of milder inflammation during longer periods of time.4 For this analysis, 59 patients with neoplasia were compared to 141 controls without neoplasia. A 6-point histologic inflammatory activity scale was developed and used for this study.4 In addition to the mean histologic inflammation score previously used by Rutter et al., a maximum histological score was reported as the maximum score for any single biopsy.
Univariate analysis demonstrated that histologic activity was positively associated with neoplasia when assessed as mean scores (OR, 2.56; 95% CI, 1.45–4.54; P =0.001) and maximum scores (OR, 1.41; 95% CI, 1.03–1.91; P =0.03). In contrast, when controlled for sex, family history of colorectal neoplasia, medication exposure, or a prior diagnosis of primary sclerosing cholangitis, multivariate conditional regression analyses demonstrated that an increase in mean scores (accounting for multiple inflammatory episodes rather than 1 single severe episode) alone remained relevant to colorectal neoplasia risk. Most strikingly a 1-unit increase in histological score was associated with a greater than 3-fold increase (OR, 3.68; 95% CI, 1.69–7.98) in neoplasia risk. Moreover, when patients with higher scores were compared to patients with quiescent or normal histology, a 7-fold increased neoplasia risk was found.4 Additionally, in contrast to the previous case-control study, this research also demonstrated that male sex was associated with increased risk for colorectal neoplasia. In contrast, prior exposure to aminosalicylates and immunomodulators was found to be chemoprotective.4
3. Cohort Studies
The 2 cohort studies were comprised of 486 patients.2,3 The first and largest of these was completed by Gupta and colleagues and was designed to understand if the degree of histologic inflammation was an independent risk factor for developing neoplasia.2 When compared to the case-control studies described previously, this cohort-based study took another approach and analyzed risk ratios by Cox proportional hazard modeling. Gupta and colleagues used inflammation scores and exposure to colonoscopy as timechanging covariate factors. In addition to an overall colonoscopy composite mean inflammation score, a separate binary score (no inflammation versus any inflammation) was also used to describe patients and was derived from the composite inflammation score. Lastly, they identified a maximum score that was derived from the highest recorded inflammation score from all colonoscopies completed per patient. Histology was assessed according to a different histology inflammation scale.2 Results from this study did not demonstrate an increased risk for neoplasia when assessed by univariate analysis.2 However, the authors reported a 3-fold increase in risk for advanced colorectal neoplasia (high-grade dysplasia and/or cancer) for each 1-point increase in mean inflammation score. This increase in relative risk was also present for and related to inflammation when it was conceptualized and analyzed as a dichotomous variable.2 In addition, maximum inflammation scores were associated with a 2-fold increased risk for advanced neoplasia.2 When analyses controlled for confounding factors by multivariate analysis, the relative risk of advanced neoplasia increased significantly and was associated with a 4-fold increase in risk for every 1-point increase in the mean inflammation score. This risk was further increased to more than 5-fold when analyses controlled for a colonoscopy frequency of more than 1 per year.
Another study by Korelitz and colleagues examined the neoplastic risk associated with histologic inflammation in a cohort of 68 patients who had at least a 10-year history of surveillance colonoscopies.3 More specifically, this study was interested in understanding the risk associated with the histology of colonoscopic exams that appeared endoscopically normal. Results indicate that a higher prevalence of histologic severity was observed on biopsies among patients who had colorectal neoplasia (n=20) when compared to patients who did not (n=48). These results further indicate that the group of patients with advanced neoplasia had more frequent histologic inflammatory activity when no endoscopic activity was found (88%, 95% CI, 72%–97%) than for the group without advanced neoplasia (59%, 95% CI, 53%–64%).3 This study specifically focused on colonoscopic outcomes as stratified by the presence of advanced neoplasia rather than by individual patient. This study did not assess outcomes by univariate or multivariate analyses.
4. Meta-Analysis
Meta-analysis of these data was attempted, but was not possible, due to heterogeneity of study design and outcome among these studies. All 4 studies used different histologic inflammation activity scales with different criteria for severity and different intervals (varying from 4 to 6 point scales) (Table 4). Additionally, the scores for these histologic assessments were summarized differently – methods utilized mean scores, a maximum score calculated per biopsy, a continuous variable per colonoscopy assessment, or a combination of these strategies. Methods also varied by analytical strategy – only 3 of 4 studies described risk ratios using univariate and/or multivariate analyses. These analyses were completed with conditional logistic regression and OR (for the case-control studies,1,4) or with proportional hazard modeling (Cox regression) (for the cohort study).3 Neoplastic outcomes were conceptualized differently across studies – some utilized an outcome of dysplasia in contrast to cancer, while others utilized development of advanced neoplasia (high-grade dysplasia and cancer) or any neoplasia as the primary outcome variable.
Table 4. Comparison of Histological Inflammation Scores.
| Numerical score | Rutter et al. (2004)1 | Gupta et al. (2007)2 | Rubin et al. (2013)4 |
|---|---|---|---|
| 0 | Normal No inflammatory cells |
Inactive/quiescent/normal No epithelial infiltration of <50% of sampled crypts or cross sections, no ulcers or erosions |
Normal (completely, uninvolved , no architectural distortion, no infiltrates) |
| 1 | Chronic inflammation only | Mildly active Neutrophil infiltration <50% of sampled crypts or cross sections, no ulcers or erosions |
Quiescent (architectural distortion, increased lamina propria lymphs, but no activity) |
| 2 | Mild active (crypts, but no crypt abscesses) | Moderately active Neutrophil infiltration ≥50% of sampled crypts or cross sections, no ulcers or erosions |
Increased lamina propria granulocytes without definite interepithelial granulocytes |
| 3 | Moderate active (few crypt abscesses) | Severely active Erosion or ulceration, irrespective of other features |
Intraepithelial granulcytes (e.g., cryptitis) without crypt abscesses |
| 4 | Severe active inflammation (numerous crypt abscesses) | - | Crypt abscesses in less than 50% of crypts |
| 5 | - | - | Crypt abscesses in greater than 50% of crypts, or erosion/ulceration |
No specific index reported for Korelitz et al.
DISCUSSION
This systematic review underscores the notion that more severe histologic inflammation is an independent risk factor for colorectal neoplasia in UC, and confirms results obtained among four individual studies. Two case control studies were included and were comprised of a total of 127 patients with neoplasia and 277 patient controls.1,4 An additional two cohort studies were included and were comprised of a total of 486 neoplastic patients.2,3
Results from the case-control studies demonstrate a significant association between risk for neoplasia and histological inflammation with a 3 to 5-fold increased in risk for colorectal neoplasia for every 1-unit increase in histological inflammation. 1,4 This risk increased substantially (between 4-fold to 5-fold) when the analyses controlled for confounding variables.1,4 The cohort-studies included in these analyses demonstrated a 3-fold increased risk for advanced colorectal neoplasia.2 Furthermore, when endoscopic and histologic outcomes were compared, patients with colorectal neoplasia had more frequent histologic inflammation without associated gross endoscopic activity than those patients who did not develop neoplasia.
Of interest, Rubin et al. was the only study that found (1) a higher neoplastic risk associated with male sex and (2) a lower neoplastic risk associated with immunomodulator use.4 That said, it's difficult to estimate the significance of these confounding factors given that each study utilized different variables to measure medication exposure. The manner by which medication exposure was categorized and recorded varied greatly. The literature has been divided about the chemoprotective or, alternatively, chemo-inducing role of immunomodulators and their relationship with colorectal neoplasia among patients with UC.7 This may be due to the fact that most of the older studies did not adjust for degree of inflammation, potentially the most significant confounder.
Of the cohort studies included, only one assessed risk ratios of neoplasia.2 The other cohort study assessed outcomes of patients with and without neoplasia and was further categorized by colonoscopic outcomes.3
All included studies were completed at major, tertiary care medical centers, which may have resulted in an increased risk for homogeneity of patients and a greater likelihood of patients with more extensive disease. While these facts might limit the generalizability of these results to the IBD population overall, this population is more severe and we believe should be stratified into a high-risk surveillance group.8
These results are corroborated by an additional large, case-control study (n=183 cases and 370 controls) that was excluded from our primary review assessed colorectal neoplasia among all IBD patients (including both UC and CD).9 While this study used the same histologic scale as Gupta et al., patients with mild-moderate disease were combined to one category. Patients with mild to moderate inflammation exhibited a 2.6-fold increased risk of neoplasia compared to patients without inflammation (95% CI, 15.6%–64.9%; P =0.0001). Furthermore, patients with high inflammatory activity exhibited an almost 32-fold increased risk compared to IBD patients with no inflammation (95% CI, 15.6%–64.9%; P <0.0001). While this study did not provide OR for the UC and CD populations individually, it underscores the need for guidelines that include risk stratification that is guided by histology and that includes both patients with UC as well as CD.8,9,10,11
In conclusion, this review identifies and confirms histologic inflammation as an independent risk factor for the development of colorectal neoplasia among patients with UC. That said, it is worth noting that this body of research was conducted among retrospective study populations alone. In addition, despite the fact that results from multiple studies pointed in the same direction, the most recent data included in these studies is a decade old.4 As there have been numerous significant advancements in our understanding of the pathogenesis of IBD-related neoplasia and its surveillance, the applicability of these results within a modern setting may be in question. While studies included in this review lack specifics about the use of advanced surveillance (such as chromoendoscopy, narrow-band imaging or confocal endomicroscopy), the role of these and other visualization enhancement techniques should be explored to assess for a shift in colorectal neoplasia risks, with perhaps earlier detection and shortened inflammation exposure.12
As research and clinical care progress and are increasingly able to accurately stratify neoplasia surveillance, future research should also focus on differences in the gut microbiome and the relative role that this factor has in the pathogenesis of colorectal neoplasia.13,14 Also important will be greater understanding of how treatment of active inflammation may — or may not — modify subsequent risk of neoplasia. Clinical considerations that factor into management these findings are important. At the current time, it seems reasonable to stratify the intensity of colonoscopic surveillance based on the presence or absence of inflammation at the time of the last examination, and to escalate treatment of active inflammation.
Footnotes
Financial support: This study was supported in part by the Digestive Diseases Research Core Center (DDRCC) (NIDDK P30DK42086).
Conflict of interest: None.
References
- 1.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korelitz BI, Sultan K, Kothari M, Arapos L, Schneider J, Panagopoulos G. Histological healing favors lower risk of colon carcinoma in extensive ulcerative colitis. World J Gastroenterol. 2014;20:4980–4986. doi: 10.3748/wjg.v20.i17.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601–1608. doi: 10.1016/j.cgh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ontario, Canada: Ottawa Hospital Research Institute; 2010. [Accessed April 1, 2014]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 7.Chapman CG, Rubin DT. The potential for medical therapy to reduce the risk of colorectal cancer and optimize surveillance in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2014;24:353–365. doi: 10.1016/j.giec.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 9.Nieminen U, Jussila A, Nordling S, Mustonen H, Färkkilä MA. Inflammation and disease duration have a cumulative effect on the risk of dysplasia and carcinoma in IBD: a case-control observational study based on registry data. Int J Cancer. 2014;134:189–196. doi: 10.1002/ijc.28346. [DOI] [PubMed] [Google Scholar]
- 10.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–651.e28. doi: 10.1053/j.gastro.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DT. Why it's time for updated U.S. colorectal cancer prevention guidelines in inflammatory bowel disease. Gastrointest Endosc. 2014;80:849–851. doi: 10.1016/j.gie.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Cleveland NK, Colman RJ, Rodriquez D, et al. Surveillance of IBD using high definition colonoscopes does not miss adenocarcinoma in patients with low-grade dysplasia. Inflamm Bowel Dis. 2016;22:631–637. doi: 10.1097/MIB.0000000000000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]