Abstract
During our ongoing investigation of neuraminidase inhibitors from medicinal fungi, we found that the fruiting bodies of Phellinus igniarius exhibited significant inhibitory activity against neuraminidase from recombinant H3N2 influenza viruses. Two active compounds were isolated from the methanolic extract of P. igniarius through solvent partitioning and Sephadex LH-20 column chromatography. The active compounds were identified as phelligridins E and G on proton nuclear magnetic resonance (1H NMR) and electrospray ionization mass measurements. These compounds inhibited neuraminidases from recombinant rvH1N1, H3N2, and H5N1 influenza viruses, with IC50 values in the range of 0.7~8.1 µM.
Keywords: Neuraminidase inhibitor, Phelligridin E, Phelligridin G, Phellinus igniarius
Neuraminidase, also known as sialidase, is an important glycoprotein in influenza viruses that cleaves sialic acid from the infected cell surface and releases virus progeny [1]. It plays a major role in viral proliferation and infecting other cells [2]. Thus, neuraminidase is an attractive therapeutic drug target for the treatment of influenza. Currently, neuraminidase inhibitors such as zanamivir (Relenza; Glaxo Wellcome Inc., Research Triangle Park, NC, USA) and oseltamivir (Tamiflu; Roche, Nutley, NJ, USA) are used for the treatment of influenza. However, several problems are associated with these drugs, including a high level of drug resistance and diverse side effects such as vomiting and nausea [3,4]. Therefore, next-generation neuraminidase inhibitors are urgently needed.
Mushrooms are both a nutritional food source and a traditional medicine. They produce various classes of bioactive secondary metabolites with unique chemical structures [5,6]. In previous studies, we reported the isolation of neuraminidase inhibitors from Phellinus baumii and P. linteus and their biological properties [7,8,9]. As part of an ongoing effort to identify neuraminidase inhibitors from medicinal fungi, we found that the methanolic extract of fruiting bodies of P. igniarius exhibited significant H3N2 neuraminidase inhibitory activity. Phellinus igniarius, belonging to the family Hymenochaetaceae, has been used to treat fester, abdominalgia, and bloody gonorrhea in traditional medicine in Korea, Japan, and China and has been reported to possess anti-oxidative, anti-proliferative, anti-metastatic, and antiinfluenza activities [10,11,12]. In this study, we describe the isolation, structure determination, and biological properties of neuraminidase inhibitors from the methanolic extract of P. igniarius.
Isolation of neuraminidase inhibitors.
In order to isolate neuraminidase inhibitors, the fruiting bodies of P. igniarius were extracted using methanol for 2 days at room temperature. The methanolic extract was concentrated under reduced pressure, and the resultant residue was partitioned twice with ethyl acetate. The ethyl acetate-soluble layer was subjected to Sephadex LH-20 column chromatography (Pharmacia, Uppsala, Sweden) eluted with methanol to yield two active fractions. One active fraction was further purified by Sephadex LH-20 column chromatography eluted with 70% aqueous methanol to provide compound 1. The other fraction was subjected to Sephadex LH-20 column chromatography with 70% aqueous methanol as the eluting solvent to yield compound 2.
Structure determination of neuraminidase inhibitors.
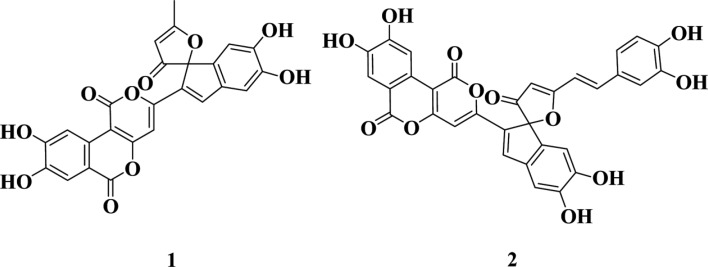
The chemical structures of compounds 1 and 2 were determined via electrospray ionization (ESI)-mass and proton nuclear magnetic resonance (1H NMR) spectrum measurements. The molecular weight of compound 1 was 474 on ESI-mass measurement, where it exhibited a quasimolecular ion peak at m/z 475 [M+H]+. The 1H NMR spectrum of 1 in DMSO-d6 demonstrated seven methine protons at δ 8.24, 7.85, 7.47, 6.97, 6.61, 6.15, and 6.09 and one methyl signal at δ 2.50. Based on literature review, compound 1 was identified as phelligridin E; the molecular weight and 1H chemical shift values were in good agreement with those previously reported for phelligridin E [13].
The chemical structure of compound 2 was also determined via ESI-mass and 1H NMR spectrum measurements. The molecular weight of compound 2 was 594 on ESI-mass measurement, and it demonstrated a quasi-molecular ion peak at m/z 595 [M+H]+. The 1H NMR spectrum of compound 2 in DMSO-d6 showed three aromatic methine signals assignable to a 1,2,4-trisubstituted benzene moiety at δ 7.17 (d, J = 2.1 Hz), 7.10 (dd, J = 8.2, 2.1 Hz), and 6.79 (d, J = 8.2 Hz), two olefinic methine peaks attributable to a trans-1,2-disubstituted double bond at δ 7.54 (d, J = 16.2 Hz) and 7.10 (d, J = 16.2 Hz), and seven methine singlets at δ 8.28, 7.86, 7.48, 7.01, 6.64, 6.25, and 6.02. These spectral data were consistent with those of phelligridin G previously reported in the literature [14]. Therefore, compounds 1 and 2 were identified as phelligridin E and phelligridin G, respectively, as shown in Fig. 1.
Fig. 1. Chemical structure of compounds 1 (phelligridin E) and 2 (phelligridin G).
Neuraminidase inhibition activity.
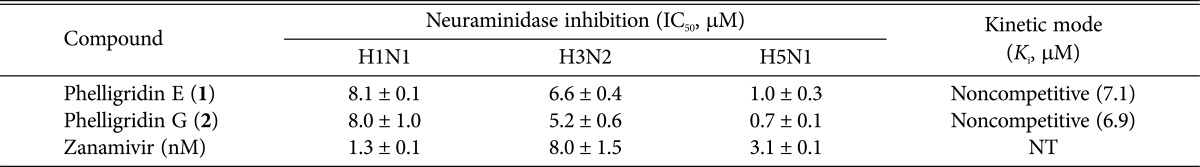
The inhibitory activity of compounds 1 and 2 against neuraminidases from recombinant rvH1N1, H3N2, and H5N1 influenza viruses was evaluated. Neuraminidase inhibition assay was performed in 96-well plates as previously described [15], with some modifications. Briefly, the substrate, 50 µL of 0.2 mM MUNANA (2-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid sodium salt; Sigma, St. Louis, MO, USA), was mixed with 90 µL of 50mM Tris buffer (containing 200 mM NaCl, 5 mM CaCl2, pH 7.5) at room temperature. Ten microliters of the sample and 50 µL of H1N1 neuraminidase (50 ng/mL) were added to each well. The mixture was then recorded at the excitation and emission wavelengths of 365 nm and 445 nm, respectively, using a POLAR OPTIMA (BMG LABTECH, Ortenberg, Germany). In the case of H3N2 and H5N1 neuraminidases, 25 mM MES buffer (containing 500 mM NaCl, 5 mM CaCl2, pH 6.5) and 50 mM MES buffer (containing 500 mM NaCl, 5 mM CaCl2, pH 6.5) were used, respectively, and H5N1 required an activation period of 24 hr at 37℃ before assay. The other methods were the same as used for H1N1 neuraminidase. A neuraminidase inhibitor, zanamivir (Relenza) was used as a positive control. Compounds 1 and 2 showed H3N2 neuraminidase inhibition activity with an IC50 value of 6.7 µM, respectively, in a dose-dependent manner. Inhibitory activity of compounds 1 and 2 against H1N1 and H5N1 neuramanidases were also evaluated. These compounds significantly inhibited the H1N1 and H5N1 neuramanidases with IC50 values in the range of 8.0~1.0 µM in a dose-dependent manner (Table 1).
Table 1. Neuraminidase inhibitory activity of compounds 1 and 2.
NT, not tested.
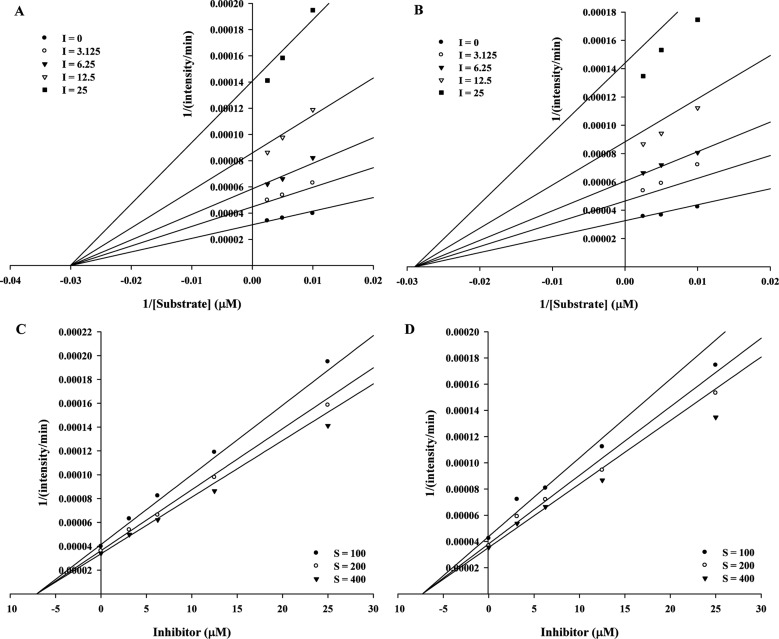
We investigated the inhibition type of compounds 1 and 2 using kinetic parameters. To study the mode of inhibition, Dixon plots were used to distinguish the mechanism of neuraminidase enzyme action and to confirm the inhibition constant (Ki). The data were measured using Sigmaplot ver. 1.3 (Systat, Chicago, IL, USA). Compounds 1 and 2 displayed noncompetitive inhibitory activity, as indicated by a decrease in Vmax while Km remained stable at increasing inhibitor concentrations. The inhibition constant (Ki) values of compounds 1 and 2 were 7.1 ± 0.3 and 6.9 ± 0.3, respectively (Fig. 2).
Fig. 2. Graphical representation of the neuraminidase inhibition of isolated compounds. A, B, Lineweaver-Burk plots of the neuraminidase inhibition of compounds 1 and 2; C, D, Dixon plots of the neuraminidase inhibition of compounds 1 and 2.
ACKNOWLEDGEMENTS
This work was supported by the Technology Development Program for Bio-industry, Ministry for Food, Agriculture, Forestry and Fisheries as well as the Korea Forest Service, Republic of Korea.
References
- 1.Dao TT, Nguyen PH, Lee HS, Kim E, Park J, Lim SI, Oh WK. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg Med Chem Lett. 2011;21:294–298. doi: 10.1016/j.bmcl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Brouillette WJ, Baipai SN, Ali SM, Velu SE, Atigadda VR, Lommer BS, Finley JB, Luo M, Air GM. Pyrrolidinobenzoic acid inhibitors of influenza virus neuraminidase: modifications of essential pyrrolidinone ring substituents. Bioorg Med Chem. 2003;11:2739–2749. doi: 10.1016/s0968-0896(03)00271-2. [DOI] [PubMed] [Google Scholar]
- 3.Dao TT, Nguyen PH, Won HK, Kim EH, Park J, Won BY, Oh WK. Curcuminoids from Curcuma longa and their inhibitory activities on influenza A neuraminidases. Food Chem. 2012;134:21–28. [Google Scholar]
- 4.Li B, Ni Y, Zhu LJ, Wu FB, Yan F, Zhang X, Yao XS. Flavonoids from Matteuccia struthiopteris and their antiinfluenza virus (H1N1) activity. J Nat Prod. 2015;78:987–995. doi: 10.1021/np500879t. [DOI] [PubMed] [Google Scholar]
- 5.Kim SE, Hwang BS, Song JG, Lee SW, Lee IK, Yun BS. New bioactive compounds from Korean native mushrooms. Mycobiology. 2013;41:171–176. doi: 10.5941/MYCO.2013.41.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindequist U, Niedermeyer TH, Jülich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeom JH, Lee IK, Ki DW, Lee MS, Seok SJ, Yun BS. Neuraminidase inhibitors from the culture broth of Phellinus linteus. Mycobiology. 2012;40:142–144. doi: 10.5941/MYCO.2012.40.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang BS, Lee MS, Lee SW, Lee IK, Seo GS, Choi HJ, Yun BS. Neuraminidase inhibitors from the fermentation broth of Phellinus linteus. Mycobiology. 2014;42:189–192. doi: 10.5941/MYCO.2014.42.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang BS, Lee IK, Choi HJ, Yun BS. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg Med Chem Lett. 2015;25:3256–3260. doi: 10.1016/j.bmcl.2015.05.081. [DOI] [PubMed] [Google Scholar]
- 10.Song TY, Lin HC, Yang NC, Hu ML. Antiproliferative and antimetastatic effects of the ethanolic extract of Phellinus igniarius (Linnearus: Fries) Quelet. J Ethnopharmacol. 2008;115:50–56. doi: 10.1016/j.jep.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Wang SJ, Mo SY, Li S, Yang YC, Shi JG. Phelligridimer A, a highly oxygenated and unsaturated 26-membered macrocyclic metabolite with antioxidant activity from the fungus Phellinus igniarius. Org Lett. 2005;7:4733–4736. doi: 10.1021/ol0520875. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Kim JI, Heo J, Lee I, Park S, Hwang MW, Bae JY, Park MS, Park HJ, Park MS. The anti-influenza virus effect of Phellinus igniarius extract. J Microbiol. 2013;51:676–681. doi: 10.1007/s12275-013-3384-2. [DOI] [PubMed] [Google Scholar]
- 13.Mo S, Wang S, Zhou G, Yang Y, Li Y, Chen X, Shi J. Phelligridins C-F: cytotoxic pyrano[4,3-c][2]benzopyran-1,6-dione and furo[3,2-c]pyran-4-one derivatives from the fungus Phellinus igniarius. J Nat Prod. 2004;67:823–828. doi: 10.1021/np030505d. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Mo SY, Wang SJ, Li S, Yang YC, Shi JG. A unique highly oxygenated pyrano[4,3-c][2]benzopyran-1,6-dione derivative with antioxidant and cytotoxic activities from the fungus Phellinus igniarius. Org Lett. 2005;7:1675–1678. doi: 10.1021/ol0475764. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Jeong HJ, Park JY, Kim YM, Park SJ, Cho JK, Park KH, Ryu YB, Lee WS. Selective and slow-binding inhibition of shikonin derivatives isolated from Liothospermum erythrorhizon on glycosyl hydrolase 33 and 34 sialidases. Bioorg Med Chem. 2012;20:1740–1748. doi: 10.1016/j.bmc.2012.01.011. [DOI] [PubMed] [Google Scholar]