Abstract
Candida spp. is an invasive infectious fungus, a major risk factor that can increase morbidity and mortality in hospitalized patients. In this study, 2,508 Candida spp. were isolated from various clinical specimens collected from university hospitals from July 2011 to October 2014. They were identified in order to determine isolation frequencies and characteristics by specimen, gender, age group, year, season, and month. The strain-specific isolation rate of Candida spp. is in the order of Candida albicans (1,218 strains, 48.56%), Candida glabrata (416 strains, 16.59%), Candida utilis (305 strains, 12.16%), Candida tropicalis (304 strains, 12.12%), and Candida parapsilosis (116 strains, 4.63%) and these five species accounted for more than 94% of the total strains. Of the specimens, Candida spp. were most frequently isolated from urine-catheter, followed by urine-voided, blood, sputum, other, open pus, vaginal discharge, Tip, ear discharge, bronchial aspiration and bile, in that order. Looking at the age distribution, the detection rate of patients in their 60s and older was significantly higher at 75.8% (1,900/2,508). The detection rate of patients in their 20s and younger was shown to be very low at 2.55% (64/2,508). By year, the detection rate of non-albicans Candida spp. showed a tendency to gradually increase each year compared with C. albicans. As isolation of Candida spp. from clinical samples at the specie level can vary depending on characteristics of the patient, sample, season, etc., continual studies are required.
Keywords: Candida albicans, Candida glabrata, Candida spp, Non-albicans Candida spp
Fungal infections by opportunistic pathogens such as Candida, Cryptococcus, and Aspergillus and the resulting morality in patients with a lowered immune system are showing a steady upward trend in the world [1]. In the United States, based on the analysis of death certificate data from 1980 to 1997, the number of deaths due to fungal infections has increased by 3.4 times from 1,577 to 6,577 people [2]. Candida spp. are the most common causative organism of fungal infections. In the United States, candidemia accounts for about 8% of pathogenic bloodstream infections, and Candida spp. are the 4th most common causative organism of nosocomial bloodstream infection, which is one of the major risk factors that increase morbidity and mortality in hospitalized patients [3,4,5]. In the past, the most causative pathogen was Candida albicans, but recently various types of Candida spp. such as Candida parapsilosis, Candida glabrata, and Candida krusei have emerged as important opportunistically infectious fungi [6,7]. Candidiasis is an infectious disease with high morbidity and mortality, of which prevalence has dramatically increased in the past 20 years [8,9], and the gradual increase in the resistance of those non-albicans Candida spp. to antifungal agents makes clinical treatment difficult. In this study, using VITEK-2 (AS-TS01; bioMérieux, Hazelwood, MO, USA), an automated analytical equipment in Dankook University Hospital located in Cheonan, Chungnam, Candida spp. were identified during the last three years and by determining the isolation frequency and characteristics by specimen, gender, age, year, month, and season, we intended to help basic research on candidiasis and clinical treatment of Candida infection.
MATERIALS AND METHODS
Clinical specimens.
The subjects of this study were 2,508 strains (1,005 patients) of Candida spp. which were isolated from the clinical specimens collected at the Dankook University Hospital for three years from July 2011 until October 2014. For suspicious infection potentially occurring nosocomially and out of the hospital, samples including sputum, bronchial wash solution, blood, pus, urine, and feces were cultured on blood agar for fungal identification. This study was approved by the Institutional Review Board Deliberations Exemption of Dankook University (IRB No. DKU 2015-10-010).
Identification of Candida spp.
The test organisms evaluated in this study included 3 American Type Culture Collection (ATCC) strains that have been established as QC strains for species identification tests: C. albicans ATCC 10231, C. tropicalis ATCC 200956, C. glabrata ATCC 90030 by Clinical and Laboratory Standards Institute (CLSI, 2008) [10] For the study, all strains were isolated from clinical samples. Tests were performed from subcultures grown for 24 to 55 hr on Sabouraud dextrose agar at 35℃. Each single colony was suspended in 5 mL of sterile distilled water and vortexed. The turbidity at a wavelength of 530nm was adjusted to a McFarland standard of 0.5 with sterile distilled water. This suspension (approximately 1 × 106 to 5 × 106 CFU/mL) was used for the broth microdilution method, after appropriate dilution according to the standardized protocol. Inoculum suspensions for use with the VITEK 2 ID-YST cards were obtained from the same overnight cultures, with the turbidity being adjusted to a 1.8 to 2.2 McFarland standard using the bioMerieux Densicheck instrument, according to the manufacturer's recommendations.
The isolated Candida spp. were identified using VITEK 2 ID-YST (bioMérieux, Durham, NC, USA), an automated analytical equipment. VITEK 2 system and ID-YST card. The VITEK ID-YST card consists of 64 wells with 47 fluorescent biochemical tests. They comprise 20 carbohydrate and six organic acid assimilation tests. The integrated VITEK 2 instrument automatically filled, sealed and transferred the cards into an incubator (incubation temperature was 35℃). Every 15 min the cards were automatically subjected to a fluorescence measurement. Each profile was interpreted according to a specific algorithm. After an incubation period of 15 hr, the profile result was compared to the ID-YST database, which led to the final identification of the microorganism [11]. The identification of the strains was performed using internal transcribed spacer (ITS) sequencing as a reference method [12]. In accordance with the classification method by International Commission of the taxonomy of Fungi (http://www.fungaltaxonomy.org/).
RESULTS
Identification of Candida. spp
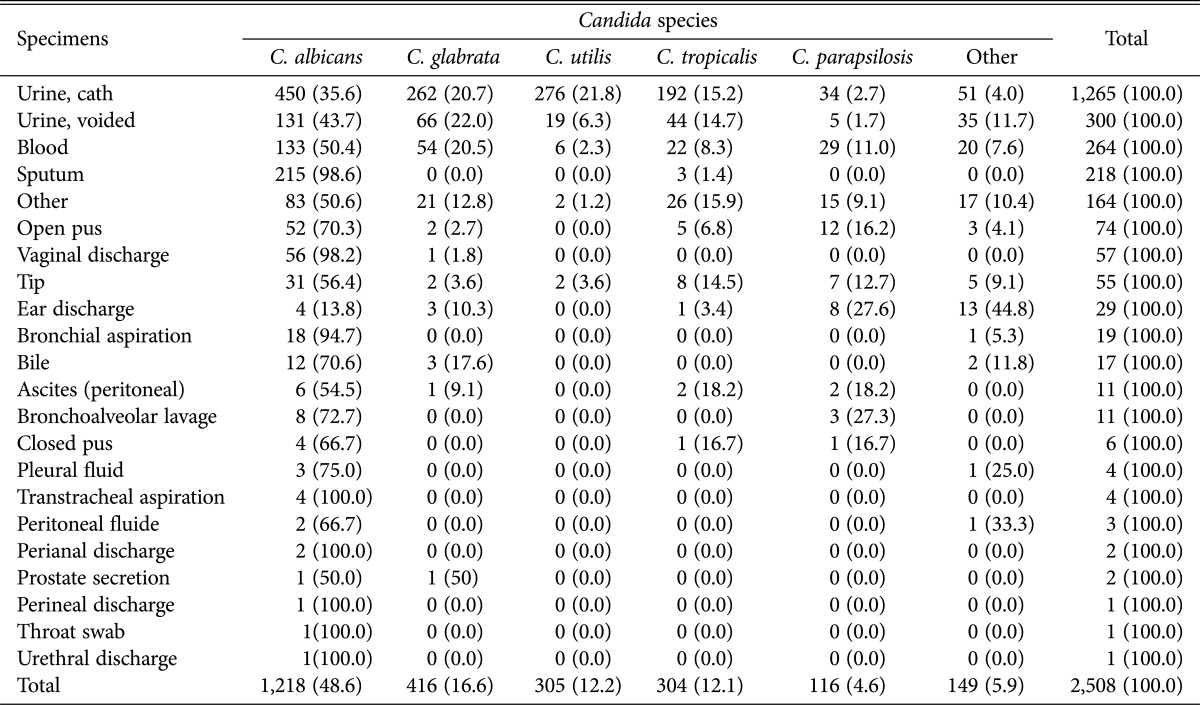
The total number of isolated Candida spp. was 2,508 strains, in which C. albicans (48.6%) was isolated the most frequently, followed by C. glabrata (16.6%), Candida utilis (12.2%), C. tropicalis (12.1%), and C. parapsilosis (4.6%) in that order. These five strains accounted for more than 94% of the total strains isolated. Consistency between the result above and of sequencing was confirmed by ITS in random samples conducted for quality control (Table 1).
Table 1. Distribution of Candida spp. in different clinical specimens.
Values are presented as number (%).
BAL, bronchoalveolar lavage.
Distribution of Candida spp. by specimen.
Looking into the isolation rate of each strain of Candida spp., which were isolated from a variety of clinical specimens, of a total of 2,508 strains, C. albicans 50.5% (1,162/2,302), C. glabrata 18.1% (416/2,303), C. utilis 13.2% (305/2,303), C. tropicalis 13,2% (304/2,303), and C. parapsilosis 5% (116/2,303) were isolated, in that order (Table 1). In particular, C. albicans was isolated most frequently from urine catheter, followed by sputum, blood, urine-voided and other in that order, C. glabrata was isolated most frequently from urine catheter, followed by urine-voided, blood and other in that order, and C. utilis was detected most frequently from urine catheter, followed by urine-voided, blood and other in that order. In addition, C. tropicalis was isolated most frequently from urine catheter, followed by urine-voided, other and blood and sputum in that order and C. parapsilosis was isolated most frequently from urine catheter, followed by blood, other, open pus and urine-voided in that order. Notable points are that C. glabrata, C. utilis, and C. parapsilosis were not detected from sputum and that C. albicans was isolated from every specimen, even though there were significant differences between each specimen.
Distribution of Candida spp. by gender and age.
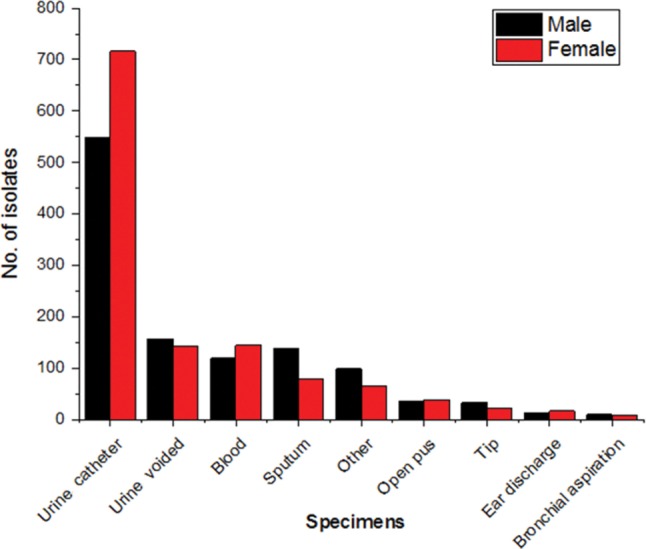
Urine catheter was the specimen from which Candida spp. were most prominently isolated, both in women and men, followed by urine-voided, blood, sputum, other, open pus, vaginal discharge, Tip, ear discharge, bronchial aspiration and bile in that order. In particular, the isolation rates from urine catheter and blood were slightly higher in men and those from urine-voided, sputum and other were slightly higher in women. Combining entire specimens, the sex ratio of males and females was 1 : 1.1 (1,197 : 1,311), and was slightly higher in women (Fig. 1).
Fig. 1. Candida spp. found in specimens of males and females.
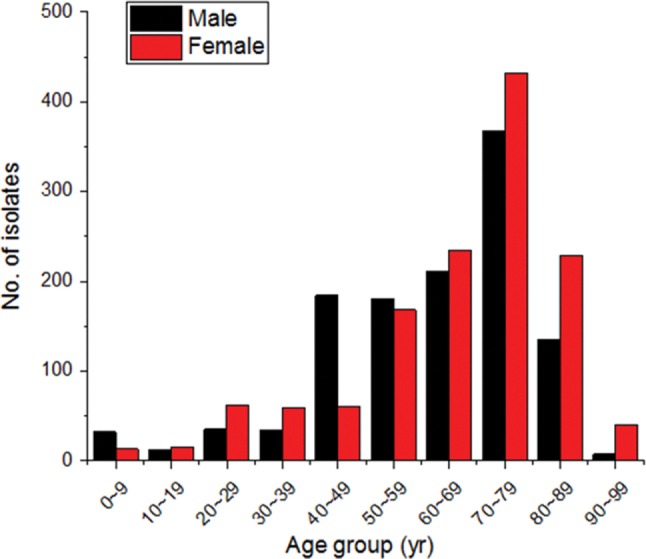
Meanwhile, the age distribution of isolated Candida spp. showed that the isolation rate of the patients in their 60s and older was significantly higher at 75.8% (1,900/2,508), of which the isolation rate of the patients in their 80s, in particular, was highest at 48.2% (1,209/2,508). For patients in their 50s, men were infected by 119 strains and women were infected by 52 strains, showing that the isolation rate of men was more than twice higher than that of women. Patients in their 20s and younger showed a low isolation rate at 2.55% (64/2,508), of which the isolation rate of the patients in their 20s, in particular, was the lowest at 0.76% (19/2,508) among the all age groups (Fig. 2).
Fig. 2. Candida spp. analysis in different age groups.
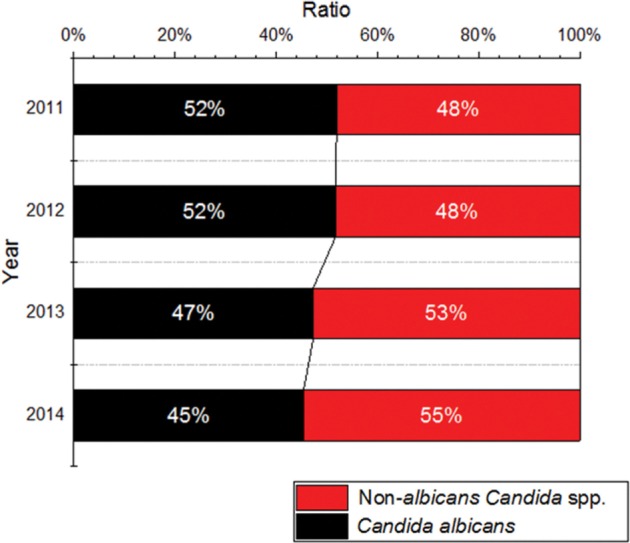
The composition ratios of Candida albicans and non-albicans Candida spp. by year.
The composition ratios of Candida albicans and non-albicans Candida spp. were analyzed and the results showed that the isolation rate in 2011 was 51.9% and 48.1%, respectively, in 2012, 51.7% and 48.3%, respectively, in 2013, 47.3% and 52.7%, respectively and in 2014, 45.4% and 54.6%, respectively. This indicates that there is a tendency for the detection rate of C. albicans to become gradually reduced each year, whereas the detection rate of non-albicans Candida spp. gradually increases (Fig. 3).
Fig. 3. Ratio of Candida albicans and non-albicans Candida spp. according to year.
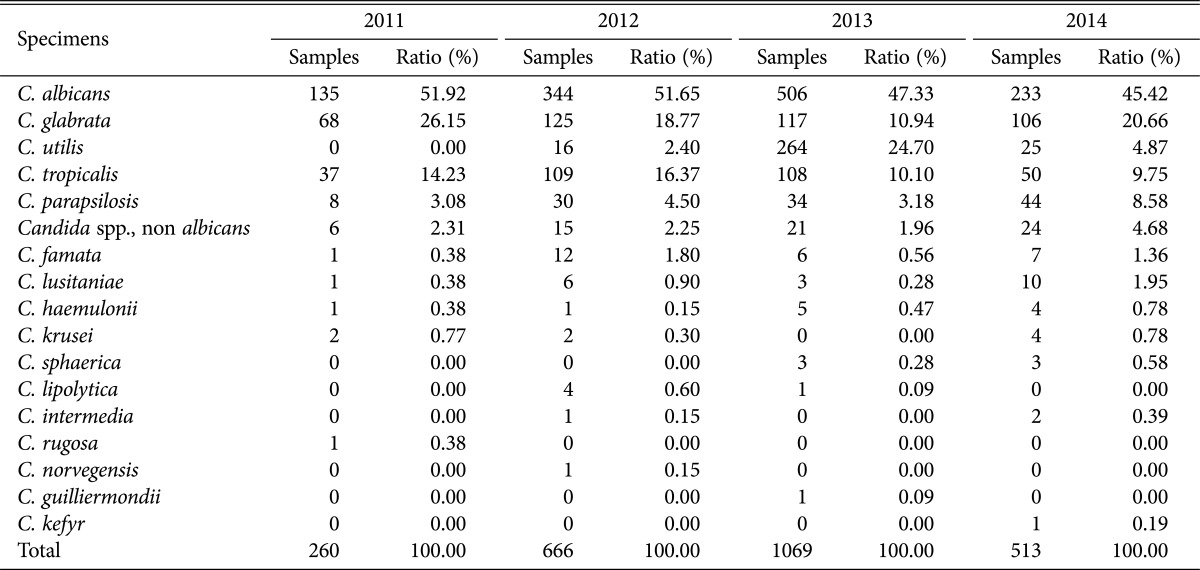
Distribution of Candida spp. by year.
Looking into the distribution of the isolated Candida spp. by year, in 2011, C. albicans was most frequently isolated at 51.92% (135/260), followed by C. glabrata at 26.15% (68/260) and C. tropicalis at 14.23% (37/260); in 2012, C. albicans was most frequently isolated at 51.65% (344/666), followed by C. glabrata at 18.77% (125/666) and C. tropicalis at 16.37% (109/666); in 2014, C. albicans was most frequently isolated at 45.42% (233/513), followed by C. glabrata at 20.66% (106/513) and C. tropicalis at 9.75% (50/513), showing a trend of similar isolation rates between strains each year. However, in 2013 it showed a very different pattern, in which C. albicans was most frequently isolated at 47.33% (506/1,069), followed by C. utilis at 24.70% (264/1,069) and C. glabrata at 10.94% (117/1,069) in that order. On the other hand, in 2011 and 2012, the isolation rate of C. albicans accounted for more than 50%, but in 2013 and 2014, it gradually began to decrease (Table 2).
Table 2. Analysis of Candida spp. by years according to specimens.
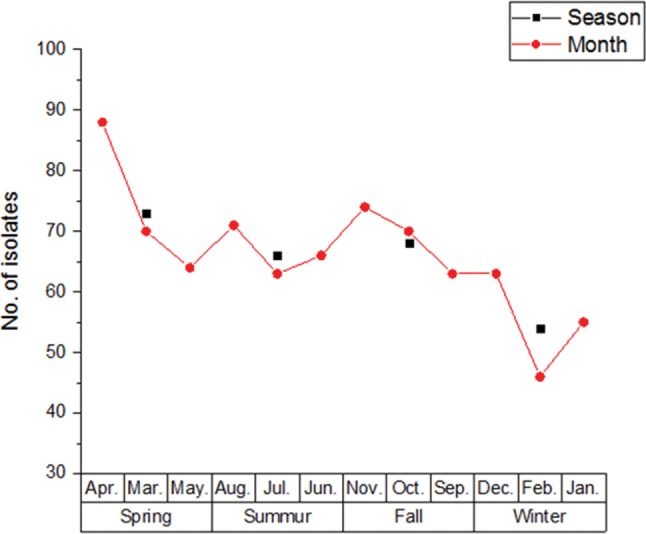
The distribution of Candida spp. by season and month.
Looking into the seasonal distribution of Candida spp., which were isolated from 2011 to 2014, they were isolated most frequently in spring (73 strains) and autumn (68 strains) in that order, and then in the winter the rate was the lowest with 54 strains isolated. Also, Candida spp. were most frequently isolated in April (88 strains) and November (74 strains), in that order; the smallest number of strains were isolated on February (46 strains) (Fig. 4).
Fig. 4. Candida spp. number isolated in different months.
DISCUSSION
There are about 100 Candida spp., of which C. albicans is the most frequently detected opportunistic pathogen. The detection rate of non-albicans Candida species (C. tropicalis, C. parapsilosis, C. krusei, C. lusitaniae, and C. glabrata), excluding C. albicans, has gradually increased [8,13,14,15,16] and the case fatality rate caused by these invasive mycoses is as high as 22.4% [5].
A total of 2,508 strains of Candida spp. were isolated in this study, of which the aforementioned five strains accounted for over 94% of the total strains isolated. Compared with the results of other researchers, it was also found that the detection rate of C. albicans was highest and the aforementioned five strains accounted for over 95%, which was consistent with the findings in this study [17]. Looking at each specimen type, C. albicans was the most frequently detected in urine, which showed a nearly similar pattern with other studies conducted at home and abroad but with some differences [18,19]. In particular, the vast majority of Candida spp. was detected from urine catheter, which showed a great difference when voiding and thus, it is thought that these fungal infections mostly resulted from nosocomial infection which was mediated through catheters. The study of Shin et al. [17] showed that the isolation rate of Candida spp. was 30.1% in sputum, 25.0% in random-urine, 15.8% in blood and 13.5% in urine catheter, which showed some slight differences in the detection rates from urine catheter, urine-voided, blood, and sputum obtained in the study. On the other hand, a domestic study reported that C. albicans (40.8%), C. parapsilosis (17.3%), C. tropicalis (16.5%), and C. glabrata (6.3%) were frequently isolated from blood, in that order [20], and the results of Uh et al.'s experiment [21] showed that the order was as follows: C. albicans (43.8%), C. parapsilosis (20.8%), C. glabrata (13.8%), and C. tropicalis (9.2%) [21]. These slight differences in the isolation rate are believed to have been resulted from the effect of seasonal, locational, environmental differences of the fungi which belong to genus Candida. The isolation rates of these fungi by specimen/gender were examined and the sex ratio of males and females was 1 : 1.1 (1,197 : 1,311) with no significant difference in both sexes. The results of other studies also showed a similar results with the present study regarding some strains and sex ratio [17]. This study showed that the infection rate by Candida spp. had a tendency to increase with increasing age, whereas other researchers reported that the number of patients 20~49 years old, especially patients 30~39 years old had the highest rate of infection [19]. On the other hand, it reported that the detection rate of teenagers, in particular, was the lowest [17,22], which showed nearly identical results with this study. It is thought that the infection rate of teenagers is relatively low because this age group of teenagers has a vigorous immune system and participation in outdoor activities is low.
Meanwhile, the isolation rate of Candida spp. was highest on average in April and lowest in February, which was consistent with the results of Shin et al. [17], However, it was the isolation rate of Candida spp. being highest in the summer [17,22,23] was somewhat different from the reports. Fungal infections by Candida occur easily in a body with a weakened immune system, which is caused by opportunistic pathogens. Based on this fact, it is thought that the immune system is significantly weakened at the change of seasons such as spring and fall, leading to a susceptible condition of infection. Winter susceptibility to these fungi is greatly lowered by reduced activity due to the decrease in temperatures. In the past, infections were mainly caused by C. albicans, but recently, infectious diseases caused by C. tropicalis and C. parapsilosis are also increasing [24,25]. Although C. albicans was most frequently detected in this study, the detection ratio of C. albicans over the entire Candida spp. has been declining year by year.
The reasons for the increased isolation rate of 16 Candida species other than C. albicans can be attributed to be the incremental factors related to the advancement of automated test equipment in hospitals as well as the increase in the opportunistic pathogens of different species according to the environmental changes and weakened immune functions in the human body. On the other hand, this study showed that the overall detection rate of C. albicans was decreased, but given that it is still an important microbial species accounting for more than 50% of the entire detection rate, it requires continuous monitoring and support of additional clinical studies.
ACKNOWLEDGEMENTS
The present research was conducted by the research fund of Dankook University in 2016.
References
- 1.Kim YK, Ki MR, Yim JS, Kim KH, Song YJ, Choi HY, Seo CW, Park JS. Epidemiological characteristic study of domestic and imported mycoses. Cheongju: Korea Centers for Disease Control and Prevention (KCDC); 2012. [Google Scholar]
- 2.McNeil MM, Nash SL, Hajjeh RA, Phelan MA, Conn LA, Plikaytis BD, Warnock DW. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin Infect Dis. 2001;33:641–647. doi: 10.1086/322606. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995;20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- 4.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 5.Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27:1138–1147. [PubMed] [Google Scholar]
- 6.Knoke M, Schulz K, Bernhardt H. Dynamics of Candida isolations from humans from 1992-1995 in Greifswald, Germany. Mycoses. 1997;40:105–110. doi: 10.1111/j.1439-0507.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 7.Shin JH, Lim WH, Shin DH, Suh SP, Ryang DW. Candida species isolated from clinical specimens and medical personnel. Korean J Infect Dis. 1999;31:481–486. [Google Scholar]
- 8.Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, Baughman WS, Reingold AL, Rothrock GA, Pfaller MA, et al. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis. 1999;29:1164–1170. doi: 10.1086/313450. [DOI] [PubMed] [Google Scholar]
- 9.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution: antifungal susceptibility testing of yeasts. Approved standard M27-A2. Wayne (PA): Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 11.Graf B, Adam T, Zill E, Göbel UB. Evaluation of the VITEK 2 system for rapid identification of yeasts and yeast-like organisms. J Clin Microbiol. 2000;38:1782–1785. doi: 10.1128/jcm.38.5.1782-1785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YC, Eisner JD, Kattar MM, Rassoulian-Barrett SL, Lafe K, Bui U, Limaye AP, Cookson BT. Polymorphic internal transcribed spacer region 1 DNA sequences identify medically important yeasts. J Clin Microbiol. 2001;39:4042–4051. doi: 10.1128/JCM.39.11.4042-4051.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St-Germain G, Laverdière M, Pelletier R, Bourgault AM, Libman M, Lemieux C, Noël G. Prevalence and antifungal susceptibility of 442 Candida isolates from blood and other normally sterile sites: results of a 2-year (1996 to 1998) multicenter surveillance study in Quebec, Canada. J Clin Microbiol. 2001;39:949–953. doi: 10.1128/JCM.39.3.949-953.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Messer SA, Hollis RJ, Jones RN, Doern GV, Brandt ME, Hajjeh RA. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn Microbiol Infect Dis. 1999;33:217–222. doi: 10.1016/s0732-8893(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 15.Arias A, Arévalo MP, Andreu A, Rodríguez C, Sierra A. In vitro susceptibility of 545 isolates of Candida spp. to four antifungal agents. Mycoses. 1994;37:285–289. doi: 10.1111/j.1439-0507.1994.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen MH, Peacock JE, Jr, Morris AJ, Tanner DC, Nguyen ML, Snydman DR, Wagener MM, Rinaldi MG, Yu VL. The changing face of candidemia: emergence of not-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 17.Shin HS, Park YB, Shin DS. Isolation frequency of Candida species from clinical specimens. Kor J Mycol. 2010;38:146–151. [Google Scholar]
- 18.Shin JH, Kim HR, Lee JN. Distribution and antifungal susceptibility of Candida species isolated from clinical specimens during the past six years. Korean J Clin Microbiol. 2004;7:164–170. [Google Scholar]
- 19.Fernandes R, Viegas A, Cerqueira F. Candida species distribution in clinical samples. Revista da Faculdade de Ciencias da Saude. Porto: Edições Universidade Fernando Pessoa; 2009. pp. 264–271. [Google Scholar]
- 20.Kang BK, Lee HJ, Suh JT. The trends of the species and antimicrobial susceptibility of bacteria and fungi isolated from blood cultures (1986-1996) Korean J Clin Pathol. 1998;18:57–64. [Google Scholar]
- 21.Uh Y, Jang IH, Yoon KJ, Kim HY. Isolation trend and antifungal susceptibility of Candida species isolated from blood cultures. Korean J Infect Dis. 2001;33:186–193. [Google Scholar]
- 22.Moon HJ, Lee JB, Kim SJ, Lee SC, Won YH. Clinical and mycological studies on dermatomycosis (1991-2000) Korean J Med Mycol. 2002;7:78–85. [Google Scholar]
- 23.Kim JS, Won YH, Chun IK, Kim YP. Clinical and mycological studies on dermatomycosis. Korean J Dermatol. 1992;30:68–75. [Google Scholar]
- 24.Shin JH, Lim WH, Shin DH, Suh SP, Ryang DW. Antifungal susceptibilities to fulconazole and itraconazole for Candida species recovered from blood cultures over a 5-year period. Korean J Infect Dis. 2000;32:179–185. [Google Scholar]
- 25.Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis. 1998;31:327–332. doi: 10.1016/s0732-8893(97)00240-x. [DOI] [PubMed] [Google Scholar]