Abstract
Background
Acute respiratory infections are the most common symptomatic reason for seeking care among patients in the US, and account for the majority of all antibiotic prescribing, yet a large fraction of antibiotic prescriptions are inappropriate.
Objective
We sought to identify the underlying factors driving variation in antibiotic prescribing across clinicians and settings.
Design, Participants
Using electronic health data for adult ambulatory visits for acute respiratory infections to a retail clinic chain and primary care practices from an integrated healthcare system, we identified a random sample of clinicians for survey.
Main Measures
We evaluated independent predictors of overall prescribing and imperfect antibiotic prescribing, controlling for clinician and site of care. We defined imperfect antibiotic prescribing as prescribing for non-antibiotic-appropriate diagnoses, failure to prescribe for an antibiotic-appropriate diagnosis, or prescribing a non-guideline-concordant antibiotic.
Key Results
Response rates were 34 % for retail clinics and 24 % for physicians’ offices (N = 187). Clinicians in physicians’ offices prescribed antibiotics less often than those in retail clinics (53 % versus 67 %; p < 0.01), but had a higher imperfect antibiotic prescribing rate (65 % versus 31 %; p < 0.01). Feeling rushed was associated with higher antibiotic prescribing (OR 1.34; 95 % CI 1.03, 1.75). Antibiotic prescribing was also associated with clinician disagreement that antibiotics are overused (OR 1.60, 95 % CI, 1.16, 2.20). Imperfect antibiotic prescribing was associated with receiving antibiotic prescribing feedback (OR 1.35, 95 % CI 1.04, 1.75) and disagreement that patient demand was a problem (OR 1.66, 95 % CI 1.00, 2.73). Imperfect antibiotic prescribing was less common with clinicians who perceived that they prescribed antibiotics less often than their peers (OR 0.63, 95 % CI 0.46, 0.87).
Conclusions
Poor-quality antibiotic prescribing was associated with feeling rushed, believing less strongly that antibiotics were overused, and believing that patient demand was not an issue, factors that can be assessed and addressed in future interventions.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-016-3643-0) contains supplementary material, which is available to authorized users.
KEY WORDS: acute respiratory infections, antibiotics, prescribing, overuse
BACKGROUND
Acute respiratory infections—including the common cold, otitis, sinusitis, pharyngitis, acute bronchitis, and pneumonia—are the most common symptomatic reason for seeking care among patients in the US, and account for the majority of all antibiotic prescriptions.1 A large fraction of antibiotic prescriptions are inappropriate, either because the antibiotic is written for a viral condition or because a broad-spectrum antibiotic is chosen in clinical situations where a narrow-spectrum antibiotic would be suitable.1 , 2
There has been some progress. Numerous interventions have been tried over the last several decades ranging from physician education to the CDC’s GET SMART patient education campaign.3 – 5 Per capita antibiotic use for acute respiratory infections has fallen by 25 %.1 While this success is laudable, several studies have found that improvements in antibiotic prescribing rates have stalled.6 , 7 Furthermore, the inappropriate use of broad-spectrum antibiotics has increased.1 We are in need of new interventions.
Variation in antibiotic prescribing between individual clinicians and clinical settings might help identify targets for new interventions. Outside of primary care physician (PCPs) offices, many patients are receiving acute respiratory infection care in settings such as retail clinics. Retail clinics, located in drug stores and grocery stores and typically staffed by nurse practitioners (NPs), are an innovative delivery model that provides walk-in care for acute respiratory infections.8 , 9 Currently there are ∼5.4 million visits to the 1200 retail clinics in the US each year, and almost two thirds of these visits are for acute respiratory infections.8 Prior work has demonstrated that retail clinic clinicians are more likely to provide guideline-concordant care.10 , 11
The goal of this study was to understand the underlying factors driving the variation in antibiotic prescribing across clinicians and settings, with the hope that these factors could be the basis of future interventions. We measured the rates of antibiotic prescribing and “imperfect” antibiotic prescribing among a sample of clinicians at physicians’ offices and providers in retail clinics. Then, using survey responses from those same clinicians, we looked for associations between antibiotic use and survey-reported measures of knowledge, attitudes, and behavior.
METHODS
Survey Development
Based on Ranji’s work on antibiotic prescribing5 and Cabana’s framework for categorizing barriers to compliance with guidelines,12 we created a conceptual framework of factors and barriers that might influence antibiotic prescribing for acute respiratory infections. These factors can affect clinicians’ behavior directly or can affect knowledge and attitudes that, in turn, affect behavior. Barriers include a lack of clinician awareness of a guideline, lack of outcome expectancy (e.g. guideline-based care will not work), self-efficacy regarding communication ability, and the perception that guideline-based care goes against patient wishes. Other factors include clinicians’ perceptions of their antibiotic prescribing compared to their peers, clinical workload, patient continuity (whether they see their own clinician), and feedback on performance.
We developed the survey using the above framework and refined the survey through cognitive testing with four clinicians and pilot testing with 31 clinicians in the spring of 2013. Please see the Online Electronic Materials for the full survey wording.
Study Population and Data Collection
We sampled clinicians in two settings: (1) PCPs’ offices in the University of Pittsburgh Medical Center—a large integrated health system with an academic affiliation; and (2) a national retail clinic chain with clinics in 19 states in the Northeast, West, South, and Midwest. We focused on these two settings because prior work has documented large differences in patterns of antibiotic prescribing.13 We strove to understand what drives that variation. Clinicians in the physicians’ offices were all physicians with an MD (doctor of medicine degree) or a DO (doctor of osteopathic medicine degree). Clinicians in the retail clinics were almost all NPs, with a small number of physician assistants. Both had an electronic health record (EHR) that allowed us to directly measure antibiotic prescribing.
We obtained electronic health record data for all adult ambulatory visits for acute respiratory infections in 2012. Based on prior work,14 we defined acute respiratory infection diagnoses using the following ICD-9 codes: streptococcal pharyngitis (034.x), otitis media (381.x, 382.x), sinusitis (461.x), pneumonia (481.x, 482.x, 483.x, 485.x, 486.x), non-specific upper respiratory infection (URI; 460.x, 465.x), non-streptococcal pharyngitis (462.x), and bronchitis (466.x, 490.x, 491.21). The physicians’ offices had 39,961 visits for acute respiratory infections in 2012, while the retail clinics had 765,667 visits. The smaller number at physicians’ offices is presumed to be due to our having surveyed within only one health system. We then excluded all visits where the patient had a comorbidity or condition requiring an antibiotic according to the ICD-9 code, based on prior literature (see Online Materials Table 1).13 We otherwise included all visits for acute respiratory infections, including those that may have been follow-up visits. Given our use of EMR data, we could not identify visits outside the health system, and therefore could not reliably identify follow-up visits.
Survey Deployment
We randomly sampled clinicians in each of the two settings from among those who had at least 25 visits for acute respiratory infections during 2012. The self-administered online survey was emailed to clinicians starting in January 2013 (physicians’ offices) and September 2013 (retail clinics). The clinicians were offered a $50 gift card as an incentive for participation. The initial email invitation was followed by two email reminders sent at 5–7-day intervals to those who had not responded. The study protocol was approved by the Human Subjects Protection Committees at the University of Pittsburgh and Harvard Medical School.
Defining Two Measures of Antibiotic Prescribing
We used EHR data to create two measures to characterize antibiotic prescribing for acute respiratory infections for each clinician. The first was overall antibiotic prescribing, which was the rate of prescribing of any antibiotic across all acute respiratory infection visits. We then created a new quality measure, called “imperfect antibiotic prescribing.” Overall acute respiratory infection antibiotic prescribing is crude, in that it does not account for acute respiratory infection conditions where antibiotics may be indicated. Thus we defined the “imperfect” antibiotic prescribing rate as prescribing an antibiotic for a non-antibiotic-appropriate diagnosis (defined as URI, bronchitis, non-streptococcal pharyngitis),13 , 14 failure to prescribe an antibiotic for an antibiotic-appropriate diagnosis (sinusitis, pneumonia, streptococcal pharyngitis, otitis media),13 , 14 or choosing a non-guideline-concordant antibiotic for an antibiotic-appropriate diagnosis. We defined non-guideline-concordant antibiotics as an antibiotic other than amoxicillin-clavulanate or amoxicillin for sinusitis,15 – 17 a macrolide (azithromycin, clarithromycin, or erythromycin) or doxycycline for pneumonia,18 amoxicillin or penicillin for streptococcal pharyngitis,19 , 20 and amoxicillin for otitis media.21 Other antibiotics that were prescribed by clinicians across all visits—none of which would be considered guideline-concordant—included fluoroquinolones (levofloxacin, ciprofloxacin, moxifloxacin, and ofloxacin), trimethoprim-sulfamethoxazole (Bactrim), first-generation cephalosporins (cefadroxil, cephalexin), second-generation cephalosporins (cefaclor, cefprozil, cefuroxime), third-generation cephalosporins (cefdinir, cefixime, cefpodoxime, ceftriaxone), and others (clindamycin, metronidazole, minocycline, tetracycline, nitrofurantoin).
Analysis
We constructed a multivariate model at the visit level, where the primary outcome was whether an antibiotic was prescribed at the visit. A secondary model was constructed identically to the first but with imperfect antibiotic prescribing as the outcome. For each model, we selected both clinician-level and visit-level covariates based on theoretical grounds. Clinician-level variables included demographic characteristics such as gender, number of years in practice, and professional degree/background (all via self-report). We also asked clinicians about the type of insurance held by their patients across all of their patients. Other clinician-level covariates included the factors from our conceptual framework collected via the survey, which included familiarity with guidelines; ability to effectively communicate; agreement that antibiotics are overused in primary care; agreement that patient demand for antibiotics is a problem; prescribing rate compared to peers; receiving feedback on prescribing; and how often the clinician felt rushed when seeing patients. From the EHR, we obtained visit-level covariates including the patient’s gender as well as the patient’s age in years at the time of the visit. Both models were run as logistic regressions with standard errors adjusted for clustering at the clinician level.
RESULTS
Among a total of 613 clinicians, 187 completed the email survey, resulting in an overall response rate of 30 %. The response rates were similar between clinicians at retail clinics (34 %) and at physicians’ offices (27 %; p = 0.08). We did not have data to compare respondents and non-respondents, except on their prescribing rates. The overall prescribing rate of 61 % for respondents was similar to that of non-respondents, at 64 % (p = 0.07). Respondents had a significantly lower imperfect antibiotic prescribing rate than non-respondents, though the absolute magnitude of difference was not large (46 % versus 52 %, p = 0.003)
Of our respondents, 29 % reported working more than 40 hours per week (Table 1). The average number of years in practice after having completed training was 13.2. Clinicians at retail clinics were more likely to be women, have less time in practice, and work part-time. Patients at both retail clinics and physicians’ offices were more likely to be women (66 %; Table 1). Patients at physicians’ offices tended to be older than those at retail clinics.
Table 1.
Demographic Characteristics
| All clinicians (N = 187) | Physicians’ offices (MD; N = 78) | Retail clinics (NP/PA; N = 109) | p value | |
|---|---|---|---|---|
| Clinician characteristics | ||||
| Gender (% female) | 72 % | 41 % | 94 % | <0.001 |
| Average number of years in practice (± SD) | 13.2 ± 9.5 | 16.8 ± 10.3 | 10.6 ± 7.9 | <0.001 |
| Hours in practice per week | ||||
| 0–25 | 3 % | 8 % | 13 % | <0.001 |
| 26–40 | 69 % | 37 % | 78 % | |
| > 40 | 29 % | 56 % | 9 % | |
| Professional background | ||||
| MD | – | 79 % | – | – |
| DO | – | 21 % | – | |
| NP | – | – | 97 % | |
| PA | – | – | 3 % | |
| Patient characteristics | ||||
| Type of insurance for patients with acute respiratory infections* | ||||
| Medicaid | 14 % | 14 % | 14 % | 0.77 |
| Medicare | 25 % | 34 % | 19 % | <0.001 |
| Private | 49 % | 42 % | 53 % | <0.001 |
| Self | 9 % | 4 % | 13 % | <0.001 |
| Sex of patients with acute respiratory infections† | ||||
| Male | 33 % | 33 % | 33 % | 0.81 |
| Female | 67 % | 67 % | 67 % | |
| Age of patients with acute respiratory infections† | ||||
| 18–24 | 13 % | 8 % | 13 % | <0.001 |
| 25–34 | 25 % | 15 % | 26 % | |
| 35–44 | 24 % | 18 % | 25 % | |
| 45–54 | 18 % | 28 % | 18 % | |
| 55-64 | 12 % | 19 % | 12 % | |
| 65+ | 7 % | 23 % | 6 % | |
*Based on physician report of patient insurance from survey
†Based on EHR data
Variation in Antibiotic Prescribing
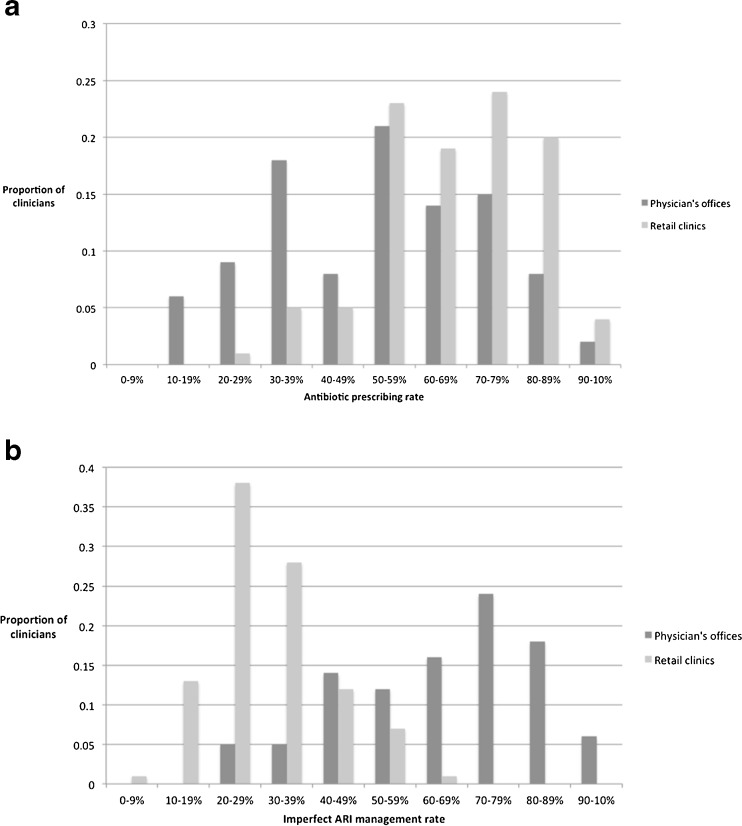
There were large variations in overall antibiotic prescribing and imperfect antibiotic prescribing rates among clinicians and between sites (Fig. 1). Overall, compared to the physicians in the physicians’ offices, clinicians in retail clinics had a higher antibiotic prescribing rate (67 % versus 53 %, p < 0.01), but a lower imperfect antibiotic prescribing rate (31 % versus 65 %, p < 0.001).
Fig. 1.
Distribution of antibiotic prescribing by site (overall antibiotic prescribing and imperfect antibiotic prescribing rates based on EHR data).
Knowledge, Attitudes, and Behaviors
Most clinicians (82 %) strongly agreed that antibiotics were overused in primary care, with no clinicians in disagreement with that statement (Table 2; results by setting can be found in Online Materials Table 2). The majority of clinicians also strongly agreed that patient demand was a problem in their practice (59 %). About a third of clinicians reported feeling rushed almost always or most of the time. Forty-three percent of respondents believed that they prescribed antibiotics at about the same rate as their peers, and 50 % reported prescribing antibiotics less often than their peers.
Table 2.
Knowledge, Attitudes, and Behaviors Reported by Survey Respondents
| All Providers, N (%) | |
|---|---|
| Familiarity with guidelines | |
| Very | 105 (57 %) |
| Somewhat | 76 (41 %) |
| Only a little | 3 (2 %) |
| Not at all | 0 (0 %) |
| Ability to effectively communicate | |
| Very | 107 (58 %) |
| Somewhat | 75 (41 %) |
| Only a little | 2 (1 %) |
| Not at all | 0 (0 %) |
| Agree that antibiotics are overused in primary care | |
| Strongly agree | 152 (82 %) |
| Somewhat agree | 33 (18 %) |
| Somewhat disagree | 0 (0 %) |
| Strongly disagree | 0 (0 %) |
| Patient demand is a problem | |
| Strongly agree | 108 (59 %) |
| Somewhat agree | 64 (35 %) |
| Somewhat disagree | 9 (5 %) |
| Strongly disagree | 3 (2 %) |
| Prescribing rate compared to peers | |
| More often | 14 (8 %) |
| About the same | 79 (43 %) |
| Less often | 92 (50 %) |
| Feedback received on prescribing | |
| No | 85 (46 %) |
| Yes | 100 (54 %) |
| How often feel rushed when seeing a patient | |
| Almost always or most of the time | 68 (37 %) |
| Sometimes, rarely or never | 116 (63 %) |
Factors Associated with Overall Antibiotic Prescribing and Imperfect Antibiotic Prescribing for Acute Respiratory Infections
Clinicians who reported feeling rushed almost always or most of the time were more likely to prescribe antibiotics (OR 1.34, 95 % CI 1.03, 1.75; Table 3). Clinicians who felt less strongly that antibiotics were overused had a higher overall antibiotic prescribing rate (OR 1.61, 95 % 1.18, 2.20). Clinicians who did not agree that patient demand was a problem in their practice were more likely to have imperfect antibiotic prescribing (OR 1.66, 95 % CI 1.00, 2.73). Clinicians who reported that they prescribed less often than their peers had a lower antibiotic prescribing rate (OR 0.63, 95 % CI 0.47, 0.86). Seemingly paradoxically, imperfect antibiotic prescribing was more likely among clinicians who reported receiving feedback (OR 1.35, 95 % CI 1.04, 1.75].
Table 3.
Independent Predictors of Different Measures of Antibiotic Prescribing*
| Antibiotic prescribed at visit (N = 65,882) | “Imperfect” antibiotic prescribing at visit (N = 65,882) | |
|---|---|---|
| Odds Ratio (95 % confidence Interval) | ||
| Familiarity with guidelines | ||
| Very | – | – |
| Somewhat, a little, or not at all | 0.91 (0.71, 1.17) | 1.08 (0.87, 1.34) |
| Ability to effectively communicate | ||
| Very | – | – |
| Somewhat, a little, or not at all | 1.10 (0.86, 1.44) | 1.10 (0.89, 1.35) |
| Overused | ||
| Strongly agree | – | – |
| Somewhat agree | 1.61 (1.18, 2.20) | 1.09 (0.83, 1.44) |
| Patient demand | ||
| Strongly agree | – | – |
| Somewhat agree | 1.03 (0.79, 1.34) | 0.98 (0.77, 1.25) |
| Somewhat or strongly disagree | 1.20 (0.86, 1.66) | 1.66 (1.00, 2.73) |
| Prescribing rate compared to peers | ||
| More often | – | – |
| About the same | 1.00 (0.53, 1.88) | 0.83 (0.58, 1.18) |
| Less often | 0.74 (0.39, 1.39) | 0.63 (0.46, 0.87) |
| Rushed | ||
| Sometimes, rarely or never | – | – |
| Almost always or most of time | 1.34 (1.03, 1.75) | 1.24 (0.99, 1.56) |
| Feedback | ||
| No | – | – |
| Yes | 1.10 (0.79, 1.52) | 1.35 (1.04, 1.75) |
| Setting | ||
| Retail clinics | – | – |
| Physicians’ offices | 0.54 (0.39, 0.74) | 2.77 (2.12, 3.64) |
The bolded text indicates results which are significant based on the confidence intervals
*Model controls for patient age and gender, as well as for clustering by clinician
DISCUSSION
To our knowledge, this is the largest and most comprehensive survey thus far of clinician antibiotic prescribing behavior related to acute respiratory infections. Consistent with prior work,13 we found notable variation among clinicians, even within single health systems, in antibiotic prescribing rates. Our goals were to exploit these differences to learn what might be driving variation in care. We found that the following factors were associated with higher rates of inappropriate prescribing: feeling rushed, less likely to perceive patient demand as a problem, clinician perception of having a higher prescribing rate than peers, and paradoxically, receiving more feedback on antibiotic prescribing. Equally notable is that knowledge of guidelines and communication ability were not predictive of inappropriate antibiotic prescribing.
Understanding what links the factors we identified in our model to actual prescribing behaviors may inform future interventions. Clinicians who are rushed may be more likely to prescribe antibiotics because they believe it takes more time to convince a patient that antibiotics are not necessary. Furthermore, the perception that antibiotic overuse is not a problem makes it easier to take the path of least resistance, which is to prescribe antibiotics. These same clinicians may then not perceive patient demand as a problem for their practice, because they more frequently prescribe antibiotics. As a result, they receive less pushback from patients and fewer conflicting demands, and thus do not perceive patient demand as a problem.
Based on these findings, future interventions could try to reduce the perceived workload associated with not prescribing antibiotics and/or increase the perceived workload with prescribing antibiotics. One set of interventions could focus on reducing the likelihood that patients will ask for antibiotics. This could be accomplished through low-cost interventions such a notification informing patients that for the majority of acute respiratory infections, the clinician does not prescribe antibiotics, a poster in the waiting room stating that the practice is committed to prescribing antibiotics only when appropriate,22 or sending patients literature on antibiotic overuse prior to the visit or providing it in the waiting room.23 Another set of interventions could focus on perceptions of feeling rushed. One example might be to give clinicians brief “talking points” about antibiotics. A third set of interventions could make it harder for clinicians to take the path of least resistance and prescribe antibiotics inappropriately. For example, being forced to provide justification in the electronic record for decisions around giving antibiotics might make prescribing antibiotics a less desirable option for the clinician who feels rushed. This would go beyond simple decision support interventions4 , 24 , 25—which have resulted in only modest reductions in antibiotic prescribing rates—to a more active review when clinicians prescribe antibiotics inappropriately.
Some of the factors that we hypothesized would be related to antibiotic prescribing either were not significant, or their effect was the opposite of what we expected. For example, receiving feedback on antibiotic prescribing, rather than improving care for acute respiratory infections, was associated with higher odds of imperfect antibiotic prescribing. Past work in the area of audit and feedback to clinicians has shown mixed effects, with some interventions demonstrating significant improvements in prescribing rates26 and others with more mixed results.14 , 27 , 28 Although we did not collect the data needed to further explore the apparently paradoxical relationship observed in this study, we wonder whether clinicians who were performing poorly in care for acute respiratory infections were also those who received feedback on their prescribing rates. In such instances, temporality cannot be assessed. Self-reported familiarity with guidelines was not associated with either prescribing outcome. This is consistent with prior work, in which self-reported familiarity was not associated with consistent guideline adherence or higher quality of care.29 And finally, self-reported communication effectiveness was not associated with quality of antibiotic prescribing. Given that very few clinicians rated themselves as poor communicators, our questions may not have been able to capture the spectrum of responses in sufficiently granular detail.
Our study had a number of limitations. We had only a fair response rate at 30 %. Not unexpectedly, we did observe differences between responders and non-responders, with responders having significantly lower rates of imperfect antibiotic prescribing. This is not entirely surprising, as those who are more aware and supportive of appropriate antibiotic prescribing may be more responsive to survey requests. Our results may thus be biased and may overestimate the quality of antibiotic prescribing in these settings. While we compared two very different settings, due to feasibility constraints, we looked at only one health system in a single state and one chain of retail clinics (though with representation across 19 states), which limits the generalizability of our findings in light of known regional differences in prescribing patterns.30 In addition, we included all visits for ARIs, and thus may have inadvertently included some follow-up visits. We were unable to reliably identify follow-up visits at retail clinics, given that the vast majority of such visits are to a different site. However, in physicians’ offices, follow-up visits within 21 days for the same condition occurred only 4.5 % of the time, and thus this inclusion is unlikely to have significantly affected our results. We did not account for antibiotic allergies in this study, both because such information was not available and because allergies are generally poorly recorded, even with the use of an EMR.31 In theory, sicker patients who might be more likely to have allergies may also be more likely to go to a physician’s office, which would bias our results towards penalizing physicians for broader antibiotic prescribing. However, we did exclude patients based on a number of comorbidities and other conditions, which may partially mitigate the issue of allergies.
An additional limitation is that our study was not designed to assess the differences between retail clinics and physicians’ offices. However, we did observe a large discrepancy in the prescribing rates at the two settings, both for overall and imperfect antibiotic prescribing. Our prescribing rates were based on ICD-9 codes, as we did not have access to laboratory or radiology data. One possibility is that clinicians at retail clinics use a smaller set of codes, and selectively choose ICD-9 codes that align with the decision to prescribe antibiotics. Unfortunately, we could not determine whether coding and true practice were similarly matched in the two settings in this study, but this would be an important area for future exploration.
In conclusion, we created a new measure of the quality of antibiotic prescribing, which is a resource that can be used, tested, and validated in future studies. In using this measure, we found that, independent of clinical discipline and site of care, poor-quality antibiotic prescribing was associated with feeling rushed, believing less strongly that antibiotics were overused, and believing that patient demand was not an issue. These factors should be considered and addressed in future interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 26 kb)
Acknowledgments
Contributors
We would like to thank the following people for their assistance: Rachel Burns, and Mark Friedberg (RAND Corporation); Stephen Strotmeyer Jr. (University of Pittsburgh); Rita Mangione-Smith (University of Washington); and Jonathan Finkelstein (Boston Children’s Hospital).
Compliance with ethical standards
Funders
This work was supported by grant # R21-AI097759 from the National Institute of Allergy and Infectious Diseases (PI: Dr. Mehrotra). Dr. Linder’s work on acute respiratory infections was also supported by grants from the National Institutes of Health (RC4 AG039115) and the Agency for Healthcare Research and Quality (R18 HS018419).
Prior presentations
None.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–66. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014;12:96. doi: 10.1186/1741-7015-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work. http://www.cdc.gov/getsmart/community/index.html (February 1 2016, date last accessed)
- 4.Holstiege J, Mathes T, Pieper D. Effects of computer-aided clinical decision support systems in improving antibiotic prescribing by primary care providers: a systematic review. J Am Med Inform Assoc. 2015;22:236–42. doi: 10.1136/amiajnl-2014-002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranji SR, Steinman MA, Shojania KG et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 4: Antibiotic Prescribing Behavior). Rockville MD; 2006 [PubMed]
- 6.Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997–2010. JAMA Intern Med. 2014;174:138–40. doi: 10.1001/jamainternmed.2013.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. JAMA. 2014;311:2020–2. doi: 10.1001/jama.2013.286141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrotra A, Wang MC, Lave JR, et al. Retail clinics, primary care physicians, and emergency departments: a comparison of patients’ visits. Health Aff (Millwood) 2008;27:1272–82. doi: 10.1377/hlthaff.27.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudavsky R, Pollack CE, Mehrotra A. The geographic distribution, ownership, prices, and scope of practice at retail clinics. Ann Intern Med. 2009;151:315–20. doi: 10.7326/0003-4819-151-5-200909010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrank WH, Krumme AA, Tong AY, et al. Quality of care at retail clinics for 3 common conditions. Am J Manag Care. 2014;20:794–801. [PubMed] [Google Scholar]
- 11.Mehrotra A, Liu H, Adams JL, et al. Comparing costs and quality of care at retail clinics with that of other medical settings for 3 common illnesses. Ann Intern Med. 2009;151:321–8. doi: 10.7326/0003-4819-151-5-200909010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabana M, Rand C, Powe N, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 13.Mehrotra A, Gidengil CA, Setodji CM, et al. Antibiotic prescribing for respiratory infections at retail clinics, physician practices, and emergency departments. Am J Manag Care. 2015;21:294–302. [PubMed] [Google Scholar]
- 14.Linder JA, Schnipper JL, Tsurikova R, et al. Electronic health record feedback to improve antibiotic prescribing for acute respiratory infections. Am J Manag Care. 2010;16:e311–9. [PubMed] [Google Scholar]
- 15.Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72–112. doi: 10.1093/cid/cis370. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work. Acute Bacterial Rhinosinusitis: Physician Information Sheet (Adults). http://www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-acute-bact-rhino.html (February 1 2016, date last accessed).
- 17.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152:S1–39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 18.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin infect Dis. 2012;55:1279–82. doi: 10.1093/cid/cis847. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work. Acute Pharyngitis in Adults. http://www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-acute-pharyngitis.html (February 1 2016, date last accessed).
- 21.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964–99. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 22.Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. 2014;174:425–31. doi: 10.1001/jamainternmed.2013.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Formoso G, Paltrinieri B, Marata AM, et al. Feasibility and effectiveness of a low cost campaign on antibiotic prescribing in Italy: community level, controlled, non-randomised trial. BMJ. 2013;347:f5391. doi: 10.1136/bmj.f5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267–73. doi: 10.1001/jamainternmed.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourgeois FC, Linder J, Johnson SA, et al. Impact of a computerized template on antibiotic prescribing for acute respiratory infections in children and adolescents. Clin Pediatr. 2010;49:976–83. doi: 10.1177/0009922810373649. [DOI] [PubMed] [Google Scholar]
- 26.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345–52. doi: 10.1001/jama.2013.6287. [DOI] [PubMed] [Google Scholar]
- 27.Baysari MT, Oliver K, Egan B, et al. Audit and feedback of antibiotic use: utilising electronic prescription data. Appl Clin Inform. 2013;4:583–95. doi: 10.4338/ACI-2013-08-RA-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurlimann D, Limacher A, Schabel M, et al. Improvement of antibiotic prescription in outpatient care: a cluster-randomized intervention study using a sentinel surveillance network of physicians. J Antimicrob Chemother. 2015;70:602–8. doi: 10.1093/jac/dku394. [DOI] [PubMed] [Google Scholar]
- 29.Linder JA, Schnipper JL, Tsurikova R, et al. Self-reported familiarity with acute respiratory infection guidelines and antibiotic prescribing in primary care. Int J Qual Health Care. 2010;22:469–75. doi: 10.1093/intqhc/mzq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172:1465–71. doi: 10.1001/archinternmed.2012.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons N, Rankin S, Sarangarm P, et al. Disparity in Patients’ Self-Reported and Charted Medication Allergy Information. South Med J. 2015;108:332–6. doi: 10.14423/SMJ.0000000000000301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 26 kb)