Abstract
Immunotherapy has been highlighted because we have obtained much evidence, which includes theoretical backborn as well as favorable results from clinical trials. As immunotherapy gives an apparently different cytotoxic mechanism and a little adverse event, the promising results are getting a lot of attention. In this article, cancer immunotherapy for gliomas is reviewed thoroughly from the literature, focusing on the clinical trial results.
Keywords: cancer immunotherapy, gliomas, cancer antigen, adoptive immunotherapy, checkpoint inhibitor
Introduction
The concept of cancer immunotherapy against glioma emerged back in 1970s and various basic researches and clinical trials have been conducted worldwide. Recently, the immunooncological approach has been highlighted because we have obtained much evidence, which theoretically supports the efficacy of the therapy. First, cytotoxic T lymphocytes (CTLs) have anti-tumor activity and a lot of cancer antigens recognized by CTLs were identified.1) Second, culture technique of dendritic cells as an antigen-presenting cells were improved and were induced well in vitro.2) Furthermore, inhibitory aspects of immune system such as checkpoint inhibitor molecule have become well studied and known.3) As immunotherapy gives an apparently different cytotoxic mechanism from other therapeutic modalities and a little adverse event, the promising results are getting lot of attention.
Immune System of Central Nervous System
Central nervous system (CNS) has been thought as immunologically tolerant. Blood brain barrier (BBB) inhibits immune cells to enter into CNS, and expression of class I/II of major histocompatibility complex (MHC) molecule is generally sparse. Also cytokines, which inactivate immune cells are produced in CNS. However, activated T cells are recently known to cross BBB and have a specific reaction with intracerebral antigens. The facts support the concept that immunotherapy can be effective in the CNS.4)
Glioma-associated Cancer Antigens
Among cancer antigens, which could be a target of CTLs, many are expressed specific in gliomas. These glioma-associated cancer antigens are classified into (1) cancer-testis (CT) antigens, expressed only in cancer tissue and normal reproductive tissue; (2) tissue-specific antigens, expressed stronger in cancer than in normal tissue; (3) mutated (neo) and overexpressed antigens, originated from cancer specific genetic alteration; (4) oncofetal antigens; and (5) viral antigens. In glioma-associated antigens, MAGE-1 as CT antigen, gp 100, and TRP-2 originally found at melanomas as tissue specific antigens and EGFR variant III (EGFRvIII) as mutated (neo) antigen are well known.5,6) Other glioma-associated antigens include WT1, IL13Ra2, SART-3, etc.
In 2009, a committee in National Cancer Institute (NCI) reviewed 75 human-specific cancer antigens for clinical availability.7) They overviewed the function of antigens, immunogenecity, contribution to oncogenecity, expression specificity, expression in cancer cells and cancer stem cells, frequency of expression, number of epitopes, and cellular location of expression in each antigen. As a result, WT1 and EGFRvIII were ranked as a top of all and was considered to be used in clinical settings.
Immunosuppression by Gliomas and Immune Regulatory Molecules
Glioma cells produce various immunosuppressive factors such as TGF-beta, VEGF, and IL-6. By its secretion, glioma regulates and suppresses local (intra-tumoral) and systemic immune function.4) TGF-beta can inhibit the proliferation of activated CTLs by proliferating regulatory T cells (Treg). VEGF can promote myeloid-derived suppressor cells (MDSCs) possessing immunosuppressive function. VEGF and IL-6 suppress macrophage activity and induce regulatory macrophages, resulting in promotion of oncogenesis.
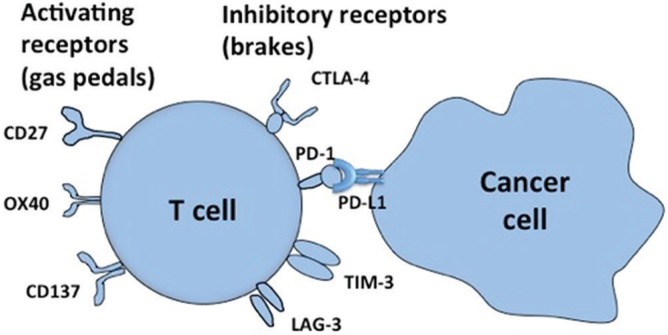
With regard to T cells, there are some immune regulatory molecules including activating receptors such as CD27, OX40, and CD137, which play very important roles for T cell activation, like “gas pedals” in a car (Fig. 1). On the other hand, there are some inhibitory receptors known to function like “brakes” in a car. These molecules have inhibitory effect on T cells and are thought to be a system that suppresses over-immune reactions, through the interaction among antigen presenting cells, activated T cells, and cancer cells. These are called immune checkpoints molecules represented by CTLA-4, PD-1, TIM-3, and LAG-3. For example, a cancer cell can inhibit T cell activation through PD-L1 molecule, which binds to PD-1 molecule on T cell surface. Clinical application of these molecules has just started.3)
Fig. 1 .
Immune regulatory molecules on T cells. Activating receptors such as CD27, OX40, and CD137 play very important roles for T cell activation, like “gas pedals” in a car. Inhibitory receptors, functioning as “brakes” in a car, including CTLA-4, PD-1, TIM-3, and LAG-3 are called immune checkpoints molecules. For example, a cancer cell can inhibit T cell activation through PD-L1 molecule, which binds to PD-1 molecule on T cell surface.
Cancer Immunotherapies for Gliomas
Cancer immunotherapy, represented by recruiting cytokines, antibodies, and activated T cells have been conducted in clinical setting and trials. Until now we do not have rigid evidence that cancer immunotherapy is effective to gliomas, proved by randomized controlled trials (RCTs). So, these are not approved in Japan and not standardized yet. However, the fact that a lot of clinical trials are ongoing, mainly in the United States and in the European countries, forces us to expect that cancer immunotherapy will be the fourth main approach following surgical reduction, radiotherapy, and chemotherapy.
We have actually various modes of action in cancer immunotherapy. One of them is “active immunotherapy” or “vaccination,” in which anti-cancer immune is induced by cancer cell itself or already identified cancer antigens. In this review, the active immunotherapy or vaccination would be mainly introduced as (1) immunotherapy recruiting cancer antigen. Then, other modes of therapy will be discussed as (2) effector cell therapy and (3) checkpoint inhibitors, which inhibit immune-inhibitory function, as mentioned in the former section.
I. Immunotherapy recruiting cancer antigen
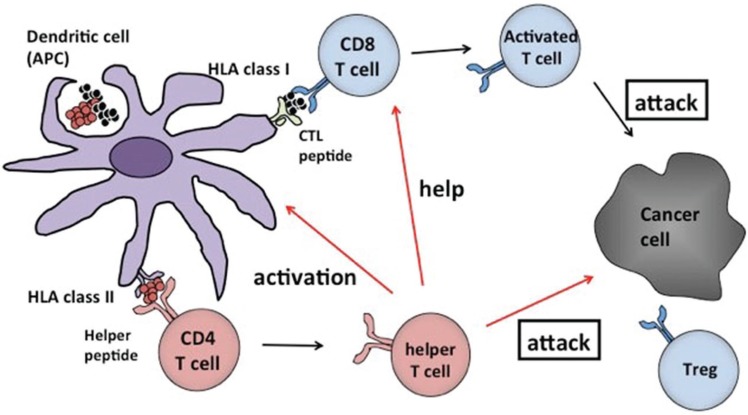
In this mode of therapy, cancer antigens are theoretically the targets of action. Minced cancer tissue itself could be used, but generally the immunogenicity is weak, though there is a merit that multiple cancer antigens are included and targeted. To enhance the immunogenicity, the cancer antigens were modified as “vaccines” and many vaccines are utilized in the clinical trials. A scheme is presented to explain the rationale of cancer antigen based-immunotherapy in Fig. 2.
Fig. 2 .
A diagrammatic representation for immunotherapy recruiting cancer antigens. Cancer antigens given to the patient in any form of peptide, protein, or tumor tissue will be combined with free human leukocyte antigen (HLA) class I molecule on the dendritic cell surface and activate the cells. Activated dendritic cells then move to the nearby lymph nodes where they activate CD8 positive cytotoxic T lymphocytes (CTLs), which can identify cancer antigen/HLA class I molecule complex. Those activated CTLs then recognize the cancer cell surface and have a killing effect (attack). By cancer antigen/HLA class II molecular complex, CD4 positive helper T cells are activated. Then those that help CTLs, activate dendritic cell (antigen presenting cell: APC) and even attack the cancer cells (attack).
1. Immunotherapy with dendritic cells (DCs)
The DCs are used for the purpose that cancer angtigens are effectively presented to immune system. A DC only can present the antigens to naïve T cells and activate them. Usually DCs obtained from the patient’s peripheral blood and induced in culture medium are exposed (pulsed) to cancer antigens in vitro. Antigens are utilized into DC and DC itself would be activated to present the peptide antigens onto MHC molecules. Such activated DCs are administered as vaccine then move to close lymph nodes, where they activate CTLs and induce a cancer-specific immune function. Antigens, which are given as a pulse to DCs, include lysates of tumor tissue and peptides retrieved from tumor tissue. Cancer antigen peptide, which is identified and artificially synthesized are utilized as well.
Clinical studies have been conducted, in which DC vaccines were pulsed with glioma tissue lysate. A phase I/II study proved the safety, but not the efficacy.8)
2. Autologous tumor vaccine
Autologous tumor vaccination is available technically, in which a cell burden retrieved from formalin fixed tumor tissue as well as some adjuvants are administered to the patients. In a clinical study with 12 glioblastoma patients, clinical responses are obtained as complete response (CR) in one patient and as partial response (PR) in one patient. There reported no severe adverse events in the trial.9) The advantage of this mode includes no necessity for culture of tumor cells or DCs, although some amount of autologous tumor tissue is necessary.
3. Peptide vaccination
A lot of cancer antigens, which can be recognized by CTLs, were identified and were utilized as targets of immunotherapy. There is class I peptide restricted (joined) with MHC or human leukocyte antigen (HLA) for human recognized CTLs (CD8 positive T cells), as well as class II peptide recognized by helper T cells (CD4 positive T cells), restricted with MHC or HLA.
Theoretically, in immunotherapy, recruiting cancer antigens can be administered as a form of peptide, protein, and gene. Also these are usually given with some adjuvant, which can activate immune system. Although various peptides joined with different types of HLA it should be identified according to HLA restriction. Nowadays in most of the clinical trials synthesized peptide vaccine is widely utilized; these are easily synthesized, cheap, and quality controlled.
A. EGFRvIII peptide vaccine
In a part of patients with glioblastomas, EGFRvIII is recognized in tumor cells. Single arm phase I/II studies with EGFRvIII peptide vaccine revealed the safety and efficacy. Adjuvants were also screened and selected. In subsequently performed multi-institutional phase II study recruiting 21 newly diagnosed glioblastoma patients, EGFRvIII vaccine was administered with keyhole limpet hemocyanin (KLH) adjuvant, just after the initial combined radiation and temozolomide therapy.10) The prolonged progression free survival (PFS) of 14.2 months and overall survival (OS) of 26.0 months in vaccination arm was confirmed statistically as compared with control arm.11
B. WT1 peptide vaccination
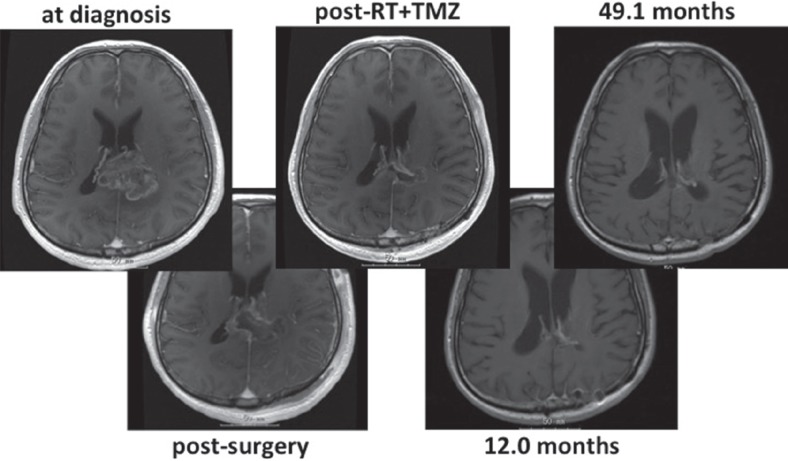
WT1 was originally found as a tumor suppressor gene, but nowadays it is widely recognized as an oncogene, as well as cancer antigen expressed in many cancers.12) A committee formerly described in NCI ranked it as a top of cancer antigen, the most promising cancer antigen for clinical use. Many types of WT1 peptide vaccination were invented and clinical phase II study revealed the safety and efficacy against recurrent glioblastomas. In a study with 21 patients, clinical response is obtained as PR in 2 patients and stable disease (SD) in 10 patients. Median PFS was 20 weeks from recurrence.13) Subsequently conducted phase I study of combined temozolomide and its vaccination revealed the safety and immunological response in seven newly-diagnosed glioblastoma patients.14) An illustrative case with combined temozolomide and WT1 vaccination is shown in Fig. 3, presenting OS over 4 years from initial diagnosis.
Fig. 3 .
Sequential magnetic resonance imagings (MRIs) of right-handed male, with left parietal glioblastoma. He underwent surgery and received radiation (RT)/temozolomide (TMZ) (Stupp regimen), and immediately went to phase I study of combined TMZ and WT1 vaccination. MRI appearance remains on complete response at 49.1 months after 24 courses of maintenance TMZ and 89 times of WT1 vaccination.
C. ITK-1 cocktail vaccine
Generally one cancer antigen can only show limited immunogenicity and multiple cocktail peptides in the tailor-made fashion was proposed. Phase I trial of ITK-1 vaccine, in which among 14 different peptides of cancer antigens, 4 was selected in each patient and injected to 12 patients with recurrent glioblastoma. Clinical response is obtained as PR in one patient and SD in seven patients. Median PFS was 10.6 months.15)
II. Effector cell therapy
A profound investigation on T cell as a final effector cell to cancer cells improved T cell culture method and lead some new modes of immunotherapy, such as adoptive immunotherapy with T cell. This therapy includes T cell transfusion using tumor infiltrating lymphocytes (TILs),16) engineered T cell therapy with T cell receptor (TCR) induction17,18) and chimeric antigen receptor (CAR) gene-induced T cell therapy.19) Clinical trial specifically targeting gliomas was not yet reported, but clinical trials on other cancers give some hint for the application to gliomas.
III. Checkpoint inhibitors
Cancer cells can induce immuno-inhibitory circumstance using checkpoint molecules and can escape from the attack of immune cells. Inhibition or regulation of such checkpoint molecules is achieved by using checkpoint inhibitor molecules, resulting in more effective attack of immune cells.3) CTLA-4, PD-1, and PD-L1 have been utilized in clinical trials in other cancers and some trials targeting gliomas are ongoing. A concern on the autoimmune activity exists when using checkpoint inhibitors. There are some reports on such autoimmune-like adverse events in clinical use of anti-CTLA-4 antibody (ipilimumab).20,21)
Current Concerns and Future Directions
Immunotherapy has been known to cause pseudoprogression. Many patients in early clinical trials dropped out because of this phenomenon.22) In addition, in immunotherapy, we cannot expect a quick response experienced in chemoradiotherapy in image-based and clinical assessments. Various assessment criteria having used in clinical trials such as RECIST, Macdonald, and RANO22–24) are considered not suitable for the evaluation. Immune-related response criteria (irRC)25) have been created for other types of cancers. Recently, immuno RANO (iRANO) was proposed by a committee in Society for Neuro-oncology,26) which exhibit a kind of combination of RANO and irRC. The use of iRANO in future clinical trials would reveal some future directions for response assessments in immunotherapy for gliomas.
For the same reason as mentioned above, the discussion for statistical issues exists; clinical trial design should be specified in immunotherapy. Other immunological issue to be addressed includes establishment of immunological monitoring and biomarkers. Disclosure of stronger adjuvants is also expected.
Because we know that one mode of action of immunotherapy is not satisfactory to kill out the gliomas, combined approach with different modes of immunotherapy as well as other cytotoxic therapies would be ideal. It is the author’s impression that the on-going clinical trials with checkpoint inhibitors may yield some satisfactory results. Then, thinking of combined checkpoint inhibitors and other immunotherapy, trials recruiting autologous tumor vaccination, or peptide vaccination would be rather realistic. Autologous tumor vaccination is not restricted for HLA as an advantage, but is somewhat complex to make it from the tumor tissue and it costs a lot. Although peptide vaccination has a restriction of HLA as a disadvantage, it can be easily synthesized, cheap, and quality controlled. At this time, we cannot conclude which is better as a counterpart of checkpoint inhibitors. It is the author’s opinion that peptide vaccination would be acceptable to patients because it can be a “medical drug” after clinical trials like other drugs purchased and used worldwide.
In this article, cancer immunotherapy for gliomas is reviewed thoroughly from the literature and self-experience. Clinical trials, especially of the combined immunotherapy, are now warranted.
Acknowledgments
The author deeply thanks Professor Haruo Sugiyama, Department of Functional Diagnostics, Osaka University Graduate School of Medicine, and Professor Toshiki Yoshimine, Department of Neurosurgery, Osaka University Graduate School of Medicine, for their kind supervision regarding the author’s immuno- and neuro-oncological work.
References
- 1). Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F: Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960– 1964, 2006. [DOI] [PubMed] [Google Scholar]
- 2). Steinman RM, Banchereau J: Taking dendritic cells into medicine. Nature 449: 419– 426, 2007. [DOI] [PubMed] [Google Scholar]
- 3). Schreiber RD, Old LJ, Smyth MJ: Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331: 1565– 1570, 2011. [DOI] [PubMed] [Google Scholar]
- 4). Chow KH, Gottschalk S: Cellular immunotherapy for high-grade glioma. Immunotherapy 3: 423– 434, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Toda M: Glioma antigen. Adv Exp Med Biol 746: 77– 84, 2012. [DOI] [PubMed] [Google Scholar]
- 6). Oji Y, Suzuki T, Nakano Y, Maruno M, Nakatsuka S, Jomgeow T, Abeno S, Tatsumi N, Yokota A, Aoyagi S, Nakazawa T, Ito K, Kanato K, Shirakata T, Nishida S, Hosen N, Kawakami M, Tsuboi A, Oka Y, Aozasa K, Yoshimine T, Sugiyama H: Overexpression of the Wilms’ tumor gene W T1 in primary astrocytic tumors. Cancer Sci 95: 822– 827, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM: The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15: 5323– 5337, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ: Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 64: 4973– 4979, 2004. [DOI] [PubMed] [Google Scholar]
- 9). Ishikawa E, Tsuboi K, Yamamoto T, Muroi A, Takano S, Enomoto T, Matsumura A, Ohno T: Clinical trial of autologous formalin-fixed tumor vaccine for glioblastoma multiforme patients. Cancer Sci 98: 1226– 1233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, for the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 10; 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 11). Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD: Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28: 4722– 4729, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Sugiyama H: Wilms’ tumor gene WT1: its oncogenic function and clinical application. Int J Hematol 73: 177– 187, 2001. [DOI] [PubMed] [Google Scholar]
- 13). Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, Kagawa N, Hashimoto N, Maruno M, Elisseeva OA, Shirakata T, Kawakami M, Oji Y, Nishida S, Ohno S, Kawase I, Hatazawa J, Nakatsuka S, Aozasa K, Morita S, Sakamoto J, Sugiyama H, Yoshimine T: Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 108: 963– 971, 2008. [DOI] [PubMed] [Google Scholar]
- 14). Hashimoto N, Tsuboi A, Kagawa N, Chiba Y, Izumoto S, Kinoshita M, Kijima N, Oka Y, Morimoto S, Nakajima H, Morita S, Sakamoto J, Nishida S, Hosen N, Oji Y, Arita N, Yoshimine T, Sugiyama H: Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: safety and impact on immunological response. Cancer Immunol Immunother 64: 707– 716, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, Sawamura Y, Kurisu K, Mineta T, Yamada A, Itoh K: Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen—A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 29: 337– 344, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Restifo NP, Dudley ME, Rosenberg SA: Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12: 269– 281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP: Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 6: 383– 393, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA: Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314: 126– 129, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Sadelain M, Brentjens R, Rivière I: The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol 21: 215– 223, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Gaudy-Marqueste C, Monestier S, Franques J, Cantais E, Richard MA, Grob JJ: A severe case of ipilimumab-induced guillain-barré syndrome revealed by an occlusive enteric neuropathy: a differential diagnosis for ipilimumab-induced colitis. J Immunother 36: 77– 78, 2013. [DOI] [PubMed] [Google Scholar]
- 21). Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 19; 363: 711– 723, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM: Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28: 1963– 1972, 2010. [DOI] [PubMed] [Google Scholar]
- 23). Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205– 216, 2000. [DOI] [PubMed] [Google Scholar]
- 24). Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277– 1280, 1990. [DOI] [PubMed] [Google Scholar]
- 25). Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS: Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15: 7412– 7420, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA: Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16: e534– e542, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]