Abstract
Cerebrospinal fluid (CSF) plays an essential role in maintaining the homeostasis of the central nervous system. The functions of CSF include: (1) buoyancy of the brain, spinal cord, and nerves; (2) volume adjustment in the cranial cavity; (3) nutrient transport; (4) protein or peptide transport; (5) brain volume regulation through osmoregulation; (6) buffering effect against external forces; (7) signal transduction; (8) drug transport; (9) immune system control; (10) elimination of metabolites and unnecessary substances; and finally (11) cooling of heat generated by neural activity. For CSF to fully mediate these functions, fluid-like movement in the ventricles and subarachnoid space is necessary. Furthermore, the relationship between the behaviors of CSF and interstitial fluid in the brain and spinal cord is important. In this review, we will present classical studies on CSF circulation from its discovery over 2,000 years ago, and will subsequently introduce functions that were recently discovered such as CSF production and absorption, water molecule movement in the interstitial space, exchange between interstitial fluid and CSF, and drainage of CSF and interstitial fluid into both the venous and the lymphatic systems. Finally, we will summarize future challenges in research. This review includes articles published up to February 2016.
Keywords: cerebrospinal fluid, interstitial fluid, Virchow-Robin space, lymphatic drainage, aquaporin
History of CSF Discovery
Edwin Smith’s surgical papyrus is a medical document from around the 17th century BC and is well known for its description of cerebrospinal fluid (CSF). It describes 48 cases, and the sixth case refers to a patient with a head injury due to a fight. This particular case contains a description of a comminuted skull fracture followed by an explanation of fluid outflow from a tear in the membrane (dura mater) that covers the occipital region of the brain. This portrayal of fluid flowing out from the occipital region is considered to be the first description of CSF. Later, great Greek scholars such as Hippocrates and Herophilus studied the structure of the brain, although direct descriptions of CSF are not found. Furthermore, although great scholars such as Herophilus, Galen, da Vinci, Vesalius, Varolius, Vieussens, Ruysch, Pacchioni, Monro, Sylvius, and Luschka presented anatomical findings of the ventricles and subarachnoid space, the existence of CSF was not known for some time. This is postulated to be because attention was not focused on the presence of fluid that fills the ventricles and subarachnoid space and because CSF had most likely leaked out along with blood when cervical decapitation was performed during autopsy or dissection.1) For detailed descriptions of these historical accounts of CSF, starting with Edwin Smith’s surgical papyrus, refer to the textbook by Deisenhammer.2)
Subsequently, Cotugno,3) Swedenborg,4) and vonHaller5) described CSF in a systematic manner (Fig. 1). Cotugno, an Italian anatomist from Naples, observed the presence of water (“liquor cotunnii”) around the ventricles and the spinal cord by conducting 20 autopsies. Another notable observation he made was that the brain decreases in size and the relative volume of water increases with increasing age. These anatomical findings were summarized in “De Ischiade Nervosa Commentarius,” which was published in Latin in 1764 in Naples3) and in English in 1775 in London.1,6,7) Many researchers consider the discovery of CSF to be the work of Cotugno. However, it should be noted that Cotugno himself gave credit to von Haller, who we introduce next, for the discovery of CSF.2) von Haller was a Swiss physiologist, who presented a revolutionary article in 1747 that described that CSF is secreted within the ventricles and that CSF is absorbed by the veins. Swedenborg, who had a unique career, graduated from the University of Uppsala and received a degree in mining engineering. After working in the coal mines, he pursued the field of anatomy in Germany, France, and Italy from 1736 to 1740, although the precise years are unknown. Swedenborg drafted his work from this time period between 1741 and 1744; however, because he was a mining engineer, he was not able to meet a medical publisher, and his manuscript was abandoned for 150 years until its discovery in Stockholm. In 1882, it was finally published as “The Brain: Considered Anatomically, Physiologically and Philosophically” (translated and edited by RL Tafel) in London.4,8) Since the first academic description of CSF by these scientists, many researchers have studied the physiology of CSF within the central nervous system. The first appearance of the term “cerebrospinal fluid” in published literature is “Le liquid cérébro-spinal” in a French document written by Magendie in 18429) (Fig. 2). This term was used to describe fluid in the ventricles and subarachnoid space and has been translated into various languages as “cerebrospinal fluid,” which has subsequently become established as a medical term.
Fig. 1 .
a: Albrecht von Haller (1708–1777) is a Swiss anatomist and physiologist. His book, Primae lineae physiologiae in usum praelectionum academicarum. Gottingae: A. Vandenhoeck (1747), is shown. Von Haller, who was given credit for the discovery of cerebrospinal fluid (CSF) by Domenico Cotugno, stated interesting anatomical findings including the observation that the consistency of CSF increases after death (Public domain). b: Domenico Felice Antonio Cotugno (1736–1822) is a Neapolitan anatomist. His book, De ischiade nervosa commentarius. Viennae: Apud Rudolphum Gräffer (1770), is shown. Cotugno reported that liquid is present and air bubbles are absent at the meninges when it is incised and opened carefully. Therefore, Cotugno postulated that the presence of CSF at the spinal cord and brain surface may have been overlooked with the conventional cervical decapitation method.1) Furthermore, Cotugno also confirmed the outflow of liquid when a drain is placed in the lumbar sac of a cadaver in the standing position. A notable aspect of the work by Cotugno is that he proved the presence of CSF in both the cranial cavity and the spinal cavity (Public domain). c: Emanuel Swedenborg (1688–1772) is an anatomist with a degree in mining engineering. Swedenborg described CSF using terms such as “spirituous lymph” and “highly gifted juice.” His book, The Cerebrum and Its Parts. London: James Speirs (1882), is shown (Public domain).
Fig. 2 .
The front cover ( left ) and part of page 8 ( right ) of “liquide céphalo-rachidien ou cérébro-spinal” described by François Jean Magendie owned by Kyoto University Library are shown. The foramen of Magendie that exists at the exit of the fourth ventricle is named after this scientist, who is well known for demonstrating the connection between the ventricular system and subarachnoid space. However, he is also famous for using the term “Le liquid cérébro-spinal” for the first time ( red underlined portion in right figure). Photos reprinted with the permission of Kyoto University Library.
Short summary
Edwin Smith’s surgical papyrus is the first description of fluid surrounding the brain that is thought to be CSF.
von Haller first described the existence of CSF systematically.
The term “cerebrospinal fluid” first appears in a document written by Magendie.
CSF Is Also Produced by Structures Other than the Choroid Plexus
The discovery of the choroid plexus by Galen and Vesalius10,11) naturally led to investigations of the choroid plexus as the production site of CSF, because choroid plexus protrusion is observed mostly at the ventricles, and some protrudes from the fourth ventricle into the subarachnoid space at the foramen of Luschka. Willis demonstrated that this choroid plexus displays a glandular structure,12) and Davson et al. concluded that this structure is ideal for CSF production.13) Later, Dandy et al. demonstrated in dogs that ventricular dilatation does not occur when the foramen of Monro is blocked and when the choroid plexus is excised from the lateral ventricle, but dilatation is observed on the side in which the choroid plexus is preserved, demonstrating that the choroid plexus is the site of CSF production.14,15) However, Hassin et al. contradicted this experiment conducted by Dandy et al. and stated from an early stage that CSF is produced by structures other than the choroid plexus.16) It is also important to note that Hassin considered that an intimate exchange between CSF and interstitial fluid occurs and that this plays an essential role in maintaining the homeostasis of the central nervous system17–19) (Footnote 1).
Next, we will introduce the mechanism of CSF production in the choroid plexus. de Rougemont et al. and Ames et al. measured the electrolytes in the fluid secreted by the choroid plexus and compared these values with the serum electrolyte concentration to examine the production of CSF.20,21) Investigations of the microstructure of the choroid plexus led to a variety of conclusions such as CSF is passively produced via hydrostatic pressure,22) CSF production is dependent on gradients of osmotic and hydrostatic pressures,23) and CSF is actively produced independent of hydrostatic pressure or colloid osmotic pressure.24) There is a well-known study by Welch regarding the amount of CSF production in the choroid plexus in which the author measured the hematocrit in the artery that drains into the choroid plexus and in the vein that drains out from the choroid plexus in an animal experiment with the goal of measuring the amount of CSF production from choroid plexus blood flow volume.25) Eventually, for measuring the amount of CSF production, methods such as extracorporeal perfusion of the choroid plexus26,27) and ventriculo-cisternal perfusion developed by the Pappenheimer group that can measure CSF production and absorption at the cerebrospinal cavity28,29) became widespread as standard techniques.23,30,31) In recent years, it has become feasible to observe the state of the choroid plexus, which changes morphologically along with the heartbeat, by inserting a micro-video probe into the lateral ventricle, and this method has been attracting attention as a novel research technique.32)
Milhorat, famous for his studies on the choroid plexus,33) had frequently questioned the view that the choroid plexus is the sole place in which CSF is produced based on the choroid plexus excision experiments he carried out in animals.34,35) Furthermore, when examining the overall amount of CSF produced in the ventricles and subarachnoid space, 58.5% of CSF is produced in the extra-ventricular CSF space in dogs,30) and 33% of CSF is produced at non-choroid plexus structures in rabbits.36) This led to the recognition that a considerable amount of CSF is produced outside the choroid plexus, and the focus on CSF production eventually shifted to non-choroid plexus sites.35,37) Hammock et al. reported that CSF production and composition do not change when the choroid plexus is excised from humans and monkeys,38) and Tamburrini et al. reported an insufficient decrease in CSF production with endoscopic bilateral choroid plexus cauterization.39) Milhorat,34) who was already skeptical that the choroid plexus is the sole site of CSF production, conducted a bilateral plexectomy in a 5-year-old child and found that CSF formation did not change after 5 years.40,41) Of course, removal of the choroid plexus is incomplete with plexectomy of the lateral ventricles alone because the choroid plexus in the third and fourth ventricles still remains. However, it has been shown clinically that eliminating the majority of choroid plexus function does not cure hydrocephalus; thus, this procedure is not established as a standard therapeutic method.39) The over-production of CSF has been detected in hyperplasia of the choroid plexus and in some choroid plexus papillomas, and it is therefore necessary to specify that, without a doubt, excessive CSF production certainly takes place at the choroid plexus.42,43)
We will now focus on the lateral and third ventricles where the majority of the choroid plexus is present. If CSF produced by the choroid plexus is not absorbed within the ventricles, then all CSF produced anatomically should flow out from the Sylvian aqueduct. To test this hypothesis, Orešković et al. placed a cannula in the Sylvian aqueduct of cats to observe CSF outflow.44) The authors monitored potential CSF outflow from the cannula inserted into the Sylvian aqueduct for a long period of time, but not a single drop of CSF emerged from the cannula.44) They consequently concluded that a balance of CSF production and absorption is maintained in the lateral and third ventricles.44) While this article by Orešković et al. is important, it does not provide the mechanism for the onset of obstructive hydrocephalus that clinicians frequently experience, indicating that their conclusion is still debatable.
We will now introduce several articles that explored non-choroid plexus structures as sites of CSF production. Some articles sought out the brain itself as the site of CSF production,45,46) whereas others claimed CSF production from the cerebral superficial subarachnoid space,37) the perivascular system,47) or the pial artery.48) Moreover, a study suggested its production by the spinal cord,49) and another has shown the presence of ependymal fluid secretion from the ependyma of the spinal cord central canal.50) Based on these reports, it is difficult at the present time to claim the choroid plexus as the sole source of CSF production. In addition, the ventriculo-cisternal perfusion method that spread due to the report by Pappenheimer et al. which was consequently utilized in studies investigating CSF production28) has been questioned in cat experiments by Maraković et al.51) as there are limitations in performing invasive procedures in animals to conduct CSF studies. Moreover, this method is invasive in humans, indicating the difficulty with its application in humans. In the future, studies using different methods are desired.
Short summary
The classical concept in which the choroid plexus is the sole production site of CSF has changed due to recent studies that have demonstrated the existence of other non-choroid plexus sites that produce CSF.
The ventriculo-cisternal perfusion method that became widespread due to the report by Pappenheimer et al. is being questioned at the present time for its interpretation.
In obstructive hydrocephalus, the ventricles dilate above the obstruction site. However, if the balance between CSF production and absorption is maintained within the ventricles, then obstructive hydrocephalus does not explain the mechanism of ventricle dilatation.
CSF Is Also Absorbed by Structures Other than Arachnoid Granulation Or villi
In the rather unique article by Milhorat et al., CSF absorption by the choroid plexus, which is normally considered to be the CSF production site, was suggested in eight patients with hydrocephalus.52) However, Wislocki et al. experimentally disproved CSF absorption by the choroid plexus,53) and additional experiments regarding CSF absorption by the choroid plexus were therefore not conducted.
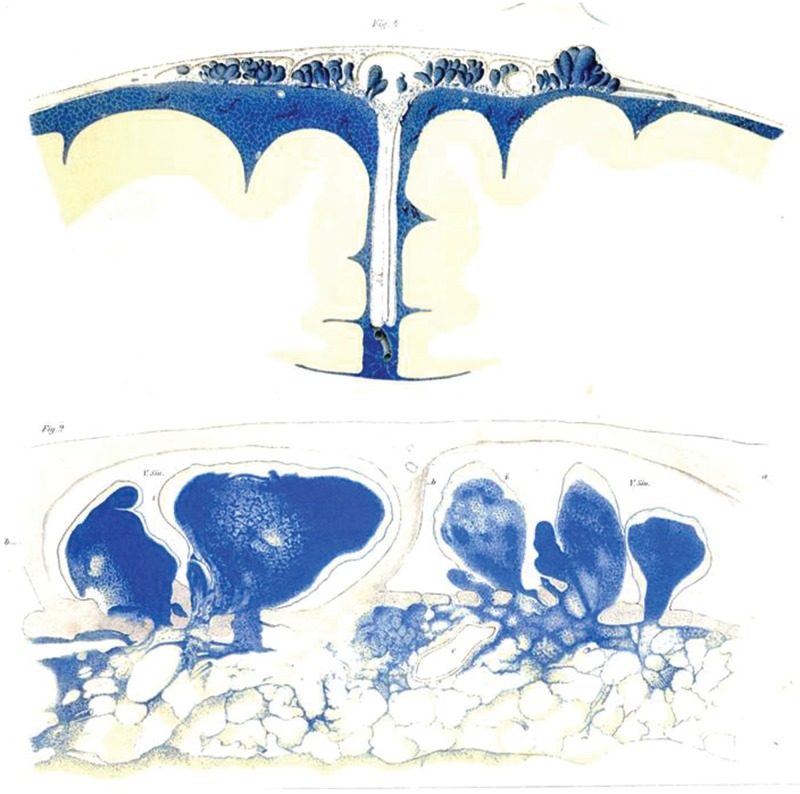
Key et al. published an extremely famous article in 1875 showing that CSF is absorbed by the arachnoid granulation or villi (Figs. 3, 4).54) Many classic textbooks cited this particular article, and for a long time thereafter it was believed that the arachnoid granulation or villi absorbs CSF.55–59) Certainly, the unique shape of the arachnoid villus in which it protrudes into the superior sagittal sinus from the subarachnoid space facilitates its interpretation as a pathway where CSF in the cerebral superficial subarachnoid space is absorbed by the venous system.60) However, there have been objections against the work by Key et al. because the pressure in which colored gelatin with soluble Berlin blue was injected into the subarachnoid space of a cadaver was high at 60 mmHg, and changes in arachnoid villus morphology may have occurred because the dye was injected in a non-physiological condition.61) Later, using radioisotopes (RIs) in an animal experiment, Davson et al. explained the circulation of CSF from the lateral ventricle to the arachnoid granulation or villi.62) With regard to CSF absorption from the arachnoid villi, this process has been demonstrated through an association between CSF pressure and venous sinus pressure63) and through light microscopic and electron microscopic observations.47,60,64–67) However, there are reports regarding the arachnoid granulation or villus, which protrudes into the lateral lacunae of the superior sagittal sinus, suggesting that open channels of communication are present between the CSF and blood through the arachnoid villi,68) that CSF communication occurs through its extracellular space,60,66) that CSF passes through the arachnoid villi via a one-way valve mechanism,69) and that the arachnoid villi are covered by endothelium and do not allow free fluid passage,70) indicating that a histological consensus has yet to be attained. It is important to note here that the human arachnoid granulation or villus is more developed compared to the rat arachnoid granulation or villus71) and that interspecies differences should be taken into consideration when interpreting the experimental results.
Fig. 3 .
The front cover of Key A, Retzius G. Studien in der anatomie des nervensystems und des bindegewebes. Stockholm: Norstedt & Söner (1875) owned by Niigata University Library is shown. This article is always referenced when cerebrospinal fluid (CSF) absorption from the arachnoid granulation or villi is described. The detailed illustrations of the cerebral superficial subarachnoid space and Virchow-Robin space are key characteristics of this article. There are illustrations in which CSF eliminated from the subarachnoid space is present in the retro-orbital tissue and cervical lymphatic system, and there are also descriptions of anatomical findings that are widely suggestive of CSF absorption. Images of the copies are taken with the permission of Niigata University Library.
Fig. 4 .
Tafel XXIX Figure 4 ( upper panel ) and Tafel XXVIII Figure 2 ( lower panel ) of the first volume of “Studien in der anatomie des nervensystems und des bindegewebes. Stockholm: Norstedt & Söner (1875) by Key A, Retzius G. owned by Niigata University Library are shown. Detailed figures of the arachnoid villi that protrude from the subarachnoid space are depicted. Images of copies are taken with the permission of Niigata University Library.
We will now shift our focus to the histological investigations that were conducted to study the human arachnoid villi. An interesting observation from studies in humans that investigated arachnoid granulation modifications that occur along with development is that although some researchers oppose this idea,72) the arachnoid granulation does not exist during prenatal and neonatal periods.73,74) If this was true, it is necessary to ascertain a CSF absorption route other than the arachnoid granulation that exists around the venous sinus during the fetal to neonatal stages.75) Aside from the venous system absorption described later, lymphatic drainage is considered to function earlier than the arachnoid granulation.76)
With regard to absorption from structures other than the arachnoid villi or granulation, there is indirect evidence from magnetic resonance imaging (MRI) that during childhood CSF is absorbed from capillaries due to hydrostatic pressure.77) On an experimental level, Bowsher administered radioactive feline serum protein into the CSF space and found that CSF migrates not only via the arachnoid granulation, but also to the pia mater capillaries in the brain and spinal cord.78)
Regarding CSF absorption at sites other than the areas around the venous sinuses, it has been reported that the compositions of aqueous humor and CSF closely resemble each other79) and that the perineural olfactory sheath is an important site of CSF outflow.80,81) Some have also reported that the areas around the optic nerve and retro-orbital tissue are involved in the absorption of CSF.82–85) It is interesting that the article by Key et al. that drew attention for describing CSF absorption by the arachnoid villi also describes the absorption of CSF from these retro-orbital tissues.54) In addition, Manzo et al. demonstrated in rabbit experiments that CSF is absorbed by the inner ear.86)
We will now discuss the absorption of CSF by the spinal canal. There are reports that discussed CSF absorption by the spinal canal49,87–89) and by the spinal nerve root.90,91) There are also reports that stated the presence of the arachnoid villi or granulation in the vicinity of the spinal nerve root92,93) and suggested that CSF is absorbed here.87) Moreover, fluid movement in the perivascular space (Virchow-Robin space) of the spinal cord94) has been demonstrated. Pollay stated that the spinal arachnoid granulation plays an auxiliary role when the intracranial arachnoid granulation becomes non-functional,76) and Weed reported that CSF absorption from the cranial subarachnoid space is faster than that from the spinal subarachnoid space.95) The above reports primarily explored the migration of CSF into the venous blood.
If the arachnoid villi, which are located at the convexity in the parietal region as a continuation of arachnoid tissue, have minimal involvement in CSF absorption, then it would be understood as a simple anatomical structure that is suspended in the brain in the perpendicular direction. The role of the arachnoid villi in CSF absorption may be minor, although there is an important article by Kida et al. who stated that the arachnoid villi are responsible for CSF absorption in a state of increased intracranial pressure.96) In addition, the discovery of red blood cells in the arachnoid granulation channels after subarachnoid hemorrhage confirms a role for these channels in CSF outflow in adults.97,98) Thus, the association between CSF absorption and the arachnoid villi cannot be completely rejected.
Next, we will shift our focus to the association between CSF and the lymphatic system. The article by Key et al., which proved absorption by the arachnoid granulation or villi and CSF migration to retro-orbital tissues, also appears here, as it clearly demonstrated the migration of dye injected into the subarachnoid space to the cervical lymph node.54) Schwalbe injected Berlin blue into the dog subarachnoid space and found that the lymphatic system is an important pathway that absorbs CSF.99) Love et al. reported that the lymph flow increases and the protein concentration in the lymph decreases when artificial CSF is injected into the cisterna magna,100) and Hasuo et al. reported that the lymph flow increases when the intracranial pressure rises,101) indicating an indirect association between lymph and CSF absorption. Bradbury et al. injected 125I- or 131I-albumin into the ventricles and caudate nucleus and found that there are drainage pathways into the cervical lymphatic vessels from the Virchow-Robin space and perivascular spaces through the subarachnoid space and also from the olfactory lobe through the submucous space of the nasal cavity.102–104) McComb et al. used 125I-albumin in cats to show that CSF drains into the lymphatic system both under normal pressure and augmented intracranial pressure conditions.105) Furthermore, Mortensen and Sullivan visually portrayed in a dog experiment that a contrast agent in the CSF migrates to the cervical lymphatic vessels.106) Mathieu et al. reported non-invasive in vivo hyperspectral imaging to identify the CSF lymphatic drainage system.107) Later, many reports proved the migration of CSF into the lymphatic system through experiments using dye, contrast agents, and RI. A pathway in which CSF re-appears in the lymphatic system is unlikely once it migrates from the arachnoid granulation or villi to the venous system. For this reason, a pathway that has been attracting attention in recent years is one in which CSF reaches the nasal mucosa from the cribriform plate through tissue around the olfactory nerve and subsequently migrates to the cervical lymph node.108) Recently, Johnston et al. injected microfil into the cisterna magna of sheep, pigs, rabbits, rats, mice, and human cadavers and demonstrated that the microfil migrates to the olfactory bulb and cribriform plate in all these mammals.109) Moreover, Di Chiro et al., who studied CSF behavior by administering an RI tracer into the CSF space,110) injected gadolinium contrast agent into the cisterna magna of dogs and found on MRI that the contrast agent aggregates in the nasal mucosa.111) Furthermore, an animal experiment, albeit a paradoxical one, has demonstrated that the prelymphatic space expands and cerebral edema develops when the cervical lymphatic vessels are ligated in cats and rabbits.112) Using an analytical modeling approach, Fard et al. showed that CSF is primarily absorbed by the lymphatic system and that impairment in the lymphatic system induces high-pressure hydrocephalus, emphasizing the importance of the lymphatic system as an absorption route of CSF.113) In addition, a study mentioned the presence of a link between the spinal cord subarachnoid space and the lymphatic system.114) Although CSF migration has been investigated carefully in other cranial nerves, it appears that except for the olfactory nerve, these nerves have minimal involvement.76)
CSF movement in the subarachnoid space and ventricles, which are the CSF reservoirs, has been considered to follow a pathway in which CSF produced by the choroid plexus descends the ventricular system of the brain and drains into the subarachnoid space via the fourth ventricle exit, ultimately reaching the arachnoid granulation or villi for absorption. However, recent research progress has illuminated different views. Greitz modified the traditional interpretation of radionuclide cisternography and negated the bulk flow theory, which states that CSF ultimately reaches the arachnoid villi for absorption.28,115) He furthermore concluded that CSF is mixed through pulsatile flow, is diluted with newly secreted CSF from the ventricular system, and is ultimately absorbed by the blood vessels. Recently published MRI analyses of CSF movement also refuted the unidirectionality of CSF and demonstrated a repetition of stirring and spreading or oscillating within reservoirs such as the ventricles and subarachnoid space.116–127)
In other words, several different views regarding the absorption of CSF were published. These included: the opinion that lymphatic drainage is the primary pathway for CSF absorption;128) an explanation in a review by Pollay that both the arachnoid granulation or villi and lymphatic system are involved in CSF absorption with a comparable balance;76) the observation that 40–48% of the CSF in the cranial compartment drains into the extracranial lymphatics according to experiments in sheep conducted by Boulton et al.;129) an explanation that the arachnoid granulation or villi play an essential role in CSF absorption and that the lymphatic system is an accessory pathway that supplements the arachnoid granulation or villi according to a review by Weed;95) and a view by Courtice et al. that very little CSF drains into the lymphatic system in experimental rabbits.130) However, Grzybowski et al. proved that fluid passage is observed when harvested human arachnoid villi are perfused and subsequently examined morphologically with electron microscopy,64) and furthermore, Welch et al. conducted a perfusion experiment of monkey arachnoid villi and concluded that substances smaller than erythrocytes can pass through the arachnoid villi.131) Thus, the results from these experiments do not completely disprove CSF absorption by the arachnoid villi. In animal experiments, it appears that the ratio of arachnoid granulation or villi to lymphatic routes for CSF absorption differs depending on the experimental method and species used, and a consensus has yet to be reached.
The next question is: is the nasal mucosa the only route in which CSF and interstitial fluid reach the cervical lymph node? The lymphatic system is present in every part of the body and plays an essential role in transporting immune response substances.132–134) For many years it was considered that the brain does not contain lymphatic vessels and that it is a special organ that is isolated from the systemic lymphatic system. Because CSF and interstitial fluid travel freely, it was considered that interstitial fluid and CSF, rather than lymphatic vessels, played a similar role as lymph to transport immune response substances in the brain. Although the nasal mucosa pathway is a well-known route in which CSF drains into the lymphatic system, recently, the presence of the lymphatic system was discovered in the dura mater and has been attracting interest. In 2015, while the Kipnis group was exploring pathways in which T cells circulate within the central nervous system, they discovered an area where immune cells aggregated around the venous sinus in the dura mater of mice, and conducted an analysis and provided an explanation using markers such as lymphatic endothelial markers.135) They subsequently named these luminal structures “meningeal lymphatic vessels” and demonstrated that these vessels connect to the cervical lymphatic system. Interestingly, they also noted that a similar tissue is found around the venous sinus of the dura mater in humans and that additional studies are necessary.135) The existence of lymphatic drainage has been postulated for some time, and an article by Kida et al. in 1993 described that the “CSF drains directly from the subarachnoid space into nasal lymphatics in the rat” and presented a figure that depicts “dural lymphatics” together with the olfactory pathway, which is worth noting (in Fig. 5: “Anatomy, histology and immunological significance”).128) Surprisingly, according to a report from Bucchieri et al.,136) the presence of lymphatics in human dura mater had already been described by Mascagni (1787) in “Vasorum lymphaticorum corporis humani historia et ichnographia.”137) However, because the dura mater arises to direct and guide the skull, some may consider it no wonder if the lymphatic system exists in the dura mater. Louveau et al. injected fluorescent tracer dye into the ventricles and demonstrated its appearance in the meningeal lymphatic vessels.135) Likewise, in 2015, Aspelund et al. discovered a similar structure in mice with findings that dural lymphatic vessels are found extensively at the base of the skull and penetrate the base of the skull along with the cranial nerves, and that tracers administered into the brain parenchyma exit into the dural lymphatic vessels.138) Thus, it is suggested that CSF reaches the meningeal lymphatic vessels through some sort of pathway; however, the precise mechanism of this pathway is yet unknown. Perhaps, because meningeal lymphatic vessels exist near the venous sinus, an anastomosis of the venous and lymphatic systems mediates this pathway. Or, perhaps CSF drainage into the lymphatic system is present around the nerve foramina at the base of the skull, similar to the cribriform plate. Although it is a fact that lymphatic vessels are absent in the brain parenchyma, additional studies regarding meningeal lymphatic vessels and the drainage of CSF and interstitial fluid are anticipated.
Fig. 5 .
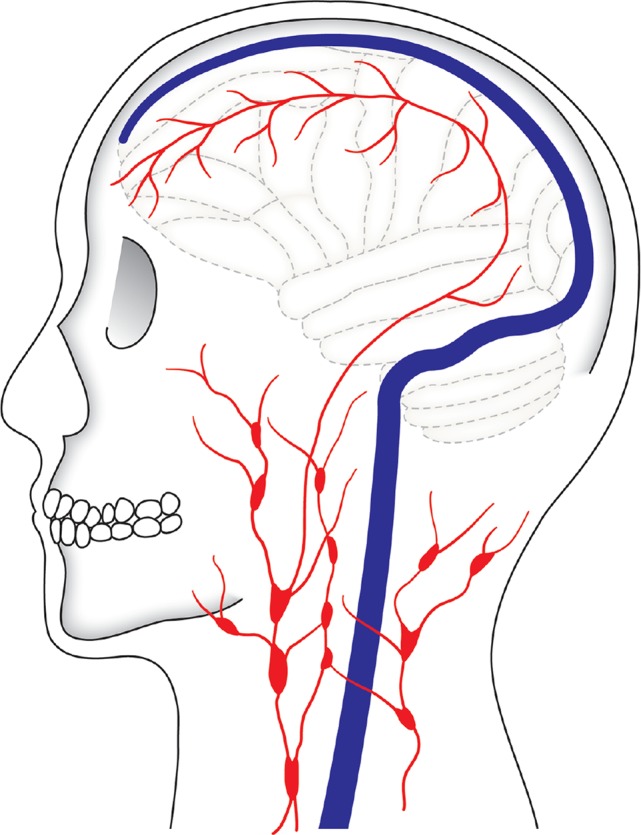
Cerebrospinal fluid (CSF) exit pathways from the subarachnoid space are shown. The first pathway shows absorption by the arachnoid granulation or villi and subsequent exit through the venous sinus. The second pathway shows CSF migration from the meningeal lymphatic vessels to the cervical lymph node. There are other routes in which CSF reaches the cervical lymph node from the cribriform plate via the nasal mucosa.
Short summary
Key et al. indicated the arachnoid granulation or villi and retro-orbital tissues as pathways for CSF absorption and also showed that dye administered into the subarachnoid space reaches the cervical lymph node.
Various locations within the central nervous system such as the arachnoid granulation or villi, periphery of some cranial nerves including the olfactory nerve, spinal nerve root, and capillaries of the brain parenchyma are postulated as routes for CSF absorption.
CSF that exits from the subarachnoid space to the dura mater and epidural space is known to travel to the systemic circulation via the venous sinus or to the cervical lymphatic system through the nasal mucosa or meningeal lymphatic vessels (Fig. 5).
The route in which CSF migrates from the subarachnoid space to the meningeal lymphatic vessel is yet unknown, and further elucidation is awaited.
Exchange between Interstitial Fluid and CSF
Until now, we have summarized previous works related to CSF production and absorption that are historically important. But, does CSF, which fills the ventricles in the deep brain and the brain surface, and interstitial fluid in the brain parenchyma ever exchange substances? And do CSF in the ventricles and CSF in the subarachnoid space maintain a completely independent existence? If the subarachnoid space and ventricles exist as a reservoir of CSF, pathways in which CSF moves in and out from this reservoir are necessary. Several studies have derived answers to these questions. Weller et al. demonstrated a clear pathway in which CSF secreted by the ventricles and subarachnoid space and interstitial fluid secreted by the brain parenchyma, spread through diffusion and further with bulk flow, and eventually reach the cervical lymph nodes.139) Bradbury and Abbott stated that interstitial fluid, which is secreted into the extracellular space through the blood-brain barrier, mixes with CSF and maintains the balance between CSF production and absorption.140,141) It has also been reported that interstitial fluid production and bulk flow in the brain are profoundly involved in brain volume regulation.142) Morphologically, the existence of the Virchow-Robin space143,144) has gained interest, and the term “Virchow-Robin space” has been used synonymously with perivascular space,145–149) periarterial space,150,151) and paravascular space,152–154) although these terms are also used distinctly at times. In this review, we will use the terms “Virchow-Robin space” or “Virchow-Robin space and perivascular spaces” (Footnote 2). In 1921, Wislocki et al. injected dye into dilated rabbit ventricles and demonstrated that the dye migrates from the walls of the ventricles to the brain parenchyma and that the granules that have taken up the dye are present at the Virchow-Robin space and perivascular spaces.53) CSF migration through the ependymal layer of the ventricles was proven by a tracer study that demonstrated the presence of its unrestricted movement in this area,155–159) and furthermore Weller et al. stated that the movement of interstitial fluid and CSF at the ventricular wall is bidirectional.160) Recently, Bedussi et al. demonstrated that interstitial fluid from the striatum migrates to the ventricles.161) A study that used electron microscopy showed that although a tight junction is present between ependymal cells, water molecules move through a paracellular pathway.162) Brightman and Palay also used electron microscopy to discover the ependymal movement of water.163,164) In addition, it was shown in an animal experiment that edema occurs around the ventricles when they dilate due to hydrocephalus.165) Furthermore, Naidich et al. used the term “periventricular CSF edema” to describe low density with a decreased computed tomography (CT) value around the ventricles on CT images, and this later changed to “periventricular lucency,” a term that is widely used today.166) Weller et al. conducted an animal experiment and found with electron microscopy that CSF that migrated from the ventricles is eliminated through the Virchow-Robin space and perivascular space.167) Additionally, there is also a report that demonstrated with CT that metrizamide leaks from the ventricles into the surrounding areas when the ependymal layer ruptures.168) Because CSF freely migrates to the brain parenchyma from the ventricular wall, it has also been reported that this CSF enters the extracellular space169) and that extracellular fluid is equivalent to CSF.170) Hassin published in 1924 that CSF communication is present at the Virchow-Robin space and perivascular spaces,18) and Zhang et al. demonstrated with electron microscopy that there is a continuity from the human pia mater to the Virchow-Robin space and perivascular spaces.149) Furthermore, although it has been shown with MRI in humans that the Virchow-Robin space and perivascular spaces connect with the ventricular wall, which is situated deep in the brain,171) it is understood by many researchers that these spaces enter the cortex from the cerebral superficial subarachnoid space together with the artery96,147,149,156,172,173) and that a cul-de-sac structure is observed at a certain point after entry.172,174) The substance that forms the periphery of the Virchow-Robin space is the glial membrane (glia limitans), which covers the brain parenchyma.175) The basement membrane of the glia joins the outer vascular membrane at the deep Virchow-Robin space, and this is where the cul-de-sac structure exists.176) The pial sheath is present on the outside of the vascular wall and migrates to the brain surface to cover the pial cells. This migration occurs at the point where the blood vessels enter the Virchow-Robin space, and the outer layer of the Virchow-Robin space ends here.177) Fenestration forms in the pial sheath around the blood vessels, and substance transport takes place at this location.149) It has been demonstrated in the Virchow-Robin space that horseradish peroxidase administered into the cerebral superficial subarachnoid space is taken up by the brain parenchyma and is subsequently cleared within 24 hours.178) A separate human study has also shown that metrizamide administered into the subarachnoid space migrates to the parenchyma of the cerebrum and cerebellum.179) These reports indicate that CSF not only circulates in the subarachnoid space and Virchow-Robin space, but also freely enters and exits the brain parenchyma. Yang et al. administered tracer into CSF and demonstrated the presence of free fluid communication between perivascular CSF and interstitial fluid.180) Furthermore, it has been demonstrated histologically that water movement occurs not only in the Virchow-Robin space, but also between the subarachnoid space and brain parenchyma via the pia mater.181) Recently, fluid motion in the Virchow-Robin space and perivascular spaces has gained vast interest, and this has led to studies that utilize mathematical models.182) This fluid motion in the Virchow-Robin space has also been attracting attention as a pathway for β-amyloid elimination.153,183–189) In 1968, Földi et al. claimed that the perivascular space functions as a lymphatic drainage system in the brain.190) In addition, impairment in peri-arterial drainage is considered to be the mechanism of onset of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and is known to be strongly associated with the onset of immunological diseases as well as age-related changes in the brain.151,186,187) Moreover, arterial pulsation is known to be profoundly involved in substance movement within the Virchow-Robin space and perivascular spaces.191) The migration of β-amyloid that has drained from the brain parenchyma into the subarachnoid space is known to change with age, and it has been reported that β-amyloid deposits are observed at the Virchow-Robin space and perivascular spaces due to age-related weakening of arterial pulsation in the perivascular space.187) Furthermore, the intimate relationship between glia and the vascular network as well as the elimination of substances through the Virchow-Robin space is called the “glymphatic system” or “garbage truck of the brain” and a review concerning the decrease in the function of this system due to age and neurodegenerative, neurovascular, and neuroinflammatory diseases has been published.192–194)
There are no tight junctions at the pia mater, which forms the outside of the brain parenchyma, and it has been shown with electron microscopy that water molecules freely migrate through this area.195) Regarding the behavior of fluid in the extracellular space of the brain parenchyma, there are several theories including bulk flow in which interstitial fluid motion occurs in a unidirectional manner through the intercellular space141,196,197) as well as diffusion.198–200) Furthermore, studies that used tracers such as 3H-water showed the presence of rapid capillary absorption of CSF after its migration to the extracellular space.155,185) A pathway in which interstitial fluid in the brain parenchyma travels to the cervical lymph node through the Virchow-Robin space and perivascular spaces has been explained using tools such as dye, contrast agents, 125I-albumin, and 3H-tritiated diisopropyl-fluorophosphate.190,201–207) Xie et al. presented an interesting report in which β-amyloid in interstitial fluid clears through the Virchow-Robin space and perivascular spaces and increases during sleep.208) Furthermore, there is an article that reported the morphology and function of the Virchow-Robin space and perivascular spaces in association with increasing age of animals, and it is interesting that the authors linked such morphology and function with the elimination of β-amyloid that increases with age.209) In addition, Bulat et al. showed that CSF is secreted through capillary filtration and absorbed by adjacent microvessels.210) They also demonstrated that ventricular dilatation does not occur even when the cerebral aqueduct of animals is blocked and that the balance between CSF production and absorption in the ventricles is maintained. On the other hand, a tracer study that showed CSF migration from the subarachnoid space to the brain parenchyma via the Virchow-Robin space and perivascular spaces demonstrated the differences in the time required to migrate to the brain parenchyma based on differences in molecular weight.153) This study also demonstrated that once CSF reaches this point, it can freely enter and exit the extracellular space from the ependyma and subarachnoid space, and also that there is a pathway in which CSF drains from the brain parenchyma into the lymphatic system. Furthermore, it is also interesting that cerebrovascular pulsation induces fluid movement in the Virchow-Robin space and perivascular spaces and plays an important role in interstitial fluid exchange.152) A similar study has also explored the association between fluid movement and the arteries in the Virchow-Robin space and perivascular spaces of the spinal cord.211) CSF that saturates the tissues of the central nervous system frequently exchanges substances with interstitial fluid and is subsequently eliminated into reservoirs such as the subarachnoid space and ventricles. Thus, intimate exchange between CSF and interstitial fluid is considered to be essential for maintaining the homeostasis of the brain.148,185) These works contrast with the traditional textbook or review concepts of CSF production and absorption,55,56,58,59,212–232) and recent reviews describe new findings related to this topic.108,150,233–248)
Short summary
The subarachnoid space and ventricles exist as a reservoir of CSF and are known to extensively exchange water and substances with the surrounding tissue.
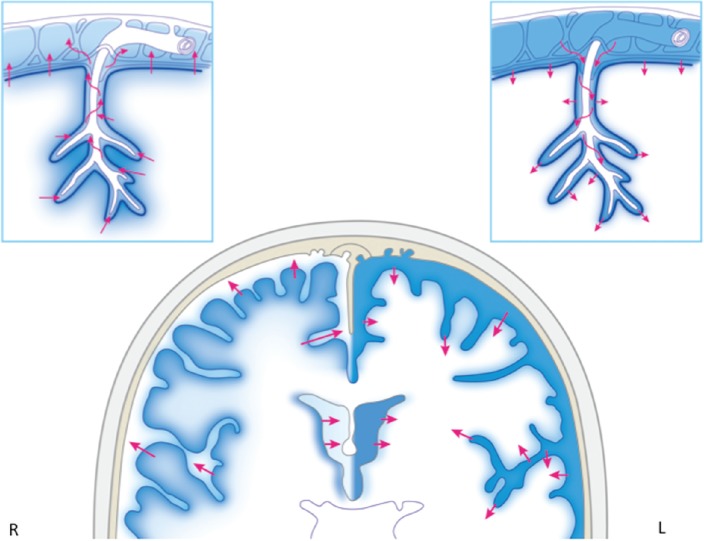
CSF enters the brain parenchyma, mixes with interstitial fluid that is produced there, and is eliminated into reservoirs such as the subarachnoid space and ventricles from the brain parenchyma as CSF again (Fig. 6).
The exchange between CSF and interstitial fluid is bidirectional (Fig. 6).
The Virchow-Robin space is an important exit route from the slightly deep area of the brain parenchyma to the subarachnoid space for interstitial fluid and CSF (Fig. 6).
CSF and interstitial fluid play an important role in the exchange of substances in the brain parenchyma and are therefore essential for maintaining the homeostasis of the brain.
Fig. 6 .
A schematic diagram of cerebrospinal fluid (CSF) and interstitial fluid exchange among the ventricle, subarachnoid space reservoir, and brain parenchyma is shown. Glia, which covers the neurovascular unit, is located at the border of the area in which water enters and exits the brain and spinal cord, and water is exchanged at the aquaporin channel of astrocyte foot processes or at other sites through the endothelium via diffusion or vesicular transport. Water movement at the ependymal layer, pia mater, and Virchow-Robin space is bidirectional. The right cerebral hemisphere has a mixing of interstitial fluid secreted within the brain parenchyma and CSF that enters the brain parenchyma, and subsequent drainage from the brain parenchyma into the CSF reservoir (subarachnoid space and ventricles). The left cerebral hemisphere shows that CSF penetrates from the ventricles and subarachnoid space into the brain parenchyma.
Water Exchange through the Aqua Pore in the Cell Membrane
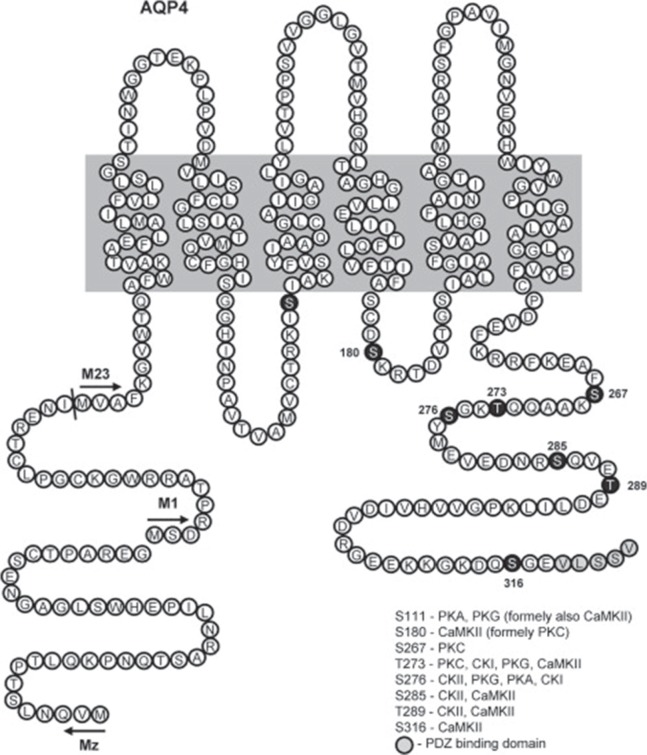
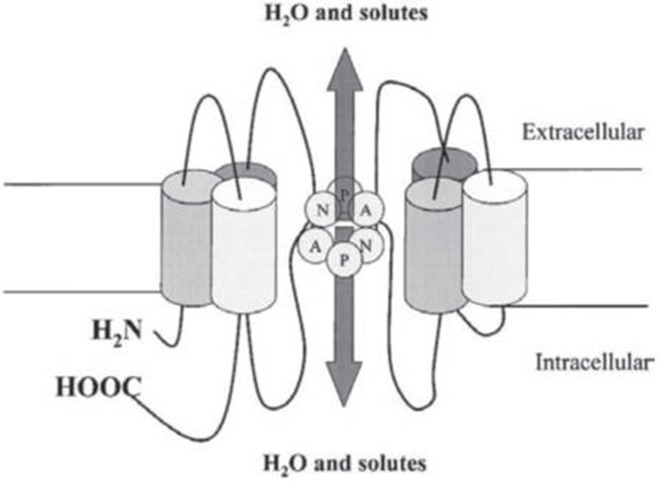
Agre, a researcher of human erythrocytes, focused on a certain unique protein that exists on the cell membrane of erythrocytes, overexpressed this protein in oocytes of African clawed frogs (Xenopus laevis), and found that water permeability is markedly enhanced.249) Agre named this protein, a pore that is present on the cell membrane and that plays an important role in passing water, “aquaporin” (AQP).250) It has been believed for a long time that a special structure is necessary for water to pass through the lipid bilayer of the cell membrane, and with the discovery and elucidation of AQP studies on the movement of water molecules have accelerated dramatically. In 2003, Agre received the Nobel Prize in Chemistry for this work. Fig. 7 shows the structure of AQP.249–253) At the narrowest portion of the AQP pore, asparagine/proline/alanine (NPA) motifs facilitate water molecules to pass through by breaking the hydrogen bond between water molecules that come in contact with each other252,254–256) (Fig. 8). At least 14 AQPs have been discovered to date.257) Some AQPs (AQP-3, AQP-7, AQP-9) are known to pass not only water, but also other molecules such as glycerol.258) AQP-1, 3, 4, 5, 8, and 9 are known to exist in the tissues of the central nervous system.259–261) Among the AQP family members, AQP-1 is known to be highly expressed in the choroid plexus,262–264) and AQP is therefore considered to be involved in CSF production and absorption.265) AQP-4 is widely distributed in the brain including the astrocyte foot processes, glia limitans, and ependymal and subependymal astrocytes, and many researchers therefore have selected AQP-4 as the target topic of research.258,260,266–271) AQP-4 is present at an important location in which interstitial fluid and CSF exchange occurs, and the presence of AQP is therefore necessary for water exchange to occur between the interstitial fluid and CSF cavity.272,273) Although interstitial fluid movement is non-specific and slow, water movement through AQP exhibits directionality and selectivity and is faster compared to interstitial fluid movement.274) Thus, AQP-4 plays a key role at the glia and ependymal layer where interstitial fluid and CSF contact each other272) and facilitates water exchange with some degree of selectivity that occurs faster than in the brain parenchyma. Recently, it has become feasible to visualize the location of AQP-4 with positron emission tomography, and this has been a major advancement for studies that aim to ascertain the movement of water that enters and exits the brain parenchyma.275) AQP-4 controls cell volume by regulating chloride and calcium276) and is also involved in potassium clearance after neural activity because it coexists with potassium channel, indicating that AQP-4 exhibits other neurophysiological effects other than a physical role of allowing water permeability.277,278) Indeed, AQP-4 is responsible for removing metabolites produced by neural activity through water permeability, thereby greatly contributing to the functional maintenance of the brain.153,258,279–282)
Fig. 7 .
Aquaporins (AQPs) are proteins with a molecular weight of 26–30 kDa and are composed of ∼250–290 amino acid residues. AQPs pass through the membrane six times in the vicinity of the pore (membrane-spanning) and allow water to travel in both directions through this pore. Reprinted with permission from Reference309) Figure 1 . (Zelenina M: Regulation of brain aquaporins. Neurochem Int 57: 468–488, 2010).
Fig. 8 .
Some aquaporins (AQPs) contain a structure called the NPA (asparagine-proline-alanine) motif. The center portion of this motif has an hourglass-shaped structure, which penetrates through the lipid bilayer. Substance transport occurs here. AQP1 only allows water to pass through. At this hourglass-shaped portion, the diameter is narrowest at approximately 2.8 Å, which is the size in which one water molecule can just pass through. Water molecules can traverse in both directions at this site. Reprinted with the permission from Reference255) Figure 1 . (Badaut J, Lasbennes F, Magistretti PJ, Regli L: Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab 22: 367–378, 2002).
Let us shift our focus to AQP studies conducted in various disease states. Peritumoral edema around gliomas is known to upregulate AQP-1, and there have been studies that endeavored to design treatments for peritumoral edema by upregulating AQP-1 via genetic modification or directly inhibiting AQP-1.283,284) AQP-4 knockout mice were created by Ma et al. in 1997.285) Subsequently, studies using AQP-4 knockout mice in various diseases became popular.278,286–288) There is also an article that investigated the relationship between cerebral blood flow and AQP and showed that the regional cerebral blood flow increases when AQP-4 is inhibited.289) In addition, the protective effect of progesterone may be related to the down-regulation of AQP-4 expression in hypoxic ischemic brain damage.290) Furthermore, it is also interesting that a review discussed the association between AQP-4 blockage and heat generated by neural activity in relation to the link between blood flow and heat diffusion.291) There are also numerous studies that used AQP as a therapeutic target.292,293) In addition, neuromyelitis optica spectrum disorder is an autoimmune disorder of the central nervous system characterized by AQP-4 autoantibodies.294–297) Regarding the diagnostic significance of AQP-4, there is a report that measured anti-AQP-4 antibody in the CSF to aid in the diagnosis of neuromyelitis optica.296,298) Bloch et al. reported that kaolin-induced hydrocephaly dilates the ventricles and augments intracranial pressure in AQP-4 null mice but does not change ventricular dilatation or intracranial pressure in wild-type mice, demonstrating that the mechanism of removing excess CSF retention in the ventricles is impaired in AQP-4 null mice.299)
In contrast, it has been reported that AQP-4 deletion mice exhibit less edema after acute water intoxication and ischemic stroke, and that the functional prognosis is also favorable.300) Furthermore, mice with AQP-4 gene deletion also do not show changes in the integrity or morphology of the blood-brain barrier.301) It has also been reported that AQP-4 null mice have small ventricles, produce less CSF, and exhibit increased water content in the brain.302) Recently, based on these contradictory study results on AQP, it has become viewed in general that AQP does not play an important role under physiologically normal conditions.280,281)
Short summary
AQP molecules present on the cell membrane play an important role in the passage of water through the cell membrane.
AQP-1 is present in the choroid plexus, and AQP-4 is distributed widely in the brain in areas such as the astrocyte foot processes, glia limitans, and ependymal and subependymal astrocytes. These AQPs play a key role in the water permeability of CSF and interstitial fluid.
The function of AQP differs between physiologically normal and abnormal conditions.
The development of diagnostic and therapeutic methods targeting AQP has been initiated.
Future Directions
1. It is necessary to focus once again on the ventricles, and this pertains to classical anatomical observations. The interesting shape of the ventricular system is visually clear and poses a scientific conundrum. The question arises: why is this unique ventricular structure, which shares common features with other mammals, built in this particular shape and size? With this particular shape, CSF and interstitial fluid communicate to exchange fluids. Tanycytes and microglia form bridges between the ependymal surface and the pial membrane, establishing an intimate relationship between neurons and endothelial cells. The solid lines in Fig. 9 indicate almost the same distance from each ventricular surface to the most adjacent subarachnoid space throughout the brain. Thus, the exchange of CSF and interstitial fluid is clearly important. Of particular importance is the existence of the distal portion of the Sylvian fissure, which is the large subarachnoid space underneath the frontal and temporal lobes. Without this fissure, the distance between the third ventricular wall and the nearby subarachnoid space (Fig. 9, dashed line) would be much longer than that between the other ventricular walls and subarachnoid spaces. As shown in Fig. 9, morphological observation shows that the Sylvian fissure extends deep between the frontal and temporal lobes. However, white matter is mostly present between the convexity of the subarachnoid space and the lateral ventricular wall, whereas the basal ganglia are interposed between the Sylvian fissure and the third ventricular wall. Generally, water moves more rapidly in the white matter than the gray matter. Thus, from the viewpoint of water movement, the subarachnoid space such as the Sylvian fissure is better positioned to more deeply enter the brain parenchyma than the subarachnoid space of the convexities. Therefore, from the point of view of CSF and interstitial fluid, investigation of the rate of water movement in both the white and gray matter is a research issue for the future.
Fig. 9 .
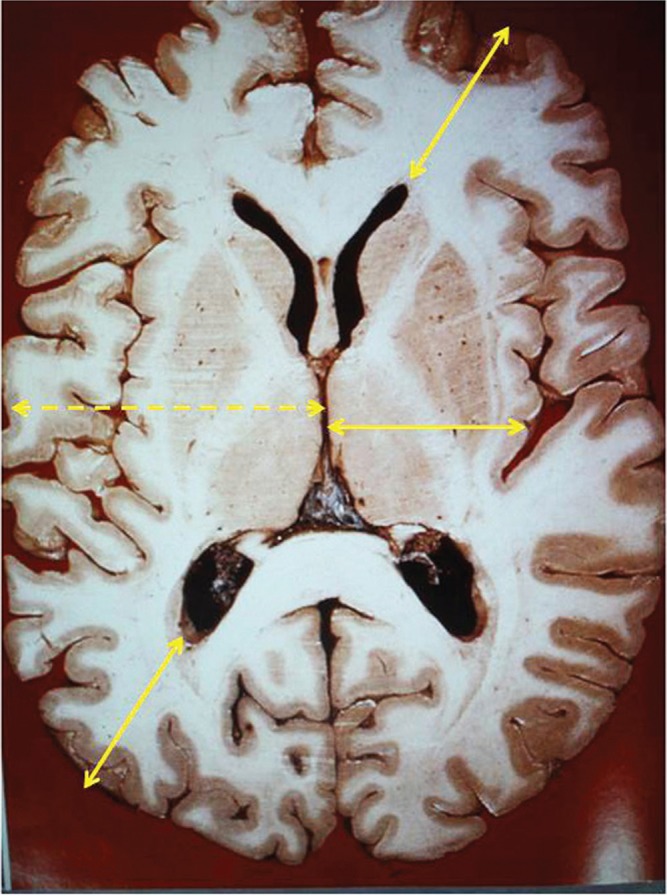
An axial slice of the cerebrum from a cadaver shows that the distance from the subarachnoid space to the lateral ventricular wall as well as the distance from the Sylvian fissure to the third ventricular wall is almost equal in each region ( solid lines ). Cerebrospinal fluid (CSF) and interstitial fluid travel a long distance when the distal part of the Sylvian fissure is not present between the third ventricle and the convexity of the subarachnoid space ( dashed line ). Reprinted with the permission from Reference310) modified Figure 1 . [Sato O: [Reconsideration of research into cerebrospinal fluid], in Arai, H, Ishikawa, M, Mori, E ( eds ): iNPH: Idiopathic Normal Pressure Hydrocephalus . Kyoto, Kinpodo, 2014, pp 8–18 (Japanese)].
Next, we discuss the shape of the rounded anterior horns of the lateral ventricle that extend into the frontal lobe. The body of the lateral ventricle curves upward from the posterior half of the frontal lobe to the parietal and occipital lobes in a manner that lifts these lobes. Furthermore, the ventricles take a form that slightly raises the temporal lobe from the trigone to the temporal horn. It is important to anatomically re-examine (1) why the ventricles display such a shape so that they are in a floating state within the brain regardless of the body position and (2) whether or not the ventricles are positioned nearly equidistant from the subarachnoid space because such form is advantageous for substance and fluid exchange in the deep white matter.
2. It is obviously necessary to consider the differences in experimental methods and in species when examining the results from animal studies. For example, with regards to CSF absorption, it is thought that the human arachnoid granulation or villi are more developed than the rat arachnoid granulation, and in contrast, human leptomeningeal anastomosis is less developed compared to other species. Taking these into consideration, it is presumable that the ratios of drainage of CSF into the venous system and lymphatic system are different among species. Thus, analyses of water molecule movements with MRI and CSF movement with non-invasive methods such as positron emission tomography targeting AQP or β-amyloid are desired, especially in humans.
3. The successful discovery of the meningeal lymphatic vessel in 2015 is a major accomplishment. However, the pathway in which CSF in the subarachnoid space reaches this meningeal lymphatic vessel is unknown, and further analysis including its mechanism is anticipated in the future.
4. AQP plays a key role in water permeability, and is important in the interstitial fluid-CSF exchange. However, a consensus regarding its role in physiologically normal conditions and in physiologically abnormal conditions is desired in the future.
5. With regard to the movement of interstitial fluid and CSF, which have gained interest as elimination routes for heat generated by neural activity and metabolites of neural activity, the promotion of studies focus not only on CSF exit from the brain, but also on entrance of nutrients into the brain parenchyma and the subsequent signal transduction, is desired.
Conclusion
Liquid that covers the brain was observed over 2000 years ago, and von Haller was the first to systematically describe its existence. Moreover, the widely accepted medical term “cerebrospinal fluid” was first used by Magendie. CSF is produced not only by the choroid plexus, but also by many other non-choroid plexus structures, and is absorbed not only by the arachnoid granulation or villi, but also by many other non-arachnoid granulation or villus structures. Glia, which covers the neurovascular unit, is located at the border of the area where water enters and exits the brain and spinal cord, and water exchange occurs through the endothelium via diffusion or vesicular transport at AQP channels on astrocyte foot processes, or at other sites. Extensive exchange of CSF and interstitial fluid occurs between CSF reservoirs (ventricles and subarachnoid space) and the brain parenchyma, and this exchange occurs in a bidirectional manner. The homeostasis of the central nervous system is maintained through CSF and interstitial fluid exchange, which facilitates processes such as adjustment of cerebrospinal volume, nutrient and drug transport, signal transduction, metabolite elimination, and dissipation of heat generated by neural activity.303) CSF eliminated from the subarachnoid space is known to move out via a pathway from the arachnoid granulation or villi to the venous system, and via a pathway from the meningeal lymphatic vessels within the dura mater and where the cranial nerve exits the dura mater to the lymphatic system.304) Concerning studies on CSF physiology, from anatomical examinations to histological assessments and from studies in ventriculo-cisternal perfusion (Pappenheimer et al.29)) to dynamic assessment of water with MRI,272,305–308) numerous new research results such as the discovery of water channels, notably AQP, as well as the discovery of a lymphatic drainage path that works along with the venous drainage path have emerged. Thus, we are diving into new territory regarding studies on the homeostasis of the central nervous system, primarily of CSF.
Footnotes
At an early stage in 1933, Hassin published a radical concept that overturned the established theory up to that point regarding the physiology of CSF at an early stage in 1933. His article claimed that CSF does not circulate, it acts as tissue fluid of the brain, it is not produced by the choroid plexus, it is not absorbed by the arachnoid granulation, it eliminates waste products that accompany neural activity, and it is eliminated through the perineural space, all of which were farseeing observations.19)
The renowned German anatomist Rudolf Virchow (1821–1902), known for discovering the Virchow-Robin space, made his greatest contribution in the dissection of the lymphatic system. In particular, he noted the relationship between cancer and lymph nodes. It should also be acknowledged that he was the first to use the terms lymphoma and lymphosarcoma.132) Furthermore, it is interesting whether it is coincidental that the Virchow-Robin space, named after Virchow, acts as an interstitial fluid drainage route in the brain and that CSF moving in the Virchow-Robin space is eventually connected to the cervical lymph node.
References
- 1). Di leva A, Yaşargil MG: Liquor cotunnii: the history of cerebrospinal fluid in Domenico Cotugno’s work. Neurosurgery 63: 352– 358; discussion 358, 2008. [DOI] [PubMed] [Google Scholar]
- 2). Deisenhammer F: The history of cerebrospinal fluid, in Deisenhammer F, Sellebjerg F, Teunissen CE, Tumani H. (eds): Cerebrospinal Fluid in Clinical Neurology. Cham, Springer International Publishing, 2015, pp 3–16 [Google Scholar]
- 3). Cotugno D: De ischiade nervosa commentarius. Viennae, Apud Rudolphum Gräffer, 1770. [Google Scholar]
- 4). Swedenborg E: The Cerebrum and its Parts, Vol 1. London, Swedenborg Library Academy of the New Church, 1882. [Google Scholar]
- 5). von Haller A: Primae lineae physiologiae in usum praelectionum academicarum. Göttinten, Vandenhoeck, 1747. [Google Scholar]
- 6). Viets HR: Domenico Cotugno: his description of the cerebrospinal fluid, with a translation of part of his De Ischiade Nervosa Commentarius (1764) and a bibliography of his important works. Bulletin of the Institute of the History of Medicine—The Johns Hopkins University 3: 701– 713, 1935. [Google Scholar]
- 7). Haymaker W, Schiller F: The Founders of Neurology: One Hundred and Thirty-three Biographical Sketches Prepared for the Fourth International Neurological Congress in Paris by Eighty-four Authors. Springfield, C.C. Thomas, 1970. [Google Scholar]
- 8). Hajdu SI: A note from history: discovery of the cerebrospinal fluid. Ann Clin Lab Sci 33: 334– 336, 2003. [PubMed] [Google Scholar]
- 9). Magendie F: Recherches physiologiques et cliniques sur le liquide céphalo-rachidien ou cérébro-spinal. Paris, Méquignon-Marvis fils, 1842. [Google Scholar]
- 10). Hildebrand R: Soemmerring’s work on the nervous system: a view on brain structure and function from the late eighteenth century. Anat Embryol 210: 337– 342, 2005. [DOI] [PubMed] [Google Scholar]
- 11). Galen C: On the brain (Book IX), in De anatomics administrationibu, Reprint. New York, Oxford University Press, 1956, pp 226–237 [Google Scholar]
- 12). Willis T: Cerebri anatome: cui accessit nervorum descriptio et usus. London, Londini, typis Jo. Flesher, impensis Jo. Martyn & Ja. Allestry apud insigne Campanae in Coemeterio D. Pauli, 1664. [Google Scholar]
- 13). Davson H, Welch K, Segal MB: Physiology and Pathophysiology of the Cerebrospinal Fluid. Edinburgh, Churchill Livingstone, 1987. [Google Scholar]
- 14). Dandy WE, Blackfan KD: Internal hydrocephalus: experimental, clinical and pathological study. Am J Dis Child 8: 406– 482, 1914. [Google Scholar]
- 15). Dandy WE, Blackfan KD: An experimental and clinical study of internal hydrocephalus. JAMA 61: 2216–2217, 1913. [Google Scholar]
- 16). Hassin GB, Oldberg E, Tinsley M: Changes in the brain in plexectomized dogs: with comments on the cerebrospinal fluid. Arch NeurPsych 38: 1224– 1239, 1937. [Google Scholar]
- 17). Hassin GB: So called circulation of the cerebrospinal fluid; chairman’s address. JAMA 101: 821– 823, 1933. [Google Scholar]
- 18). Hassin GB: Notes on the nature and origin of the cerebrospinal fluid. Journal of Nervous & Mental Disease 59: 113– 121, 1924. [Google Scholar]
- 19). Hassin GB: Circulation of cerebrospinal fluid. Journal of Nervous & Mental Disease 79: 465, 1934. [Google Scholar]
- 20). de Rougemont, Ames A, 3rd, Nesbett FB, Hofmann HF: Fluid formed by choroid plexus; a technique for its collection and a comparison of its electrolyte composition with serum and cisternal fluids. J Neurophysiol 23: 485– 495, 1960. [DOI] [PubMed] [Google Scholar]
- 21). Ames A, 3rd, Sakanoue M, Endo S: Na, K, Ca, Mg, and Cl concentrations in choroid plexus fluid and cisternal fluid compared with plasma ultrafiltrate. J Neurophysiol 27: 672– 681, 1964. [DOI] [PubMed] [Google Scholar]
- 22). Pollay M, Stevens FA, Roberts PA: Alteration in choroid-plexus blood flow and cerebrospinal-fluid formation by increased ventricular pressure , in Wood J. (ed): Neurobiology of Cerebrospinal Fluid 2. New York, Plenum Press, 1983, pp 687–695 [Google Scholar]
- 23). Maraković J, Orešković D, Rados M, Vukić M, Jurjević I, Chudy D, Klarica M: Effect of osmolarity on CSF volume during ventriculo-aqueductal and ventriculo-cisternal perfusions in cats. Neurosci Lett 484: 93– 97, 2010. [DOI] [PubMed] [Google Scholar]
- 24). O’Connell JE: Cerebrospinal fluid mechanics. Proc R Soc Med 63: 507– 518, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Welch K: Secretion of cerebrospinal fluid by choroid plexus of the rabbit. Am J Physiol 205: 617– 624, 1963. [DOI] [PubMed] [Google Scholar]
- 26). Pollay M, Stevens A, Estrada E, Kaplan R: Extracorporeal perfusion of choroid plexus. J Appl Physiol 32: 612– 617, 1972. [DOI] [PubMed] [Google Scholar]
- 27). Pollay M: Formation of cerebrospinal fluid. Relation of studies of isolated choroid plexus to the standing gradient hypothesis. J Neurosurg 42: 665– 673, 1975. [DOI] [PubMed] [Google Scholar]
- 28). Heisey SR, Held D, Pappenheimer JR: Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol 203: 775– 781, 1962. [DOI] [PubMed] [Google Scholar]
- 29). Pappenheimer JR, Heisey SR, Jordan EF, Downer deC: Perfusion of the cerebral ventricular system in unanesthetized goats. Am J Physiol 203: 763– 774, 1962. [Google Scholar]
- 30). Sato O, Bering EA, Jr, Yagi M, Tsugane R, Hara M, Amano Y, Asai T: Bulk flow in the cerebrospinal fluid system of the dog. Acta Neurol Scand 51: 1– 11, 1975. [DOI] [PubMed] [Google Scholar]
- 31). Lorenzo AV, Page LK, Watters GV: Relationship between cerebrospinal fluid formation, absorption and pressure in human hydrocephalus. Brain 93: 679– 692, 1970. [DOI] [PubMed] [Google Scholar]
- 32). Zheng W, Chodobski A: The Blood-Cerebrospinal Fluid Barrier. Boca Raton, CRC Press, 2005. [Google Scholar]
- 33). Milhorat TH: Structure and function of the choroid plexus and other sites of cerebrospinal fluid formation. Int Rev Cytol 47: 225– 288, 1976. [DOI] [PubMed] [Google Scholar]
- 34). Milhorat TH: Choroid plexus and cerebrospinal fluid production. Science 166: 1514– 1516, 1969. [DOI] [PubMed] [Google Scholar]
- 35). Milhorat TH, Hammock MK, Fenstermacher JD, Levin VA: Cerebrospinal fluid production by the choroid plexus and brain. Science 173: 330– 332, 1971. [DOI] [PubMed] [Google Scholar]
- 36). Pollay M, Curl F: Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am J Physiol 213: 1031– 1038, 1967. [DOI] [PubMed] [Google Scholar]
- 37). Bering EA, Jr, Sato O: Hydrocephalus: changes in formation and absorption of cerebrospinal fluid within the cerebral ventricles. J Neurosurg 20: 1050– 1063, 1963. [DOI] [PubMed] [Google Scholar]
- 38). Hammock MK, Milhorat TH: Recent studies on the formation of cerebrospinal fluid. Dev Med Child Neurol Suppl 15: 27– 34, 1973. [DOI] [PubMed] [Google Scholar]
- 39). Tamburrini G, Caldarelli M, Di Rocco F, Massimi L, D’Angelo L, Fasano T, Di Rocco C: The role of endoscopic choroid plexus coagulation in the surgical management of bilateral choroid plexuses hyperplasia. Childs Nerv Syst 22: 605– 608, 2006. [DOI] [PubMed] [Google Scholar]
- 40). Milhorat TH, Hammock MK, Chien T, Davis DA: Normal rate of cerebrospinal fluid formation five years after bilateral choroid plexectomy. Case report. J Neurosurg 44: 735– 739, 1976. [DOI] [PubMed] [Google Scholar]
- 41). Milhorat TH: Failure of choroid plexectomy as treatment for hydrocephalus. Surg Gynecol Obstet 139: 505– 508, 1974. [PubMed] [Google Scholar]
- 42). Anei R, Hayashi Y, Hiroshima S, Mitsui N, Orimoto R, Uemori G, Saito M, Sato M, Wada H, Hododuka A, Kamada K: Hydrocephalus due to diffuse villous hyperplasia of the choroid plexus. Neurol Med Chir (Tokyo) 51: 437–441, 2011. [DOI] [PubMed] [Google Scholar]
- 43). Safaee M, Clark AJ, Bloch O, Oh MC, Singh A, Auguste KI, Gupta N, McDermott MW, Aghi MK, Berger MS, Parsa AT: Surgical outcomes in choroid plexus papillomas: an institutional experience. J Neurooncol 113: 117– 125, 2013. [DOI] [PubMed] [Google Scholar]
- 44). Orešković D, Klarica M, Vukić M: The formation and circulation of cerebrospinal fluid inside the cat brain ventricles: a fact or an illusion? Neurosci Lett 327: 103– 106, 2002. [DOI] [PubMed] [Google Scholar]
- 45). Weed LH: The development of the cerebrospinal spaces in pig and in man. Contrib Embryol Carnegie Inst 5: 1– 116, 1917. [Google Scholar]
- 46). Bering EA: Cerebrospinal fluid production and its relationship to cerebral metabolism and cerebral blood flow. Am J Physiol 197: 825– 828, 1959. [DOI] [PubMed] [Google Scholar]
- 47). Weed LH: Studies on cerebro-spinal fluid. No. IV: the dual source of cerebro-spinal fluid. J Med Res 31: 93– 117, 1914. [PMC free article] [PubMed] [Google Scholar]
- 48). Hassin GB: The morphology of the pail blood vessels and its bearing on the formation and absorption of the cerebrospinal fluid. J Neuropathol Exp Neurol 7: 432– 438, 1948. [DOI] [PubMed] [Google Scholar]
- 49). Sato O, Asai T, Amano Y, Hara M, Tsugane R, Yagi M: Formation of cerebrospinal fluid in spinal subarachnoid space. Nature 233: 129– 130, 1971. [DOI] [PubMed] [Google Scholar]
- 50). Sonnenberg H, Solomon S, Frazier DT: Sodium and chloride movement into the central canal of cat spinal cord. Proc Soc Exp Biol Med 124: 1316– 1320, 1967. [DOI] [PubMed] [Google Scholar]
- 51). Maraković J, Orešković D, Jurjević I, Rados M, Chudy D, Klarica M: Potential error in ventriculocisternal perfusion method for determination of cerebrospinal fluid formation rate in cats. Coll Antropol 35(Suppl 1): 73– 77, 2011. [PubMed] [Google Scholar]
- 52). Milhorat TH, Mosher MB, Hammock MK, Murphy CF: Evidence for choroid-pleux absorption in hydrocephalus. N Engl J Med 283: 286– 289, 1970. [DOI] [PubMed] [Google Scholar]
- 53). Wislocki GB, Putnam TJ: Absorption from the ventricles in experimentally produced internal hydrocephalus. Am J Anat 29: 313– 320, 1921. [Google Scholar]
- 54). Key A, Retzius G: Studien in der Anatomie des Nervensystems und des Bindegewebes. Stockholm, Norstedt & Söner, 1875. [Google Scholar]
- 55). Marburg O: Hydrocephalus: Its Symptomatology, Pathology, Pathogenesis and Treatment. New York, Oskar Piest, 1940. [Google Scholar]
- 56). Millen JW, Woollam DHM: The Anatomy of the Cerebrospinal Fluid. London, Oxford University Press, 1962. [Google Scholar]
- 57). Shapiro K, Marmarou A, Portnoy H: Hydrocephalus. New York, Raven Press, 1984. [Google Scholar]
- 58). Turner L: The structure and relationships of arachnoid granulations , in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd, 1958, pp 32–54 [Google Scholar]
- 59). Selverstone B: Studies of the formation and absorption of the cerebrospinal fluid using radioactive isotopes: a critical evaluation of data and conclusions , in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd, 1958, pp 147–167 [Google Scholar]
- 60). Kida S, Yamashima T, Kubota T, Ito H, Yamamoto S: A light and electron microscopic and immunohistochemical study of human arachnoid villi. J Neurosurg 69: 429– 435, 1988. [DOI] [PubMed] [Google Scholar]
- 61). Weed LH: Studies on cerebro-spinal fluid. No. II: the theories of drainage of cerebro-spinal fluid with an analysis of the methods of investigation. J Med Res 31: 21– 49, 1914. [PMC free article] [PubMed] [Google Scholar]
- 62). Davson H, Domer FR, Hollingsworth JR: The mechanism of drainage of the cerebrospinal fluid. Brain 96: 329– 336, 1973. [DOI] [PubMed] [Google Scholar]
- 63). Shulman K, Yarnell P, Ransohoff J: Dural sinus pressure. In normal and hydrocephalic dogs. Arch Neurol 10: 575– 580, 1964. [DOI] [PubMed] [Google Scholar]
- 64). Grzybowski DM, Holman DW, Katz SE, Lubow M: In vitro model of cerebrospinal fluid outflow through human arachnoid granulations. Invest Ophthalmol Vis Sci 47: 3664– 3672, 2006. [DOI] [PubMed] [Google Scholar]
- 65). Welch K, Friedman V: The relation between the structure of arachnoid villi and their functions. Surgical Forum 10: 767– 769, 1960. [Google Scholar]
- 66). Yamashima T: Ultrastructural study of the final cerebrospinal fluid pathway in human arachnoid villi. Brain Res 384: 68– 76, 1986. [DOI] [PubMed] [Google Scholar]
- 67). Tripathi BJ, Tripathi RC: Vacuolar transcellular channels as a drainage pathway for cerebrospinal fluid. J Physiol (Lond) 239: 195–206, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Shabo AL, Maxwell DS: Electron microscopic observations on the fate of particulate matter in the cerebrospinal fluid. J Neurosurg 29: 464– 474, 1968. [Google Scholar]
- 69). Levine JE, Povlishock JT, Becker DP: The morphological correlates of primate cerebrospinal fluid absorption. Brain Res 241: 31– 41, 1982. [DOI] [PubMed] [Google Scholar]
- 70). Alksne JF, Lovings ET: The role of the arachnoid villus in the removal of red blood cells from the subarachnoid space. An electron microscope study in the dog. J Neurosurg 36: 192– 200, 1972. [DOI] [PubMed] [Google Scholar]
- 71). Weller RO, Kida S, Zhang ET: Pathways of fluid drainage from the brain—morphological aspects and immunological significance in rat and man. Brain Pathol 2: 277– 284, 1992. [DOI] [PubMed] [Google Scholar]
- 72). Gomez DG, Ehrmann JE, Gordon Potts D, Pavese AM, Gilanian A: The arachnoid granulations of the newborn human: an ultrastructural study. Int J Dev Neurosci 1: 139– 147, 1983. [DOI] [PubMed] [Google Scholar]
- 73). Osaka K, Handa H, Matsumoto S, Yasuda M: Development of the cerebrospinal fluid pathway in the normal and abnormal human embryos. Childs Brain 6: 26– 38, 1980. [DOI] [PubMed] [Google Scholar]
- 74). Oi S, Di Rocco C: Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Childs Nerv Syst 22: 662– 669, 2006. [DOI] [PubMed] [Google Scholar]
- 75). Papaiconomou C, Bozanovic-Sosic R, Zakharov A, Johnston M: Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol Regul Integr Comp Physiol 283: R869– R876, 2002. [DOI] [PubMed] [Google Scholar]
- 76). Pollay M: The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res 7: 9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77). Bateman GA, Napier BD: External hydrocephalus in infants: six cases with MR venogram and flow quantification correlation. Childs Nerv Syst 27: 2087– 2096, 2011. [DOI] [PubMed] [Google Scholar]
- 78). Bowsher D: Pathways of absorption of protein from the cerebrospinal fluid: an autoradiographic study in the cat. Anat Rec 128: 23– 39, 1957. [DOI] [PubMed] [Google Scholar]
- 79). Bito LZ, Davson H, Fenstermacher JD: The ocular and cerebrospinal fluids, Proceedings of a Fogarty International Center Symposium, Bethesda, Md 3–6 May 1976 , Vol 25, Supplement 1. London, Academic Press, 1977. [Google Scholar]
- 80). Foltz E, Blanks J, Morton ME: Experimental transcerebral fistula. Perineural olfactory CSF flow in the normal, hydrocephalic, and postoperative hydrocephalic dog shown by radionuclide ventriculography. J Neurosurg 61: 355– 364, 1984. [DOI] [PubMed] [Google Scholar]
- 81). Arnold W, Ritter R, Wagner WH: Quantitative studies on the drainage of the cerebrospinal fluid into the lymphatic system. Acta Otolaryngol 76: 156– 161, 1973. [DOI] [PubMed] [Google Scholar]
- 82). Tripathi RC: The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res 25 Suppl: 65– 116, 1977. [DOI] [PubMed] [Google Scholar]
- 83). Erlich SS, McComb JG, Hyman S, Weiss MH: Ultrastructure of the orbital pathway for cerebrospinal fluid drainage in rabbits. J Neurosurg 70: 926– 931, 1989. [DOI] [PubMed] [Google Scholar]
- 84). Field EJ, Brierley JB: The retro-orbital tissues as a site of outflow of cerebrospinal fluid. Proc R Soc Med 42: 447– 450, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85). Gomez DG, Manzo RP, Fenstermacher JD, Potts DG: Cerebrospinal fluid absorption in the rabbit. Optic pathways. Graefes Arch Clin Exp Ophthalmol 226: 1– 7, 1988. [DOI] [PubMed] [Google Scholar]
- 86). Manzo RP, Gomez DG, Potts DG: Cerebrospinal fluid absorption in the rabbit. Inner ear pathways. Acta Otolaryngol 109: 389– 396, 1990. [DOI] [PubMed] [Google Scholar]
- 87). Edsbagge M, Tisell M, Jacobsson L, Wikkelso C: Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol 287: R1450– R1455, 2004. [DOI] [PubMed] [Google Scholar]
- 88). Sato O, Asai T, Amano Y, Hara M, Tsugane R, Yagi M: Extraventricular origin of the cerebrospinal fluid: formation rate quantitatively measured in the spinal subarachnoid space of dogs. J Neurosurg 36: 276– 282, 1972. [DOI] [PubMed] [Google Scholar]
- 89). Tubbs RS, Hansasuta A, Stetler W, Kelly DR, Blevins D, Humphrey R, Chua GD, Shoja MM, Loukas M, Oakes WJ: Human spinal arachnoid villi revisited: immunohistological study and review of the literature. J Neurosurg Spine 7: 328– 331, 2007. [DOI] [PubMed] [Google Scholar]
- 90). Gomez DG, Chambers AA, Di Benedetto AT, Potts DG: The spinal cerebrospinal fluid absorptive pathways. Neuroradiology 8: 61– 66, 1974. [DOI] [PubMed] [Google Scholar]
- 91). Hassin GB: The cerebrospinal fluid pathways (a critical note). J Neuropathol Exp Neurol 6: 172– 176, 1947. [DOI] [PubMed] [Google Scholar]
- 92). Elman R: Spinal arachnoid granulations with especial reference to the cerebrospinal fluid. Johns Hopkins Hosp Bull 34: 99– 104, 1923. [Google Scholar]
- 93). Welch K, Pollay M: The spinal arachnoid villi of the monkeys Cercopithecus aethiops sabaeus and Macaca irus. Anat Rec 145: 43– 48, 1963. [DOI] [PubMed] [Google Scholar]
- 94). Bilston LE, Stoodley MA, Fletcher DF: The influence of the relative timing of arterial and subarachnoid space pulse waves on spinal perivascular cerebrospinal fluid flow as a possible factor in syrinx development. J Neurosurg 112: 808– 813, 2010. [DOI] [PubMed] [Google Scholar]
- 95). Weed LH: Studies on cerebro-spinal fluid. No. III: the pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J Med Res 31: 51– 91, 1914. [PMC free article] [PubMed] [Google Scholar]
- 96). Kida S, Weller RO: Morphological basis for fluid transport through and around ependymal, arachnoidal, and glial cells , in Raimondi AJ, Choux M, Di Rocco C. (eds): Intracranial Cyst Lesions. New York, Springer Verlag, 1993, pp 37–52 [Google Scholar]
- 97). Benito-León J, León PG, Ferreiro A, Martinez J: Intracranial hypertension syndrome as an unusual form of presentation of spinal subarachnoid haemorrhage and subdural haematoma. Acta Neurochir (Wien) 139: 261–262, 1997. [DOI] [PubMed] [Google Scholar]
- 98). Brinker T, Seifert V, Stolke D: Acute changes in the dynamics of the cerebrospinal fluid system during experimental subarachnoid hemorrhage. Neurosurgery 27: 369– 372, 1990. [DOI] [PubMed] [Google Scholar]
- 99). Schwalbe G: Der arachnoidalraum ein lympharaum und sein zusammenhang mir den perichoroidalraum. Zentralbl Med Wiss 7: 465– 467, 1869. [Google Scholar]
- 100). Love JA, Leslie RA: The effects of raised ICP on lymph flow in the cervical lymphatic trunks in cats. J Neurosurg 60: 577– 581, 1984. [DOI] [PubMed] [Google Scholar]
- 101). Hasuo M, Asano Y, Teraoka M, Ikeyama A, Kageyama N: Cerebrospinal fluid absorption into the lymphatic system in increased intracranial pressure , in Ishii S, Nagai H, Brock M. (eds): Intracranial Pressure V. Berlin, Springer Verlag, 1983, pp 611– 617 [Google Scholar]
- 102). Bradbury MW, Cole DF: The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol (Lond) 299: 353– 365, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103). Bradbury MW: Proportion of cerebrospinal fluid draining into jugular lymphatic trunks of the cat [proceedings]. J Physiol 276: 67P– 68P, 1978. [PubMed] [Google Scholar]
- 104). Bradbury MW, Cserr HF, Westrop RJ: Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol 240: F329– F336, 1981. [DOI] [PubMed] [Google Scholar]
- 105). McComb JG, Hyman S, Weiss MH: Lymphatic drainage of cerebrospinal fluid in the cat, in Shapiro K, Marmarou A. (eds): Hydrocephalus. New York, Raven Press, 1984, pp 83– 98 [Google Scholar]
- 106). Mortensen OA, Sullivan WE: The cerebrospinal fluid and the cervical lymph nodes. The Anat Rec 56: 359– 363, 1933. [Google Scholar]
- 107). Mathieu E, Gupta N, Macdonald RL, Ai J, Yücel YH: In vivo imaging of lymphatic drainage of cerebrospinal fluid in mouse. Fluids Barriers CNS 10: 35, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108). Chen L, Elias G, Yostos MP, Stimec B, Fasel J, Murphy K: Pathways of cerebrospinal fluid outflow: a deeper understanding of resorption. Neuroradiology 57: 139– 147, 2015. [DOI] [PubMed] [Google Scholar]
- 109). Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D: Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1: 2, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110). Di Chiro G: Movement of the cerebrospinal fluid in human beings. Nature 204: 290– 291, 1964. [DOI] [PubMed] [Google Scholar]
- 111). Di Chiro G, Girton ME, Frank JA, Dietz MJ, Gansow OA, Wright DC, Dwyer AJ: Cerebrospinal fluid rhinorrhea: depiction with MR cisternography in dogs. Radiology 160: 221– 222, 1986. [DOI] [PubMed] [Google Scholar]
- 112). Casley-Smith JR, Földi-Börsök E, Földi M: The prelymphatic pathways of the brain as revealed by cervical lymphatic obstruction and the passage of particles. Br J Exp Pathol 57: 179– 188, 1976. [PMC free article] [PubMed] [Google Scholar]
- 113). Fard PJ, Tajvidi MR, Gharibzadeh S: High-pressure hydrocephalus: a novel analytical modeling approach. J Theor Biol 248: 401– 410, 2007. [DOI] [PubMed] [Google Scholar]
- 114). Brierley JB, Field EJ: The connexions of the spinal sub-arachnoid space with the lymphatic system. J Anat 82: 153– 166, 1948. [PubMed] [Google Scholar]
- 115). Greitz D, Hannerz J: A proposed model of cerebrospinal fluid circulation: observations with radionuclide cisternography. AJNR Am J Neuroradiol 17: 431– 438, 1996. [PMC free article] [PubMed] [Google Scholar]
- 116). Hayashi N, Matsumae M, Yatsushiro S, Hirayama A, Abdullah A, Kuroda K: Quantitative analysis of cerebrospinal fluid pressure gradients in healthy volunteers and patients with normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 55: 657– 662, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117). Matsumae M, Hirayama A, Atsumi H, Yatsushiro S, Kuroda K: Velocity and pressure gradients of cerebrospinal fluid assessed with magnetic resonance imaging. J Neurosurg 120: 218– 227, 2014. [DOI] [PubMed] [Google Scholar]
- 118). Yatsushiro S, Hirayama A, Matsumae M, Kuroda K: Visualization of pulsatile CSF motion separated by membrane-like structure based on four-dimensional phase-contrast (4D-PC) velocity mapping. Conf Proc IEEE Eng Med Biol Soc 2013: 6470– 6473, 2013. [DOI] [PubMed] [Google Scholar]
- 119). Stadlbauer A, Salomonowitz E, Brenneis C, Ungersböck K, van der Riet W, Buchfelder M, Ganslandt O: Magnetic resonance velocity mapping of 3D cerebrospinal fluid flow dynamics in hydrocephalus: preliminary results. Eur Radiol 22: 232– 242, 2012. [DOI] [PubMed] [Google Scholar]
- 120). Howden L, Giddings D, Power H, Aroussi A, Vloeberghs M, Garnett M, Walker D: Three-dimensional cerebrospinal fluid flow within the human ventricular system. Comput Methods Biomech Biomed Engin 11: 123– 133, 2008. [DOI] [PubMed] [Google Scholar]
- 121). Bradley WG, Jr: Magnetic resonance imaging in the evaluation of cerebrospinal fluid flow abnormalities. Magn Reson Q 8: 169– 196, 1992. [PubMed] [Google Scholar]
- 122). Bradley WG, Jr, Scalzo D, Queralt J, Nitz WN, Atkinson DJ, Wong P: Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 198: 523– 529, 1996. [DOI] [PubMed] [Google Scholar]
- 123). Greitz D: Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography. Acta Radiol Suppl 386: 1– 23, 1993. [PubMed] [Google Scholar]
- 124). Naidich TP, Altman NR, Gonzalez-Arias SM: Phase contrast cine magnetic resonance imaging: normal cerebrospinal fluid oscillation and applications to hydrocephalus. Neurosurg Clin N Am 4: 677– 705, 1993. [PubMed] [Google Scholar]
- 125). Greitz D, Franck A, Nordell B: On the pulsatile nature of intracranial and spinal CSF-circulation demonstrated by MR imaging. Acta Radiol 34: 321– 328, 1993. [PubMed] [Google Scholar]
- 126). Hirayama A, Matsumae M, Yatsushiro S, Abdulla A, Atsumi H, Kuroda K: Visualization of pulsatile csf motion around membrane-like structures with both 4D velocity mapping and time-SLIP technique. Magn Reson Med Sci 14: 263– 273, 2015. [DOI] [PubMed] [Google Scholar]
- 127). Yamada S: Cerebrospinal fluid physiology: visualization of cerebrospinal fluid dynamics using the magnetic resonance imaging Time-Spatial Inversion Pulse method. Croat Med J 55: 337– 346, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128). Kida S, Pantazis A, Weller RO: CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19: 480– 488, 1993. [DOI] [PubMed] [Google Scholar]
- 129). Boulton M, Flessner M, Armstrong D, Hay J, Johnston M: Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol 274: R88– R96, 1998. [DOI] [PubMed] [Google Scholar]
- 130). Courtice FC, Simmonds WJ: The removal of protein from the subarachnoid space. Aust J Exp Biol Med Sci 29: 255– 263, 1951. [DOI] [PubMed] [Google Scholar]
- 131). Welch K, Pollay M: Perfusion of particles through arachnoid villi of the monkey. Am J Physiol 201: 651– 654, 1961. [DOI] [PubMed] [Google Scholar]
- 132). Loukas M, Bellary SS, Kuklinski M, Ferrauiola J, Yadav A, Shoja MM, Shaffer K, Tubbs RS: The lymphatic system: a historical perspective. Clin Anat 24: 807– 816, 2011. [DOI] [PubMed] [Google Scholar]
- 133). Clouse ME: History, in Clouse ME, Wallace S. (eds): Lymphatic Imaging Lymphography, Computed Tomography and Scintigraphy, ed 2 Baltimore, Williams & Wilkins, 1985, pp 1– 14 [Google Scholar]
- 134). Harrison DA, Clouse ME: Normal anatomy, in Clouse ME, Wallace S. (eds): Lymphatic Imaging Lymphography, Computed Tomography and Scintigraphy, ed 2 Baltimore, Williams & Wilkins, 1985, pp 15– 94 [Google Scholar]
- 135). Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J: Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337– 341, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136). Bucchieri F, Farina F, Zummo G, Cappello F: Lymphatic vessels of the dura mater: a new discovery? J Anat 227: 702– 703, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137). Mascagni P: Vasorum lymphaticorum corporis humani historia et ichnographia. Siena, Pazzini Carli, 1787 [Google Scholar]
- 138). Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K: A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212: 991– 999, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139). Weller RO, Galea I, Carare RO, Minagar A: Pathophysiology of the lymphatic drainage of the central nervous system: implications for pathogenesis and therapy of multiple sclerosis. Pathophysiology 17: 295– 306, 2010. [DOI] [PubMed] [Google Scholar]
- 140). Bradbury M: Physiopathology of the blood-brain barrier, in Levi G, Battistin L, Lajtha A. (eds): Transport Phenomena in the Nervous System. New York, Plenum Press, 1976, pp 507– 516 [DOI] [PubMed] [Google Scholar]
- 141). Abbott NJ: Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45: 545– 552, 2004. [DOI] [PubMed] [Google Scholar]
- 142). Cserr HF: Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci 529: 9– 20, 1988. [DOI] [PubMed] [Google Scholar]
- 143). Virchow R: Ueber die Erweiterung kleinerer Gefäfse. Arch Pathol Anat Physiol Klin Med 3: 427– 462, 1851. [Google Scholar]
- 144). Robin C: Recherches sur quelques particularités de la structure des capillaires de l’encéphale. J Physiol Homme 2: 537– 548, 1859. [Google Scholar]
- 145). Bechmann I, Priller J, Kovac A, Böntert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R: Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 14: 1651– 1658, 2001. [DOI] [PubMed] [Google Scholar]
- 146). Bilston LE, Fletcher DF, Brodbelt AR, Stoodley MA: Arterial pulsation-driven cerebrospinal fluid flow in the perivascular space: a computational model. Comput Methods Biomech Biomed Engin 6: 235– 241, 2003. [DOI] [PubMed] [Google Scholar]
- 147). Ichimura T, Fraser PA, Cserr HF: Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res 545: 103– 113, 1991. [DOI] [PubMed] [Google Scholar]
- 148). Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO: Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 238: 962– 974, 2006. [DOI] [PubMed] [Google Scholar]
- 149). Zhang ET, Inman CB, Weller RO: Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat 170: 111– 123, 1990. [PMC free article] [PubMed] [Google Scholar]
- 150). Hladky SB, Barrand MA: Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11: 26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151). Carare RO, Hawkes CA, Weller RO: Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav Immun 36: 9– 14, 2014. [DOI] [PubMed] [Google Scholar]
- 152). Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M: Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33: 18190– 18199, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153). Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M: A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154). Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA: Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326: 47– 63, 1985. [DOI] [PubMed] [Google Scholar]
- 155). Bulat M, Lupret V, Orehković D, Klarica M: Transventricular and transpial absorption of cerebrospinal fluid into cerebral microvessels. Coll Antropol 32(Suppl 1): 43– 50, 2008. [PubMed] [Google Scholar]
- 156). Zhang ET, Richards HK, Kida S, Weller RO: Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol 83: 233– 239, 1992. [DOI] [PubMed] [Google Scholar]
- 157). Brightman MW: The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. I. Ependymal distribution. J Cell Biol 26: 99– 123, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158). Brightman MW: The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice. Prog Brain Res 29: 19– 40, 1968. [DOI] [PubMed] [Google Scholar]
- 159). Bulat M: Dynamics and statics of the cerebrospinal fluid: the classical and a new hypothesis, in Avezaat CJJ, van Eijndhoven JHM, Maas AIR, Tans JTJ. (eds): Intracranial Pressure VIII. Springer; Berlin Heidelberg, 1993, pp 726– 730 [Google Scholar]
- 160). Weller RO, Kida S, Harding BN: Aetiology and pathology of hydrocephalus, in Schurr PH, Polkey CE. (eds): Hydrocephalus. Oxford, Oxford University Press, 1993, pp 48– 99 [Google Scholar]
- 161). Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E, Bakker EN: Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids and Barriers CNS 12: 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162). Crone C: The blood-brain barrier as a tight epithelium: where is information lacking? Ann N Y Acad Sci 481: 174– 185, 1986. [DOI] [PubMed] [Google Scholar]
- 163). Brightman MW, Palay SL: The fine structure of ependyma in the brain of the rat. J Cell Biol 19: 415– 439, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164). Brightman MW: The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. II. Parenchymal distribution. Am J Anat 117: 193– 219, 1965. [DOI] [PubMed] [Google Scholar]
- 165). Sahar A, Hochwald GM, Ransohoff J: Alternate pathway for cerebrospinal fluid absorption in animals with experimental obstructive hydrocephalus. Exp Neurol 25: 200– 206, 1969. [DOI] [PubMed] [Google Scholar]
- 166). Naidich TP, Epstein F, Lin JP, Kricheff II, Hochwald GM: Evaluation of pediatric hydrocephalus by computed tomography. Radiology 119: 337– 345, 1976. [DOI] [PubMed] [Google Scholar]
- 167). Weller RO, Mitchell J: Cerebrospinal fluid edema and its sequelae in hydrocephalus. Adv Neurol 28: 111– 123, 1980. [PubMed] [Google Scholar]
- 168). Drayer BP, Rosenbaum AE, Higman HB: Cerebrospinal fluid imaging using serial metrizamide CT cisternography. Neuroradiology 13: 7– 17, 1977. [DOI] [PubMed] [Google Scholar]
- 169). Bering EA, Jr: Water exchange of central nervous system and cerebrospinal fluid. J Neurosurg 9: 275– 287, 1952. [DOI] [PubMed] [Google Scholar]
- 170). Williams MA, McAllister JP, Walker ML, Kranz DA, Bergsneider M, Del Bigio MR, Fleming L, Frim DM, Gwinn K, Kestle JR, Luciano MG, Madsen JR, Oster-Granite ML, Spinella G: Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. J Neurosurg 107: 345– 357, 2007. [DOI] [PubMed] [Google Scholar]
- 171). Tsutsumi S, Ito M, Yasumoto Y, Tabuchi T, Ogino I: The Virchow-Robin spaces: delineation by magnetic resonance imaging with considerations on anatomofunctional implications. Childs Nerv Syst 27: 2057– 2066, 2011. [DOI] [PubMed] [Google Scholar]
- 172). Jones EG: On the mode of entry of blood vessels into the cerebral cortex. J Anat 106: 507– 520, 1970. [PMC free article] [PubMed] [Google Scholar]
- 173). Brumback RA: Anatomic and physiologic aspects of the cerebrospinal fluid space, in Herndon R, Brumback R. (eds): The Cerebrospinal Fluid. Boston, Kluwer Academic Publishers, 1989, pp 15– 43 [Google Scholar]
- 174). Woollam DH, Millen JW: The perivascular spaces of the mammalian central nervous system and their relation to the perineuronal and subarachnoid spaces. J Anat 89: 193– 200, 1955. [PMC free article] [PubMed] [Google Scholar]
- 175). Krahn V: The pia mater at the site of the entry of blood vessels into the central nervous system. Anat Embryol 164: 257– 263, 1982. [DOI] [PubMed] [Google Scholar]
- 176). Ge S, Song L, Pachter JS: Where is the blood-brain barrier ... really? J Neurosci Res 79: 421– 427, 2005. [DOI] [PubMed] [Google Scholar]
- 177). Krisch B, Leonhardt H, Oksche A: Compartments and perivascular arrangement of the meninges covering the cerebral cortex of the rat. Cell Tissue Res 238: 459– 474, 1984. [DOI] [PubMed] [Google Scholar]
- 178). Turner PT, Harris AB: Ultrastructure of exogenous peroxidase in cerebral cortex. Brain Res 74: 305– 326, 1974. [DOI] [PubMed] [Google Scholar]
- 179). Drayer BP, Rosenbaum AE: Metrizamide brain penetrance. Acta Radiol Suppl 355: 280– 293, 1977. [PubMed] [Google Scholar]
- 180). Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, Nedergaard M: Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 11: 107, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181). Hutchings M, Weller RO: Anatomical relationships of the pia mater to cerebral blood vessels in man. J Neurosurg 65: 316– 325, 1986. [DOI] [PubMed] [Google Scholar]
- 182). Wang P, Olbricht WL: Fluid mechanics in the perivascular space. J Theor Biol 274: 52– 57, 2011. [DOI] [PubMed] [Google Scholar]
- 183). Weller RO, Subash M, Preston SD, Mazanti I, Carare RO: Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 18: 253– 266, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184). Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO: Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol 29: 106– 117, 2003. [DOI] [PubMed] [Google Scholar]
- 185). Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO: Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34: 131– 144, 2008. [DOI] [PubMed] [Google Scholar]
- 186). Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO: Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol 39: 593– 611, 2013. [DOI] [PubMed] [Google Scholar]
- 187). Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO: Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121: 431– 443, 2011. [DOI] [PubMed] [Google Scholar]
- 188). Suzuki Y, Nakamura Y, Yamada K, Igarashi H, Kasuga K, Yokoyama Y, Ikeuchi T, Nishizawa M, Kwee IL, Nakada T: Reduced CSF water influx in Alzheimer’s disease supporting the β-amyloid clearance hypothesis. PLoS One 10: e0123708, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189). Carare RO, Teeling JL, Hawkes CA, Püntener U, Weller RO, Nicoll JA, Perry VH: Immune complex formation impairs the elimination of solutes from the brain: implications for immunotherapy in Alzheimer’s disease. Acta Neuropathol Commun 1: 48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190). Földi M, Csillik B, Zoltán OT: Lymphatic drainage of the brain. Experientia 24: 1283– 1287, 1968. [DOI] [PubMed] [Google Scholar]
- 191). Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, Park JW, Bankiewicz K: The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther 14: 69– 78, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192). Simon MJ, Iliff JJ: Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 1862: 442– 451, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193). Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, Nedergaard M: Ultra-fast magnetic resonance encephalography of physiological brain activity—glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194). Nedergaard M: Neuroscience. Garbage truck of the brain. Science 340: 1529– 1530, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195). Alcolado R, Weller RO, Parrish EP, Garrod D: The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol Appl Neurobiol 14: 1– 17, 1988. [DOI] [PubMed] [Google Scholar]
- 196). Cserr HF, Ostrach LH: Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol 45: 50– 60, 1974. [DOI] [PubMed] [Google Scholar]
- 197). Cserr HF: Bulk flow of cerebral extracellular fluid as a possible mechanism of CSF-brain exchange, in Cserr HF, Fenstermacher JD, Fencl V. (eds): Fluid Environment of the Brain. New York, Academic Press Inc., 1975, pp 215– 224 [Google Scholar]
- 198). Fenstermacher J, Kaye T: Drug “diffusion” within the brain. Ann N Y Acad Sci 531: 29– 39, 1988. [DOI] [PubMed] [Google Scholar]
- 199). Patlak CS, Fenstermacher JD: Measurements of dog blood-brain transfer constants by ventriculocisternal perfusion. Am J Physiol 229: 877– 884, 1975. [DOI] [PubMed] [Google Scholar]
- 200). Syková E, Nicholson C: Diffusion in brain extracellular space. Physiol Rev 88: 1277– 1340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201). Kozma M, Zoltãn OT, Csillik B: Die anatomischen Grundlagen des prälymphatischen systems im Gehirn. Cells Tissues Organs 81: 409– 420, 1972. [PubMed] [Google Scholar]
- 202). Oehmichen W, Gencic M: Experimental studies on kinetics and functions of monuclear phagozytes of the central nervous system. Acta Neuropathol Suppl Suppl 6: 285– 290, 1975. [DOI] [PubMed] [Google Scholar]
- 203). Cserr HF: Convection of brain interstitial fluid and its drainage into deep cervical lymph. Wiss Z Karl Marx Univ Leipzig Math Naturwiss R 36: 127– 130, 1987. [Google Scholar]
- 204). Cserr HF, Knopf PM: Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 13: 507– 512, 1992. [DOI] [PubMed] [Google Scholar]
- 205). Cserr HF: Relationship between cerebrospinal fluid and interstitial fluid of brain. Fed Proc 33: 2075– 2078, 1974. [PubMed] [Google Scholar]
- 206). Chikly B: Is human CSF reabsorbed by lymph? Lymph drainage therapy (LDT) and manual drainage of the central nervous system. The Academy of Osteopathy Journal 8: 28– 34, 1998. [Google Scholar]
- 207). Fenstermacher JD, Patlak CS: The exchange of material between cerebrospinal fluid and brain, in Cserr HF, Fenstermacher JD, Fencl V. (eds): Fluid Environment of the Brain. New York, Academic Press Inc., 1975, pp 201– 214 [Google Scholar]
- 208). Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M: Sleep drives metabolite clearance from the adult brain. Science 342: 373– 377, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209). Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M: Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: 845– 861, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210). Bulat M, Klarica M: Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev 65: 99– 112, 2011. [DOI] [PubMed] [Google Scholar]
- 211). Martin BA, Reymond P, Novy J, Balédent O, Stergiopulos N: A coupled hydrodynamic model of the cardiovascular and cerebrospinal fluid system. Am J Physiol Heart Circ Physiol 302: H1492– H1509, 2012. [DOI] [PubMed] [Google Scholar]
- 212). Davson H: Physiology of the Cerebrospinal Fluid. London, Churchill, 1967 [Google Scholar]
- 213). Cushing H: Studies on the cerebro-spinal fluid: I. Introduction. J Med Res 31: 1– 19, 1914. [PMC free article] [PubMed] [Google Scholar]
- 214). Bowsher D: Cerebrospinal Fluid Dynamics in Health and Disease. Springfield, C.C. Thomas, 1960 [Google Scholar]
- 215). Davson H: Formation and drainage of the cerebrospinal fluid. Sci Basis Med Annu Rev 238– 259, 1966 [PubMed] [Google Scholar]
- 216). Brodbelt A, Stoodley M: CSF pathways: a review. Br J Neurosurg 21: 510– 520, 2007. [DOI] [PubMed] [Google Scholar]
- 217). Craven J: Cerebrospinal fluid and its circulation. Anaesth Intens Care Med 11: 355– 356, 2010. [Google Scholar]
- 218). Clarke E, O’Malley CD: The ventricular system and cerebrospinal fluid, in The Human Brain and Spinal Cord: A Historical Study Illustrated by Writings from Antiquity to the Twentieth Century. San Francisco, Norman Publishing, 1996, pp 708– 755 [Google Scholar]
- 219). Hammock MK, Milhorat TH, Davis DA: Isotope cisternography and ventriculography in the diagnosis of hydrocephalus. Dev Med Child Neurol 16: 58– 71, 1974. [DOI] [PubMed] [Google Scholar]
- 220). Han CY, Backous DD: Basic principles of cerebrospinal fluid metabolism and intracranial pressure homeostasis. Otolaryngol Clin North Am 38: 569– 576, 2005. [DOI] [PubMed] [Google Scholar]
- 221). Herndon RM, Brumback R: The Cerebrospinal Fluid. Boston, Kluwer Academic Publishers, 1989 [Google Scholar]
- 222). Kappers JA: Structural and functional changes in the telencephalic choroid plexus during human ontogenesis, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption . Chichester, John Wiley & Sons Ltd., 1958, pp 3– 31 [Google Scholar]
- 223). Wislocki GB, Ladman AJ: The fine structure of the mammalian choroid plexus, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 55– 79 [Google Scholar]
- 224). Cooper ERA: Nerves of the meninges and choroid plexuses, in Wolstenholme GEW, O’connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 80– 96 [Google Scholar]
- 225). Woollam DHM, Millen JW: Observations on the production and circulation of the cerebrospinal fluid, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 124– 146 [Google Scholar]
- 226). Davson H: Some aspects of the relationship between the cerebrospinal fluid and the central nervous system, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 189– 208 [Google Scholar]
- 227). Herlin L: The existence of a barrier between the cerebrospinal fluid and the boundary of the brain; Including experimental investigations on rabbits, using bilirubinaemia, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 209– 229 [Google Scholar]
- 228). Zülch KJ: Neuropathological observations on the cerebrospinal fluid pathway, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 230– 245 [Google Scholar]
- 229). Dott NM, Gillingham JF: Mechanical aspects of the cerebrospinal fluid circulation—physiological, pathological, surgical, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 246– 264 [Google Scholar]
- 230). Johnson KM: Clinicopathological aspects of the cerebrospinal fluid circulation, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 265– 281 [Google Scholar]
- 231). Bowsher D: A possible mechanism of hydrocephalus: the osmotic regulation of cerebrospinal fluid volume, in Wolstenholme GEW, O’Connor CM. (eds): Ciba Foundation Symposium—The Cerebrospinal Fluid: Production, Circulation and Absorption. Chichester, John Wiley & Sons Ltd., 1958, pp 282– 301 [Google Scholar]
- 232). Potts G: Hydrocephalus. J Neuroradiol 8: 195– 206, 1981. [PubMed] [Google Scholar]
- 233). Brinker T, Stopa E, Morrison J, Klinge P: A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11: 10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234). Orešković D, Klarica M: The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev 64: 241– 262, 2010. [DOI] [PubMed] [Google Scholar]
- 235). Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD: Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5: 10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236). Raybaud C: MR assessment of pediatric hydrocephalus: a road map. Childs Nerv Syst 32: 19– 41, 2016. [DOI] [PubMed] [Google Scholar]
- 237). Johanson C: Choroid plexus—cerebrospinal fluid circulatory dynamics: impact on brain growth, metabolism, and repair, in Conn PM. (ed): Neuroscience in Medicine. Totowa, Humana Press, 2008, pp 173– 200 [Google Scholar]
- 238). Irani DN: Cerebrospinal Fluid in Clinical Practice. Elsevier Health Sciences, 2008 [Google Scholar]
- 239). Johnston I, Teo C: Disorders of CSF hydrodynamics. Childs Nerv Syst 16: 776– 799, 2000. [DOI] [PubMed] [Google Scholar]
- 240). Johanson CE: Ventricles and cerebrospinal fluid, Chapter 10, in Conn PM. (ed): Neuroscience in Medicine. Philadelphia, Lippincott, 1995, pp 171– 196 [Google Scholar]
- 241). Koh L, Zakharov A, Johnston M: Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res 2: 6, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242). Liddelow SA: Fluids and barriers of the CNS: a historical viewpoint. Fluids Barriers CNS 8: 2, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243). Sato O, Takei F, Yamada S: Hydrocephalus: is impaired cerebrospinal fluid circulation only one problem involved? Childs Nerv Syst 10: 151– 155, 1994. [DOI] [PubMed] [Google Scholar]
- 244). Sakka L, Coll G, Chazal J: Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128: 309– 316, 2011. [DOI] [PubMed] [Google Scholar]
- 245). Tumani H: Anatomy of CSF-related spaces and barriers between blood, CSF, and brain, in Deisenhammer F, Sellebjerg F, Teunissen CE, Tumani H. (eds): Cerebrospinal Fluid in Clinical Neurology. Cham, Springer International Publishing, 2015, pp 17– 24 [Google Scholar]
- 246). Tumani H: Physiology and constituents of CSF, in Deisenhammer F, Sellebjerg F, Teunissen CE, Tumani H. (eds): Cerebrospinal Fluid in Clinical Neurology. Cham, Springer International Publishing, 2015, pp 25– 34 [Google Scholar]
- 247). Chikly B, Quaghebeur J: Reassessing cerebrospinal fluid (CSF) hydrodynamics: a literature review presenting a novel hypothesis for CSF physiology. J Bodyw Mov Ther 17: 344– 354, 2013. [DOI] [PubMed] [Google Scholar]
- 248). Spector R, Robert Snodgrass S, Johanson CE: A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol 273: 57– 68, 2015. [DOI] [PubMed] [Google Scholar]
- 249). Preston GM, Agre P: Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88: 11110– 11114, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250). Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S: Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol 265: F463– F476, 1993. [DOI] [PubMed] [Google Scholar]
- 251). Nielsen S, Kwon TH, Frøkiaer J, Agre P: Regulation and dysregulation of aquaporins in water balance disorders. J Intern Med 261: 53– 64, 2007. [DOI] [PubMed] [Google Scholar]
- 252). Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y: Structural determinants of water permeation through aquaporin-1. Nature 407: 599– 605, 2000. [DOI] [PubMed] [Google Scholar]
- 253). Kozono D, Yasui M, King LS, Agre P: Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine. J Clin Invest 109: 1395– 1399, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254). de Groot BL, Grubmüller H: The dynamics and energetics of water permeation and proton exclusion in aquaporins. Curr Opin Struct Biol 15: 176– 183, 2005. [DOI] [PubMed] [Google Scholar]
- 255). Badaut J, Lasbennes F, Magistretti PJ, Regli L: Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab 22: 367– 378, 2002. [DOI] [PubMed] [Google Scholar]
- 256). Ishibashi K: Aquaporin superfamily with unusual npa boxes: S-aquaporins (superfamily, sip-like and subcellular-aquaporins). Cell Mol Biol (Noisy-legrand) 52: 20– 27, 2006 [PubMed] [Google Scholar]
- 257). Day RE, Kitchen P, Owen DS, Bland C, Marshall L, Conner AC, Bill RM, Conner MT: Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta 1840: 1492– 1506, 2014. [DOI] [PubMed] [Google Scholar]
- 258). Tait MJ, Saadoun S, Bell BA, Papadopoulos MC: Water movements in the brain: role of aquaporins. Trends Neurosci 31: 37– 43, 2008. [DOI] [PubMed] [Google Scholar]
- 259). Yang M, Gao F, Liu H, Yu WH, He GQ, Zhuo F, Qiu GP, Sun SQ: Immunolocalization of aquaporins in rat brain. Anat Histol Embryol 40: 299– 306, 2011. [DOI] [PubMed] [Google Scholar]
- 260). Yamamoto N, Yoneda K, Asai K, Sobue K, Tada T, Fujita Y, Katsuya H, Fujita M, Aihara N, Mase M, Yamada K, Miura Y, Kato T: Alterations in the expression of the AQP family in cultured rat astrocytes during hypoxia and reoxygenation. Brain Res Mol Brain Res 90: 26– 38, 2001. [DOI] [PubMed] [Google Scholar]
- 261). Chai RC, Jiang JH, Wong AY, Jiang F, Gao K, Vatcher G, Hoi Yu AC: AQP5 is differentially regulated in astrocytes during metabolic and traumatic injuries. Glia 61: 1748– 1765, 2013. [DOI] [PubMed] [Google Scholar]
- 262). Praetorius J, Nielsen S: Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol, Cell Physiol 291: C59– C67, 2006. [DOI] [PubMed] [Google Scholar]
- 263). Speake T, Freeman LJ, Brown PD: Expression of aquaporin 1 and aquaporin 4 water channels in rat choroid plexus. Biochim Biophys Acta 1609: 80– 86, 2003. [DOI] [PubMed] [Google Scholar]
- 264). Akdemir G, Kaymaz F, Gursoy-Özdemir Y, Akalan N, Akdemir ES: The time course changes in expression of aquaporin 4 and aquaporin 1 following global cerebral ischemic edema in rat. Surg Neurol Int 7: 4, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265). Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS: Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem 269: 5497– 5500, 1994. [PubMed] [Google Scholar]
- 266). Ishibashi K, Kuwahara M, Sasaki S: Molecular biology of aquaporins, in Reviews of Physiology Biochemistry and Pharmacology, Vol 141 Berlin, Springer-Verlag, 2000, pp 1– 32 [DOI] [PubMed] [Google Scholar]
- 267). Sobue K, Yamamoto N, Yoneda K, Fujita K, Miura Y, Asai K, Tsuda T, Katsuya H, Kato T: Molecular cloning of two bovine aquaporin-4 cDNA isoforms and their expression in brain endothelial cells. Biochim Biophys Acta 1489: 393– 398, 1999. [DOI] [PubMed] [Google Scholar]
- 268). Venero JL, Vizuete ML, Machado A, Cano J: Aquaporins in the central nervous system. Prog Neurobiol 63: 321– 336, 2001. [DOI] [PubMed] [Google Scholar]
- 269). Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP: Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17: 171– 180, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270). Di Benedetto B, Malik VA, Begum S, Jablonowski L, Gómez-González GB, Neumann ID, Rupprecht R: Fluoxetine requires the endfeet protein aquaporin-4 to enhance plasticity of astrocyte processes. Front Cell Neurosci 10: 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271). Ibata K, Takimoto S, Morisaku T, Miyawaki A, Yasui M: Analysis of aquaporin-mediated diffusional water permeability by coherent anti-stokes Raman scattering microscopy. Biophys J 101: 2277– 2283, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272). Igarashi H, Tsujita M, Kwee IL, Nakada T: Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. Neuroreport 25: 39– 43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273). Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S: Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA 95: 11981– 11986, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274). Agre P: The aquaporin water channels. Proc Am Thorac Soc 3: 5– 13, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275). Suzuki Y, Nakamura Y, Yamada K, Huber VJ, Tsujita M, Nakada T: Aquaporin-4 positron emission tomography imaging of the human brain: first report. J Neuroimaging 23: 219– 223, 2013. [DOI] [PubMed] [Google Scholar]
- 276). Benfenati V, Ferroni S: Water transport between CNS compartments: functional and molecular interactions between aquaporins and ion channels. Neuroscience 168: 926– 940, 2010. [DOI] [PubMed] [Google Scholar]
- 277). Guadagno E, Moukhles H: Laminin-induced aggregation of the inwardly rectifying potassium channel, Kir4.1, and the water-permeable channel, AQP4, via a dystroglycan-containing complex in astrocytes. Glia 47: 138– 149, 2004. [DOI] [PubMed] [Google Scholar]
- 278). Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC: Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta 1758: 1085– 1093, 2006. [DOI] [PubMed] [Google Scholar]
- 279). Yukutake Y, Yasui M: Regulation of water permeability through aquaporin-4. Neuroscience 168: 885– 891, 2010. [DOI] [PubMed] [Google Scholar]
- 280). Papadopoulos MC, Verkman AS: Aquaporin water channels in the nervous system. Nat Rev Neurosci 14: 265– 277, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281). MacAulay N, Zeuthen T: Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience 168: 941– 956, 2010. [DOI] [PubMed] [Google Scholar]
- 282). Owler BK, Pitham T, Wang D: Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res 7: 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283). Oshio K, Binder DK, Liang Y, Bollen A, Feuerstein B, Berger MS, Manley GT: Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery 56: 375– 381; discussion 375–381, 2005. [DOI] [PubMed] [Google Scholar]
- 284). Oshio K, Binder DK, Bollen A, Verkman AS, Berger MS, Manley GT: Aquaporin-1 expression in human glial tumors suggests a potential novel therapeutic target for tumor-associated edema. Acta Neurochir Suppl 86: 499– 502, 2003. [DOI] [PubMed] [Google Scholar]
- 285). Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS: Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest 100: 957– 962, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286). Verkman AS: Knock-out models reveal new aquaporin functions. Handb Exp Pharmacol 359– 381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287). Badaut J, Fukuda AM, Jullienne A, Petry KG: Aquaporin and brain diseases. Biochim Biophys Acta 1840: 1554– 1565, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288). Hirt L, Fukuda AM, Ambadipudi K, Rashid F, Binder D, Verkman A, Ashwal S, Obenaus A, Badaut J: Improved long-term outcome after transient cerebral ischemia in aquaporin-4 knockout mice. J Cereb Blood Flow Metab 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289). Igarashi H, Tsujita M, Suzuki Y, Kwee IL, Nakada T: Inhibition of aquaporin-4 significantly increases regional cerebral blood flow. Neuroreport 24: 324– 328, 2013. [DOI] [PubMed] [Google Scholar]
- 290). Li X, Bai R, Zhang J, Wang X: Effect of progesterone intervention on the dynamic changes of AQP-4 in hypoxic-ischaemic brain damage. Int J Clin Exp Med 8: 18831– 18836, 2015. [PMC free article] [PubMed] [Google Scholar]
- 291). Nakada T: Virchow-Robin space and aquaporin-4: new insights on an old friend. Croat Med J 55: 328– 336, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292). Zador Z, Stiver S, Wang V, Manley GT: Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol 159– 170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293). Patil RV, Xu S, van Hoek AN, Rusinko A, Feng Z, May J, Hellberg M, Sharif NA, Wax MB, Irigoyen M, Carr G, Brittain T, Brown P, Colbert D, Kumari S, Varadaraj K, Mitra AK: Rapid identification of novel inhibitors of the human aquaporin-1 water channel. Chem Biol Drug Des 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294). Hokari M, Yokoseki A, Arakawa M, Saji E, Yanagawa K, Yanagimura F, Toyoshima Y, Okamoto K, Ueki S, Hatase T, Ohashi R, Fukuchi T, Akazawa K, Yamada M, Kakita A, Takahashi H, Nishizawa M, Kawachi I: Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann Neurol 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 295). Kim W, Lee JE, Kim SH, Huh SY, Hyun JW, Jeong IH, Park MS, Cho JY, Lee SH, Lee KS, Kim HJ: Cerebral cortex involvement in neuromyelitis optica spectrum disorder. J Clin Neurol 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296). Kariya Y, Kariya Y, Saito T, Nishiyama S, Honda T, Tanaka K, Yoshida M, Fujihara K, Hashimoto Y: Increased cerebrospinal fluid osteopontin levels and its involvement in macrophage infiltration in neuromyelitis optica. BBA Clin 3: 126– 134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297). Pandit L: Neuromyelitis optica spectrum disorders: an update. Ann Indian Acad Neurol 18: S11– S15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298). Pereira WL, Reiche EM, Kallaur AP, Kaimen-Maciel DR: Epidemiological, clinical, and immunological characteristics of neuromyelitis optica: a review. J Neurol Sci 355: 7– 17, 2015. [DOI] [PubMed] [Google Scholar]
- 299). Bloch O, Auguste KI, Manley GT, Verkman AS: Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab 26: 1527– 1537, 2006. [DOI] [PubMed] [Google Scholar]
- 300). Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS: Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6: 159– 163, 2000. [DOI] [PubMed] [Google Scholar]
- 301). Saadoun S, Tait MJ, Reza A, Davies DC, Bell BA, Verkman AS, Papadopoulos MC: AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience 161: 764– 772, 2009. [DOI] [PubMed] [Google Scholar]
- 302). Li X, Kong H, Wu W, Xiao M, Sun X, Hu G: Aquaporin-4 maintains ependymal integrity in adult mice. Neuroscience 162: 67– 77, 2009. [DOI] [PubMed] [Google Scholar]
- 303). Kuriyama N, Yamada K, Sakai K, Tokuda T, Akazawa K, Tomii Y, Tamura A, Kondo M, Watanabe I, Ozaki E, Matsui D, Nakagawa M, Mizuno T, Watanabe Y: Ventricular Temperatures in Idiopathic Normal Pressure Hydrocephalus (iNPH) Measured with DWI-based MR Thermometry. Magn Reson Med Sci 14: 305– 312, 2015. [DOI] [PubMed] [Google Scholar]
- 304). Iliff JJ, Goldman SA, Nedergaard M: Implications of the discovery of brain lymphatic pathways. Lancet Neurol 14: 977– 979, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305). Ohno N, Miyati T, Mase M, Osawa T, Kan H, Kasai H, Hara M, Shibamoto Y, Hayashi N, Gabata T, Matsui O: Idiopathic normal-pressure hydrocephalus: temporal changes in ADC during cardiac cycle. Radiology 261: 560– 565, 2011. [DOI] [PubMed] [Google Scholar]
- 306). Mase M, Yamada K, Banno T, Miyachi T, Ohara S, Matsumoto T: Quantitative analysis of CSF flow dynamics using MRI in normal pressure hydrocephalus. Acta Neurochir Suppl 71: 350– 353, 1998. [DOI] [PubMed] [Google Scholar]
- 307). Miyati T, Mase M, Banno T, Kasuga T, Yamada K, Fujita H, Koshida K, Sanada S, Onoguchi M: Frequency analyses of CSF flow on cine MRI in normal pressure hydrocephalus. Eur Radiol 13: 1019– 1024, 2003. [DOI] [PubMed] [Google Scholar]
- 308). Le Bihan D, Moonen CT, van Zijl PC, Pekar J, DesPres D: Measuring random microscopic motion of water in tissues with MR imaging: a cat brain study. J Comput Assist Tomogr 15: 19– 25, 1991. [DOI] [PubMed] [Google Scholar]
- 309). Zelenina M: Regulation of brain aquaporins. Neurochem Int 57: 468– 488, 2010. [DOI] [PubMed] [Google Scholar]
- 310). Sato O: [Reconsideration of research into cerebrospinal fluid], in Arai H, Ishikawa M, Mori E. (eds): iNPH: Idiopathic Normal Pressure Hydrocephalus. Kyoto, Kinpodo, 2014, pp 8– 18 (Japanese) [Google Scholar]