Abstract
Noise-induced hearing loss (NIHL) is a major unresolved public health problem. Here, we investigate pathomechanisms of sensory hair cell death and suggest a novel target for protective intervention. Cellular survival depends upon maintenance of energy homeostasis, largely by AMP-activated protein kinase (AMPK). In response to a noise exposure in CBA/J mice, the levels of phosphorylated AMPKα increased in hair cells in a noise intensity-dependent manner. Inhibition of AMPK via siRNA or the pharmacological inhibitor compound C attenuated noise-induced loss of outer hair cells (OHCs) and synaptic ribbons, and preserved auditory function. Additionally, noise exposure increased the activity of the upstream AMPK kinase liver kinase B1 (LKB1) in cochlear tissues. The inhibition of LKB1 by siRNA attenuated the noise-increased phosphorylation of AMPKα in OHCs, reduced the loss of inner hair cell synaptic ribbons and OHCs, and protected against NIHL. These results indicate that noise exposure induces hair cell death and synaptopathy by activating AMPK via LKB1-mediated pathways. Targeting these pathways may provide a novel route to prevent NIHL.
SIGNIFICANCE STATEMENT Our results demonstrate for the first time that the activation of AMP-activated protein kinase (AMPK) α in sensory hair cells is noise intensity dependent and contributes to noise-induced hearing loss by mediating the loss of inner hair cell synaptic ribbons and outer hair cells. Noise induces the phosphorylation of AMPKα1 by liver kinase B1 (LKB1), triggered by changes in intracellular ATP levels. The inhibition of AMPK activation by silencing AMPK or LKB1, or with the pharmacological inhibitor compound C, reduced outer hair cell and synaptic ribbon loss as well as noise-induced hearing loss. This study provides new insights into mechanisms of noise-induced hearing loss and suggests novel interventions for the prevention of the loss of sensory hair cells and cochlear synaptopathy.
Keywords: activation of AMPK, cochlear synaptopathy, noise-induced hearing loss, protection of noise-induced hearing loss, sensory hair cells
Introduction
Exposure to damaging levels of noise is a major cause of hearing loss worldwide. Animal research has delineated pathomechanisms related to oxidative stress and calcium influx, and potential approaches to pharmacological intervention (Yamane et al., 1995; Yamasoba et al., 1998; Ohlemiller et al., 1999; Ohinata et al., 2000; Le Prell et al., 2007; Fetoni et al., 2013; Yuan et al., 2015). However, there is no clinical treatment to prevent or mitigate the pathology of noise-induced hearing loss (NIHL), which includes loss of sensory hair cells and synaptic connections to the auditory nerve and, consequently, auditory function (Wang et al., 2002; Kujawa and Liberman, 2009; Liberman and Liberman, 2015). Our laboratory has recently reported (Chen et al., 2012) that transient intracellular ATP reduction is one of the initial responses of cochlear tissues to noise exposure in mice, suggesting the involvement of AMP-activated protein kinase (AMPK)-related mechanisms in NIHL.
The survival of essentially all organisms depends on the dynamic control of energy metabolism during short-term or long-term exposure to various stressors (Viollet et al., 2010). AMPK is a key cellular energy sensor that is able to detect and react to the increased intracellular levels of AMP that accompany reductions in ATP (Winder and Thomson, 2007). This kinase regulates energy homeostasis by directing the cell to switch off energy-consuming pathways and to turn on energy-generating pathways (Hardie et al., 2003; Hardie, 2003; Viollet et al., 2010). The binding of AMP to the AMPKγ subunit allosterically activates AMPK twofold to fivefold (Hardie et al., 1999). In addition, the binding of AMP exposes the threonine 172 (T172) residue on the catalytic α subunit to reversible phosphorylation by upstream kinases, such as liver kinase B1 (LKB1). This combined activation via allosteric and phosphorylation mechanisms causes a 1000-fold increase in the kinase activity of AMPK, allowing a high sensitivity in response to small changes in the intracellular energy status (Suter et al., 2006).
Although the activation of AMPK is initially an adaptive response to cellular stress, there are diverse consequences of AMPK activity on cell death and survival. In neuronal cells, AMPK has a neuroprotective effect against glutamate excitotoxicity by elevating glucose transporter 3 trafficking in response to a decrease in cellular ATP levels (Weisová et al., 2011). On the other hand, the prolonged elevation of phosphorylated (p)-AMPK triggers the chronic activation of c-Jun N-terminal protein kinase (JNK), the upregulation of proapoptotic Bim, and the subsequent apoptosis in neuronal and pancreatic cells (Kefas et al., 2004; Yun et al., 2005; Weisová et al., 2011). AMPK is also able to inhibit JNK activity in neuronal cells, suggesting that the regulation of cell fate by AMPK is complex and may depend on the cell type and insult (Schulz et al., 2008; Yun et al., 2009; Weisová et al., 2011).
Despite the central functions of AMPK in the regulation of cell fate, the role of AMPK and its mode of activation in sensory hair cells after inner ear insults have yet to be established. To investigate these questions, we examined the contribution of AMPK and LKB1 to noise-induced hearing loss in CBA/J mice by using siRNA silencing and pharmacological modulators. These studies are the first to explore the role of AMPK and LKB1 in the pathogenesis of noise-induced sensory hair cells loss, cochlear synaptopathy, and subsequent hearing loss.
Materials and Methods
Animals.
Male CBA/J mice at the age of 10 weeks were purchased from The Jackson Laboratory. All mice had free access to water and a regular mouse diet (catalog #2918; Harlan Sprague Dawley), and were kept at 22 ± 1°C under a standard 12 h light/dark cycle to acclimate for at least 1 week before the experiments. Noise exposure was conducted at the age of 12 weeks. All research protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Animal care was under the supervision of the Division of Laboratory Animal Resources at the Medical University of South Carolina.
Noise exposure.
Unrestrained CBA/J male mice at 12 weeks of age were exposed to a broadband noise with a frequency spectrum from 2 to 20 kHz for 2 h at either 106 dB SPL, to induce severe permanent threshold shifts (PTS) that range from 50 to 65 dB at high frequencies with a loss of both outer hair cells (OHCs) and inner hair cells (IHCs), or 98 dB SPL, to induce PTS that range from 40 to 50 dB with the loss of OHCs only examined 2 weeks after the exposure (Yuan et al., 2015). Generally, four mice (one mouse per stainless steel wire cage) were located in the center of the sound chamber, and the levels of sound were consistent throughout the field during the noise exposure. Age- and gender-matched control mice were kept in silence within the same chamber and cages for 2 h without noise exposure. The sound exposure chamber was the same as previously described (Chen et al., 2012; Zheng et al., 2014; Yuan et al., 2015). Briefly, the chamber was fitted with a loudspeaker (model 2450H; JBL) driven by a power amplifier (model XLS 202D; Crown Audio) fed from a CD player (model Tascam CD-200; TEAC America). Audio CD sound files were created and equalized with audio editing software (Audition 3; Adobe Systems). Sound levels were calibrated with a sound level meter (model 1200; Quest Technologies) at multiple locations within the chamber to ensure the uniformity of the sound field among the four cages, and were measured before and after exposure to ensure stability.
Auditory brainstem responses.
Mice were anesthetized with an injection of xylazine (20 mg/kg, i.p.) and ketamine (100 mg/kg, i.p.), and then placed in a sound-isolated and electrically shielded booth (Acoustic Systems). Body temperature was maintained near 37°C with a heating pad. Acoustic stimuli were delivered monaurally to a Beyer earphone attached to a customized plastic speculum inserted into the ear canal. Subdermal electrodes were inserted at the vertex of the skull, under the left ear and under the right ear (ground). Auditory brainstem responses (ABRs) were measured at 8, 16, and 32 kHz. Tucker Davis Technology System III hardware and SigGen/Biosig software were used to present the stimuli (15 ms duration tone bursts with 1 ms rise–fall time) and record the response. Up to 1200 responses were averaged for each stimulus level. Thresholds were determined for each frequency by reducing the intensity in 10 dB increments and then in 5 dB steps near threshold until no organized responses were detected. ABR waves I and II were monitored to assess thresholds. Thresholds were estimated between the lowest stimulus level where a response was observed and the highest level without a response. All ABR measurements were conducted by the same experimenter. The ABR thresholds were assessed by an expert who was blinded to the treatment conditions.
Drug administration via intraperitoneal route.
Compound C (CC; catalog #P5499; Sigma-Aldrich) was dissolved in dimethylsulfoxide (DMSO) as a stock solution (31.25 mg/ml) and stored at −20°C. The stock solution was diluted in 0.9% saline solution immediately before injection. Each animal received a total of three intraperitoneal injections of compound C at a dose of 20 or 10 mg/kg per injection. The three intraperitoneal injections were administered 24 h before, 2 h before, and immediately after noise exposure. The animals were decapitated 1 h after noise exposure, and the temporal bones were removed to dissect the cochlea for immunohistochemistry assays. The mice used for experiments to observe the progression of ABR thresholds received an additional intraperitoneal injection 24 h after noise exposure.
Intratympanic delivery of siRNA.
AMPKα1 siRNA (siAMPKα1; catalog #s98535; Invitrogen), siLKB1 (catalog #s74497; Invitrogen), or scrambled siRNA (siControl; Invitrogen) was locally delivered via intratympanic application, which allows the entry of siRNA specifically within sensory hair cells with minimal uptake in other cochlear cell types, as previously described (Chen et al., 2013; Oishi et al., 2013). Briefly, after anesthesia, a retroauricular incision was made to approach the temporal bone. The otic bulla was identified ventral to the facial nerve, and a shallow hole was made in the thin part of the otic bulla with a 30 ga needle and enlarged with a dental drill to a diameter of 2 mm to visualize the round window. A customized sterile micro medical tube was inserted into the hole just above the round window to slowly deliver 10 μl (0.3, 0.6, or 0.9 μg) of a single siRNA type via syringe. Although the volume of the mouse middle ear cavity is 5–8 μl, we slowly flushed 10 μl of the siRNA solution into the cavity, as some of the solution flows out through the 2 mm hole made for access to the round window niche. After siRNA delivery, the hole was covered with surrounding muscle and glued with tissue adhesive. Last, the skin incision was closed with tissue adhesive, and the mouse was kept in the surgical position for 1 h. Based on our previous experiments (Oishi et al., 2013; Zheng et al., 2014), local intratympanic delivery of siRNA results in temporary elevation of thresholds that completely recovers by 72 h. Therefore, noise exposure was performed 72 h after siRNA delivery.
Surface preparations and diaminobenzidine staining of cochlear epithelia for hair cell counts.
The procedures for surface preparations and diaminobenzidine (DAB) staining of cochlear epithelia were followed as previously described (Chen et al., 2012). Briefly, the temporal bones were removed immediately following the decapitation of the mice, and were perfused through the cochlear scala media with a solution of 4% paraformaldehyde in PBS, pH 7.4, and kept in this fixative overnight at 4°C. The cochleae were then rinsed in PBS. Before decalcification in a 4% solution of sodium EDTA (adjusted with HCl to pH 7.4), the apical otic capsule was removed from each cochlea. The EDTA solution was changed daily for 3 d and maintained at 4°C. Following decalcification, the cochleae were placed in 3% hydrogen peroxide for 2.5 h to quench endogenous peroxidases. After incubation in a solution for blocking nonspecific antibody binding overnight at 4°C, the tissues were incubated with a primary antibody (rabbit polyclonal anti-myosin VIIa; catalog #25-6790; Proteus Biosciences) at a 1:100 dilution for 4 d at 4°C on a Nutator mixer, washed in PBS, and then incubated overnight at 4°C with secondary antibody (biotinylated goat anti-rabbit) at a 1:100 dilution. The specimens were rinsed again and then incubated in ABC solution (catalog #PK-4001; Vector Laboratories) overnight. Following another washing, the cochleae were incubated in DAB for 3 h, as necessary for sufficient staining intensity, followed by washing to stop the DAB reaction. Finally, the cochleae were microdissected under a microscope into equally sized apical, middle, and basal segments, and mounted on slides with Fluoromount-G mounting medium. Images were taken with Zeiss AxioCam MRc5 camera with Axioplan 2 imaging software with a Zeiss microscope for hair cell counts. Hair cells were counted from captured images using the 40× magnification lens on the Zeiss microscope from the apex through the base of the DAB-stained surface preparations. The lengths of the cochlear epithelia were measured and recorded in millimeters. Both outer and inner hair cells were counted from the apex to the base along the entire length of the mouse cochlear epithelium. The percentages of hair cell loss in each 0.5 mm length of epithelium were plotted as a function of the cochlear length as a cytocochleogram (Chen et al., 2012).
Immunohistochemistry for cochlear paraffin sections.
Following decalcification with 4% EDTA, each cochlea was transferred to 70% ethanol and embedded in paraffin for sections. Five-micrometer formalin-fixed paraffin-embedded (FFPE) sections were routinely deparaffinized in xylene and rehydrated in alcohol. The sections were incubated with target retrieval solution (catalog #S2367; Dako) in a steamer (catalog #CKSTSTMD5-W; Oster) for 10 min, and then 3% hydrogen peroxide for 10 min and protein block (catalog #0909; Dako) for 20 min at room temperature. A monoclonal primary antibody [p-AMPKα at 1:100 (catalog #2535; Cell Signaling Technology); AMPKα1 at 1:200 (catalog #ab32047; Abcam); AMPKα2 at 1:100 (catalog #ab3760; Abcam); p-LKB1 (S428) at 1:500 (catalog #SAB4504034; Sigma-Aldrich)] was incubated overnight in a humid chamber at 4°C, followed by biotinylated secondary antibody (Vector Laboratories) for 30 min and ABC reagent (Vector Laboratories) for 30 min. Immunocomplexes of horseradish peroxidase were visualized by DAB (Dako) reaction, and sections were counterstained with hematoxylin before mounting.
Immunohistochemistry of cochlear surface preparations.
Following decalcification with 4% EDTA, each cochlea for immunocytochemistry of surface preparations was dissected under a microscope by removing the softened otic capsule, stria vascularis, Reissner's membrane, and tectorial membrane. The remaining tissue, including the modiolus and cochlear sensory epithelium, was permeabilized in 3% Triton X-100 solution for 30 min at room temperature. The specimens were washed three times with PBS and blocked with 10% goat serum for 30 min at room temperature. For immunolabeling of hair cell proteins, the tissues were incubated at 4°C overnight with the following primary antibodies: monoclonal rabbit anti-p-AMPKα (T177) at 1:50 (catalog #2535S; Cell Signaling Technology); or monoclonal rabbit anti-p-LKB1 at 1:50 (catalog #3482; Cell Signaling Technology). After washing three times, the tissues were incubated with the Alexa Fluor 594-conjugated secondary antibody at a concentration of 1:200 at 4°C overnight in darkness. After washing, specimens were then incubated with Alexa Fluor 488 phalloidin at a 1:1000 at room temperature (25°C) for 20 min in darkness. For immunolabeling of synaptic ribbons and glutamate receptors, the cochleae were fixed for 45 min. After the decalcification, tissues were incubated at 37°C overnight with the following primary antibodies: monoclonal mouse anti-carboxyl-terminal binding protein 2 (CtBP2) IgG1 at 1:200 (#612044; BD Biosciences) and monoclonal mouse anti-GluR2 IgG2a at 1:2000 (catalog MAB397; Millipore). After washing three times, the tissues were incubated with the Alexa Fluor 594- and Alexa Fluor 488-conjugated secondary antibodies at a concentration of 1:1000 at 37°C for 1 h in darkness. After washing three times, the tissues were reincubated with Alexa Fluor-conjugated secondary antibodies for an additional 1 h at 37°C to increase the immunolabeling for GluR2 (Wan et al., 2014). After washing three times, the tissues were incubated in darkness at 4°C overnight with polyclonal rabbit anti-myosin VIIa at 1:200 (catalog #25-6790; Proteus Biosciences). Following washing steps, the tissues were incubated with Alexa Fluor 350-conjugated secondary antibody at a concentration of 1:200 at 4°C overnight in darkness. For all immunolabeling samples, after the final wash with PBS, the tissue was dissected in PBS by removing the modiolus. The epithelia were divided equally into three segments (apex, middle, and base). Specimens were mounted on slides with Fluoro-gel with Tris buffer (catalog #17985-10; Electron Microscopy Sciences). Control incubations were routinely processed without primary antibody treatments. Immunolabeled images were taken using a laser confocal microscope (model LSM 510, Zeiss; or TCS SP5 AOBS, Leica).
Quantification of the immunolabeled signals from outer hair cells of surface preparations.
Immunolabeled signals of OHCs on surface preparations were quantified from original confocal images, each taken with a 63× magnification lens under identical conditions and equal parameter settings for laser gains and photomultiplier tube (PMT) gains, using ImageJ software (National Institutes of Health). The cochleae from the different groups were fixed and stained simultaneously with identical solutions and processed in parallel. All of the surface preparations were counterstained with Alexa Fluor 488 phalloidin (green) to label hair cell structure. The borders of each individual OHC were outlined with the circle tool based on the phalloidin staining. The immunolabeling for the target proteins was measured in the basal region of cochlear surface preparations in 0.12 mm segments, each containing ∼60 OHCs. The intensity of the background fluorescence was subtracted, and the average fluorescence per cell was calculated. The fluorescence was quantified by normalizing the ratio of average fluorescence of noise-exposed hair cells to the average fluorescence of the unexposed hair cells. In these studies, there were no differences in the levels of p-AMPKα and LKB1 molecules in OHCs of the apical and middle turns between ears of control mice and those harvested 1 h after noise.
Quantification of the immunolabeled ribbons from z-projections on surface preparations.
Immunolabeling of CtBP2 on surface preparations was quantified from original confocal images, each taken with a 63× magnification lens under identical z-stack conditions with 0.25 intervals and equal parameter settings for laser gains and PMT. The z-stack images in each 0.12 mm segment (containing ∼16 IHCs) were captured from cochlear surface preparations. The number of synaptic ribbons was counted using ImageJ software. Briefly, the background of the images was subtracted and the threshold was set to isolate the immunolabeling of signals from IHCs. The image was converted to a binary file and the number of ribbon particles was counted using the 3D Object Counter. The binary file was converted to a 3D z–y projection to measure ribbon dispersion. The number of functional synapses, identified by juxtaposed CtBP2 and GluR2, were manually counted by visualizing the presence of GluR2 colocalization with CtBP2.
Extraction of total cochlear protein.
Cochleae were rapidly removed and dissected in ice-cold PBS, pH 7.4, containing complete mini EDTA-free protease inhibitor cocktail tablets (catalog #11836170001; Roche Diagnostics). To extract total protein, tissues from the cochleae of a single mouse were homogenized in ice-cold RIPA lysis buffer containing RIPA lysis buffer (catalog #R0278; Sigma-Aldrich) plus phosphatase inhibitor cocktails II and III, and Roche protease inhibitor by using a glass/glass microtissue grind pestle and vessel for 30 s. Tissue debris were removed by centrifugation at 10,000 × g at 4°C for 10 min, and the supernatants were retained as the total protein fractions. Protein concentrations were determined using the Bio-Rad Protein Assay dye reagent with bovine serum albumin as a protein standard. Two cochleae from the same mouse were pooled for each sample. Unless otherwise specified, all chemicals and reagents used were purchased from Sigma-Aldrich.
Extraction of protein from formalin-fixed sensory epithelia.
Cochleae were rapidly removed and perfused with 4% paraformaldehyde through the cochlear scala media and incubated for 2 h at room temperature (25°C). The cochleae were then rinsed in PBS and decalcified in a 4% solution of sodium EDTA for 3 d at 4°C, with the EDTA solution changed daily. Following decalcification, the dissected sensory epithelia from three mice were placed in 1.5 ml collection tubes with 100 μl of extraction buffer EXB Plus (Qproteome FFPE Tissue Kit #37623; Qiagen) supplemented with β-mercaptoethanol. Glass microgrinder pestles were used to grind the tissue for 3 min. The tubes were sealed with a sealing clip and vortexed. The samples were incubated on ice for 5 min, followed by repeat vortexing. The tubes were then incubated for 20 min at 100°C on a heating block. After this incubation, the tubes were incubated for 2 h at 80°C with agitation at 750 rpm (Eppendorf) and then allowed to cool at 4°C for 1 min. Finally, the samples were centrifuged at 14,000 × g at 4°C for 15 min. The supernatant containing the extracted proteins were transferred to a new tube. Protein concentrations were determined using the Bio-Rad RC DC protein assay (catalog #500-0119; Invitrogen) with bovine serum albumin as a protein standard.
Western blot analysis.
Protein samples (30 μg) were separated by SDS-PAGE. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (Pierce) and blocked with 5% nonfat dry milk in PBS plus 0.1% Tween 20 (PBS-T). The membranes were incubated with anti-p-LKB1 (1:1000; catalog #3482; Cell Signaling Technology), anti-LKB1 (1:1000), or anti-GAPDH (1:10,000; catalog #MAB374; Millipore) at 4°C overnight, and then washed three times (10 min each) with PBS-T buffer. Membranes were incubated with an appropriate secondary antibody at a concentration of 1:2500 for 1 h. Following extensive washing of the membrane, the immunoreactive bands were visualized by SuperSignal West Dura Extended Duration Substrate or Thermo Scientific Pierce ECL Western Blotting Substrate (ThermoFisher Scientific). The x-ray films of Western blots were scanned and analyzed using ImageJ software. The band densities were first normalized to background. Next, the probing protein/GAPDH ratio was calculated from the band densities run on the same gel. Finally, the difference in the ratio of the control and experimental bands was tested for statistical significance.
Extraction of total cochlear RNA for quantitative PCR.
Cochleae were rapidly removed and isolated in RNAlater (Invitrogen). Cochleae from a single mouse were placed in a 2 ml tube with 100 μl of RNAlater and immediately crushed with forceps. A 1 ml volume of TRIzol (Invitrogen) was added to each sample, followed by homogenization with an RNase-free Polytron Tissue Grinder (Kinematica). The samples were incubated for 5 min at room temperature before the addition of 0.2 ml of chloroform and vigorous shaking. After 2–3 min of incubation, the samples were centrifuged at 12,000 × g at 4°C for 15 min, and the upper colorless aqueous phase was retained and transferred to phase-lock gel heavy tubes (Eppendorf). A total of 0.2 ml of chloroform was added to each sample and followed by centrifugation at 12,000 × g at 4°C for 10 min. The upper aqueous phase was transferred to a fresh RNase-free tube for RNA precipitation with 1.5 μl of glycogen and 0.5 ml of isopropanol, followed by centrifugation at 12,000 × g at 4°C for 10 min. The RNA pellet was washed in 75% ethanol and dissolved in DEPC water. RNA concentrations were determined using a NanoDrop spectrophotometer. Relative expression levels were analyzed using the Light Cycler 480 (Roche). Unless otherwise specified, all chemicals and reagents used were purchased from Sigma-Aldrich.
Statistical analysis.
Data were analyzed using SYSTAT and GraphPad software for Windows. The group size (n) in vivo was determined by the variability of measurements and the magnitude of the differences between groups. Statistical methods used include one-way ANOVA with Tukey's multiple comparisons, repeated-measures ANOVA with post hoc testing, unpaired t tests, and one-sample t tests. All tests were two tailed, and a p value of <0.05 was considered to be statistically significant.
Results
AMPKα is phosphorylated in basal OHCs in a noise intensity-dependent manner
Based on our previously characterized parameters for CBA/J mice, we used specific noise conditions for temporary threshold shifts without OHC loss (92 dB SPL), PTS with only OHC loss (98 dB SPL), and severe PTS with both outer and IHC loss (106 dB SPL) to evaluate the p-AMPKα (Chen et al., 2012; Yuan et al., 2015). Under the 106 dB noise exposure condition, there was no IHC loss noted at 1 h after the exposure, but apoptotic and necrotic outer hair cell death appeared in the lower basal region (beginning ∼5 mm from the apex; Chen et al., 2012; Zheng et al., 2014). Immunolabeling for p-AMPKα at threonine 172 in OHCs 1 h after 92 dB exposure was similar to age-matched controls without the exposure; however, after 98 dB exposure, immunolabeling for p-AMPKα in OHCs at basal turn was increased by 60% (p = 0.013, t(10) = 2.994) and 230% after 106 dB exposure (p = 0.003, t(3) = 8.971). Furthermore, the increase in p-AMPKα by 106 dB exposure was significantly higher than after a 98 dB exposure (p = 0.004, t(5) = −5.119; Fig. 1a,a′). Since changes in AMPKα phosphorylation were detected only following 98 and 106 dB exposures leading to PTS, these conditions were used for the rest of the study.
Figure 1.
Noise exposure increased p-AMPKα in basal outer and inner hair cells in a noise intensity-dependent manner. a, The p-AMPKα immunolabeling (red) was stronger in basal OHCs (green) 1 h after exposure to 98 or 106 dB noise than unexposed controls. Representative images were taken from the basal turn. a′, Quantification of p-AMPKα immunolabeling in basal OHCs confirmed significant increases in a noise intensity-dependent fashion. Data are presented as the mean ± SD. **p < 0.01, *p < 0.05. Control, n = 4; 98 dB, n = 3; 106 dB, n = 4. b, Western blot using sensory epithelium tissues displayed antibody specificity for p-AMPKα and total AMPKα, but no alteration in band density for p-AMPKα and total AMPKα examined 1 h after 98 or 106 dB exposure compared with unexposed controls. GAPDH served as the loading control; n = 4. c, Sections of the adult CBA/J mouse cochlea showed increased DAB-stained immunolabeling for p-AMPKα (brown) in IHCs (arrow) and OHCs (arrowheads) of the organ of Corti (OC), but no obvious changes in SGN or the stria vascularis (Stria) 1 h after 98 dB exposure. There was strong immunolabeling for p-AMPKα in outer and inner pillar cells in both control and noise-exposed mice. Representative images were taken from the upper basal turn. Scale bars: a, c, 10 μm.
To evaluate the localization of this increased p-AMPKα in three key regions of the cochlea, the organ of Corti, spiral ganglion neurons (SGNs), and stria vascularis, p-AMPKα was immunolabeled in cochlear paraffin sections. In nonexposed control animals, immunolabeling for p-AMPKα was weak in OHCs (arrowheads) and IHCs (arrow), but was stronger in both outer and inner pillar cells. One hour after a 98 dB noise exposure, the immunolabeling for p-AMPKα was changed only in IHCs and OHCs with a stronger change in IHCs and marginal elevation in OHCs; no obvious changes were detected in either outer or inner pillar cells, and p-AMPKα immunolabeling remained relatively weak within Deiters cells of the organ of Corti, the SGNs, and the stria vascularis (Fig. 1c). The strong immunolabeling for p-AMPKα in outer and inner pillar cells in both control and exposed mice is in agreement with previous observations (Nagashima et al., 2011). Western blots, using formalin-fixed tissues of the sensory epithelia, detected a single band for p-AMPKα at 63 kDa, indicating the specificity of the antibody. Changes in band density of p-AMPKα with noise exposure were not detectable (Fig. 1b).
Since noise exposure increased the levels of phosphorylated AMPKα in basal OHCs by immunohistochemistry analysis from confocal images, we also examined the expression of total AMPKα in cochlear cell types by cochlear paraffin sections. In the control animals, immunolabeling for total AMPKα1 was primarily localized to Deiters and pillar cells of the organ of Corti. One hour after a 98 dB noise exposure, AMPKα1 was increased in the cytosol of OHCs, IHCs, and pillar cells in the organ of Corti, in SGNs, and in the stria vascularis, including marginal and basal cells, and fibrocytes in the spiral ligament (Fig. 2a). Likewise, AMPKα2 immunolabeling was increased in the nuclei and cytosol of IHCs, OHCs, supporting cells, SGNs, and lateral wall structures (Fig. 2b). In contrast, Western blots for the analysis of total AMPKα1 and AMPKα2 protein levels (Fig. 2c) and qRT-PCR assessing mRNA levels of AMPKα1 and AMPKα2 (data not shown) using total cochlear homogenates were unchanged between control and noise-exposed cochlear samples examined 1 h after noise exposure.
Figure 2.
Noise exposure increased total AMPKα1 and AMPKα2 in the organ of Corti. a, b, Paraffin sections of the adult CBA/J mouse cochlea revealed localization of DAB-stained immunolabeling for total AMPKα1 (a) or AMPKα2 (b; brown) in the organ of Corti (OC), including in OHCs, IHCs, inner and outer pillar cells, SGNs, and marginal and basal cells of the stria vascularis (Stria) 1 h after 98 dB noise exposure. AMPKα1 was mainly localized in the cytosol, while AMPKα2 was predominantly localized in the nuclei. Representative images were taken from the basal turn. Scale bar, 10 μm. c, Western blots for analysis of total AMPKα1 and AMPKα2 revealed no changes after the noise exposure. GAPDH served as the sample loading control. n = 3.
Silencing AMPKα1 or treatment with pharmacological inhibitor compound C protects against noise-induced outer hair cell loss and permanent hearing loss
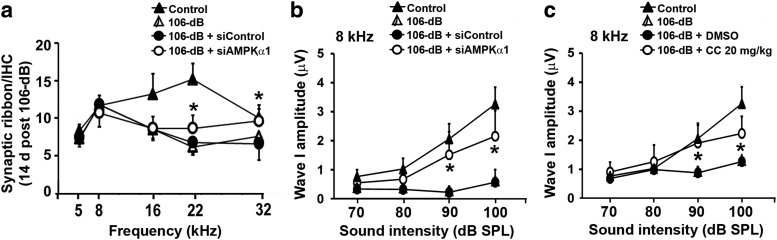
To determine the contribution of AMPKα to NIHL, AMPKα1 siRNA (siAMPKα1) was used. Three concentrations (0.3, 0.6, and 0.9 μg) of siAMPKα1 were tested in preliminary in vivo experiments; 0.6 μg was selected for use in this study due to silencing efficiency in hair cells. Seventy-two hours after intratympanic delivery of siAMPKα1, the 98 dB noise-induced elevation of immunolabeling for p-AMPKα in OHCs was attenuated by 30% compared with the OHCs of mice that received scrambled siRNA (siControl) examined 1 h after the noise exposure (p = 0.0127, t(2) = 8.781; Fig. 3a,a′). However, Western blots for the analysis of p-AMPKα and total AMPKα1 showed no difference between siControl- and siAMPKα1-treated groups (data not shown). Furthermore, pretreatment with siAMPKα1 reduced noise-induced OHC loss measured 14 d after 98 dB exposure (p = 0.001, F(1,3) = 176.966; Fig. 3d). The reduction of OHC loss was to control levels at 3–3.5 mm (p = 0.028) and by >55% at 4–5.5 mm (4 mm, p = 0.004; 4.5 mm, p < 0.001; 5 mm, p < 0.001; 5.5 mm, p < 0.001) from the apex of the sensory epithelium. Consequently, auditory threshold shifts of the siAMPKα1-treated group recovered to near-baseline levels at 8 kHz (p = 0.009, F(2,33) = 5.418) and were attenuated from 50 to 10 dB at both 16 kHz (p < 0.001, F(2,33) = 82.882) and 32 kHz (p < 0.001, F(2,33) = 135.791; Fig. 3b). In addition, the siAMPKα1 pretreatment significantly reduced auditory threshold shifts 14 d after exposure to 106 dB noise by 30 dB at 8 kHz (p = 0.023, F(2,11) = 5.226), and by 20 dB at 16 kHz (p = 0.05, F(2,11) = 3.48) and 32 kHz (p < 0.001, F(2,11) = 21.23; Fig. 3c).
Figure 3.
Inhibition of AMPKα1 via siRNA and compound C protected against noise-induced hearing loss and outer hair cell loss. a, a′, Pretreatment with siAMPKα1 decreased immunolabeling for p-AMPKα in OHCs compared with siControl treatment 1 h after 98 dB exposure; n = 3. Representative images (a) were taken from the basal turn. Scale bar, 10 μm. b, c, siAMPKα1 pretreatment reduced 98 dB (b) and 106 dB (c) noise-induced permanent threshold shifts. 98 dB, n = 18; 98 dB + siControl, n = 8; 98 dB + siAMPKα1, n = 7; 106 dB, n = 6; 106 dB + siControl, n = 3; 106 dB + siAMPKα1, n = 4. d, Noise-induced (98 dB) OHC loss was reduced by siAMPKα1 pretreatment; n = 3. e, Compound C treatment reduced 98 dB noise-induced permanent threshold shifts in a dose-dependent fashion. 98 dB + DMSO, n = 20; 98 dB + CC 10 mg/kg, n = 4; 98 dB + CC 20 mg/kg, n = 6. f, Compound C treatment (20 mg/kg) reduced 106 dB noise-induced permanent threshold shifts; n = 6. g, h, Compound C treatment (20 mg/kg) decreased 98 dB (g) and 106 dB (h) noise-induced OHC loss. 98 dB + DMSO, n = 7; 98 dB + CC 20 mg/kg, n = 6; 106 dB + DMSO, n = 3; 106 dB + CC 20 mg/kg, n = 6. i, Noise-induced IHC loss was unchanged by compound C treatment. n = 4. j, A representative image (basal turn) displays DAB-stained myosin VIIa immunolabeling of sensory hair cells of a control mouse. Data are presented as the mean ± SD. Data were collected 14 d after exposure, unless otherwise stated. ***p < 0.001, **p < 0.01, *p < 0.05.
Since silencing AMPKα1 protected against noise-induced hair cell loss and NIHL, we next examined the effects of compound C, a selective ATP-competitive inhibitor of AMPK. Based on the findings of previous studies (Shen et al., 2008), we tested two concentrations (10 and 20 mg/kg) in adult CBA/J mice. Our preliminary results showed that mice receiving five doses of either concentration maintained normal body weights and hearing thresholds. The fur of treated mice also appeared as shiny as that of control mice, and the mice displayed no overt signs of illness. Treatment with either concentration of compound C significantly reduced the auditory threshold shifts 2 weeks after a 98 dB exposure in a dose-dependent fashion at 8 kHz (F(2,27) = 4.427, p = 0.022), 16 kHz (F(2,27) = 60.052, p < 0.001), and 32 kHz (F(2,27) = 44.294, p < 0.001; Fig. 3e). Further analysis confirmed significant protection by pretreatment with either siAMPKα or compound C (for detailed statistical values, see Table 1). Since the higher dose of compound C (20 mg/kg) elicited greater protection, OHC loss was examined at this concentration. OHC loss remained <10% throughout the basal region of the cochlea in compound C-treated mice 14 d after 98 dB exposure, in contrast to the vehicle (DMSO) group in which 10%, 40%, 90%, and 100% OHC loss occurred at 3.5 mm (p = 0.046), 4 mm (p < 0.001), 4.5 mm (p < 0.001), 5 mm (p < 0.001), and 5.5 mm (p < 0.001) from the apex, respectively (p < 0.001, F(1,10) = 53.51; Fig. 3g). Furthermore, treatment with compound C reduced 106 dB-induced OHC loss by 20–50% at 4 mm (p < 0.001), 4.5 mm (p = 0.004), and 5 mm (p = 0.041) from the apex (p < 0.001, F(1,10) = 53.51; Fig. 3h); and attenuated auditory thresholds by 30, 15, and 20 dB at 8 kHz (p < 0.001, t(8) = −6.559), 16 kHz (p = 0.006, t(8) = −3.651), and 32 kHz (p = 0.001, t(8) = −5.22), respectively, measured 14 d after the exposure (Fig. 3f). However, treatment with compound C did not alter 106 dB-noise-induced IHC loss (Fig. 3i).
Table 1.
Post hoc tests for data in Figure 3, b, c, e, and f
| Noise (dB SPL) | Groups | Frequency (kHz) | p value |
|---|---|---|---|
| 98 | siControl vs siAMPKα1 | 8 | 0.009 |
| 16 | <0.001 | ||
| 32 | <0.001 | ||
| DMSO vs CC 20 mg/kg | 8 | 0.027 | |
| 16 | <0.001 | ||
| 32 | 0.001 | ||
| DMSO vs CC 10 mg/kg | 8 | NA | |
| 16 | <0.001 | ||
| 32 | 0.011 | ||
| CC 10 mg/kg vs CC 20 mg/kg | 8 | NA | |
| 16 | 0.001 | ||
| 32 | 0.001 | ||
| 106 | siControl vs siAMPKα1 | 8 | 0.023 |
| 16 | 0.05 | ||
| 32 | <0.001 | ||
| DMSO vs CC 20 mg/kg | 8 | <0.001 | |
| 16 | 0.006 | ||
| 32 | 0.001 |
NA, Not applicable.
Silencing AMPKα1 or treatment with pharmacological inhibitor compound C prevents noise-induced loss of inner hair cell presynaptic ribbons and wave I amplitude
To determine whether blockade of AMPKα1 activation by siRNA or compound C could attenuate noise-induced loss of IHC synaptic connections, we first quantified ribbons at 1 h, and 1, 3, 7, and 14 d after a 98 dB noise. Exposure to the noise significantly reduced synaptic ribbon counts by 40% 1 h after the exposure at 22 kHz region (p = 0.005, t(8) = −3.906). The ribbon loss remained stable and significantly lower than those of unexposed controls for at least 14 d, the latest time measured (Fig. 4d). We also identified functional IHC synapses as juxtaposed presynaptic ribbons and postsynaptic glutamate receptors examined 1 h and 14 d after the 98 dB noise exposure. In agreement with the loss of ribbons, the synapses were also reduced at 22 kHz (p = 0.022, t(8) = −2.848; Fig. 4e), but there was no difference between loss of ribbons and synapses. Furthermore, a 106 dB noise exposure induced a greater amount of synaptic ribbon loss at the high-frequency regions (22 kHz: F(2,9) = 57.412, p < 0.001; 32 kHz: F(2,8) = 16.561, p = 0.02) after the exposure evaluated at 1 h, which also did not recover 14 d after the exposure (Fig. 4b). In addition, the 106 dB noise exposure reduced functional IHC synapses at high-frequency regions 1 h and 14 d after exposure (control vs 1 h after 106 dB noise exposure: F(2,9) = 57.398, p < 0.001; control vs 14 d after 106 dB noise exposure: F(2,8) = 16.561, p = 0.001; Fig. 4c). Further analysis revealed that the differences between the control group and 1 h after 106 dB noise exposure groups at 22 kHz (p < 0.001) and 32 kHz (p = 0.001) were significant. The loss of synapses at 22 kHz at 14 d was less than those lost at 1 h after a 106 dB noise exposure (p = 0.0485; Fig. 4c). Additionally, we observed a difference only in the level of noise-induced presynaptic ribbon loss and the number of functional synapses lost at 1 h after the 106 dB noise exposure at 32 kHz, at which time there was a greater loss of synapses (p = 0.028, t(5) = 3.052). However, the number of functional synapses recovered to a level comparable to that of the presynaptic ribbons by 14 d after exposure. Furthermore, to confirm whether synapses with juxtaposed CtBP2 and GluA2 indeed represented functional synapses, we conducted regression analysis that showed a correlation between synapse number and auditory function (R2 = 0.7514; i.e., more synapses correlated with good hearing function; Fig. 4f).
Figure 4.
Noise-induced synapse loss partially recovers 14 d after exposure. a, Representative images of 106 dB noise-induced IHC synaptic ribbon and glutamate receptor loss at 22 kHz. Blue, myosin-VIIa labeled IHCs; red, CtBP2-labeled synaptic ribbons; green, GluA2-labeled postsynaptic terminals. Scale bar, 10 μm. b, Ribbons per IHC were reduced 1 h and 14 d after 106 dB exposure at 22 and 32 kHz compared with unexposed controls. c, Synapses (juxtaposed ribbons and glutamate receptors) per IHC were reduced 1 h and 14 d after 106 dB exposure at 22 and 32 kHz compared with controls. Synapses at 22 kHz significantly increased at 14 d compared with 1 h after exposure. d, Ribbons per IHC at 22 kHz were reduced 1 h and maintained 14 d after 98 dB exposure compared with controls. Since there are no changes in the synaptic ribbons within 14 d in normal control adult CBA/J mice (without noise exposure), synaptic ribbons at each time point after the noise exposure were compared with a single control group analyzed without noise exposure. e, Synapses per IHC at 22 kHz were reduced 1 h and 14 d after 98 dB exposure compared with controls. The distance along the cochlear duct that correlated with the frequency regions is indicated below e. f, Synapse number negatively correlates with auditory threshold shifts 14 d after exposure. n = 8. Data are presented as the mean ± SD. ***p < 0.001, **p < 0.01, */#p < 0.05. Control, n = 7; 1 h after 106 dB, n = 4; 14 d after 106 dB, n = 4; 1 h after 98 dB, n = 5; 14 d after 98 dB, n = 5. *Control vs 14 d postexposure; #14 d vs 1 h.
The effect of inhibition of AMPK by pretreatment with siAMPKα1 and compound C on the protection of synaptic ribbons was examined 1 h and 14 d after a 106 dB noise exposure, and ABR wave I amplitudes were measured at 14 d after exposure. One hour after exposure, CtBP2-immunolabeled synaptic ribbons of IHCs decreased on surface preparations in the 22 kHz region (Fig. 5a, corresponding to a location 3 mm from the apex) by 70% compared with unexposed controls (F(2,13) = 4.034, p < 0.001; Fig. 5a′). Pretreatment with siAMPKα1 prevented the loss of synaptic ribbons by 50% (F(2,13) = 4.034, p < 0.001; Fig. 5a′). Additionally, noise exposure dispersed the synaptic ribbons along the IHC y-axis (p = 0.017), which was prevented by siAMPKα1 pretreatment (F(2,9) = 7.03, p = 0.014; Fig. 5b). Furthermore, significant protection with siAMPKα1 pretreatment against synaptic ribbon loss was still evident 14 d after noise exposure at higher-frequency regions of the cochlea corresponding to 22 kHz (t(13) = 2.16, p = 0.0492) and 32 kHz (t(12) = 2.67, p = 0.02; Fig. 6a). Additionally, treatment with compound C at 20 mg/kg also prevented noise-induced loss of synaptic ribbons in IHCs (Fig. 5c) 1 h after 106 dB noise exposure (F(2,11) = 15.072, p = 0.04) by 50% compared with DMSO treatment (p = 0.001; Fig. 5c′).
Figure 5.
Inhibition of AMPK-attenuated noise-induced inner hair cell synaptic ribbon loss at 22 kHz 1 h after 106 dB noise exposure. a, Noise-induced loss of immunolabeled CtBP2 (red) in IHCs was prevented by pretreatment with siAMPKα1. The immunolabeling for CtBP2 in IHC nuclei was removed from the figure, and the nuclei are illustrated with circles for clear visualization of the synaptic ribbons. A rough outline of an IHC is indicated by dashed lines. a′, Quantification of CtBP2-immunolabeled ribbon particles in IHCs confirmed that noise-induced reduction of ribbons was partially prevented with siAMPKα1 pretreatment; n = 4. b, 106 dB noise-induced dispersion of CtBP2-immunolabeled ribbons along the IHC y-axis returned to baseline levels with siAMPKα1 pretreatment; n = 4. c, Compound C treatment (20 mg/kg) prevented the noise-induced loss of CtBP2-immunolableled (red) synaptic ribbons in IHCs. c′, Quantification of CtBP2-immunolabeled ribbons in IHCs confirmed significant increases with compound C (20 mg/kg) treatment; n = 4. Representative images (a, c) were taken from the 22 kHz region. Scale bar, 10 μm. Data (a′, b, c′) are presented as the mean ± SD. ***p < 0.001, **p < 0.01, *p < 0.05.
Figure 6.
Inhibition of AMPK protected from noise-induced synaptopathy 14 d after 106 dB exposure. a, 106 dB noise-induced synaptic ribbon loss remained significantly lower than unexposed controls at 16, 22, and 32 kHz 14 d after the exposure. Pretreatment with siAMPKα1 increased the number of synaptic ribbons at 22 and 32 kHz regions of the sensory epithelium. Control, n = 4; 106 dB, n = 5; 106 dB + siControl, n = 5; 106 dB + siAMPKα1, n = 5. b, Pretreatment with siAMPKα1 significantly elevated 106 dB noise-induced reduction in wave I amplitude at sound intensities of 90 and 100 dB SPL at 8 kHz. Control, n = 10; 106 dB + siControl, n = 8; 106 dB + siAMPKα1, n = 4. c, The decrease in wave I amplitude after 106 dB noise was prevented by compound C treatment (20 mg/kg) at sound intensities of 90 and 100 dB SPL at 8 kHz. Control, n = 10; 106 dB + DMSO, n = 14; 106 dB + CC 20 mg/kg, n = 4. Data are presented as the mean ± SD. *p < 0.05 was 106 dB + siControl vs 106 dB + siAMPKα1 or 106 dB + DMSO vs 106 dB + CC 20 mg/kg.
Since ABR wave I amplitudes indicate the activity of the auditory nerve, we measured the amplitude of ABR wave I. The amplitudes were only examined at 8 kHz (1 mm from the apex) to avoid regions of outer hair cell loss (Müller et al., 2005). Fourteen days after exposure to 106 dB noise, the noise-induced decrease in wave I amplitudes was significantly elevated at 8 kHz either by pretreatment with siAMPKα (siAMPKa1 vs siControl: F(1,6) = 8.489, p = 0.027) or compound C (CC vs DMSO: F(1,10) = 7.498, p = 0.021). Further analysis confirmed significant elevation of wave I amplitude at 90 and 100 dB stimulus (Fig. 6b,c; for statistical data, see Table 2).
Table 2.
Post hoc tests for data in Figure 6, b and c
| Frequency (kHz) | Groups | Sound intensity (dB) | p value |
|---|---|---|---|
| 8 | 106 dB + siAMPKα vs 106 dB + siControl | 90 | 0.027 |
| 100 | 0.038 | ||
| 106 dB + CC (20 mg/kg) vs 106 dB + DMSO | 90 | 0.035 | |
| 100 | 0.023 |
Noise exposure increases LKB1 activity in cochlear cells
LKB1 serves as the primary upstream kinase of AMPK; therefore, we examined p-LKB1, a signal for nuclear translocation of the activated LKB1 complex. Immunolabeling for p-LKB1 was increased in the nuclei and cytosol of OHCs, IHCs, Deiters and pillar cells, SGNs, the basal cells of the stria vascularis, and the fibrocytes of the spiral ligament 1 h following a 98 dB noise exposure (Fig. 7a). The increased immunolabeling for p-LKB1 in the cytosol of OHCs was quantified from surface preparations, which showed a more than twofold increase in basal OHCs 1 h after 98 dB exposure compared with unexposed controls (p = 0.005, t(3) = 7.585). In addition, immunoblots of total cochlear homogenates showed a single band for p-LKB1 at the molecular weight of 54 kDa, which was significantly increased 1 h after the exposure (p = 0.017, t(4) = 3.947). No change was detected in total LKB1 levels (Fig. 7c,c′).
Figure 7.
Noise exposure increased p-LKB1 in the cochlea. a, Cochlear sections showed increased DAB-stained immunolabeling of p-LKB1 (brown) in the cytosol and nuclei of OHCs, IHCs, and supporting cells of the organ of Corti (OC), SGNs, basal cells of the stria vascularis, and fibrocytes of the spiral ligament (Stria) 1 h after 98 dB exposure compared with unexposed controls. Representative images were taken from the basal turn. Scale bar, 10 μm. b, b′, Western blots using total cochlear homogenates showed increased p-LKB1 1 h after 98 dB exposure compared with unexposed controls. GAPDH served as the sample loading control; n = 5. Data are presented as the mean ± SD. *p < 0.05.
Pretreatment with LKB1 siRNA diminishes noise-elevated p-AMPKα and reduces noise-induced outer hair cell loss, synaptopathy, and hearing loss
We also evaluated the role of LKB1 in NIHL using siRNA-silencing techniques. Pretreatment with 0.6 μg of siLKB1 reduced 98 dB noise-elevated immunolabeling for p-LKB1 by 25% in OHCs at basal turn compared with groups treated with scrambled siRNA (siControl: p = 0.014, t(4) = −4.155). Pretreatment with siLKB1 also significantly reduced the 98 dB noise-induced immunolabeling for p-AMPKα in OHCs at basal turn (Fig. 8a) by 20% (p = 0.0451, t(2) = 4.5466; Fig. 8a′), indicating that LKB1 phosphorylates AMPKα1. However, Western blots for analysis of p-AMPKα and total AMPKα1 from homogenates of whole cochleae showed no changes between siControl- and siLKB-treated groups (data not shown).
Figure 8.
Inhibition of LKB1 reduced noise-induced p-AMPKα in outer hair cells, auditory threshold shifts, and loss of outer hair cells and inner hair cell synaptic ribbons. a, Pretreatment with siLKB1 decreased p-AMPKα immunolabeling (red) in OHCs (green) 1 h after 98 dB exposure. Representative images were taken from the basal turn. Scale bar, 10 μm. a′, Quantification of p-AMPKα immunolabeling confirms a significant 20% decrease in siLKB1-treated cochleae 1 h after 98 dB noise exposure; n = 3. b, Pretreatment with siLKB1 reduced 98 dB noise-induced threshold shifts at 16 and 32 kHz 14 d after the exposure; n = 5. c, Quantitative analysis of OHCs revealed significant protection from OHC loss 14 d after the exposure with siLKB1 pretreatment. 98 dB + siControl, n = 12; 98 dB + siLKB1, n = 3. d, Pretreatment with siLKB1 did not attenuate 106 dB noise-induced threshold shifts at 16 and 32 kHz 14 d after the exposure; n = 6. e, Pretreatment with siLKB1 recovered wave I amplitudes at 8 kHz. Control, n = 10; 106 dB + siControl, n = 11; 106 dB + siLKB1, n = 6. *106 dB + siControl vs 106 dB + siLKB1. f, Pretreatment with siLKB1 increased the CtBP2 immunolabeled synaptic ribbons in IHCs 1 h after 106 dB exposure; n = 4. Data (a′ to f) are presented as the mean ± SD. ***p < 0.001, **p < 0.01, *p < 0.05.
Furthermore, siLKB1 pretreatment completely prevented 98 dB noise-induced OHC loss at 4 mm (p = 0.013), and significantly reduced loss by 50% at 4.5 mm (p = 0.003) and 5 mm (p < 0.001) from the apex (p < 0.001, F(1,12) = 21.618; Fig. 8c). Subsequently, 98 dB noise-induced auditory threshold shifts were reduced from 15 dB to baseline levels at 8 kHz, and from 40 to 50 dB to 5 dB at both 16 kHz (p < 0.001, t(8) = 7.246) and 32 kHz (p < 0.001, t(8) = 10.695) 14 d after the exposure (Fig. 8b). Unfortunately, siLKB treatment did not significantly attenuate 106 dB noise-induced hearing loss (Fig. 8d), but it protected against the reduction in ABR wave I amplitudes caused by 106 dB noise exposure. The height of ABR wave I amplitudes was restored to baseline levels for a 70 dB stimulus (F(2,27) = 51.786, p = 0.004) and an 80 dB stimulus (F(2,29) = 41.782, p < 0.001) at 8 kHz (Fig. 8e). Moreover, we counted synaptic ribbons 1 h after 106 dB noise exposure. Since 106 dB noise exposure resulted in the loss of synaptic ribbons at high frequencies (Fig. 4b), we counted synaptic ribbons only at 22 and 32 kHz. In agreement with the results of siAMPKα treatment, pretreatment with siLKB1 also prevented noise-induced loss of synaptic ribbons (F(2,9) = 29.044, p < 0.001), which increased from five to eight ribbons per IHC (siControl vs 106 dB plus siControl, p < 0.001; 106 dB plus siControl vs 106 dB plus siLKB1, p = 0.044; Fig. 8f).
Discussion
AMPK activity contributes to the pathogenesis of noise-induced hearing loss by mediating loss of outer hair cells and inner hair cell synaptic ribbons
Our results demonstrate that exposure to traumatic levels of noise leads to sustained AMPK activation, resulting in outer hair cell death and cochlear synaptopathy with subsequent NIHL. Immunoreactivity of p-AMPKα on both surface preparations and cochlear sections are in agreement, showing that immunolabeling for p-AMPKα increases in sensory hair cells at the basal turn in a noise intensity-dependent manner. In contrast, immunoblots were generated from cochlear epithelial tissue homogenates that contain multiple cochlear cell types. Therefore, changes in p-AMPKα in the basal sensory hair cells are diluted by other cochlear cell types, such as supporting cells and spiral ganglion cells, in which the levels of p-AMPKα are unchanged. It may seem surprising that pretreatment with siAMPKα1 reduces 98 dB-induced threshold shifts by 80% at both 16 and 32 kHz, with only a modest 30% reduction in noise-elevated p-AMPKα levels in OHCs. This suggests that a partial inhibition of AMPKα1 that occurs specifically in OHCs is sufficient for the prevention of NIHL. Treatment with the pharmacological AMPK inhibitor compound C provides even greater reduction of noise-induced OHC loss. This is logical, since compound C inhibits the phosphorylation of both α1 and α2 isoforms of AMPK, while AMPKα siRNA targets only the α1 isoform. Furthermore, our results are in line with those from a recent report (McKay et al., 2015) showing that precise genetic reduction of the enzyme AMPK can provide rescue from deafness in a model of profound mitochondrial genetic nonsyndromic hearing loss. Still, in our study neither treatment was fully protective from 106 dB noise-induced hearing loss and OHC loss, suggesting that such a high-level exposure may trigger additional cell death pathways and that pharmacological protection has to be directed at multiple targets. As a case in point, we have shown that the inhibition of noise-induced apoptosis in OHCs shifts the predominant cell death pathway to necrosis (Zheng et al., 2014).
Our results are in agreement with the notion that cochlear pathology is noise intensity dependent, not only for OHC loss, but also for the degree of loss of IHC presynaptic ribbons and functional synapses, with 106 dB noise exposure being more severe than 98 dB noise exposure. Functional synapses can be visualized by the juxtaposition of presynaptic ribbons (immunolabeling for CtBP2 protein) and postsynaptic terminals (immunolabeling for Glu2A protein). Our results confirm that the remaining number of functional synapses correlates with preserved auditory function. Although noise-induced loss of presynaptic ribbons remains unchanged over time, the loss of functional synapses is initially more severe than presynaptic ribbon loss. The number of functional synapses recovers over time to levels comparable to the number of initially remaining presynaptic ribbons, suggesting that postsynaptic nerve terminals may be able to re-establish connections with the remaining presynaptic ribbons (Wan et al., 2014; Liberman and Liberman, 2015). Additionally, our results on noise-induced synaptic ribbon dispersion examined at 1 h after noise exposure are in line with the previous observation indicating a disruption in the juxtaposition of presynaptic and postsynaptic terminals resulting in nonfunctional synapses (Liberman and Liberman, 2015). The prevention of cochlear synaptopathy by the inhibition of AMPK (via siAMPKα1 or compound C) is supported not only by attenuation of the loss of IHC ribbons and synapses, but also by diminution of the dispersion of synaptic ribbons. Furthermore, noise-induced reduction of ABR wave I amplitudes is also prevented by the inhibition of AMPK, suggesting the preservation of neuron firing.
Based on cochlear frequency maps, the area coding to 8 kHz is located ∼1 mm from the apex of the cochlear sensory epithelium (Chen and Fechter, 2003; Müller et al., 2005). In line with our previous reports, we observed ∼40 dB threshold shifts at 8 kHz after 106 dB noise exposure, but without losses of OHCs and ribbon synapses in that region. This may be due, in part, to the fused or collapsed stereocilia (Hu et al., 2002). Our results showing attenuation of NIHL by inhibition of AMPK activation via silencing or pharmacological inhibitor compound C differ from the findings with AMPKα knock-out mice previously reported (Föller et al., 2012). The difference between results following the silencing of AMPKα and the knockout of AMPKα points to the intrinsic disparities between the two methods of inhibition, as siRNA silencing transiently inhibits the expression of a gene, while a knockout eliminates gene function for the entire lifespan of the animal. A partial reduction of AMPK, modeled by silencing and pharmacological inhibition, may prevent the noise-induced imbalance of AMPKα signaling, whereas knockout of AMPKα may alter other molecular events, such as changes in BK channels, which may exacerbate hearing loss (Föller et al., 2012). In addition, our current study highlights a pathological function of the activation of AMPK in sensory hair cells in response to traumatic noise insults, which is contradictory to the traditional prosurvival view of AMPK. We can postulate that the role of AMPK varies with noise intensity. When noise levels cause temporary threshold shifts, AMPK may promote cell preservation through its energy-conserving downstream pathways, such as autophagy (Yuan et al., 2015). However, exposure to noise that causes permanent hearing loss resulted in sustained activation of AMPK, which may upregulate autophagy to eliminate the irreparably damaged cells or lead to mitochondrial dysfunction (Vicente-Torres and Schacht, 2006; Chen et al., 2013). Determining the balance between AMPK activation and inhibition, and identifying the key timing or circumstances in which AMPK switches from a protective to a pathological function may be key to designing future therapies.
Finally, the concept that LKB1 mediates the contribution of AMPK to the progression of NIHL is supported by our results showing pretreatment with siLKB1-diminished immunolabeling for p-AMPKα. Although the siLKB1 pretreatment reduced p-AMPKα by only 20%, its reduction was similar to that detected with siAMPKα pretreatment, making a strong case for the phosphorylation of AMPK by LKB1 following noise exposure.
Changes in AMPK and LKB1 signaling molecules in sensory hair cells
Our results reveal that noise exposure resulted in increased immunolabeling for total AMPKα1 and AMPKα2 in both sensory hair cells and supporting cells, with AMPKα1 primarily localizing in the cytosol and AMPKα2 localizing in both the cytosol and nuclei. Since AMPKα2 is localized to the nuclei, it is possible that this isoform is able to directly phosphorylate transcription factors and alter gene expression, while the α1 isoform may have less of an impact on gene expression and more of an effect on short-term changes, such as the post-translational modulation of protein activity. This notion is in agreement with a report detecting expression of AMPKα1 mRNA in OHCs, but not in IHCs (Föller et al., 2012), suggesting that noise-induced elevation of AMPKα1 in IHCs may not be related to gene expression. Although noise exposure increases total AMPKα1 and AMPKα2 by immunohistochemistry, the levels of total AMPKα protein detected by Western blots and mRNA assessed by qRT-PCR remain unchanged, possibly due to the fact that nonsensory cells in cochlear tissue may dominate the population of cells that respond to AMPKα. On the other hand, the unchanged levels of mRNA point to a decrease in protein degradation after noise exposure.
In line with a previous report, phosphorylation of LKB1 is localized to both the nuclei and cytosol in the cells of the organ of Corti (Song et al., 2008), but p-AMPKα increases only in sensory hair cells after the noise exposure. This discrepancy may be owed to an effect of intrinsic differences in homeostatic defense systems against metabolic stress, as supporting cells are resistant to inner ear insults, while sensory hair cells are vulnerable (Tiede et al., 2009; Jensen-Smith et al., 2012; May et al., 2013). It is possible that the maintenance of homeostasis and the correction of ion imbalances in supporting cells is more effective and efficient due to the presence of gap junctions between supporting cells that allow for the diffusion and flux of ions and second messengers providing enhanced resilience compared with sensory hair cells (Denoyelle et al., 1998; Xia et al., 1998; Zhang et al., 2005; Anselmi et al., 2008; Lahne and Gale, 2010). Moreover, the antioxidant abilities of supporting cells may be stronger than those of sensory hair cells, as supporting cells secrete HSP70 (May et al., 2013), which increases the aptitude of supporting cells to recover from a metabolic disruption and prevents proapoptotic signaling pathways. In addition, LKB1 activity may play divergent roles in hair cells and supporting cells. LKB1 is known to phosphorylate a total of 14 AMPK-related kinases, including the microtubule affinity-regulating kinases, which are involved in the regulation of cellular polarity and the cytoskeleton (Lizcano et al., 2004; Jansen et al., 2009). Sensory hair cells are composed primarily of actin cytoskeleton (Han et al., 2015), making them more vulnerable than supporting cells.
Conclusion
This study proposes AMPKα as a novel therapeutic target to prevent NIHL. Several noise-induced events, including calcium influx, ATP depletion, and oxidative stress play a role in initiating or exacerbating the pathological responses of sensory cells. AMPK is a determining factor at a crossroads between these events and thus may constitute a more effective target to address the complexities of NIHL signaling pathways. This project evaluated losses of sensory hair cells and synaptic ribbons, as well as functional deficits in auditory brainstem response and wave I amplitudes, possibly providing an example for future evaluation of otoprotective agents.
Footnotes
This research was supported by Grant R01-DC-009222 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. This work was conducted in the WR Building at the Medical University of South Carolina (MUSC) in a renovated space supported by Grant C06-RR-014516. Animals were housed in the MUSC Children' Research Institute animal facilities, which were supported by Grant C06-RR-015455 from the Extramural Research Facilities Program of the National Center for Research Resources. We thank Dr. Jochen Schacht for valuable comments on this manuscript. We also thank the MUSC Biorepository & Tissue Analysis Shared Resource for technical assistance with cochlear paraffin sections and Andra Talaska for proofreading this manuscript.
The authors declare no competing financial interests.
References
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FQ, Zheng HW, Hill K, Sha SH. Traumatic noise activates rho-family GTPases through transient cellular energy depletion. J Neurosci. 2012;32:12421–12430. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FQ, Zheng HW, Schacht J, Sha SH. Mitochondrial peroxiredoxin 3 regulates sensory cell survival in the cochlea. PLoS One. 2013;8:e61999. doi: 10.1371/journal.pone.0061999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Fechter LD. The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res. 2003;177:81–90. doi: 10.1016/S0378-5955(02)00802-X. [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Lina-Granade G, Plauchu H, Bruzzone R, Chaïb H, Lévi-Acobas F, Weil D, Petit C. Connexin 26 gene linked to a dominant deafness. Nature. 1998;393:319–320. doi: 10.1038/30639. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, De Bartolo P, Eramo SL, Rolesi R, Paciello F, Bergamini C, Fato R, Paludetti G, Petrosini L, Troiani D. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci. 2013;33:4011–4023. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Föller M, Jaumann M, Dettling J, Saxena A, Pakladok T, Munoz C, Ruth P, Sopjani M, Seebohm G, Rüttiger L, Knipper M, Lang F. AMP-activated protein kinase in BK-channel regulation and protection against hearing loss following acoustic overstimulation. FASEB J. 2012;26:4243–4253. doi: 10.1096/fj.12-208132. [DOI] [PubMed] [Google Scholar]
- Han Y, Wang X, Chen J, Sha SH. Noise-induced cochlear F-actin depolymerization is mediated via ROCK2/p-ERM signaling. J Neurochem. 2015;133:617–628. doi: 10.1111/jnc.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338:717–722. doi: 10.1042/bj3380717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/S0014-5793(03)00560-X. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. F-actin cleavage in apoptotic outer hair cells in chinchilla cochleas exposed to intense noise. Hear Res. 2002;172:1–9. doi: 10.1016/S0378-5955(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89:777–798. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- Jensen-Smith HC, Hallworth R, Nichols MG. Gentamicin rapidly inhibits mitochondrial metabolism in high-frequency cochlear outer hair cells. PLoS One. 2012;7:e38471. doi: 10.1371/journal.pone.0038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefas BA, Cai Y, Kerckhofs K, Ling Z, Martens G, Heimberg H, Pipeleers D, Van de Casteele M. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem Pharmacol. 2004;68:409–416. doi: 10.1016/j.bcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Gale JE. Damage-induced cell-cell communication in different cochlear cell types via two distinct ATP-dependent Ca waves. Purinergic Signal. 2010;6:189–200. doi: 10.1007/s11302-010-9193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42:1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Suzuki J, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol. 2015;16:205–219. doi: 10.1007/s10162-015-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LA, Kramarenko II, Brandon CS, Voelkel-Johnson C, Roy S, Truong K, Francis SP, Monzack EL, Lee FS, Cunningham LL. Inner ear supporting cells protect hair cells by secreting HSP70. J Clin Invest. 2013;123:3577–3587. doi: 10.1172/JCI68480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay SE, Yan W, Nouws J, Thormann MJ, Raimundo N, Khan A, Santos-Sacchi J, Song L, Shadel GS. Auditory pathology in a transgenic mtTFB1 mouse model of mitochondrial deafness. Am J Pathol. 2015;185:3132–3140. doi: 10.1016/j.ajpath.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, von Hünerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Nagashima R, Yamaguchi T, Kuramoto N, Ogita K. Acoustic overstimulation activates 5′-AMP-activated protein kinase through a temporary decrease in ATP level in the cochlear spiral ligament prior to permanent hearing loss in mice. Neurochem Int. 2011;59:812–820. doi: 10.1016/j.neuint.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–173. doi: 10.1016/S0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Oishi N, Chen FQ, Zheng HW, Sha SH. Intra-tympanic delivery of short interfering RNA into the adult mouse cochlea. Hear Res. 2013;296:36–41. doi: 10.1016/j.heares.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Münzel T, Keaney JF., Jr Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen QW, Gerrard DE, Du M. Compound C, an inhibitor of AMP-activated protein kinase, inhibits glycolysis in mouse longissimus dorsi postmortem. Meat Sci. 2008;78:323–330. doi: 10.1016/j.meatsci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Song P, Xie Z, Wu Y, Xu J, Dong Y, Zou MH. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J Biol Chem. 2008;283:12446–12455. doi: 10.1074/jbc.M708208200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- Tiede L, Steyger PS, Nichols MG, Hallworth R. Metabolic imaging of the organ of corti—a window on cochlea bioenergetics. Brain Res. 2009;1277:37–41. doi: 10.1016/j.brainres.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J Neurosci Res. 2006;83:1564–1572. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife. 2014;3:e03564. doi: 10.7554/eLife.03564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisová P, Dávila D, Tuffy LP, Ward MW, Concannon CG, Prehn JH. Role of 5′-adenosine monophosphate-activated protein kinase in cell survival and death responses in neurons. Antioxid Redox Signal. 2011;14:1863–1876. doi: 10.1089/ars.2010.3544. [DOI] [PubMed] [Google Scholar]
- Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:332–347. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- Xia JH, Liu CY, Tang BS, Pan Q, Huang L, Dai HP, Zhang BR, Xie W, Hu DX, Zheng D, Shi XL, Wang DA, Xia K, Yu KP, Liao XD, Feng Y, Yang YF, Xiao JY, Xie DH, Huang JZ. Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet. 1998;20:370–373. doi: 10.1038/3845. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–508. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998;784:82–90. doi: 10.1016/S0006-8993(97)01156-6. [DOI] [PubMed] [Google Scholar]
- Yuan H, Wang X, Hill K, Chen J, Lemasters J, Yang SM, Sha SH. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal. 2015;22:1308–1324. doi: 10.1089/ars.2014.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Lee M, Kim SS, Ha J. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J Biol Chem. 2005;280:9963–9972. doi: 10.1074/jbc.M412994200. [DOI] [PubMed] [Google Scholar]
- Yun H, Kim HS, Lee S, Kang I, Kim SS, Choe W, Ha J. AMP kinase signaling determines whether c-Jun N-terminal kinase promotes survival or apoptosis during glucose deprivation. Carcinogenesis. 2009;30:529–537. doi: 10.1093/carcin/bgn259. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P, Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci U S A. 2005;102:15201–15206. doi: 10.1073/pnas.0501859102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HW, Chen J, Sha SH. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014;5:e1262. doi: 10.1038/cddis.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]