Abstract
Ischaemic heart disease, stroke, and other cardiovascular diseases (CVDs) are responsible for an estimated 17.5 million annual deaths in the world. If account is taken of population aging, death rates from CVDs are estimated to be steadily decreasing in the world as a whole, and in regions with reliable trend data. The declines in high-income countries and some countries in Latin America have been ongoing for decades with no indication of slowing. In high-income countries, these positive trends have broadly coincided with, and benefited from, declines in smoking and physiological risk factors like blood pressure and serum cholesterol. Improvements in medical care, including effective primary prevention through management of physiological risk factors, better diagnosis and treatment of acute CVDs, and post-hospital care of those with prior CVDs, are also likely to have contributed to declining CVD event and death rates, especially in the past 40 years. However, the measured risk factor and treatment variables neither explain why the decline began when it did, nor much of the similarities and differences in the start time and rate of the decline across countries or between men and women. There have been sharp changes and fluctuations in CVDs in the former communist countries of Europe and the Soviet Union since the fall of communism in the early 1990s, with changes in volume and patterns of alcohol drinking, as a major cause of the rise in Russia and some other former Soviet countries. The challenge of reaching more definitive conclusions concerning the drivers of what constitutes one of the most remarkable international trends in adult mortality in the past half-century in part reflects the paucity of time trend data not only on disease incidence, risk factors, and clinical care, but also on other potential drivers, including infection and associated inflammatory processes throughout the lifecourse.
Introduction
Ischaemic heart disease (IHD) and stroke are the two most common causes of death in the world. Together they are estimated to be responsible for 14 million annual deaths in the world, a quarter of all global deaths.1 Other cardiovascular diseases (CVDs) are responsible for an additional 3.5 million deaths. The absolute numbers of deaths from CVDs is increasing in the world and in most countries. What is not widely appreciated is that the rise in the number of deaths is occurring mostly in people older than 70 years of age, and is due to increased longevity and the associated population aging.2,3 If account is taken of population aging, death rates from CVDs are steadily decreasing in the world as a whole, and in regions with reliable trend data (Figure 1).2–4
Figure 1.
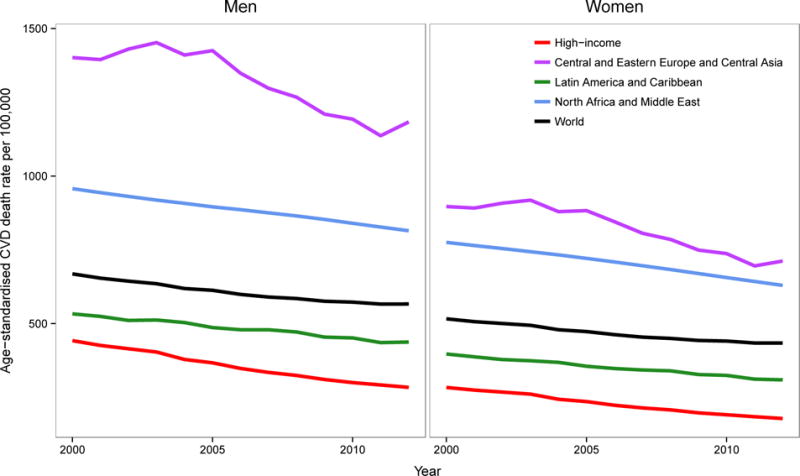
Trends in age-standardised death rates from cardiovascular diseases for adults aged 30 years and older, by region and sex between 2000 and 2012. Death rates are age-standardized to the WHO standard population. Source: World Health Organization (WHO) Global Health Estimates.1
Note: WHO estimates mortality trends using on available data from vital statistics, from sample registration of deaths, and from representative verbal autopsy surveys.310 These data are used together with demographic and epidemiological models, to reconstruct trends.310 Trends are not shown for sub-Saharan Africa and for East, South, and Southeast Asia and the Pacific (with the exception of Japan, Singapore, and South Korea which are included in the high-income group) because mortality data from these regions are very limited, especially for estimating time trends. As described in the text, studies from selected countries and communities in these regions show a modest decline in total CVD mortality and in mortality from stroke and hypertensive disease.
A natural question of scientific and public health interest is how much trends in known risk factors, individually and in combination, are responsible for trends in CVD mortality and its variations across countries. The answer will help anticipate likely trends in future CVD mortality under specific projected risk factor scenarios.3 We review the major studies that have examined the role of trends in preventable risk factors as drivers of trends in CVD mortality. Our focus is not whether a factor is a cause of CVD occurrence or death, which is the subject of epidemiological studies in individual participants. Rather, we summarise and critically evaluate the evidence on whether change in risk factor levels in whole communities and populations is associated with change in mortality. Although our primary focus is on risk factors, we briefly consider the role of improved medical care for two reasons. First, as we describe below, trends in major physiological risk factors for CVDs are due to a combination of changes in behaviour and pharmacological treatment. Second, we find it incomplete, if not impossible, to attribute change to specific risk factors without any consideration of other major determinants of CVDs which have changed markedly at the same time.
The paper begins with a short review of the existing data on trends in CVD mortality and risk factors. We focus primarily on IHD and stroke when data allow, and on all CVDs when factors like changes in classification of causes of death make trends in specific diseases less reliable. We then review studies on the role of risk factors in CVD trends, organised largely by how the scientific knowledge has evolved. While socio-economic inequalities are an important feature of CVDs, and of their trends, we do not deal with them as they have been addressed elsewhere, including in a recent review by Harper et al.5–8
Worldwide CVD mortality trends
Rise and fall in high-income countries of Asia, Australasia, North America, and Western Europe
As early as the middle of 20th century, trends in deaths from CVDs were being discussed in the literature. Studies from Australia, Europe, and the United States (US) found that, with the exception of some countries during World War II (WWII), CVD death rates rose in the first few decades of the 20th century.9–14 Some of these early studies systematically considered whether changes in diagnostic and classification criteria or the expanding geographical coverage of death registration within countries had influenced the observed trends and concluded that, while present, these factors were unlikely to explain the rising trend. Importantly, some of these studies noted that a substantial proportion of the rise in crude death rates was due to the aging of the population, which, as stated above, continues to be overlooked even today. A rising trend was nonetheless seen in age-specific and age-standardised death rates.10–13 In the US, trends were diverging among population subgroups as early as the 1940s, rising among white men, while declines were already evident at working ages for white women and blacks.15 By the 1960s, CVD mortality was declining in most western high-income countries.16,17 This reversal in the trend was initially missed or even dismissed by some people but was noted by more discerning reports.18,19
Data on total CVD mortality mask the fact that for most of the first half of the 20th Century, IHD and stroke had opposite trends in high-income countries like the UK and the US, with stroke (and hypertensive disease) mortality declining decades before the IHD decline began.12,16,17,20–26 Of the two main types of stroke, ischaemic stroke shares many risk factors with IHD. The earlier decline of stroke as a whole might reflect that haemorrhagic stroke may have begun declining much earlier in the 20th Century, as has been recently suggested in an analysis of data from England and Wales.23
Japan, the only country in Asia with reliable long-term mortality data, seems to also have experienced rising IHD mortality (albeit from very low levels) from 1950 until the 1970s and rising stroke until the 1960s, after which death rates began to decline.27–32 IHD death rates in Japan never reached the levels seen in western high-income countries. As in western countries, haemorrhagic stroke began its decline well before ischaemic stroke, at least in the 1950s.29
Following pioneering works in the 1950s and 1960s to document the rise in IHD mortality, seeking explanations for the decline in IHD mortality in western countries became a focus of research efforts from the 1970s.33–35 This focus is exemplified by two major milestones in research on CVD mortality trends. First, data and perspectives on the topic came together at the 1978 Bethesda Conference on the Decline in Coronary Heart Disease Mortality (Panel 1).24 Second, the MONICA (Multinational MONItoring of trends and determinants in CArdiovascular disease) Project was initiated to assess prospectively the influence of changes in major risk factors on change in IHD mortality over a period of ten years in the 1980s and early 1990s in 21 countries (Panel 2).36 Decades after these pioneering investigations, we use national mortality and risk factor data to revisit these trends with the advantage of having data from much longer period of time and a larger, and more diverse, set of countries than has been previously considered (Panel 3).37
Continued decline in high-income countries of Asia, Australasia, North America, and Western Europe
Figure 2 shows per cent decline in total CVD mortality for 20 western high-income countries with reliable vital statistics in each of the past six decades. The figure reveals two important features of CVD mortality trends: first, the decline in CVD mortality had already begun in the 1950s among women in these 20 countries, especially in those under the age of 70 years. Although the decline in men became fully established in later years, in some countries, like Canada, Sweden, and Switzerland, declining trends were seen in the 1950s (Figure 3). By the 1970s, total CVD death rates were declining in most high-income countries, including in Japan (Figure 3). Greece was possibly the only western high-income country where CVDs continued to rise until the 1980s before joining the wave of declines. Second, there was substantial variation across countries in the rate of decline with the best and worst performing country in each decade about 30% apart.21,22 While these points were apparent to some earlier investigators, what is so extraordinary today is that the CVD mortality decline shows no signs of slowing; in fact the proportional rate of decline in the most recent decade seems even larger than previous decades. Taken over the nearly 60 years since it began, this decline in CVD mortality is arguably one of the most notable health phenomena of the late 20th and early 21st centuries, resulting in over 70% lower death rates, relative to their peak, from this group of causes among men and over 75% lower among women in countries like Australia, Canada, Finland, France, and Switzerland. Total CVD mortality in Japan began to decline in the early 1960s, due to the decline in total stroke deaths while IHD continued to rise as stated earlier.
Figure 2.
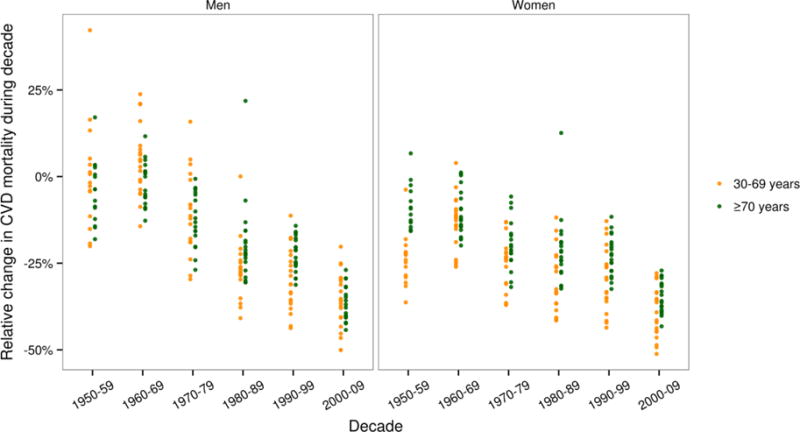
Relative (per cent) decline in death rates from cardiovascular diseases by decade, sex, and age group in 20 western high-income countries. Within each age group, death rates were age-standardised using the WHO standard population. The countries are English-speaking high-income countries (Australia, Canada, Ireland, New Zealand, UK, and USA) and countries in Western Europe (Austria, Belgium, Denmark, Finland, France, Germany, Greece, Italy, the Netherlands, Norway, Portugal, Spain, Sweden, and Switzerland). Germany was excluded because its boundaries changed during the period of display. We also have not shown data for smaller countries like Cyprus, Iceland, Luxemburg, and Malta.
Figure 3.
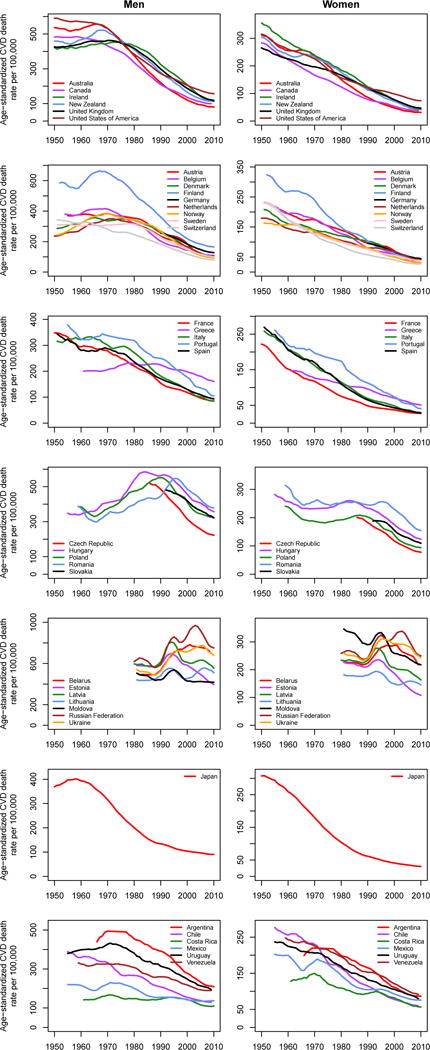
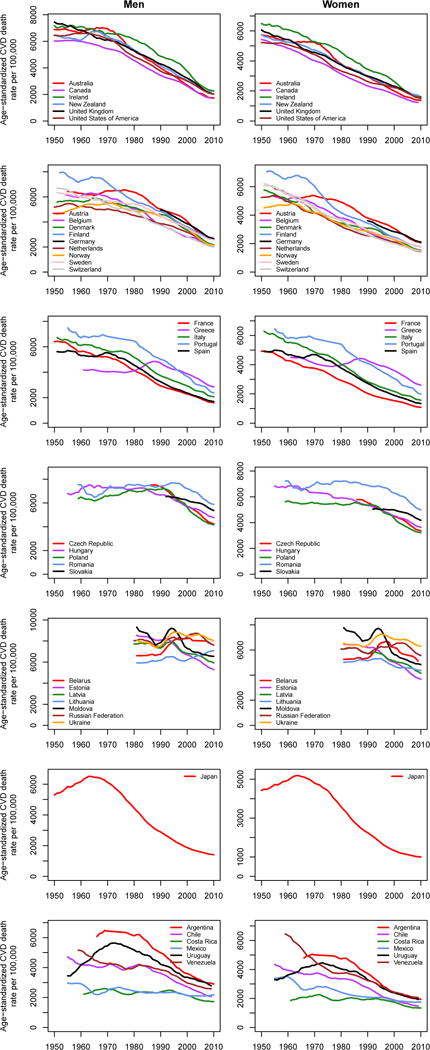
Trends in death rates from cardiovascular diseases for adults (A) aged 30–69 years and (B) 70 years and older. Within each age group, death rates are age-standardized to the WHO standard population. Trends are smoothed using a 5-year moving average. See Figure S1 for results for 30 years and older combined.
Countries in this figure were selected based on recent work by Mathers et al. on trends in life expectancy and cause-specific mortality in ageing populations.213 Of the countries analysed by Mathers et al.,213 we have shown trends for Japan, English-speaking high-income countries, and countries in Western Europe (excluding the above-mentioned smaller countries and Germany), in Central and Eastern Europe, and in Latin America.
Latin America
Historical mortality data with details on age and cause of death are available for fewer countries in Latin America than in the high-income world although efforts are ongoing to collate such data (see https://www.ssc.wisc.edu/cdha/latinmortality/). Among those with better quality data, CVD mortality has been declining at least since the 1970s in Argentina, Chile, Costa Rica, Uruguay, and Venezuela with the pace of decline only slightly slower than in high-income countries, with declines in Mexico (and Brazil which is not shown in the figure) beginning around the same time or shortly after (Figure 3).38–40 More recently, CVD death rates seem to be declining in the region as a whole, again at a slower pace than in high-income countries (Figure 1).
Former communist countries of Central and Eastern Europe
The former communist countries include those of Central and Eastern Europe such as Hungary and Poland as well as those of the former Soviet Union. While the precise nature of the political regimes varied, and some may describe them as having being socialist rather than communist, as a group they manifest trends that are quite different to those of other industrialised and high-income countries. Most importantly, declines in CVD mortality had a far later onset than in high-income countries of Asia, Australasia, North America, and Western Europe. As early as the Bethesda conference it was noted that IHD mortality was increasing in many Central and Eastern European countries, especially among men (Figure 3).21,22,41,42 The increases in CVD death rates in countries such as Czech Republic, Hungary, Poland, Romania, and Slovakia continued for decades after the decline started in high-income countries, and only reversed in the mid-late 1980s and early-mid 1990s, with the decline starting earlier in women than in men. However, once the CVD decline began in these countries, the rates of decline rapidly converged with those in other high-income countries (Figure 3).43 Like high-income countries of Asia, Australasia, North America, and Western Europe, there were variations in when the declines began and its rate in these countries.
The countries of the former Soviet Union including Belarus, Moldova, Russia, Ukraine and the Baltic states of Estonia, Latvia, and Lithuania show a more complex trajectory. Unlike high-income countries, CVD mortality in these countries was on a shallow upward trend among men and stagnant among women in the last few decades of the Soviet Union. Death rates declined in both sexes after Gorbachev’s anti-alcohol campaign in 1985 in some of these populations, only to rise steeply from the early 1990s coinciding with the collapse of the Soviet Union. These fluctuations, which were different from relatively gradual changes seen elsewhere, were particularly dramatic for working age men.44–46 In Russia and Ukraine, CVD death rates declined between 1994 and 1998, and increased again until the mid-2000s since when they have declined.47 In contrast, in Estonia and Latvia the decline was established from the early-mid 1990s. The dramatic fluctuations in CVD mortality in Russia were parallel to fluctuations in external causes of death from injuries and acute alcohol poisonings (Figure 4).48 There is some suggestion that the apparent fluctuations in deaths attributed to IHD might in fact be due to misclassification of sudden cardiac deaths induced by heavy drinking (for which death rates from acute alcohol poisoning act as proxy),48 rather than being classic manifestations of atherosclerotic disease such as myocardial infarction.49
Figure 4.
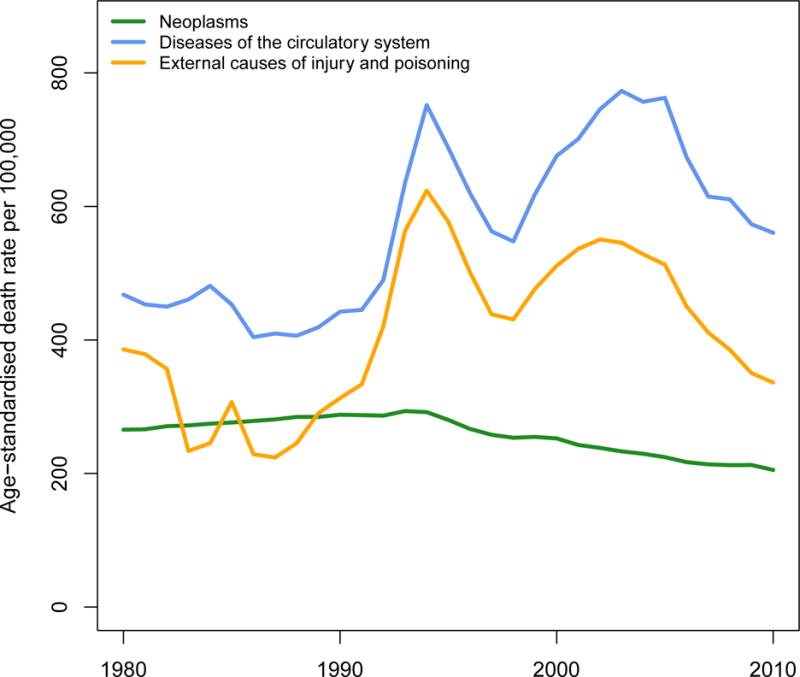
Trends in death rates from cardiovascular diseases, compared with external causes and cancers, in adults aged 25–64 years of age in Russia.
China
The absence of complete vital registration makes data on trends in China less reliable. Data from the MONICA study and from other community studies seem to indicate a decline in total stroke death rates (driven by relatively large declines in haemorrhagic stroke), similar to earlier trends in Japan, and possibly a small rise in IHD death rates.50–52 In Hong Kong, where there are high-quality data, IHD seems to have plateaued and even begun declining in the 1980s, especially at younger ages.53
Sub-Saharan Africa
There are very few data on CVD trends from low-and-middle-income countries because vital registration systems do not exist, or are incomplete and have limited information on causes of death. A notable exception is high-quality vital registration data with medical certification of causes of death in Seychelles, a middle-income country in Africa. These data show steady declines in death rates from stroke, IHD, and other CVDs.54 South Africa, another country with somewhat complete vital statistics also seems to have had declining age-standardised death rates from IHD and stroke.55
Trends in CVD mortality sex ratios
One of the striking features of the CVD mortality trends is the difference in their start time and pace for men and women. Figure 5 shows the sex ratios in CVD death rates for people aged 30–69 years, an age range commonly considered as premature death, for the same countries as in Figure 3. The figure shows that historically, premature CVD death rates in men were 1–2 times those in women in high-income countries. The ratio increased over time, reaching 2–3 in most countries, and nearly 4 in Finland. It has plateaued or is decreasing in English-speaking and North-Western Europe.26 The male-to-female ratio of premature CVD death rates is still increasing in Central and Eastern Europe, Japan, and Latin America.
Figure 5.
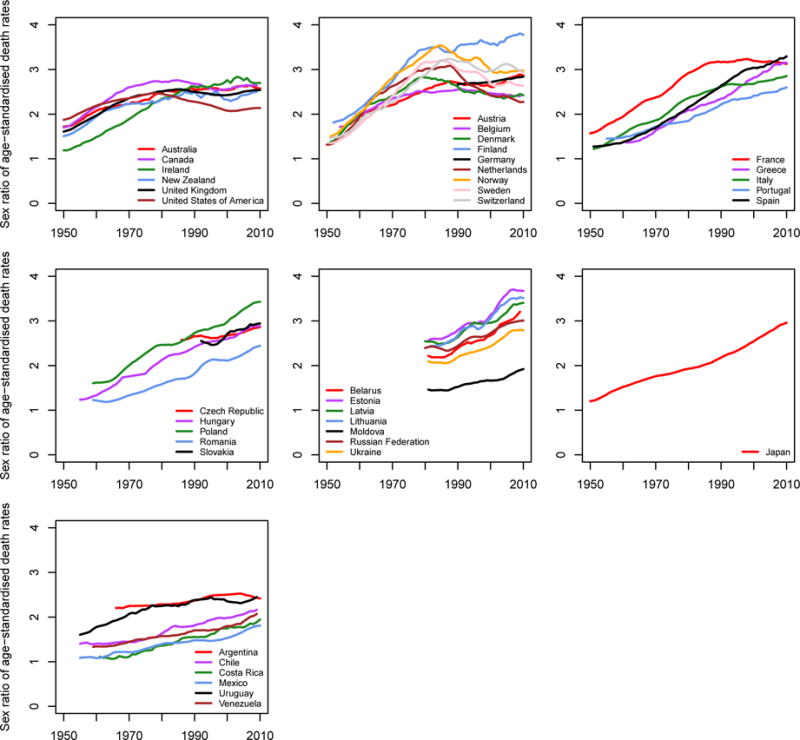
Trends in male-to-female ratio of cardiovascular disease death rates for people aged 30–69 years in selected countries with reliable mortality data. Within this age group, death rates are age-standardized to the WHO standard population. Trends are smoothed using a 5-year moving average.
CVD risk factors
Observational studies and randomised trials have identified numerous behavioural, environmental, nutritional, physiological, and psychosocial risk factors for CVDs. Today there are hundreds of risk factors above and beyond the “classic” risks (smoking, blood pressure, serum cholesterol, and blood glucose or diabetes) considered in studies such as the Framingham Heart Study and the MONICA Project.56–58 These risks span the life course and include factors such as impaired fetal and childhood growth and nutrition, adult height and lung function, psycho-social factors including social gradient and status, and numerous environmental and occupational exposures and components of diet. They also include an increasing number of physiological risk factors such as persistent low-grade inflammation (including that related to infections) and a putative role for the gut microbiome, which may in turn be affected by diet and biophysical environment.59,60
Despite the proliferation of putative risk factors, we will focus on the established risk factors for which there is extensive evidence from observational epidemiology and/or randomised trials, and for which trend data are available and reliable for an appreciable number of countries. We also look at trends in adiposity, aspects of diet, and alcohol use. Before considering trends in each of these we briefly discuss some fundamental issues concerning the conceptualisation of the link between risk factors and CVD mortality trends.
Any explanatory framework for changes over time in CVD incidence and death has to assume that the factors considered are either directly causal or are proxy markers for causal factors. Causal influences can be along a continuum from the most proximal (usually physiological) to the most distal (such as large scale social or economic changes that can set in train changes in behaviour).61,62 This is represented very simply in the following:
In reviewing which factors might be responsible for trends in CVD, it is necessary to take into account this hierarchy of influences. Changes in the intermediate trait of blood pressure and serum cholesterol might be the result of a range of behavioural factors such as exercise and diet. These physiological traits should be regarded in this sense as mediators of behavioural or societal changes. However, there is a further level of complexity for several intermediate traits, which is that trends in these can be influenced by medical interventions (e.g., decline in lipids or blood pressure as a result of statin use and anti-hypertensive medications). In this regard, any role that trends in blood pressure or serum cholesterol levels may have in trends in CVD morbidity or mortality does not neatly fall into either lifestyle or treatment effects, the apparent objective of analyses that seek to apportion the more recent declines in CVD mortality into these two categories. The usual, but important caveat concerning genetic influences needs to be made. While the huge postgenome effort to identify genetic determinants of cardiovascular traits and disease is showing some progress, it sheds little light upon the dramatic and largely simultaneous temporal trends we have described.
As discussed briefly below, the extent to which some the less-established risk factors are truly causal is controversial. Even for those risk factors that are believed to be causally related to CVDs, such as blood pressure, serum lipids, and smoking, which measure best captures the causal element is open to question. For example, total cholesterol appears to be a poorer marker of risk than LDL, HDL, or their ratio (or ratio of ApoB to ApoA1).63 Finally, when considering the likely role played by different risk factors as determinants of CVD trends, there is the question of the time of changes in disease rates with respect to changes in risk factors.3 For a number of risk factors a combination of observational and trial evidence suggests that risk of IHD and stroke falls relatively rapidly following reductions in risk factor levels. For example, among people who give up smoking, the risk of CVD falls to that of non-smokers within as short a time as up to five years, although other estimates suggest something closer to ten years (Figure 6).3,64 This is in contrast to lung cancer and chronic obstructive pulmonary disease, where the risk for former smokers decays over a much longer time period of many decades.3,64 There is also evidence that following smoking bans in public places, there were rapid falls in hospitalisation rates for acute coronary syndromes.65,66 For cholesterol and blood pressure, evidence from randomised trials suggests that reductions in CVD risk occur within five years.67 For alcohol, body mass index (BMI), and dietary salt there is relatively little evidence available on which to estimate the temporal responsiveness of CVDs, although effects on intermediate traits like blood pressure occur rapidly.68–72 Moreover, there is scant evidence for any of these factors about the induction period – time from when increases in exposure levels result in increased risk of a CVD event.73 What is known, however, is that the development of atherosclerotic plaques or the hardening of the arteries is a gradual life-long process.74,75 In contrast, risk of a fatal obstruction of the coronary arteries may be reduced relatively abruptly, particularly if it affects late-stage factors in the process such as clotting and thrombus formation. Although not directly related to CVD, mortality from alcoholic liver cirrhosis well illustrates this sort of temporal asymmetry. Cirrhosis risk increases among moderate to heavy drinkers only after many years of alcohol consumption. However, at a population level, declines in alcohol consumption (typically those that are the result of governmental regulation of availability and price) can result in very abrupt reductions in liver cirrhosis mortality, as those with compromised livers have a reduced risk of experiencing a final, life-threatening binge. This complexity of risk factors is summarised in Table 1.
Figure 6.
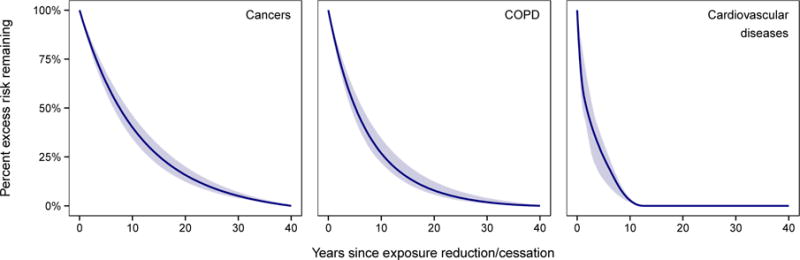
Proportion (percentage) of excess relative risk (relative risk minus one) of cardiovascular diseases remaining over time since smoking cessation, compared to cancers and chronic obstructive pulmonary disease (COPD). The shaded area shows the uncertainty of the fitted curve. Source: Kontis et al.3
In summary, data on changes in disease-specific mortality RRs after cessation were from a re-analysis of American Cancer Society Cancer Prevention Study II (CPS-II) data.64 A convex constrained b-spline (fitted using the library “cobs” in statistical software R) was fitted to the raw RR data (which can be seen in Figure 2 of Oza and colleagues64) to obtain a smooth continuous relationship of RR over time since smoking cessation. The proportion (percentage) of excess relative risk over time since cessation as , where RRt is the RR t years after quitting smoking and RR0 is the RR of smokers.
Table 1.
Characteristics of important cardiovascular risk factors which may be partly responsible for trends in CVD mortality.
| Risk factor | Type | Temporal responsiveness |
|---|---|---|
| Smoking | Behavioural | Observational evidence that following cessation risk begins to fall immediately and reaches that of non-smokers within 5–10 years.3,64 |
| Alcohol | Behavioural | Little good observational evidence,68 with the exception of Russia and other former communist countries which suggests abrupt changes in CVD mortality. The changes might be due to sudden cardiac deaths misclassified to IHD. |
| Adiposity | Intermediate trait; mediators include blood pressure, lipids, inflammation, and glycaemia and diabetes | Limited evidence that weight management (accompanied by broader lifestyle change) reduces the risk of diabetes within a few years in people with impaired glucose tolerance,315 but no good evidence on temporal responsiveness for CVDs.316 |
| Blood pressure | Intermediate trait with direct effect | Evidence primarily from randomised trials that decline in blood pressure results in fall in IHD and stroke risk to start immediately. |
| Serum cholesterol | Intermediate trait with direct effect | Evidence from randomised trials that decline in serum cholesterol results in fall in IHD risk to start immediately with risk reversibility largely complete in 5 years.67 |
| Glycaemia and diabetes | Intermediate trait with direct effect | Little evidence on temporal responsiveness of CVDs to glycaemic control or rise because most trials have focused on intensity of glucose lowering, and have had short duration.266 |
| Diet and nutrition | Behavioural; potential mediators include BMI, blood pressure, lipids, inflammation, and oxidation | Some evidence from randomised trials that changes in salt or the type of fats/oils can result in changes in mediators or CVD risk, with declines starting immediately. Less is known about how long before the full benefits are achieved because of the limited duration of the trials. |
Trends in CVD risk factors
Historical population-based data on CVD risk factors, especially at the national level, are less plentiful than mortality data which are collected routinely where there is vital registration. Health surveys, food and alcohol production and trade data, and sales receipts for tobacco and alcohol have all been used to reconstruct trends in behavioural and dietary risks. Reconstructing trends in physiological risk factors, including adiposity, blood pressure, cholesterol, and glycaemia and diabetes, require population-based measurement data as self-reports or measurements from groups attending clinics or hospitals are biased. The longest-duration nationally representative health examination survey (HES) began in 1948 in Japan and has been conducted virtually every year since then.76,77 Subsequently, other countries developed HES programmes, with regular or periodic surveys that allow direct measurement of more recent trends in physiological risk factors and diet in specific countries. More recently, WHO has coordinated a series of HES through its STEPwise approach to Surveillance (STEPS) programme in countries where traditionally there have been fewer data on CVD risks. In addition to studies of trends in individual countries, there are a small number of multi-country comparative studies. The MONICA Project provided a picture of change in CVD risk factors in 21 countries in the 1980s and 1990s. More recently, the Metabolic Risk Factors of Chronic Diseases Collaboration (www.imperial.ac.uk/medicine/globalmetabolics/) has collated much of the available population-based data on major cardio-metabolic risk factors throughout the world and has used advanced statistical methods to reconstruct trends in risk factors for all countries.78–83 Despite these efforts, data on trends in important CVD risk factors are more limited than on mortality in most countries, and the estimated trends to have large uncertainty.
Smoking
There has been a great deal of attention to trends in smoking with summaries of available data presented every decade or so.84,85 These studies show that there was a steady rise in smoking among men in English-speaking and Northern European high-income countries throughout much of 20th century, with the trend reversing in the 1960s; the rise, and to a lesser extent the decline in smoking, had a strong birth-cohort pattern.86–90 Subsequent to the start of the smoking epidemic in English-speaking and Northern European countries, smoking rates rose among men in Central, Eastern, and Southern Europe; in Japan; in some countries in Latin America (although not to the same levels as European and English-speaking countries); and eventually in other countries in Asia and Middle East.84 Smoking prevalence is still relatively low among men in Africa, where smokers smoke fewer cigarettes than in other regions.
The dynamics of the smoking epidemic in women were somewhat different, with the increases and declines in women occurring decades after those in men. The rise in smoking in women began after WWII, first in English-speaking high-income countries and in Northern European countries and continued to the 1980s but did not become as prevalent as it was among men. Countries varied considerably in sex differences in smoking prevalence. For example, in Denmark and the UK women as well as men historically had relatively high prevalences of smoking, with similar sex ratios seen later in Southern Europe and some Latin American (e.g., Argentina and Chile) and Central European (e.g., Austria, Czech Republic, and Hungary) countries. In contrast, Smoking remains relatively rare among women in much of Asia and Africa, with regional prevalences less than 5%;86,91,92. Elsewhere, e.g., in Russia and Ukraine where 50–60% of adult men and 15–25% of adult women smoke, there are intermediate sex ratios of smoking.86,91,92
In addition to changes in the prevalence of smoking and amount smoked by smokers, over the past 60 years there have been changes in the type of cigarettes available, including the introduction of “low-tar” and “light” cigarettes that aimed to reduce the tar and carbon monoxide yields of cigarettes.93,94 Some studies have associated these factors with differences in the risk of CVD events,95 making their changes a candidate explanation for some portion of the decline in CVD mortality (and its timing). However, recent Reports of the Surgeon General concluded that changes in cigarette design, including low-yield cigarettes, had not reduced overall disease risk, and that focus should be on preventing smoking initiation and facilitating quitting among current smokers.96,97
Alcohol use
Trends in alcohol use have been more heterogeneous than those of smoking, and highly influenced by cultural, socioeconomic, and political factors; by the larger diversity of alcohol types, scale, and ownership of production; and by alcohol policies and regulations. We will not review the full trends in alcohol use here as these have been done elsewhere,86,98–102 but note a few important features. First, alcohol consumption has declined steadily in traditional wine drinking countries (mainly Southern Europe and a few countries in South America) for decades (Figure 7).86,103,104 In other western countries, trends have been more variable, downwards in the presence of alcohol control policies like taxes and regulation that raise prices and restrict access, e.g., in Australia and Canada in the 1980s and 1990s, and upwards where there has been little policy intervention, e.g., Denmark, Finland, and the UK. Alcohol use is rising steadily in many countries in Asia, reflecting higher purchasing power coupled with low taxes and very little regulation.
Figure 7.
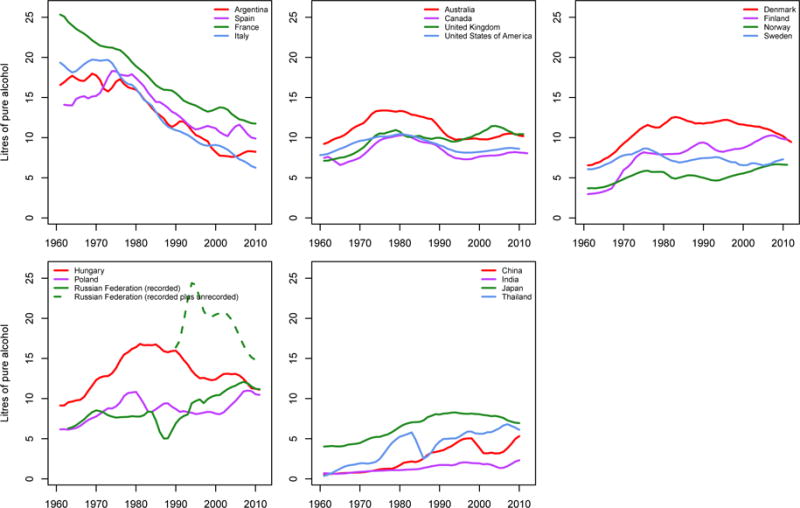
Trends in recorded per-capita alcohol consumption by adults aged 15 years and older in selected countries. Data were smoothed using a 3-year moving average. Data on recorded consumption are from the WHO Global Information System on Alcohol and Health (http://apps.who.int/gho/data/node.main.GISAH).
Note: In addition to recorded consumption, there is unrecorded consumption in some countries. For example, adult per capita unrecorded consumption is estimated to be less than 0.5 litres per year in Japan and France, and 1 to 2 litres per year in China, Sweden, the United Kingdom, and the United States.102 The unexpected drop followed by rise in Chinese trends may be due to change in the source of data on alcohol consumption from industry reports to government reports around 2000.
One of the most notable worldwide trends in alcohol use, with major consequences for CVDs, has been massive fluctuations in Russia and some of its neighbouring countries from the mid-1980s to the end of the 1990s.105 Even what constitutes alcohol sales is complex in these countries and includes much illegal and otherwise unrecorded consumption, making it difficult to estimate total consumption levels.106–109 What is clear, however, is that patterns of consumption in Russia and neighbouring countries are particularly hazardous, with high fraction of ethanol drunk as spirits and in intense drinking spells. Trends in such drinking patterns are reflected in the substantial fluctuations in mortality from acute alcohol poisonings as mentioned above.110 Although some debate remains about whether light or moderate drinking is cardio-protective,111 agreement is emerging that harmful patterns of alcohol use, especially heavy drinking, is associated with increased CVD risk.112 Yet, much less has been written on trends in drinking patterns elsewhere (in fact, the first international conference focusing specifically on patterns was only held in the mid 1990s,113,114 and systematic data collection on patterns of consumption only began afterwards).115
Diet
The early evidence on the effects of dietary factors on CVDs was largely from observational studies in humans and animal experimental studies.34 Given the strong correlations between diet and other behaviours, and among various features of diet themselves, there was and remains significant potential for confounding of effects in observational studies. However, well-designed randomised trials are increasingly common in nutritional epidemiology, have confirmed the effects of some dietary factors on CVDs and their intermediate physiological risks, including the harms associated of high salt intake on blood pressure (and in one case with additional follow-up on CVDs),116–119 and the benefits of replacing saturated fats with unsaturated fats, and of diets rich in fruits, vegetables, whole grains, low fat dairy, and nuts.120–127
Among dietary traits, trends in fats, especially animal-source and saturated fats, have received the most attention, because they have been considered an important risk factor for IHD.9 Using food supply, Antar and Kahn each concluded that there had been a rise in fat content of the American diet but that the rise was too small to alone explain the rising IHD mortality in the early part of the 20th century (rather, Antar hypothesised a role for the replacement of complex carbohydrates by processed ones).128,129 Similar analyses using data on food supply or dietary surveys associated reductions in saturated and animal-source fats as a driver of IHD decline in the last few decades of the 20th century in Finland, Germany, New Zealand, Poland, Sweden, the UK, and the US (with some also documenting a decline in salt intake and a rise in consumption of fruits).19,26,130–135 Addressing a longer period, Stephen and colleagues fitted a quadratic relationship to all available nutrition surveys and concluded that fat intake increased in the US until the 1960s before declining; the peak in the UK was in the 1970s.136,137 They associated these peaks with the timing of IHD mortality decline (but overlooked the fact that the peak for women had occurred at an entirely different time). The analysis of Japan’s impressive time-series of surveys also found an increase in fats while there was a rise in IHD, but concluded that trends in fats, when converted to cholesterol using Keys’ relationships, could not alone account for the observed change in IHD.27,30,76,77
Animal-source foods as well as vegetable oils seem to have increased in China for decades, as have the former in some Mediterranean countries like Greece.86,138 Pooling of worldwide nutrition surveys and analysis of food availability data from the UN Food and Agricultural Organization (FAO) both found that there has been little change in the availability or intake of animal fats at the global level, balancing a decline in high-income countries and a rise in the developing world.139,140
Trans fats, which are made by hydrogenating edible oils and are harmful for CVDs, were common in many countries because of reduced storage cost. Their use seems to have decreased in high-income countries, at least partially in response to regulation,141,142 and in some Central and Eastern European countries after the introduction of market economy;143,144 there may have been an increase in India and parts of Middle East.140,145
High levels of salt intake are now recognised as major risk factor for CVDs, especially for stroke, largely through effects on blood pressure.72,116,146,147 While partly related to cultural and geographical factors, economic development also influences salt intake, in two opposite ways: it reduces the need to use salt for preserving food through enhanced access to refrigeration and more regular availability of fresh foods, and it changes (mostly but not necessarily upwards) salt intake through the use of packaged and prepared foods which tend to use salt, often in high doses, for flavouring. Dietary salt in Japan, one of the countries with the best historical data, changed little from 1950s to 1970s, but declined subsequently.148,149 Similar reductions are occurring in parts of China but were not seen in South Korea.150 Among western countries, there have been long-term reductions in Finland, associated with lower deaths from stroke (and from stomach cancer), and more recently in the UK as a result of specific efforts to engage the food industry in reducing salt content of their products.132,133,151–155 Analyses of worldwide surveys have found high salt intake, with little change over time, in many regions,156,157 but the estimated trends have likely been affected by very limited data availability.
Less has been done on trends in the consumption of fruits and vegetables, and whether they are consumed fresh, cooked, or processed into juices; at least in their fresh form, fruit and vegetable consumption is linked to lower blood pressure and CVD risk.120,158,159 The limited available studies are divided between those that have reported increasing absolute intakes, at least in some high-income western and Asian countries (with a possible recent plateau)129,132,151,160–163 and those that have focused on their decreasing share of total energy,160,164 which is simply a reflection of the increase in intake of other foods and hence total calories. There is also some recent attention to trends in overall food and diet patterns, broadly concluding higher intake of vegetable fats (although not necessarily the healthy unsaturated fats) in low-income countries; higher availability of animal-source foods and dairy in middle-income countries and lower consumption in western high-income countries; higher use of refined carbohydrates and sugars and less of whole grains and foods with high fibre content; more fruits; and higher dietary diversity.86,139,160,165,166
Adiposity, glycaemia, and diabetes
BMI and adiposity are risk factors for IHD and stroke.167 The systematic pooling and analysis of data by the Metabolic Risk Factors of Chronic Diseases Collaboration showed that since 1980, age-standardized mean BMI increased among men in every region except Central Africa and South Asia, and among women in every region except Central and Eastern Europe and possibly Japan and South Korea.79 These trends resulted in a doubling of the prevalence of obesity in the world between 1980 and 2008.83
Largely associated with rising BMI, there has been a rise in blood glucose and diabetes in the world and in most regions, with the largest rise occurring in some Pacific islands and in countries in the Middle East and North Africa and in Central America.81 When compared to the increasing trend in BMI, the rise in diabetes was slower in Western Europe relative to other regions; in South Asia, it occurred faster than other regions. As we describe below, unlike diabetes, trends in blood pressure and serum cholesterol in high-income countries (and in many low- and middle-income countries with reliable data) have been opposite those of BMI, although the declines in blood pressure and serum cholesterol were smaller where BMI rose more.168
Blood pressure
Between 1980 and 2008, systolic blood pressure (SBP) declined slightly globally,80 with the largest declines occurring in high-income countries of Australasia, North America, and Western Europe. SBP rose in Oceania, East Africa, and South and Southeast Asia for both sexes, and in West Africa for women. The few longer term country studies also show decades of decline in blood pressure in a few high-income countries like the USA, Japan, and Finland.30,148,149,151,169,170 The declining blood pressures seem to be a result of both cohort and period effects.169,171 In other words, blood pressure has been lower in subsequent birth cohorts when they reach their 3rd and 4th decade of life with the improvement persisting through their lifecourse; above and beyond this cohort effect, people have experienced lower blood pressure at every age as time has passed, regardless of when they were born. Further, the reductions happened through the full distribution of blood pressures but there were additional benefits to those with hypertension.169,172,173
Little is known about the reasons for these trends and even analyses of the rich time-series Japanese data could only explain a part of the observed trend.148,149 The drivers however are likely to include lower intake of salt in some countries and higher and/or year-around use of fruits and vegetables which are a source of potassium (even in their less healthy form of juices), and the use of antihypertensive medications especially at middle and older ages. Additional reasons for cohort patterns can only be hypothesised and may include changes in early life nutrition or infection that persist through a cohort’s life. Importantly, these reductions in mean blood pressure occurred despite rising adiposity in high-income countries. More recently, similar declines have been recorded in Seychelles, a middle-income country in Africa, again despite rising BMI.174As a result, while in 1980 population mean blood pressure was directly associated with mean BMI, by 2008 the association had become null for men and inverse for women.175
Cholesterol
There has been a long history of interest in trends in serum cholesterol, due to its role as a cause of IHD,9 although it shows a surprisingly weak association with stroke risk.167 Researchers have used time series food data to estimate upward trends in (dietary) cholesterol in the US for the first 60 years of the 20th century and in Japan between 1949 and 1960s, although neither was sufficiently large to have alone led to the observed changes in IHD death rates27,128. Studies starting in the 1960s or 1970s using HES data have also shown rising serum cholesterol levels in Japan,170,176,177 and declines in western high-income countries.19,132,151,178,179 Pooling of global HES data showed that global serum total cholesterol levels have been virtually constant since 1980, as a result of a decline in Australasia, North America and Europe offset by a rise in East and Southeast Asia and Pacific, including in China, Japan, and Thailand.82 The decline in Western countries began well before widespread use of lipid lowering drugs such as statins.179 There was little evidence of change in total cholesterol in Latin America and Caribbean, North Africa and Middle East, South Asia, and sub-Saharan Africa, partly due to limited historical data in these regions.
The early signals and studies on risk factors and CVD mortality trends
Once researchers noted trends in CVD mortality, they also attempted to understand the drivers of these trends, both the rise and the subsequent decline. Moriyama and colleagues, who were among the first to note the long-term trends and its differences by sex, sought to find hypotheses by asking experts about explanations for these trends.33 The hypothesised explanations included changes in diet especially higher fat, energy imbalance and obesity (which they submitted as being worse in men because “the emergence in the early 1920s of the slim figure fashion” would have led to lower obesity in women), psychosocial stresses and strains (which was believed to affect men more due to the nature of their jobs hence partially accounting for differences in trends by sex), decline in infections and related CVDs, improvements in pregnancy care, and improvements in women’s economic and social status due to more job opportunities and because “by virtue of smaller families and mechanical aids, women had been liberated from prolonged, exhausting household drudgery.”33 The use of postmenopausal oestrogen – a topic to which attention returned nearly half a century later after the Women’s Health Initiative showed an associated increase in IHD risk180 – was deemed too recent and still too limited to have contributed to the trend.
Moriyama’s hypothesis-generating study aside, the earliest studies of the drivers of CVD trends focused almost solely on fats, saturated fats, and cholesterol in diet, followed by studies that also considered a role for trends in blood pressure, smoking, and eventually obesity. Occasionally other factors, such as the type of carbohydrates, were considered.129 Some of these studies found coinciding trends in risk factors and CVD mortality; others did not find an association or found inconsistent evidence on the role of the same risk factor between men and women (or other subgroups – for example Utah Mormons, who rarely smoked, had similar declines in CVD mortality as the general US population around when smoking began to decline).10,19,20,26,27,30,129,181–184 Walker went as far as attributing the decline in coronary disease to specific policy acts including the 1964 Report of the Surgeon General on Smoking and Health and the American Heart Association statement on diet.20
Taken altogether, two features of these studies emerge: First, there was substantially more focus on IHD than on stroke or other CVDs which were also declining. Second, the epidemiological studies (and strong beliefs) about the importance of specific risk factors, especially dietary fats, may have led to additional possible explanations being overlooked. A more careful look at countries with long time-series of data indicates that decline in CVD mortality in at least some countries, may have begun before its classical risk factors started to decline,53,168,185,186 raising the possibility that other important factors, and their combinations, may have been partly responsible for its initiation.
The Bethesda conference and the MONICA Project
The decade-long work on the determinants of decline came together at the Bethesda Conference on the Decline in Coronary Heart Disease Mortality (Panel 1).24 It also contributed to initiating the MONICA Project (Panel 2) which measured IHD risk factors (BMI, blood pressure, serum cholesterol, and smoking), incidence, treatment, case fatality, and mortality in 38 communities in 21 countries from mid-1980s to mid-1990s, aiming to quantify the contributions of risk factors, and subsequently medical care, to trends in IHD.36
IHD death rates declined in most MONICA centres, with an average cross-centre decline of 2·7% per year in men and 2.1% per year in women across centres, although they rose in most of the former communist countries of Europe and China. Change at specific centres ranged between a >7% per year decline in some Australian and Western European centres and a 4% per year rise in some of the Central and Eastern European centres.187 IHD (fatal plus non-fatal) event rates fell by 2.1% per year in men and 1.4% in women; 28-day case-fatality (which broadly represents hospital-related care) fell by 0.8% and 0.6%, respectively. Based on these results, the MONICA collaborators concluded that declining event rates contributed about twice as much as improvements in 28-day case-fatality to mortality decline; we will return to the role of medical care later in this article. The two components had heterogeneous effects across countries and in some places were working in opposite directions.
Despite its pioneering, visionary, and unique character the MONICA study is limited in how much light it can throw on the contribution of individual risk factors to changes in event rates. With only a few dozen data points and four risk factors, the estimates of effects had considerable imprecision, and there is a concern that there may have been over-fitting which might make findings not replicable.188 Nevertheless after mutual adjustment, change in IHD event rate was found to be positively associated with changes in blood pressure and cholesterol, but inversely associated with change in BMI, for both men and women.189 Moreover, while the association with change in smoking was direct for men, for women it was inverse in adjusted analysis. In addition to the above factors, this implausible result may be due to under-adjustment for other factors that confound the effects of smoking in women, which increased in many European countries where IHD was declining rapidly. Inverse associations of change in IHD with change in BMI may be due to similar reasons, or because the effects of rising BMI were countered by declining blood pressure and cholesterol, which are important mediators of its effects on IHD190 (importantly, these two risks, and smoking, declined more steeply in overweight and obese people than in those with normal weight;191 such within-population correlations of risk factors were not taken into account in the MONICA analysis and in similar subsequent studies).168
The magnitudes of associations aside, risk factor trends only explained about one half of the cross-country variation in IHD decline in men, and less than 20% in women, across the MONICA centres. While this may be partly due to measurement error in risk factors, it also implies that other important factors were acting to reduce IHD rates at the population level. The important role of these other factors is also apparent from the negative intercept of the association between IHD change and risk factor changes, with the implication that even if the risk factors considered had not changed, IHD would have declined in the MONICA study populations.
Other community-based studies
Even before the Bethesda Conference, some researchers had begun to empirically investigate why heart disease mortality was declining. Notable among these, Pell began to investigate incidence and shorter (24-hour and 30-day) and longer term survival from myocardial infarction among employees of Du Pont Company in 1957.192,193 As the employees had regular medical examinations and their health and vital status was known, this investigation was among the few to document trends in both incidence and survival.193 This analysis found that incidence had declined since 1957, when data collection began. Case-fatality began to decline in the early 1970s, which continued steadily to 1983, the last year of data.
Nonetheless, most of the activities in this area began around and after the Bethesda Conference. In addition to the MONICA Project the Bethesda Conference motivated a number of community-based studies in the US, including the Minnesota Heart Study, the Olmsted County Study, Worcester Community Study, and the Atherosclerosis Risk in Communities (ARIC) Study. These studies collected population-based data on trends in hospitalisation for CVDs, treatment, survival of hospitalised patients, and in some cases risk factors.194–198 Most of these studies used hospital records to track trends in hospital admissions and discharges with IHD (some confirmed by chart review – typically symptomatology, electrocardiogram features, and an ever-evolving set of cardiac enzymes – and sometimes with additional efforts to find cases that either did not appear in these databases or whose cause of admission or death was coded incorrectly). Minnesota Heart Study and ARIC also used vital statistics to monitor out-of-hospital IHD deaths. The British Regional Heart Study had similar aims but followed individuals vs. whole communities.199,200 More recently, the availability of electronic data on hospitalisation and the ability to link these data to death records has allowed monitoring trends in CVD event rates, and in- and out-of-hospital survival.201–206 Bureaucratic and legal obstacles to linkage, packaged as privacy concerns, however have so far not permitted the potential of such data to be fully utilised, or are threatening them where there have been advances.
Risk factor attribution modelling studies
Around the same time as the MONICA Project, numerous prospective cohort studies were initiated, and helped identify new risk factors for CVDs (but could not assess how their changes affect disease trends in populations) and randomised trials showed the efficacy of new treatments and led to changes in clinical guidelines (but could not examine their uptake and effectiveness in whole populations). A different group of studies used estimates of the size of aetiological effects of risk factors and treatment from these individual-level studies to attribute, using modelling, either levels or changes in CVD mortality to specific risk factors and to treatment.7,62,207–213 These studies all attributed relatively large proportions of current CVD mortality or change in mortality, to risk factor levels and trends. They also found that treatment, either acute or long-term secondary prevention among people with a history of CVD, was partly responsible for the CVD mortality decline. The number of risk factors in such studies has increased over time,214,215 including the effects of multiple risks.210,211,216,217
An important difference between these modelling studies and the analyses done in the MONICA Project is that the contributions of risk factors in MONICA were directly estimated as associations between the data on change in risk factors and in CVD disease or mortality across populations (which of course might introduce different sources of error compared to the modelling studies). The attribution modelling studies, on the other hand, by necessity assume that the individual-level associations apply to the populations considered, which may be different from the epidemiological cohorts that generated them (there is some effort to evaluate the evidence for such similarity or its absence in relation to region and ethnicity).167,190,218 Some of these studies have accounted for the fact that the effects of different risk factors, and of risk factors and treatment, cannot be simply added,3,216,217 i.e., when multiple risk factors improve and treatment improves, the reductions in some CVD deaths may be attributed to more than one factor.219,220 Others did not, and constrained the contributions to add to 100% (theoretically, the sum of individual contributions, if not considered jointly, could add to more than 100%). By their nature, such “additive” models predict a rise in CVDs when risk factors rise, as has been predicted for adiposity221–223 – as described earlier, such predictions are the opposite of the actual trend data, which have shown a consistent decline in CVD mortality even as BMI rose. More recently, these models have been extended to model the future impacts of continued risk factor trends or alternative scenarios.3,221–223 A recent study analysed the impacts of achieving the globally-agreed targets on risk factors on future trends in “premature” (i.e. under 70 years of age) mortality from various noncommunicable diseases (NCDs) (targets were set following the 2011 UN High Level Meeting on NCDs).3 The study found that, under a so-called business-as-usual trajectory, premature CVD mortality (i.e., projections based on current trends with no additional action) are expected to decline by 18% between 2010 and 2025. Achieving the globally-agreed targets on smoking, alcohol use, salt intake, obesity, and raised blood pressure and glucose will nearly double the decline, to 34%.
Recent studies on risk factors and CVD mortality trends
More recently, some studies have returned to the empirical traditions of 1970s and 1980s, and have implicated trends in a specific risk factor in explaining those of CVDs, often making use of convenient natural experiments which had engendered a change in risk factors in some populations but not others. The decline in CVDs in some countries in Eastern Europe was attributed to transition from hydrogenated fats to non-hydrogenated vegetable oils after the fall of communism,143,144 although there was no attempt to deal with the numerous other socioeconomic, behavioural, and nutritional changes in these countries. Careful analysis of trends was instrumental in establishing the fall of alcohol consumption in the Soviet Union during Gorbachev years, and the subsequent rise in harmful and heavy drinking, as the most important determinant of decline and sharp rise in CVD mortality in Russia and some other former Soviet republics.46,224,225 This role was initially challenged by epidemiologists in view of the apparent CVD benefits of moderate drinking in Western Europe, until individual-level case-control and cohort studies confirmed the massive hazardous effects in Russia.226–229
Recently, researchers credited a decade of salt reduction in the UK as an important driver of the decline in blood pressure, and by extension in stroke and IHD deaths, above and beyond other factors such as medication and increased consumption of fruits and vegetables in the UK.153 In a recent study from Cuba, researchers associated changes (both decline and increase) in body weight during periods of economic crisis and recovery with decline and increase in diabetes and changes in the rate of CVD decline (i.e., CVD continued to decline regardless of whether weight declined or increased).230
Finally, in an analysis similar to the MONICA Project in terms of included risk factors and approach, data on population blood pressure, serum total cholesterol, BMI, and smoking (measured by lung cancer to account for cumulative effects)231,232 were used to examine empirically the association of changes in risk factors with those of cardiometabolic (CVDs plus diabetes) mortality;168 Figure 8 shows the results of this study for CVDs alone. Similar to the MONICA Project, change in CVD mortality was positively associated with those of blood pressure and cholesterol; unlike MONICA results, there was also positive association with change in smoking for both sexes, and in BMI for women, after adjustment for other risk factors (when CVDs and diabetes were used together, the association with change in BMI was positive for both sexes).168 The magnitudes of the associations were consistent with those found in prospective cohorts of individual participants. Importantly, and as in the MONICA Project, change in risk factors only explained a relatively small share of cross-country variation in mortality change demonstrating the crucial role of other determinants at the population level. The above-mentioned caveats in terms of measurement error and temporal features of change also apply to these results.
Figure 8.
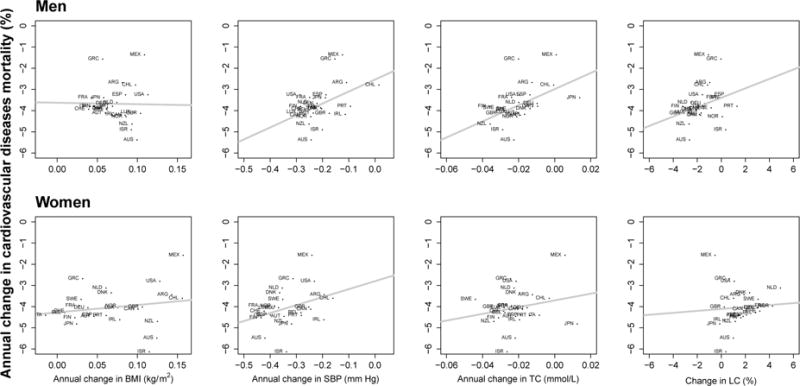
The cross-country associations between change in risk factors and change in cardiovascular death rates between 1980 and 2008 in 26 industrialised countries. Each point shows one country. All variables were age-standardized. The lines show the fitted linear associations. Source: Di Cesare et al.168
Note: Lung cancer (LC) death rate was used to measure cumulative population exposure to smoking using because data on lung cancer mortality trends are more widely available and more reliable than on smoking prevalence and intensity.231,232
ARG: Argentina; AUS: Australia; AUT: Austria; BEL: Belgium; CAN: Canada; CHE: Switzerland; CHL: Chile; DEU: Germany; DNK: Denmark; ESP: Spain; FIN: Finland; FRA: France; GBR: United Kingdom; GRC: Greece; IRL: Ireland; ISR: Israel; ITA: Italy; JPN: Japan; LUX: Luxembourg; MEX: Mexico; NLD: Netherlands; NOR: Norway; NZL: New Zealand; PRT: Portugal; SWE: Sweden; USA: United States of America
The role of medical care
When analysing trends in CVDs and eliciting their drivers, it is inevitable to wonder about the relative contributions of risk factor prevention and medical care. Modelling studies aside, the Bethesda conference, the MONICA Project, and some community studies remain among the few attempts to investigate the contributions of prevention and medical care to CVD mortality decline (noting that sometimes this was only in the form of data on incidence and case-fatality which do not map completely to the underlying questions of interest because treatment can affect risk factors like blood pressure and serum lipids, and some lifestyle changes can improve post-event survival).
The period following the Bethesda Conference coincided with a multiplication of effective (and some less effective) treatments for acute coronary syndromes and stroke, as well as management of those who survived the acute events (although in his study of Du Pont employees Pell documented better survival starting from early 1970s).193,233 The enthusiasm for coronary care units appears to have been misplaced, as they improved diagnosis and in-hospital management without a corresponding change in actual deaths at the population level which were dominated by out-of-hospital deaths.19,234 On the other hand, the efficacy of anti-thrombotic and reperfusion therapies – including aspirin, systemic thrombolytics, and more recently percutaneous coronary interventions – was demonstrated in randomized clinical trials in the mid-late 1980s. These interventions were subsequently taken up in clinical guidelines and practice in high-income countries (albeit at varying degrees and speeds as described below) with emphasis on reducing time to access to acute treatments.235–240
In high-income countries, following approximately the same time course as advances in hospital care and post-hospital secondary prevention for acute cardiovascular events, evidence was accrued from randomized controlled trials for the benefits of primary prevention through pharmacological treatments (anti-hypertensive medications, statins, and low-dose aspirin). Following the first Veterans Administration Trial in the US, which reported a large reduction in cardiovascular events (mainly stroke) from anti-hypertensive medication,241 placebo-controlled trials in Australia and the UK confirmed the benefits of anti-hypertensive medication for people with moderately-elevated blood pressures.242,243 More recent studies, including in the elderly, and meta-analyses, have shown reductions in cardiac events as well as all cardiovascular events from blood pressure lowering medicines.244–248 Since the 1970s, treatment of high blood pressure has increased steadily in high-income countries of Asia, Australasia, North America, and Western Europe, and subsequently in upper-middle-income countries in Latin America and elsewhere.30,148,149,249–255
The epidemiologic and animal evidence on the role of cholesterol as an important CVD risk factor produced intense interest in cholesterol-lowering treatments, with the first of HMG-CoA reductase inhibitors (statins) released in 1987.179,256,257 The evidence from randomised trials that the benefits of statins depend on baseline risk of CVD disease, regardless of baseline cholesterol levels, led to changes in treatment guidelines and a rapid increase in their use, especially in the US (uptake in other countries was slower due to some debates regarding the generalizability of early findings on mortality reductions, which have been settled in more recent trials).256,258–263
Inevitably, pharmacological treatment has been responsible for some of the reductions in blood pressure, especially at older ages, and for more recent reductions in cholesterol. The extent of such contributions are difficult to establish due to simultaneous and partially correlated trends in various known (as well as unknown) determinants of blood pressure and cholesterol in even the best population-based data, such as those from Japan.148 Further, if treatment is based on absolute risk of disease, vs. the level of risk factor, it can have large contributions to CVD disease and mortality without much effect on mean population exposure. Preventive treatments remain relatively uncommon in low-income countries, even among people with a history of CVD events.255,264
Although it has been known for decades that hyperglycaemia and diabetes are associated with higher risk of CVDs,265 the contribution of pharmacological glycaemic control to the CVD decline is less clear, both because of the paucity of evidence from population-based studies and the mixed signals from clinical trials in terms of CVD benefits (vs. on microvascular complications, like retinopathy and nephropathy, for which there is more evidence).266 For example, the United Kingdom Prospective Diabetes Study (UKPDS) trial found a minor effect of glycaemic control on MI and mortality during the trial, which became significant at 10-year follow-up.267 The ACCORD study, on the other hand, was stopped early because of increased all-cause and cardiovascular mortality in the intensive treatment arm.268 These conflicting results likely represent a trade-off between the benefits of good glycaemic control on long-term cardiovascular outcomes, and the risks of hypoglycaemia, especially in patients with advanced diabetes or prior CVD.269–271 Combined with such equivocal results in trials, rates of treatment and glycaemic control are low even in high-income countries.272 Therefore, one may speculate that changes in glycaemic management have perhaps made only a modest impact on CVD mortality trends.
Given this rise in effective treatments, the MONICA Project provided an opportunity for examining the contributions of both risk factors and treatment in the same study. The role of medical care was initially presented only as changes in short-term case-fatality which measures only the role of acute care.187 Subsequently, MONICA data were analysed to also examine how much changes in measured medical care and treatment, including acute and sub-acute coronary event management and secondary prevention in people with a history of IHD, had affected changes in coronary-event rates and case-fatality, and in IHD mortality (even then the contributions of changes in risk factors and medical care were reported in separate papers, possibly to avoid over-fitting, with relatively few participating centres (n) and a large number of explanatory variables (p), a problem that is more tractable today than it was in the 1990s owing to methodological advances related to the analysis of ‘omic data in which so-called ‘p > n’ problems are common).273,274
The MONICA investigators analysed the coverage of a variety of treatments that could prevent acute events and improve both short- and long-term survival (antiplatelet therapy, antihypertensive medications, and acute coronary reperfusion procedures) separately, and also aggregated them into an overall score measuring treatment intensity/quality. They then correlated changes in these measures to changes in study outcomes.273 As perhaps expected, improvements in treatment intensity were strongly associated with lower case-fatality, with treatment change accounting for 51% of variation in case-fatality change across MONICA sites. Geographically, treatment improved more in Western Europe, North America, Australia, and New Zealand than MONICA centres from in Eastern Europe and China, with the improvements in the best-performing countries associated with about 25% larger decline in case-fatality than those in those with the smallest improvement – little has been done on the reasons for this variation in the uptake of effective treatment, which is seen even among high-income countries.
More surprisingly, they also found strong associations between change in treatment intensity and change in event rates, with treatment change explaining 41% of change in coronary event rates and 64% of change in overall IHD mortality rate – both larger than the corresponding numbers for the proportion explained by changes in risk factors (although as noted above the investigators made no formal comparison between the contributions of risk factors and treatment towards lower event and death rates).273
The apparent difference in contributions of treatment vs. prevention to decline in event rates in MONICA could have been affected by better measurement of treatment coverage than risk factors and by differences in how fast they act (immediate for treatment and more gradual for risk factor changes), biasing the estimated effect of the latter towards zero. However, while the correlation between treatment intensity and CVD trends in the MONICA Project could be confounded by other, as yet unmeasured, factors, the expansion of evidence-based acute care and secondary prevention measures over this period provide a fairly convincing mechanistic and temporal explanation for an important part of the decline.274
Further support for the important contribution of treatment to declines in mortality from CVDs, especially from IHD, came from the above-mentioned community studies in the US (and before that from the above-mentioned detailed study among Du Pont employees by Pell). All of these studies found declines in IHD deaths and in case-fatality of hospitalized patients, alongside striking increases in treatment intensity, between the 1980s and 1990s; in those studies that had measured both hospitalisation and out-of-hospital deaths, the share of events that were admitted to hospitals increased.194–198,275,276 The Minnesota Heart Survey, which also collected data on risk factors in the same community, found significant but modest declines in cholesterol (by 3–5 mg/dl), smoking (2–3%), and blood pressure (1 mm Hg) between 1985–87 and 1990–92. In light of these findings, the authors generally attributed the decline in mortality largely to improvements in treatment.196
The interpretation of results from some of these studies was complicated by evolving diagnosis of MI over the study period, with use of troponin-based assays increasing from 8% to 98% between 1996 and 2001 in the ARIC Study. This change led to higher estimated incidence over time and a changing composition of hospital-based cases (towards those with more minor infarctions).277–279 Adjusting for changes in diagnosis reversed the estimated incidence trends (from increasing to declining), and led to lower, but nonetheless significant, estimates of the contribution of treatment.274,280 At the same time, better diagnosis (and hence treatment) of milder cases who would have been missed without sensitive tests should count towards the contributions of advances in medical care towards lower CVD mortality because the milder cases, once detected and treated, have an improved prognosis.281,282
Infectious diseases and CVD trends
Going back a century or more, prior to countries passing through the epidemiologic transition, the global profile of cardiovascular disease mortality would likely have been dominated by conditions associated with infection including rheumatic heart disease (RHD), infective endocarditis, and cardiomyopathies.283 Today, although IHD and stroke are the two largest CVD causes of death globally, RHD, cardiomyopathy, myocarditis, and endocarditis account for an estimated 800,000 deaths worldwide.1 Infection-related CVDs remain major contributors to CVDs in many developing countries, especially in children and young adults.1,284,285 Trends in RHD, and its underlying risk factor of rheumatic fever, over the past 150 years have been characterized by four important features.286,287 First, the incidence of rheumatic fever began to decline before the introduction of antibiotics in industrialized countries,288 and virtually disappeared by the 1970s or 1980s.16,289–291 The reasons for this early fall are unknown but are likely to be general improvements in living and housing conditions, and in nutrition and general healthcare. The decline in RHD was a likely contributor to the above-mentioned decline in total CVD mortality, even as IHD death rates were increasing. Despite the overall downward trend in high-income nations, RHD remains an important public health problem in the aboriginal communities of Australia, Canada and New Zealand.292,293 Second, there was a post-antibiotic fall in rheumatic fever in countries such as Costa Rica, Cuba, Guadeloupe, and Martinique which implemented comprehensive programmes for the prevention of rheumatic fever, through both social and environmental interventions and the use of antibiotics. Third, unlike this latter group, the incidence of rheumatic fever remains high in sub-Saharan Africa and South Asia, where there are no national programmes for the prevention and treatment.294 Finally, there has been a resurgence of rheumatic fever in the former Soviet Republics in Central Asia following the collapse of the Soviet Union. Kyrgyzstan, Tajikistan, Turkmenistan, Kazakhstan, and Uzbekistan have some of the highest rates of RHD in the world.295,296 Less is known on trends in cardiomyopathies, which are endemic in tropical countries.297–299
More broadly, populations exposed to a chronic burden of infections, with associated profiles of inflammation, may also be at a resultant increased risk for IHD, especially in the presence of other behavioural and environmental risk factors.300 It is therefore reasonable to hypothesise that lower incidence of infections, and more treatment, may also have been partly responsible for decreasing IHD mortality as countries develop. Although there are no data on trends in inflammatory markers to evaluate this possibility, the increasing use of antibiotics and the resultant decline in the burden of infections in many countries from the late 1940s onwards, came just before the rising trends of IHD slowed and then reversed.
An area of increasing importance is the potential role of the HIV/AIDS epidemic, and its treatment, on the future course of CVDs in countries and communities affected by it. Untreated HIV infection is associated not only with an overall shortening in life expectancy but also with weight loss and a fall in systolic blood pressure.301 By contrast, in infected individuals there is higher incidence of inflammatory cardiovascular disorders, resulting in cardiomyopathy, tuberculous pericarditis, pulmonary hypertension, stroke, and vasculopathy. The use of some antiretroviral treatments is associated with an increase in insulin resistance, dyslipidaemia, and lipodystrophy. These atherogenic complications of antiretroviral therapy will be of increasing relevance as more people receive long-term antiretroviral treatment and reach middle and older ages.302,303
Conclusions
As we have reviewed, since the middle of the 20th century, there has been attention to trends in CVD deaths, and a desire to understand its drivers. We found that with the exception of the well-designed and carefully executed MONICA Project and a few other within- or cross-country studies, most analyses of the role of risk factors or treatment in CVD mortality trends relied on qualitative observations or basic quantitative tests of whether the two variables changed together, with limited attention to other explanations for the decline.
This seemingly critical assessment is however at least partly an outcome of both the changing epidemiological and clinical knowledge on CVD causes and treatments and of the changing nature of CVDs themselves. All of the major empirical studies on determinants of CVD mortality decline collected data over a period in which many aspects of CVD aetiology and survival were changing rapidly in high-income countries: partially correlated trends in some risk factors and opposite trends in others; changes in demography of people with CVD events with average age increasing and changes in sex composition of CVD events and deaths (issues that MONICA was less able to capture given that it did not assess people over 65 years old and that fewer of the events were in women than in men); and dramatic changes in how AMI was defined, diagnosed, and treated with increased awareness on the part of physicians and the general public about symptoms and the importance of early in-hospital treatment, increasingly sensitive biomarkers capable of detecting minor events that would not be captured by symptoms alone or the electrocardiogram, and uptake of more effective treatments both at the hospital and among survivors. As a result of the multiplicity of other changes, measured and unmeasured, over this period, and the paucity of high-quality longitudinal population-based data (many of which had been proposed at the Bethesda Conference but were not pursued), the answer to the question of what set into motion the decline in mortality, and the relative roles of treatment and prevention, is likely to remain largely correlational and somewhat speculative, rather than causal. Despite these limitations, the vast body of research on CVD mortality trends also allows some conclusions with some confidence:
First, in high-income countries and in some middle-high-income countries in Latin America, once the CVD mortality decline began, it never stopped or even slowed down, a situation that was hard to envision in the 1970s and 1980s. Even today, there is little indication that the relative rate of decline in CVD mortality in high income countries is reducing; if anything, it is increasing proportionally in many countries. If current downwards trends continue, IHD and stroke will become uncommon causes of death under 70 years of age in high-income countries. Recent data and modelled mortality trends by WHO and others seem to indicate that currently middle-income and upper-middle-income countries have begun similar trends. This decline in turn will lead to an increase in longevity and life expectancy, because CVDs are a major cause of mortality worldwide.213
Second, trends since the 1970s have almost certainly benefited from a mixture of continued declines in key behavioural risk factors such as smoking; in physiological risk factors such as blood pressure and serum cholesterol, part of which is likely to be due to changes in diet (e.g., lower intake of salt and saturated fats, and year-around availability of fruits and vegetables), and due to widespread use of effective pharmacological treatments for hypertension and dyslipidaemia; and in improvements in acute care including time before reaching hospital, better diagnosis, and acute care. Blood pressure seems to have been declining for an even longer time in high-income countries, a phenomenon whose drivers are not known but which may have been responsible for the much earlier start of the decline in stroke mortality compared to IHD. While the contributions of both risk factors and treatments to the decline in CVDs is likely real, none of the measured drivers explains the similarities and differences across countries or between men and women in when the decline began.184 This leaves room for hypotheses related to other phenomena – related to subtle changes in diet or health systems, to reductions in infections and resulting inflammatory response, and to improved fetal and childhood nutrition and health – as possible determinants of the decline.
Third, the nearly universal increase in adiposity does not seem so far to have attenuated the long-term declining trend of CVD mortality in high income countries since the 1960s. It may be that the rate of decline in these countries might have been even steeper if the obesity epidemic had not occurred, but to date the correlation and the magnitude of such an effect seems modest (see Figure 8).168,189 This contrast with the relatively important effect of obesity in individual-level epidemiological studies may have been partly due to more aggressive management of physiological risks that mediate the effects of adiposity among overweight and obese people,190 leading to a “decoupling” of BMI from blood pressure and lipids (but not diabetes) both across populations and in individuals.175,191 Nevertheless in low-income countries, where maternal and child under-nutrition are still widespread and where the thinly stretched healthcare system may be unable to screen and treat physiological risk factors,304 rising rates of obesity might result in increased population risk of CVDs beyond the relatively modest impacts in high-income countries; reliable mortality trend data to better envision this possibility are however not available.
Finally, whatever the explanations for a century of rise and fall in CVD mortality in the high-income world, former communist countries in Central and Eastern Europe, defy their universal generalisation. The extraordinary patterns of change in these countries appear to be closely synchronised with massive political and social changes following the collapse of communism starting in the early 1990s. Although an important role for hazardous alcohol drinking has been identified, especially in Russia, there remains an uncertainty about the precise mechanisms involved.
Going forward, it is reasonable to advocate more systematic population-based data to better understand why the decades-long decline in CVDs continues in high-income countries, and to document its early and subsequent stages in middle-income countries. Such knowledge may help further accelerate the decline in high-income countries and extend it to other regions where they remain the leading cause of mortality (and, paradoxically, their absolute mortality and morbidity burden is set to rise due to population aging). The availability of electronic population-based data on hospitalisation and mortality, and the ability to link across them, as well as regular health and nutrition surveys mean that such data can be obtained with relatively modest additional investments to bring together existing data sources and complement them with additional ones. These data have national coverage, and also allow time trends and subnational variations and inequalities to be studied.5,8,305 Such data exist in most high-income and some middle-income countries but their use requires overcoming the increasing bureaucratic and legal obstacles to using and linking across administrative data on health risks and outcomes. In lower income countries in Africa and Asia, the first step may be to assess trends in CVD mortality and subsequently incidence without the current overreliance on models. This in turn requires strengthening vital registration and disease and risk factor surveillance systems in these settings.
While a fully comprehensive and convincing account of the precise drivers of the declines we have described in CVD for many countries remains elusive, we return to the fact that these declines are enormous and are making important contributions to improvements in life expectancy in most regions. For some countries and populations, therefore, it is perhaps reasonable to argue that whatever the drivers of the decline in CVDs, the continued impressive pace of decline should be a reason to give more attention to other health conditions that are either not declining as fast or rising such as cancers, diabetes and neuro-psychiatric conditions.
Supplementary Material
Key points.
Death rates from ischaemic heart disease (IHD), stroke and other cardiovascular diseases (CVDs) have declined for decades in high-income countries and in many countries in Latin America. There is no indication that the decline is slowing.
These trends have likely benefited from declines in some key behavioural risk factors including smoking and physiological risk factors such as blood pressure and serum cholesterol.
The nearly universal increase in adiposity does not so far seem to have modified the long-term declining trend of CVD mortality in high income countries. However, the rate of decline might have been slightly steeper if the obesity epidemic had not occurred.
Improvements in medical care, including effective treatment of physiological risk factors, better diagnosis and treatment of acute CVDs, and post-hospital care of those with prior CVDs, have also contributed to declining CVD event and death rates.
Measured risk factor and treatment variables, while important, neither explain why the decline began when it did, nor much of the similarities and differences in the start time and rate of the decline across countries or between men and women.
Trends in CVDs in the former communist countries of Europe and the Soviet Union, whether declining or increasing, appear to be closely synchronised with massive political and social changes following the collapse of communism in the early 1990s, with hazardous alcohol use as an important behavioural mediator.
Panel 1: The Bethesda Conference on the Decline in Coronary Heart Disease Mortality.
The Bethesda Conference on the Decline in Coronary Heart Disease Mortality was organised by the National Heart, Long, and Blood Institute (NHLBI) and held in October 1978, a decade after the decline in IHD had begun. The meeting aimed “(1) to consider whether the greater than 20% decline in coronary heart disease mortality since 1968 is real, (2) to discuss possible causes, and (3) to recommend further studies to elucidate the causes.”24 The meeting systematically considered a large number of potential explanations from artefact, to the classic risk factors of smoking, cholesterol, and blood pressure, to new risk factors such as ambient air pollution and physical activity,306,307 to pre-hospital and in-hospital treatment and survival. The papers and discussion from the Bethesda Conference were exhaustive and comprehensive, involving some of the best epidemiologists, statisticians, and clinical scientists of the day. However, their conclusion was stark: “Although there was general agreement that the decline in coronary heart disease is real, the probable cause or causes could not be precisely identified”.24
Three key areas for research were identified: (1) improve data collection systems on morbidity and mortality for IHD; (2) evaluate contributions, and future potential, of preventive and therapeutic “patient management”; and (3) continue to undertake basic clinical and non-clinical research into pathogenesis, risk factors, and clinical management of IHD.24 The Bethesda Conference was an important catalyst for the initiation of the MONICA Project and a number of US community-based studies discussed later in this review. Nonetheless, population-based data on outcomes and on risk factor and treatment determinants remained weaker than recommended at the Conference.
Panel 2: The MONICA Project.
Partly motivated by the discussions and recommendations of the Bethesda Conference, the World Health Organization (WHO) coordinated the initiation of the MONICA (Multinational MONItoring of trends and determinants in CArdiovascular disease) Project in 1979.36,308 The MONICA Project aimed to assess prospectively the influence of changes in major CVD risk factors including serum cholesterol, blood pressure, cigarette smoking, and later on body-mass index, plus in 28-day case-fatality, on change in IHD mortality. Change in treatment was subsequently analysed as one of the variables of interest. IHD risk factors, incidence, case fatality and mortality were measured in 38 communities in 21 countries, mainly in Europe but also in Australasia, China, and North America.36,308 Data for the main study were collected between mid-1980s and mid-1990s although many MONICA centres have continued to collect data and monitor trends. The focus was on premature mortality, at that time defined as death before 65 years of age. The key defining feature of the MONICA protocol was the use of identical or very similar protocols in all centres including standardised case definitions for incident IHD. The criteria remained in use for population-based surveillance years after the study ended.309
Panel 3: Analysing and reporting trends in cardiovascular disease (CVD) mortality.
The early works on CVD trends, like those of Moriyama and colleagues,11,12 pointed out and attempted to address a series challenge of analysing and reporting trends in cause-specific mortality at the population level that continue to persist today.26,213,310,311 These issues include the differences over time in relation to the geographical areas or even social and ethnic groups covered by death registration, completeness of death registration among the covered population, and assignment of medical cause of death. In many countries, death registration begins in certain areas, often urban centres or a subset of provinces, before incorporating other parts of the country. Whether immigrants and/or emigrants are covered differs across countries and changes over time. Limitations in the civil registration and statistical systems lead to incomplete death registration, with completeness changing over time. And combinations of changes in the in International Classification of Disease (ICD) system, and clinical culture and training, lead to both inappropriate assignment of cause of death and inconsistently and incomparability in how this is done over space and time. The methods used for dealing with these issues, by the World Health Organization (WHO) and researchers, have become increasingly more complex and sophisticated over time.285,310,312,313 Nonetheless, fundamentally they involve a combination of clinical and epidemiological judgements and assumptions plus analytical methods that formalise these judgements and assumptions. The trends in Figures 1–3 are based on data and work at the WHO, and are described in various WHO publications.213,310,314 In brief, the countries shown in Figures 2 and 3 have complete or near-complete registration of deaths with medical certification of causes of death. WHO uses demographic methods to test and, if needed, adjust for completeness of death registration, and redistributes ill-defined and improbable causes of death (e.g., deaths assigned to senility and to signs and symptoms such as pain) based on patterns in countries with high-quality cause of death assignment.
Acknowledgments
We thank Colin Mathers, Gretchen Stevens, and Vasilis Kontis for data and figures on mortality trends, Juergen Rehm for data on trends in alcohol consumption in Russia, and Mohammed Ali, Perviz Asaria, Robert Beaglehole, Peter Burney, Zhengming Chen, Goodarz Danaei, Darwin Labarathe, Juergen Rehm, and Jonathan Samet for discussions on materials covered in the review. ME is supported by a strategic grant from the UK Medical Research Council (MRC), and by the MRC and Public Health England (PHE) though support to the MRC-PHE Centre for Environment and Health. PE acknowledges funding from the National Institute for Health Research (NIHR) Biomedical Research Centre at Imperial College Healthcare NHS Trust and Imperial College London, the NIHR Health Protection Research Unit on the Impact of Environmental Hazards, and from the MRC and PHE in support of the MRC-PHE Centre for Environment and Health.
Biographies
Majid Ezzati is Professor of Global Environmental Health at Imperial College London. He completed a PhD at Princeton University, and was a Global Health Leadership Fellow at WHO, where he led the Comparative Risk Assessment (CRA) Study – the first consistent global analysis of behavioural, environmental, nutritional, psychosocial, and metabolic risk factors, which formed the scientific core of World Health Report 2002: Reducing Risks, Promoting Healthy Life. He currently leads the NCD Risk Factor Collaboration, a worldwide scientific collaboration to strengthen the evidence on exposure and health effects of cardiometabolic risks. He is a Fellow of the UK Academy of Medical Sciences.
Ziad Obermeyer is Assistant Professor of Emergency Medicine and Health Care Policy at Harvard Medical School, and an emergency physician at the Brigham and Women’s Hospital. After training in history of science at Harvard and Cambridge, he worked as a consultant for McKinsey & Co. before receiving his MD from Harvard. He completed his clinical residency in emergency medicine at the Brigham and Women’s, Massachusetts General, and Boston Children’s Hospitals. He is the recipient of an Early Independence Award from the Office of the Director of the National Institutes of Health.
Ioanna Tzoulaki is Senior Lecturer in Epidemiology at Imperial College London. She completed her PhD at the University of Edinburgh on cardiovascular disease epidemiology in 2006. Her research interests include cardiovascular disease risk factors, cardiovascular biomarkers, and cardiovascular risk prediction models as well as empirical research methods and evidence-based medicine.
Bongani M Mayosi is Professor and Head of the Department of Medicine at Groote Schuur Hospital and University of Cape Town, South Africa. He qualified in medicine from the University of KwaZulu-Natal in Durban, trained in internal medicine and cardiology in Cape Town, and graduated with a DPhil from the University of Oxford in 2003. His research interests include the genetics of cardiovascular traits, treatment of tuberculous pericarditis, and prevention of rheumatic fever. He is president of the Pan-African Society of Cardiology, and associate editor for Africa of Circulation.
Paul Elliott is Professor of Epidemiology and Public Health Medicine at Imperial College London. After completing a PhD at the London School of Hygiene & Tropical Medicine, he went on to become Head of the Environmental Epidemiology Unit. Since 2009, he has been Director of the MRC-PHE Centre for Environment and Health, leading a programme of research into environmental and genetic risk factors. His research interests include the epidemiology of high blood pressure and its non-pharmacologic treatment. He is a co-principal investigator of the UK MRC-NIHR National Phenome Centre and a founding member of the UK Biobank Steering Committee. He is a Fellow of the UK Academy of Medical Sciences and an NIHR Senior Investigator.
David A Leon is Professor of Epidemiology at the London School of Hygiene & Tropical Medicine, from which he also obtained his PhD. He also holds a position at the Arctic University of Norway, UiT. He has worked in a range of research areas over the past 30 years, but now focuses on a series of innovatory studies of cardiovascular disease in Russia.
Footnotes
Contributions
ME developed the review concept and coordinated the review and writing of the article. All authors contributed to the review materials and writing, and discussed the contents of the article.
Competing interest
None
References
- 1.World Health Organization. Global Health Estimates: Deaths by Cause, Age, Sex and Country, 2000–2012. WHO; 2014. [Google Scholar]
- 2.Moran AE, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483–1492. doi: 10.1161/CIRCULATIONAHA.113.004042. CIRCULATIONAHA.113.004042 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontis V, et al. Contribution of six risk factors to achieving the 25×25 non-communicable disease mortality reduction target: a modelling study. Lancet. 2014;384:427–437. doi: 10.1016/S0140-6736-(14)60616-4. S0140-6736(14)60616-4 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaria P, et al. Trends and inequalities in cardiovascular disease mortality across 7932 English electoral wards, 1982–2006: Bayesian spatial analysis. Int J Epidemiol. 2012;41:1737–1749. doi: 10.1093/ije/dys151. doi:dys151 [pii] 10.1093/ije/dys151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Cesare M, et al. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381:585–597. doi: 10.1016/S0140-6736(12)61851-0. S0140-6736(12)61851-0 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Harper S, Lynch J, Smith GD. Social determinants and the decline of cardiovascular diseases: understanding the links. Annu Rev Public Health. 2011;32:39–69. doi: 10.1146/annurev-publhealth-031210-101234. [DOI] [PubMed] [Google Scholar]
- 8.Danaei G, et al. The promise of prevention: the effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States. PLoS Med. 2010;7:e1000248. doi: 10.1371/journal.pmed.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keys A. Coronary heart disease–the global picture. Atherosclerosis. 1975;22:149–192. doi: 10.1016/0021-9150(75)90001-5. [DOI] [PubMed] [Google Scholar]
- 10.Strom A, Jensen RA. Mortality from circulatory diseases in Norway 1940–1945. Lancet. 1951;1:126–129. doi: 10.1016/s0140-6736(51)91210-x. [DOI] [PubMed] [Google Scholar]
- 11.Morris JN. Recent history of coronary disease. Lancet. 1951;1:69–73. doi: 10.1016/s0140-6736(51)91163-4. [DOI] [PubMed] [Google Scholar]
- 12.Woolsey TD, Moriyama IM. Statistical studies of heart diseases; important factors in heart disease mortality trends. Public Health Rep. 1948;63:1247–1273. [PMC free article] [PubMed] [Google Scholar]
- 13.Moriyama IM, Gover M. Heart diseases and allied causes of death in relation to age changes in the population. Public Health Rep. 1948;63:537–545. [PubMed] [Google Scholar]
- 14.Stallones RA. The rise and fall of ischemic heart disease. Sci Am. 1980;243:53–59. doi: 10.1038/scientificamerican1180-53. [DOI] [PubMed] [Google Scholar]
- 15.Moriyama IM, Woolsey TD. Statistical studies of heart disease. IX. Race and sex differences in the trend of mortality from the major cardiovascular-renal diseases. Public Health Rep. 1951;66:355–368. [PMC free article] [PubMed] [Google Scholar]
- 16.Klebba AJ, Maurer JD, Glass EJ. Mortality trends for leading causes of death: United States, 1950–69. Report No 0083-2022 (Print) 0083-2022 (Linking) 1974 [PubMed] [Google Scholar]
- 17.Borhani NO, Hechter HH. Recent Changes in CVR Disease Mortality in California. Public Health Rep. 1964;79:147–160. [PMC free article] [PubMed] [Google Scholar]
- 18.Walker WJ. Coronary mortality: what is going on? JAMA. 1974;227:1045–1046. [PubMed] [Google Scholar]
- 19.Stern MP. The recent decline in ischemic heart disease mortality. Ann Intern Med. 1979;91:630–640. doi: 10.7326/0003-4819-91-4-630. [DOI] [PubMed] [Google Scholar]
- 20.Walker WJ, Changing United. States life-style and declining vascular mortality: cause or coincidence? The New England journal of medicine. 1977;297:163–165. doi: 10.1056/NEJM197707212970311. [DOI] [PubMed] [Google Scholar]
- 21.Uemura K, Pisa Z. Recent trends in cardiovascular disease mortality in 27 industrialized countries. World Health Stat Q. 1985;38:142–162. [PubMed] [Google Scholar]
- 22.Uemura K, Pisa Z. Trends in cardiovascular disease mortality in industrialized countries since 1950. World Health Stat Q. 1988;41:155–178. [PubMed] [Google Scholar]
- 23.Lawlor DA, Smith GD, Leon DA, Sterne JA, Ebrahim S. Secular trends in mortality by stroke subtype in the 20th century: a retrospective analysis. Lancet. 2002;360:1818–1823. doi: 10.1016/S0140-6736(02)11769-7. [DOI] [PubMed] [Google Scholar]
- 24.Havlik RJ, Feinleib M. National Heart, Lung and Blood Institute. US Department of Health, Education, and Welfare; Washington DC: 1979. [Google Scholar]
- 25.Stallones RA. Epidemiology of cerebrovascular disease. A review. Journal of chronic diseases. 1965;18:859–872. doi: 10.1016/0021-9681(65)90022-6. [DOI] [PubMed] [Google Scholar]
- 26.Charlton J, Murphy M, Khaw KT, Ebrahim S, Davey Smith G. In: The Health of Adult Britain 1841–1994. Charlton J, Murphy M, editors. Vol. 2. Stationary Office; 1997. pp. 60–81. [Google Scholar]
- 27.Wen CP, Gershoff SN. Changes in serum cholesterol and coronary heart disease mortality associated with changes in the postwar Japanese diet. Am J Clin Nutr. 1973;26:616–619. doi: 10.1093/ajcn/26.6.616. [DOI] [PubMed] [Google Scholar]
- 28.Kodama K. Stroke trends in Japan. Ann Epidemiol. 1993;3:524–528. doi: 10.1016/1047-2797(93)90109-h. [DOI] [PubMed] [Google Scholar]
- 29.Ueshima H. Explanation for the Japanese paradox: prevention of increase in coronary heart disease and reduction in stroke. Journal of atherosclerosis and thrombosis. 2007;14:278–286. doi: 10.5551/jat.e529. [DOI] [PubMed] [Google Scholar]
- 30.Ueshima H, Tatara K, Asakura S. Declining mortality from ischemic heart disease and changes in coronary risk factors in Japan, 1956–1980. Am J Epidemiol. 1987;125:62–72. doi: 10.1093/oxfordjournals.aje.a114512. [DOI] [PubMed] [Google Scholar]
- 31.Sarti C, Rastenyte D, Cepaitis Z, Tuomilehto J. International trends in mortality from stroke, 1968 to 1994. Stroke. 2000;31:1588–1601. doi: 10.1161/01.str.31.7.1588. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Ikeda K, Yamori Y. Changes in stroke mortality rates for 1950 to 1997: a great slowdown of decline trend in Japan. Stroke. 2001;32:1745–1749. doi: 10.1161/01.str.32.8.1745. [DOI] [PubMed] [Google Scholar]
- 33.Moriyama IM, Woolsey TD, Stamler J. Observations on possible factors responsible for the sex and race trends in cardiovascular-renal mortality in the United States. Journal of chronic diseases. 1958;7:401–412. doi: 10.1016/0021-9681(58)90104-8. [DOI] [PubMed] [Google Scholar]
- 34.Stamler J. Lectures on Preventive Cardiology. Grune & Stratton; 1967. [Google Scholar]
- 35.Katz LN, Stamler J, Pick R. Nutrition and arteriosclerosis. Lea & Febiger; 1958. [Google Scholar]
- 36.The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 37.Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88:119–124. doi: 10.1136/heart.88.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curioni C, Cunha CB, Veras RP, Andre C. The decline in mortality from circulatory diseases in Brazil. Rev Panam Salud Publica. 2009;25:9–15. doi: 10.1590/s1020-49892009000100002. [DOI] [PubMed] [Google Scholar]
- 39.Andre C, Curioni CC, Braga da Cunha C, Veras R. Progressive decline in stroke mortality in Brazil from 1980 to 1982, 1990 to 1992, and 2000 to 2002. Stroke. 2006;37:2784–2789. doi: 10.1161/01.STR.0000244768.46566.73. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez T, et al. Trends in mortality from coronary heart and cerebrovascular diseases in the Americas: 1970–2000. Heart. 2006;92:453–460. doi: 10.1136/hrt.2004.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein FH, Pisa Z. In: Proceedings of the conference on the decline in coronary heart disease mortality October 24–25, 1978. Havlik RJ, Feinleib M, editors. National Heart, Lung and Blood Institute, US Department of Health, Education, and Welfare; Washington DC: 1979. pp. 58–90. (NIH Publication No. 79-1610). [Google Scholar]
- 42.Mesle F, Shkolnikov V, Vallin J. Mortality by cause in the USSR in 1970–1987: the reconstruction of time series. European journal of population = Revue europeenne de demographie. 1992;8:281–308. doi: 10.1007/BF01796624. [DOI] [PubMed] [Google Scholar]
- 43.Kesteloot H, Sans S, Kromhout D. Dynamics of cardiovascular and all-cause mortality in Western and Eastern Europe between 1970 and 2000. Eur Heart J. 2006;27:107–113. doi: 10.1093/eurheartj/ehi511. [DOI] [PubMed] [Google Scholar]
- 44.Shkolnikov V, Mesle F, Vallin J. Health crisis in Russia. II. Changes in causes of death: a comparison with France and England and Wales (1970 to 1993) Population English selection. 1996;8:155–189. [PubMed] [Google Scholar]
- 45.Shkolnikov V, Mesle F, Vallin J. Health crisis in Russia. I. Recent trends in life expectancy and causes of death from 1970 to 1993. Population English selection. 1996;8:123–154. [PubMed] [Google Scholar]
- 46.Leon DA, et al. Huge variation in Russian mortality rates 1984–94 artefact, alcohol, or what? Lancet. 1997;350:383–388. doi: 10.1016/S0140-6736(97)03360-6. doi:S0140-6736(97)03360-6 [pii] 10.1016/S0140-6736(97)03360-6. [DOI] [PubMed] [Google Scholar]
- 47.Grigoriev P, et al. The Recent Mortality Decline in Russia: Beginning of the Cardiovascular Revolution? Population and Development Review. 2014;40:107–129. doi: 10.1111/j.1728-4457.2014.00652.x. [DOI] [Google Scholar]
- 48.Razvodovsky YE. Alcohol consumption and ischemic heart disease mortality in Russia. Adicciones. 2012;24:23–29. [PubMed] [Google Scholar]
- 49.Leon DA, Shkolnikov VM, McKee M, Kiryanov N, Andreev E. Alcohol increases circulatory disease mortality in Russia: acute and chronic effects or misattribution of cause? Int J Epidemiol. 2010;39:1279–1290. doi: 10.1093/ije/dyq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Truelsen T, et al. Trends in stroke and coronary heart disease in the WHO MONICA Project. Stroke. 2003;34:1346–1352. doi: 10.1161/01.STR.0000069724.36173.4D. [DOI] [PubMed] [Google Scholar]
- 51.Jiang B, et al. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke. 2006;37:63–68. doi: 10.1161/01.STR.0000194955.34820.78. [DOI] [PubMed] [Google Scholar]
- 52.Jiang G, et al. Coronary heart disease mortality in China: age, gender, and urban-rural gaps during epidemiological transition. Rev Panam Salud Publica. 2012;31:317–324. doi: 10.1590/s1020-49892012000400008. [DOI] [PubMed] [Google Scholar]
- 53.Yu TS, Wong SL, Lloyd OL, Wong TW. Ischaemic heart disease: trends in mortality in Hong Kong, 1970–89. J Epidemiol Community Health. 1995;49:16–21. doi: 10.1136/jech.49.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stringhini S, et al. Declining Stroke and Myocardial Infarction Mortality Between 1989 and 2010 in a Country of the African Region. Stroke. 2012 doi: 10.1161/STROKEAHA.112.658468. doi:STROKEAHA.112.658468 [pii] 10.1161/STROKEAHA.112.658468. [DOI] [PubMed] [Google Scholar]
- 55.Mayosi BM, et al. Health in South Africa: changes and challenges since 2009. Lancet. 2012;380:2029–2043. doi: 10.1016/S0140-6736(12)61814-5. [DOI] [PubMed] [Google Scholar]
- 56.Dawber TR, Kannel WB, Revotskie N, Kagan A. The epidemiology of coronary heart disease–the Framingham enquiry. Proc R Soc Med. 1962;55:265–271. doi: 10.1177/003591576205500403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyle JT, Dawber TR, Kannel WB, Heslin AS, Kahn HA. Cigarette smoking and coronary heart disease. Combined experience of the Albany and Framingham studies. The New England journal of medicine. 1962;26:796–801. doi: 10.1056/nejm196204192661602. [DOI] [PubMed] [Google Scholar]
- 58.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease–six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 59.Howitt MR, Garrett WS. A complex microworld in the gut: gut microbiota and cardiovascular disease connectivity. Nat Med. 2012;18:1188–1189. doi: 10.1038/nm.2895. [DOI] [PubMed] [Google Scholar]
- 60.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 61.Murray CJ, Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S. Comparative quantification of health risks conceptual framework and methodological issues. Popul Health Metr. 2003;1:1. doi: 10.1186/1478-7954-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ezzati M, Lopez AD, Rodgers A, Murray CJL. 2248. World Health Organization; Geneva: 2004. [Google Scholar]
- 63.Emerging Risk, Factors C, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oza S, Thun MJ, Henley SJ, Lopez AD, Ezzati M. How many deaths are attributable to smoking in the United States? Comparison of methods for estimating smoking-attributable mortality when smoking prevalence changes. Prev Med. 2011;52:428–433. doi: 10.1016/j.ypmed.2011.04.007. doi:S0091-7435(11)00160-5 [pii] 10.1016/j.ypmed. [DOI] [PubMed] [Google Scholar]
- 65.Pell JP, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. The New England journal of medicine. 2008;359:482–491. doi: 10.1056/NEJMsa0706740. [DOI] [PubMed] [Google Scholar]
- 66.Bartecchi C, et al. Reduction in the Incidence of Acute Myocardial Infarction Associated With a Citywide Smoking Ordinance. Circulation. 2006;114:1490–1496. doi: 10.1161/circulationaha.106.615245. [DOI] [PubMed] [Google Scholar]
- 67.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367–372. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmes J, Meier PS, Booth A, Guo Y, Brennan A. The temporal relationship between per capita alcohol consumption and harm: a systematic review of time lag specifications in aggregate time series analyses. Drug Alcohol Depend. 2012;123:7–14. doi: 10.1016/j.drugalcdep.2011.12.005. S0376-8716(11)00527-8 [pii] [DOI] [PubMed] [Google Scholar]
- 69.Law MR, Frost CD, Wald NJ. By how much does dietary salt reduction lower blood pressure? III–Analysis of data from trials of salt reduction. BMJ. 1991;302:819–824. doi: 10.1136/bmj.302.6780.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin X, et al. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38:1112–1117. doi: 10.1161/hy1101.093424. [DOI] [PubMed] [Google Scholar]
- 71.Whelton PK, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 72.Aburto NJ, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rose G. Incubation period of coronary heart disease. Br Med J (Clin Res Ed) 1982;284:1600–1601. doi: 10.1136/bmj.284.6329.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McNamara JJ, Molot MA, Stremple JF, Cutting RT. Coronary artery disease in combat casualties in Vietnam. JAMA. 1971;216:1185–1187. [PubMed] [Google Scholar]
- 75.Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. J Am Med Assoc. 1953;152:1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- 76.Katanoda K, Matsumura Y. National Nutrition Survey in Japan–its methodological transition and current findings. Journal of nutritional science and vitaminology. 2002;48:423–432. doi: 10.3177/jnsv.48.423. [DOI] [PubMed] [Google Scholar]
- 77.Yoshiike N, Matsumura Y, Iwaya M, Sugiyama M, Yamaguchi M. National Nutrition Survey in Japan. Journal of epidemiology/Japan Epidemiological Association. 1996;6:S189–200. doi: 10.2188/jea.6.3sup_189. [DOI] [PubMed] [Google Scholar]
- 78.Finucane MM, Paciorek CJ, Danaei G, Ezzati M. Bayesian Estimation of Population-Level Trends in Measures of Health Status. Statistica Science. 2014:18–25. doi: 10.1214/13-sts427. [DOI] [Google Scholar]
- 79.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. doi:S0140-6736(10)62037-5 [pii] 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Danaei G, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. doi:S0140-6736(10)62036-3 [pii] 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 81.Danaei G, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(11)60679-X. doi:S0140-6736(11)60679-X [pii] 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 82.Farzadfar F, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. doi:S0140-6736(10)62038-7 [pii] 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 83.Stevens GA, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. 1478-7954-10-22 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.World Health Organization (WHO) Tobacco or Health: A Global Status Report. World Health Organization; 1997. [Google Scholar]
- 85.Shafey O, Dolwick S, Guindon G. American Cancer Society. Atlanta, GA: 2003. [Google Scholar]
- 86.Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. The New England journal of medicine. 2013;369:954–964. doi: 10.1056/NEJMra1203528. [DOI] [PubMed] [Google Scholar]
- 87.Burns DM, et al. In: Changes in Cigarette-Related Disease Risks and Their Implications for Prevention and Control. Burns DM, Garfinkel L, Samet JM, editors. National Cancer Institute; 1997. pp. 13–112. (Smoking and Tobacco Control Monograph No. 8). [Google Scholar]
- 88.Kluger R. Ashes to Ashes: America’s Hundred-Year Cigarette War, the Public Health, and the Unabashed Triumph of Philip Morris. Vintage Books; 1997. [Google Scholar]
- 89.Brandt AM. The Cigarette Century: The Rise, Fall, and Deadly Persistence of the Product That Defined America. Basic Books; 2007. [Google Scholar]
- 90.Wald N, Nicolaides-Bouman A. UK smoking statistics. Oxford University Press; 1991. [Google Scholar]
- 91.World Health Organization. Global status report on noncommunicable diseases 2010. World Health Organization; 2011. [Google Scholar]
- 92.World Health Organization. Global status report on noncommunicable diseases 2014: Attaining the nine global noncommunicable diseases targets; a shared responsibility. World Health Organization; 2014. [Google Scholar]
- 93.Wald N, Doll R, Copeland G. Trends in tar, nicotine, and carbon monoxide yields of UK cigarettes manufactured since 1934. Br Med J (Clin Res Ed) 1981;282:763–765. doi: 10.1136/bmj.282.6266.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jarvis MJ. Trends in sales weighted tar, nicotine, and carbon monoxide yields of UK cigarettes. Thorax. 2001;56:960–963. doi: 10.1136/thorax.56.12.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parish S, et al. Cigarette smoking, tar yields, and non-fatal myocardial infarction: 14,000 cases and 32,000 controls in the United Kingdom. The International Studies of Infarct Survival (ISIS) Collaborators. BMJ. 1995;311:471–477. doi: 10.1136/bmj.311.7003.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.U.S. Department of Health and Human Services. A Report of the Surgeon General How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease, 2010. USDHHS, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. (Report No. DHHS Publication No. (CDC) 89-8411). [Google Scholar]
- 97.U.S. Department of Health and Human Services. A Report of the Surgeon General The health consequences of smoking – 50 years of progress. USDHHS, Public Health Service, Centers for Disease Control and Prevention,, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. (Report No. DHHS Publication No. (CDC) 89-8411). [Google Scholar]
- 98.Simpura J. Mediterranean mysteries: mechanisms of declining alcohol consumption. Addiction. 1998;93:1301–1304. doi: 10.1080/09652149834784. [DOI] [PubMed] [Google Scholar]
- 99.Simpura J. Trends in alcohol consumption and drinking patterns: sociological and economic explanations and alcohol policies. Nordic Studies on Alcohol and Drugs. 2001;18:S3–S13. [Google Scholar]
- 100.Mäkelä K, et al. Alcohol, society, and the state: 1 A comparative history of alcohol control. Addiction Research Foundation; 1981. [Google Scholar]
- 101.Rehm J, et al. In: Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. World Health Organization; 2004. pp. 959–1108. [Google Scholar]
- 102.World Health Organization. Global status report on alcohol and health 2014. World Health Organization; 2014. [Google Scholar]
- 103.Allamani A, Prina F. Why the decrease in consumption of alcoholic beverages in Italy between the 1970s and the 2000s? Shedding light on an Italian mystery. Contemporary Drug Problems. 2007;34:187–198. [Google Scholar]
- 104.Cipriani F, Prina F. The Research outcome: Summary and Conclusions on the Reduction in Wine Consumption in Italy. Contemporary Drug Problems. 2007;34:361–378. [Google Scholar]
- 105.Nemtsov AV. Estimates of total alcohol consumption in Russia, 1980–1994. Drug Alcohol Depend. 2000;58:133–142. doi: 10.1016/s0376-8716(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 106.Shield KD, et al. Global alcohol exposure estimates by country, territory and region for 2005–a contribution to the Comparative Risk Assessment for the 2010 Global Burden of Disease Study. Addiction. 2013;108:912–922. doi: 10.1111/add.12112. [DOI] [PubMed] [Google Scholar]
- 107.Poznyak V, et al. The world health organization’s global monitoring system on alcohol and health. Alcohol research: current reviews. 2013;35:244–249. doi: 10.35946/arcr.v35.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rehm J, et al. A systematic review of the epidemiology of unrecorded alcohol consumption and the chemical composition of unrecorded alcohol. Addiction. 2014;109:880–893. doi: 10.1111/add.12498. [DOI] [PubMed] [Google Scholar]
- 109.Rehm J, Kanteres F, Lachenmeier DW. Unrecorded consumption, quality of alcohol and health consequences. Drug and alcohol review. 2010;29:426–436. doi: 10.1111/j.1465-3362.2009.00140.x. [DOI] [PubMed] [Google Scholar]
- 110.Stickley A, et al. Alcohol poisoning in Russia and the countries in the European part of the former Soviet Union, 1970 2002. Eur J Public Health. 2007;17:444–449. doi: 10.1093/eurpub/ckl275. [DOI] [PubMed] [Google Scholar]
- 111.Bergmann MM, et al. The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int J Epidemiol. 2013;42:1772–1790. doi: 10.1093/ije/dyt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rehm J, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. doi:ADD2899 [pii] 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rehm J, et al. On the emerging paradigm of drinking patterns and their social and health consequences. Addiction. 1996;91:1615–1621. [PubMed] [Google Scholar]
- 114.Roerecke M, Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am J Epidemiol. 2010;171:633–644. doi: 10.1093/aje/kwp451. doi:kwp451 [pii] 10.1093/aje/kwp451. [DOI] [PubMed] [Google Scholar]
- 115.Makela P, et al. Episodic heavy drinking in four Nordic countries: a comparative survey. Addiction. 2001;96:1575–1588. doi: 10.1080/09652140120080714. [DOI] [PubMed] [Google Scholar]
- 116.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;4:CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mozaffarian D, et al. Global sodium consumption and death from cardiovascular causes. The New England journal of medicine. 2014;371:624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 118.Cook NR, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169:32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cook NR, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sacks FM, Campos H. Dietary therapy in hypertension. The New England journal of medicine. 2010;362:2102–2112. doi: 10.1056/NEJMct0911013. doi:362/22/2102 [pii] 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 121.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–2891. doi: 10.1161/CIRCULATIONAHA.110.968735. doi:123/24/2870 [pii] 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Howard BV, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 124.Chowdhury R, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 125.Nordmann AJ, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. doi:166/3/285 [pii] 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 126.Estruch R, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 127.Estruch R, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. New England Journal of Medicine. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 128.Kahn HA. Change in serum cholesterol associated with changes in the United States civilian diet, 1909–1965. Am J Clin Nutr. 1970;23:879–882. doi: 10.1093/ajcn/23.7.879. [DOI] [PubMed] [Google Scholar]
- 129.Antar MA, Ohlson MA, Hodges RE. Changes in Retail Market Food Supplies in the United States in the Last Seventy Years in Relation to the Incidence of Coronary Heart Disease, with Special Reference to Dietary Carbohydrates and Essential Fatty Acids. Am J Clin Nutr. 1964;14:169–178. doi: 10.1093/ajcn/14.3.169. [DOI] [PubMed] [Google Scholar]
- 130.Jackson R, Beaglehole R. Trends in dietary fat and cigarette smoking and the decline in coronary heart disease in New Zealand. Int J Epidemiol. 1987;16:377–382. doi: 10.1093/ije/16.3.377. [DOI] [PubMed] [Google Scholar]
- 131.Winkler G, Doring A, Keil U. Trends in dietary sources of nutrients among middle-aged men in southern Germany. Results of the MONICA Project Augsburg: dietary surveys 1984/1985 and 1994/1995. MONItoring trends and determinants in CArdiovascular disease. Appetite. 2000;34:37–45. doi: 10.1006/appe.1999.0273. [DOI] [PubMed] [Google Scholar]
- 132.Pietinen P, Vartiainen E, Seppanen R, Aro A, Puska P. Changes in diet in Finland from 1972 to 1992: impact on coronary heart disease risk. Prev Med. 1996;25:243–250. doi: 10.1006/pmed.1996.0053. doi:S0091-7435(96)90053-5 [pii] 10.1006/pmed.1996.0053. [DOI] [PubMed] [Google Scholar]
- 133.Laatikainen T, et al. Sodium in the Finnish diet: 20-year trends in urinary sodium excretion among the adult population. Eur J Clin Nutr. 2006;60:965–970. doi: 10.1038/sj.ejcn.1602406. doi:1602406 [pii] 10.1038/sj.ejcn.1602406. [DOI] [PubMed] [Google Scholar]
- 134.Krachler B, Eliasson MC, Johansson I, Hallmans G, Lindahl B. Trends in food intakes in Swedish adults 1986–1999: findings from the Northern Sweden MONICA (Monitoring of Trends and Determinants in Cardiovascular Disease) Study. Public Health Nutr. 2005;8:628–635. doi: 10.1079/phn2004710. [DOI] [PubMed] [Google Scholar]
- 135.Waskiewicz A, Piotrowski W, Sygnowska E, Rywik S, Jasinski B. Did favourable trends in food consumption observed in the 1984–2001 period contribute to the decrease in cardiovascular mortality? – Pol-MONICA Warsaw Project. Kardiologia polska. 2006;64:16–23. discussion 24–15. [PubMed] [Google Scholar]
- 136.Stephen AM, Sieber GM. Trends in individual fat consumption in the UK 1900–1985. Br J Nutr. 1994;71:775–788. doi: 10.1079/bjn19940183. [DOI] [PubMed] [Google Scholar]
- 137.Stephen AM, Wald NJ. Trends in individual consumption of dietary fat in the United States, 1920–1984. Am J Clin Nutr. 1990;52:457–469. doi: 10.1093/ajcn/52.3.457. [DOI] [PubMed] [Google Scholar]
- 138.Du SF, Wang HJ, Zhang B, Zhai FY, Popkin BM. China in the period of transition from scarcity and extensive undernutrition to emerging nutrition-related non-communicable diseases, 1949–1992. Obes Rev. 2014;15(Suppl 1):8–15. doi: 10.1111/obr.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wolmarans P. Background paper on global trends in food production, intake and composition. Ann Nutr Metab. 2009;55:244–272. doi: 10.1159/000229005. [DOI] [PubMed] [Google Scholar]
- 140.Micha R, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ. 2014;348:g2272. doi: 10.1136/bmj.g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leth T, Jensen HG, Mikkelsen AA, Bysted A. The effect of the regulation on trans fatty acid content in Danish food. Atheroscler Suppl. 2006;7:53–56. doi: 10.1016/j.atherosclerosissup.2006.04.019. doi:S1567-5688(06)00038-9 [pii] 10.1016/j.atherosclerosissup.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 142.Angell SY, et al. Cholesterol control beyond the clinic: New York City’s trans fat restriction. Ann Intern Med. 2009;151:129–134. doi: 10.7326/0003-4819-151-2-200907210-00010. [DOI] [PubMed] [Google Scholar]
- 143.Zatonski W, Campos H, Willett W. Rapid declines in coronary heart disease mortality in Eastern Europe are associated with increased consumption of oils rich in alpha-linolenic acid. Eur J Epidemiol. 2008;23:3–10. doi: 10.1007/s10654-007-9195-1. [DOI] [PubMed] [Google Scholar]
- 144.Zatonski WA, Willett W. Changes in dietary fat and declining coronary heart disease in Poland: population based study. BMJ. 2005;331:187–188. doi: 10.1136/bmj.331.7510.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Butt MS, Sultan MT. Levels of trans fats in diets consumed in developing economies. Journal of AOAC International. 2009;92:1277–1283. [PubMed] [Google Scholar]
- 146.Institute of Medicine. Sodium intake in populations: assessment of evidence. National Academies Press; 2013. [PubMed] [Google Scholar]
- 147.Cobb LK, et al. Methodological Issues in Cohort Studies That Relate Sodium Intake to Cardiovascular Disease Outcomes: A Science Advisory From the American Heart Association. Circulation. 2014;129:1173–1186. doi: 10.1161/cir.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 148.Ikeda N, Gakidou E, Hasegawa T, Murray CJ. Understanding the decline of mean systolic blood pressure in Japan: an analysis of pooled data from the National Nutrition Survey, 1986–2002. Bull World Health Organ. 2008;86:978–988. doi: 10.2471/BLT.07.050195. doi:S0042-96862008001200016 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ueshima H, Tatara K, Asakura S, Okamoto M. Declining trends in blood pressure level and the prevalence of hypertension, and changes in related factors in Japan, 1956–1980. Journal of chronic diseases. 1987;40:137–147. doi: 10.1016/0021-9681(87)90065-8. [DOI] [PubMed] [Google Scholar]
- 150.Du S, et al. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr. 2014;99:334–343. doi: 10.3945/ajcn.113.059121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vartiainen E, et al. Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol. 2010;39:504–518. doi: 10.1093/ije/dyp330. doi:dyp330 [pii] 10.1093/ije/dyp330. [DOI] [PubMed] [Google Scholar]
- 152.He FJ, MacGregor GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. 2010;52:363–382. doi: 10.1016/j.pcad.2009.12.006. doi:S0033-0620(09)00127-3 [pii] 10.1016/j.pcad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 153.He FJ, Pombo-Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ open. 2014;4:e004549. doi: 10.1136/bmjopen-2013-004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He FJ, Brinsden HC, MacGregor GA. Salt reduction in the United Kingdom: a successful experiment in public health. J Hum Hypertens. 2014;28:345–352. doi: 10.1038/jhh.2013.105. [DOI] [PubMed] [Google Scholar]
- 155.Tuomilehto J, Geboers J, Joossens JV, Salonen JT, Tanskanen A. Trends in stomach cancer and stroke in Finland. Comparison to northwest Europe and USA. Stroke. 1984;15:823–828. doi: 10.1161/01.str.15.5.823. [DOI] [PubMed] [Google Scholar]
- 156.Powles J, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ open. 2013;3:e003733. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. doi: 10.1093/ije/dyp139. [DOI] [PubMed] [Google Scholar]
- 158.Sacks FM, Kass EH. Low blood pressure in vegetarians: effects of specific foods and nutrients. Am J Clin Nutr. 1988;48:795–800. doi: 10.1093/ajcn/48.3.795. [DOI] [PubMed] [Google Scholar]
- 159.He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 160.Drewnowski A, Popkin BM. The nutrition transition: new trends in the global diet. Nutrition Reviews. 1997;55:31–43. doi: 10.1111/j.1753-4887.1997.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 161.Pan WH, et al. Diet and health trends in Taiwan: comparison of two nutrition and health surveys from 1993–1996 and 2005–2008. Asia Pac J Clin Nutr. 2011;20:238–250. [PubMed] [Google Scholar]
- 162.Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Prev Chronic Dis. 2008;5:A35. [PMC free article] [PubMed] [Google Scholar]
- 163.Welsh SO, Marston RM. Review of trends in food use in the United States, 1909 to 1980. J Am Diet Assoc. 1982;81:120–128. [PubMed] [Google Scholar]
- 164.Cavadini C, Siega-Riz AM, Popkin BM. US adolescent food intake trends from 1965 to 1996. West J Med. 2000;173:378–383. doi: 10.1136/ewjm.173.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang DD, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee S, et al. Trends in diet quality for coronary heart disease prevention between 1980–1982 and 2000–2002: The Minnesota Heart Survey. J Am Diet Assoc. 2007;107:213–222. doi: 10.1016/j.jada.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 167.Singh GM, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. PONE-D-12-31608 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Di Cesare M, et al. The contributions of risk factor trends to cardiometabolic mortality decline in 26 industrialized countries. Int J Epidemiol. 2013;42:838–848. doi: 10.1093/ije/dyt063. [DOI] [PubMed] [Google Scholar]
- 169.Goff DC, Howard G, Russell GB, Labarthe DR. Birth cohort evidence of population influences on blood pressure in the United States, 1887–1994. Ann Epidemiol. 2001;11:271–279. doi: 10.1016/s1047-2797(00)00224-6. [DOI] [PubMed] [Google Scholar]
- 170.Sakata K, Labarthe DR. Changes in cardiovascular disease risk factors in three Japanese national surveys 1971–1990. Journal of epidemiology/Japan Epidemiological Association. 1996;6:93–107. doi: 10.2188/jea.6.93. [DOI] [PubMed] [Google Scholar]
- 171.McCarron P, Smith GD, Okasha M. Secular changes in blood pressure in childhood, adolescence and young adulthood: systematic review of trends from 1948 to 1998. J Hum Hypertens. 2002;16:677–689. doi: 10.1038/sj.jhh.1001471. [DOI] [PubMed] [Google Scholar]
- 172.Tunstall-Pedoe H, Connaghan J, Woodward M, Tolonen H, Kuulasmaa K. Pattern of declining blood pressure across replicate population surveys of the WHO MONICA project, mid-1980s to mid-1990s, and the role of medication. Bmj. 2006;332:629–635. doi: 10.1136/bmj.38753.779005.BE. doi:bmj.38753.779005.BE [pii] 10.1136/bmj.38753.779005.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hardoon SL, et al. Assessing the impact of medication use on trends in major coronary risk factors in older British men: a cohort study. Eur J Cardiovasc Prev Rehabil. 2010;17:502–508. doi: 10.1097/HJR.0b013e3283378865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bovet P, et al. Divergent fifteen-year trends in traditional and cardiometabolic risk factors of cardiovascular diseases in the Seychelles. Cardiovasc Diabetol. 2009;8:34. doi: 10.1186/1475-2840-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Danaei G, et al. The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 2013;127:1493–1502. doi: 10.1161/CIRCULATIONAHA.113.001470. CIRCULATIONAHA.113.001470 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Okayama A, et al. Changes in total serum cholesterol and other risk factors for cardiovascular disease in Japan 1980–1989. Int J Epidemiol. 1993;22:1038–1047. doi: 10.1093/ije/22.6.1038. [DOI] [PubMed] [Google Scholar]
- 177.Okayama A, et al. Different trends in serum cholesterol levels among rural and urban populations aged 40–59 in Japan from 1960 to 1990. J Clin Epidemiol. 1995;48:329–337. doi: 10.1016/0895-4356(94)00146-h. [DOI] [PubMed] [Google Scholar]
- 178.Sjol A, Grunnet K, Schroll M. Secular trends in serum cholesterol, high density lipoproteins and triglycerides 1964–1987. Int J Epidemiol. 1991;20:105–113. doi: 10.1093/ije/20.1.105. [DOI] [PubMed] [Google Scholar]
- 179.Capewell S, Ford ES. Why have total cholesterol levels declined in most developed countries? BMC Public Health. 2011;11:641. doi: 10.1186/1471-2458-11-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Manson JE, et al. Estrogen plus progestin and the risk of coronary heart disease. The New England journal of medicine. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 181.Sigfusson N, et al. Decline in ischaemic heart disease in Iceland and change in risk factor levels. BMJ. 1991;302:1371–1375. doi: 10.1136/bmj.302.6789.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dwyer T, Hetzel BS. A comparison of trends of coronary heart disease mortality in Australia, USA and England and Wales with reference to three major risk factors-hypertension, cigarette smoking and diet. Int J Epidemiol. 1980;9:65–71. doi: 10.1093/ije/9.1.65. [DOI] [PubMed] [Google Scholar]
- 183.Williams RR, Lyon JL, Brockert JE, Maness T. In: Proceedings of the conference on the decline in coronary heart disease mortality October 24–25, 1978. Havlik RJ, Feinleib M, editors. National Heart, Lung and Blood Institute, US Department of Health, Education, and Welfare; Washington DC: 1979. pp. 48–57. (NIH Publication No. 79-1610). [Google Scholar]
- 184.Why the American decline in coronary heart-disease? Lancet. 1980;1:183–184. doi: 10.1016/s0140-6736(80)90666-2. [DOI] [PubMed] [Google Scholar]
- 185.Nicolosi A, Casati S, Taioli E, Polli E. Death from cardiovascular disease in Italy, 1972–1981: decline in mortality rates and possible causes. Int J Epidemiol. 1988;17:766–772. doi: 10.1093/ije/17.4.766. [DOI] [PubMed] [Google Scholar]
- 186.Menotti A, Scanga M. Trends in coronary risk factors in Italy. Responsible Investigators of the RF2, OB43 and MICOL Research Groups. Int J Epidemiol. 1992;21:883–892. doi: 10.1093/ije/21.5.883. [DOI] [PubMed] [Google Scholar]
- 187.Tunstall-Pedoe H, et al. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–1557. doi: 10.1016/s0140-6736(99)04021-0. doi:S0140673699040210 [pii] [DOI] [PubMed] [Google Scholar]
- 188.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic medicine. 2004;66:411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 189.Kuulasmaa K, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355:675–687. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- 190.Lu Y, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. S0140-6736(13)61836-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gregg EW, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. Jama. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 192.Pell S, D’Alonzo GA. Immediate Mortality and Five-Year Survival of Employed Men with a First Myocardial Infarction. New England Journal of Medicine. 1964;270:915–922. doi: 10.1056/NEJM19640430270.1801. [DOI] [PubMed] [Google Scholar]
- 193.Pell S, Fayerweather WE. Trends in the incidence of myocardial infarction and in associated mortality and morbidity in a large employed population, 1957–1983. The New England journal of medicine. 1985;312:1005–1011. doi: 10.1056/NEJM198504183121601. [DOI] [PubMed] [Google Scholar]
- 194.Rosamond WD, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. The New England journal of medicine. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 195.McGovern PG, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota heart survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 196.McGovern PG, et al. Recent trends in acute coronary heart disease–mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. The New England journal of medicine. 1996;334:884–890. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 197.Roger VL, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 198.Goldberg RJ, Yarzebski J, Lessard D, Gore JM. A two-decades (1975 to 1995) long experience in the incidence, in-hospital and long-term case-fatality rates of acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;33:1533–1539. doi: 10.1016/s0735-1097(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 199.Morris RW, et al. Geographic variation in incidence of coronary heart disease in Britain: the contribution of established risk factors. Heart. 2001;86:277–283. doi: 10.1136/heart.86.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hardoon SL, Whincup PH, Petersen I, Capewell S, Morris RW. Trends in longer-term survival following an acute myocardial infarction and prescribing of evidenced-based medications in primary care in the UK from 1991: a longitudinal population-based study. J Epidemiol Community Health. 2011;65:770–774. doi: 10.1136/jech.2009.098087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Capewell S, et al. Short-term and long-term outcomes in 133,429 emergency patients admitted with angina or myocardial infarction in Scotland, 1990–2000 population-based cohort study. Heart (British Cardiac Society) 2006;92:1563–1570. doi: 10.1136/hrt.2005.085399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grey C, et al. Twenty-eight day and one-year case fatality after hospitalisation with an acute coronary syndrome: a nationwide data linkage study. Australian and New Zealand journal of public health. 2014;38:216–220. doi: 10.1111/1753-6405.12241. [DOI] [PubMed] [Google Scholar]
- 203.Hammar N, et al. A national record linkage to study acute myocardial infarction incidence and case fatality in Sweden. International journal of epidemiology. 2001;30(Suppl 1):S30–34. doi: 10.1093/ije/30.suppl_1.s30. [DOI] [PubMed] [Google Scholar]
- 204.Krumholz HM, Normand SLT, Wang Y. Trends in Hospitalizations and Outcomes for Acute Cardiovascular Disease and Stroke: 1999–2011. Circulation. 2014:1999–2011. doi: 10.1161/CIRCULATIONAHA.113.007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schmidt M, Bonde J, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;356:1–12. doi: 10.1136/bmj.e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ. 2012;344:d8059–d8059. doi: 10.1136/bmj.d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 208.Hunink MG, et al. The recent decline in mortality from coronary heart disease, 1980–1990. The effect of secular trends in risk factors and treatment. Jama. 1997;277:535–542. [PubMed] [Google Scholar]
- 209.Goldman L, et al. The effect of risk factor reductions between 1981 and 1990 on coronary heart disease incidence, prevalence, mortality and cost. J Am Coll Cardiol. 2001;38:1012–1017. doi: 10.1016/s0735-1097(01)01512-1. [DOI] [PubMed] [Google Scholar]
- 210.Unal B, Critchley JA, Capewell S. Modelling the decline in coronary heart disease deaths in England and Wales, 1981–2000 comparing contributions from primary prevention and secondary prevention. Bmj. 2005;331:614. doi: 10.1136/bmj.38561.633345.8F. doi:bmj.38561.633345.8F [pii] 10.1136/bmj.38561.633345.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Unal B, Capewell S, Critchley JA. Coronary heart disease policy models: a systematic review. BMC Public Health. 2006;6:213. doi: 10.1186/1471-2458-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goldman L, Cook EF. The decline in ischemic heart disease mortality rates. An analysis of the comparative effects of medical interventions and changes in lifestyle. Ann Intern Med. 1984;101:825–836. doi: 10.7326/0003-4819-101-6-825. [DOI] [PubMed] [Google Scholar]
- 213.Mathers CD, Stevens GA, Boerma T, White RA, Tobias MI. Causes of international increases in older age life expectancy. Lancet. 2014 doi: 10.1016/S0140-6736(14)60569-9. [DOI] [PubMed] [Google Scholar]
- 214.Lim SS, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010 a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. S0140-6736(12)61766-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Danaei G, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. The Lancet Diabetes & Endocrinology. 2014 doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ezzati M, et al. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet. 2003;362:271–280. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- 218.Woodward M, et al. A comparison of the associations between risk factors and cardiovascular disease in Asia and Australasia. Eur J Cardiovasc Prev Rehabil. 2005;12:484–491. doi: 10.1097/01.hjr.0000170264.84820.8e. [DOI] [PubMed] [Google Scholar]
- 219.Walter SD. Prevention of multifactorial disease. Am J Epidemiol. 1980;112:409–416. doi: 10.1093/oxfordjournals.aje.a113007. [DOI] [PubMed] [Google Scholar]
- 220.Yerushalmy J, Palmer CE. On the Methodology of Investigations of Etiologic Factors in Chronic Diseases. Journal of Chronic Disease. 1959;108:27–40. doi: 10.1016/0021-9681(59)90015-3. [DOI] [PubMed] [Google Scholar]
- 221.Thorolfsdottir RB, et al. Population assessment of future trajectories in coronary heart disease mortality. PLoS One. 2014;9:e85800. doi: 10.1371/journal.pone.0085800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. doi:S0140-6736(11)60814-3 [pii] 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 223.Webber L, et al. The future burden of obesity-related diseases in the 53 WHO European-Region countries and the impact of effective interventions: a modelling study. BMJ open. 2014;4:e004787. doi: 10.1136/bmjopen-2014-004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leon DA, Shkolnikov VM, McKee M. Alcohol and Russian mortality: a continuing crisis. Addiction. 2009;104:1630–1636. doi: 10.1111/j.1360-0443.2009.02655.x. ADD2655 [pii] [DOI] [PubMed] [Google Scholar]
- 225.McKee M, Shkolnikov V, Leon DA. Alcohol is implicated in the fluctuations in cardiovascular disease in Russia since the 1980s. Ann Epidemiol. 2001;11:1–6. doi: 10.1016/s1047-2797(00)00080-6. [DOI] [PubMed] [Google Scholar]
- 226.Leon DA, et al. Hazardous alcohol drinking and premature mortality in Russia: a population based case-control study. Lancet. 2007;369:2001–2009. doi: 10.1016/S0140-6736(07)60941-6. [DOI] [PubMed] [Google Scholar]
- 227.Tomkins S, et al. Identifying the determinants of premature mortality in Russia: overcoming a methodological challenge. BMC Public Health. 2007;7:343. doi: 10.1186/1471-2458-7-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zaridze D, et al. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet. 2009;373:2201–2214. doi: 10.1016/S0140-6736(09)61034-5. doi:S0140-6736(09)61034-5 [pii] 10.1016/S0140-6736(09)61034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zaridze D, et al. Alcohol and mortality in Russia: prospective observational study of 151,000 adults. Lancet. 2014;383:1465–1473. doi: 10.1016/S0140-6736(13)62247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Franco M, et al. Population-wide weight loss and regain in relation to diabetes burden and cardiovascular mortality in Cuba 1980–2010 repeated cross sectional surveys and ecological comparison of secular trends. BMJ. 2013;346:f1515. doi: 10.1136/bmj.f1515. [DOI] [PubMed] [Google Scholar]
- 231.Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. doi:0140-6736(92)91600-D [pii] [DOI] [PubMed] [Google Scholar]
- 232.Preston SH, Glei DA, Wilmoth JR. A new method for estimating smoking-attributable mortality in high-income countries. Int J Epidemiol. 39:430–438. doi: 10.1093/ije/dyp360. dyp360 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Beaglehole R. Medical management and the decline in mortality from coronary heart disease. Br Med J (Clin Res Ed) 1986;292:33–35. doi: 10.1136/bmj.292.6512.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rose G. The contribution of intensive coronary care. British journal of preventive & social medicine. 1975;29:147–150. doi: 10.1136/jech.29.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) Lancet. 1986;1:397–402. [PubMed] [Google Scholar]
- 236.Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 237.Gunnar RM, et al. Guidelines for the early management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee to Develop Guidelines for the Early Management of Patients with Acute Myocardial Infarction) J Am Coll Cardiol. 1990;16:249–292. doi: 10.1016/0735-1097(90)90575-a. [DOI] [PubMed] [Google Scholar]
- 238.Rogers WJ, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J Am Coll Cardiol. 2000;36:2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 239.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. The New England journal of medicine. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 240.Kumbhani DJ, et al. Temporal trends for secondary prevention measures among patients hospitalized with coronary artery disease. The American journal of medicine. 2014 doi: 10.1016/j.amjmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 241.Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 242.MRC trial of treatment of mild hypertension: principal results. Medical Research Council Working Party. Br Med J (Clin Res Ed) 1985;291:97–104. doi: 10.1136/bmj.291.6488.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261–1267. [PubMed] [Google Scholar]
- 244.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 245.Beckett NS, et al. Treatment of hypertension in patients 80 years of age or older. The New England journal of medicine. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 246.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stott DJ. In: Handbook of Hypertension, Vol 20, Epidemiology of Hypertension. Bulpitt CJ, editor. Elsevier; Amsterdam: 2000. [Google Scholar]
- 248.Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. The Lancet. 362:1527–1535. doi: 10.1016/S0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 249.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 250.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. Jama. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 251.Chobanian AV, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 252.Andersen UO, Jensen GB. Trends and determinant factors for population blood pressure with 25 years of follow-up: results from the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2010;17:655–659. doi: 10.1097/HJR.0b013e328336ec59. [DOI] [PubMed] [Google Scholar]
- 253.Andersen UO, Jensen GB. Trends and determinant factors in hypertension control in a population study with 25 years of follow-up. J Hypertens. 2010;28:1091–1096. doi: 10.1097/HJH.0b013e328335fa81. [DOI] [PubMed] [Google Scholar]
- 254.Fasce E, et al. Trends in prevalence, awareness, treatment and control of hypertension in urban communities in Chile. J Hypertens. 2007;25:1807–1811. doi: 10.1097/HJH.0b013e328244e481. [DOI] [PubMed] [Google Scholar]
- 255.Chow CK, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 256.Tobert JA. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat Rev Drug Discov. 2003;2:517–526. doi: 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- 257.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2010;140:226–235. doi: 10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 258.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% Bmj. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cholesterol Treatment, Trialists C, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.National Cholesterol Education Program Expert Panel on Detection, E. & Treatment of High Blood Cholesterol in, A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 261.Carleton RA, et al. Report of the Expert Panel on Population Strategies for Blood Cholesterol Reduction. A statement from the National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health. Circulation. 1991;83:2154–2232. doi: 10.1161/01.cir.83.6.2154. [DOI] [PubMed] [Google Scholar]
- 262.Roth GA, et al. High total serum cholesterol, medication coverage and therapeutic control: an analysis of national health examination survey data from eight countries. Bull World Health Organ. 2011;89:92–101. doi: 10.2471/BLT.10.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. The Lancet. 366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 264.Yusuf S, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. doi:S0140-6736(11)61215-4 [pii] 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 265.Pell S, D’Alonzo CA. Factors associated with long-term survival of diabetics. JAMA. 1970;214:1833–1840. [PubMed] [Google Scholar]
- 266.Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. The Lancet. 2014;383:2008–2017. doi: 10.1016/S0140-6736(14)60794-7. [DOI] [PubMed] [Google Scholar]
- 267.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. The New England journal of medicine. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 268.Riddle MC, et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–990. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Skyler JS, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 270.Ismail-Beigi F, et al. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154:554–559. doi: 10.7326/0003-4819-154-8-201104190-00007. [DOI] [PubMed] [Google Scholar]
- 271.Cefalu WT. Glycemic targets and cardiovascular disease. The New England journal of medicine. 2008;358:2633–2635. doi: 10.1056/NEJMe0803831. [DOI] [PubMed] [Google Scholar]
- 272.Ali MK, et al. Achievement of goals in U.S. diabetes care, 1999–2010. The New England journal of medicine. 2013;368:1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 273.Tunstall-Pedoe H, et al. Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet. 2000;355:688–700. doi: 10.1016/s0140-6736(99)11181-4. doi:S0140673699111814 [pii] [DOI] [PubMed] [Google Scholar]
- 274.Tunstall-Pedoe H. In: Coronary Heart Disease Epidemiology: From Aetiology to Public Health. Marmot M, Elliott P, editors. Oxford University Press; 2005. pp. 850–864. [Google Scholar]
- 275.Arciero TJ, et al. Temporal trends in the incidence of coronary disease. The American journal of medicine. 2004;117:228–233. doi: 10.1016/j.amjmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 276.Levy D, Thom TJ. Death rates from coronary disease–progress and a puzzling paradox. The New England journal of medicine. 1998;339:915–917. doi: 10.1056/NEJM199809243391309. [DOI] [PubMed] [Google Scholar]
- 277.Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 278.Tunstall-Pedoe H. Comment on the ESC/ACC redefinition of myocardial infarction by a consensus dissenter. Eur Heart J. 2001;22:613–616. doi: 10.1053/euhj.2000.2579. [DOI] [PubMed] [Google Scholar]
- 279.Myerson R, Rosenfield LC, Lim SS, Murray CJL. Safe pregnancy and delivery: a systematic analysis of trends in the coverage of antenatal and intrapartum care [abstract]. In GHME Conference Organizing Committee. March 14–16, 2011 in Seattle, WA, USA. The Lancet. 2011;377:969–970. Abstract nr 985. [Google Scholar]
- 280.Rosamond WD, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hamm CW, et al. The prognostic value of serum troponin T in unstable angina. The New England journal of medicine. 1992;327:146–150. doi: 10.1056/NEJM199207163270302. [DOI] [PubMed] [Google Scholar]
- 282.Mills NL, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA. 2011;305:1210–1216. doi: 10.1001/jama.2011.338. [DOI] [PubMed] [Google Scholar]
- 283.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 284.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–1194. doi: 10.1016/j.ijcard.2012.11.065. S0167-5273(12)01548-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. S0140-6736(12)61728-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mayosi BM. In: Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th. Mann DL, Zipes DP, Libby P, Bonow RO, editors. Elsevier Saunders; 2015. [Google Scholar]
- 287.Kaplan ELT. Duckett Jones Memorial Lecture. Global assessment of rheumatic fever and rheumatic heart disease at the close of the century. Influences and dynamics of populations and pathogens: a failure to realize prevention? Circulation. 1993;88:1964–1972. doi: 10.1161/01.cir.88.4.1964. [DOI] [PubMed] [Google Scholar]
- 288.Moriyama IM, Baum WS, Haenszel WM, Mattison BF. Inquiry into diagnostic evidence supporting medical certifications of death. American journal of public health and the nation’s health. 1958;48:1376–1387. doi: 10.2105/ajph.48.10.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tibazarwa KB, Volmink JA, Mayosi BM. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart. 2008;94:1534–1540. doi: 10.1136/hrt.2007.141309. [DOI] [PubMed] [Google Scholar]
- 290.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clinical epidemiology. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Gordis L. The virtual disappearance of rheumatic fever in the United States: lessons in the rise and fall of disease. T. Duckett Jones memorial lecture. Circulation. 1985;72:1155–1162. doi: 10.1161/01.cir.72.6.1155. [DOI] [PubMed] [Google Scholar]
- 292.Madden S, Kelly L. Update on acute rheumatic fever: it still exists in remote communities. Canadian family physician Medecin de famille canadien. 2009;55:475–478. [PMC free article] [PubMed] [Google Scholar]
- 293.White H, et al. Rheumatic heart disease in indigenous populations. Heart, lung & circulation. 2010;19:273–281. doi: 10.1016/j.hlc.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 294.Karthikeyan G, Mayosi BM. Is primary prevention of rheumatic fever the missing link in the control of rheumatic heart disease in Africa? Circulation. 2009;120:709–713. doi: 10.1161/CIRCULATIONAHA.108.836510. [DOI] [PubMed] [Google Scholar]
- 295.Omurzakova NA, et al. Rheumatologic services in Central Asian countries: current state of development of rheumatology in Central Asia. International journal of rheumatic diseases. 2009;12:288–292. doi: 10.1111/j.1756-185X.2009.01442.x. [DOI] [PubMed] [Google Scholar]
- 296.Omurzakova NA, et al. High incidence of rheumatic fever and rheumatic heart disease in the republics of Central Asia. International journal of rheumatic diseases. 2009;12:79–83. doi: 10.1111/j.1756-185X.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 297.Alfieri O, Mayosi BM, Park SJ, Sarrafzadegan N, Virmani R. Exploring unknowns in cardiology. Nat Rev Cardiol. 2014;11:664–670. doi: 10.1038/nrcardio.2014.123. [DOI] [PubMed] [Google Scholar]
- 298.Mocumbi AO, Falase AO. Recent advances in the epidemiology, diagnosis and treatment of endomyocardial fibrosis in Africa. Heart. 2013;99:1481–1487. doi: 10.1136/heartjnl-2012-303193. [DOI] [PubMed] [Google Scholar]
- 299.Sliwa K, Mayosi BM. Recent advances in the epidemiology, pathogenesis and prognosis of acute heart failure and cardiomyopathy in Africa. Heart. 2013;99:1317–1322. doi: 10.1136/heartjnl-2013-303592. [DOI] [PubMed] [Google Scholar]
- 300.Roivainen M, et al. Infections, inflammation, and the risk of coronary heart disease. Circulation. 2000;101:252–257. doi: 10.1161/01.cir.101.3.252. [DOI] [PubMed] [Google Scholar]
- 301.Barnighausen T, et al. Hiding in the shadows of the HIV epidemic: obesity and hypertension in a rural population with very high HIV prevalence in South Africa. J Hum Hypertens. 2008;22:236–239. doi: 10.1038/sj.jhh.1002308. [DOI] [PubMed] [Google Scholar]
- 302.Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: an African perspective. Nature clinical practice Cardiovascular medicine. 2009;6:120–127. doi: 10.1038/ncpcardio1437. [DOI] [PubMed] [Google Scholar]
- 303.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–741. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 304.Farzadfar F, et al. Effectiveness of diabetes and hypertension management by rural primary health-care workers (Behvarz workers) in Iran: a nationally representative observational study. Lancet. 2012;379:47–54. doi: 10.1016/S0140-6736(11)61349-4. doi:S0140-6736(11)61349-4 [pii] 10.1016/S0140-6736(11)61349-4. [DOI] [PubMed] [Google Scholar]
- 305.Ezzati M, Friedman AB, Kulkarni SC, Murray CJ. The reversal of fortunes: trends in county mortality and cross-county mortality disparities in the United States. PLoS Med. 2008;5:e66. doi: 10.1371/journal.pmed.0050066. doi:07-PLME-RA-0862 [pii] 10.1371/journal.pmed.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Paffenbarger RSJ. In: Proceedings of the conference on the decline in coronary heart disease mortality October 24–25, 1978. Havlik RJ, Feinleib M, editors. National Heart, Lung and Blood Institute, US Department of Health, Education, and Welfare; Washington DC: 1979. pp. 298–311. (NIH Publication No. 79-1610). [Google Scholar]
- 307.Kuller LH, Laporte RE, Weinberg GB. In: Proceedings of the conference on the decline in coronary heart disease mortality October 24–25, 1978. Havlik RJ, Feinleib M, editors. National Heart, Lung and Blood Institute, US Department of Health, Education, and Welfare; Washington DC: 1979. pp. 312–339. (NIH Publication No. 79-1610). [Google Scholar]
- 308.Luepker RV. WHO MONICA project: what have we learned and where to go from here? Public Health Reviews. Public Health Reviews. 2012;33:373–396. [Google Scholar]
- 309.Tunstall-Pedoe H, et al. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 310.World Health Organization. WHO methods and data sources for global causes of death, 2000–2012. WHO; 2014. (available at http://www.who.int/healthinfo/global_burden_disease/GlobalCOD_method_2000_2012.pdf) [Google Scholar]
- 311.Griffiths C, Brock A, Rooney C. The impact of introducing ICD-10 on trends in mortality from circulatory diseases in England and Wales. Health statistics quarterly/Office for National Statistics. 2004:14–20. [PubMed] [Google Scholar]
- 312.Murray CJ, Kulkarni SC, Ezzati M. Understanding the coronary heart disease versus total cardiovascular mortality paradox: a method to enhance the comparability of cardiovascular death statistics in the United States. Circulation. 2006;113:2071–2081. doi: 10.1161/CIRCULATIONAHA.105.595777. [DOI] [PubMed] [Google Scholar]
- 313.Stevens GA, King G, Shibuya K. Deaths from heart failure: using coarsened exact matching to correct cause-of-death statistics. Popul Health Metr. 2010;8:6. doi: 10.1186/1478-7954-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.World Health Organization. The global burden of disease: 2004 update. WHO; 2008. [Google Scholar]
- 315.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. 346/6/393 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Look Ahead Research Group et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


