Abstract
We conducted a systematic review and 3-part meta-analysis to characterize the relationship between smoking and perinatal death, defined as the combination of stillbirth and neonatal death. The PubMed database was searched (1956–August 31, 2011) with keywords, and manual reference searches of included articles and Surgeon Generals' reports were conducted. The full texts of 1,713 articles were reviewed, and 142 articles that examined the associations between active or passive smoking and perinatal death were included in the meta-analyses. Data were abstracted by 2 reviewers. Any active maternal smoking was associated with increased risks of stillbirth (summary relative risk (sRR) = 1.46, 95% confidence interval (CI): 1.38, 1.54 (n = 57 studies)), neonatal death (sRR = 1.22, 95% CI: 1.14, 1.30 (n = 28)), and perinatal death (sRR = 1.33, 95% CI: 1.25, 1.41 (n = 46)). The risks of stillbirth, neonatal death, and perinatal death increased with the amount smoked by the mother. Biases in study publication, design, and analysis were present but did not significantly affect the results. These findings strengthen the evidence that women should not smoke while pregnant, and all women of reproductive age should be warned that smoking increases the risks of stillbirth, neonatal death, and perinatal death.
Keywords: neonatal death, perinatal death, perinatal mortality, pregnancy, smoking, stillbirth, tobacco
The relationship between smoking during pregnancy and risk of perinatal death has undergone extensive scrutiny since the 1960s (1–3). Perinatal death includes 2 components: stillbirth (death of the fetus in utero, starting at 20–28 weeks' gestation) and neonatal death (death within 1 month after birth at any gestational age). The scientific and medical communities' acceptance of smoking as a cause of perinatal death was hindered by the statistical phenomenon of the “birth weight paradox” (4). Although babies of smokers are less likely to survive than are babies of nonsmokers, low-birth-weight babies of smokers are more likely to survive than are low-birth-weight babies of nonsmokers. This counterintuitive pattern was explained by the finding that babies of smokers have a different birth weight distribution than babies of nonsmokers, invalidating birth-weight-specific comparisons between the 2 groups (5). Approximately 11% of smokers' babies are considered “low birth weight” by the criterion of birth weight less than 2,500 g, as compared with 6% of nonsmokers' babies (6). After decades of controversy and resolution of the paradox (5, 6), smoking was gradually established as a cause of perinatal death (7).
Surgeon Generals' reports have cited an increased risk of perinatal mortality among babies of smokers (7–9). The 2001 report (Women and Smoking) stated, “The risk for perinatal mortality—both stillbirth and neonatal deaths—and the risk for sudden infant death syndrome (SIDS) are increased among the offspring of women who smoke during pregnancy” (7, p. 15), and subsequent Surgeon Generals' reports affirmed this conclusion (10–12). Despite this assessment, the complete literature on smoking and perinatal mortality has not been consolidated, and the magnitude of the association, the dose-response relationship, and effect modifiers have not been characterized. One barrier to understanding the impact of smoking on perinatal death is that multiple papers from the same data sets have been published, and the impact of such duplicated data, as well as other biases, has never been examined. To synthesize the literature, examine trends, and explore heterogeneity and sources of bias, we conducted a systematic review and 3-part meta-analysis of the association between smoking and perinatal death.
METHODS
We carried out the systematic review and meta-analysis using the guidelines of the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) consensus statement (13) and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (14).
Inclusion criteria
Studies eligible for inclusion in the meta-analyses were original observational or experimental studies. Eligible studies compared risks of perinatal death between women exposed to tobacco smoke from cigarettes and women not exposed. Relevant exposures were smoking of cigarettes by the mother and secondhand smoke (SHS) exposure in pregnant women. Articles written in any language were eligible, and foreign-language articles were translated into English as necessary using Google Translate (Google Inc., Mountain View, California) (15). We excluded duplicate publications and articles presenting duplicated data (e.g., studies conducted using data from the same registry with overlapping years). Quality measures were not used to select studies for inclusion.
Literature search strategy
Two reviewers independently searched PubMed (National Library of Medicine, Bethesda, Maryland; 1956–August 31, 2011) for articles relevant to smoking and adverse pregnancy outcomes, using the search terms (smoking OR tobacco) AND pregnancy. From the chosen articles, those relevant to perinatal death were selected for inclusion and/or review of references. We conducted manual searches by checking references of the articles identified in the PubMed searches. The papers referenced by all relevant articles (original articles, reviews, and letters) were searched by at least 1 reviewer, and the papers referenced by included articles and all Surgeon Generals' reports regarding tobacco and health were searched by 2 reviewers. Disagreements about final inclusion status were resolved by discussion.
Data abstraction
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools (16). Two reviewers independently extracted from all articles data on study type, country, calendar years of the pregnancies in the studies, population characteristics, participant inclusion and exclusion criteria, recruitment method, participation and follow-up rates, exposure and outcome definitions, features of data collection, numbers of participants, effect sizes, and statistical significance tests. Differences in item coding were resolved through discussion between the reviewers, and the κ statistic was computed to assess agreement between reviewers. The median Strout-Fleiss reliability statistic for continuous variables was 1.00, and the median κ statistic for covariates analyzed was 0.73.
Definition of outcomes
Stillbirth, neonatal death, and perinatal death were analyzed separately. Stillbirth was variably defined across the studies. Common lower thresholds for gestational age were between 20 and 28 weeks, and some studies used minimum birth weights of 400–1,000 g. Many reports did not provide a definition of stillbirth; for these studies, any outcome described as “stillbirth” or “fetal death” without any other description was used and combined with other stillbirth outcomes. Some studies examined antenatal and intrapartum stillbirth or explained and unexplained stillbirth. These were combined and entered into the main analyses (17).
Neonatal death was generally defined as death after live birth within 1, 7, 28, or 30 days of birth. When no definition was provided, outcomes termed “neonatal death” or “early neonatal death” were included. The broadest definition in each study was used for the main analysis, and early neonatal death (death within 6–8 days after birth) was examined separately.
Perinatal death is the combination of stillbirth and neonatal death. As with the other outcomes, only some studies provided detailed definitions of perinatal death, but all deaths identified as such were included. Reproductive lifetime history of perinatal death (ever having had at least 1 perinatal death over a woman's lifetime) was analyzed separately from perinatal death in an individual pregnancy. Outcomes stratified by birth weight categories other than minima of ≤1,000 g were excluded.
Grouping of exposures
Any study that used as its exposure indicator “smoking,” “smoker,” “secondhand smoke,” “environmental tobacco smoke,” “passive smoking,” “lives with a smoker,” or “partner/husband is a smoker” was considered eligible. Exposures were categorized by type of smoking (active/passive), timing in relation to pregnancy, amount of exposure, and source of exposure if given. Amount of exposure was given as number of cigarettes or packs smoked per day by the mother or father, hours or general semiquantitative frequency of maternal SHS exposure, or maternal serum cotinine concentration. Reference exposures included “0 cigarettes per day,” “nonsmoker,” “never smoker,” “no SHS exposure,” and other designations indicating no exposure to tobacco smoke. Studies that collected data on smoking exposure before or during pregnancy were categorized as prospective, and case-control studies or other studies that collected smoking exposure data after pregnancy were categorized as retrospective. The window of smoking exposure was categorized as follows: 1) smoking before pregnancy; 2) smoking during pregnancy; 3) lifetime exposure or current smoking after all studied pregnancies; 4) ex-smoker at the time of pregnancy; 5) quit smoking during pregnancy; and 6) not specified. “Any active smoking” refers to definitions of smoking as at least 1 cigarette per day and to the undefined terms of “smoker” and “smoking.”
Analysis
A random-effects model was used to account for heterogeneity of study populations and designs (18). The estimate of relative risk used was the odds ratio, risk ratio, or hazard ratio, as given in the original article. For studies without a relative risk estimate, the risk ratio or odds ratio was calculated from available data as appropriate.
Relative risk estimates for “any active smoking” were combined, as were those for categories of 1–10, 11–20, and ≥21 cigarettes smoked per day (19). Because too few studies that examined SHS or history of perinatal death gave results by amount of exposure, dose-response analyses were not conducted for this exposure and outcome, respectively. Instead, all studies that included summary relative risks (sRRs) for various categories of exposure were included in “any exposure” analyses after combining the multiple sRRs into a single estimate for each study (17). Analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina).
Dose-response analysis
All sRRs given for the risk of stillbirth, neonatal death, or perinatal death based on number of cigarettes smoked per day, regardless of categorization scheme, were analyzed in a dose-response meta-regression, separately for each outcome. We used a SAS macro for meta-analysis of linear and nonlinear dose-response relationships that combines studies of the same relationship that have different exposure levels (20, 21). For closed-ended categories, the midpoints were taken as the dose. For open-ended categories (those specified with minimum but not maximum numbers of cigarettes smoked per day, such as “20 or more”), we imputed a maximum number of cigarettes per day based on the category minimum, and then took the midpoint of the minimum and the imputed maximum as the dose. The imputed maximum was based on the approximate mean number and categorical distribution of cigarettes smoked per day among women in the National Health Interview Survey (22). Categories with minimum cigarettes per day of 40, 30–39, 25–29, 20–24, 15–19, and 2–14 were given maxima of 45, 40, 35, 30, 25, and 20, respectively.
Heterogeneity and risk of bias
Heterogeneity between studies was assessed using the I2 statistic, which represents the percentage of total variation that is true between-studies heterogeneity (17). The statistical significance of the heterogeneity was analyzed with the Q statistic. When heterogeneity was statistically significant and high, sources of the heterogeneity were examined using random-effects meta-regression for both continuous and dichotomous variables (23, 24). Subgroups were contrasted on the basis of exposure timing in relation to pregnancy (exposure during pregnancy vs. not specified or other). If unexplained heterogeneity remained, post hoc meta-regression and subgroup analyses were conducted on other variables for which data were collected. We examined the effect of duplicated data in multiple publications by including all studies in a sensitivity analysis.
Risk of bias assessment was undertaken using indicators to address information bias, confounding, selection bias, and violation of statistical assumptions. Studies that were conducted prospectively, analyzed only 1 pregnancy per woman, and adjusted for appropriate confounders were considered to have the lowest risk of bias. Appropriate adjustment for potential confounders was considered to be adjustment for at least maternal age, race, and socioeconomic status (various measures including education, occupation, and home environment variables) and no adjustment for birth weight or for prior pregnancy loss. Studies with the highest risk of bias were retrospective, with no control for confounders, and with an unspecified number of pregnancies per woman or more than 1 pregnancy per woman analyzed. Publication bias was analyzed for each outcome through visual analysis of funnel plots, Egger's regression, Begg rank correlation, funnel plot regression, and trim-and-fill tests using the PUB_BIAS SAS macro (25).
Imputation
Three studies required imputation of data for variance estimation. For 2 of these, the P value was specified as greater than 0.05, and to use a conservative estimate, we imputed the mean of 0.05 and 1 (26, 27). One other study required imputation of the numbers of subjects in subgroups of boys and girls, for which we used the given number of subjects and approximated the sex ratio as 1.04 (28). This article was also missing the percentage of subjects who were smokers, but we obtained this percentage from another article presenting results from the same study (29) and assumed that the prevalence of smoking did not vary by the sex of the baby. Imputation of the covariates “national smoking prevalence” and “national cigarettes per capita” was also performed for each study based on year and country. Year of publication and midpoint year of study pregnancies were used for the year value. Smoking prevalence data were mostly obtained from the World Health Organization Global InfoBase (complete reference list available upon request) (30). Data on cigarettes per capita were obtained from several reports (31–33).
RESULTS
Studies included in the systematic review and meta-analysis
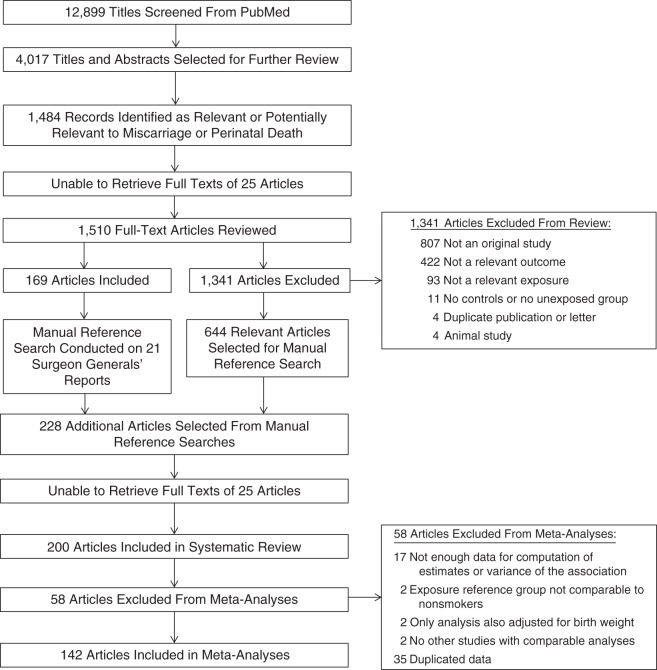
From the initial 12,899 articles identified by keywords for tobacco and pregnancy, 200 articles published between 1948 and 2011 were selected for inclusion in the systematic review and 142 for inclusion in the meta-analyses (Figure 1; also see Web Table 1, available at http://aje.oxfordjournals.org/). Articles were excluded if the studies described therein were not original (n = 807), were not studying stillbirth, neonatal death, or perinatal death (n = 422), were not analyzing smoking in relation to stillbirth, neonatal death, or perinatal death (n = 93), were lacking a control group or unexposed group (n = 11), were duplicate publications or letters (n = 4), or were studies of nonhuman animals (n = 4). Articles included in the systematic review were excluded from the meta-analyses if they lacked information for computation of estimates or variance of the association (n = 17), used a reference group not comparable to nonsmokers (n = 2), only presented an analysis adjusted for birth weight (n = 2), had no other reviewed studies with comparable analyses (n = 2), or contained duplicated study data (n = 35) (see Web Table 2). Among studies included in the meta-analyses, the outcome analyzed in relation to smoking was stillbirth (n = 97 articles), neonatal death (n = 45), and/or perinatal death (n = 63). Ten studies used history of stillbirth as the outcome, and 138 used stillbirth, neonatal death, and/or perinatal death in an individual pregnancy. All results refer to stillbirth, neonatal death, or perinatal death in an individual pregnancy unless otherwise specified. Ten studies examined SHS exposure of the mother, and 138 examined active maternal smoking. Twenty-six percent of the studies were conducted in the United States, and the remainder were conducted in 28 other countries or sets of countries. For 20 studies, articles were written in languages other than English.
Figure 1.
Selection of published studies (1948–August 31, 2011) included in a systematic review and 3-part meta-analysis of smoking and risk of perinatal death.
Some sources of data used by the studies were not unique to each publication. These duplicated data were identified on the basis of studies conducted in the same country, using the same registry, hospital, or city population, and during the same years. Some were duplicate publications by the same authors, and others were registry studies with overlapping years, such as a set of articles all based on data from the Swedish Medical Birth Register (Web Table 3). Ninety-five articles included in the systematic review and eligible for inclusion in the meta-analyses were placed into 24 sets of duplicated data with 2–23 articles per set. One article was separated into 2 studies with duplicated data due to use of both cohort and case-control study designs. All studies remained in the analytical data set to maximize the number of studies per analysis, because even 2 studies with identical data sometimes presented the data differently or could be used in different analyses of heterogeneity (e.g., smoker/nonsmoker and categories of cigarettes per day). To prevent analysis of duplicated data, we chose only 1 study from each set for a single analysis, and 35 studies from these sets were not used in any analysis (Web Table 3).
Active maternal smoking
Results of the meta-analysis
The sRR of perinatal death among women who smoked was 1.33 (95% confidence interval (CI): 1.25, 1.41 (46 studies); Table 1, Web Figure 1). The risk was greater for stillbirth than for neonatal death (sRR = 1.46 vs. sRR = 1.22 (P< 0.001); Table 1, Web Figures 2 and 3). No significant differences were found among comparisons of odds ratios and risk/rate/hazard ratios or among comparisons of crude and adjusted relative risks (P's > 0.1; Tables 2–4). Thus, for the main analyses, crude and adjusted estimates of odds ratios, risk ratios, rate ratios, and hazard ratios were combined. The inclusion of duplicated data did not significantly affect the sRRs for stillbirth (sRR = 1.47, 95% CI: 1.41, 1.53 (80 studies)), neonatal death (sRR = 1.21, 95% CI: 1.16, 1.26 (42 studies)), or perinatal death (sRR = 1.32, 95% CI: 1.25, 1.38 (55 studies)). All further analyses excluded duplicated data. Few studies analyzed ex-smokers and persons who quit smoking during pregnancy, so we combined these studies after finding no significant differences between them (P's > 0.1). Ex-smokers and women who quit smoking during pregnancy had similar risks of stillbirth, neonatal death, and perinatal death in comparison with nonsmokers (sRRs were 1.02, 1.12, and 0.98, respectively; Table 1).
Table 3.
Results From Heterogeneity and Study-Quality Analyses for Any Active Smoking During Pregnancy and Risk of Neonatal Death, 1948–2011
| No. of Studies |
Odds Ratioa | 95% Confidence Interval | |
|---|---|---|---|
| Year of publication (per 10 years) | 28 | 1.00b | 0.95, 1.05 |
| Midpoint year of study pregnancies (per 10 years) | 27 | 0.99b | 0.95, 1.03 |
| Exposure prevalence in the study population (prevalence in controls for case-control studies) (per percentage point) | 28 | 1.00b | 0.57, 1.76 |
| Neonatal death rate among nonsmokers in cohort studies (per log unit of the rate in thousands) | 24 | 0.94b | 0.87, 1.02 |
| Per-capita cigarette consumption (per thousand, imputed) | 27 | 0.98b | 0.92, 1.04 |
| Study design | |||
| Case-control studies | 3 | 1.23 | 0.98, 1.54 |
| Cohort studies | 26 | 1.21 | 1.14, 1.30 |
| Definition of timing of exposure | |||
| Studies which specified that the smoking exposure used was smoking during the pregnancy after which neonatal death risk was measured | 26 | 1.21 | 1.13, 1.30 |
| Studies with exposures without a specified time or exposure specified as smoking before pregnancy, excluding former smoking | 2 | 1.28 | 0.88, 1.87 |
| Country of study | |||
| United States | 10 | 1.17 | 1.08, 1.26 |
| Other | 18 | 1.25 | 1.15, 1.36 |
| Definition of neonatal death used in study | |||
| Death within 6–8 days of birth or “early neonatal death” | 13 | 1.18 | 1.04, 1.34 |
| Death within 28–31 days of birth or not specified | 17 | 1.24 | 1.15, 1.33 |
| Biased self-report of smoking | |||
| Prospective or biochemical measurement of smoking | 14 | 1.18 | 1.07, 1.30 |
| Retrospective, nonbiochemical measurement | 14 | 1.24 | 1.13, 1.36 |
| Control for confounding | |||
| Control by statistical adjustment or matching | 5 | 1.13 | 1.07, 1.19 |
| No control for confounders (crude estimates) | 26 | 1.27 | 1.19, 1.35 |
| Type of risk estimate | |||
| Odds ratios | 9 | 1.23 | 1.13, 1.34 |
| Risk, rate, or hazard ratios | 26 | 1.24 | 1.17, 1.32 |
| Independence of outcomes | |||
| Analysis of only 1 pregnancy per woman or statistical adjustment for nonindependence of outcomes and among only singleton births | 10 | 1.24 | 1.15, 1.33 |
| Not specified or >1 pregnancy per woman analyzed, or twins included in analysis | 23 | 1.19 | 1.11, 1.27 |
| Overall risk of bias | |||
| Lowest risk: prospective, best statistical model, 1 pregnancy per woman analyzed | 1 | 1.20 | 1.01, 1.42 |
| Highest risk: retrospective, no control for confounders, not specified, or >1 pregnancy per woman analyzed | 13 | 1.28 | 1.18, 1.40 |
a Summary relative risk of neonatal death for smokers versus nonsmokers in the subgroup, except where specified.
b Change in the summary relative risk of neonatal death for smokers versus nonsmokers with each increment of the continuous variable, as specified.
Table 1.
Summary Relative Risks of Stillbirth, Neonatal Death, and Perinatal Death Among Women Who Smoked During Pregnancy As Compared With Nonsmokers, 1948–2011
| Stillbirth |
Neonatal Death |
Perinatal Death |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| sRR | 95% CI | No. of Studies |
sRR | 95% CI | No. of Studies |
sRR | 95% CI | No. of Studies |
|
| Any active smokinga | 1.46 | 1.38, 1.54 | 57 | 1.22 | 1.14, 1.30 | 28 | 1.33 | 1.25, 1.41 | 46 |
| Smoking category, cigarettes/daya | |||||||||
| 1–10 | 1.10 | 0.98, 1.24 | 20 | 1.06 | 0.90, 1.26 | 10 | 1.17 | 1.05, 1.31 | 16 |
| 11–20 | 1.30 | 1.22, 1.38 | 9 | 1.30 | 1.00, 1.68 | 4 | 1.35 | 1.18, 1.53 | 6 |
| >20 | 1.24 | 1.03, 1.50 | 10 | 1.31 | 1.11, 1.55 | 5 | 1.41 | 1.32, 1.50 | 11 |
| Former smokinga | 1.12 | 0.91, 1.37 | 6 | 0.98 | 0.81, 1.18 | 4 | 1.02 | 0.88, 1.19 | 6 |
| Secondhand smoke exposure | 1.40 | 1.06, 1.85 | 7 | Not calculated | 1.42 | 1.10, 1.85 | 2 | ||
| Evidence for publication biasb | Strong | None | Moderate | ||||||
Abbreviations: CI, confidence interval; sRR, summary relative risk.
a Risk of perinatal death, stillbirth, or neonatal death in a single pregnancy, excluding duplicated data.
b Evidence of publication bias was assessed using studies included in the analysis of the association between any active smoking (excluding ex-smokers and those who quit smoking during pregnancy) and the risk of perinatal death, stillbirth, or neonatal death.
Table 2.
Results From Heterogeneity and Study-Quality Analyses for Any Active Smoking During Pregnancy and Risk of Stillbirth, 1948–2011
| No. of Studies |
Odds Ratioa | 95% Confidence Interval | |
|---|---|---|---|
| Year of publication (per 10 years) | 57 | 1.02b | 0.98, 1.06 |
| Midpoint year of study pregnancies (per 10 years) | 55 | 1.02b | 0.98, 1.06 |
| Exposure prevalence in the study population (prevalence in controls for case-control studies) (per percentage point) | 54 | 0.80b | 0.50, 1.27 |
| Stillbirth rate among nonsmokers in cohort studies (per log unit of the rate in thousands) | 30 | 0.92b | 0.82, 1.03 |
| Per-capita cigarette consumption (per thousand, imputed) | 48 | 0.93b | 0.87, 1.00 |
| Study design | |||
| Case-control studies | 20 | 1.42 | 1.32, 1.53 |
| Cohort studies | 38 | 1.47 | 1.36, 1.59 |
| Definition of timing of exposure | |||
| Studies which specified that the smoking exposure used was smoking during the pregnancy in which stillbirth risk was measured | 50 | 1.45 | 1.37, 1.53 |
| Studies with exposures without a specified time or exposure specified as smoking before pregnancy, excluding former smoking | 10 | 1.60 | 1.26, 2.04 |
| Country of study | |||
| United States | 15 | 1.36 | 1.25, 1.47 |
| Other | 42 | 1.51 | 1.39, 1.64 |
| Definition of stillbirth used in study | |||
| ≥20–23 weeks’ gestation | 24 | 1.48 | 1.38, 1.58 |
| ≥24 weeks’ gestation | 15 | 1.62 | 1.35, 1.95 |
| Not specified | 19 | 1.41 | 1.21, 1.65 |
| Biased self-report of smoking | |||
| Prospective or biochemical measurement of smoking | 27 | 1.58 | 1.38, 1.80 |
| Retrospective, nonbiochemical measurement | 31 | 1.43 | 1.34, 1.52 |
| Control for confounding | |||
| Control by statistical adjustment or matching | 23 | 1.47 | 1.34, 1.61 |
| No control for confounders (crude estimates) | 37 | 1.46 | 1.37, 1.56 |
| Type of risk estimate | |||
| Odds ratios | 36 | 1.43 | 1.35, 1.52 |
| Risk, rate, or hazard ratios | 32 | 1.53 | 1.39, 1.68 |
| Independence of outcomes | |||
| Analysis of only 1 pregnancy per woman or statistical adjustment for nonindependence of outcomes and among only singleton births | 13 | 1.65 | 1.44, 1.89 |
| Not specified or >1 pregnancy per woman analyzed, or twins included in analysis | 47 | 1.43 | 1.34, 1.52 |
| Overall risk of bias | |||
| Lowest risk: prospective, best statistical model, 1 pregnancy per woman analyzed | 10 | 1.57 | 1.27, 1.93 |
| Highest risk: retrospective, no control for confounders, not specified, or >1 pregnancy per woman analyzed | 21 | 1.43 | 1.33, 1.55 |
| Studies that analyzed risk of stillbirth in the second of 2 pregnancies in which smoking was measured (reference group: women who did not smoke during either pregnancy) | |||
| Smoking in first pregnancy only | 2 | 1.06 | 0.88, 1.28 |
| Smoking in second (index) pregnancy only | 2 | 1.08 | 0.70, 1.68 |
| Smoking in both first pregnancy and second (index) pregnancy | 2 | 1.27 | 1.13, 1.44 |
a Summary relative risk of stillbirth for smokers versus nonsmokers in the subgroup, except where specified.
b Change in the summary relative risk of stillbirth for smokers versus nonsmokers with each increment of the continuous variable, as specified.
Table 4.
Results From Heterogeneity and Study-Quality Analyses for Any Active Smoking During Pregnancy and Risk of Perinatal Death, 1948–2011
| No. of Studies |
Odds Ratioa | 95% Confidence Interval | |
|---|---|---|---|
| Year of publication (per 10 years) | 46 | 1.02b | 0.98, 1.06 |
| Midpoint year of study pregnancies (per 10 years) | 45 | 1.02b | 0.98, 1.07 |
| Smoking prevalence in the study population (prevalence in controls for case-control studies) (per percentage point) | 44 | 0.87b | 0.56, 1.36 |
| Perinatal death rate among nonsmokers in cohort studies (per log unit of the rate in thousands) | 39 | 0.96b | 0.87, 1.07 |
| Per-capita cigarette consumption (per thousand, imputed) | 36 | 0.96b | 0.91, 1.02 |
| Study design | |||
| Case-control studies | 4 | 1.40 | 1.33, 1.48 |
| Cohort studies | 43 | 1.33 | 1.25, 1.42 |
| Definition of timing of exposure | |||
| Studies which specified that the smoking exposure used was smoking during the pregnancy in which perinatal death risk was measured | 39 | 1.32 | 1.24, 1.40 |
| Studies with exposures without a specified time or exposure specified as smoking before pregnancy, excluding former smoking | 7 | 1.45 | 1.11, 1.88 |
| Country of study | |||
| United States | 10 | 1.22 | 1.06, 1.39 |
| Other | 36 | 1.36 | 1.27, 1.45 |
| Definition of perinatal death used in study | |||
| ≥20–23 weeks’ gestation | 12 | 1.37 | 1.27, 1.49 |
| ≥24 weeks’ gestation | 9 | 1.28 | 1.18, 1.39 |
| Not specified | 26 | 1.37 | 1.19, 1.57 |
| Biased self-report of smoking | |||
| Prospective or biochemical measurement of smoking | 23 | 1.31 | 1.20, 1.44 |
| Retrospective, nonbiochemical measurement | 23 | 1.36 | 1.27, 1.45 |
| Control for confounding | |||
| Control by statistical adjustment or matching | 12 | 1.29 | 1.20, 1.39 |
| No control for confounders (crude estimates) | 35 | 1.33 | 1.23, 1.45 |
| Type of risk estimate | |||
| Odds ratios | 16 | 1.32 | 1.23, 1.42 |
| Risk, rate, or hazard ratios | 38 | 1.32 | 1.24, 1.41 |
| Independence of outcomes | |||
| Analysis of only 1 pregnancy per woman or statistical adjustment for nonindependence of outcomes and among only singleton births | 10 | 1.41 | 1.29, 1.55 |
| Not specified or >1 pregnancy per woman analyzed, or twins included in analysis | 38 | 1.28 | 1.20, 1.37 |
| Overall risk of bias | |||
| Lowest risk: prospective, best statistical model, 1 pregnancy per woman analyzed | 2 | 1.24 | 1.18, 1.30 |
| Highest risk: retrospective, no control for confounders, not specified, or >1 pregnancy per woman analyzed | 17 | 1.34 | 1.23, 1.45 |
a Summary relative risk of perinatal death for smokers versus nonsmokers in the subgroup, except where specified.
b Change in the summary relative risk of perinatal death for smokers versus nonsmokers with each increment of the continuous variable, as specified.
History of perinatal death
Smoking was associated with an increased risk of having a history of stillbirth (sRR = 1.28, 95% CI: 0.81, 2.02 (8 studies)). No studies examined the risks of having a history of perinatal death or neonatal death (at least 1 death in the woman's lifetime) on the basis of smoking status.
Dose-response analysis
The relative risk of stillbirth in smokers compared with nonsmokers increased with number of cigarettes smoked per day up to the level of 15 cigarettes per day and then decreased (data not shown). Exclusion of open-ended categories resulted in a nonlinear, monotonic curve in which the relative risk of stillbirth increased with the number of cigarettes per day (Figure 2). The relative risk of neonatal death increased by a factor of 1.01 for each additional cigarette smoked (95% CI: 1.01, 1.02), and this relationship did not depart significantly from linearity (P = 0.06; Figure 3). The relative risk of perinatal death increased nonlinearly with the number of cigarettes smoked per day (Web Figure 4) (34). Excluding open-ended categories did not affect the curves for perinatal death or neonatal death. Using only the most common categories of 1–10, 11–20, and >20 cigarettes per day, the sRR of perinatal death increased as the number of cigarettes smoked per day increased, but the sRRs for stillbirth and neonatal death did not continue to increase above the level of 11–20 cigarettes per day (Table 1).
Figure 2.
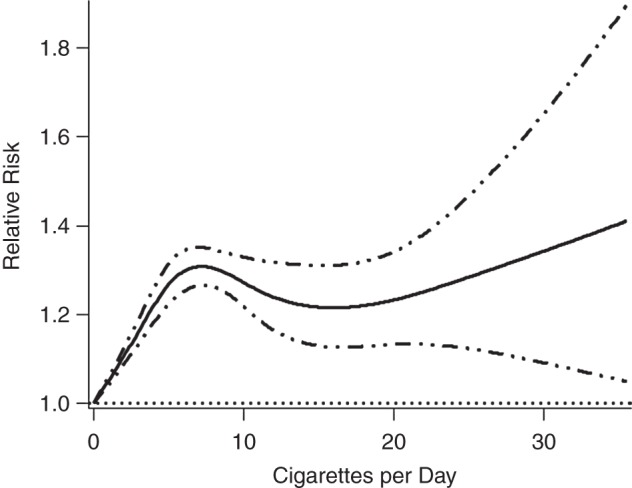
Relative risk of stillbirth according to number of cigarettes smoked per day during pregnancy, calculated using 22 published studies and 65 data points and excluding open-ended categories of cigarettes per day (1 study and 24 data points). Solid line, relative risk; dotted-dashed lines, 95% confidence interval.
Figure 3.
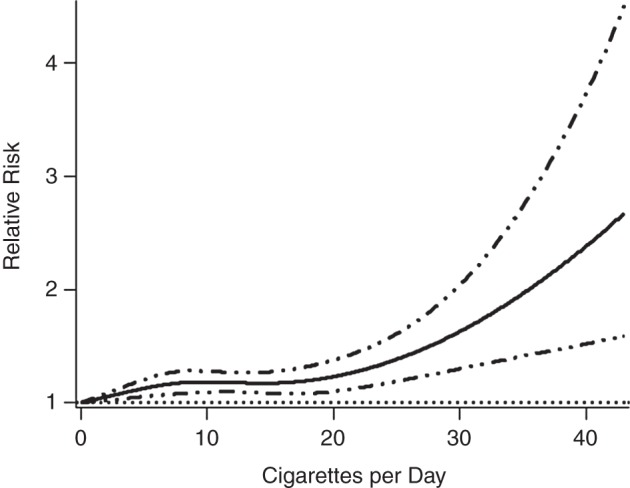
Relative risk of neonatal death according to number of cigarettes smoked per day during pregnancy, calculated using 10 studies and 32 data points and excluding open-ended categories of cigarettes per day (0 studies and 10 data points). Solid line, relative risk; dotted-dashed lines, 95% confidence interval.
Assessment of heterogeneity
Among studies that evaluated any active smoking, heterogeneity was highest among studies examining stillbirth (I2 = 67%, P < 0.0001), moderate among those examining perinatal death (I2 = 60%, P < 0.0001), and lower for those examining neonatal death (I2 = 39%, P < 0.05; see Tables 2–4 for detailed results). Not associated with the sRRs for any outcomes were year of article publication, midpoint year of the study pregnancies, smoking prevalence, perinatal death rate, per-capita cigarette consumption, study design, country of study, and outcome definition by gestational age.
Assessment of bias
Measures of risk of bias did not significantly affect the sRRs for any outcome (Tables 2–4). No studies analyzing any outcome met a priori criteria for lowest risk of bias, and appropriate adjustment for potential confounders was redefined as adjustment for at least maternal age and no adjustment for birth weight or for prior pregnancy loss (Tables 2–4). Publication bias was demonstrated by several formal statistical tests in analyses of any active smoking and perinatal death or stillbirth (P's < 0.05 for Egger's regression, Begg rank correlation, and trim-and-fill tests; Web Figures 5–7). For stillbirth, removing the 8 studies with the highest relative risks corrected the publication bias, and the sRR after elimination of these studies was 1.41 (95% CI: 1.34, 1.49). For perinatal death, removing the 3 studies with the highest relative risks corrected the publication bias, and the sRR after elimination of these studies was 1.30 (95% CI: 1.24, 1.37). The same tests did not demonstrate bias among studies used in analyses of any active smoking for neonatal death (P's > 0.1). Excluding the largest study from each dose-response analysis made the curves more linear but did not affect the per-cigarette relative risks (data not shown).
Maternal SHS exposure
Women who were exposed to SHS had an increased risk of stillbirth (sRR = 1.40, 95% CI: 1.06, 1.85 (7 studies); Web Figure 8). SHS was also associated with a significantly increased risk of perinatal death (sRR = 1.42, 95% CI: 1.10, 1.85 (2 studies)). The only study to analyze SHS exposure in relation to risk of neonatal death found that, compared with infants with nonsmoking parents, the adjusted relative risk of neonatal death was 1.16 when the father smoked 1–10 cigarettes per day (95% CI: 0.93, 1.45) and 1.53 when the father smoked more than 10 cigarettes per day (95% CI: 1.15, 2.04). Publication bias was found among the studies that analyzed stillbirth in relation to SHS exposure (P's < 0.05 for Egger's regression and Begg rank correlation; Web Figure 9). Removing the study with the largest relative risk corrected the publication bias, and the sRR after elimination of these studies was 1.37 (95% CI: 1.04, 1.82). Because of small numbers, publication bias was not examined among the studies that analyzed perinatal death or neonatal death in relation to SHS exposure.
DISCUSSION
The results of these meta-analyses support prior conclusions that maternal smoking is a cause of perinatal death (7).
Our review had several limitations reflecting the reliance on secondary data gathered from publications. First, we were unable to fully investigate the association of timing of smoke exposure across pregnancy with perinatal death risk. In the small group of studies examined, ex-smokers and women who quit smoking during pregnancy were not at increased risk, but the point at which the women quit in relation to pregnancy varied widely and was not always specified. Therefore, we still do not know the optimal time for pregnant women to quit smoking and lower the risk of perinatal death. Second, most articles included only categories of number of cigarettes smoked per day instead of the actual number of cigarettes smoked, which may vary from day to day. We used midpoints of these categories, which may not have accurately represented the number of cigarettes smoked per day. The imputation used for open-ended categories may also have created misclassified representations of the actual number of cigarettes smoked per day. This source of bias may have contributed to the unexpected finding that the relative risk of stillbirth increased with amount smoked only up to 15 cigarettes per day and then decreased to the null at 40 cigarettes per day when including open-ended categories. Another explanation is that very few women smoke heavily during pregnancy, and these women may have other unusual characteristics. Even among studies published in the 1960s, when 40%–50% of women smoked during pregnancy, only about 10%–15% of pregnant smokers reported smoking more than 20 cigarettes per day (35, 36).
Biases in study design are unlikely to fully account for the results. Grouping of the studies on the basis of study design and analysis methods in order to study variation by risk of bias showed that studies with high and low risks for confounding and misclassification of smoking gave similar sRRs. Studies with appropriate statistical considerations, such as analysis of only 1 pregnancy per woman and exclusion of twins, gave results similar to those of studies with potential nonindependence of outcomes. Publication bias among studies that examined active smoking or SHS and the risks of perinatal death and stillbirth may have inflated the sRRs. However, the results were robust to trim-and-fill adjustment for publication bias, so the effect of publication bias would have been minor. The stability of the results is underlined by their insensitivity to the inclusion of duplicated data despite large changes in the number of estimates included.
Active smoking and SHS exposure conferred similar risks of stillbirth (sRRs were 1.46 and 1.40, respectively). The similarity is not readily explained, given the monotonic dose-response relationship between number of cigarettes smoked daily by the mother and risk of stillbirth. An important caveat to the similarity is the difference in precision. The 95% confidence interval for active smoking was 1.38, 1.54 (n = 19,628,348), whereas that for SHS exposure was 1.06, 1.85 (n = 25,526). Another issue is the paucity of studies in the SHS analysis, giving us low power to detect publication bias.
This study had several strengths. We compiled 200 reports on the association between smoking and perinatal death. Many covariates were analyzed to examine heterogeneity and the potential for biased results. We did not select studies on the basis of quality, language, or database indexing, which allowed analysis by quality measures and reduced bias in study selection (37). Studies that had relative risks of perinatal death given for varied categories of cigarettes smoked per day were combined, allowing a quantitative and visual analysis of the dose-response relationships. We demonstrated that allowing only 1 publication per study for each analysis to prevent overrepresentation of highly used data sets did not affect the results; and publication bias, while present, did not significantly affect the results.
Overall, the evidence in our systematic review supports the established causal explanation for the association between smoking and perinatal death. There was general consistency of results, despite significant overall heterogeneity of the relative risks that was not explained by the examined covariates. The relative risks in the 19 subgroups for heterogeneity and risk of bias analyses were consistent, never varying more than 15% from the main results of 46%, 22%, and 33% increases among smokers in the risks of stillbirth, neonatal death, and perinatal death, respectively. The studies were carried out in 28 countries over a period of observation that spanned more than a half century. During this time, both the incidence of perinatal death and the prevalence of smoking declined significantly based on both the included studies (data not shown) and reports from the literature (38). In the included studies, the rate of perinatal death per 1,000 births ranged from 0.4 to 44, and the prevalence of smoking during pregnancy varied between 0.7% and 52% of women. Thus, the relative consistency of the results is remarkable given the variation in populations, study designs, and frequencies of perinatal death and smoking.
Smoking had a stronger association with the risk of stillbirth than with the risk of neonatal death. This was supported by the finding that the sRR for perinatal death (sRR = 1.33) was between the relative risks for stillbirth (sRR = 1.46) and neonatal death (sRR = 1.22). Postneonatal infant death is largely caused by sudden infant death syndrome, which has a strong correlation with maternal smoking during pregnancy (odds ratio = 2.25, 95% CI: 2.03, 2.50) (39). Why smoking confers higher relative risks of stillbirth and sudden infant death syndrome than of neonatal death is unclear.
Our findings for perinatal death are consistent with the dose-dependence of the well-known increased risk of low birth weight with smoking (11). However, though low birth weight is strongly associated with both smoking and perinatal mortality, whether it lies along the causal pathway linking maternal smoking to perinatal mortality is debated (6, 40). Smoking can lead to perinatal death through placental abruption, an uncommon but serious condition in which the placenta detaches from the uterus before delivery of the fetus, causing maternal hemorrhage and fetal asphyxia (41). Placentation abnormalities are thought to be responsible for abruption, but the mechanism by which smoking causes abruption is unknown, because findings regarding the effects of smoking on the placenta have been inconsistent (11).
This analysis of 200 articles confirmed that smokers have risk increases of 46%, 33%, and 22% for stillbirth, perinatal death, and neonatal death, respectively. The analyses of heterogeneity and risk of bias provided further evidence that these associations are probably causal. Given the few small studies of the relationship between SHS and perinatal death (leading to wide confidence intervals around the relative risk estimates), more work is needed to assess the association.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California (Beth L. Pineles, Sarah Hsu, Edward Park, Jonathan M. Samet).
All authors contributed equally to this work.
This project was supported by grant 5F30DA032190 (to B.L.P.) from the National Institute on Drug Abuse.
We thank Tiffany Chen for her help in obtaining the articles and study data.
The funding sources played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Drug Abuse.
Conflict of interest: none declared.
REFERENCES
- 1.Underwood P, Hester LL, Laffitte T Jr et al. The relationship of smoking to the outcome of pregnancy. Am J Obstet Gynecol. 1965;912:270–276. [DOI] [PubMed] [Google Scholar]
- 2.Downing GC, Chapman WE. Smoking and pregnancy—a statistical study of 5,659 patients. Calif Med. 1966;1043: 187. [PMC free article] [PubMed] [Google Scholar]
- 3.Comstock GW, Lundin FE Jr. Parental smoking and perinatal mortality. Am J Obstet Gynecol. 1967;985:708–718. [DOI] [PubMed] [Google Scholar]
- 4.Yerushalmy J. The relationship of parents’ cigarette smoking to outcome of pregnancy—implications as to the problem of inferring causation from observed associations. Am J Epidemiol. 1971;936:443–456. [DOI] [PubMed] [Google Scholar]
- 5.Terrin M, Meyer MB. Birth weight-specific rates as a bias in the effects of smoking and other perinatal hazards. Obstet Gynecol. 1981;585:636–638. [PubMed] [Google Scholar]
- 6.Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;16411:1115–1120. [DOI] [PubMed] [Google Scholar]
- 7.Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. Women and Smoking: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2001. [Google Scholar]
- 8.Public Health Service, US Department of Health, Education, and Welfare. The Health Consequences of Smoking: A Report of the Surgeon General. Washington, DC: US Department of Health, Education, and Welfare; 1971. [Google Scholar]
- 9.Office on Smoking and Health, Office of the Assistant Secretary for Health, Public Health Service, US Department of Health and Human Services. The Health Consequences of Smoking for Women: A Report of the Surgeon General. Washington, DC: US Department of Health and Human Services; 1980. [Google Scholar]
- 10.Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 11.Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2010. [PubMed] [Google Scholar]
- 12.Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;28315:2008–2012. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;67:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balk EM, Chung M, Chen ML et al. Data extraction from machine-translated versus original language randomized trial reports: a comparative study. Syst Rev. 2013;2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;422:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JPT et al. Introduction to Meta-Analysis. Vol. 1 (Statistics in Practice). Chichester, United Kingdom: John Wiley & Sons Ltd.; 2009. [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;73:177–188. [DOI] [PubMed] [Google Scholar]
- 19.Hertzmark E, Spiegelman D. The SAS METAANAL macro. Cambridge, MA: Harvard School of Public Health; 2012. http://www.hsph.harvard.edu/donna-spiegelman/software/metaanal/ Updated May 24, 2012. Accessed May 31, 2012. [Google Scholar]
- 20.Orsini N, Li R, Wolk A et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;1751:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li R, Spiegelman D. The SAS %METADOSE macro. Cambridge, MA: Harvard School of Public Health; 2010. http://www.hsph.harvard.edu/donna-spiegelman/software/metadose/ Updated August 26, 2010. Accessed May 31, 2012. [Google Scholar]
- 22.Burns DM, Major JM, Shanks TJ. Changes in number of cigarettes smoked per day: cross-sectional and birth cohort analyses using NHIS. In: Amarcher RH, Marcus SE, eds. Those Who Continue to Smoke: Is Achieving Abstinence From Smoking Harder and Do We Need to Change Our Interventions? Bethesda, MD: National Cancer Institute; 2003:83–99. [Google Scholar]
- 23.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. Fairfax, VA: George Mason University; 2010. http://mason.gmu.edu/~dwilsonb/ma.html Updated August 11, 2010. Accessed June 30, 2012. [Google Scholar]
- 24.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 25.Rendina-Gobioff G, Kromrey JD. PUB_BIAS: A SAS Macro for Detecting Publication Bias in Meta-Analysis. Tampa, FL: University of South Florida; 2006. http://analytics.ncsu.edu/sesug/2006/PO04_06.PDF Updated 2006. Accessed June 13, 2012. [Google Scholar]
- 26.Schramm WF. Smoking during pregnancy: Missouri longitudinal study. Paediatr Perinat Epidemiol. 1997;11(suppl 1):73–83. [DOI] [PubMed] [Google Scholar]
- 27.Hafez AS, Fahim HI, Badawy HA. Socioenvironmental predictors of abortion and stillbirths in an industrial community in Egypt. J Egypt Public Health Assoc. 2001;76(1-2):1–16. [PubMed] [Google Scholar]
- 28.Xu B, Järvelin MR, Rantakallio P. Maternal smoking in pregnancy and sex differences in perinatal death between boys and girls. Soc Biol. 1998;45(3-4):273–277. [DOI] [PubMed] [Google Scholar]
- 29.Rantakallio P. The effect of maternal smoking on birth weight and the subsequent health of the child. Early Hum Dev. 1978;24:371–382. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO Global InfoBase. NCD indicators: tobacco use prevalence [database] Geneva, Switzerland: World Health Organization; 2011. http://www.who.int/ncd_surveillance/infobase/web/InfoBaseCommon/ Updated January 20, 2011. Accessed August 16, 2012. [Google Scholar]
- 31.Guindon GE, Boisclair D. Past, Current and Future Trends in Tobacco Use. (Health, Nutrition and Population (HNP) Discussion Paper). (Economics of Tobacco Control paper no. 6) Geneva, Switzerland: Tobacco Free Initiative, World Health Organization; 2003:21–43. [Google Scholar]
- 32.La Vecchia C, Harris RE, Wynder EL. Comparative epidemiology of cancer between the United States and Italy. Cancer Res. 1988;4824:7285–7293. [PubMed] [Google Scholar]
- 33.National Center for Health Statistics. VitalStats [database] Atlanta, GA: Centers for Disease Control and Prevention; 2012. http://www.cdc.gov/nchs/vitalstats/index.htm. Accessed March 16, 2013. [Google Scholar]
- 34.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;85:551–561. [DOI] [PubMed] [Google Scholar]
- 35.Savel LE, Roth E. Effects of smoking in pregnancy: a continuing retrospective study. Obstet Gynecol. 1962;203:313–316. [PubMed] [Google Scholar]
- 36.Yerushalmy J. Mother's cigarette smoking and survival of infant. Am J Obstet Gynecol. 1964;88:505–518. [DOI] [PubMed] [Google Scholar]
- 37.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;3167124:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaudino JA, Hoyert DL, MacDorman MF et al. Fetal deaths. In: Wilcox LS, Marks JS, eds. From Data to Action: CDC's Public Health Surveillance for Women, Infants, and Children. Atlanta, GA: Centers for Disease Control and Prevention; 1994:163–178. [Google Scholar]
- 39.Zhang K, Wang X. Maternal smoking and increased risk of sudden infant death syndrome: a meta-analysis. Leg Med (Tokyo). 2013;153:115–121. [DOI] [PubMed] [Google Scholar]
- 40.Platt RW, Ananth CV, Kramer MS. Analysis of neonatal mortality: is standardizing for relative birth weight biased? BMC Pregnancy Childbirth. 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tikkanen M, Surcel HM, Bloigu A et al. Self-reported smoking habits and serum cotinine levels in women with placental abruption. Acta Obstet Gynecol Scand. 2010;8912:1538–1544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.