Abstract
In a population-based case-control study, we investigated whether familial confounding influenced the associations between maternal overweight/obesity and risks of stillbirth and infant mortality by including both population and sister controls. Using nationwide data from the Swedish Medical Birth Register (1992–2011), we included all primiparous women with singleton births who also had a sister with a first birth during that time period. We used logistic regression analyses to calculate odds ratios (and 95% confidence intervals) adjusted for maternal age, height, smoking habits, education, and time period (5-year groups) of child's birth. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Compared with population controls with a normal BMI (18.5–24.9), stillbirth risk increased with increasing BMI (BMI 25–29.9: odds ratio (OR) = 1.51 (95% confidence interval (CI): 1.21, 1.89); BMI 30–34.9: OR = 1.77 (95% CI: 1.24, 2.50); BMI ≥35: OR = 3.16 (95% CI: 2.10, 4.76)). The sister case-control analyses revealed similar results. Offspring of obese women (BMI ≥30) had an increased risk of infant mortality when population controls were used (OR = 2.41, 95% CI: 1.83, 3.16), and an even higher risk was obtained when sister controls were used (OR = 4.04, 95% CI: 2.25, 7.25). We conclude that obesity in early pregnancy is associated with increased risks of stillbirth and infant mortality independently of genetic and early environmental risk factors shared within families.
Keywords: body mass index, familial confounding, infant mortality, neonatal mortality, postneonatal mortality, sibling-design studies, stillbirth
Editor's note:An invited commentary on this article appears on page 106.
The body mass index (BMI; weight (kg)/height (m)2) of the general population has increased worldwide during recent decades, and in 2008 there were an estimated 300 million obese (BMI ≥30) women in the world (1). In Sweden, the prevalences of overweight (BMI 25–<30) and obesity (BMI ≥30) in early pregnancy (usually the first trimester) increased from 20% and 6%, respectively, in 1992 to 25% and 13%, respectively, in 2013 (2). In the United States in 2011–2012, 53% of gravid women were reported to be overweight or obese in early pregnancy (3).
Maternal obesity is associated with increased risks of gestational diabetes, large-for-gestational-age birth, preterm birth (<37 weeks’ gestation), and congenital malformations (4–8). Risks of stillbirth and infant mortality also increase with maternal overweight and obesity (9–13). However, the possible influence of genetic or early environmental factors has not been accounted for in previous studies.
Twin and family studies show that heritability plays a substantial role in determining BMI (14). Genetic factors may also influence risks of stillbirth and infant mortality, and there is a recurrence risk of stillbirth in successive pregnancies (15). Fetal growth restriction is one of the main causes of stillbirth (16), and there is a strong familial aggregation of small-for-gestational-age births (17, 18). Preterm birth is a major cause of infant mortality (9) and has been shown to be clustered in families (19, 20). Quantitative genetic analyses show that maternal genetic factors are of importance for preterm birth (21). Congenital anomalies are commonly seen in both stillbirths and infant mortality (22, 23) and may be caused by genetic factors (24).
Full sisters have approximately 50% of their genomes in common and are usually brought up together. Thus, results from a study of risks related to maternal BMI within sister pairs discordant for the outcome would be controlled for familial factors (i.e., shared genetic and early environmental factors) by design. In this population-based study, we investigated associations between BMI in early pregnancy and risks of stillbirth and infant mortality using 2 reference groups: population controls and sister controls.
METHODS
Data sources
In this study, data from nationwide Swedish registries were linked using the individually unique personal registration number assigned to all Swedish residents (25). The Swedish Medical Birth Register was established in 1973 and includes information collected prospectively during pregnancy, delivery, and the neonatal period (26). Information on early-pregnancy BMI has been included in the Register since 1992. The Cause of Death Register, which provides information on dates and causes of death in Sweden, was used to identify cases of infant mortality (27). Information about the mother's highest achieved educational level was retrieved from the Swedish Register of Education (28). The Multigeneration Register includes data on all subjects born in 1932 and later (index persons) and their first-degree relatives (parents, full and half siblings, and children) and was used to identify full sisters of mothers included in the Medical Birth Register (29).
Study population
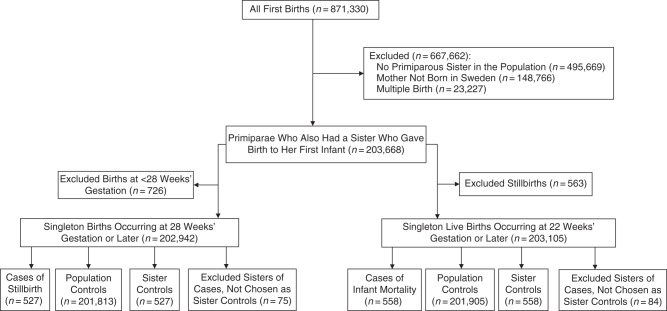
From 1992 through 2011, there were 2,038,185 births recorded in the Swedish Medical Birth Register. Because parity influences both BMI and rates of stillbirth and infant mortality (9, 30, 31), we included only births to primiparous mothers (n = 871,330). We excluded births to women without a primiparous sister (n = 495,669), births to women not born in Sweden (as their full sisters were less likely to be identified in the Multigeneration Register (n = 148,766)), and twin births (singletons and twins differ with respect to risk factors and rates of stillbirth and infant mortality (n = 23,227)). Consequently, the study population was restricted to 203,668 singleton births to primiparous women who also had a primiparous sister with a singleton birth between the beginning of 1992 and the end of 2011 (Figure 1).
Figure 1.
Selection of participants for a study of associations between early-pregnancy body mass index and risks of stillbirth and infant mortality using 2 reference groups: population controls and sister controls. The study population included all singleton first births taking place in Sweden during 1992–2011 to mothers born in Sweden who had a sister who also gave birth to her first child during that time period.
Outcomes
Stillbirth was defined as fetal death occurring at or after 28 completed weeks of gestation. In 2008, the definition of stillbirth in the Medical Birth Register was changed from 28 weeks or later to 22 weeks or later, and we decided to use the same stillbirth definition throughout the study period (i.e., intrauterine deaths occurring at ≥28 weeks). Therefore, in the analysis of stillbirth, we excluded 726 births (stillbirths and live births) that took place at 22–27 weeks’ gestation. Infant mortality was defined as the death of a liveborn infant during the first year of life. Analyses of infant mortality were based on all live births occurring from 22 completed weeks’ gestation onward; 563 stillbirths were excluded, of which 36 occurred at 22–27 weeks’ gestation (Figure 1). Neonatal mortality was defined as infant mortality occurring within the first 27 completed days after birth, and postneonatal mortality was defined as infant mortality occurring 28–364 completed days after birth.
Study design
We selected 2 control groups: population controls and sister controls, both having their first singleton birth during the study period. If a case mother had more than 1 suitable sister control, the sister who gave birth closest in time to the case mother was selected as the control (Figure 1). For the stillbirth case-control study, we identified 527 cases of stillbirth, 201,813 population controls, and 527 sister controls with live births at 28 weeks’ gestation or later (Figure 1). For the case-control analyses of infant mortality, we identified 558 cases of infant mortality, 201,905 population controls, and 558 sister controls for which infants in both control groups survived the first year of life. Of the 558 cases of infant mortality, there were 390 neonatal deaths and 168 postneonatal deaths.
Exposures
Information on maternal height and weight was collected at the first antenatal visit, which occurs within the first trimester for more than 90% of Swedish women (26). Height was self-reported, while weight was measured by the midwife. BMI was calculated as weight in kilograms divided by height in meters squared. The World Health Organization's classification was used to categorize BMI as underweight (BMI <18.5), normal-weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), obese grade 1 (BMI 30–34.9), or obese grade 2–3 (BMI ≥35) (32). Self-reported information on smoking in early pregnancy was also collected at the first antenatal visit. Information on maternal age at delivery was calculated from the mother's birth date (included in the personal registration number) and the date of delivery. Information on the mother's highest level of education attained (by December 31, 2011) was retrieved from the Education Register. Maternal age, height, smoking, and educational level and period of child's birth (in 5-year groups) were categorized as presented in Table 1.
Table 1.
Distribution of Maternal Characteristics for Cases of Stillbirth, Population Controls, and Sister Controls Among Singleton Births in Sweden, 1992–2011
| Stillbirth Cases |
Controls |
|||||
|---|---|---|---|---|---|---|
| Population |
Sisters |
|||||
| No. | % | No. | % | No. | % | |
| Total | 527 | 100.0 | 201,813 | 100.0 | 527 | 100.0 |
| Maternal body mass indexa | ||||||
| <18.5 | 14 | 2.7 | 4,675 | 2.3 | 15 | 2.9 |
| 18.5–24.9 | 244 | 46.3 | 120,962 | 59.9 | 308 | 58.4 |
| 25–29.9 | 120 | 22.8 | 37,398 | 18.5 | 102 | 19.4 |
| 30–34.9 | 38 | 7.2 | 10,198 | 5.1 | 24 | 4.6 |
| ≥35 | 26 | 4.9 | 3,832 | 1.9 | 13 | 2.5 |
| Missing data | 85 | 16.3 | 24,748 | 12.3 | 65 | 12.3 |
| Maternal age, years | ||||||
| ≤24 | 143 | 27.1 | 52,569 | 26.1 | 153 | 29.0 |
| 25–29 | 188 | 35.7 | 80,862 | 40.1 | 195 | 37.0 |
| 30–34 | 137 | 26.0 | 53,081 | 26.3 | 139 | 26.4 |
| ≥35 | 59 | 11.2 | 15,301 | 7.6 | 40 | 7.6 |
| Maternal height, cm | ||||||
| ≤154 | 14 | 2.7 | 2,712 | 1.3 | 6 | 1.1 |
| 155–164 | 171 | 32.5 | 59,112 | 29.3 | 173 | 32.8 |
| 165–174 | 246 | 46.7 | 105,518 | 52.3 | 262 | 49.7 |
| ≥175 | 46 | 8.7 | 21,326 | 10.6 | 56 | 10.6 |
| Missing data | 50 | 9.5 | 13,145 | 6.5 | 30 | 5.7 |
| Smoking in early pregnancy | ||||||
| Yes | 71 | 13.5 | 18,939 | 9.4 | 66 | 12.5 |
| No | 414 | 78.6 | 172,818 | 85.6 | 441 | 83.7 |
| Missing data | 42 | 8.0 | 10,056 | 5.0 | 20 | 3.8 |
| Education, years | ||||||
| ≤9 | 42 | 7.9 | 10,944 | 5.4 | 44 | 8.4 |
| 10–11 | 88 | 16.7 | 28,448 | 14.1 | 87 | 16.5 |
| 12 | 155 | 29.4 | 56,414 | 28.0 | 137 | 26.0 |
| 13–14 | 77 | 14.9 | 30,129 | 14.9 | 89 | 16.9 |
| ≥15 | 161 | 30.6 | 74,994 | 37.2 | 165 | 31.3 |
| Missing data | 4 | 0.8 | 884 | 0.4 | 5 | 1.0 |
| Time period of child's birth | ||||||
| 1992–1996 | 118 | 22.4 | 46,089 | 22.8 | 133 | 25.2 |
| 1997–2001 | 145 | 27.5 | 47,283 | 23.4 | 117 | 22.2 |
| 2002–2006 | 149 | 28.3 | 56,757 | 28.1 | 159 | 30.2 |
| 2007–2011 | 115 | 21.8 | 51,684 | 25.6 | 118 | 22.4 |
a Weight (kg)/height (m)2.
The study protocol was approved by the Research Ethics Committee at Karolinska Institutet (Stockholm, Sweden).
Statistical analyses
In order to control for familial confounding (i.e., genetic and family environmental factors), we compared results from case-control analyses using population controls and sister controls. If an association is seen using population controls but not when using sister controls, the association is confounded by familial factors. We used unconditional logistic regression for the population case-control analyses of associations between maternal BMI and risks of stillbirth and infant, neonatal, and postneonatal mortality. The dependency in the data (due to the fact that all mothers had a sister in the population) was handled with a generalized estimating equations model. In the analyses of case-control sister pairs, we used conditional logistic regression. Odds ratios and 95% confidence intervals were calculated, and results were adjusted for maternal age, height, smoking, education, and time period (5-year groups) of child's birth. In the analyses of infant mortality, there were too few sister controls in the obesity grade 2–3 category (BMI ≥35) for analysis (n = 8); therefore, all women with obesity (BMI ≥30) were collapsed into 1 category. In analyses of neonatal and postneonatal mortality, underweight mothers were excluded because of small numbers (7 cases of neonatal mortality and 5 cases of postneonatal mortality).
Because data on maternal BMI were missing for up to 19% of participants, we also performed analyses using multiple imputation. Missing values for BMI, maternal height, smoking, and education were imputed by means of ordinal logistic regression, using available information on maternal age, time period of child's birth, stillbirth, and/or infant mortality. Ten data sets were created and were analyzed with the same logistic regression models as in the complete-case analyses. Parameter estimates and covariance matrices were analyzed with the MIANALYZE procedure in SAS (SAS Institute, Inc., Cary, North Carolina). These estimates were used in sensitivity analyses, and the results were compared with those of the complete-case analyses (Figures 2 and 3).
Figure 2.
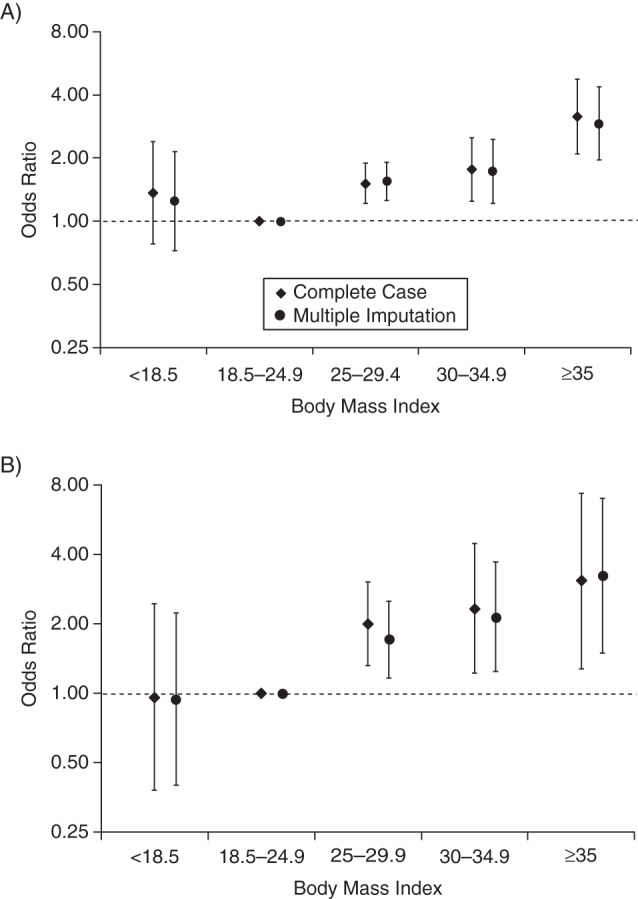
Risk of stillbirth in singleton first births according to early-pregnancy body mass index (weight (kg)/height (m)2) among mothers born in Sweden who gave birth during 1992–2011, derived using population controls (A) and sister controls (B). Odds ratios were estimated using complete covariate information (n = 174,199 in the population control analysis and n = 889 in the sister control analysis) and after multiple imputation of missing values. Odds ratios were adjusted for maternal age, maternal height, smoking, education, and time period (5-year groups) of child's birth. Bars, 95% confidence intervals.
Figure 3.
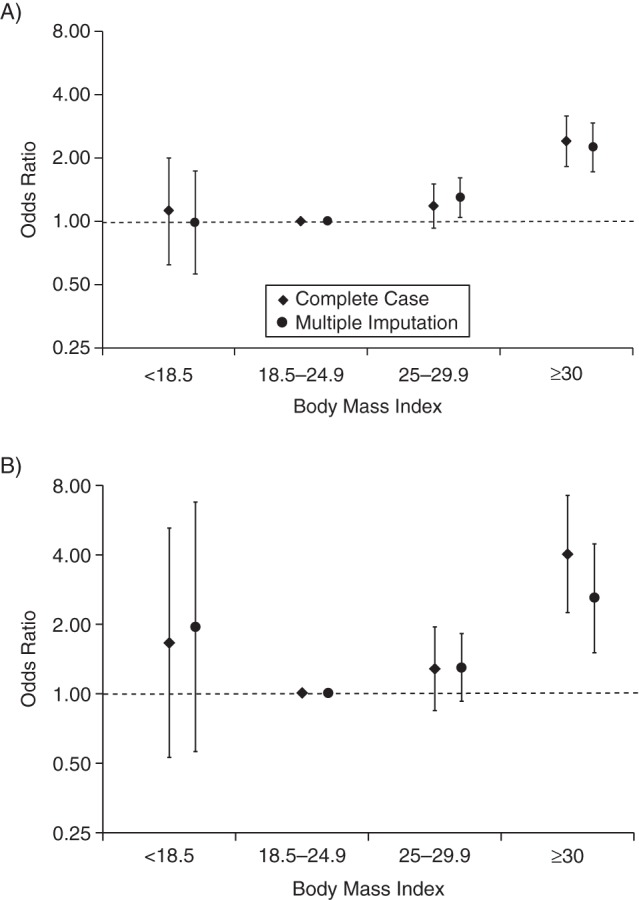
Risk of infant mortality in singleton first births according to early-pregnancy body mass index (weight (kg)/height (m)2) among mothers born in Sweden who gave birth during 1992–2011, derived using population controls (A) and sister controls (B). Odds ratios were estimated using complete covariate information (n = 174,141 in the population control analysis and n = 917 in the sister control analysis) and after multiple imputation of missing values. Odds ratios were adjusted for maternal age, maternal height, smoking, education, and time period (5-year groups) of child's birth. Bars, 95% confidence intervals.
We also performed trend tests with BMI included in the analyses as a continuous variable. P values for neonatal mortality and postneonatal mortality are presented. SAS software, version 9.4, was used for all analyses.
RESULTS
Among the stillbirth case mothers, rates of overweight (BMI 25–29.9), obesity grade 1 (BMI 30–34.9), and obesity grade 2–3 (BMI ≥35) were higher than corresponding rates among population controls and sister controls. The stillbirth case mothers were slightly older and were more often smokers than population controls and sister controls (Table 1).
Among the infant mortality case mothers, 17.6% were overweight and 12.9% were obese. Corresponding rates were 18.5% and 6.9%, respectively, in population control mothers and 20.6% and 7.0%, respectively, among sister control mothers (Appendix Table 1). Smoking during pregnancy was equally common among case mothers and their sisters (13%), as compared with 9.4% among population controls.
In adjusted analyses, we found positive associations between maternal BMI and stillbirth risks, both when using population controls (Figure 2A) and when using sister controls (Figure 2B). Compared with normal weight, the stillbirth risk increased with BMI in the population case-control analyses (overweight: odds ratio (OR) = 1.51 (95% confidence interval (CI): 1.21, 1.89); obesity grade 1: OR = 1.77 (95% CI: 1.24, 2.50); obesity grade 2–3: OR = 3.16 (95% CI: 2.10, 4.76)) (Figure 2A). In the sister case-control analyses, corresponding odds ratios and 95% confidence intervals were similar (overweight: OR = 2.00 (95% CI: 1.32, 3.03); obesity grade 1: OR = 2.33 (95% CI: 1.22, 4.46); obesity grade 2–3: OR = 3.08 (95% CI: 1.28, 7.40)) (Figure 2B). We found no statistically significant association between being underweight (BMI <18.5) in early pregnancy and stillbirth risk, either when using population controls (OR = 1.36, 95% CI: 0.78, 2.39) or when using sister controls (OR = 0.96, 95% CI: 0.38, 2.45). The estimates from the multiple imputation analyses were similar to those from the complete-case analyses (Figures 2A and 2B).
In analyses of BMI and infant mortality, we also found a similar risk pattern regardless of whether we used population controls (Figure 3A) or sister controls (Figure 3B) as the reference group. In the population case-control analyses, infants of obese women (BMI ≥30) had a more than doubled risk of infant death (OR = 2.41, 95% CI: 1.83, 3.16) compared with infants of normal-weight women. Infant mortality risk was not increased among infants of overweight women (OR = 1.18, 95% CI: 0.93, 1.50) or underweight women (OR = 1.12, 95% CI: 0.62, 2.00) (Figure 3A). In the sister case-control analyses, infants of obese women had a 4-fold higher risk of infant death (OR = 4.04, 95% CI: 2.25, 7.25) than infants of normal-weight women. The odds ratios for infant mortality were 1.29 (95% CI: 0.85, 1.95) for infants of overweight women and 1.67 (95% CI: 0.53, 5.24) for infants of underweight women (Figure 3B). The estimates from the multiple imputation analyses showed results similar to those of the complete-case analyses (Figure 3A and 3B).
Finally, we estimated risks of neonatal and postneonatal mortality, respectively (Table 2). Most infant deaths occurred during the neonatal period, and the association between maternal BMI and risk of neonatal mortality was similar to that in the analyses of BMI and infant mortality. Compared with infants of normal-weight mothers, the adjusted risk of neonatal mortality was higher in infants of obese mothers, both when using population controls (OR = 2.52, 95% CI: 1.83, 3.47) and when using sister controls (OR = 4.86, 95% CI: 2.33, 10.14). Compared with infants of normal-weight mothers, the risk of postneonatal mortality was doubled among infants of obese mothers in the crude analyses. In the adjusted analyses, although point estimates were similarly elevated, a statistically increased risk was achieved only when population controls were used (Table 2).
Table 2.
Maternal Body Mass Index in Early Pregnancy and Risks of Neonatal Mortality and Postneonatal Mortality Derived Using Both Population Controls and Sister Controls, Sweden, 1992–2011
| Population Control Analyses |
Sister Control Analyses |
|||||||
|---|---|---|---|---|---|---|---|---|
| Crude |
Adjusteda |
Crude |
Adjusteda |
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Neonatal Mortality b | ||||||||
| Maternal BMIc | ||||||||
| 18.5–24.9 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 25.0–29.9 | 1.23 | 0.93, 1.61 | 1.20 | 0.90, 1.59 | 1.17 | 0.73, 1.88 | 1.14 | 0.67, 1.96 |
| ≥30.0 | 2.35 | 1.72, 3.21 | 2.52 | 1.83, 3.47 | 4.00 | 2.05, 7.81 | 4.86 | 2.33, 10.14 |
| P for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Postneonatal Mortality d | ||||||||
| Maternal BMIe | ||||||||
| 18.5–24.9 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 25.0–29.9 | 1.08 | 0.70, 1.67 | 1.12 | 0.72, 1.75 | 1.53 | 0.80, 2.92 | 1.76 | 0.83, 3.74 |
| ≥30.0 | 2.24 | 1.38, 3.61 | 2.03 | 1.20, 3.42 | 2.45 | 1.02, 5.87 | 2.41 | 0.82, 7.09 |
| P for trend | 0.0006 | 0.0028 | 0.0068 | 0.0150 | ||||
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
a ORs were adjusted for maternal age, maternal height, smoking, education, and time period (5-year groups) of child's birth.
b Neonatal mortality was defined as death occurring during the first 27 completed days of life (n = 381 cases, n = 197,228 population controls, and n = 381 sister controls).
c BMI was defined as weight (kg)/height (m)2. Women with a BMI below 18.5 were excluded because of very low numbers of cases and sister controls (n = 7 cases, n = 2 sister controls, and n = 4,677 population controls).
d Postneonatal mortality was defined as death occurring within the first year of life, after the first 27 completed days of life (n = 160 cases, n = 197,228 population controls, and n = 160 sister controls).
e Women with a BMI below 18.5 were excluded because of very low numbers among cases and sister controls (n = 5 cases, n = 5 sister controls, and n = 4,677 population controls).
DISCUSSION
In this large population-based study, we found that risk of stillbirth increased with overweight and increasing obesity, both when using population controls as the reference group and when using sister controls. Moreover, in the analyses of infant mortality, we found that obesity but not overweight in early pregnancy was associated with increased infant mortality risk irrespective of control group. These findings indicate that the associations between BMI and stillbirth and infant mortality are not confounded by familial factors.
Many earlier studies found an association between maternal overweight/obesity and risks of stillbirth and infant mortality (9, 10, 16, 33). In a large meta-analysis, a dose-response association between BMI and stillbirth was shown, with an increased relative risk of 24% per 5-unit increase in BMI (34). Maternal weight gain between the first and second pregnancies also increases risks of stillbirth and infant mortality in secondborn offspring (31, 35). Weight loss in overweight or obese women during pregnancy seems to decrease the risk of gestational diabetes, large-for-gestational-age birth, and neonatal mortality (31, 35, 36). These studies strengthen the possibility of a causal relationship between maternal overweight/obesity and risks of stillbirth and infant mortality.
The mechanisms behind the associations between maternal BMI above the normal range and stillbirth and infant mortality are probably multifactorial. Inflammation and infection have been associated with stillbirth, and obesity may contribute to dysregulation of inflammatory responses and increase the risk of infection, leading to fetal loss (37). Adipose tissue in obese individuals mainly releases proinflammatory cytokines (e.g., tumor necrosis factor α, interleukin-6, leptin), while adipose tissue in lean individuals secretes antiinflammatory adipokines (adiponectin, transforming growth factor β, interleukin-10) (38). Histopathological investigations of placentas have shown a higher prevalence of both acute and chronic placental inflammation among stillbirths (39, 40). Genetic studies have shown an association between stillbirth and heritable thrombophilias. One example is a common association between stillbirth, fetal growth restriction, and placental abruption and 4G/4G homozygosity for the hypofibrinolytic plasminogen activator inhibitor type 1 gene (PAI-1) (41, 42). Proinflammatory cytokines may also be responsible for the obesity-related increased risk of spontaneous extremely preterm birth, which is a main cause of infant mortality (5, 9). Further, maternal obesity increases the risk of preeclampsia, which may increase risks of stillbirth and infant mortality through fetal growth restriction and preterm birth (43, 44). Obesity is also a risk factor for gestational diabetes, which increases risks of antepartum stillbirth (45), large-for-gestational-age birth, and neonatal infant mortality due to asphyxia (9, 46, 47). Moreover, congenital anomalies, which may cause stillbirth and infant mortality, are also more common in pregnancies of obese women (7, 9, 23).
Strengths of this study include the large population-based study base. The study base included more than 870,000 births to primiparous women, recorded in reliable health registries. The subsidized health-care system in Sweden gives all mothers equal access to antenatal and delivery care free of charge. We were able to perform linkage between sisters’ data using individually unique Swedish personal registration numbers and the Multigeneration Register. We were also able to control for additional important confounding factors, such as smoking, education, and maternal age.
Limitations of this study included a lack of statistical power to use finer BMI categories in the analyses of infant mortality and neonatal and postneonatal mortality. The mothers included in our study might have had a more stable family background than the general population, as we only included full sisters who gave birth during a limited time period. However, the same inclusion criteria were applied to both sibling and population controls, and our focus was to compare associations between maternal BMI and stillbirth and infant mortality when cases were compared with either sibling or population controls, rather than generalizability. Maternal BMIs were calculated from self-assessed height and from weight measured at the first antenatal care visit. Self-assessed height tends to be overestimated (48), and differences in time of weight measurement in early pregnancy might under- or overestimate BMI in early pregnancy. However, these data were recorded prospectively and would be unlikely to lead to systematic misclassification of BMI between cases and controls. We had up to 19% missing information on BMI in our data, and the rates of missing values were higher among cases than among sister or population controls. We used multiple imputation to assess the risk of selection bias due to missing data, and found that the estimates from the multiple imputations were similar to those from the complete-case analyses (Figures 2 and 3).
Although the sibling case-control study design controls for factors shared by the siblings, including genetic and early environmental factors, it may be sensitive to bias due to nonshared confounding (49). Moreover, if the sibling pairs differ more with regard to the confounders than with regard to the exposure of interest, a spurious association due to nonshared confounding bias may occur. In our study, the largest differences between the sisters were seen in BMI and not in the other confounders (Table 1 and Appendix Table 1), so there seems little reason to assume the presence of confounding bias. However, this does not include unmeasured confounding bias.
In conclusion, we found that obesity in early pregnancy is associated with increased risks of stillbirth and infant mortality independently of genetic and early environmental risk factors shared within families.
ACKNOWLEDGMENTS
Author affiliations: Clinical Epidemiology Unit, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden (Anna Lindam, Stefan Johansson, Olof Stephansson, Anna-Karin Wikström, Sven Cnattingius); Department of Clinical Science and Education, Södersjukhuset, Karolinska Institutet, Stockholm, Sweden (Stefan Johansson); Division of Epidemiology, School of Public Health, University of California, Berkeley, Berkeley, California (Olof Stephansson); and Department of Women's and Children's Health, Faculty of Medicine, Uppsala University, Uppsala, Sweden (Anna-Karin Wikström).
Financial support was provided by the Swedish Research Council for Health, Working Life and Welfare (grant 2014-0073) and a Distinguished Professor Award to S.C. from the Karolinska Institutet (grant 2368/10-221).
Parts of this study were orally presented at the 28th Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Denver, Colorado, June 15–16, 2015.
Conflict of interest: none declared.
Appendix Table 1.
Distribution of Maternal Characteristics for Cases of Infant Mortality, Population Controls, and Sister Controls Among Singleton Births in Sweden, 1992–2011
| Infant Mortality Cases |
Controls |
|||||
|---|---|---|---|---|---|---|
| Population |
Sisters |
|||||
| No. | % | No. | % | No. | % | |
| Total | 558 | 100.0 | 201,905 | 100 | 558 | 100 |
| Maternal body mass indexa | ||||||
| <18.5 | 12 | 2.2 | 4,677 | 2.3 | 7 | 1.3 |
| 18.5–24.9 | 268 | 48.0 | 120,918 | 59.9 | 335 | 60.0 |
| 25–29.9 | 98 | 17.6 | 37,389 | 18.5 | 115 | 20.6 |
| ≥30 | 72 | 12.9 | 14,017 | 6.9 | 39 | 7.0 |
| Missing data | 108 | 19.4 | 24,904 | 12.3 | 62 | 11.1 |
| Maternal age, years | ||||||
| ≤24 | 183 | 32.8 | 52,582 | 26.0 | 174 | 31.2 |
| 25–29 | 201 | 36.0 | 80,872 | 40.1 | 213 | 38.2 |
| 30–34 | 127 | 22.8 | 53,134 | 26.3 | 122 | 21.9 |
| ≥35 | 47 | 8.4 | 15,317 | 7.6 | 49 | 8.8 |
| Maternal height, cm | ||||||
| ≤154 | 7 | 1.3 | 2,714 | 1.3 | 12 | 2.2 |
| 155–164 | 185 | 33.2 | 59,118 | 29.3 | 161 | 28.9 |
| 165–174 | 241 | 43.2 | 105,463 | 52.2 | 292 | 52.3 |
| ≥175 | 48 | 8.6 | 21,314 | 10.6 | 57 | 10.2 |
| Missing data | 77 | 13.8 | 13,296 | 6.6 | 36 | 6.5 |
| Smoking in early pregnancy | ||||||
| Yes | 74 | 13.3 | 18,916 | 9.4 | 75 | 13.4 |
| No | 416 | 74.6 | 172,783 | 85.6 | 457 | 81.9 |
| Missing data | 68 | 12.2 | 10,206 | 5.1 | 26 | 4.7 |
| Education, years | ||||||
| ≤9 | 48 | 8.6 | 10,928 | 5.4 | 58 | 10.4 |
| 10–11 | 106 | 19.0 | 28,472 | 14.1 | 87 | 15.6 |
| 12 | 155 | 27.8 | 56,453 | 28.0 | 152 | 27.2 |
| 13–14 | 68 | 12.2 | 30,168 | 14.9 | 83 | 14.9 |
| ≥15 | 174 | 31.2 | 75,002 | 37.2 | 173 | 31.0 |
| Missing data | 7 | 1.3 | 882 | 0.4 | 5 | 0.9 |
| Time period of child's birth | ||||||
| 1992–1996 | 176 | 31.5 | 46,073 | 22.8 | 145 | 26.0 |
| 1997–2001 | 128 | 22.9 | 47,301 | 23.4 | 143 | 25.6 |
| 2002–2006 | 147 | 26.3 | 56,798 | 28.1 | 144 | 25.8 |
| 2007–2011 | 107 | 19.2 | 51,733 | 25.6 | 126 | 22.6 |
a Weight (kg)/height (m)2.
REFERENCES
- 1. Finucane MM, Stevens GA, Cowan MJ et al. . National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;3779765:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Board of Health and Welfare. Pregnancies, deliveries and newborn infants. The Swedish Medical Birth Register 1973–2013. Assisted reproduction, treatment 1991–2012 [in Swedish]. http://www.socialstyrelsen.se/publikationer2014/2014-12-19/Sidor/default.aspx Published 2014. Accessed January 8, 2016.
- 3. Dalenius K, Brindley P, Smith B et al. . Pregnancy Nutrition Surveillance 2010 Report. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2012. http://www.cdc.gov/pednss/pdfs/2010-PNSS-Summary-Report-Text%20File.pdf Accessed January 8, 2016. [Google Scholar]
- 4. Berntorp K, Anderberg E, Claesson R et al. . The relative importance of maternal body mass index and glucose levels for prediction of large-for-gestational-age births. BMC Pregnancy Childbirth. 2015;15:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cnattingius S, Villamor E, Johansson S et al. . Maternal obesity and risk of preterm delivery. JAMA. 2013;30922:2362–2370. [DOI] [PubMed] [Google Scholar]
- 6. Singh J, Huang CC, Driggers RW et al. . The impact of pre-pregnancy body mass index on the risk of gestational diabetes. J Matern Fetal Neonatal Med. 2012;251:5–10. [DOI] [PubMed] [Google Scholar]
- 7. Stothard KJ, Tennant PW, Bell R et al. . Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;3016:636–650. [DOI] [PubMed] [Google Scholar]
- 8. Surkan PJ, Hsieh CC, Johansson AL et al. . Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;1044:720–726. [DOI] [PubMed] [Google Scholar]
- 9. Johansson S, Villamor E, Altman M et al. . Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden. BMJ. 2014;349:g6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cnattingius S, Bergström R, Lipworth L et al. . Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;3383:147–152. [DOI] [PubMed] [Google Scholar]
- 11. Chen A, Feresu SA, Fernandez C et al. . Maternal obesity and the risk of infant death in the United States. Epidemiology. 2009;201:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tennant PW, Rankin J, Bell R. Maternal body mass index and the risk of fetal and infant death: a cohort study from the North of England. Hum Reprod. 2011;266:1501–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kristensen J, Vestergaard M, Wisborg K et al. . Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;1124:403–408. [DOI] [PubMed] [Google Scholar]
- 14. Elks CE, den Hoed M, Zhao JH et al. . Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne). 2012;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhattacharya S, Prescott GJ, Black M et al. . Recurrence risk of stillbirth in a second pregnancy. BJOG. 2010;11710:1243–1247. [DOI] [PubMed] [Google Scholar]
- 16. Flenady V, Koopmans L, Middleton P et al. . Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;3779774:1331–1340. [DOI] [PubMed] [Google Scholar]
- 17. Svensson AC, Pawitan Y, Cnattingius S et al. . Familial aggregation of small-for-gestational-age births: the importance of fetal genetic effects. Am J Obstet Gynecol. 2006;1942:475–479. [DOI] [PubMed] [Google Scholar]
- 18. Selling KE, Carstensen J, Finnström O et al. . Intergenerational effects of preterm birth and reduced intrauterine growth: a population-based study of Swedish mother-offspring pairs. BJOG. 2006;1134:430–440. [DOI] [PubMed] [Google Scholar]
- 19. Klebanoff MA, Schulsinger C, Mednick BR et al. . Preterm and small-for-gestational-age birth across generations. Am J Obstet Gynecol. 1997;1763:521–526. [DOI] [PubMed] [Google Scholar]
- 20. Porter TF, Fraser AM, Hunter CY et al. . The risk of preterm birth across generations. Obstet Gynecol. 1997;901:63–67. [DOI] [PubMed] [Google Scholar]
- 21. Svensson AC, Sandin S, Cnattingius S et al. . Maternal effects for preterm birth: a genetic epidemiologic study of 630,000 families. Am J Epidemiol. 2009;17011:1365–1372. [DOI] [PubMed] [Google Scholar]
- 22. Hobbs CA, Chowdhury S, Cleves MA et al. . Genetic epidemiology and nonsyndromic structural birth defects: from candidate genes to epigenetics. JAMA Pediatr. 2014;1684:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith GC, Fretts RC. Stillbirth. Lancet. 2007;3709600:1715–1725. [DOI] [PubMed] [Google Scholar]
- 24. Lozić B, Krželj V, Kuzmić-Prusac I et al. . The OSR1 rs12329305 polymorphism contributes to the development of congenital malformations in cases of stillborn/neonatal death. Med Sci Monit. 2014;20:1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU et al. . The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;2411:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Board of Health and Welfare. The Swedish Medical Birth Register—A Summary of Content and Quality. Stockholm, Sweden: National Board of Health and Welfare; 2003. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/10655/2003-112-3_20031123.pdf Accessed January 8, 2016. [Google Scholar]
- 27. National Board of Health and Welfare. Causes of death 2012 [in Swedish] http://www.socialstyrelsen.se/publikationer2013/2013-8-6 Published 2013. Accessed January 8, 2016.
- 28. Statistics Sweden. Evaluation of the Swedish Register of Education [in Swedish] Stockholm, Sweden: Statistics Sweden; 2006. http://www.scb.se/statistik/_publikationer/BE9999_2006A01_BR_BE96ST0604.pdf Accessed January 8, 2016. [Google Scholar]
- 29. Statistics Sweden. Multi-Generation Register 2009. A Description of Contents and Quality. Stockholm, Sweden: Statistics Sweden; 2010. http://www.scb.se/statistik/_publikationer/BE9999_2009A01_BR_BE96BR1003.pdf Accessed January 8, 2016. [Google Scholar]
- 30. Waldenström U, Cnattingius S, Norman M et al. . Advanced maternal age and stillbirth risk in nulliparous and parous women. Obstet Gynecol. 2015;1262:355–362. [DOI] [PubMed] [Google Scholar]
- 31. Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;3689542:1164–1170. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization. Global Database on Body Mass Index. An interactive surveillance tool for monitoring nutrition transition. http://apps.who.int/bmi/index.jsp?introPage=intro.html Published 2006. Updated January 8, 2016. Accessed January 8, 2016.
- 33. Meehan S, Beck CR, Mair-Jenkins J et al. . Maternal obesity and infant mortality: a meta-analysis. Pediatrics. 2014;1335:863–871. [DOI] [PubMed] [Google Scholar]
- 34. Aune D, Saugstad OD, Henriksen T et al. . Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;31115:1536–1546. [DOI] [PubMed] [Google Scholar]
- 35. Cnattingius S, Villamor E. Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet. 2016;38710018:558–565. [DOI] [PubMed] [Google Scholar]
- 36. Bogaerts A, Ameye L, Martens E et al. . Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstet Gynecol. 2015;1253:566–575. [DOI] [PubMed] [Google Scholar]
- 37. Blackwell C. The role of infection and inflammation in stillbirths: parallels with SIDS? Front Immunol. 2015;6:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;22:139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Varli IH, Petersson K, Kublickas M et al. . Both acute and chronic placental inflammation are overrepresented in term stillbirths: a case-control study. Infect Dis Obstet Gynecol. 2012;2012:293867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pinar H, Goldenberg RL, Koch MA et al. . Placental findings in singleton stillbirths. Obstet Gynecol. 2014;1232:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ernst LM, Rand CM, Bao R et al. . Stillbirth: genome-wide copy number variation profiling in archived placental umbilical cord samples with pathologic and clinical correlation. Placenta. 2015;368:783–789. [DOI] [PubMed] [Google Scholar]
- 42. Glueck CJ, Phillips H, Cameron D et al. . The 4G/4G polymorphism of the hypofibrinolytic plasminogen activator inhibitor type 1 gene: an independent risk factor for serious pregnancy complications. Metabolism. 2000;497:845–852. [DOI] [PubMed] [Google Scholar]
- 43. Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;1122:359–372. [DOI] [PubMed] [Google Scholar]
- 44. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;3659461:785–799. [DOI] [PubMed] [Google Scholar]
- 45. Stephansson O, Dickman PW, Johansson A et al. . Maternal weight, pregnancy weight gain, and the risk of antepartum stillbirth. Am J Obstet Gynecol. 2001;1843:463–469. [DOI] [PubMed] [Google Scholar]
- 46. Kaaja R, Rönnemaa T. Gestational diabetes: pathogenesis and consequences to mother and offspring. Rev Diab Studies. 2008;54:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Persson M, Johansson S, Villamor E et al. . Maternal overweight and obesity and risks of severe birth-asphyxia-related complications in term infants: a population-based cohort study in Sweden. PLoS Med. 2014;115:e1001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;112:137–144. [DOI] [PubMed] [Google Scholar]
- 49. Frisell T, Öberg S, Kuja-Halkola R et al. . Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;235:713–720. [DOI] [PubMed] [Google Scholar]