Abstract
Neuronal elements distributed throughout the cardiac nervous system, from the level of the insular cortex to the intrinsic cardiac nervous system, are in constant communication with one another to ensure that cardiac output matches the dynamic process of regional blood flow demand. Neural elements in their various ‘levels’ become differentially recruited in the transduction of sensory inputs arising from the heart, major vessels, other visceral organs and somatic structures to optimize neuronal coordination of regional cardiac function. This White Paper will review the relevant aspects of the structural and functional organization for autonomic control of the heart in normal conditions, how these systems remodel/adapt during cardiac disease, and finally how such knowledge can be leveraged in the evolving realm of autonomic regulation therapy for cardiac therapeutics.
Abbreviations
- AF
atrial fibrillation
- ART
autonomic regulatory therapy
- CHF
congestive heart failure
- DRG
dorsal root ganglia
- HF
heart failure
- LV
left ventricle
- MI
myocardial infarction
- NTS
nucleus of the solitary tract
- SCS
spinal cord stimulation
- TRPV1
transient receptor potential vanilloid 1
- VNS
vagus nerve stimulation
Structural and functional organization of the cardiac nervous system: afferent signalling
Cardiac afferent neurons
Cardiac afferent neurons have been classified as being (i) mechanosensory, (ii) chemosensory or (iii) multimodal (transducing both modalities) in nature (Thoren et al. 1976; Thoren, 1977; Brown, 1979; Malliani & Lombardi, 1982; Foreman, 1999; Thompson et al. 2000; Kember et al. 2001; Armour, 2004; Fu & Longhurst, 2009). Afferent neuronal somata associated with sensory neurites in atrial, ventricular and intrathoracic major intravascular tissues are located in nodose and dorsal root ganglia (DRG) (Vance & Bowker, 1983; Armour et al. 1994; Hoover et al. 2008), as well as in intrathoracic extracardiac (Armour, 1983, 1986 a) and intrinsic cardiac (Ardell et al. 1991; Beaumont et al. 2013) ganglia (Figure 1). Mechanosensory neurons are also associated with neurites embedded in the carotid sinus and thoracic aorta (Kirchheim, 1976; Zucker & Gilmore, 1991; Chapleau et al. 2001; Andresen et al. 2004). The sensory neurites associated with these somata transduce their local mechanical and/or chemical milieu in a differential manner, depending on the cardiac region transduced and the location of the ganglion in which their somata reside (Armour & Kember, 2004).
Figure 1. Network interactions occurring within and between peripheral ganglia and the central nervous system for control of the heart .
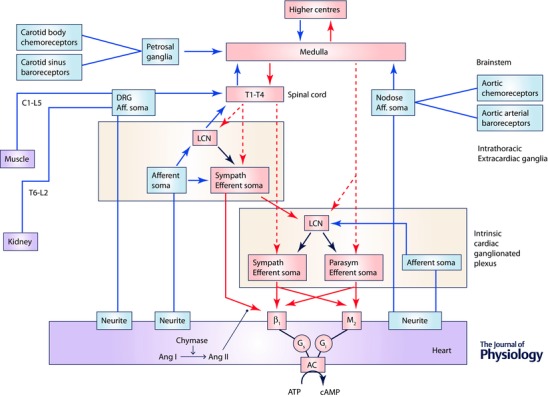
The cardiac nervous system is composed of multiple (distributed) processing centres from which independent and interdependent neural feedback and feed‐forward neural circuits interact to control regional cardiac electrical and mechanical function. Afferent projections are indicated in blue and efferent projections in red (dashed lines, preganglionic; continuous lines, postganglionic). The intrinsic cardiac nervous system (ICNS) possesses sympathetic (Sympath) and parasympathetic (Parasym) efferent postganglionic neurons, local circuit neurons (LCN) and afferent neurons. Extracardiac intrathoracic ganglia contain afferent neurons, LCN and sympathetic efferent postganglionic neurons. Neurons in intrinsic cardiac and extracardiac networks form nested feedback loops that act in concert with CNS feedback loops (spinal cord, brainstem, hypothalamus and forebrain) to coordinate cardiac function on a beat to beat basis. These systems demonstrate plasticity which underlies adaptations to acute and chronic stressors. Ang I, angiotensin I; Ang II, angiotensin II; AC, adenylate cyclase.
In the heart, a dense network of both myelinated and non‐myelinated sensory fibres project to somata in the DRG of the thoracic spinal cord. These endings respond to many substances including hydrogen ions (Uchida & Murao, 1975), potassium (Uchida & Murao, 1975), bradykinin (Uchida & Murao, 1974), oxygen radicals (Ustinova & Schultz, 1994 a; Huang et al. 1995), adenosine (Arora & Armour, 2003), ATP (Katchanov et al. 1996) and arachidonic acid metabolites (Staszewska‐Barczak, 1983; Nerdrum et al. 1986; Sun et al. 2001; Fu et al. 2008). While single fibre recordings from afferents entering the thoracic DRG suggest that most of these endings are chemically sensitive,they may also respond to mechanical deformation, especially during intense ventricular contraction (Malliani et al. 1983). Studies by Zipes and coworkers (Inoue et al. 1988; Ito & Zipes, 1994) have demonstrated that one can interrupt transmission via these fibres to abrogate reflex responses to bradykinin and nicotine by painting solutions of phenol on the surface of the heart, especially around the atrioventricular groove. Although the detailed distribution of these endings has not been elucidated, studies by Zipes, along with Zucker suggest that at least a large proportion of these fibres run near the surface of the left ventricle (Brandle et al. 1994; Zucker et al. 1995 a,b). The use of new techniques such as CLARITY (Chung & Deisseroth, 2013) may help in the composition of a comprehensive cardiac map of the distribution and anatomical relationships of these nerve endings to other structures in the myocardium. CLARITY is a method for making tissue transparent using acrylamide‐based hydrogels (Chung & Deisseroth, 2013). When used in conjunction with antibody labelling, CLARITY allows for highly detailed characterization of 3‐D organization of heart and its innervation.
While some cardiac afferent somata reside in nodose and thoracic DRG, others are located in intrathoracic extracardiac and intrinsic cardiac ganglia (Armour & Ardell, 1994, 2004; Armour, 2008) suggesting a potential role for peripheral cardiocentric modulation of efferent autonomic neurons by local circuit neurons therein. Because their cardiac sensory endings transduce a variety of chemicals, including various neuropeptides (e.g. substance P, bradykinin and calcitonin gene related peptide), such primary afferents can also participate in ‘axon reflexes’ that initiate local inflammatory, vascular and permeability changes in the heart (Franco‐Cereceda et al. 1993; White et al. 1993; Yaoita et al. 1994). While chronic activation of such sensory endings in cardiovascular disease states may not necessarily evoke symptoms (Foreman, 1999; Foreman et al. 2004), they may be important in initiating cardiac remodelling via a pro‐inflammatory mechanism (Wang et al. 2014; Jänig, 2014 a).
Glutamatergic transmission communicates information from the primary DRG sensory fibres arising in the heart to the dorsal horn of the thoracic spinal neurons (Foreman, 1999; Oliveira et al. 2003; Armour & Kember, 2004). This signalling can be importantly supplemented by the release of substance P, especially during cardiac stress (Hua et al. 2004; Ding et al. 2008 a). Experimental studies have shown that occlusion of the coronary artery increased the release of substance P located in the superficial dorsal laminae I and II and deeper laminae including laminae III–VII, a change that was sustained for the duration of the occlusion (Hua et al. 2004). Furthermore, increased release of substance P during coronary artery occlusion was eliminated after bilaterally transecting the T2–T5 dorsal roots. Furthermore, molecular and morphological profiles have shown that substance P and preprotachykinin mRNA were upregulated in the T1–T5 dorsal horn during occlusion of the coronary artery (Guo et al. 2007), an effect that in part requires the involvement of transient receptor potential vanilloid 1 (TRPV1) receptors (Steagall et al. 2012). Thus, these data provide evidence that substance P released in the spinal cord dorsal horn is a key neuropeptide mediator that corresponds with the increased transmission of nociceptive information that results from myocardial ischaemia and is partially dependent on a TRPV1 mechanism.
The central pathways by which cardiac sympathetic afferent neurons participate in both pain and sympathetic responses are probably more complex than is appreciated currently. However, we do know that they ascend, in part, via the dorsal columns, spinothalamic and spinoreticular tracts to cortical terminations (Fig. 2) (Foreman, 1991, 1999). Along this path they project to mid‐ and hindbrain autonomic integrative areas. Recent studies have shown that cardiac afferent neurons influence neuronal activity in the paraventricular nucleus (Reddy et al. 2005; Affleck et al. 2012; Xu et al. 2013) and nucleus of the solitary tract (NTS) (Wang et al. 2006, 2007). These neuronal collections are ideally suited to modulate multiple cardiovascular reflexes, such as the arterial baroreflex and the chemoreflex (Reddy et al. 2005; Gao et al. 2007; Wang et al. 2007; Chen et al. 2015). As depicted below, activation of these endings during coronary ischaemia probably initiates cardiac sympathetic efferent reflexes that evoke lethal arrhythmias (Fukuda et al. 2015), an adverse response that can be mitigated by thoracic dorsal rhizotomy (Schwartz et al. 1976) or stellectomy (Bourke et al. 2010; Vaseghi et al. 2014). Activation of these cardiac afferents likewise is central to triggering neural remodelling associated with heart failure (HF) (Zucker et al. 2012; Wang et al. 2014). Neural remodelling is defined herein as induced changes in neuronal morphology, interconnectivity, phenotype and/or plasticity indicative of changes in active and/or passive membrane electrical properties on soma, dendrites and synapses.
Figure 2. A schematic diagram of cardiac sympathetic afferents (T1–T6) transmitting nociceptive information in response to myocardial ischaemia that is relayed to ascending pathways terminating in supraspinal nuclei that participate in the signalling of cardiac pain .
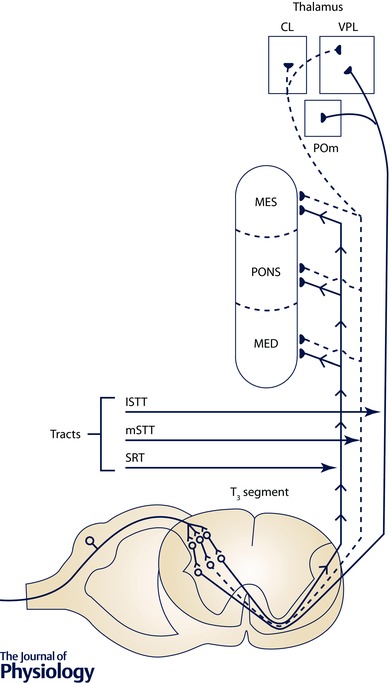
The spinoreticular tract (SRT), lateral spinothalamic tract (lSTT) and medial spinothalamic tract (mSTT) ascend to the reticular formation (medulla (MED), PONS, mesencephalon (MES)) and the ventral posterior lateral (VPL), central lateral (CL) and medial posterior (POm) nuclei of the thalamus. The cardiac sympathetic afferents also mediate sympatho‐excitatory responses when stimulated by a variety of substances. Adapted from Foreman (1991) with permission.
Arterial baroreceptors
The arterial baroreflex is fundamental to dynamic regulation of blood pressure, both in response to everyday environmental stressors and for overall homeostasis (Kirchheim, 1976; Jänig, 2006; Sleight, 2014). Mechanosensory neurites, embedded in the outer walls of the aorta and carotid arteries, transduce local wall stretch. Their grouped firing yields activity patterns that sensitively track local arterial wall dynamics as reflective of arterial pressure waves (Kirchheim, 1976; Chapleau et al. 1995; Andresen et al. 2004). The larger myelinated fibres preferentially transduce dynamic changes in blood pressure; the small myelinated and C‐fibres provide critical input on baseline levels of pressure (Seagard et al. 1990). This baroreceptor information is carried to the brainstem by central projections to evoke integrated reflex responses described below (see central reflexes).
Arterial chemoreceptors
Arterial chemoreflexes are subserved by peripheral chemosensory endings located in the carotid and aortic bodies, along with central chemoreceptors located in the medulla (Kumar & Prabhakar, 2012; Guyenet, 2014; Schultz et al. 2015 b). The peripheral chemoreceptors preferentially respond to hypoxaemia; central chemoreceptors respond primarily to hypercapnia, a distinction that is not absolute (Kumar & Prabhakar, 2012; Guyenet, 2014). The sensitivity of these chemoreceptors can be modulated by a wide array of neurally derived and circulating substances; however, to date the exact molecular mechanisms of activation remain unclear (Kumar & Prabhakar, 2012; Guyenet, 2014).
These chemoreflexes, in addition to playing a significant role in the control of alveolar ventilation for CO2 and O2 homeostasis, also contribute to cardiovascular control (Kumar & Prabhakar, 2012; Guyenet, 2014). For instance, chemoreflex activation by hypoxia or hypercapnia increases blood pressure due to sympathetic efferent neuronal activation of the vascular beds (Kumar & Prabhakar, 2012; Guyenet, 2014). Reflex hyperventilation also increases central neuronal inputs from thoracic wall stretch receptor afferents. These act in a negative feedback manner to blunt the sympathetic activation discussed above (Somers et al. 1989 a). There is also additional negative feedback on sympathetic outflow from arterial baroreceptors which are activated by chemoreflex‐mediated increases in blood pressure (Somers et al. 1991). Such negative feedback mechanisms become more prominent in responses mediated by peripheral compared to central chemoreceptors (Somers et al. 1989 b). Thus, chemoreceptor effects on the heart are influenced by cardiopulmonary and arterial baroreflex feedback. Conversely, there is the potential for feed‐forward reflex control of peripheral chemoreflex function by sympathetic efferent neurons responding to peripheral chemoreception. As such, surgical removal of the right stellate ganglion reduces the reflex responses elicited by aortic chemoreceptors to hypoxia and hypotension (Anand, 1996). This excitatory effect of sympathetic nerves on peripheral chemoreceptors is thought to be mediated by ischaemic hypoxia secondary to vasoconstriction and reduction in blood flow to the chemoreceptor glomus (Anand, 1996).
Chronotropic responses to chemoreflex activation are more variable than the vasculature sympathetic responses when compared among species (Marshall, 1994). In humans, hypercapnia and hypoxia elicit a reflex increase in sympathetic efferent neuronal activity with a corresponding decrease in parasympathetic efferent neuronal inputs; a tachycardia thus accompanies the hyperventilation (Kara et al. 2003). However, during periods of apnoea, peripheral chemoreflex activation with hypoxia elicits a bradycardia (Kara et al. 2003). Such chemoreflex‐mediated bradycardia, and corresponding peripheral sympathetically mediated vasoconstriction, is thought to contribute to the ‘diving’ reflex prominent in seals and evident in many mammals including primates (Marshall, 1994; Kara et al. 2003). These responses reduce myocardial energy demands, while maintaining normal arterial pressure in the face of the peripheral vasodilatory effects of hypoxia elicited by the periods of apnoea. In humans, in pathophysiological states, when apnoea becomes frequent and recurring, these responses are detrimental, resulting in sustained sympathetic efferent neuronal activation to the periphery and autonomic imbalance to the heart (Fletcher, 2001).
Renal afferent neurons
Renal sensory neurons are subclassified as mechanosensitive or chemosensitive (Booth et al. 2015). Their mechanoreceptors are localized primarily to the renal parenchyma and wall of the renal pelvis (Niijima, 1975). Stimulation of these receptors evokes a centrally mediated reno‐renal reflex (Ueda et al. 1967; Francisco et al. 1980; Kopp et al. 1985). Renal chemoreceptors (R1 and R2 types) are activated by the chemical environment in the intrarenal tissue and pelvis (Recordati et al. 1978, 1980). Stimulation of these receptors likewise evokes central‐mediated sympatho‐excitation (Recordati et al. 1982; Rogenes, 1982). Renal afferent neurons are primarily associated with unmyelinated C‐fibres plus a much smaller population of Aδ‐fibres (Knuepfer & Schramm, 1987). Their afferent inputs, projecting centrally via dorsal root ganglia (Donovan et al. 1983; Knuepfer & Schramm, 1987), can reflexly modulate sympathetic efferent neuronal outflows via spinal, medullary and higher centres (Calaresu & Ciriello, 1981; Wyss & Donovan, 1984; Xu et al. 2015). Thus, ablation of renal afferent inputs has the potential to alter autonomic outflows, both in the setting of hypertension (Esler et al. 2010; Bakris et al. 2015; Blankestijn et al. 2015) and HF (Booth et al. 2015).
Muscle afferent neurons
Skeletal muscle afferent neurons transduce the mechanical and chemical milieu of the musculature (Kaufman & Rybicki, 1987). Mechanical events in the musculature are transduced by mechanoreceptors associated with thinly myelinated group III fibres (Kaufman & Rybicki, 1987). On the other hand, local metabolic by‐products are transduced by afferent neurons with unmyelinated (group IV) axons (Kaufman & Rybicki, 1987). Because these muscle receptors display polymodal transduction (Kaufman & Rybicki, 1987), exercise induced sensory activation can elicit substantial changes in autonomic neuronal cardiorespiratory adjustments, even during relatively mild exercise (Amann et al. 2011; Dempsey et al. 2014). Signals from muscle afferents integrate at multiple nexus points along the cardiac neuraxis and in humans can influence the activity of key autonomic midbrain nuclei such as the periaqueductal grey (Basnayake et al. 2011). Such sensory reflex activation preferentially increases sympathetic drive to the heart compared to the peripheral vasculature such that pressor responses are primarily due to increasing cardiac efferent neuronal output concomitant with central blood volume mobilization (Wyss et al. 1983; Sheriff et al. 1998; Crisafulli et al. 2003; Sala‐Mercado et al. 2006).
Structural/functional organization of cardiac nervous system: cardiac motor neurons
Cardiac myocytes and coronary vessels are ultimately modulated by sympathetic and parasympathetic motor neurons (Randall et al. 1972; Randall, 1994), along with circulating hormones (Dell'Italia, 2011; Zucker et al. 2012). Efferent neuronal outflows from the central autonomic nervous system to the heart depend on both central (preganglionic) and peripheral neuronal mediated (postganglionic) reflexes (Zucker & Gilmore, 1991; Randall, 1994; Armour & Ardell, 2004; Coote, 2013). Within each nexus point of the neuronal hierarchy for cardiac control, from the central nervous system (CNS) to intrinsic cardiac ganglia (see Fig. 1), network interactions occurring within and between its levels are fundamental to network output control of cardiac motor neurons (Ardell, 2004; Armour, 2008; Herring & Paterson, 2009). The concept that the two efferent limbs of the cardiac nervous system function in a ‘reciprocal’ fashion wholly under central neuronal command (Levy, 1971; Levy & Martin, 1979; Dampney et al. 2002; Billman, 2006; Williamson et al. 2006) has been revised since it is now known that: (i) neurons in either of these motor limbs can be activated or suppressed concurrently (Armour, 2004; Coote, 2013); (ii) cardiocentric control exists within the thorax (Armour, 1983, 2008; Ardell et al. 1991) such that (iii) intrathoracic reflexes act to maintain bidirectional control of regional cardiac indices that is independent of the central nervous system (Armour et al. 1998; Schwartz, 2014; Vaseghi et al. 2014). That being said, a major scientific challenge remains to understand the essential elements of these interactions in determining not only the response to acute stressors, but also in the long‐term adaptations associated with ageing and progressive cardiac disease.
Cardiac sympathetic efferent neurons
The sympathetic circuit features a core of pre‐sympathetic efferent neurons in the brainstem that project to cardiac sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord (Guyenet, 2006; Guyenet et al. 2013). In humans, there is now functional evidence that mid‐brain circuits also contribute to the modulation of sympathetic outflow. In particular, stimulation of the subthalamic (Thornton et al. 2002) and the periaqueductal grey (Green et al. 2005; Sverrisdottir et al. 2014) can drive sympathetic outflow. These caudal cervical and cranial thoracic spinal cardiac sympathetic efferent preganglionic neurons, in turn, project axons to sympathetic efferent postganglionic neurons in ganglia located in the neck and thorax (Norris et al. 1974, 1977; Buckley et al. 2016). While there is a degree of laterality in postganglionic projections arising from extracardiac sympathetic ganglia to the heart, there are substantial inputs to all chambers arising from somata in each major peripheral ganglion (Ardell et al. 1988; Vaseghi et al. 2012; Ajijola et al. 2013). In addition, sympathetic efferent postganglionic neurons localized in each major intrinsic cardiac ganglionated plexus exert control over electrical and mechanical indices throughout the atria and ventricles (Butler et al. 1990 a,b; Cardinal et al. 2009). That functional anatomy preserves cardiac control even when one or more intrinsic cardiac ganglionated plexuses are compromised (McGuirt et al. 1997; Randall et al. 1998, 2003; Leiria et al. 2011).
Cardiac parasympathetic efferent neurons
Cardiac parasympathetic efferent preganglionic neurons within the medulla oblongata (e.g. primarily nucleus ambiguus) (Hopkins & Armour, 1982; Massari et al. 1995; Dergacheva et al. 2014) target efferent postganglionic neurons distributed throughout the atrial and ventricular ganglionated plexuses. In turn, neurons in each of these distributed intrinsic cardiac ganglia exert control over electrical and mechanical function throughout both atria and ventricles (Yuan et al. 1994; Arora et al. 2003 b; Cardinal et al. 2009). Such divergence of control also includes, in part, the bilateral nature of vagal preganglionic neuronal projections to all major atrial or ventricular ganglionated plexuses (Ardell & Randall, 1986; McGuirt et al. 1997; Yamakawa et al. 2014). Taken together, these data support the thesis that the intrinsic cardiac nervous system, as a collective, acts to coordinate regional cardiac electrical and mechanical function (Armour, 2008; Kember et al. 2011), even when operating chronically disconnected from higher centres (Ardell et al. 1991; Murphy et al. 2000; Vaseghi et al. 2009).
Local circuit neurons
Local circuit neurons are located in all intrathoracic ganglia, including those distributed on the heart. They subserve interactive processing of information by neurons in and among peripheral autonomic ganglia (Ardell, 2004; Armour, 2004, 2008). The cardiac and intrathoracic vascular mechano‐ and chemosensory inputs discussed above are processed within intrinsic cardiac and intrathoracic extracardiac ganglia primarily by these local circuit neurons (Armour, 1983, 1994; Ardell et al. 1991; Murphy et al. 2000). They also transduce inputs from the central nervous system (Armour, 1985, 1986 a,b; Beaumont et al. 2013). As such, peripheral interactive local circuit neurons are fundamental to coordinating motor neuronal outputs to the heart (Armour & Ardell, 2004; Armour, 2008).
Structural/functional organization of cardiac nervous system: reflex control
Peripheral intrinsic cardiac reflexes
The entire cardiac neuronal hierarchy acts as a distributive processor, employing multiple nested feedback control loops to modulate cardiac function throughout each cardiac cycle that together initiate both fast‐acting, short‐loop as well as longer‐latency reflex control over regional cardiac indices (Armour, 2008). In such a scenario, intrinsic cardiac neurons are ultimately under the control of more centrally located intrathoracic extracardiac neurons along with the central nervous system (Ardell, 2004). The population of intrinsic cardiac neurons that receive obligatory pre‐ to postganglionic efferent inputs is less than 15% of the total population (Gagliardi et al. 1988; Armour & Hopkins, 1990 a,b; Beaumont et al. 2013). A substantial population of the local circuit neurons responds to both sympathetic and/or parasympathetic inputs (Beaumont et al. 2013; Rajendran et al. 2016). Being involved in inter‐ganglionic interactions, the local circuit neurons subserve critical roles in integrated control of cardiac function among peripheral ganglia and between peripheral and central components of the cardiac nervous system. These local circuit neurons may serve as the primary targets for pharmacological and/or bioelectric therapeutic strategies to mitigate the hyperdynamic cardiac reflex responses accompanying progression of cardiac disease (Armour, 2008; Gibbons et al. 2012; Beaumont et al. 2015; Hardwick et al. 2015). The intrinsic cardiac nervous system is the cornerstone of cardiocentric reflexes; that is, reflexes that are confined to the periphery and with the heart as their target organ.
Peripheral intrathoracic, extracardiac reflexes
Cardiocentric reflexes are also dependent upon neuronal somata in superior cervical and intrathoracic (superior and middle cervical, stellate and mediastinal) sympathetic ganglia, forming intrathoracic, extracardiac reflexes that control intrinsic cardiac motor and local circuit neurons (Armour & Ardell, 2004; Armour, 2010). Intrathoracic extracardiac reflexes receive both excitatory and inhibitory inputs from spinal cord neurons that, in particular, influence the regulation of regional cardiac electrical and mechanical function (Armour, 1985, 1986 a,b; Ardell et al. 2009).
Peripheral somato‐autonomic reflexes
Muscle sensory neurites transduce changes in skeletal mechanical events, along with the concentration of metabolic by‐products (Kaufman & Rybicki, 1987). Activation of these afferents can change spinal driven efferent autonomic outputs, and thereby contribute to the cardiac adjustments elicited by dynamic exercise (Amann et al. 2011; Dempsey et al. 2014). Normally, during exercise, activation of these afferents preferentially increases sympathetic efferent neuronal inputs to the heart, thereby mediating a pressor response associated with increasing cardiac output (Sheriff et al. 1998; Crisafulli et al. 2003; Sala‐Mercado et al. 2006). Increase in ventricular function occurs despite restraint involving metabolite induced coronary arterial vasodilatation that can overcome increased sympathetic drive that targets coronary arterial α‐adrenergic receptors (Coutsos et al. 2010). These changes involve at least three different control mechanisms: (i) feed‐forward effects of central command, (ii) feedback initiated by the exercise‐induced pressor reflex (secondary to activation of skeletal muscle afferents), and (iii) feedback effects mediated by arterial and cardiopulmonary baroreflexes (Rowell et al. 1996).
Central integration
Tonic activity of autonomic neurons reflects the output of an integration process within central neuronal networks that set a base level of regulatory influence even in the unstressed, resting cardiovascular state. The sympathetic network contains a central sympathetic pattern generator with a high level of intrinsic, tonic activity (Guyenet, 2006; Jänig, 2014 b). At rest, inhibitory mechanisms (GABA) activated by ongoing arterial baroreceptor afferent discharge restrains central sympathetic network activity and thus sympathetic output to the periphery (Schreihofer & Guyenet, 2002; Guyenet, 2006). In contrast, cardiac parasympathetic preganglionic neurons display lower levels of basal activity (McAllen & Spyer, 1978; Mendelowitz, 1999; McAllen et al. 2011). For cardiac directed parasympathetic preganglionic efferent neurons, these neurons have no intrinsic pattern generators but at rest are activated by ongoing excitatory inputs (Kunze, 1972; Mendelowitz, 1999). This resting primary afferent input to cardiac vagal preganglionic neurons arises primarily from arterial baroreceptors with lightly myelinated, A‐type axons, since these have pressure thresholds somewhat below normal prevailing mean arterial pressure at rest (Andresen & Kunze, 1994; Andresen et al. 2012). Cardiac parasympathetic preganglionic efferent neuron activity activates subsets of parasympathetic efferent postganglionic neurons distributed throughout the intrinsic cardiac nervous system that ultimately act as conduits to cardiac myocytes such as those of the sinoatrial node to control heart rate and the rest of the heart (Edwards et al. 1995; McAllen et al. 2011; Beaumont et al. 2013; Hardwick et al. 2014).
Peripheral reflexes
Perhaps less recognized in such control is the impact of dual cranial and spinal paths of primary cardiovascular sensory afferent neurons (Armour & Kember, 2004; Kember et al. 2011, 2013 a). With respect to overall control, the heart as well as the vascular system provide two basic sources of primary afferent inputs: (i) those directed to the brainstem at the NTS, and (ii) those projecting to spinal cord neurons (thus, cranial visceral afferent neurons such as in nodose vs. spinal dorsal root ganglia, respectively). The cranial nerve activated pathways, in most instances, suppress cardiac functions in a negative feedback fashion (Guyenet, 2006; Browning & Travagli, 2011; Guyenet et al. 2013). Conversely, spinal visceral afferent neurons can elicit deleterious positive feedback to sympathetic effector neurons – a form of positive feedback (Brown, 1979; Ardell et al. 1982; Malliani & Montano, 2002; Grundy, 2004). Activation of cardiac afferent inputs to NTS generally are not consciously perceived, whereas activation of cardiac spinal afferent inputs can activate perceptible pain along with indirect pathways that impact brainstem autonomic centres (Foreman, 1999).
Cardiac primary afferent axons are subclassified as belonging to two distinct cellular phenotypes that are indicated by differences in axon conduction velocity: (i) fast conducting myelinated A‐fibres and (ii) slowly conducting unmyelinated C‐fibres. The cardiovascular sensors have characteristically different sensitivities with A‐fibre afferent axons associated with lower physiological thresholds and higher discharge rates compared to C‐fibre afferent axons with more limited and often irregular discharge (Armour & Kember, 2004). C‐class axons constitute the overwhelming majority of these cardiovascular primary afferents. For instance, 80–90% of arterial baroreceptor neurons are of the C‐fibre phenotype (Andresen et al. 2012). C‐fibre activation elicits strong cardiovascular reflexes at low frequencies of activation compared to A‐fibre afferents which require higher frequencies for discernible reflex responses (Fan et al. 1999). While A‐ and C‐fibre cardiovascular afferent neurons synaptically terminate in similar regions of the NTS, the afferent information generally does not mix to excite the same NTS neuron (Donoghue et al. 1981; Mifflin, 1996). As such, these neural circuits provide highly separate reflexes that are dependent upon either A‐fibre or C‐fibre afferent neural inputs to the medulla (Jin et al. 2004; Andresen & Peters, 2008; Peters et al. 2011). Many of these neurons receiving afferent inputs then directly send axons to project out of NTS to other destinations within the brainstem such as nucleus ambiguus (e.g. cardiac parasympathetic preganglionics) or beyond to areas such as the periventricular nucleus of the hypothalamus (Bailey et al. 2006). Some NTS neurons are only indirectly connected to the primary afferents and these higher order sensory neurons often also connect to different brainstem or forebrain areas including those in the ventromedial and raphe nuclei as well as key hypothalamic nuclei, regions which strongly impact autonomic efferent control (Dampney et al. 2005).
To date, the gains for parasympathetic and sympathetic baroreflex appear to be independent of one another and when they change with pathophysiology no direct correlation has been found, even though cranial baroreceptor afferents contribute to both (Rudas et al. 1999). Most measures of baroreflex gain, especially those used clinically or for in vivo chronic recordings, depend primarily on activating myelinated reflex pathways because only afferents with lower afferent transduction thresholds are engaged at normal pressures (Andresen et al. 2012). Similar independence appears in chronic modification of autonomic pathways in congestive heart failure where exercise training improves parasympathetic, but not sympathetic, baroreflex control of rabbit (Liu et al. 2002) and human hearts (Sheldahl et al. 1994). The presence and release of neuropeptides onto their G‐protein coupled receptors in central autonomic circuits may well be responsible for acute and chronic plasticity of the system (Stern et al. 2012). Beyond NTS, interconnections between anatomical sites show broad pairings of reciprocal connections; but little is known about their specific functions or even broad points such as A‐fibre/C‐fibre convergence. Only fragments of these relationships suggest potential impacts of these interactions between NTS and key brainstem or supramedullary regions, such as the paraventricular nucleus of the hypothalamus. Thus, growing evidence suggests that central pathways are likely to be much more discrete in organization than currently appreciated with specific contributions to the integrated whole in cardiovascular control.
Questions
Circuit map for normal cardiac control
What are the critical neural elements (peripheral vs. central) for cardiac control that translate from animal models to the human condition?
What are the critical inherent differences in cardiac neural control that predispose individuals to cardiac disease and, as such, what is/are the appropriate biomarker(s) to assess this potential?
What are the effects of ageing on integrated neural control of the heart?
Neuraxial transduction of cardiac pathology
Overview
Cardiac disease involves maladaptive interactions that occur not only at the level of the cardiomyocyte, but also among both local and more remote neurons regulating cardiac function (Zipes et al. 2006; Houser et al. 2012; Park et al. 2012; Shinohara et al. 2012; Ajijola et al. 2015). In the presence of cardiac pathology the cardiac nervous system adapts in a reactive attempt to maintain adequate cardiac electrical and mechanical function (Armour, 2008; Fukuda et al. 2015). While cardiac hierarchical control readily reorganizes in response to normal physiological perturbations (Kember et al. 2011), it can be compromised as it responds to the demands of deteriorating cardiac function over longer time scales (e.g. heart failure) or sudden shifts in demand initiated by events such a myocardial ischaemia (Vaseghi & Shivkumar, 2008; Kember et al. 2013 b; Florea & Cohn, 2014; Fukuda et al. 2015). This section deals primarily with adaptations in the neural processing associated with ischaemic and non‐ischaemic heart disease, focusing on translating information derived from preclinical studies. Figure 3 schematically represents specific aspects of these adjustments, focusing on adaptation to myocardial infarction. The reader is referred to the companion White Papers on the neural–myocyte microenvironment (Habecker et al. 2016) and clinical manifestations (Shivkumar et al. 2016) for additional information. Across all three White Papers, the fundamental premise is that the progression of cardiac disease reflects the dynamic interplay between neurohumoral and cardiac end‐effectors. Specifically, multiple factors are involved in disease progression including cardiac substrates, the neural–myocyte interface, myocyte energetics, vascular and interstitial hormonal systems, peripheral and central mediated reflexes of the cardiac nervous system, and the interplay with higher centre neurons.
Figure 3. A schematic overview of a potential role for cardiac sympathetic afferents in mediating both sympatho‐excitatory and cardiac remodelling effects in heart failure and the use of resiniferatoxin (RTX) to antagonize these effects by ablation of TRPV1‐expressing afferents .
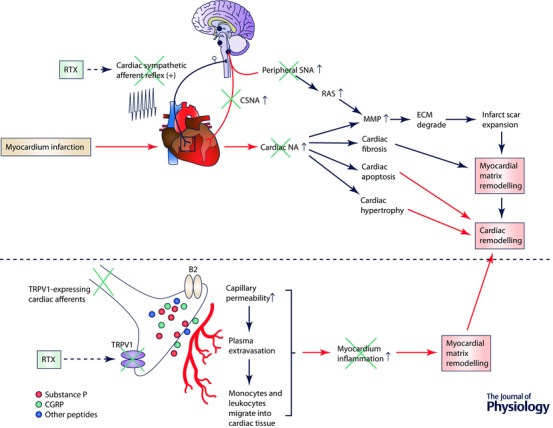
SNA, sympathetic nerve activity; CSNA, cardiac sympathetic nerve activity; MMP, matrix metalloprotease; ECM, extracellular matrix; NA, noradrenaline. RAS, renin angiotensin system; CGRP, calcitonin gene‐related peptide.
Cardiac pathology can induce both short‐term and long‐term effects on neural networks involved in cardiac control, some giving rise to exaggerated reflex responses in some while others are blunted (Zucker et al. 2012; Schultz et al. 2013; Florea & Cohn, 2014; Fukuda et al. 2015). Such neuronal remodelling has the potential to develop conflicts between central and peripheral reflexes of the cardiac nervous system (Kember et al. 2013 b), a condition predisposing to arrhythmia formation and/or deterioration of contractile function (Armour, 2008; Florea & Cohn, 2014; Fukuda et al. 2015). Central sensitization can occur because of plasticity of neurons within the spinal grey matter. Continual intense transmission of action potentials arising from a cardiac site of injury or inflammation, via nociceptive afferent neurons, can elicit cellular processes that increase the excitability of cell membranes, facilitate synaptic strength, and decrease inhibitory transmission within the nervous system (Latremoliere & Woolf, 2009). These cellular changes can enhance the responsiveness of spinal neurons and neurons that transmit nociceptive information to supraspinal levels with a resultant increase in pain sensation (Woolf & Salter, 2000).
The relevance of the cardiac neuronal hierarchy in myocardial ischaemia
Evidence exists indicating that central neuronal sensitization may be associated with changes in glial function during myocardial ischaemic episodes. Marked increases in the central neuronal release of glutamate, substance P and other neurotransmitters resulting from the bombardment of spinal afferent neurons may trigger enhanced excitation of glia in neuronal domains (Milligan & Watkins, 2009). In addition, numerous inflammatory mediators can be released from glia, including tumour necrosis factor α (TNF‐α), which has been shown to be associated with myocardial ischaemia. In fact, occlusion of the rat left anterior descending coronary artery was found to upregulate TNF‐α in the dorsal horn 30 min after onset; such upregulation could be sustained for 6 h (Niu et al. 2009). Myocardial ischaemia engages multiple afferent pathways, impacting both peripheral and central reflex arcs (Ustinova & Schultz, 1994 b; Schultz & Ustinova, 1996; Armour, 1999; Malliani & Montano, 2002). The cardiac nervous system exhibits short‐term memory (Armour et al. 2002; Ardell et al. 2009) and longer‐term plasticity (Kember et al. 2013 a; Wang et al. 2014; Fukuda et al. 2015). Such neural influences represent a major determinant for cardiac control in the setting of progressive cardiovascular disease.
The relevance of the cardiac neuronal hierarchy to cardiac arrhythmia induction
It has been recognized for some time that autonomic neuronal dysfunction plays a significant role in the induction and maintenance of atrial or ventricular arrhythmias (Armour et al. 1972; Gelband et al. 1977; Scherlag et al. 2006; Chen et al. 2014; Fukuda et al. 2015; Zipes, 2015). Pathological stressors have the potential to disrupt nested feedback loops within the cardiac neural hierarchy, sometimes with lethal consequences (Schwartz, 1984, 2001; Chen et al. 2001; Billman, 2006; Scherlag & Po, 2006; Shen & Zipes, 2014; Ajijola et al. 2015; Fukuda et al. 2015; Gardner et al. 2015). In fact, derangement of neural processing throughout the cardiac neural hierarchy in response to transducing pathologies such as regional ventricular ischaemia frequently give rise to altered efferent neuronal outputs to different cardiac regions (Armour, 1999; Kember et al. 2013 b). This includes alterations in reflex processing within intrinsic cardiac (Huang et al. 1993; Foreman et al. 2000) and extracardiac intrathoracic ganglia (Armour et al. 1998; Ardell et al. 2009; Ajijola et al. 2015) as well with central (spinal and supraspinal) reflexes (Malliani & Montano, 2002; Ding et al. 2008 a; Fu & Longhurst, 2009; Zucker et al. 2012; Wang et al. 2014).
Enhanced cardiac sympathetic drive promotes myocyte calcium influx and increases the inotropic state of the heart, which results in increased myocardial oxygen demand (Levy & Martin, 1979; Randall, 1994; Zucker et al. 2012). This can exacerbate the harmful effects of pre‐existing cardiac ischaemia to precipitate life‐threatening ventricular arrhythmias (Opie & Clusin, 1990; Lubbe et al. 1992). This is particularly prevalent when there is an abnormal cardiac structural phenotype to support re‐entrant pathways, such as in ischaemic and dilated cardiomyopathies, hypertrophic cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy (Florea & Cohn, 2014; Fukuda et al. 2015). Excessive adrenergic activity is unsurprisingly a negative prognostic indicator post myocardial infarction (MI) (Kleiger et al. 1987; La Rovere et al. 1998), as well as during congestive cardiac failure (Cohn et al. 1984; Nolan et al. 1998). High sympathetic drive to the heart can also precipitate arrhythmia, particularly when there is an abnormal cardiac electrophysiological substrate such as in long QT syndrome (LQTS) types 1 and 2 (Shen & Zipes, 2014).
In randomized controlled trials β‐blockers, introduced over 50 years ago, represent the only anti‐arrhythmic pharmacological agent known to improve mortality post MI or in chronic congestive HF (ISIS‐1, 1986; CIBIS‐II, 1999). Moreover, improving cardiac cholinergic neurotransmission is notoriously difficult to achieve pharmacologically, although gene transfer of neuronal nitric oxide synthase (nNOS) into intracardiac ganglia can facilitate cholinergic transmission (Heaton et al. 2007). Thus, recently there has been a surge in interest of utilizing interventional procedures that are aimed at modulating cardiac sympathovagal balance. In fact, recent case series have advocated stellectomy (Ajijola et al. 2012; Schwartz, 2014), thoracic epidural anaesthesia (Bourke et al. 2010) or renal sympathetic nerve denervation (Bradfield et al. 2014), as well as vagal nerve stimulation (Huang et al. 2015) as potential approaches to various cardiac pathologies.
Under normal conditions the cardiac cholinergic signalling prevents intracellular calcium overload and slows heart rate. Its activation can raise the threshold for ventricular fibrillation induction (Ng et al. 2007) and this appears to be dependent on an nNOS, muscarinic receptor dependent pathway (Kalla et al. 2016). Conditions that promote high vagal tone such as exercise training (Danson & Paterson, 2003; Billman, 2006) protect against cardiac mortality (Cole et al. 1999). On the other hand, impaired vagal function is a negative prognostic indicator in congestive cardiac failure patients (La Rovere et al. 1998). It is important to note that in conditions associated with the SCN5A mutation causing LQT3 or Brugada's syndrome, vagal tone can paradoxically trigger ventricular arrhythmias; the mechanistic basis of this observation remains ill‐understood (Shen & Zipes, 2014).
Cholinergic stimulation targeting cardiac myocyte muscarinic receptors counters sympathetic changes by inhibiting adrenergic and cyclic adenosine monophosphate (cAMP)‐protein kinase A dependent increases in L‐type calcium current I CaL via direct and indirect pathways. Direct inhibition of β‐adrenergic receptor function occurs via interactions of adenylate cyclase with the α subunit of pertussis toxin (PTX)‐sensitive Gi/Go proteins of the M2 receptor (Sunahara et al. 1996). Indirect effects may also be dependent upon the generation of NO by endothelial nitric oxide synthase (eNOS), thereby leading to cGMP production and increased phosphodiesterase‐2 activity that initiate breakdown of cAMP and subsequent reduction in I CaL (Balligand et al. 1993; Han et al. 1994) – a controversial concept (Vandecasteele et al. 1999). The main functional role for NO appears to be presynaptic at the level of the cholinergic ganglia in modulating acetylcholine release (Herring & Paterson, 2001; Herring et al. 2002; Paton et al. 2002) (see accompanying White Paper Habecker et al. 2016 for additional details). These cellular mechanisms contribute to changes in ventricular electrical events, as evidenced by action potential duration (APD) prolongation and flattening of the electrical restitution curve – along with reduction in spatial heterogeneity of these variables (Ng et al. 2001). Recent evidence has also shown an increase in myocyte connexin‐43 expression in response to chronic vagal nerve stimulation, a change that appears to promote homogeneity in conduction velocity (Ando et al. 2005).
Given the above, excessive activation of subpopulations of intrinsic cardiac neurons by co‐activating their extracardiac cholinergic and adrenergic inputs is known to initiate cardiac arrhythmias (Schwartz et al. 1992; Chen et al. 2001; Armour et al. 2005; Billman, 2006; Lujan et al. 2010; Gibbons et al. 2012; Beaumont et al. 2013). In fact, this propensity appears to reside with excessive, stochastic interplay initiated among select neuronal elements within the intrinsic cardiac nervous system – specifically its local circuit neurons (Gibbons et al. 2012; Beaumont et al. 2013). Although our knowledge of the role that local circuit neurons play in intrinsic cardiac and intrathoracic extracardiac neuronal interactions remains limited, it appears that this population of neurons is responsible for most integrative control that occurs in the periphery (Armour et al. 1998; Waldmann et al. 2006; Armour, 2008; McAllen et al. 2011). As such, it has been suggested that targeting this population therapeutically might act to normalize information processing within the intrathoracic nervous system to reduce excessive imbalance and thereby mitigate this arrhythmogenic substrate (Gibbons et al. 2012).
The cardiac neuronal hierarchy in heart failure
In the failing heart changes occur not only in the cardiac musculature, but also in the neurohumoral control system that modulates its musculature (Zipes et al. 2006; Armour, 2008; Vaseghi & Shivkumar, 2008; Mill et al. 2011; Zucker et al. 2012). With respect to the cardiac neuronal hierarchy, in HF remodelling can occur at multiple levels from the intrinsic cardiac nervous system (Bibevski & Dunlap, 1999, 2004, 2011; Arora et al. 2003 a; Hardwick et al. 2008, 2009, 2014; Shinohara et al. 2012) to intrathoracic extracardiac neurons (Tallaj et al. 2003; Hankes et al. 2006; Han et al. 2012; Nguyen et al. 2012; Ajijola et al. 2015), extending up to central neural processing circuits associated with the arterial baroreflex (Zucker et al. 2012). Alterations in neurohumoral control also include the renin–angiotensin–aldosterone system (RAAS) and circulating catecholamines (Dell'Italia, 2011; Mill et al. 2011).
Altered intrathoracic reflex control in heart failure
Altered afferent neuronal feedback to intrathoracic (intrinsic cardiac and stellate/middle cervical ganglion) neurons in the transduction of myocardial infarction leads to remodelling of the intrathoracic cardiac nervous system, both intrinsic cardiac and extracardiac. The altered afferent input so induced transduces the (i) ventricular substrate subsequent to infarction (Crow et al. 2004; Glukhov et al. 2010, 2012; Belevych et al. 2012), (ii) local efferent neural sprouting (Cao et al. 2000; Zhou et al. 2004; Ewert et al. 2008; Gardner et al. 2015), (iii) stress‐induced changes in the collagen matrix (Dobaczewski et al. 2010; Ulasova et al. 2011; Wei et al. 2012), and (iv) disruptions in the regional cardiac milieu as reflective of local contractility and associated metabolic changes (Mollema et al. 2007; Antoni et al. 2011). Taken together, these adaptations lead to afferent dependent disruptions in central and peripheral reflexes for cardiac control (Ahonen et al. 1975; Armour, 1999, 2008; Chen et al. 2001; Zucker et al. 2012; Florea & Cohn, 2014; Fukuda et al. 2015).
Altered baroreflex function in heart failure
In HF patients, haemodynamic, metabolic, humoral (e.g. renin–angiotensin system) and inflammatory response alterations are involved in initating excessive sympatho‐excitation (Dell'Italia, 2011; Zucker et al. 2012; Florea & Cohn, 2014; Jänig, 2014 a). A prolonged sympatho‐excitatory state exacerbates HF (Zucker et al. 2012; Florea & Cohn, 2014). Reduced baroreflex control of heart rate, a dynamic baroreflex function, has been shown to correlate closely with severity and, as a consequence, poor prognosis in HF patients with reduced ejection fraction (HFrEF) (Florea & Cohn, 2014). Blunted baroreflex control of heart rate also occurs in HF patients with preserved ejection fraction (HFpEF) (Borlaug et al. 2006; Borlaug, 2014). In fact, compromised baroreflex control of cardiac function is fundamental to the evolution of HF (Zucker et al. 2012; Floras & Ponikowski, 2015).
One major component of altered baroreflex control in heart failure is induced changes in the end‐organ innervation profile. The hyperdynamic sympatho‐excitation (Zucker et al. 2012; Florea & Cohn, 2014; Fukuda et al. 2015), with resultant changes in end‐organ receptor coupling (Lefkowitz, 2013), also includes alterations in regional sympathetic innervation. Specifically, there is progressive loss of end‐terminus nerve terminals and their functional apparatus to release and re‐uptake catecholamines (Himura et al. 1993). Preserving the integrity and function of sympathetic nerve terminals may be an important approach in the management of heart failure (Liang, 2003). The reader is referred to the companion White Paper by Habecker et al. for an expanded discussion on cellular and molecular adaptions to heart disease (Habecker et al. 2016).
In combination with influencing cardiac indices, the baroreflex exerts a substantial control over the peripheral vasculature (Kirchheim, 1976; Zucker & Gilmore, 1991). Funakoshi et al. (2014) examined the impact of baroreflex function on volume tolerance in rats. They demonstrated that rats maintained with constant carotid sinus pressure levels lost the ability to buffer elevation of left atrial pressure over time as well as arterial pressure changes in response to intravenous volume infusion. Sakamoto et al. (2016) demonstrated that sinoaortic denervation, which is an established animal model of impaired baroreflex, destabilizes left atrial pressure dynamics along with arterial pressure regulation in freely moving rats, thereby inducing recurrent episodes of high left atrial pressure. Additionally, they demonstrated that high salt intake exacerbates such volume intolerance (Sakamoto et al. 2016).
Sakamoto et al. (2015) reported that integrated baroreflex control of blood pressure depends in large part on vascular properties that contribute to altered baroreflex regulation of heart rate (4 ± 2%), arterial vascular resistance (32 ± 4%), end‐systolic elastance (14 ± 4%) and stressed blood volume (39 ± 4%). Thus, it appears that the baroreflex markedly influences vascular capacitance to buffer blood pressure changes. These data indicate the dynamic nature that baroreflex function plays in blood volume shifts in normal and evolving pathological states. Given sufficient stress and time, such hyperdynamic compensatory responses transition to a decompensated state in end‐stage pathology (Zucker et al. 2012; Florea & Cohn, 2014; Fukuda et al. 2015). Understanding the mechanistic basis for such adaptions as well as their pathological outcomes provides fertile ground for evolving new targeted therapies to mitigate cardiac disease processes associated with altered baroceptor function.
Altered skeletal muscle reflex function in heart failure
Activation of skeletal muscle afferents in heart failure often causes marked increases in sympathetic activity and circulating levels of vasoactive hormones which elicits substantial peripheral vasoconstriction (Hammond et al. 2000; Smith et al. 2003; Crisafulli et al. 2007; Koba et al. 2008). Increased α‐adrenergic coronary vasoconstriction contributes to the limited ability to raise ventricular function (Coutsos et al. 2013). This shift in the reflex pressor responses, from raising cardiac output to increased peripheral vasoconstriction, may involve depressed buffering by the arterial baroreflex in heart failure (Kim et al. 2005).
Altered chemoreflex function in heart failure
There is compelling evidence that cardiovascular chemoreflexes, particularly those involving peripheral chemoreflexes, are tonically activated in several disease states, including hypertension, renal failure, HF, diabetes and sleep apnoea, with detrimental effects on cardiorespiratory function (Hering et al. 2007; McBryde et al. 2013; Conde et al. 2014; Schultz et al. 2015 b). Although the specific mechanisms responsible for enhancing chemoreflexes in these disease states are by no means fully defined, it appears likely that they differ based upon the aetiology of the disease. As such, to date there is no clear unifying molecular, cellular or integrative process delineating their response characteristics in such pathologies.
However, it is clear that the pathophysiological effects of tonic chemoreflex activation share many common features across various cardiac diseases. Given the above, current evidence indicates that it is likely that changes occur at multiple levels of reflex transduction. These include: (i) increased sensitivity of the sensory chemoreceptors to local tissue excitatory mediators; (ii) altered central integration of these increased chemoreceptor inputs; and (iii) altered integration of efferent sympathetic and parasympathetic neuronal outflows to the heart, vasculature, lungs and other organs. The hallmark of such pathological control is defined by increases in chemoreflex activity that can override negative feedback mechanisms such as arise as a consequence of cardiac, major vasculature and pulmonary milieu transduction with resultant hyperdynamic activation of sympathetic outflows to the heart and vasculature (Hering et al. 2007; Paton et al. 2013; Schultz et al. 2013; Conde et al. 2014). Such sympatho‐excitation not only contributes to increased cardiac stress and arrhythmia formation, but also hypertension, impaired renal function and the development of insulin resistant diabetes. Tonic chemoreflex activation also destabilizes breathing to increase apnoea incidence, which can then act in a feed‐forward manner to propagate this exaggerated chemoreflex state (Dempsey & Smith, 2014). Impairment in baroreflex function, a common feature of these diseases, further amplifies maladaptive chemoreflex effects on cardiovascular sympathetic drive (Zucker et al. 2012). Obviously, all of these reflex interactions must be taken into account if one is to devise targeted neuromodulatory therapies to treat the various pathologies delineated above.
Perspectives on disease induced remodelling of neural hierarchy for cardiac control
The thesis presented here is that the cardiac neural hierarchy functions as a distributive processor with multiple nested feedback control loops involving peripheral and central aspects of the autonomic nervous system. It is becoming increasingly evident that control depends first upon the capacity of the neural networks to transduce alterations in regional cardiovascular mechanical and chemical milieus to its various neuronal elements in the reflex control of cardiac motor (adrenergic and cholinergic) neurons. It is further becoming evident that there are inherent and acquired differences in neural network interactions between individuals that impact the progression of cardiac disease to sudden onset (e.g. myocardial infarction) vs. chronic disease conditions (e.g. hypertension, diabetes) (Chen et al. 2014; Florea & Cohn, 2014; Fukuda et al. 2015). It is by understanding the mechanisms by which hierarchical neural networks remodel/adapt in combination with the induced changes in the neural/myocyte interface that rational neuromodulation based therapies can be evolved.
Questions
Remodelling/adaptions in cardiac control with cardiovascular disease
What are the primary events triggering neural remodelling of peripheral vs. central aspects of the cardiac nervous system in cardiovascular disease?
How do neurochemical and neuroimmune environments of the dorsal root ganglia, spinal networks and supraspinal networks and nuclei contribute to the various cardiac diseases in patients and animal models?
The mechanisms of typical angina observed in male patients vs. atypical angina pectoris as observed in female patients are relatively unknown. What are the pathophysiological differences between sexes?
Autonomic regulation therapy for cardiac disease
Evolution of cardiac disease involves alterations in both cardiac myocytes and the cardiac neuroaxis. Autonomic imbalance, characterized by vagal withdrawal and sustained sympathetic excitation, has been shown to play a significant role in aggravating cardiac disease. Restoration of such balance could, for instance, enhance cardiac function in the presence of cardiac pathologies. Evolving autonomic regulatory therapy (ART; that involves targeting the cardiac neuronal hierarchy) may stabilize such control in the support of cardiac function in disease.
Endogenous ART: exercise
Endurance exercise training represents an effective way to provide relatively safe, non‐pharmacological therapy for mitigating subclasses of cardiac diseases (Keteyian et al. 2010, 2012; Belardinelli et al. 2012), including lethal ventricular arrhythmias (Billman, 2006). It has been known for a long time that exercise training effects parasympathetic (Billman & Kukielka, 2006; Kukielka et al. 2006; Billman, 2009) and sympathetic (Billman et al. 2006; Holycross et al. 2007; Billman, 2009) regulation of the normal heart as well as hearts of patients experiencing myocardial ischaemia. In animal studies, myocardial ischaemia responses have been stratified into (i) susceptible vs. (ii) resistant states with respect to ventricular fibrillation induction in pre‐training conditions (Billman, 2006). It was found that brief (2 min) periods of coronary artery occlusion can provoke significantly greater increases in heart rate (accompanied by reductions in heart rate variability – an index of cardiac vagal tone) in animals susceptible to sudden cardiac death compared to disease resistant animals (Billman, 2006; Billman & Kukielka, 2006). Exercise training significantly reduced the heart rate response to exercise onset and enhanced (accelerated) return of heart rate to baseline at exercise offset (Billman & Kukielka, 2007). In sedentary animals, heart rate responses to all phases of exercise remain consistent over time (Billman & Kukielka, 2007).
Exercise training improves baroreceptor sensitivity (Liu et al. 2001; Zucker et al. 2001). In fact, animals that exhibit the greatest increases in heart rate in response to baroreflex challenges proved to be resistant to ischaemia‐induced arrhythmias (Billman et al. 1982; Billman, 2006). Most importantly, exercise training transformed animals with chronic myocardial infarction that were at high risk of sudden cardiac death to animals that were resistent to ventricular fibrillation even in response to the extreme stress imposed by a second ischaemic event during the end‐stages of an exercise stress test (Billman et al. 1984; Billman, 2006, 2009). While enhanced parasympathetic activity is a principal manifestation of exercise training, a mitigating of sympathetic tone is also a primary benefit (Liu et al. 2001; Zucker et al. 2001, 2012).
In order to evaluate the cardiac parasympathetic contribution to the anti‐arrhythmic effects of exercise training, studies were repeated after the adminstration of atropine to abolish any exercise training‐induced enhancement of cardiac vagal tone. This intervention increased heart rate (Billman & Kukielka, 2006) and provoked reductions in the various indices of heart rate variability but only induced ventricular fibrillation in one of eight trained susceptible dogs (Billman & Kukielka, 2006). Thus, exercise training‐induced increases in cardiac vagal tone was not solely responsible for the training‐induced protection from ventricular fibrillation. Other factors, including alterations in the β‐adrenergic receptor regulation of the heart, must also have contributed to the protection from ventricular fibrillation.
Progression of cardiac disease alters signal transduction in autonomic neural circuits and at the end‐terminus of cardiac neural projections (Armour, 2008; Florea & Cohn, 2014; Fukuda et al. 2015). β‐Adrenergic receptors are a critical aspect of such alterations, reflective of the hyperdynamic sympathetic response (Lefkowitz, 2013; Florea & Cohn, 2014; Fukuda et al. 2015). Regular endurance exercise improves β‐adrenergic receptor responsiveness in normal (Spina et al. 1992; Barbier et al. 2004), aged (Mazzeo et al. 1995), as well as hypertensive (MacDonnell et al. 2005) subjects with little or no change occuring in cardiomyocyte β1‐adrenergic receptor density (Hammond et al. 1987; Mazzeo et al. 1995; MacDonnell et al. 2005). In vitro studies indicate that β2‐adrenergic receptor agonists elicited significantly smaller increases in isotonic shortening of ventricular myocytes derived from susceptible dogs after training than those of sedentary animals (Billman et al. 2006). In vivo studies further demonstrated that before exercise training the β2‐adrenergic receptor antagonist ICI 118,551 significantly reduces peak contractile responses to isoproterenol (isoprenaline) more in susceptible compared to resistant dogs (Billman et al. 2006). After exercise training, resistant and susceptible dogs exhibited similar responses to a β2‐adrenergic receptor antagonist (Billman et al. 2006). These data indicate that exercise training acts to restore cardiac β‐adrenergic receptor balance (by reducing β2‐adrenergic receptor responsiveness) in stabilizing cardiac responsiveness to the stress of exercise. In conjunction with changes in integrated network function within the hierarchy for cardiac control (Zucker et al. 2012), these changes in the neural–myocyte interface are fundamental to the cardioprotective effects associated with exercise training.
Vagus nerve stimulation (VNS)
VNS and heart failure. Vagal stimulation activates multiple signalling pathways that involve (i) afferent‐mediated reflexes (Ardell et al. 2015; Yamakawa et al. 2016) and (ii) direct efferent neuronal targeting of cardiac muscarinic M2 and M3 receptors as well as inhibition of pro‐inflammatory cytokines (Tracey, 2007; Jänig, 2014 a) and normalization of nitric oxide signalling (Sabbah, 2011; Sabbah et al. 2011 b). VNS increases the release of the acetylcholine from the cholinergic efferent postganglionic neurons that innervate the mammalian heart. Acetylcholine, in turn, activates cardiomyocyte M2 muscarinic receptors to induce negative chronotropic, dromotropic and inotropic effects (Levy & Martin, 1979). VNS likewise exerts anti‐adrenergic effects mediated within the intrinsic cardiac ganglia (Furukawa et al. 1996; McGuirt et al. 1997; Randall et al. 2003), at the neural–myocyte interface (Levy et al. 1966; Levy, 1971; Levy & Martin, 1979) and centrally via afferent mediated changes in sympathetic outflow (Saku et al. 2014). Recent data indicate that VNS may also impact myocyte energetics to render myocytes stress resistant (Beaumont et al. 2015). Together, such changes restore a physiological balance between energy demands and energy supply of the failing myocardium (Sabbah et al. 2011 b; De Ferrari, 2014; Rhee et al. 2015; Buckley et al. 2015).
VNS impacts the microenvironment on the heart. First, vagal input inhibits local cytokine release to prevent tissue injury and cell death (Tracey, 2007; Jänig, 2014 a). These effects appear to be mediated via activation of the α‐7 nicotinic acetylcholine receptor (Wang et al. 2004) that inhibits the release from macrophages of a mediator of inflammation, namely, high mobility group box 1 (HMGB1) (Wang et al. 2004). In fact, long‐term VNS in dogs with HF reduces plasma HMGB1 levels along with left ventricle (LV) tissue TNF‐α and interleukin‐6 (Sabbah, 2011). Secondly, VNS impacts nitric oxide signalling. There are three isoforms of NOS identified to date that are involved in regulation of the heart: endothelial NOS (eNOS), inducible NOS (iNOS) and neuronal NOS (nNOS) (Kelly et al. 1996; Feng et al. 2002; Mungrue et al. 2002; Bendall et al. 2004; Nisoli & Carruba, 2006). Coronary artery microembolization‐induced HF in canines up‐regulates mRNA and protein expression of nNOS (Ruble et al. 2010; Sabbah, 2011). In dogs, mRNA and protein expression of myocardial eNOS is significantly down‐regulated in HF (Sabbah, 2011), whereas inducible NOS is up‐regulated (Ruble et al. 2010; Sabbah, 2011). VNS therapy normalizes the expression of nNOS in the failing dog LV and improves iNOS and eNOS expression (Ruble et al. 2010; Sabbah et al. 2011 a,b). Moreover, exercise training upregulates nNOS and facilitates vagal responsiveness (Danson & Paterson, 2003). These responses are lost in the nNOS knockout mouse, but can be rescued with nNOS gene transfer (Danson et al. 2004). Together, these effects serve to augment cardiac energetics, autonomic function and myocardial function leading to improved contractility (Zhang et al. 2009 b; Sabbah et al. 2011 a,b; Shinlapawittayatorn et al. 2013, 2014; Beaumont et al. 2015). VNS has been likewise associated with an improvement in biomarker assessments (e.g. plasma levels of noradrenaline (norepinephrine), angiotensin II and C‐reactive protein) (Zhang et al. 2009 b). Finally, VNS can confer a marked survival benefit, at least in the setting of chronic ischaemic heart disease (Li et al. 2004).
Based on such preclinical evidence, three clinical studies of VNS have reported the effects of VNS in patients with New York Heart Association (NYHA) classes II–IV chronic reduced ejection HF (HFrEF) (De Ferrari et al. 2011, 2014; Premchand et al. 2014, 2015; Zannad et al. 2015). VNS treatment proved to be feasible, safe and well tolerated, with improvements of quality of life, exercise capacity and left ventricular ejection fraction. Preliminary data from these trials likewise indicated that therapeutic levels of VNS can be delivered from either the right or left vagus (Premchand et al. 2014, 2015). To date, two of three reported trials (De Ferrari et al. 2011; De Ferrari, 2014; Premchand et al. 2014, 2015) have indicated improvements in ejection fraction and decreases in cardiac size with VNS, the third study reporting neutral effects (Zannad et al. 2015).
What is clear from these ongoing clinical studies is that the stimulus protocol for VNS is not yet optimized, that patient selection is probably indicated, and the potential interactions with standard of care pharmacological therapies still need to be defined. Recent data further indicate that ‘the stronger the better’ may not be applicable to VNS therapy, specifically, that therapeutic effects can be achieved without evident bradycardia during on‐phase stimulation (Kember et al. 2014; Ardell et al. 2015). This point, defined as the neural fulcrum, reflects an operating point where bioelectric activation of afferent and efferent projections are balanced such that evoked heart responses are null (Kember et al. 2014; Ardell et al. 2015) and disease induced imbalances within the cardiac neuronal hierarchy are blunted (Sabbah et al. 2011 b; Beaumont et al. 2015).
VNS and ventricular arrhythmias. Animal experiments and clinical studies have demonstrated that VNS imparts anti‐arrhythmic protection against both induced and spontaneously occurring ventricular arrhythmias (Billman, 2006; Zipes, 2015). This occurs by both direct (efferent motor) and reflex (afferent) activation of the cardiac neural hierarchy (Kember et al. 2014; Ardell et al. 2015; Yamakawa et al. 2015). Parasympathetic efferent mediated release of ACh that activates end‐organ muscarinic receptors (M2 and M3) coupled with antagonism of sympathetic efferent outflows to the heart have the net effect to reduce heart rate and prolong APD, reduce APD dispersion and effect ventricular restitution/refractoriness (Brack et al. 2007, 2013; Chen et al. 2014; Fukuda et al. 2015; Herring, 2015). In accord with that, infusion of the non‐selective muscarinic receptor antagonist atropine increases the occurrence of induced ventricular fibrillation (VF) (De Ferrari et al. 1991).
VNS and atrial arrhythmias. Atrial fibrillation (AF) affects more than three million people a year in the United States (Naccarelli et al. 2009). Despite such prevalence, the underlying mechanisms of AF are not fully understood. Current treatments consist of pharmacological therapies that have been combined with localized atrial catheter‐based or surgical ablation (Chen et al. 2014; Shen & Zipes, 2014). Success rates for such therapy are suboptimum (Cappato et al. 2010; Weerasooriya et al. 2011). Active neuromodulation therapies for AF represent an emerging therapeutic approach for disease management.
Vagal stimulation has the potential to either increase or decrease the propensity to arrhythmias (Nadeau et al. 2007; Lee et al. 2013; Chen et al. 2014). Higher intensity stimulations tend to increase atrial fibrillation inducibility (Zhang et al. 2009 a,c); lower intensity vagal stimulation can stabilize atrial electrical function (Stavrakis et al. 2015; Chinda et al. 2016). Moreover, recent studies have demonstrated that the anti‐arrhythmic effects of VNS can be elicited with minimal adverse effects (Zhang et al. 2009 a; Sheng et al. 2011; Zhang & Mazgalev, 2011). It has also been reported that low level VNS therapy suppresses AF induced by cholinergic neuronal activation in ambulatory dogs concomitant with suppression of stellate ganglion hyperactivity (Shen et al. 2011; Chinda et al. 2016). It has also been hypothesized that obtunding intrinsic cardiac neuronal transduction might represent a mechanistic basis of such benefit (Yu et al. 2011; Gibbons et al. 2012).
These data indicate that VNS therapy can favourably modify the underlying pathophysiology of ischaemic and non‐ischaemic heart disease. It can also prevent the development of malignant ventricular arrhythmias responsible for sudden cardiac death. These benefits apparently are the result of targeting multiple components of the cardiac neuroaxis to: (i) reduce heart rate, (ii) normalize sympathetic inputs to the heart, (iii) suppress pro‐inflammatory cytokines; (iv) normalize nitric oxide signalling pathways; and (v) to alter myocyte energetics. Future studies on the efficacy of VNS for cardiac therapeutics should focus on optimization of stimulation parameters, while focusing on patient selection and therapeutic transition where indicated in the standard of care. In view of the fact that VNS engages multiple levels of autonomic control (Ardell et al. 2015; Yamakawa et al. 2015), future preclinical and clinical studies should be designed to employ the entire cardiac nervous system in order to achieve long‐term therapeutic benefits while minimizing off‐target side effects.
Autonomic regulation therapy: spinal cord stimulation
Spinal cord stimulation (SCS) has a 20 year history for the treatment of refractory angina pectoris (Mannheimer et al. 2002; Foreman & Linderoth, 2012; Zhang et al. 2014). Beyond its well characterized anti‐anginal effects, SCS exerts multifactorial cardioprotective influences that include suppression of atrial and ventricular arrhythmias (Cardinal et al. 2006; Lopshire et al. 2009; Gibbons et al. 2012) while minimizing apoptotic changes (Southerland et al. 2007, 2012) to preserve contractile function (Lopshire et al. 2009; Lopshire & Zipes, 2012). A body of preclinical work has demonstrated that SCS fundamentally alters peripheral ganglia neural processing (Armour et al. 2002; Ardell et al. 2009; Gibbons et al. 2012) along with the neural–end‐organ interface (Cardinal et al. 2006; Southerland et al. 2007).
SCS preclinical studies. Cardiac sympathetic afferent neurons transduce the mechanical and chemical milieu of the heart via paravertebral sympathetic ganglia (C8–T6) to the DRG and subsequently to the spinal cord and thereby to higher centres (Foreman, 1999; Armour & Kember, 2004; Foreman & Linderoth, 2012; Yamakawa et al. 2016). The spinal cell bodies that convey sympathetic afferent visceral inputs to the brain stem are located in laminae I, V, VII and X in the C8–T9 dorsal horn (Foreman, 1999; Armour & Kember, 2004; Foreman & Linderoth, 2012). Both central and the peripheral reflex processing of such afferent signalling contributes to sympatho‐excitation that enhances progression into heart failure (Zucker et al. 2012; Florea & Cohn, 2014) and the potential for arrhythmias including sudden cardiac death (Fukuda et al. 2015).
SCS impacts autonomic reflexes at multiple levels of the cardiac neuroaxis to minimize sympathetic reflex responses to imposed stress. At the spinal cord itself, SCS induces the release of neuromodulators such as dynorphin that blunt the release of primary afferent related neurotransmitters (such as substance P), and it alters basal activity with sympathetic preganglionic neurons (Ding et al. 2008 a,b). Within intrathoracic extracardiac sympathetic ganglia, reflex sympatho‐excitation imposed by transient cardiac ischaemic stress is blunted by SCS (Kingma et al. 2001; Ardell et al. 2009). Likewise, within the intrinsic cardiac nervous system, reflex responses to transient ischaemic stress are blunted by SCS (Foreman et al. 2000; Armour et al. 2002), the protective effects extending for up to 1 h after SCS offset (Armour et al. 2002). The neural memory, subsequent to SCS, has important implications for intermittent SCS, both for cardiac control and angina management (Armour, 2008; Foreman & Linderoth, 2012; Kember et al. 2013 b).
For both intrathoracic extracardiac and intrinsic cardiac ganglia, local circuit neurons appear to be primary targets of SCS therapy (Ardell et al. 2009; Gibbons et al. 2012). SCS modifies synaptic function without directly altering active and passive transmembrane properties of neuronal somata (Ardell et al. 2014). As a consequence of intrathoracic neural targeting, SCS reduces aberrant and heterogeneous electrophysiological activity within the myocardium – even in animal models with chronic ischaemic heart disease (Cardinal et al. 2004). Furthermore, in a canine rapid pace HF model it reduced propensities to ventricular arrhythmias, while improving left ventricular contractile function (Lopshire et al. 2009; Lopshire & Zipes, 2012). Finally, the SCS‐induced effects on autonomic and end‐organ function are positionally dependent. High thoracic SCS (T1–T4) targets thoracic elements for autonomic control of the heart (Foreman et al. 2000; Southerland et al. 2007; Ardell et al. 2009; Lopshire & Zipes, 2014). High cervical SCS (C1–C2) can target both thoracic and visceral autonomic neural networks (Foreman et al. 2004; Foreman & Linderoth, 2012; Southerland et al. 2012). This has important implications for multi‐organ pathologies.
SCS clinical studies. The efficacy of SCS has been found to be optimum when applied pre‐emptively (Foreman et al. 2000; Southerland et al. 2007). Yet, it has demonstrable cardioprotective effects even when applied chronically after the event (Lopshire et al. 2009; Ardell et al. 2014). Defeat‐HF trial (NCT01112579) was a randomized, multicentre, single blind study of 66 patients with systolic HF that failed to show improvement in left ventricular function (Wang et al. 2015; Zipes et al. 2016). It should be noted that in this study SCS was cycled: 12 h on and 12 h off. In contrast, The Spinal Cord Stimulation Heart study, a multicentre, prospective, pilot trial involving SCS in patients with systolic HF (ejection fraction 20–30% and NYHA class III), reported that continuous T1–T3 SCS (50 Hz for 24 h a day) improved NYHA classification, quality of life, left ventricular end systolic volume and peak oxygen consumption (Tse et al. 2015). Such preclinical and clinical studies substantiate the safety of SCS for management of both cardiac arrhythmias and progression into HF. It is evident that additional mechanistic studies are required to delineate the precise mechanisms whereby SCS exerts its effects on (i) central vs. (ii) peripheral components of the cardiac neuroaxis in rendering cardiomyocytes stress resistant (Ardell, 2016).
Autonomic regulation therapy: carotid body ablation
Carotid body chemoreceptor activity is elevated in many cardiovascular disease states to negatively impact autonomic balance. Recent studies have demonstrated that the peripheral chemoreceptors, particularly those located in the carotid bodies, play a key role in increasing sympathetic drive in both hypertension and HF (Paton et al. 2013; Schultz et al. 2015 b). The maladaptive role of carotid body chemoreceptors in cardiovascular disease is driven primarily by tonic increases in carotid body afferent neuronal discharge (Sun et al. 1999; McBryde et al. 2013; Schultz et al. 2015 a). Such enhancement of tonic afferent inputs to the brainstem results in a tonic reflex drive that initiaties sympathetic efferent neuronal hyperactivity (McBryde et al. 2013; Schultz et al. 2015 a). This response, as a consequence, induces in part the autonomic imbalance characteristic of cardiac disease.
Recent studies have demonstrated that ablation of the carotid bodies improves cardiovascular end points in hypertensive, pre‐diabetic and HF animal models (Del Rio et al. 2013; McBryde et al. 2013; Ribeiro et al. 2013; Marcus et al. 2014). Carotid body ablation prevents excessive sympathetic motor drive and thus the development of hypertension in rats (Abdala et al. 2012; McBryde et al. 2013; Ribeiro et al. 2013). In spontaneously hypertensive (SHR) rats, carotid body ablation decreases heart rate and increases cardiac baroreflex gain – indicative of the relevance of these bodies to overall cardiovascular control (McBryde et al. 2013). In accord with that, hypertensive patients exhibited long‐term reductions in systolic arterial pressure (with little change in heart rate) after unilateral carotid body tumour resection (Fudim et al. 2015). It has also been shown that carotid body removal in patients results in dissociation of heart rate and blood pressure responses to hypoxia (Niewinski et al. 2014). Whereas there was significant attenuation of the hypertensive blood pressure response to hypoxia after carotid body resection, the tachycardia response remained (Niewinski et al. 2014). These results suggest that, in humans, the carotid bodies are responsible for ventilatory as well as blood pressure control. In contrast, it appears that chemoreflex control of heart rate might reside primarily with the aortic body chemotransduction.
Thus, carotid bodies play an important role in autonomic control of cardiovascular function – in addition to respiratory instability associated with HF (Del Rio et al. 2013; Marcus et al. 2014). HF rats (Del Rio et al. 2013) and rabbits (Marcus et al. 2014) displayed marked shifts in the heart rate variability (HRV) index towards augmented sympathetic tone and reduced parasympathetic tone. In fact carotid body inputs contribute to the cardiac autonomic imbalance and the cardiac sympathetic neuronal excessive activation seen in congestive heart failure (CHF). Chronic carotid body activation has also been shown to contribute to increased arrhythmia incidence in rabbits with HF (Marcus et al. 2014) and in rats leads to cardiac remodelling/fibrosis after myocardial infarction (Del Rio et al. 2013). Arrhythmias were reversed and cardiac fibrosis prevented by carotid body ablation. In rabbits, carotid body ablation is known to attenuate decreases in the LV ejection fraction while reversing increases in systolic and diastolic ventricular volumes attending chronic cardiac pacing (Marcus et al. 2014). Carotid body ablations can also delay progressive left ventricular ejection fraction deterioration after myocardial infarction in rats (Del Rio et al. 2013). Taken together, these data indicate the relevant involvement of carotid bodies in controlling cardiac contractile and electrical indices.
Autonomic regulation therapy: carotid sinus stimulation
Baroreceptors can be stimulated in different regions of the arterial tree; the easiest point of stimulation is at the level of the carotid sinus. While early studies with carotid sinus implants were associated with structural damage to implanted areas (Braunwald et al. 1970), evolving biotechnology has overcome such problems. Current devices are implanted with electrodes positioned in the carotid perivascular space around the sinus of the carotid arteries (Sabbah et al. 2011 a; Abraham et al. 2015). With time, the interface to the carotid sinus has been miniaturized with unilateral placement (Abraham et al. 2015). The premise for such therapy is that stimulation of the peripheral baroreceptor fibres increases afferent activity transduced to the NTS, which is interpreted as an increase in blood pressure. In reflex response to that baroreceptor afferent signal sympathetic efferent are reflexly decreased with a corresponding reflex augmentation in parasympathetic activity, thereby leading to reductions in blood pressure and heart rate. With respect to therapy, minimizing the potential for concurrent activation of the carotid body chemoreceptors with baroreceptor stimulation is imperative given their role in progression of HF by contributing to respiratory instability and oscillatory breathing (changes in tidal volume and respiratory frequency) (Marcus et al. 2014).
Baroreceptor activation: preclinical studies. Preclinical studies support proof‐of‐concept for utilizing bioelectric approaches using carotid sinus stimulation to treat cardiac disease. Such bioelectric stimulation was correlated with reduced plasma angiotensin II and noradrenaline levels and was associated with reduced mortality in a canine model of HF (Zucker et al. 2007). Improvements in left ventricular function have been demonstrated in chronic canine HF models with such stimulation. Carotid sinus nerve stimulation improved left ventricle systolic and diastolic function and reduced heart rate compared to untreated heart failure controls (Sabbah et al. 2011 a). Adverse structural remodelling was also mitigated in the treated group.
Autonomic regulation therapy is primarily delivered in an open‐loop configuration with an interactive titration phase to the final target levels of therapy (Zipes, 2015; Buckley et al. 2015). With the advent of technologies to record relevant biomarkers, the potential to implement closed‐loop therapy is now possible. Sunagawa and collegues have recently reported on such methodology, where a sensed arterial pressure signal was utilized to control electrical stimuli applied to the aortic depressor nerve, an aggregate of axons that derive from baroreceptors on the aortic arch (Hosokawa et al. 2011, 2012). With such technology they were able to emulate normal dynamic baroreflex function. Further optimization of such approaches would allow for controllable gain and setpoint in a closed‐loop setting.
Baroreceptor activation: clinical studies. Clinical trials have been taking place to determine the outcomes of baroreceptor stimulation therapy in both reduced (HFrEF) and preserved (HFpEF) ejection fraction heart failure. The use of implantable carotid sinus stimulator devices (Rheos System), in patients with drug resistant hypertension, showed a sustained blood pressure drop up to 4 years out and improvement in cardiac function (Scheffers et al. 2010; Bisognano et al. 2011 a). This was followed by the Rheos Pivotal Trial, a double‐blind randomized placebo‐controlled trial, that showed 88% maintenance of blood pressure reduction response during a 12 month trial period (Bisognano et al. 2011 b). A recent study investigated baroreflex activation therapy in advanced HFrEF (Abraham et al. 2015). Compared to standard medical therapy, patients treated with baroreflex activation therapy showed improved quality of life scores, NYHA classification and N‐terminal ‐pro‐brain natriuretic peptide (NT‐proBNP); however, it did not show any significant change in left ventricular function (Abraham et al. 2015). Thus the use of carotid sinus nerve stimulation to modulate baroreflex control mechanisms has received proof‐of‐concept safety approval for therapy in heart disease. Future studies will be required to improve electrode interfaces, stimulus protocols, and the potential for closed‐loop feedback.
Autonomic regulation therapy: renal denervation
Preclinical studies. Afferent signalling from somatic, visceral and thoracic sites contribute to integrated cardiac control. Owing to the central role that the renal circulation plays in volume regulation and in control of the renin–angiotension system, autonomic regulation therapy targeted to the renal circulation has received extensive interest. Targeting renal arterial nerves has been accomplished by catheter ablation delivered to the renal arteries under fluoroscopic and/or electro‐anatomic mapping guidance. For instance, renal sympathetic axonal modulation in a rat model with compensated high output HF resulted in attenuated sodium excretion after sodium loading (Villarreal et al. 1994). In rats with chronic myocardial infarction, renal neural ablation increased sodium excretion, decreased LV filling pressure and improved left ventricular function (Nozawa et al. 2002). In a rabbit model of pacing induced HF, renal denervation modified angiotensin II receptor expression and preserved renal function (Clayton et al. 2011). In the canine model of pacing‐induced HF, renal nerve ablation reduced circulating angiotensin II, aldosterone, brain natriuretic peptide, endothelin‐1, transforming growth factor β (TGF‐β) and renalase in conjunction with reduced ventricular substrate remodelling compared to untreated controls (Dai et al. 2014; Zhao et al. 2014). With such renal sympathetic modulation, substrate and electrical remodelling were correspondingly mitigated and inducibility of ventricular fibrillation decreased (Guo et al. 2014).
Renal axonal modulation: clinical studies. Small case studies have demonstrated a reduction of ventricular arrhythmia potential post renal nerve denervation (Bradfield et al. 2014; Remo et al. 2014). Although the beneficial effect for arrhythmia burden did not specifically improve left ventricular function, frequent lethal ventricular arrhythmias in the setting of LV dysfunction were attenuated (Bradfield et al. 2014; Remo et al. 2014). There is mounting evidence that suggests benefits in preserved ejection fraction, probably as a result of the anti‐hypertensive effects of such therapy, thereby resulting in less structural remodelling (Brandt et al. 2012). What is clear from recent experience with renal ablation for hypertension, Symplicity HTN‐1 to HTN‐3 (Krum et al. 2009; Esler et al. 2010; Kandzari et al. 2012; Bhatt et al. 2014; Blankestijn et al. 2015), is that effective implementation of any type of autonomic regulation therapy requires an objective index for efficacy of treatment delivery. For Symplicity HTN‐3, suboptimum nerve ablation was probably responsible in part for trial failure (Blankestijn et al. 2015; Epstein & de Marchena, 2015).
Autonomic regulation therapy: cardiac afferent targets
Several studies have now shown that there is an enhanced input from cardiac sympathetic afferents in experimental CHF. Wang et al. (Wang & Zucker, 1996) carried out experiments in anaesthetized dogs with pacing‐induced CHF. Epicardial surface or left atrial administration of bradykinin or capsaicin enhanced renal sympathetic nerve activity and arterial pressure in the CHF state, compared to sham operated animals. Furthermore, in CHF animals cardiac afferent axons that transduce local epicardial application of capsaicin show enhanced discharge (Zucker et al. 1996). Perhaps the most convincing evidence for enhanced tonic input from cardiac sympathetic afferent neurons to the spinal cord is the observation that in anaesthetized dogs with the vagi sectioned application of epicardial lidocaine resulted in a significant reduction in renal sympathetic nerve activity that is not seen in sham animals (Zucker et al. 1996). Furthermore, in the CHF state the cardiac sympathetic afferent reflex has been shown to be modulated by central angiotensin II through the AT1 receptor and oxidative stress (Zhu et al. 2002, 2004 a,b; Wang et al. 2007, 2008; Gao et al. 2008), thus indicating defects at multiple sites in the neuraxis in animals with CHF.
Because the sensory endings of DRG neurons release a variety of neuropeptides (Steinhoff et al. 2014), sensitization of these endings in the diseased heart may have a profound effect on their potential to transduce local ventricular function and to initiate an inflammatory process that participates in the cardiac remodelling following myocardial infarction. In recent work by Wang et al. (Wang et al. 2014) the role of cardiac sympathetic afferent neurons in such sympatho‐excitation, baroreflex function and cardiac remodelling post MI has been demonstrated. In fact, many cardiac sympathetic afferent neurites express the TRPV1 receptor that is normally activated by agents such as capsaicin that participate in pain transduction (Wu & Pan, 2007).
Employing the highly selective TRPV1 agonist resiniferatoxin (RTX) (Lee et al. 2012) Wang et al. were able to ablate sensory neurites on or near the epicardium at the time of coronary artery ligation (Wang et al. 2014). Twelve weeks later, rats were evaluated for sympathetic function and cardiac remodelling. At this time there was a clear reduction in baseline cardiac function while renal sympathetic neuronal outflow response to epicardial application of capsaicin was completely abolished. In fact, there was a reduction in global sympathetic tone, as evidenced by reduced noradrenaline excretion. This intervention also reduced myocardial fibrosis in regions remote from the scar. Furthermore, markers of the fibrotic process such as fibronectin and TGF‐β receptor expression were normalized in these RTX treated animals – a finding of considerable clinical relevance. As such, there was improvement in LV diastolic function (the slope of the end‐diastolic pressure–volume relationship (EDPVR) was decreased) and increased responsiveness to isoproterenol in the presence of maintained basal LV systolic function. The fundamental premise here is that aberrant afferent activation is central to the evolution of cardiac disease and that mitigation of that afferent signal is an emerging therapeutic target.
Decentralization of the peripheral cardiac nervous system
As depicted above, patients with structural heart disease are known to be at risk of ventricular arrhythmias that progress to sudden cardiac death (SCD) (Vaseghi & Shivkumar, 2008). They are usually treated with medication and/or catheter ablation (Florea & Cohn, 2014; Fukuda et al. 2015). Despite that, subsets of patients remain who are refractory to these therapies and continue to experience incessant ventricular arrhythmias with a high risk for SCD. In that population, direct targeting of selective nodes of the autonomic nervous system is emerging as an effective adjunct therapy (Vaseghi & Shivkumar, 2012). This includes surgical approaches to the cranial thoracic paravertebral chain to modulate autonomic imbalance and reduce cardiac arrhythmias (Schwartz, 2014). In fact, resection of the stellate caudal to the T4 paravertebral ganglia bilaterally has been shown to effectively impart anti‐arrhythmic effects in patients with ventricular tachycardia that are refractory to other forms of therapy (Ajijola et al. 2012; Vaseghi et al. 2013, 2014). For further details on this critical neuromodulation methodology, see the companion White Paper by Shivkumar et al. (2016).
Questions
Autonomic regulation therapy for cardiovascular disease
What are the preferential sites for ART in the cardiac neuraxis? Do they differ depending on the aetiology of the cardiovascular disease? How does it relate to patient selection?
What is/are the critical neural targets for ART – afferent, efferent or local circuit neurons – intrathoracic vs. central?
What are the appropriate biomarkers to assess the efficacy of differing forms of ART in the short‐, mid‐ and long‐term?
Concluding perspectives
Neurocardiology is based on the premise that cardiac control must be evaluated in the context of the end‐organ substrate, interdependent interactions within multiple levels of the cardiac nervous system (peripheral and central), and relevant neuro‐humoral responses. It is clear that there are inherent differences that impact the autonomic response to acquired stressors and that such differences are critical to final outcomes. Imbalances in autonomic evoked responses, while beneficial in the short term, are often hyper‐dynamic and ultimately contribute to the evolution of cardiac pathology. What is also clear is that by a mechanistic understanding of these interactions, novel neural based therapeutic targets have emerged to restore a more balanced autonomic response. Since cardiac pathology is multifaceted, it is also likely that the optimum neural target will be influenced by the underlying stressor (e.g. myocardial infarction, hypertension). As our understanding of where to target emerges, there is also a need to identify appropriate biomarkers such that closed‐loop autonomic regulation therapies can evolve. The ultimate goal is to work with endogenous control systems, rather than in opposition to them, to optimize outcomes.
Additional information
Competing interests
None declared.
Funding
The following authors were supported by the US Department of Health and Human Services (HHS) and the National Institutes of Health (NIH): J.L.A. (U18 EB021799; HL071830), M.C.A. (HL10573), G.E.B. (HL086700), P.‐S.C. (HL078931; HL071140; HL124741), R.D.F. (NS035471; HL075524), D.S.O'L. (HL126706; HL‐55473), H.N.S. (HL074237), H.D.S. (HL62222) and I.H.Z. (HL62222). N.H. was supported by the British Heart Foundation (FS/15/8/31155). K.S. was supported by the Japan Society for the Promotion of Science (JSPS) (S23220013; 15K19386).
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV & Paton JF (2012). Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 590, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, Halbach M, Klug D, Lovett EG, Müller‐Ehmsen J, Schafer JE, Senni M, Swarup V, Wachter R & Little WC (2015). Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail 3, 487–496. [DOI] [PubMed] [Google Scholar]
- Affleck VS, Coote JH & Pyner S (2012). The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience 219, 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen A, Harkonen M, Juntunen J, Kormano M & Penttila A (1975). Effects of myocardial infarction on adrenergic nerves of the rat heart muscle, a histochemical study. Acta Physiol Scand 93, 336–344. [DOI] [PubMed] [Google Scholar]
- Ajijola OA, Lellouche N, Bourke T, Tung R, Ahn S, Mahajan A & Shivkumar K (2012). Bilateral cardiac sympathetic denervation for the management of electrical storm. J Am Coll Cardiol 59, 91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajijola OA, Vaseghi M, Zhou W, Yamakawa K, Benharash P, Hadaya J, Lux RL, Mahajan A & Shivkumar K (2013). Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle. Am J Physiol Heart Circ Physiol 304, H579–H588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL & Shivkumar K (2015). Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: Neuropeptide and morphologic changes. Heart Rhythm 12, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR & Richardson RS (2011). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A (1996). Reflex stimulation of aortic chemoreceptors through the stellate ganglion during hypoxia and hypotension in cats. J Physiol 491, 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K & Sato T (2005). Efferent vagal nerve stimulation protects heart against ischemia‐induced arrhythmias by preserving connexin43 protein. Circulation 112, 164–170. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Hofmann ME & Fawley JA (2012). The unsilent majority‐TRPV1 drives ‘spontaneous’ transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regul Integr Comp Physiol 303, R1207–R1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC & Kunze DL (1994). Nucleus tractus solitarius – gateway to neural circulatory control. Annu Rev Physiol 56, 93–116. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL & Mendelowitz D (2004). Central nervous system regulation of the heart In Basic and Clincial Neurocardiology, ed. Armour JA. & Ardell JL, pp. 187–219. Oxford University Press, New York. [Google Scholar]
- Andresen MC & Peters JH (2008). Comparison of baroreceptive to other afferent synaptic transmission to the medial solitary tract nucleus. Am J Physiol Heart Circ Physiol 295, H2032–H2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni ML, Boden H, Hoogslag GE, Ewe SH, Auger D, Holman ER, van der Wall EE, Schalij MJ, Bax JJ & Delgado V (2011). Prevalence of dyssynchrony and relation with long‐term outcome in patients after acute myocardial infarction. Am J Cardiol 108, 1689–1696. [DOI] [PubMed] [Google Scholar]
- Ardell JL (2004). Intrathoracic neuronal regulation of cardiac function In Basic and Clinical Neurocardiology, ed. Armour JA. & Ardell JL, pp. 118–152. Oxford University Press, New York. [Google Scholar]
- Ardell JL (2016). Heart failure: Mechanisms of spinal cord neuromodulation for heart disease. Nat Rev Cardiol 13, 127–128. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Barman SM & Gebber GL (1982). Sympathetic nerve discharge in chronic spinal cat. Am J Physiol 243, H463–H470. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Butler CK, Smith FM, Hopkins DA & Armour JA (1991). Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol 260, H713–H721. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Cardinal R, Beaumont E, Vermeulen M, Smith FM & Armour JA (2014). Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Auton Neurosci 186, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell JL, Cardinal R, Vermeulen M & Armour JA (2009). Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia. Am J Physiol Regul Integr Comp Physiol 297, R470–R477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell JL, Rajendran PS, Nier HA, KenKnight BH & Armour JA (2015). Central‐peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am J Physiol Heart Circ Physiol 309, H1740–H1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell JL & Randall WC (1986). Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol 251, H764–H773. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Randall WC, Cannon WJ, Schmacht DC & Tasdemiroglu E (1988). Differential sympathetic regulation of automatic, conductile, and contractile tissue in dog heart. Am J Physiol 255, H1050–H1059. [DOI] [PubMed] [Google Scholar]
- Armour JA (1983). Synaptic transmission in the chronically decentralized middle cervical and stellate ganglia of the dog. Can J Physiol Pharmacol 61, 1149–1155. [DOI] [PubMed] [Google Scholar]
- Armour JA (1985). Activity of in situ middle cervical ganglion neurons in dogs, using extracellular recording techniques. Can J Physiol Pharmacol 63, 704–716. [DOI] [PubMed] [Google Scholar]
- Armour JA (1986. a). Activity of in situ stellate ganglion neurons of dogs recorded extracellularly. Can J Physiol Pharmacol 64, 101–111. [DOI] [PubMed] [Google Scholar]
- Armour JA (1986. b). Neuronal activity recorded extracellularly in chronically decentralized in situ canine middle cervical ganglia. Can J Physiol Pharmacol 64, 1038–1046. [DOI] [PubMed] [Google Scholar]
- Armour JA (1994). Peripheral autonomic neuronal interactions in cardiac regulation In Neurocardiology, ed. Armour JA. & Ardell JL, pp. 219–244. Oxford University Press, New York. [Google Scholar]
- Armour JA (1999). Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res 41, 41–54. [DOI] [PubMed] [Google Scholar]
- Armour JA (2004). Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 287, R262–R271. [DOI] [PubMed] [Google Scholar]
- Armour JA (2008). Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93, 165–176. [DOI] [PubMed] [Google Scholar]
- Armour JA (2010). Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm 7, 994–996. [DOI] [PubMed] [Google Scholar]
- Armour JA & Ardell JL (1994). Neurocardiology. Oxford University Press, New York. [Google Scholar]
- Armour JA & Ardell JL (2004). Basic and Clinical Neurocardiology. Oxford University Press, New York. [Google Scholar]
- Armour JA, Collier K, Kember G & Ardell JL (1998). Differential selectivity of cardiac neurons in separate intrathoracic autonomic ganglia. Am J Physiol 274, R939–R949. [DOI] [PubMed] [Google Scholar]
- Armour JA, Hageman GR & Randall WC (1972). Arrhythmias induced by local cardiac nerve stimulation. Am J Physiol 223, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Armour JA & Hopkins DA (1990. a). Activity of canine in situ left atrial ganglion neurons. Am J Physiol 259, H1207–H1215. [DOI] [PubMed] [Google Scholar]
- Armour JA & Hopkins DA (1990. b). Activity of in vivo canine ventricular neurons. Am J Physiol 258, H326–H336. [DOI] [PubMed] [Google Scholar]
- Armour JA, Huang MH, Pelleg A & Sylven C (1994). Responsiveness of in situ canine nodose ganglion afferent neurones to epicardial mechanical or chemical stimuli. Cardiovasc Res 28, 1218–1225. [DOI] [PubMed] [Google Scholar]
- Armour JA & Kember G (2004). Cardiac sensory neurons In Basic and Clinical Neurocardiology, ed. Armour JA. & Ardell JL, pp. 79–117. Oxford University Press, New York. [Google Scholar]
- Armour JA, Linderoth B, Arora RC, DeJongste MJ, Ardell JL, Kingma JG Jr, Hill M & Foreman RD (2002). Long‐term modulation of the intrinsic cardiac nervous system by spinal cord neurons in normal and ischaemic hearts. Auton Neurosci 95, 71–79. [DOI] [PubMed] [Google Scholar]
- Armour JA, Richer LP, Page P, Vinet A, Kus T, Vermeulen M, Nadeau R & Cardinal R (2005). Origin and pharmacological response of atrial tachyarrhythmias induced by activation of mediastinal nerves in canines. Auton Neurosci 118, 68–78. [DOI] [PubMed] [Google Scholar]
- Arora RC & Armour JA (2003). Adenosine A1 receptor activation reduces myocardial reperfusion effects on intrinsic cardiac nervous system. Am J Physiol Regul Integr Comp Physiol 284, R1314–R1321. [DOI] [PubMed] [Google Scholar]
- Arora RC, Cardinal R, Smith FM, Ardell JL, Dell'Italia LJ & Armour JA (2003. a). Intrinsic cardiac nervous system in tachycardia induced heart failure. Am J Physiol Regul Integr Comp Physiol 285, R1212–R1223. [DOI] [PubMed] [Google Scholar]
- Arora RC, Waldmann M, Hopkins DA & Armour JA (2003. b). Porcine intrinsic cardiac ganglia. Anat Rec A Discov Mol Cell Evol Biol 271, 249–258. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC & Aicher SA (2006). Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target‐specific synaptic reliability and convergence patterns. J Neurosci 26, 11893–11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakris GL, Townsend RR, Flack JM, Brar S, Cohen SA, D'Agostino R, Kandzari DE, Katzen BT, Leon MB, Mauri L, Negoita M, O'Neill WW, Oparil S, Rocha‐Singh K & Bhatt DL; SYMPLICITY HTN‐3 Investigators (2015). 12‐month blood pressure results of catheter‐based renal artery denervation for resistant hypertension: the SYMPLICITY HTN‐3 trial. J Am Coll Cardiol 65, 1314–1321. [DOI] [PubMed] [Google Scholar]
- Balligand JL, Kelly RA, Marsden PA, Smith TW & Michel T (1993). Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci USA 90, 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier J, Rannou‐Bekono F, Marchais J, Berthon PM, Delamarche P & Carre F (2004). Effect of training on beta1 beta2 beta3 adrenergic and M2 muscarinic receptors in rat heart. Med Sci Sports Exerc 36, 949–954. [DOI] [PubMed] [Google Scholar]
- Basnayake SD, Hyam JA, Pereira EA, Schweder PM, Brittain JS, Aziz TZ, Green AL & Paterson DJ (2011). Identifying cardiovascular neurocircuitry involved in the exercise pressor reflex in humans using functional neurosurgery. J Appl Physiol (1985) 110, 881–891. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA & Ardell JL (2013). Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591, 4515–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA & Ardell JL (2015). Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol 309, H1198–H1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G & Purcaro A (2012). 10‐year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol 60, 1521–1528. [DOI] [PubMed] [Google Scholar]
- Belevych AE, Terentyev D, Terentyeva R, Ho HT, Gyorke I, Bonilla IM, Carnes CA, Billman GE & Györke S (2012). Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res 110, 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, Milliez P, Robidel E, Marotte F, Samuel JL & Heymes C (2004). Role of myocardial neuronal nitric oxide synthase‐derived nitric oxide in β‐adrenergic hyporesponsiveness after myocardial infarction‐induced heart failure in rat. Circulation 110, 2368–2375. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha‐Singh K, Townsend RR & Bakris GL; SYMPLICITY HTN‐3 Investigators (2014). A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370, 1393–1401. [DOI] [PubMed] [Google Scholar]
- Bibevski S & Dunlap ME (1999). Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation 99, 2958–2963. [DOI] [PubMed] [Google Scholar]
- Bibevski S & Dunlap ME (2004). Prevention of diminished parasympathetic control of the heart in experimental heart failure. Am J Physiol Heart Circ Physiol 287, H1780–H1785. [DOI] [PubMed] [Google Scholar]
- Bibevski S & Dunlap ME (2011). Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev 16, 129–135. [DOI] [PubMed] [Google Scholar]
- Billman GE (2006). A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti‐arrhythmic drug development. Pharmacol Ther 111, 808–835. [DOI] [PubMed] [Google Scholar]
- Billman GE (2009). Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol 297, H1171–H1193. [DOI] [PubMed] [Google Scholar]
- Billman GE & Kukielka M (2006). Effects of endurance exercise training on heart rate variability and susceptibility to sudden cardiac death: protection is not due to enhanced cardiac vagal regulation. J Appl Physiol (1985) 100, 896–906. [DOI] [PubMed] [Google Scholar]
- Billman GE & Kukielka M (2007). Effect of endurance exercise training on heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J Appl Physiol (1985) 102, 231–240. [DOI] [PubMed] [Google Scholar]
- Billman GE, Kukielka M, Kelley R, Moustafa‐Bayoumi M & Altschuld RA (2006). Endurance exercise training attenuates cardiac β2‐adrenoceptor responsiveness and prevents ventricular fibrillation in animals susceptible to sudden death. Am J Physiol Heart Circ Physiol 290, H2590–H2599. [DOI] [PubMed] [Google Scholar]
- Billman GE, Schwartz PJ & Stone HL (1982). Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation 66, 874–880. [DOI] [PubMed] [Google Scholar]
- Billman GE, Schwartz PJ & Stone HL (1984). The effects of daily exercise on susceptibility to sudden cardiac death. Circulation 69, 1182–1189. [DOI] [PubMed] [Google Scholar]
- Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW & Sica DA (2011. a). Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double‐blind, randomized, placebo‐controlled rheos pivotal trial. J Am Coll Cardiol 58, 765–773. [DOI] [PubMed] [Google Scholar]
- Bisognano JD, Kaufman CL, Bach DS, Lovett EG & de Leeuw P; DEBuT‐HT and Rheos Feasibility Trial Investigators (2011. b). Improved cardiac structure and function with chronic treatment using an implantable device in resistant hypertension: results from European and United States trials of the Rheos system. J Am Coll Cardiol 57, 1787–1788. [DOI] [PubMed] [Google Scholar]
- Blankestijn PJ, Alings M, Voskuil M & Grobbee DE (2015). The complexity after simplicity: how to proceed with renal denervation in hypertension? Eur J Prev Cardiol 22, 412–414. [DOI] [PubMed] [Google Scholar]
- Booth LC, May CN & Yao ST (2015). The role of the renal afferent and efferent nerve fibers in heart failure. Front Physiol 6, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug BA (2014). The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 11, 507–515. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC & Kass DA (2006). Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114, 2138–2147. [DOI] [PubMed] [Google Scholar]
- Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y & Shivkumar K (2010). Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 121, 2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Patel VH, Coote JH & Ng GA (2007). Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol 583, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Winter J & Ng GA (2013). Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation – tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmias in heart failure. Heart Fail Rev 18, 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield JS, Vaseghi M & Shivkumar K (2014). Renal denervation for refractory ventricular arrhythmias. Trends Cardiovasc Med 24, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandle M, Wang W & Zucker IH (1994). Ventricular mechanoreflex and chemoreflex alterations in chronic heart failure. Circ Res 74, 262–270. [DOI] [PubMed] [Google Scholar]
- Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Bohm M & Hoppe UC (2012). Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 59, 901–909. [DOI] [PubMed] [Google Scholar]
- Braunwald NS, Epstein SE & Braunwald E (1970). Carotid sinus nerve stimulation for the treatment of intractable angina pectoris: surgical technic. Ann Surg 172, 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM (1979). Cardiac reflexes In Handbook of Physiology, The Cardiovascular System, ed. Berne RM, Sperelakis N. & Geiger SR, pp. 677–689. American Physiological Society, Williams and Wilkins, Bethesda. [Google Scholar]
- Browning KN & Travagli RA (2011). Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci 161, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley U, Shivkumar K & Ardell JL (2015). Autonomic regulation therapy in heart failure. Curr Heart Fail Rep 12, 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley U, Yamakawa K, Takamiya T, Armour JA, Shivkumar K & Ardell JL (2016). Targeted stellate decentralization: implications for sympathetic control of ventricular electrophysiology. Heart Rhythm 13, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CK, Smith FM, Cardinal R, Murphy DA, Hopkins DA & Armour JA (1990. a). Cardiac responses to electrical stimulation of discrete loci in canine atrial and ventricular ganglionated plexi. Am J Physiol 259, H1365–H1373. [DOI] [PubMed] [Google Scholar]
- Butler CK, Smith FM, Nicholson J & Armour JA (1990. b). Cardiac effects induced by chemically activated neurons in canine intrathoracic ganglia. Am J Physiol 259, H1108–H1117. [DOI] [PubMed] [Google Scholar]
- Calaresu FR & Ciriello J (1981). Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst 3, 311–320. [DOI] [PubMed] [Google Scholar]
- Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC & Chen PS (2000). Nerve sprouting and sudden cardiac death. Circ Res 86, 816–821. [DOI] [PubMed] [Google Scholar]
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F & Biganzoli E (2010). Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 3, 32–38. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Ardell JL, Linderoth B, Vermeulen M, Foreman RD & Armour JA (2004). Spinal cord activation differentially modulates ischaemic electrical responses to different stressors in canine ventricles. Auton Neurosci 111, 37–47. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Pagé P, Vermeulen M, Ardell JL & Armour JA (2009). Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci 145, 55–62. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Pagé P, Vermeulen M, Bouchard C, Ardell JL, Foreman RD & Armour JA (2006). Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol 291, R1369–R1375. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Hajduczok G, Sharma RV, Wachtel RE, Cunningham JT, Sullivan MJ & Abboud FM (1995). Mechanisms of baroreceptor activation. Clin Exp Hypertens 17, 1–13. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Li Z, Meyrelles SS, Ma X & Abboud FM (2001). Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann NY Acad Sci 940, 1–19. [DOI] [PubMed] [Google Scholar]
- Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS & Fishbein MC (2001). Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 50, 409–416. [DOI] [PubMed] [Google Scholar]
- Chen PS, Chen LS, Fishbein MC, Lin SF & Nattel S (2014). Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 114, 1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM & Zhu GQ (2015). Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf) 213, 778–794. [DOI] [PubMed] [Google Scholar]
- Chinda K, Tsai WC, Chan YH, Lin AY, Patel J, Zhao Y, Tan AY, Shen MJ, Lin H, Shen C, Chattipakorn N, Rubart‐von der Lohe M, Chen LS, Fishbein MC, Lin SF, Chen Z & Chen PS (2016). Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm 13, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K & Deisseroth K (2013). CLARITY for mapping the nervous system. Nat Methods 10, 508–513. [DOI] [PubMed] [Google Scholar]
- CIBIS‐II (1999). The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 353, 9–13. [PubMed] [Google Scholar]
- Clayton SC, Haack KK & Zucker IH (2011). Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am J Physiol Renal Physiol 300, F31–F39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB & Rector T (1984). Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311, 819–823. [DOI] [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE & Lauer MS (1999). Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Conde SV, Sacramento JF, Guarino MP, Gonzalez C, Obeso A, Diogo LN, Monteiro EC & Ribeiro MJ (2014). Carotid body, insulin, and metabolic diseases: unraveling the links. Front Physiol 5, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH (2013). Myths and realities of the cardiac vagus. J Physiol 591, 4073–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutsos M, Sala‐Mercado JA, Ichinose M, Li Z, Dawe EJ & O'Leary DS (2010). Muscle metaboreflex‐induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol (1985) 109, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutsos M, Sala‐Mercado JA, Ichinose M, Li Z, Dawe EJ & O'Leary DS (2013). Muscle metaboreflex‐induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304, H1029–H1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P & Concu A (2007). Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292, H2988–H2996. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJ, Concu A & Piepoli MF (2003). Muscle metaboreflex‐induced increases in stroke volume. Med Sci Sports Exerc 35, 221–228; Discussion 229. [DOI] [PubMed] [Google Scholar]
- Crow MT, Mani K, Nam YJ & Kitsis RN (2004). The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 95, 957–970. [DOI] [PubMed] [Google Scholar]
- Dai Z, Yu S, Zhao Q, Meng Y, He H, Tang Y, Wang X, Xiao J, Wang X & Huang C (2014). Renal sympathetic denervation suppresses ventricular substrate remodelling in a canine high‐rate pacing model. Eurointervention 10, 392–399. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD & Tagawa T (2002). Central mechanisms underlying short‐ and long‐term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol 29, 261–268. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS & McDowall LM (2005). Long‐term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32, 419–425. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Mankia KS, Golding S, Dawson T, Everatt L, Cai S, Channon KM & Paterson DJ (2004). Impaired regulation of neuronal nitric oxide synthase and heart rate during exercise in mice lacking one nNOS allele. J Physiol 558, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson EJ & Paterson DJ (2003). Enhanced neuronal nitric oxide synthase expression is central to cardiac vagal phenotype in exercise‐trained mice. J Physiol 546, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GM (2014). Vagal stimulation in heart failure. J Cardiovasc Transl Res 7, 310–320. [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K & Schwartz PJ; CardioFit Multicenter Trial Investigators (2011). Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32, 847–855. [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, Tuinenburg AE, Ruble S, Brugada J, Klein H, Butter C, Wright DJ, Schubert B, Solomon S, Meyer S, Stein K, Ramuzat A & Zannad F (2014). Rationale and study design of the NEuroCardiac TherApy foR Heart Failure Study: NECTAR‐HF. Eur J Heart Fail 16, 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GM, Vanoli E, Stramba‐Badiale M, Hull SS Jr, Foreman RD & Schwartz PJ (1991). Vagal reflexes and survival during acute myocardial ischemia in conscious dogs with healed myocardial infarction. Am J Physiol 261, H63–H69. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ & Schultz HD (2013). Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62, 2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Italia LJ (2011). Translational success stories: angiotensin receptor 1 antagonists in heart failure. Circ Res 109, 437–452. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Blain GM & Amann M (2014). Are type III–IV muscle afferents required for a normal steady‐state exercise hyperpnoea in humans? J Physiol 592, 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA & Smith CA (2014). Pathophysiology of human ventilatory control. Eur Respir J 44, 495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Weigand LA, Dyavanapalli J, Mares J, Wang X & Mendelowitz D (2014). Function and modulation of premotor brainstem parasympathetic cardiac neurons that control heart rate by hypoxia‐, sleep‐, and sleep‐related diseases including obstructive sleep apnea. Prog Brain Res 212, 39–58. [DOI] [PubMed] [Google Scholar]
- Ding X, Ardell JL, Hua F, McAuley RJ, Sutherly K, Daniel JJ & Williams CA (2008. a). Modulation of cardiac ischemia‐sensitive afferent neuron signaling by preemptive C2 spinal cord stimulation: effect on substance P release from rat spinal cord. Am J Physiol Regul Integr Comp Physiol 294, R93–R101. [DOI] [PubMed] [Google Scholar]
- Ding X, Hua F, Sutherly K, Ardell JL & Williams CA (2008. b). C2 spinal cord stimulation induces dynorphin release from rat T4 spinal cord: potential modulation of myocardial ischemia‐sensitive neurons. Am J Physiol Regul Integr Comp Physiol 295, R1519–R1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M, Gonzalez‐Quesada C & Frangogiannis NG (2010). The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48, 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S, Fox RE, Kidd C & Koley BN (1981). The distribution in the cat brain stem of neurones activated by vagal nonmyelinated fibres from the heart and lungs. Q J Exp Physiol 66, 391–404. [DOI] [PubMed] [Google Scholar]
- Donovan MK, Wyss JM & Winternitz SR (1983). Localization of renal sensory neurons using the fluorescent dye technique. Brain Res 259, 119–122. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Klemm MF & Steele PA (1995). Different types of ganglion cell in the cardiac plexus of guinea‐pigs. J Physiol 486, 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M & de Marchena E (2015). Is the failure of SYMPLICITY HTN‐3 trial to meet its efficacy endpoint the ‘end of the road’ for renal denervation? J Am Soc Hypertens 9, 140–149. [DOI] [PubMed] [Google Scholar]
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE & Bohm M (2010). Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet 376, 1903–1909. [DOI] [PubMed] [Google Scholar]
- Ewert TJ, Gritman KR, Bader M & Habecker BA (2008). Post‐infarct cardiac sympathetic hyperactivity regulates galanin expression. Neurosci Lett 436, 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Schild JH & Andresen MC (1999). Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol 277, R748–R756. [DOI] [PubMed] [Google Scholar]
- Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T & Yee SP (2002). Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 106, 873–879. [DOI] [PubMed] [Google Scholar]
- Fletcher EC (2001). Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol (1985) 90, 1600–1605. [DOI] [PubMed] [Google Scholar]
- Floras JS & Ponikowski P (2015). The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 36, 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea VG & Cohn JN (2014). The autonomic nervous system and heart failure. Circ Res 114, 1815–1826. [DOI] [PubMed] [Google Scholar]
- Foreman RD (1991). The neurological basis for cardiac pain In Reflex Control of the Circulation, ed. Zucker IH. & Gilmore JP, pp. 311–316. CRC Press, Inc, Boca Raton, Florida. [Google Scholar]
- Foreman RD (1999). Mechanisms of cardiac pain. Annu Rev Physiol 61, 143–167. [DOI] [PubMed] [Google Scholar]
- Foreman RD & Linderoth B (2012). Neural mechanisms of spinal cord stimulation. Int Rev Neurobiol 107, 87–119. [DOI] [PubMed] [Google Scholar]
- Foreman RD, DeJongste MJL & Linderoth B (2004). Integrative control of cardiac function by cervical and thoracic spinal neurons In Basic and Clinical Neurocardiology, ed. Armour JA. & Ardell JL, pp. 153–186. Oxford University Press, New York. [Google Scholar]
- Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS Jr, TerHorst GJ, DeJongste MJ & Armour JA (2000). Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res 47, 367–375. [DOI] [PubMed] [Google Scholar]
- Francisco LL, Hoversten LG & DiBona GF (1980). Renal nerves in the compensatory adaptation to ureteral occlusion. Am J Physiol 238, F229–F234. [DOI] [PubMed] [Google Scholar]
- Franco‐Cereceda A, Kallner G & Lundberg JM (1993). Capsazepine‐sensitive release of calcitonin gene‐related peptide from C‐fibre afferents in the guinea‐pig heart by low pH and lactic acid. Eur J Pharmacol 238, 311–316. [DOI] [PubMed] [Google Scholar]
- Fu LW, Guo ZL & Longhurst JC (2008). Undiscovered role of endogenous thromboxane A2 in activation of cardiac sympathetic afferents during ischaemia. J Physiol 586, 3287–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LW & Longhurst JC (2009). Regulation of cardiac afferent excitability in ischemia. Handb Exp Pharmacol, 185–225. [DOI] [PubMed] [Google Scholar]
- Fudim M, Groom KL, Laffer CL, Netterville JL, Robertson D & Elijovich F (2015). Effects of carotid body tumor resection on the blood pressure of essential hypertensive patients. J Am Soc Hypertens 9, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kanazawa H, Aizawa Y, Ardell JL & Shivkumar K (2015). Cardiac innervation and sudden cardiac death. Circ Res 116, 2005–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi K, Hosokawa K, Kishi T, Ide T & Sunagawa K (2014). Striking volume intolerance is induced by mimicking arterial baroreflex failure in normal left ventricular function. J Card Fail 20, 53–59. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Hoyano Y & Chiba S (1996). Parasympathetic inhibition of sympathetic effects on sinus rate in anesthetized dogs. Am J Physiol 271, H44–H50. [DOI] [PubMed] [Google Scholar]
- Gagliardi M, Randall WC, Bieger D, Wurster RD, Hopkins DA & Armour JA (1988). Activity of in vivo canine cardiac plexus neurons. Am J Physiol 255, H789–H800. [DOI] [PubMed] [Google Scholar]
- Gao L, Pan YX, Wang WZ, Li YL, Schultz HD, Zucker IH & Wang W (2007). Cardiac sympathetic afferent stimulation augments the arterial chemoreceptor reflex in anesthetized rats. J Appl Physiol (1985) 102, 37–43. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang WZ, Wang W & Zucker IH (2008). Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension 52, 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RT, Wang L, Lang BT, Cregg JM, Dunbar CL, Woodward WR, Silver J, Ripplinger CM & Habecker BA (2015). Targeting protein tyrosine phosphatase σ after myocardial infarction restores cardiac sympathetic innervation and prevents arrhythmias. Nat Commun 6, 6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband H, Rosen MR, Myerburg RJ, Bush HL, Bassett AL & Hoffman BF (1977). Restorative effect of epinephrine on the electrophysiologic properties of depressed human atrial tissue. J Electrocardiol 10, 313–320. [DOI] [PubMed] [Google Scholar]
- Gibbons DD, Southerland EM, Hoover DB, Beaumont E, Armour JA & Ardell JL (2012). Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 302, R357–R364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhov AV, Fedorov VV, Kalish PW, Ravikumar VK, Lou Q, Janks D, Schuessler RB, Moazami N & Efimov IR (2012). Conduction remodeling in human end‐stage nonischemic left ventricular cardiomyopathy. Circulation 125, 1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N & Efimov IR (2010). Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res 106, 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Wang S, Owen SL, Xie K, Liu X, Paterson DJ, Stein JF, Bain PG & Aziz TZ (2005). Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport 16, 1741–1745. [DOI] [PubMed] [Google Scholar]
- Grundy D (2004). What activates visceral afferents? Gut 53 (Suppl. 2), ii5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Niu YL, Zhang JW & Yao TP (2007). Coronary artery occlusion alters expression of substance P and its mRNA in spinal dorsal horn in rats. Neuroscience 145, 669–675. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhao Q, Deng H, Tang Y, Wang X, Dai Z, Xiao J, Wan P, Wang X, Huang H & Huang C (2014). Renal sympathetic denervation attenuates the ventricular substrate and electrophysiological remodeling in dogs with pacing‐induced heart failure. Int J Cardiol 175, 185–186. [DOI] [PubMed] [Google Scholar]
- Guyenet PG (2006). The sympathetic control of blood pressure. Nat Rev Neurosci 7, 335–346. [DOI] [PubMed] [Google Scholar]
- Guyenet PG (2014). Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4, 1511–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG & Abbott SB (2013). C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol 305, R187–R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habecker BA, Anderson ME, Birren SJ, Fukada K, Herring N, Hoover DB, Kanazawa H, Paterson DJ & Ripplinger CM (2016). Molecular and cellular neurocardiology: development, cellular and molecular adaptations to heart disease. J Physiol 594, 3853–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond HK, White FC, Brunton LL & Longhurst JC (1987). Association of decreased myocardial β‐receptors and chronotropic response to isoproterenol and exercise in pigs following chronic dynamic exercise. Circ Res 60, 720–726. [DOI] [PubMed] [Google Scholar]
- Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K & O'Leary DS (2000). Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278, H818–H828. [DOI] [PubMed] [Google Scholar]
- Han S, Kobayashi K, Joung B, Piccirillo G, Maruyama M, Vinters HV, March K, Lin SF, Shen C, Fishbein MC, Chen PS & Chen LS (2012). Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol 59, 954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Shimoni Y & Giles WR (1994). An obligatory role for nitric oxide in autonomic control of mammalian heart rate. J Physiol 476, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankes GH, Ardell JL, Tallaj J, Wei CC, Aban I, Holland M, Rynders P, Dillon R, Cardinal R, Hoover DB, Armour JA, Husain A & Dell'Italia LJ (2006). β 1‐Adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol 291, H147–H151. [DOI] [PubMed] [Google Scholar]
- Hardwick JC, Baran CN, Southerland EM & Ardell JL (2009). Remodeling of the guinea pig intrinsic cardiac plexus with chronic pressure overload. Am J Physiol Regul Integr Comp Physiol 297, R859–R866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JC, Ryan SE, Beaumont E, Ardell JL & Southerland EM (2014). Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci 181, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JC, Ryan SE, Powers EN, Southerland EM & Ardell JL (2015). Angiotensin receptors alter myocardial infarction‐induced remodeling of the guinea pig cardiac plexus. Am J Physiol Regul Integr Comp Physiol 309, R179–R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JC, Southerland EM & Ardell JL (2008). Chronic myocardial infarction induces phenotypic and functional remodeling in the guinea pig cardiac plexus. Am J Physiol Regul Integr Comp Physiol 295, R1926–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton DA, Li D, Almond SC, Dawson TA, Wang L, Channon KM & Paterson DJ (2007). Gene transfer of neuronal nitric oxide synthase into intracardiac ganglia reverses vagal impairment in hypertensive rats. Hypertension 49, 380–388. [DOI] [PubMed] [Google Scholar]
- Hering D, Zdrojewski Z, Krol E, Kara T, Kucharska W, Somers VK, Rutkowski B & Narkiewicz K (2007). Tonic chemoreflex activation contributes to the elevated muscle sympathetic nerve activity in patients with chronic renal failure. J Hypertens 25, 157–161. [DOI] [PubMed] [Google Scholar]
- Herring N (2015). Autonomic control of the heart: going beyond the classical neurotransmitters. Exp Physiol 100, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring N, Danson EJ & Paterson DJ (2002). Cholinergic control of heart rate by nitric oxide is site specific. News Physiol Sci 17, 202–206. [DOI] [PubMed] [Google Scholar]
- Herring N & Paterson DJ (2001). Nitric oxide–cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea‐pig in vitro . J Physiol 535, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring N & Paterson DJ (2009). Neuromodulators of peripheral cardiac sympatho‐vagal balance. Exp Physiol 94, 46–53. [DOI] [PubMed] [Google Scholar]
- Himura Y, Felten SY, Kashiki M, Lewandowski TJ, Delehanty JM & Liang CS (1993). Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation 88, 1299–1309. [DOI] [PubMed] [Google Scholar]
- Holycross BJ, Kukielka M, Nishijima Y, Altschuld RA, Carnes CA & Billman GE (2007). Exercise training normalizes β‐adrenoceptor expression in dogs susceptible to ventricular fibrillation. Am J Physiol Heart Circ Physiol 293, H2702–H2709. [DOI] [PubMed] [Google Scholar]
- Hoover DB, Shepherd AV, Southerland EM, Armour JA & Ardell JL (2008). Neurochemical diversity of afferent neurons that transduce sensory signals from dog ventricular myocardium. Auton Neurosci 141, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DA & Armour JA (1982). Medullary cells of origin of physiologically identified cardiac nerves in the dog. Brain Res Bull 8, 359–365. [DOI] [PubMed] [Google Scholar]
- Hosokawa K, Funakoshi K, Tanaka A, Sakamoto T, Onitsuka K, Sakamoto K, Tobushi T, Fujino T, Saku K, Murayama Y, Ide T & Sunagawa K (2011). Artificial baroreflex system restores volume tolerance in the absence of native baroreflex. Conf Proc IEEE Eng Med Biol Soc 2011, 697–699. [DOI] [PubMed] [Google Scholar]
- Hosokawa K, Ide T, Tobushi T, Sakamoto K, Onitsuka K, Sakamoto T, Fujino T, Saku K & Sunagawa K (2012). Bionic baroreceptor corrects postural hypotension in rats with impaired baroreceptor. Circulation 126, 1278–1285. [DOI] [PubMed] [Google Scholar]
- Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ; American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology (2012). Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111, 131–150. [DOI] [PubMed] [Google Scholar]
- Hua F, Ricketts BA, Reifsteck A, Ardell JL & Williams CA (2004). Myocardial ischemia induces the release of substance P from cardiac afferent neurons in rat thoracic spinal cord. Am J Physiol Heart Circ Physiol 286, H1654–H1664. [DOI] [PubMed] [Google Scholar]
- Huang HS, Pan HL, Stahl GL & Longhurst JC (1995). Ischemia‐ and reperfusion‐sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. Am J Physiol 269, H888–H901. [DOI] [PubMed] [Google Scholar]
- Huang J, Qian J, Yao W, Wang N, Zhang Z, Cao C, Song B & Zhang Z (2015). Vagus nerve stimulation reverses ventricular electrophysiological changes induced by hypersympathetic nerve activity. Exp Physiol 100, 239–248. [DOI] [PubMed] [Google Scholar]
- Huang MH, Ardell JL, Hanna BD, Wolf SG & Armour JA (1993). Effects of transient coronary artery occlusion on canine intrinsic cardiac neuronal activity. Integr Physiol Behav Sci 28, 5–21. [DOI] [PubMed] [Google Scholar]
- Inoue H, Skale BT & Zipes DP (1988). Effects of ischemia on cardiac afferent sympathetic and vagal reflexes in dog. Am J Physiol 255, H26–H35. [DOI] [PubMed] [Google Scholar]
- ISIS‐1 (1986). Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS‐1. First International Study of Infarct Survival Collaborative Group. Lancet 2, 57–66. [PubMed] [Google Scholar]
- Ito M & Zipes DP (1994). Efferent sympathetic and vagal innervation of the canine right ventricle. Circulation 90, 1459–1468. [DOI] [PubMed] [Google Scholar]
- Jänig W (2006). The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. Cambridge University Press, New York. [Google Scholar]
- Jänig W (2014. a). Autonomic nervous system and inflammation. Auton Neurosci 182, 1–3. [DOI] [PubMed] [Google Scholar]
- Jänig W (2014. b). Sympathetic nervous system and inflammation: a conceptual view. Auton Neurosci 182, 4–14. [DOI] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Li BY, Schild JH & Andresen MC (2004). Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24, 4709–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla M, Chotalia M, Coughlan C, Hao G, Crabtree MJ, Tomek J, Bub G, Paterson DJ & Herring N (2016). Protection against ventricular fibrillation via cholinergic receptor stimulation and the generation of nitric oxide. J Physiol 594, 3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandzari DE, Bhatt DL, Sobotka PA, O'Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha‐Singh K, Straley C, Townsend RR & Bakris G (2012). Catheter‐based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN‐3 Trial. Clin Cardiol 35, 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K & Somers VK (2003). Chemoreflexes – physiology and clinical implications. Acta Physiol Scand 177, 377–384. [DOI] [PubMed] [Google Scholar]
- Katchanov G, Xu J, Hurt CM & Pelleg A (1996). Electrophysiological‐anatomic correlates of ATP‐triggered vagal reflex in the dog. III. Role of cardiac afferents. Am J Physiol 270, H1785–H1790. [DOI] [PubMed] [Google Scholar]
- Kaufman MP & Rybicki KJ (1987). Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61, I60–65. [PubMed] [Google Scholar]
- Kelly RA, Balligand JL & Smith TW (1996). Nitric oxide and cardiac function. Circ Res 79, 363–380. [DOI] [PubMed] [Google Scholar]
- Kember G, Ardell JL, Armour JA & Zamir M (2014). Vagal nerve stimulation therapy: what is being stimulated? PLoS One 9, e114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kember G, Armour JA & Zamir M (2011). Neural control of heart rate: the role of neuronal networking. J Theor Biol 277, 41–47. [DOI] [PubMed] [Google Scholar]
- Kember G, Armour JA & Zamir M (2013. a). Dynamic neural networking as a basis for plasticity in the control of heart rate. J Theor Biol 317, 39–46. [DOI] [PubMed] [Google Scholar]
- Kember G, Armour JA & Zamir M (2013. b). Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol Genomics 45, 638–644. [DOI] [PubMed] [Google Scholar]
- Kember GC, Fenton GA, Armour JA & Kalyaniwalla N (2001). Competition model for aperiodic stochastic resonance in a Fitzhugh‐Nagumo model of cardiac sensory neurons. Phys Rev E Stat Nonlin Soft Matter Phys 63, 041911. [DOI] [PubMed] [Google Scholar]
- Keteyian SJ, Fleg JL, Brawner CA & Pina IL (2010). Role and benefits of exercise in the management of patients with heart failure. Heart Fail Rev 15, 523–530. [DOI] [PubMed] [Google Scholar]
- Keteyian SJ, Leifer ES, Houston‐Miller N, Kraus WE, Brawner CA, O'Connor CM, Whellan DJ, Cooper LS, Fleg JL, Kitzman DW, Cohen‐Solal A, Blumenthal JA, Rendall DS, Piña IL; HF‐ACTION Investigators (2012). Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol 60, 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Sala‐Mercado JA, Hammond RL, Rodriguez J, Scislo TJ & O'Leary DS (2005). Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol 289, H2416–H2423. [DOI] [PubMed] [Google Scholar]
- Kingma JG Jr, Linderoth B, Ardell JL, Armour JA, DeJongste MJ & Foreman RD (2001). Neuromodulation therapy does not influence blood flow distribution or left‐ventricular dynamics during acute myocardial ischemia. Auton Neurosci 91, 47–54. [DOI] [PubMed] [Google Scholar]
- Kirchheim HR (1976). Systemic arterial baroreceptor reflexes. Physiol Rev 56, 100–177. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Miller JP, Bigger JT Jr & Moss AJ (1987). Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59, 256–262. [DOI] [PubMed] [Google Scholar]
- Knuepfer MM & Schramm LP (1987). The conduction velocities and spinal projections of single renal afferent fibers in the rat. Brain Res 435, 167–173. [DOI] [PubMed] [Google Scholar]
- Koba S, Xing J, Sinoway LI & Li J (2008). Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol Heart Circ Physiol 294, H311–H321. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Smith LA & DiBona GF (1985). Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am J Physiol 249, F507–F517. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT & Esler M (2009). Catheter‐based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet 373, 1275–1281. [DOI] [PubMed] [Google Scholar]
- Kukielka M, Seals DR & Billman GE (2006). Cardiac vagal modulation of heart rate during prolonged submaximal exercise in animals with healed myocardial infarctions: effects of training. Am J Physiol Heart Circ Physiol 290, H1680–H1685. [DOI] [PubMed] [Google Scholar]
- Kumar P & Prabhakar NR (2012). Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2, 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze DL (1972). Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 222, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A & Schwartz PJ (1998). Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351, 478–484. [DOI] [PubMed] [Google Scholar]
- Latremoliere A & Woolf CJ (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10, 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Huh BK, Choi SS, Lee DK, Lim BG & Lee M (2012). The effect of epidural resiniferatoxin in the neuropathic pain rat model. Pain Physician 15, 287–296. [PubMed] [Google Scholar]
- Lee S, Sahadevan J, Khrestian CM, Durand DM & Waldo AL (2013). High density mapping of atrial fibrillation during vagal nerve stimulation in the canine heart: restudying the Moe hypothesis. J Cardiovasc Electrophysiol 24, 328–335. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ (2013). A brief history of G‐protein coupled receptors (Nobel Lecture). Angew Chem Int Ed Engl 52, 6366–6378. [DOI] [PubMed] [Google Scholar]
- Leiria TL, Glavinovic T, Armour JA, Cardinal R, de Lima GG & Kus T (2011). Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Auton Neurosci 161, 68–74. [DOI] [PubMed] [Google Scholar]
- Levy MN (1971). Sympathetic‐parasympathetic interactions in the heart. Circ Res 29, 437–445. [DOI] [PubMed] [Google Scholar]
- Levy MN & Martin PJ (1979). Neural control of the heart In Handbook of Physiology, section 2, The Cardiovascular System, vol. I, The Heart, ed. Berne RM, pp. 581–620. The American Physiological Society, Bethesda. [Google Scholar]
- Levy MN, Ng M, Martin P, Zieske H & Rogoff T (1966). Sympathetic and parasympathetic interactions upon the left ventricle of the dog. Circ Res 19, 5–10. [Google Scholar]
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M & Sunagawa K (2004). Vagal nerve stimulation markedly improves long‐term survival after chronic heart failure in rats. Circulation 109, 120–124. [DOI] [PubMed] [Google Scholar]
- Liang CS (2003). Sympatholysis and cardiac sympathetic nerve function in the treatment of congestive heart failure. J Am Coll Cardiol 42, 549–551. [DOI] [PubMed] [Google Scholar]
- Liu JL, Kulakofsky J & Zucker IH (2002). Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J Appl Physiol (1985) 92, 2403–2408. [DOI] [PubMed] [Google Scholar]
- Liu JL, Pliquett RU, Brewer E, Cornish KG, Shen YT & Zucker IH (2001). Chronic endothelin‐1 blockade reduces sympathetic nerve activity in rabbits with heart failure. Am J Physiol Regul Integr Comp Physiol 280, R1906–R1913. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Zhou X, Dusa C, Ueyama T, Rosenberger J, Courtney N, Ujhelyi M, Mullen T, Das M & Zipes DP (2009). Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation 120, 286–294. [DOI] [PubMed] [Google Scholar]
- Lopshire JC & Zipes DP (2012). Device therapy to modulate the autonomic nervous system to treat heart failure. Curr Cardiol Rep 14, 593–600. [DOI] [PubMed] [Google Scholar]
- Lopshire JC & Zipes DP (2014). Spinal cord stimulation for heart failure: preclinical studies to determine optimal stimulation parameters for clinical efficacy. J Cardiovasc Transl Res 7, 321–329. [DOI] [PubMed] [Google Scholar]
- Lubbe WF, Podzuweit T & Opie LH (1992). Potential arrhythmogenic role of cyclic adenosine monophosphate (AMP) and cytosolic calcium overload: implications for prophylactic effects of beta‐blockers in myocardial infarction and proarrhythmic effects of phosphodiesterase inhibitors. J Am Coll Cardiol 19, 1622–1633. [DOI] [PubMed] [Google Scholar]
- Lujan HL, Palani G, Zhang L & DiCarlo SE (2010). Targeted ablation of cardiac sympathetic neurons reduces the susceptibility to ischemia‐induced sustained ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 298, H1330–H1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Salo LM, Paton JF & Pickering AE (2011). Processing of central and reflex vagal drives by rat cardiac ganglion neurones: an intracellular analysis. J Physiol 589, 5801–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM & Spyer KM (1978). Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol 282, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA & Paton JF (2013). The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun 4, 2395. [DOI] [PubMed] [Google Scholar]
- MacDonnell SM, Kubo H, Crabbe DL, Renna BF, Reger PO, Mohara J, Smithwick LA, Koch WJ, Houser SR & Libonati JR (2005). Improved myocardial β‐adrenergic responsiveness and signaling with exercise training in hypertension. Circulation 111, 3420–3428. [DOI] [PubMed] [Google Scholar]
- McGuirt AS, Schmacht DC & Ardell JL (1997). Autonomic interactions for control of atrial rate are maintained after SA nodal parasympathectomy. Am J Physiol 272, H2525–H2533. [DOI] [PubMed] [Google Scholar]
- Malliani A & Lombardi F (1982). Consideration of the fundamental mechanisms eliciting cardiac pain. Am Heart J 103, 575–578. [DOI] [PubMed] [Google Scholar]
- Malliani A & Montano N (2002). Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension 39, 63–68. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Pizzinelli P, Furlan R & Guzzetti S (1983). Cardiovascular reflexes mediated by sympathetic afferent fibers. J Auton Nerv Syst 7, 295–301. [DOI] [PubMed] [Google Scholar]
- Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Luscher T, Pasic M & Thelle D (2002). The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J 23, 355–370. [DOI] [PubMed] [Google Scholar]
- Marcus NJ, Del Rio R, Schultz EP, Xia XH & Schultz HD (2014). Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM (1994). Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74, 543–594. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Johnson TA & Gatti PJ (1995). Cardiotopic organization of the nucleus ambiguus? An anatomical and physiological analysis of neurons regulating atrioventricular conduction. Brain Res 679, 227–240. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Podolin DA & Henry V (1995). Effects of age and endurance training on β‐adrenergic receptor characteristics in Fischer 344 rats. Mech Ageing Dev 84, 157–169. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D (1999). Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14, 155–161. [DOI] [PubMed] [Google Scholar]
- Mifflin SW (1996). Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol 271, R870–R880. [DOI] [PubMed] [Google Scholar]
- Mill JG, Stefanon I, dos Santos L & Baldo MP (2011). Remodeling in the ischemic heart: the stepwise progression for heart failure. Braz J Med Biol Res 44, 890–898. [DOI] [PubMed] [Google Scholar]
- Milligan ED & Watkins LR (2009). Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollema SA, Liem SS, Suffoletto MS, Bleeker GB, van der Hoeven BL, van de Veire NR, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Gorcsan J 3rd & Bax JJ (2007). Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. J Am Coll Cardiol 50, 1532–1540. [DOI] [PubMed] [Google Scholar]
- Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ & Husain M (2002). Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 109, 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Thompson GW, Ardell JL, McCraty R, Stevenson RS, Sangalang VE, Cardinal R, Wilkinson M, Craig S, Smith FM, Kingma JG & Armour JA (2000). The heart reinnervates after transplantation. Ann Thorac Surg 69, 1769–1781. [DOI] [PubMed] [Google Scholar]
- Naccarelli GV, Varker H, Lin J & Schulman KL (2009). Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 104, 1534–1539. [DOI] [PubMed] [Google Scholar]
- Nadeau R, Cardinal R, Armour JA, Kus T, Richer LP, Vermeulen M, Yin Y & Page P (2007). Cervical vagosympathetic and mediastinal nerves activation effects on atrial arrhythmia formation. Anadolu Kardiyol Derg 7 (Suppl. 1), 34–36. [PubMed] [Google Scholar]
- Nerdrum T, Baker DG, Coleridge HM & Coleridge JC (1986). Interaction of bradykinin and prostaglandin E1 on cardiac pressor reflex and sympathetic afferents. Am J Physiol 250, R815–R822. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE & Coote JH (2001). Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart – a novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol 86, 319–329. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Patel VH & Coote JH (2007). Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 73, 750–760. [DOI] [PubMed] [Google Scholar]
- Nguyen BL, Li H, Fishbein MC, Lin SF, Gaudio C, Chen PS & Chen LS (2012). Acute myocardial infarction induces bilateral stellate ganglia neural remodeling in rabbits. Cardiovasc Pathol 21, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Jazwiec P, Banasiak W, Sobotka PA, Hart EC, Paton JF & Ponikowski P (2014). Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp Physiol 99, 552–561. [DOI] [PubMed] [Google Scholar]
- Niijima A (1975). Observation on the localization of mechanoreceptors in the kidney and afferent nerve fibres in the renal nerves in the rabbit. J Physiol 245, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E & Carruba MO (2006). Nitric oxide and mitochondrial biogenesis. J Cell Sci 119, 2855–2862. [DOI] [PubMed] [Google Scholar]
- Niu YL, Guo Z & Zhou RH (2009). Up‐regulation of TNF‐α in neurons of dorsal root ganglia and spinal cord during coronary artery occlusion in rats. Cytokine 47, 23–29. [DOI] [PubMed] [Google Scholar]
- Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM & Fox KA (1998). Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK‐heart). Circulation 98, 1510–1516. [DOI] [PubMed] [Google Scholar]
- Norris JE, Foreman RD & Wurster RK (1974). Responses of the canine heart to stimulation of the first five ventral thoracic roots. Am J Physiol 227, 9–12. [DOI] [PubMed] [Google Scholar]
- Norris JE, Lippincott D & Wurster RD (1977). Responses of canine endocardium to stimulation of the upper thoracic roots. Am J Physiol 233, H655–H659. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H & Inoue H (2002). Effects of long‐term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart Vessels 16, 51–56. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S & Meister B (2003). Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 50, 117–129. [DOI] [PubMed] [Google Scholar]
- Opie LH & Clusin WT (1990). Cellular mechanism for ischemic ventricular arrhythmias. Annu Rev Med 41, 231–238. [DOI] [PubMed] [Google Scholar]
- Park HW, Shen MJ, Lin SF, Fishbein MC, Chen LS & Chen PS (2012). Neural mechanisms of atrial fibrillation. Curr Opin Cardiol 27, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Kasparov S & Paterson DJ (2002). Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci 25, 626–631. [DOI] [PubMed] [Google Scholar]
- Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA & Narkiewicz K (2013). Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep 15, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA & Andresen MC (2011). TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLoS One 6, e25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH & Anand IS (2014). Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: Results of the ANTHEM‐HF Trial. J Card Fail 20, 808–816. [DOI] [PubMed] [Google Scholar]
- Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH & Anand IS (2015). Extended follow‐up of patients with heart failure receiving autonomic regulation therapy in the ANTHEM‐HF Study. J Card Fail DOI: http://dx.doi.org/10.1016/j.cardfail.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL & Shivkumar K (2016). Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 594, 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DC, Brown DR, Li SG, Olmstead ME, Kilgore JM, Sprinkle AG, Randall WC & Ardell JL (1998). Ablation of posterior atrial ganglionated plexus potentiates sympathetic tachycardia to behavioral stress. Am J Physiol 275, R779–R787. [DOI] [PubMed] [Google Scholar]
- Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA & Ardell JL (2003). Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol 285, R1066–R1075. [DOI] [PubMed] [Google Scholar]
- Randall WC (1994). Efferent sympathetic innervation of the heart In Neurocardiology, ed. Armour JA. & Ardell JL, pp. 77–94. Oxford University Press, New York. [Google Scholar]
- Randall WC, Armour JA, Geis WP & Lippincott DB (1972). Regional cardiac distribution of the sympathetic nerves. Fed Proc 31, 1199–1208. [PubMed] [Google Scholar]
- Recordati G, Genovesi S & Cerati D (1982). Renorenal reflexes in the rat elicited upon stimulation of renal chemoreceptors. J Auton Nerv Syst 6, 127–142. [DOI] [PubMed] [Google Scholar]
- Recordati GM, Moss NG, Genovesi S & Rogenes PR (1980). Renal receptors in the rat sensitive to chemical alterations of their environment. Circ Res 46, 395–405. [DOI] [PubMed] [Google Scholar]
- Recordati GM, Moss NG & Waselkov L (1978). Renal chemoreceptors in the rat. Circ Res 43, 534–543. [DOI] [PubMed] [Google Scholar]
- Reddy MK, Patel KP & Schultz HD (2005). Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol 289, R789–R797. [DOI] [PubMed] [Google Scholar]
- Remo BF, Preminger M, Bradfield J, Mittal S, Boyle N, Gupta A, Shivkumar K, Steinberg JS & Dickfeld T (2014). Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm 11, 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KS, Hsueh CH, Hellyer JA, Park HW, Lee YS, Garlie J, Onkka P, Doytchinova AT, Garner JB, Patel J, Chen LS, Fishbein MC, Everett T 4th, Lin SF & Chen PS (2015). Cervical vagal nerve stimulation activates the stellate ganglion in ambulatory dogs. Korean Circ J 45, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC & Conde SV (2013). Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62, 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogenes PR (1982). Single‐unit and multiunit analyses of renorenal reflexes elicited by stimulation of renal chemoreceptors in the rat. J Auton Nerv Syst 6, 143–156. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS & Kellogg DL Jr (1996). Chapter 17. Integration of cardiovascular control systems in dynamic exercise In Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB. & Shepherd J. pp. 770–838. Oxford Press, New York. [Google Scholar]
- Ruble SB, Hamann JJ, Gupta RC, Mishra S & Sabbah HN (2010). Chronic vagus nerve stimulation impacts biomarkers of heart failure in canines. J Am Coll Cardiol 55, A16.E153. [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA & Eckberg DL (1999). Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol 276, H1691–H1698. [DOI] [PubMed] [Google Scholar]
- Sabbah HN (2011). Electrical vagus nerve stimulation for the treatment of chronic heart failure. Cleve Clin J Med 78 (Suppl. 1), S24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah HN, Gupta RC, Imai M, Irwin ED, Rastogi S, Rossing MA & Kieval RS (2011. a). Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail 4, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M & Gupta RC (2011. b). Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 16, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Hosokawa K, Saku K, Sakamoto T, Tobushi T, Oga Y, Kishi T, Ide T & Sunagawa K (2016). Baroreflex failure increases the risk of pulmonary edema in conscious rats with normal left ventricular function. Am J Physiol Heart Circ Physiol 310, H199–H205. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kakino T, Sakamoto K, Tobushi T, Tanaka A, Saku K, Hosokawa K, Onitsuka K, Murayama Y, Tsutsumi T, Ide T & Sunagawa K (2015). Changes in vascular properties, not ventricular properties, predominantly contribute to baroreflex regulation of arterial pressure. Am J Physiol Heart Circ Physiol 308, H49–H58. [DOI] [PubMed] [Google Scholar]
- Saku K, Kishi T, Sakamoto K, Hosokawa K, Sakamoto T, Murayama Y, Kakino T, Ikeda M, Ide T & Sunagawa K (2014). Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiol Rep 2, e12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala‐Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW & O'Leary DS (2006). Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290, H751–H757. [DOI] [PubMed] [Google Scholar]
- Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T & de Leeuw PW (2010). Novel baroreflex activation therapy in resistant hypertension: results of a European multi‐center feasibility study. J Am Coll Cardiol 56, 1254–1258. [DOI] [PubMed] [Google Scholar]
- Scherlag BJ, Patterson E & Po SS (2006). The neural basis of atrial fibrillation. J Electrocardiol 39, S180–183. [DOI] [PubMed] [Google Scholar]
- Scherlag BJ & Po S (2006). The intrinsic cardiac nervous system and atrial fibrillation. Curr Opin Cardiol 21, 51–54. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM & Guyenet PG (2002). The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29, 514–521. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ & Del Rio R (2013). Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep 15, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ & Del Rio R (2015. a). Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp Physiol 100, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ & Del Rio R (2015. b). Role of the carotid body chemoreflex in the pathophysiology of heart failure: A perspective from animal studies. Adv Exp Med Biol 860, 167–185. [DOI] [PubMed] [Google Scholar]
- Schultz HD & Ustinova EE (1996). Cardiac vagal afferent stimulation by free radicals during ischaemia and reperfusion. Clin Exp Pharmacol Physiol 23, 700–708. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ (1984). Sympathetic imbalance and cardiac arrhythmias In Nervous Control of Cardiovascular Functions, ed. Randall WC, pp. 225–252. Oxford University Press, New York. [Google Scholar]
- Schwartz PJ (2001). QT prolongation, sudden death, and sympathetic imbalance: the pendulum swings. J Cardiovasc Electrophysiol 12, 1074–1077. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ (2014). Cardiac sympathetic denervation to prevent life‐threatening arrhythmias. Nat Rev Cardiol 11, 346–353. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Foreman RD, Stone HL & Brown AM (1976). Effect of dorsal root section on the arrhythmias associated with coronary occlusion. Am J Physiol 231, 923–928. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, La Rovere MT & Vanoli E (1992). Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post‐myocardial infarction risk stratification. Circulation 85, I77–91. [PubMed] [Google Scholar]
- Seagard JL, van Brederode JF, Dean C, Hopp FA, Gallenberg LA & Kampine JP (1990). Firing characteristics of single‐fiber carotid sinus baroreceptors. Circ Res 66, 1499–1509. [DOI] [PubMed] [Google Scholar]
- Sheldahl LM, Ebert TJ, Cox B & Tristani FE (1994). Effect of aerobic training on baroreflex regulation of cardiac and sympathetic function. J Appl Physiol (1985) 76, 158–165. [DOI] [PubMed] [Google Scholar]
- Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF & Chen PS (2011). Continuous low‐level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 123, 2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MJ & Zipes DP (2014). Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114, 1004–1021. [DOI] [PubMed] [Google Scholar]
- Sheng X, Scherlag BJ, Yu L, Li S, Ali R, Zhang Y, Fu G, Nakagawa H, Jackman WM, Lazzara R & Po SS (2011). Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low‐level vagosympathetic nerve stimulation. J Am Coll Cardiol 57, 563–571. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Augustyniak RA & O'Leary DS (1998). Muscle chemoreflex‐induced increases in right atrial pressure. Am J Physiol 275, H767–H775. [DOI] [PubMed] [Google Scholar]
- Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Kumfu S, Kumphune S, Chattipakorn S, KenKnight BH & Chattipakorn N (2014). Vagus nerve stimulation initiated late during ischemia, but not reperfusion, exerts cardioprotection via amelioration of cardiac mitochondrial dysfunction. Heart Rhythm 11, 2278–2287. [DOI] [PubMed] [Google Scholar]
- Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Thunsiri K, Weerateerangkul P, Chattipakorn S, KenKnight BH & Chattipakorn N (2013). Low‐amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia‐reperfusion injury. Heart Rhythm 10, 1700–1707. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Shen MJ, Han S, Maruyama M, Park HW, Fishbein MC, Shen C, Chen PS & Lin SF (2012). Heart failure decreases nerve activity in the right atrial ganglionated plexus. J Cardiovasc Electrophysiol 23, 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen P‐S, Esler M, De Ferrari G, Fishbein MC, Goldberger JJ, Harper RM, Joyner MJ, Khalsa SS, Kumar R, Lane R, Mahajan A, Po S, Schwartz PJ, Somers VK, Valderrabano M, Vaseghi M & Zipes DP (2016). Clinical neurocardiology defining the value of neuroscience‐based cardiovascular therapeutics. J Physiol 594, 3911–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight P (2014). A historical perspective on peripheral reflex cardiovascular control from animals to man. Exp Physiol 99, 1017–1026. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mammen PP, Mitchell JH & Garry MG (2003). Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL & Abboud FM (1991). Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87, 1953–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Zavala DC & Abboud FM (1989. a). Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol (1985) 67, 2095–2100. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Zavala DC & Abboud FM (1989. b). Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985) 67, 2101–2106. [DOI] [PubMed] [Google Scholar]
- Southerland EM, Gibbons DD, Smith SB, Sipe A, Williams CA, Beaumont E, Armour JA, Foreman RD & Ardell JL (2012). Activated cranial cervical cord neurons affect left ventricular infarct size and the potential for sudden cardiac death. Auton Neurosci 169, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southerland EM, Milhorn DM, Foreman RD, Linderoth B, DeJongste MJ, Armour JA, Subramanian V, Singh M, Singh K & Ardell JL (2007). Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia‐induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol 292, H311–H317. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Ogawa T, Coggan AR, Holloszy JO & Ehsani AA (1992). Exercise training improves left ventricular contractile response to beta‐adrenergic agonist. J Appl Physiol (1985) 72, 307–311. [DOI] [PubMed] [Google Scholar]
- Staszewska‐Barczak J (1983). Prostanoids and cardiac reflexes of sympathetic and vagal origin. Am J Cardiol 52, 36A–45A. [DOI] [PubMed] [Google Scholar]
- Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R & Po SS (2015). Low‐level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 65, 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steagall RJ, Sipe AL, Williams CA, Joyner WL & Singh K (2012). Substance P release in response to cardiac ischemia from rat thoracic spinal dorsal horn is mediated by TRPV1. Neuroscience 214, 106–119. [DOI] [PubMed] [Google Scholar]
- Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C & Bunnett NW (2014). Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 94, 265–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Sonner PM, Son SJ, Silva FC, Jackson K & Michelini LC (2012). Exercise training normalizes an increased neuronal excitability of NTS‐projecting neurons of the hypothalamic paraventricular nucleus in hypertensive rats. J Neurophysiol 107, 2912–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Wang W & Schultz HD (2001). Activation of cardiac afferents by arachidonic acid: relative contributions of metabolic pathways. Am J Physiol Heart Circ Physiol 281, H93–H104. [DOI] [PubMed] [Google Scholar]
- Sun SY, Wang W, Zucker IH & Schultz HD (1999). Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol (1985) 86, 1273–1282. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW & Gilman AG (1996). Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36, 461–480. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir YB, Green AL, Aziz TZ, Bahuri NF, Hyam J, Basnayake SD & Paterson DJ (2014). Differentiated baroreflex modulation of sympathetic nerve activity during deep brain stimulation in humans. Hypertension 63, 1000–1010. [DOI] [PubMed] [Google Scholar]
- Tallaj J, Wei CC, Hankes GH, Holland M, Rynders P, Dillon AR, Ardell JL, Armour JA, Lucchesi PA & Dell'Italia LJ (2003). β1‐Adrenergic receptor blockade attenuates angiotensin II‐mediated catecholamine release into the cardiac interstitium in mitral regurgitation. Circulation 108, 225–230. [DOI] [PubMed] [Google Scholar]
- Thompson GW, Horackova M & Armour JA (2000). Chemotransduction properties of nodose ganglion cardiac afferent neurons in guinea pigs. Am J Physiol Regul Integr Comp Physiol 279, R433–R439. [DOI] [PubMed] [Google Scholar]
- Thoren PN (1977). Characteristics of left ventricular receptors with nonmedullated vagal afferents in cats. Circ Res 40, 415–421. [DOI] [PubMed] [Google Scholar]
- Thoren PN, Donald DE & Shepherd JT (1976). Role of heart and lung receptors with nonmedullated vagal afferents in circulatory control. Circ Res 38, 2–9. [DOI] [PubMed] [Google Scholar]
- Thornton JM, Aziz T, Schlugman D & Paterson DJ (2002). Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol 539, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ (2007). Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse HF, Turner S, Sanders P, Okuyama Y, Fujiu K, Cheung CW, Russo M, Green MD, Yiu KH, Chen P, Shuto C, Lau EO & Siu CW (2015). Thoracic spinal cord stimulation for heart failure as a restorative treatment (SCS HEART study): First‐in‐man experience. Heart Rhythm 12, 588–595. [DOI] [PubMed] [Google Scholar]
- Uchida Y & Murao S (1974). Bradykinin‐induced excitation of afferent cardiac sympathetic nerve fibers. Jpn Heart J 15, 84–91. [DOI] [PubMed] [Google Scholar]
- Uchida Y & Murao S (1975). Acid‐induced excitation of afferent cardiac sympathetic nerve fibers. Am J Physiol 228, 27–33. [DOI] [PubMed] [Google Scholar]
- Ueda H, Uchida Y & Kamisaka K (1967). Mechanism of the reflex depressor effect by the kidney in dog. Jpn Heart J 8, 597–606. [DOI] [PubMed] [Google Scholar]
- Ulasova E, Gladden JD, Chen Y, Zheng J, Pat B, Bradley W, Powell P, Zmijewski JW, Zelickson BR, Ballinger SW, Darley‐Usmar V & Dell'Italia LJ (2011). Loss of interstitial collagen causes structural and functional alterations of cardiomyocyte subsarcolemmal mitochondria in acute volume overload. J Mol Cell Cardiol 50, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova EE & Schultz HD (1994. a). Activation of cardiac vagal afferents by oxygen‐derived free radicals in rats. Circ Res 74, 895–903. [DOI] [PubMed] [Google Scholar]
- Ustinova EE & Schultz HD (1994. b). Activation of cardiac vagal afferents in ischemia and reperfusion. Prostaglandins versus oxygen‐derived free radicals. Circ Res 74, 904–911. [DOI] [PubMed] [Google Scholar]
- Vance WH & Bowker RC (1983). Spinal origins of cardiac afferents from the region of the left anterior descending artery. Brain Res 258, 96–100. [DOI] [PubMed] [Google Scholar]
- Vandecasteele G, Eschenhagen T, Scholz H, Stein B, Verde I & Fischmeister R (1999). Muscarinic and β‐adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat Med 5, 331–334. [DOI] [PubMed] [Google Scholar]
- Vaseghi M, Ajijola O, Mahajan A & Shivkumar K (2013). Sympathetic innervations, denervation, and cardiac arrhythmias In Cardiac Electrophysiology: From Cell to Bedside, ed. Zipes DP. & Jalifa J, pp. 391–403. Saunders Elsevier, Philadelphia. [Google Scholar]
- Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A & Shivkumar K (2014). Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long‐term follow‐up. Heart Rhythm 11, 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi M, Lellouche N, Ritter H, Fonarow GC, Patel JK, Moriguchi J, Fishbein MC, Kobashigawa JA & Shivkumar K (2009). Mode and mechanisms of death after orthotopic heart transplantation. Heart Rhythm 6, 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi M, Lux RL, Mahajan A & Shivkumar K (2012). Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol 302, H1838–H1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi M & Shivkumar K (2008). The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50, 404–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi M & Shivkumar K (2012). Neuraxial modulation for ventricular arrhythmias: a new hope. Heart Rhythm 9, 1888–1889. [DOI] [PubMed] [Google Scholar]
- Villarreal D, Freeman RH, Johnson RA & Simmons JC (1994). Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am J Physiol 266, R1599–R1604. [DOI] [PubMed] [Google Scholar]
- Waldmann M, Thompson GW, Kember GC, Ardell JL & Armour JA (2006). Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol (1985) 101, 413–419. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al‐Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ & Ulloa L (2004). Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10, 1216–1221. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Wang W, Cornish KG, Rozanski GJ & Zucker IH (2014). Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhou X, Wang Z, Huang B, Zhou L, Chen M, Yu L & Jiang H (2015). DEFEAT‐HF Trial: The potential causes for the negative result. Int J Cardiol 191, 271–272. [DOI] [PubMed] [Google Scholar]
- Wang W & Zucker IH (1996). Cardiac sympathetic afferent reflex in dogs with congestive heart failure. Am J Physiol 271, R751–R756. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Gao L, Pan YX, Zucker IH & Wang W (2006). Differential effects of cardiac sympathetic afferent stimulation on neurons in the nucleus tractus solitarius. Neurosci Lett 409, 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WZ, Gao L, Pan YX, Zucker IH & Wang W (2007). AT1 receptors in the nucleus tractus solitarii mediate the interaction between the baroreflex and the cardiac sympathetic afferent reflex in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 292, R1137–R1145. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Gao L, Wang HJ, Zucker IH & Wang W (2008). Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol 295, H1216–H1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M & Jais P (2011). Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow‐up? J Am Coll Cardiol 57, 160–166. [DOI] [PubMed] [Google Scholar]
- Wei CC, Chen Y, Powell LC, Zheng J, Shi K, Bradley WE, Powell PC, Ahmad S, Ferrario CM & Dell'Italia LJ (2012). Cardiac kallikrein‐kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss. PLoS One 7, e40110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CB, Roberts AM & Joshua IG (1993). Arteriolar dilation mediated by capsaicin and calcitonin gene‐related peptide in rats. Am J Physiol 265, H1411–H1415. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Fadel PJ & Mitchell JH (2006). New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol 91, 51–58. [DOI] [PubMed] [Google Scholar]
- Woolf CJ & Salter MW (2000). Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769. [DOI] [PubMed] [Google Scholar]
- Wu ZZ & Pan HL (2007). Role of TRPV1 and intracellular Ca2+ in excitation of cardiac sensory neurons by bradykinin. Am J Physiol Regul Integr Comp Physiol 293, R276–R283. [DOI] [PubMed] [Google Scholar]
- Wyss CR, Ardell JL, Scher AM & Rowell LB (1983). Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol 245, H481–H486. [DOI] [PubMed] [Google Scholar]
- Wyss JM & Donovan MK (1984). A direct projection from the kidney to the brainstem. Brain Res 298, 130–134. [DOI] [PubMed] [Google Scholar]
- Xu B, Zheng H, Liu X & Patel KP (2015). Activation of afferent renal nerves modulates RVLM‐projecting PVN neurons. Am J Physiol Heart Circ Physiol 308, H1103–H1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zheng H & Patel KP (2013). Relative contributions of the thalamus and the paraventricular nucleus of the hypothalamus to the cardiac sympathetic afferent reflex. Am J Physiol Regul Integr Comp Physiol 305, R50–R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K, Howard‐Quijano K, Zhou W, Rajendran PS, Yagishita D, Vaseghi M, Ajijola OA, Armour JA, Shivkumar K, Ardell JL & Mahajan A (2016). Central vs. peripheral neuraxial sympathetic control of porcine ventricular electrophysiology. Am J Physiol Regul Integr Comp Physiol 310, R414–R421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K, Rajendran PS, Takamiya T, Yagishita D, So EL, Mahajan A, Shivkumar K & Vaseghi M (2015). Vagal nerve stimulation activates vagal afferent fibers that reduce cardiac efferent parasympathetic effects. Am J Physiol Heart Circ Physiol 309, H1579–H1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K, So EL, Rajendran PS, Hoang JD, Makkar N, Mahajan A, Shivkumar K & Vaseghi M (2014). Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium. Am J Physiol Heart Circ Physiol 307, H722–H731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita H, Sato E, Kawaguchi M, Saito T, Maehara K & Maruyama Y (1994). Nonadrenergic noncholinergic nerves regulate basal coronary flow via release of capsaicin‐sensitive neuropeptides in the rat heart. Circ Res 75, 780–788. [DOI] [PubMed] [Google Scholar]
- Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, Zhang Y, Jackman WM, Lazzara R, Jiang H & Po SS (2011). Low‐level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol 22, 455–463. [DOI] [PubMed] [Google Scholar]
- Yuan BX, Ardell JL, Hopkins DA, Losier AM & Armour JA (1994). Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec 239, 75–87. [DOI] [PubMed] [Google Scholar]
- Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, Ramuzat A, Schubert B, Daum D, Neuzil P, Botman C, Castel MA, D'Onofrio A, Solomon SD, Wold N & Ruble SB (2015). Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR‐HF) randomized controlled trial. Eur Heart J 36, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TC, Janik JJ & Grill WM (2014). Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res 1569, 19–31. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ilsar I, Sabbah HN, Ben David T & Mazgalev TN (2009. a). Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm 6, 244–250. [DOI] [PubMed] [Google Scholar]
- Zhang Y & Mazgalev TN (2011). Arrhythmias and vagus nerve stimulation. Heart Fail Rev 16, 147–161. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR & Mazgalev TN (2009. b). Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high‐rate pacing model. Circ Heart Fail 2, 692–699. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Scherlag BJ, Lu Z, Niu GD, Yamanashi WS, Hogan C, Fields J, Ghias M, Lazzara R, Jackman WM & Po S (2009. c). Comparison of atrial fibrillation inducibility by electrical stimulation of either the extrinsic or the intrinsic autonomic nervous systems. J Interv Card Electrophysiol 24, 5–10. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Huang H, Wang X, Wang X, Dai Z, Wan P, Guo Z, Yu S, Tang Y & Huang C (2014). Changes of serum neurohormone after renal sympathetic denervation in dogs with pacing‐induced heart failure. Int J Clin Exp Med 7, 4024–4030. [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B & Chen PS (2004). Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95, 76–83. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH & Wang W (2004. a). AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 287, H1828–H1835. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Gao L, Patel KP, Zucker IH & Wang W (2004. b). ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol (1985) 97, 1746–1754. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Patel KP, Zucker IH & Wang W (2002). Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol 282, H2039–H2045. [DOI] [PubMed] [Google Scholar]
- Zipes DP (2015). Antiarrhythmic therapy in 2014: Contemporary approaches to treating arrhythmias. Nat Rev Cardiol 12, 68–69. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL & Zamorano JL; American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines (2006). ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 48, e247–346. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Neuzil P, Theres H, Caraway D, Mann DL, Mannheimer C, Van Buren P, Linde C, Linderoth B, Kueffer F, Sarazin SA & DeJongste MJ; DEFEAT‐HF Trial Investigators (2016). Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: The DEFEAT‐HF study. JACC Heart Fail 4, 129–136. [DOI] [PubMed] [Google Scholar]
- Zucker IH & Gilmore JP (1991). Reflex Control of the Circulation. CRC Press, Boca Raton. [Google Scholar]
- Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD & Rossing MA (2007). Chronic baroreceptor activation enhances survival in dogs with pacing‐induced heart failure. Hypertension 50, 904–910. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Patel KP & Schultz HD (2012). Neurohumoral stimulation. Heart Fail Clin 8, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Wang W, Brandle E & Schultz HD (1996). Baroreflex and cardiac reflex control of the circulation in pacing‐induced heart failure In Pathophysiology of Tachycardia‐Induced Heart Failure, ed. Spinale FG, pp. 193–226. Futura Publishing, Armonk, New York. [Google Scholar]
- Zucker IH, Wang W, Brandle M, Schultz HD & Patel KP (1995. a). Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis 37, 397–414. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Wang W, Pliquett RU, Liu JL & Patel KP (2001). The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann NY Acad Sci 940, 431–443. [PubMed] [Google Scholar]
- Zucker IH, Wang W & Schultz HD (1995. b). Cardiac receptor activity in heart failure: implications for the control of sympathetic nervous outflow. Adv Exp Med Biol 381, 109–124. [PubMed] [Google Scholar]


