Abbreviations
- AVN
atrioventricular node
- CM
cardiomotor
- GIT
gastrointestinal tract
- ICNS
intrinsic cardiac nervous system
- NTS
nucleus of the solitary tract
- SAN
sinoatrial node
- WHBP
working heart–brain stem preparation
Pre‐ and postganglionic neurones of the autonomic nervous system are functionally differentiated according to their target tissues. Individual sympathetic pre‐ and postganglionic neurones can be activated or inhibited reflexly by appropriate physiological stimuli as has been shown in anaesthetized animals (mainly cats and rats) for neurones of the lumbar sympathetic outflow to skeletal muscle, skin and pelvic viscera and for neurones of the thoracic sympathetic outflow to the head and neck (Jänig, 2006; Jänig & McLachlan, 2013) and in humans for postganglionic neurones projecting in muscle or skin nerves (Jänig & Häbler, 2003; Wallin, 2013). The reflexes correspond to the effector responses that are induced by changes in activity of these neurones. The reflex patterns are characteristic and therefore represent the physiological ‘fingerprints’ for each type of sympathetic pathway. They are the functional expression of the neural circuits in spinal cord, brain stem and hypothalamus connected to the peripheral sympathetic pathways.
The same types of reflex patterns have been observed in both preganglionic and postganglionic neurones. The neurones in most of these pathways (e.g. the vasoconstrictor, sudomotor, motility‐regulating pathways, and other pathways) have ongoing activity whereas the neurones in other pathways are normally silent in anaesthetized animals (e.g. pilomotor and vasodilator pathways, pathways to sexual organs). It is likely that other target cells are similarly innervated by functionally distinct groups of sympathetic neurones that have not been systematically studied so far. These include heart (pacemaker, myocytes, coronary arteries), kidney (blood vessels, juxtaglomerular cells, tubules), urogenital tract, hindgut, spleen, and brown and white adipose tissue (Verberne & Sartor, 2010; Morrison, 2011, 2013).
Several systematic studies have been made on the functional properties of parasympathetic pre‐ and postganglionic neurones. The principle of organization into functionally discrete pathways is likely to be the same as in the sympathetic nervous system, the main difference being that some targets of the sympathetic system are widely distributed throughout the body (e.g. blood vessels, sweat glands, erector pili muscles, fat tissue) whereas the targets of the parasympathetic pathways are more restricted (Jänig, 2006; Jänig & McLachlan, 2013).
The centrally generated signals are faithfully transmitted from the preganglionic neurones to the postganglionic neurones in the autonomic ganglia and from the postganglionic neurones to the effector tissues at the neuroeffector junctions or at the sites where the varicosities of the postganglionic axons are in close proximity with the tissue (e.g. non‐excitable tissues). This signal transmission is function‐specific and the basis for the precise regulation of autonomic effector tissues by the brain.
A few effector tissues also contain intrinsic neuronal networks that include the components for reflex control within the periphery. The most prominent is the enteric nervous system, which contains afferent neurones, interneurones and motoneurones that regulate various target tissues (smooth musculature, secretory epithelia, endocrine cells) and even modulate postganglionic sympathetic neurones in prevertebral ganglia. The preganglionic parasympathetic and sympathetic pathways regulate the activity of these intrinsic pathways in an integrative manner to determine organ function. Some peripheral afferents from the visceral organs project as far as prevertebral sympathetic ganglia and amplify postganglionic activity (e.g. Szurszewski, 1981; Jänig & McLachlan, 1987; McLachlan & Meckler, 1989). Peripheral neuronal reflex systems have been identified in the heart and pancreas, and possibly other organs. However, other than the important control of peristalsis and secretion in the gut (Furness, 2006; Furness et al. 2014), the operation of these peripheral reflex pathways in determining organ function remains largely unknown and needs further investigation.
The conclusions to be drawn from these neurophysiological studies are important for the discussion of the neural regulation of the heart (Jänig, 2006; Jänig & McLachlan, 2013):
The peripheral autonomic (parasympathetic and sympathetic) systems consist of several separate neuronal channels transmitting the central messages to the autonomic target tissues. This conclusion is supported by morphological studies using tracers and by studies of neuropeptides co‐localized in postganglionic and preganglionic neurones with the classical transmitters acetylcholine or noradrenaline (Gibbins, 1995, 2004). Peripheral circuits may also modulate the central activity patterns related to some visceral organs.
The distinct reflex patterns generated in autonomic neurones by physiological stimulation of afferent neurones innervating visceral, skin or deep somatic tissues indicate that each autonomic pathway is connected to specific neural circuits in spinal cord, brain stem and hypothalamus that are involved in autonomic regulation.
We have some knowledge about the central circuits involved in cardiovascular regulation and thermoregulation. However, the central circuits, including the spinal ones, are largely unknown for most peripheral final autonomic pathways (see Jänig, 2006; Llewellyn‐Smith & Verberne, 2011; Paton & Spyer, 2013).
The brain–heart axis
The brain–heart axis is involved in the neural regulation of the heart via the autonomic cardiomotor (CM) pathways. This axis was already present in vertebrates some 500 million years ago in elasmobranch and teleost fishes. The heart of elasmobranchs is under parasympathetic (vagal) CM control and the heart of teleosts under both parasympathetic and sympathetic CM control (Nilsson, 2011; Jänig, 2013). This shows that the regulation of the heart by the brain is phylogenetically old, as is the case for the foregut, but probably went through various changes in the central and peripheral nervous system, although the basic principles of the organization of the peripheral CM pathways remained the same throughout the vertebrate kingdom (transmitters and their receptors, excitatory effects, inhibitory effects, etc.). This shows that the brain–heart axis is biologically rather important. However, our knowledge about the mechanisms underlying the functioning of this axis under biological conditions remains still amazingly poor in view of the fact that the pathophysiology of the neural regulation of the heart and its consequences for therapy has a high representation in medicine. Knowledge about these peripheral and central mechanisms is the gate to understanding the pathophysiology underlying various diseases related to the heart in humans (Floras, 2012; Golombek, 2012; Robertson & Sato, 2012; Bajpai & Camm, 2013; Francis & Cohn, 2013; Hainsworth & Claydon, 2013; Samuels, 2013). Thus, research in neurocardiology should primarily focus on the neurobiology of the regulation of the heart, covering the field from neuroeffector transmission to telencephalic control of the heart.
Here I will discuss research questions related to the brain–heart axis. This discussion is based on my personal opinion and does not imply any priority of the type of basic research involved and of the level of integration at which the neural control of the heart occurs. However, it should be kept in mind that the ‘neural machineries’ of the peripheral CM pathways (neuroeffector transmission, transmission and integration of impulse activity in cardiac and possibly stellate ganglia) are fully integrated in the central integrative processes, so that the peripheral neural machineries are ‘used’ by the brain as tools to regulate the functioning of the heart.
Neuroeffector transmission (1 in Fig. 1)
Figure 1. Schematic figure showing the neural regulation of the heart: levels of integration and afferent feedback (spinal, vagal) .
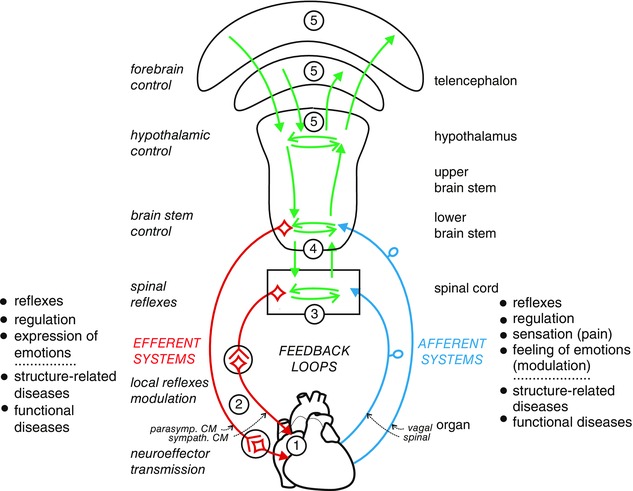
Possible extracentral feedback loops between intrinsic primary afferent neurones and local circuit neurones of the intrinsic cardiac ganglionated plexus or extrinsic spinal cardiac afferent neurones and postganglionic neurones in the stellate ganglion have not been included (see Ardell et al. 2016; Rajendran et al. 2016). 1, neuroeffector transmission. 2, transmission and integration in autonomic ganglia. 3, integration in spinal cord. 4, integration in lower brain stem. 5, integration in hypothalamus and telencephalon. Pathophysiological changes in the heart result in structure‐related cardiac diseases, including functional and structural neural remodelling. Changes in neural regulation of the heart by the brain may produce functional cardiac diseases mediated by the autonomic innervation. CM, cardiomotor.
To unravel the transmission of the neural signals in the postganglionic cardiomotor (CM) neurones to the effector cells (cells of the sinoatrial node (SAN) and the atrioventricular node (AVN), myocytes, coronary vascular cells) is of utmost importance. This transmission of neural signals occurs at rather small areas of the surface of the target cell syncytia (<0.5%). The idea that exogenous application of transmitter (acetylcholine, noradrenaline, neuropeptides) to a preparation (e.g. blood vessels, heart) mimics the effect of neurally released transmitter is a misconception. Therefore, it is not far‐fetched to assume that the intracellular pathways connecting junctional and extrajunctional membrane receptors for acetylcholine or noradrenaline with the cellular effector mechanisms (ionic channels, contractile machinery) are different for the two groups of receptors. The function(s) of the extrajunctional receptors under physiological conditions is (are) largely unclear. However, this may radically change under pathophysiological conditions, as far as the functions of the extrajunctional receptors are concerned (Jänig, 2011). The experimental work of Hirst and co‐workers (Bywater et al. 1989, 1990; Campbell et al. 1989; Bramich et al. 1990; Klemm et al. 1992; Choate et al. 1993; Edwards et al. 1993, 1995; Hirst et al. 1991, 1996) and others has to be reproduced and extended. Clarification of the neurobiology will lead to a better understanding of the pathophysiology (e.g. after myocardial infarction (Rajendran et al. 2016), atrial and ventricular fibrillation, myocardial ischaemia, sudden cardiac death, ventricular tachycardia, in diabetic neuropathy) and to new mechanism‐based therapeutical strategies. Neurobiological research (e.g. in vitro experimentation as well as quasi in vivo experimentation using the working heart–brain stem preparation (Paton, 1996) and associated techniques) should concentrate on the following topics:
Neuroeffector transmission to the SAN cell syncytium (parasympathetic, sympathetic) and to cardiac myocytes (sympathetic).
Role of junctional and extrajunctional cholinergic muscarinic receptors and adrenoreceptors and their connections via intracellular pathways to the cellular effectors (Ca2+, Na+, K+ channels, contractile mechanism).
Role of neuropeptides and their receptors in neuroeffector transmission to the heart.
Postganglionic parasympathetic CM neurones do not only innervate atria, SAN and AVN but also the ventricles. The parasympathetic innervation of the cardiac ventricles may act on the myocytes either directly or via the sympathetic innervation or via interaction with neurones of the intrinsic cardiac plexus (Coote, 2013). How dense is this parasympathetic innervation of the ventricles compared to the atria? Quantitative immune histochemical studies of atria and ventricles have to be done on the same sections using tyrosine hydroxylase (as a noradrenergic marker) and vesicular acetylcholine transporter (as a cholinergic marker).
The long‐held story about the physiological interaction between sympathetic postganglionic CM axons and parasympathetic postganglionic CM axons, based on pharmacological investigations, is largely open and unclear (Levy, 1984; Manabe et al. 1991). This interaction involves acetylcholine and prejunctional muscarinic receptors in sympathetic terminals and possibly neuropeptides. A better understanding of such interactions could provide novel targets for treatment in postinfarct ventricular tachycardia, such as pharmacological stimulation of the parasympathetic axons (Coote, 2013).
Transmission of activity in cardiac ganglia (2 in Fig. 1)
The cardiac ganglia have not been studied extensively, the main reason being that these studies are technically demanding. It is silently assumed [with some exceptions, Edwards et al. 1995; Armour, 2008; Ardell et al. 2016 (in this issue)] that transmission of activity through these ganglia follows the simple rules of relay and distribution of activity. However, this may not be true.
What are the functions of 60% of neurones in parasympathetic ganglia that cannot be activated by preganglionic CM neurones and are suspected to be local afferent neurones or local motoneurones without preganglionic synaptic input (based on in vivo and in vitro experimentation and on experiments using the working heart–brain stem preparation (WHBP); Edwards et al. 1995; Jänig, 2006, 2011; McAllen et al. 2011)? Are these neurones functionally unimportant under normal conditions but play a role in the preganglionically denervated heart? Based on the available data, we plainly cannot know whether integrative processes in the parasympathetic ganglia are important under physiological or pathophysiological conditions. Would activity in the parasympathetic ganglia be expected to counter‐regulate the influence of sympathetic nerves on the injured heart? Could a dysregulation in parasympathetic ganglia play a role in the detrimental effect of sympathetic excitation on the injured heart (Ardell et al. 2016; Rajendran et al. 2016)? One postganglionic parasympathetic CM neurone is innervated and driven by one strong preganglionic parasympathetic input. Can this safe synaptic transmission be modulated by intraganglionic synaptic inputs (McAllen et al. 2011)?
What is the nature of synaptic transmission from sympathetic preganglionic CM neurones to sympathetic postganglionic CM neurones in the stellate ganglion and in the superior cervical ganglion? The activity of the postganglionic CM neurones seem to be dominated by one or a few strong synaptic inputs (E. M. McLachlan & L. V. Melnitchenko, unpublished observations) as is the case in ganglia of the sympathetic chain (Lichtman et al. 1980; McLachlan et al. 1997, 1998; Bratton et al. 2010). In the stellate ganglion cardiac and non‐cardiac neurones are not different in morphology or electrophysiological properties (Mo et al. 1994).
How are synaptic connectivity and strength within cardiac ganglia set and adjusted under physiological and pathophysiological situations (Rajendran et al. 2016)? What is the feedback that is used in this process, and where and how is this feedback registered?
What is the nature of putative local afferent neurones or interneurones in the stellate ganglion? It is important to show whether integration based on local excitatory neurones principally exists (Bosnjak & Kampine, 1982, 1989), whether it is functionally important during normal neural regulation of the heart, or whether it is some sort of developmental left‐over. Do peptidergic spinal afferent neurones innervating the heart form synapses by collaterals with sympathetic postganglionic neurones innervating the heart? Under which physiological or pathophysiological conditions is this peptidergic synaptic transmission functioning?
Which data support the interesting concept of the ‘intrinsic cardiac nervous system’ (ICNS) (‘little brain of the heart’) as propagated by Armour and co‐workers (Armour, 2008; Beaumont et al. 2013; Rajendran et al. 2016; Ardell et al. 2016) and others? Is the ICNS functioning in a similar manner to the enteric nervous system (ENS) (Furness, 2006; Furness et al. 2014)? Is there evidence for intrinsic reflexes mediated by the ICNS in analogy to the ENS? Do intrinsic afferent neurones form peripheral feedback loops with postganglionic cardiomotor neurones?
Clinical and experimental evidence suggest that neurones of the ICNS undergo plastic changes (described by the concept of ‘structural and functional remodelling’) under pathophysiological conditions (Rajendran et al. 2016). Changes of the cardiac target tissue of the cardiomotor neurones under pathophysiological conditions may lead to plastic changes of the autonomic innervation of the heart. Thus, the intrinsic afferent, the intrinsic efferent and the preganglionic neurones may start to sprout and form new synapses (Kepper & Keast, 1998; Keast, 2004).
Sympathetic preganglionic CM neurones, spinal afferent feedback and spinal integration (3 in Fig. 1)
Do functionally separate sympathetic CM pathways exist, e.g. to the SAN or AVN and to the myocytes? Is the sympathetic pathway to the coronary arteries (cardiovasoconstrictor neurones) separate from the sympathetic pathway(s) to the SAN, AVN or myocytes? If functionally separate sympathetic pathways to the heart do exist this should be reflected in functionally characteristic discharge patterns as functional markers for different populations of sympathetic neurones supplying the heart (Jänig, 2006; Jänig & McLachlan, 2013). This neurophysiological work should be combined with morphological work (intracellular labelling of functionally identified postganglionic neurones with reconstruction of their dendrites and axons). Intracellular recording from postganglionic neurones using the WHBP with attached spinal cord (McAllen et al. 2011) and in vitro experimentation using the SAN and other parts of the heart with attached nerves should be done.
Which sympathetic neuronal subtype mediates the detrimental effects on the heart in pathophysiological conditions such as atrial or ventricular fibrillation, myocardial ischaemia, myocardial infarction, sudden cardiac death and the like? Is it possible that the changes in the coronary innervation regulating blood flow is important in these pathological conditions?
The heart is innervated by spinal visceral afferent neurones which are involved in cardiac nociception and pain as well in the regulation of cardiac output during body exercise. It is hypothesized that sympathetic cardiomotor neurones, spinal visceral afferent cardiac neurones and spinal cord circuits, including excitatory and inhibitory interneurones, form cardio‐cardiac reflexes. These reflex circuits are normally involved in adaptation of cardiac output during exercise but may form, under pathophysiologically deleterious conditions, a positive feedback loop with the heart. Which types of sympathetic CM neurones are involved in these reflexes? Which classes of spinal interneurones are involved (Deuchars, 2011)?
Muscle vasoconstrictor neurones are inhibited by stimulation of muscle nociceptors of the same extremity but not by stimulation of cutaneous nociceptors. Cutaneous vasoconstrictor neurones are inhibited by stimulation of cutaneous nociceptors of the same extremity but not by stimulation of muscle nociceptors (Kirillova‐Woytke et al. 2014). These protective inhibitory nociceptive reflexes are most likely organized at the level of the spinal cord and under powerful supraspinal control. Do these types of protective nociceptive reflexes exist for the heart (e.g. in the vasoconstrictor neurones innervating the coronary blood vessels)?
What are the functions and underlying mechanisms of the descending control of spinal cardiac circuits involving brain stem, hypothalamus and telencephalon? This important question should also be studied in humans using brain imaging of grey and white matter changes under resting state activity and under cardiac load.
What are the mechanisms underlying the referral of cardiac diseases (e.g. coronary disease) to deep somatic tissues (myotomes, sclerotomes), superficial somatic tissues (skin, subcutaneous zone, dermatomes) and other visceral organs (oesophagus, upper gastrointestinal tract (GIT), airways) involving spinal circuits and their supraspinal control (King et al. 2011)?
Can functional cardiac diseases be diagnosed by manual palpation (and other procedures) of the deep somatic and superficial referred zone of the heart and discriminated from functional diseases of other visceral organs innervated by the same spinal (thoracic) segments (e.g. lungs and airways, lower oesophagus, stomach and duodenum, pancreas and liver) as propagated in the field of osteopathic medicine (Cox et al. 1983; Beal, 1985; Nicholas et al. 1985)? Brain imaging approaches should be used to identify abnormal central processing in functional heart diseases. Data obtained in this way should be correlated with quantitative data describing the changes in the referred body zones (changes of blood flow, sweating, tissue consistency (trophic changes)) (Jänig et al. 2015).
Can functional diseases of the heart be treated by manual interventions and other interventions in the referred superficial and deep somatic zone of the heart as propagated in osteopathic medicine and other subdisciplines of manual medicine? This idea is based on the concept (philosophy) that there exist special somato‐visceral and viscero‐somatic neural relations between visceral organs and somatic tissues (deep somatic, body surface) involving spinal circuits and their control by supraspinal centres (Jänig, 2006; King et al. 2011).
What are the interactions between different visceral organs (heart, lungs, airways, GIT) via the spinal circuits, leading to referred viscero‐visceral hyperalgesia and referred functional changes involving spinal circuits, sympathetic outflows and afferent feedback (Giamberardino et al. 2010; Jänig, 2010)?
Parasympathetic preganglionic cardiomotor neurones (4 in Fig. 1)
Are the parasympathetic preganglionic CM neurones in the nucleus ambiguus functionally uniform?
What are the types and organization of reflex arcs and their modulation by supramedullary centres connected to the parasympathetic preganglionic CM neurones?
What are the functions of the parasympathetic preganglionic CM neurones with unmyelinated fibres located in the lateral part of the dorsal motor nucleus of the vagus? These preganglionic neurones are normally silent or exhibit irregular non‐respiration‐related discharges. They appear to be unaffected by stimulation of arterial baro‐ or chemoreceptors (Jones, 2001). Recent experiments on rats demonstrate that these preganglionic neurones may be involved in the ventricular excitability via a nitrergic mechanism (Machhada et al. 2015). However, evidence that these parasympathetic neurones innervate the cardiac ventricles is lacking (but see Coote, 2013).
What is the evidence for parasympathetic vasodilator neurones supplying the coronary arteries of the heart? Investigation of the vasculature of the rat heart using immunohistochemical techniques shows a much denser innervation of atrial than ventricular coronary arteries by noradrenergic and cholinergic efferent nerve fibres, whereas nitrergic fibres are sparse. Peptidergic sensory nerve fibres are uniformly distributed to atrial and ventricular vessels (Schäfer et al. 1998; Sequeira et al. 2005).
How do reflex arcs in the lower brain stem connect to parasympathetic CM pathways, to preganglionic neurones innervating airways (Canning, 2011), to preganglionic neurones innervating the proximal GIT (including liver; Jänig, 2006; Travagli et al. 2006) interact? These reflex arcs include the second‐order neurones in the nucleus of the solitary tract (NTS), the nucleus ambiguus and the dorsal motor nucleus of the vagus, and their multiple supramedullary controls (Jänig, 2006; Andresen & Paton, 2011; Jordan, 2011), and are closely associated with the respiratory ponto‐medullary network (Spyer & Gourine, 2009; Guyenet, 2014; Guyenet & Bayliss, 2015).
Cardio‐respiratory integration and its control (4 in Fig. 1)
Neural regulation of the cardiovascular system (blood vessels, heart) and of respiration are closely integrated in the lower brain stem (pons, medulla oblongata) (Jänig, 2006; Guyenet, 2011; Paton & Pickering, 2012).
Knowledge of neural cardio‐respiratory integration is still in its infancy. Unravelling this integration in the lower brain stem (medulla oblongata and pons) is essential for understanding the neural regulation of both systems under various functional conditions (Dampney, 1994; Jänig, 2006; Spyer & Gourine, 2009; Guyenet, 2011, 2014) and may give us a better lead to the so‐called cardioprotective effects of the activity in parasympathetic cardiomotor neurones.
What are the mechanisms underlying the integration between the respiratory neural network and the preganglionic CM neurones (Jänig, 2006; Paton & Pickering, 2012)?
How are the reflex circuits related to parasympathetic CM neurones (baroreceptor reflexes, peripheral chemoreceptor reflexes, central chemoreceptor reflexes, trigeminal reflexes, nociceptor reflexes, other) and the respiratory network integrated under various functional conditions (e.g. diving, exercise, mental activity) (Guyenet, 2011, 2014; Paton & Spyer, 2013)? Are these reflex circuits affected by cardiac pathology?
Neuroanatomical and neurophysiological experiments show that centres in the lower brain stem that are involved in cardiovascular regulation, such as the NTS, the rostral ventrolateral medulla and the caudal ventrolateral medulla, are also influenced by the vestibular system (in particular the vestibular otolith organs). This influence serves to adjust arterial blood pressure and organ blood flow during movement and changes in posture (Yates et al. 2014). Mechanisms underlying the regulation of the heart via parasympathetic and sympathetic CM neurones during these adjustments are almost unknown.
Regulation of heart, blood flow through resistance vessels of skeletal muscle and viscera, and blood flow through skin are under powerful cortical control, as shown during diving, exercise and other behavioural conditions (Elsner et al. 1966; Goodwin et al. 1972; Casson & Ronald, 1975; Gandevia et al. 1993). Where in the ponto‐medullary cardio‐vascular‐respiratory network do the cortical command signals interfere to adjust the cardiovascular system during these behaviours? Knowing the neural mechanisms underlying this coupling would give us a better lead to the neural control of the heart by the forebrain under pathophysiological conditions.
Heart, interoception and emotions (5 in Fig. 1)
In humans and higher vertebrates, expression of emotions signals the state of behaviour to conspecifics, being involved in the regulation of social behaviour, whereas the feeling of emotions is involved in internal signalling and regulation of their own behaviour. The generation and modulation of the emotions are believed to be dependent on the state of the body tissues, in particular visceral organs and deep somatic tissues (e.g. skeletal muscle), and therefore on the functions and on the spatio‐temporal patterns of the activity in the afferent feedback from these body tissues to their central representations. The afferent neurones have small‐diameter myelinated or unmyelinated axons and encode in their activity the mechanical, thermal, metabolic and inflammatory states of the body tissues. They project to lamina I of the spinal or trigeminal dorsal horn (spinal/trigeminal afferent neurones, Craig, 2003 b) or to the NTS (vagal afferent neurones). Both lamina I and NTS are the interoceptive interface between body tissues and brain. Their tract neurones project, in addition to various nuclei in brain stem and hypothalamus, via specific thalamic nuclei (the posterior part of the ventromedial nucleus (VMpo) for the lamina I tract neurones and the basal part of the ventromedial nucleus (VMb) for the tract neurones in the NTS) to the dorsal posterior insular cortex. This cortex is according to Craig the primary interoceptive cortex or limbic sensory cortex (Craig, 2003 a,b, 2015, 2016).
Activity in these afferent feedback systems is also or mainly dependent on the activity in the efferent (autonomic, somatomotor) systems innervating the body tissues. Thus, efferent systems, body tissues and afferent interoceptive feedback form body loops which are hypothesized to be essential in the development (after birth), generation, maintenance and modulation of emotional expression as well as emotional feelings (James, 1884; Damasio, 1999, 2003). What is fact and what fiction?
The heart is traditionally believed to play an important role in body interoception [see Shivkumar et al. 2016 (in this issue)] as well as in the generation or shaping of emotions. The efferent limbs of the body loop involving the heart are formed by the parasympathetic and sympathetic cardiomotor neurones. The afferent limbs are formed by arterial baroreceptor afferent neurones and cardiac vagal afferent neurones, both projecting to the NTS, as well as spinal cardiac afferent neurones projecting to the dorsal horn of the thoracic spinal cord.
How important is the efferent–afferent body loop involving the heart to shape and/or generate emotional feelings in comparison to potential loops involving other visceral organs or somatic tissue (e.g. gastrointestinal tract, pelvic organs, skeletal musculature)? This question should be explored carefully using imaging methods focusing on spinal cord, brain stem and forebrain combined with psychophysics and other methods. After all it should be kept in mind (1) that the afferent feedback from different tissues is functionally different, (2) that the peripheral target tissues are innervated by many functionally different peripheral autonomic pathways, which is reflected in the distinct discharge patterns of their neurones, and dependent on the distinct central organization of the autonomic systems, and (3) that the activity in the afferent feedback is also or mainly dependent on the activity in the peripheral autonomic pathways.
Various cortical and subcortical areas of the forebrain (e.g. insular cortex, anterior cingulate cortex, medial and ventral prefrontal cortex, amygdala) are involved, via autonomic ‘centres’ in hypothalamus and brain stem, in autonomic regulation of the heart and in the processing of the afferent feedback from the heart. What are the mechanisms underlying this forebrain control?
Experimental work addressing the role of the heart in shaping and generating emotions will be conducted on the basis of the James–Lange–Damasio theory of emotions (James, 1884; Damasio, 1999, 2003). This theory suggests that the efferent–afferent body loop via the heart, as well as the corresponding centrally organized ‘as if’ loop that is vicarious for the real body loop, to the central representations of the body tissues (Damasio, 1996) are essential to generate emotional feelings. Based on their experimental investigations of various groups of human subjects Ekman and co‐workers have shown that the basic emotions are characterized by distinct autonomic patterns (expressed as change in heart rate, change in skin conductance, change in skin temperature). Thus, subjective emotional feelings, expression of emotions mediated by the somatomotor system (facial expression) and activation of autonomic systems are highly correlated. They conclude that ‘there is an innate affect program for each emotion that once activated directs for each emotion changes in the organism's biological state by providing instructions to multiple response systems, including facial skeletal muscles, other skeletal muscles and autonomic nervous system [to various effectors, one being the heart]’ (Levenson et al. 1990, 1991; Ekman, 1992).
Research on the autonomic body loop (real and ‘as if’) in human subjects should be performed using modern imaging techniques combined with measurements of autonomic parameters, psychophysical parameters, etc. Independent of the biological importance of this basic research (we learn who we are), this experimental approach will suggest leads to various functional diseases of the heart (e.g. related to the effect of bodily and psychological stress on the heart). Therefore experimental work on this topic should have a high priority (Emani & Binkley, 2010; Taggart et al. 2011; Garfinkel & Critchley, 2016).
Additional information
Acknowledgements
I thank Elspeth McLachlan for her valuable comments and suggestions.
References
- Andresen MC & Paton JFR (2011). The nucleus of the solitary tract processing information from viscerosensory afferents In Central Regulation of Autonomic Functions, 2nd edn, ed. Llewellyn‐Smith IJ. & Verberne AJM, pp. 23–46. Oxford University Press, New York. [Google Scholar]
- Ardell JL, Andresen MC, Armour JA, Billman GE, Chen P‐S, Foreman RD, Herring N, O'Leary DS, Sabbah HN, Schultz HD, Sunagawa K & Zucker IH (2016). Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 594, 3877–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour JA (2008). Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93, 165–176. [DOI] [PubMed] [Google Scholar]
- Bajpai A & Camm AJ (2013). Cardiac causes of syncope In Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th edn, ed. Mathias CJ. & Bannister R, pp. 751–763. Oxford University Press, Oxford. [Google Scholar]
- Beal MC (1985). Viscerosomatic reflexes: a review. J Am Osteopath Assoc 85, 786–801. [PubMed] [Google Scholar]
- Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA & Ardell JL (2013). Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591, 4515–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnjak ZJ & Kampine JP (1982). Intracellular recordings from the stellate ganglion of the cat. J Physiol 324, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnjak ZJ & Kampine JP (1989). Cardiac sympathetic afferent cell bodies are located in the peripheral nervous system of the cat. Circ Res 64, 554–562. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Edwards FR & Hirst GD (1990). Sympathetic nerve stimulation and applied transmitters on the sinus venosus of the toad. J Physiol 429, 349–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton B, Davies P, Jänig W & McAllen R (2010). Ganglionic transmission in a vasomotor pathway studied in vivo . J Physiol 588, 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RA, Campbell GD, Edwards FR & Hirst GD (1990). Effects of vagal stimulation and applied acetylcholine on the arrested sinus venosus of the toad. J Physiol 425, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RA, Campbell G, Edwards FR, Hirst GD & O'Shea JE (1989). The effects of vagal stimulation and applied acetylcholine on the sinus venosus of the toad. J Physiol 415,35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GD, Edwards FR, Hirst GD & O'Shea JE (1989). Effects of vagal stimulation and applied acetylcholine on pacemaker potentials in the guinea‐pig heart. J Physiol 415, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ (2011). Central autonomic regulation of the airways In Central Regulation of Autonomic Functions, 2nd edn, ed. Llewellyn‐Smith IJ. & Verberne AJM, pp. 238–258. Oxford University Press, New York. [Google Scholar]
- Casson DM & Ronald K (1975). The harp seal, Pagophilus groenlandicus (Erxleben, 1777). XIV. Cardiac arrythmias. Comp Biochem Physiol A Comp Physiol 50, 307–314. [DOI] [PubMed] [Google Scholar]
- Choate JK, Klemm M & Hirst GD (1993). Sympathetic and parasympathetic neuromuscular junctions in the guinea‐pig sino‐atrial node. J Auton Nerv Syst 44, 1–15. [DOI] [PubMed] [Google Scholar]
- Coote JH (2013). Myths and realities of the cardiac vagus. J Physiol 591, 4073–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JM, Gorbis S, Dick LM, Rogers JC & Rogers FJ (1983). Palpable musculoskeletal findings in coronary artery disease: results of a double‐blind study. J Am Osteopath Assoc 82, 832–836. [PubMed] [Google Scholar]
- Craig AD (2003. a). Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13, 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003. b). Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26, 1–30. [DOI] [PubMed] [Google Scholar]
- Craig AD (2015). How do you Feel? An Interoceptive Moment with your Neurobiological Self. Princeton University Press, Princeton, Oxford. [Google Scholar]
- Craig AD (2016). Interoception and emotion: A Neuroanatomical perspective In Handbook of Emotions, 4th edn, ed. Barrett LF, Lewis M. & Haviland‐Jones JM, pp. 215–234. The Guilford Press, New York. [Google Scholar]
- Damasio AR (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351, 1413–1420. [DOI] [PubMed] [Google Scholar]
- Damasio A (1999). The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace, New York, San Diego, London. [Google Scholar]
- Damasio A (2003). Looking for Spinoza. Joy, Sorrow, and the Feeling Brain. Harcourt, Orlando. [Google Scholar]
- Dampney RA (1994). Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74, 323–364. [DOI] [PubMed] [Google Scholar]
- Deuchars SA (2011). Spinal interneurons in the control of autonomic function In Central Regulation of Autonomic Functions, 2nd edn, ed. Llewellyn‐Smith IJ. & Verberne AJM, pp. 140–160. Oxford University Press, New York. [Google Scholar]
- Edwards FR, Bramich NJ & Hirst GD (1993). Analysis of the effects of vagal stimulation on the sinus venosus of the toad. Philos Trans R Soc Lond B Biol Sci 341, 149–162. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Klemm MF & Steele PA (1995). Different types of ganglion cell in the cardiac plexus of guinea‐pigs. J Physiol 486, 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P (1992). Facial expression of emotion: new findings, new questions. Psychol Sci 3, 34–38. [Google Scholar]
- Elsner R, Franklin DL, Van Citters RL & Kenney DW (1966). Cardiovascular defense against asphyxia. Science 153, 941–949. [DOI] [PubMed] [Google Scholar]
- Emani S & Binkley PF (2010). Mind‐body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 3, 715–725. [DOI] [PubMed] [Google Scholar]
- Floras JS (2012). Heart failure In Primer on the Autonomic Nervous System, 3rd edn, ed. Robertson D, Biaggioni I, Burnstock G, Low PA. & Paton JFR, pp. 367–370. Academic Press, London, Waltham, San Diego. [Google Scholar]
- Francis GS & Cohn JN (2013). Cardiac failure and the autonomic nervous system In Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th edn, ed. Mathias CJ. & Bannister R, pp. 761–769. Oxford University Press, Oxford. [Google Scholar]
- Furness JB (2006). The Enteric Nervous System. Blackwell Science Ltd, Oxford. [Google Scholar]
- Furness JB, Callaghan BP, Rivera LR & Cho HJ (2014). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 817, 39–71. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Killian K, McKenzie DK, Crawford M, Allen GM, Gorman RB & Hales JP (1993). Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol 470, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN & Critchley HD (2016). Threat and the body: how the heart supports fear processing. Trends Cogn Sci 20,34–46. [DOI] [PubMed] [Google Scholar]
- Giamberardino MA, Costantini R, Affaitati G, Fabrizio A, Lapenna D, Tafuri E & Mezzetti A (2010). Viscero‐visceral hyperalgesia: characterization in different clinical models. Pain 151, 307–322. [DOI] [PubMed] [Google Scholar]
- Gibbins IL (1995). Chemical neuroanatomy of sympathetic ganglia In Autonomic Ganglia, ed. McLachlan EM, pp. 73–122. Harwood Academic Publishers, Luxembourg. [Google Scholar]
- Gibbins IL (2004). Peripheral autonomic pathways In The Human Nervous System, 2nd edn, ed. Paxinos G. & Mai JK, pp. 134–189. Elsevier Academic Press, Amsterdam, San Diego, London. [Google Scholar]
- Golombek DA (2012). Circadian rhythms and autonomic function In Primer on the Autonomic Nervous System, 3rd edn, ed. Robertson D, Biaggioni I, Burnstock G, Low PA. & Paton JFR, pp. 157–159. Academic Press, London, Waltham, San Diego. [Google Scholar]
- Goodwin GM, McCloskey DI & Mitchell JH (1972). Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG (2011). Cardiorespiratory integration In Central Regulation of Autonomic Functions, 2nd edn, ed. Llewellyn‐Smith IJ. & Verberne AJM, pp. 180–201. Oxford University Press, New York. [Google Scholar]
- Guyenet PG (2014). Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4, 1511–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG & Bayliss DA (2015). Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R & Claydon VE (2013). Syncope and fainting: classification and pathophysiological basis In Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th edn, ed. Mathias CJ. & Bannister R, pp. 690–700. Oxford University Press, Oxford. [Google Scholar]
- Hirst GD, Choate JK, Cousins HM, Edwards FR & Klemm MF (1996). Transmission by post‐ganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience 73, 7–23. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Edwards FR, Bramich NJ & Klemm MF (1991). Neural control of cardiac pacemaker potentials. News Physiol Sci 6, 185–190. [Google Scholar]
- James W (1884). What is an emotion? Mind 9, 188–205. [Google Scholar]
- Jänig W (2006). The Integrative Action of the Autonomic Nervous System. Neurobiology of Homeostasis. Cambridge University Press, Cambridge, New York. [Google Scholar]
- Jänig W (2010). Visceral pain – still an enigma? Pain 151, 239–240. [DOI] [PubMed] [Google Scholar]
- Jänig W (2011). Transmission of impulses in the parasympathetic cardiomotor pathway to the sino‐atrial node. J Physiol 589, 5911–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W (2013). Autonomic nervous system In Neurosciences ‐ From Molecule to Behavior. A University Textbook, ed. Galizia CG. & Lledo P‐M, pp. 179–211. Springer‐Verlag, Berlin, Heidelberg. [Google Scholar]
- Jänig W, Böhni U & von Heymann W (2015). Interozeption, Schmerz und vegetatives Nervensystem In Manuelle Medizin 1, ed. Böhni U, Lauper M. & Locher H, pp. 69–100. Thieme Verlag, Stuttgart, New York. [Google Scholar]
- Jänig W & Häbler HJ (2003). Neurophysiological analysis of target‐related sympathetic pathways – from animal to human: similarities and differences. Acta Physiol Scand 177, 255–274. [DOI] [PubMed] [Google Scholar]
- Jänig W & McLachlan EM (1987). Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev 67, 1332–1404. [DOI] [PubMed] [Google Scholar]
- Jänig W & McLachlan EM (2013). Neurobiology of the autonomic nervous system In Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th edn, ed. Mathias CJ. & Bannister R, pp. 21–34. Oxford University Press, Oxford. [Google Scholar]
- Jones JF (2001). Vagal control of the rat heart. Exp Physiol 86, 797–801. [DOI] [PubMed] [Google Scholar]
- Jordan JF (2011). Parasympathetic preganglionic neurons In Central Regulation of Autonomic Functions, 2nd edn, ed. Llewellyn‐Smith IJ. & Verberne AJM, pp. 120–139. Oxford University Press, New York. [Google Scholar]
- Keast JR (2004). Remodelling of connections in pelvic ganglia after hypogastric nerve crush. Neuroscience 126, 405–414. [DOI] [PubMed] [Google Scholar]
- Kepper ME & Keast JR (1998). Specific targeting of ganglion cell sprouts provides an additional mechanism for restoring peripheral motor circuits in pelvic ganglia after spinal nerve damage. J Neurosci 18, 7987–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HH, Jänig W. & Patterson MM. (eds) (2011). The Science and Clinical Application of Manual Therapy. Churchill Livingstone Elsevier, Edinburgh. [Google Scholar]
- Kirillova‐Woytke I, Baron R & Jänig W (2014). Reflex inhibition of cutaneous and muscle vasoconstrictor neurons during stimulation of cutaneous and muscle nociceptors. J Neurophysiol 111, 1833–1845. [DOI] [PubMed] [Google Scholar]
- Klemm M, Hirst GD & Campbell G (1992). Structure of autonomic neuromuscular junctions in the sinus venosus of the toad. J Auton Nerv Syst 39, 139–150. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV & Ekman P (1991). Emotion, physiology, and expression in old age. Psychol Aging 6, 28–35. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ekman P & Friesen WV (1990). Voluntary facial action generates emotion‐specific autonomic nervous system activity. Psychophysiology 27, 363–384. [DOI] [PubMed] [Google Scholar]
- Levy MN (1984). Cardiac sympathetic‐parasympathetic interactions. Fed Proc 43, 2598–2602. [PubMed] [Google Scholar]
- Lichtman JW, Purves D & Yip JW (1980). Innervation of sympathetic neurones in the guinea‐pig thoracic chain. J Physiol 298, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn‐Smith IJ & (eds Verberne AJM) (2011). Central Regulation of Autonomic Functions, 2nd edn. Oxford University Press, New York. [Google Scholar]
- McAllen RM, Salo LM, Paton JF & Pickering AE (2011). Processing of central and reflex vagal drives by rat cardiac ganglion neurones: an intracellular analysis. J Physiol 589, 5801–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machhada A, Ang R, Ackland GL, Ninkina N, Buchman VL, Lythgoe MF, Trapp S, Tinker A, Marina N & Gourine AV (2015). Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm 12, 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Davies PJ, Häbler HJ & Jamieson J (1997). On‐going and reflex synaptic events in rat superior cervical ganglion cells. J Physiol 501, 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Häbler HJ, Jamieson J & Davies PJ (1998). Analysis of the periodicity of synaptic events in neurones in the superior cervical ganglion of anaesthetized rats. J Physiol 511, 461–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM & Meckler RL (1989). Characteristics of synaptic input to three classes of sympathetic neurone in the coeliac ganglion of the guinea‐pig. J Physiol 415, 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe N, Foldes FF, Torocsik A, Nagashima H, Goldiner PL & Vizi ES (1991). Presynaptic interaction between vagal and sympathetic innervation in the heart: modulation of acetylcholine and noradrenaline release. J Auton Nerv Syst 32, 233–242. [DOI] [PubMed] [Google Scholar]
- Mo N, Wallis DI & Watson A (1994). Properties of putative cardiac and non‐cardiac neurones in the rat stellate ganglion. J Auton Nerv Syst 47, 7–22. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2011). 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol (1985) 110, 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF (2013). Central thermoregulation In Primer on the Autonomic Nervous System, 3rd edn, ed. Robertson D, Biaggioni I, Burnstock G. & Paton JFR, pp. 243–247. Academic Press, London, Waltham, San Diego. [Google Scholar]
- Nicholas AS, DeBias DA, Ehrenfeuchter W, England KM, England RW, Greene CH, Heilig D & Kirschbaum M (1985). A somatic component to myocardial infarction. Br Med J (Clin Res Ed) 291, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S (2011). Comparative anatomy of the autonomic nervous system. Auton Neurosci 165, 3–9. [DOI] [PubMed] [Google Scholar]
- Paton JFR (1996). A working heart‐brainstem preparation of the mouse. J Neurosci Methods 65, 63–68. [DOI] [PubMed] [Google Scholar]
- Paton JFR & Pickering AE (2012). Cross‐talk between body systems: respiratory–cardiovascular coupling in health and disease In Primer on the Autonomic Nervous System, 3rd edn, ed. Robertson D, Biaggioni I, Burnstock G, Low PA. & Paton JFR, pp. 151–155. Academic Press, London, Waltham, San Diego. [Google Scholar]
- Paton JFR & Spyer KM (2013). Central nervous control of the cardiovascular system In Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th edn, ed. Mathias CJ. & Bannister R, pp. 35–51. Oxford University Press, Oxford. [Google Scholar]
- Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL & Shivkumar K (2016). Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 594, 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D & Sato K (2012). Stress cardiomyopathy and Takotsubo syndrome In Primer on the Autonomic Nervous System, 3rd edn, ed. Robertson D, Biaggioni I, Burnstock G, Low PA. & Paton JFR, pp. 371–375. Academic Press, London, Waltham, San Diego. [Google Scholar]
- Samuels MA (2013). Introduction to neurocardiology In Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th edn, ed. Mathias CJ. & Bannister R, pp. 669–676. Oxford University Press, Oxford. [Google Scholar]
- Schäfer MK, Eiden LE & Weihe E (1998). Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. I. Central nervous system. Neuroscience 84, 331–359. [DOI] [PubMed] [Google Scholar]
- Sequeira IM, Haberberger RV & Kummer W (2005). Atrial and ventricular rat coronary arteries are differently supplied by noradrenergic, cholinergic and nitrergic, but not sensory nerve fibres. Ann Anat 187, 345–355. [DOI] [PubMed] [Google Scholar]
- Shivkumar K, Ajijola O, Anand A, Armour JA, Chen P‐S, Esler M, De Ferrari GM, Fishbein MC, Goldberger J, Harper RK, Joyner MJ, Khalsa SS, Kumar R, Lane R, Mahajan A, Po S, Schwartz P, Somers V, Valderrabano M, Vaseghi M & Zipes DP (2016). Clinical neurocardiology defining the value of neuroscience‐based cardiovascular therapeutics. J Physiol 594, 3911–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM & Gourine AV (2009). Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond B Biol Sci 364, 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH (1981). Physiology of mammalian prevertebral ganglia. Annu Rev Physiol 43, 53–68. [DOI] [PubMed] [Google Scholar]
- Taggart P, Critchley H & Lambiase PD (2011). Heart‐brain interactions in cardiac arrhythmia. Heart 97, 698–708. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN & Rogers RC (2006). Brainstem circuits regulating gastric function. Annu Rev Physiol 68, 279–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne AJ & Sartor DM (2010). Rostroventrolateral medullary neurons modulate glucose homeostasis in the rat. Am J Physiol Endocrinol Metab 299, E802–E807. [DOI] [PubMed] [Google Scholar]
- Wallin BG (2013). Intraneural recordings of normal and abnormal sympathetic activity in humans In Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th edn, ed. Mathias CJ. & Bannister R, pp. 323–331. Oxford University Press, Oxford. [Google Scholar]
- Yates BJ, Bolton PS & Macefield VG (2014). Vestibulo‐sympathetic responses. Compr Physiol 4, 851–887. [DOI] [PMC free article] [PubMed] [Google Scholar]


