Abstract
Background
Investigation on incidence and mortality of anaerobic bacteremia (AB) is clinically relevant in spite of its infrequent occurrence and not often explored, which report varies according to period and institutions. Therefore, it is necessary to analyze the incidence and risk factors related to mortality and assess clinical outcomes of AB in current aspect.
Materials and Methods
Characteristics of AB patients and anaerobic bacteria from blood culture at a university hospital in 2012 were reviewed retrospectively. The correlation between risk factors and 28-day patient mortality was analyzed.
Results
A total of 70 non-duplicated anaerobic bacteria were isolated from blood of 70 bacteremia patients in 2012. The history of cardiovascular disease as host's risk factor was statistically significant (P = 0.0344) in univariate and multivariate analysis. Although the inappropriate therapy was not statistically significant in univariate and multivariate analysis, the survival rate of bacteremia was significantly worse in patients who had inappropriate therapy compared with those underwent appropriate therapy (hazard ratio, 5.4; 95% confidence interval, 1.7–6.9; P = 0.004). The most frequently isolated organism was Bacteroides fragilis (32 isolates, 46%), followed by Bacteroides thetaiotaomicron (10, 14%), and non-perfringens Clostridium (7, 10%).
Conclusion
The incidence of AB in 2012 was 2.3% (number of AB patients per 100 positive blood culture patients) and the mortality rate in patients with clinically significant AB was 21.4%. In addition, AB was frequently noted in patients having malignancy and the survival rate of AB was significantly worse in patients who received inappropriate therapy compared with those underwent appropriate therapy.
Keywords: Anaerobic bacteremia, Mortality, Survival rate, Inappropriate therapy
Introduction
Anaerobic bacteremia (AB) is occasionally encountered in 0.5-11.8% of all positive blood cultures [1,2] depending on the institution. In spite of its infrequent occurrence, the fatal rate remains high from 25% to 44% [3,4]. Due to such significant mortality, characterizing the risk factors associated with mortality in patients with AB was previously attempted from the 1970s. Generally, increasing age, polymicrobial infection, and underlying diseases were considered as factors contributing the mortality [5]. The significance of inappropriate antimicrobial treatment in mortality of AB patients was discussed in number of studies [6,7], however, the association was not conclusive. Given the changes in antimicrobial therapy, clinical characteristic of patients, and antimicrobial susceptibilities, it is difficult to apply former studies to current circumstances. Moreover, to our knowledge, there have been limited studies defining factors associated with mortality of AB and whether the appropriate therapy contributed the survival of AB patients, based on bacterial susceptibilities. To these limitations, the aims of the present study were to determine the clinical significance of anaerobes isolated from blood and assess the risk factors related to mortality of patients with AB at a tertiary-care hospital in Korea.
Materials and Methods
1. Patients
Seventy patients who experienced clinically relevant bacteremia between January and December of 2012 and hospitalized at Severance Hospital (a 2,000-bed university tertiary referral hospital in Seoul, Korea) were analyzed retrospectively by reviewing laboratory records and electronic medical records.
2. Microbiology
Blood cultures were obtained by general venipuncture guidelines to draw no less than 10 ml of blood when possible. Blood was injected to BacT/ALERT FA and BacT/ALERT FN (bioMérieux, Marcy-l'Etoile, France) bottles. The positive blood cultures were inoculated on Brucella agar and phenylethyl alcohol blood agar, and then incubated in an anaerobic chamber (Forma Scientific, Marietta, OH, USA) at 35℃ for 48 hours. All isolates were identified by either conventional methods [8], the Vitek Anaerobe and Corynebacterium (ANC) identification card (bioMérieux), or the VITEK MS (bioMérieux) matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) system. An episode with positive culture of Propionibacterium acnes was excluded, which was considered as a common skin commensal. The antimicrobial susceptibility testing was performed for 44 isolates of the Bacteroides fragilis group following the CLSI agar dilution method [9].
3. Definitions
Bacteremia was considered clinically relevant when a patient had one or more positive blood cultures for anaerobes and met one of the following SIRS (Systemic inflammatory response syndrome) critera for documented or suspected infection: leukocyte count greater than 12,000/mm3 or less than 4,000/mm3, body temperature higher than 38.3℃ or lower than 36℃, pulse rate faster than 90/min, elevated C-reactive protein (CRP), hypotension or clinical evidence consistent with infection such as symptoms and signs of organ dysfunction [10].
The presence of an underlying disease was based on the description made by the physician. A patient was said to have cardiovascular disease when one or more of the following terms was used: chronic heart disase, coronary artery disease, hypertension, or cerebrovascular disease.
The source of the infection was assessed using imaging studies, clinical symptoms and signs. Each episode was classified as community acquired (CA), hospital acquired (HA) and healthcare-associated (HCA) community onset infection by reviewing the patient's medical records according to guidelines suggested by Lenz et al. [11]. The infection was deemed to be HA if a positive culture was drawn after more than 48 hours following admission or within 48 hours of discharge from hospital. A HCA was defined as first culture obtained less than 48 hours of admission and at least of: 1) visit to a hospital clinic or emergency department within the prior 5-30 days before onset of bloodstream infection; 2) receipt of dialysis; or 3) active cancer. CA are those with first positive culture obtained less than 48 hours of admission and not fulfilling criteria for HCA.
A delay in starting antimicrobials even after objective data confirmed an infection or the use of an antimicrobial agent to which the pathogen is resistant (by known susceptibility results) was considered inappopriate therapy [12,13]. However, some treatments could not be classified in those patients discharged without any prescriptions. The patients' outcome was considered as either alive or dead within 28 days of the onset of bacteremia.
4. Statistics
Statistical analyses were performed using the SPSS software (version 23.0; SPSS Inc., Chicago, IL, USA). Means of non-normally distributed quantitative values were compared using the Mann-Whitney U test and correlation between nonparametric characteristics and mortality was determined by Pearson Chi-square and Fisher's exact test. A two-tailed value of P < 0.05 was considered statistically significant. Multivariate logistic regression analysis was used to determine associations between characteristics which were significant in univariate analysis with the value of P < 0.2 and mortality. Survival data were collected for 28 days following the onset of bacteremia, using Cox proportional-hazards regression model.
Results
1. Demographics and underlying diseases
A total of 70 non-duplicated subjects were included in the analysis, which incidence was 2.3% (Number of AB patients per 100 positive blood culture patients) of 3,050 positive blood culture patients in 2012. Thirty-eight (54.3%) patients were male. The median age of the patients was 61 (first quartile 50 yrs; third quartile 69 yrs) years.
Thirty-five patients were > 60 years old, accounting for half of total patients. Fifty-six patients (70.0%) had malignancy as an underlying disease, 27 patients (38.6%) had histoty of surgical procedure, 14 patients had liver disease (20.0%) and 11 patients had cardiovascular disease (15.7%). The presence of cardiovascular disease was statistically significant (P = 0.049) in 28-day mortality, whereas other underlying diseases were not statistically significant in univariate analysis. The five significant factors (P < 0.2) were examined using multivariate logistic regression analysis to determine which variables were acting independently. Cardiovascular disease was statistically significant in the multivariate analysis.
2. Source and onset of infection
The most common sources of bacteremia among those were available to be determined were the intra-abdominal area (42 patients, 60.0%), skin barrier penetration (5 patients, 7.1%) and the respiratory tract (2 patients, 2.9%). Forty-four patients (62.9%) were HA, 24 patients (34.3%) were HCA, while two patients (2.9%) were CA. Neither the source of infection nor where the infection was acquired was statistically related to 28-day mortality (Table 1).
Table 1. Host or microbial characteristics associated with 28-day mortality in 70 patients of anaerobic bacteremia.
| Characteristics/factors | No. (%) of patients | P-value | |||
|---|---|---|---|---|---|
| All (No=70) | Alive (No =55) | Dead (No =15) | Univariatea | Multivariateb | |
| Age (median, interquartile range, years) | 61 (50-69) | 61 (48-69) | 60 (50-77) | 0.897 | |
| Age > 60 years | 35 (50.0) | 28 (50.9) | 7 (46.7) | 0.771 | |
| Male/Female (male, %) | 38/32 (54.3) | 33/22 (60.0) | 5/10 (33.3) | 0.066 | 0.050 |
| Underlying diseases, n (%) | |||||
| Hematologic malignancy | 6 (8.6) | 4 (7.3) | 2 (13.3) | 1.000 | |
| Non-hematologic malignancy | 50 (71.4) | 39 (70.9) | 11 (73.3) | 0.749 | |
| Cardiovascular disease | 11 (15.7) | 6 (10.9) | 5 (33.3) | 0.049 | 0.045 |
| Previous surgery | 27 (38.6) | 21 (38.2) | 6 (40.0) | 0.898 | |
| Diabetes mellitus | 9 (12.9) | 7 (12.7) | 2 (13.3) | 1.000 | |
| Liver disease | 14 (20.0) | 10 (18.2) | 4 (26.7) | 0.480 | |
| Source of infection, n (%) | |||||
| Intra-abdominal | 42 (60.0) | 34 (61.8) | 8 (53.3) | 1.000 | |
| Skin barrier penetration | 5 (7.1) | 5 (9.1) | 0 (0.0) | 0.577 | |
| Respiratory | 2 (2.9) | 1 (1.8) | 1 (6.7) | 1.000 | |
| Origin of infection, n (%) | |||||
| Hospital-acquired infection | 44 (62.9) | 32 (58.2) | 12 (80.0) | 0.1210 | 1.000 |
| Community-acquired infection | 2 (2.9) | 2 (3.6) | 0 (0.0) | 1.000 | |
| Healthcare-associated community onset infection | 24 (34.3) | 21 (38.2) | 3 (20.0) | 0.189 | 1.000 |
| Polymicrobial infection | 9 (12.9) | 3 (5.5) | 6 (40.0) | 0.392 | |
| Bacteroides spp. infection | 50 (71.4) | 37 (67.3) | 13 (86.7) | 0.202 | |
| Inappropriate therapy | 16 (22.9) | 10 (18.2) | 6 (40.0) | 0.139 | 0.168 |
aThe Pearson χ2 test was used for categorical variables and the Mann-Whitney U test was used for continuous variables.
bThe multivariate logistic regression analysis was used to determine which variables were acting independently.
3. Microbiology
The most frequently isolated organisms were B. fragilis (32 isolates, 46%), Bacteroides thetaiotaomicron (10, 14%), and non-perfringens Clostridium (7, 10%) (Table 2). In 61 patients (87.1%), bacteremia was caused by a single organism. Nine patients (12.9%) had a polymicrobial infection, most commonly with coagulase-negative staphylococci, Escherichia coli, and Klebsiella pneumoniae (in order of frequency), however, the mortality was not significantly associated with mixed infections. Among the 70 patients, 50 patients had bacteremia due to Bacteroides spp., although the mortality was not statistically significant. On an antibiogram (Table 3) for B. fragilis, the susceptibility rate to clindamycin, piperacillin and moxifloxacin were 50%, 75% and 78%, respectively, whereas the susceptibility rate to cefoxitin, piperacillin-tazobactam, imipenem, meropenem, chloramphenicol, and metronidazole were 91% to 100%. However, for other species of the B. fragilis group, the susceptibility rate to clindamycin, piperacillin, cefoxitin, and piperacillin-tazobactam, were 17%, 0%, 25%, and 75%, respectively.
Table 2. Anaerobic organisms isolated from blood culture.
| Organisms | No.(%) of patients |
|---|---|
| Bacteroides fragilis | 32 (45.7) |
| Bacteroides thetaiotaomicron | 10 (14.3) |
| Bacteroides ovatus | 4 (5.7) |
| Bacteroides caccae | 2 (2.9) |
| Bacteroides vulgatus | 2 (2.9) |
| Fusobacterium nucleatum | 1 (1.4) |
| Prevotella buccae | 1 (1.4) |
| Clostiridium spp. | 9 (12.9) |
| Bifidobacterium sp. | 1 (1.4) |
| Eubacterium lentum | 1 (1.4) |
| Veillonella parvula | 3 (4.3) |
| Parvimonas micra | 4 (5.7) |
| Total | 70 (100.0) |
Table 3. Antimicrobial activities against 44 Bacteroides fragilis group isolates in 2012.
| Antimicrobial agents | Bacteroides fragilis (32) | B. fragilis group, other species (12)b | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | % Susceptibilitya | MIC (µg/mL) | % Susceptibilitya | |||||
| Range | 90% | S | R | Range | 90% | S | R | |
| Piperacillin | 2->256 | 128 | 75 | 13 | 128->256 | >256 | 0 | 100 |
| Piperacillin-tazobactam | 0.25-128 | 2 | 97 | 3 | 0.03-256 | 128 | 75 | 17 |
| Cefoxitin | 8-128 | 16 | 91 | 9 | 4-64 | 32 | 25 | 8 |
| Cefotetan | 4-256 | 32 | 81 | 9 | 8-256 | 256 | 17 | 67 |
| Imipenem | 0.06-32 | 1 | 97 | 3 | 0.06-8 | 8 | 83 | 0 |
| Meropenem | 0.12-256 | 1 | 91 | 6 | 0.125-4 | 2 | 100 | 0 |
| Clindamycin | 0.03-256 | 256 | 50 | 50 | 2-256 | 256 | 17 | 67 |
| Moxifloxacin | 0.25-32 | 8 | 78 | 16 | 0.5-64 | 32 | 75 | 25 |
| Chloramphenicol | 4-8 | 8 | 100 | 0 | 4-8 | 8 | 100 | 0 |
| Metronidazole | 0.5-4 | 4 | 100 | 0 | 1-2 | 2 | 100 | 0 |
aS, susceptible; R, resistant.
bBacteroides thetaiotaomicron (n=6), B. caccae (n=2), B. ovatus (n=2), B. vulgatus (n=2).
4. Therapy and outcome
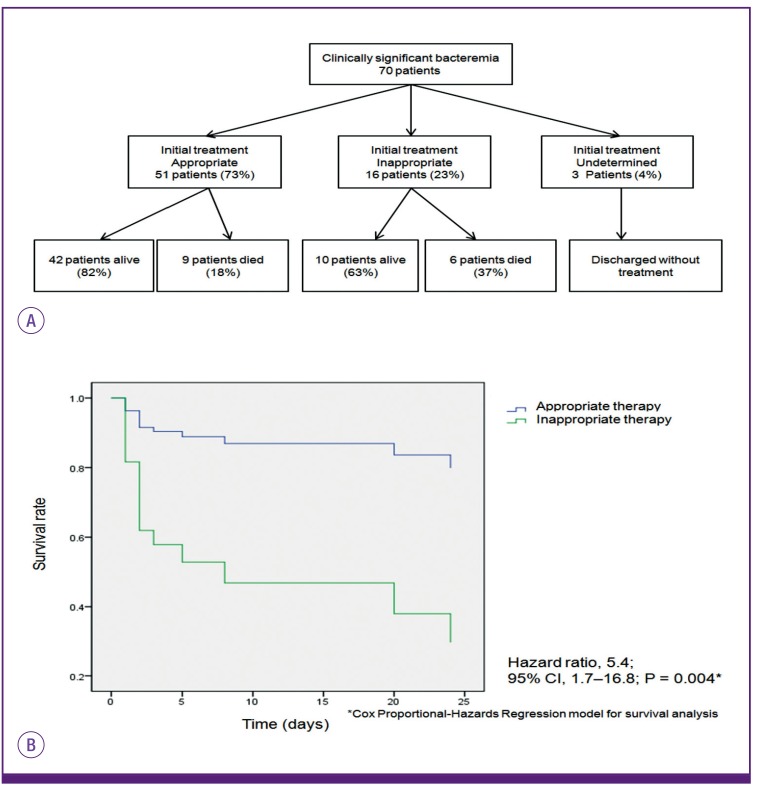
During the study period, patients were hospitalized for (13.5 [4, 36]) days (median, [first quartile, third quartile], range 1-166 days) and 15 patients (21.4%) died within 28 days of the onset of bacteremia. Fifty-one patients (72.9%) received appropriate antimicrobial therapy: piperacillin-tazobactam in 24 patients, carbapenem in 20 patients, and metronidazole in six patients. Sixteen patients received inappropriate therapy with either ineffective antimicrobials or an inadequate time of initiation (Fig. 1A). Six of the 16 patients who received inappropriate therapy died, and 4 of these 6 patients underwent therapy that included third-generation cephalosporin and other agents known to be ineffective against AB. Five of the 6 patients were deemed to be expired due to bacteremia within 1 to 5 days after the blood culture were drawn. Among the ten live patients of the 16 patients who received inappropriate therapy, six patients were treated with third-generation cephalosporins and other agents known to be ineffective against AB, three patients did not receive any antimicrobial therapy, and one patient underwent delayed therapy with carbapenem. The appropriateness of therapy was not significant in mortality by univariate and multivariate analysis, however, survival rate of bacteremia was significantly worse in patients who received inappropriate therapy compared with those underwent appropriate therapy by Cox proportional-hazards regression analysis (hazard ratio, 5.4; 95% confidence interval, 1.7–6.9; P = 0.004) (Fig. 1B).
Figure 1. Clinical outcome and survival rate of 70 patients with anaerobic bacteremia according to appropriateness of treatment(A, B).
Discussion
Clinical decision making is more difficult in anaerobic infections than aerobic infections, because of its limitations in isolating and susceptibility testing, such as slow growth of organisms, complexity of testing method and predictable susceptibility patterns among the anaerobes [14]. However, AB has reemerged as a significant clinical problem, owing to multiple reasons, such as change of medical practices and increasing number of patients with complex underlying diseases [15]. Similarly, from the trends in AB that we have followed since 1974, the incidence of AB has gradually increased in the last four decades, from 1.39% in 1974-1983 to 1.96% in 2007-2008 [16,17,18]. There were technological advances in blood culture detection systems in our institution, blood culture systems from conventional anaerobic broth blood systems to BACTEC 9240 systems (Becton Dickinson Diagnostic Instrument Systems, Sparks, MA, USA) in 1997 and to BacT/Alert 3D systems (bioMérieux) in 2005, however, the steady rather than sudden increase was noted during the decades. According to an annual report from the Korean Ministry of Health and Welfare [19], the number of elderly patients and patients with underlying malignancies is increasing and we assume that the change in patient population attributed to this trend we observed. Indeed, patients admitted to the Severance Hospital, a tertiary care referral center, often present with comorbidities and advanced disease.
To our knowledge, this is the first study in Korea to demonstrate a correlation between risk factors and mortality, using χ2 test and Cox proportional-hazards regression model for statistical analysis. In this study of AB, there were three findings: 1) mortality of patients with AB is associated with cardiovascular disease as an underlying disease; and 2) more than half of AB is derived from HA infection; and 3) the survival was significantly worse in patients who received inappropriate therapy compared with those underwent appropriate therapy.
The cardiovascular disease as an underlying condition was significantly associated with mortality, which is a novel finding in this study. Yang et al. [20] reported this comorbidity as not being a significant risk factor for 30-day mortality, however, the limitation in scope to a single species such as Clostridium perfringens, and different assessment of patient demographics may have contributed to discordant results. The relationship between cardiovascular disease and mortality in AB patients has not been demonstrated, so further investigation is needed.
The nosocomial acquisition of AB was commonly estimated in previous studies, from 25% to 64% of AB cases [20,21]. The nosocomial bacteremia was defined as a positive blood culture obtained more than 48 h after admission in previous studies. However, due to a complexity in the delivery of healthcare services, the category and definition of blood stream infection has changed. Based on the proposed definition of CA, HA, and HCA infection, 62.9% of cases were HA infection in this study.
Inappropriate therapy has been regarded as a prognostic factor in patient outcomes [6,22], and was also found to be statistically significant for the survival rate in this study. In the period of this study, 54 patients (77.1%) received initial therapy that was appropriately effective against AB, which is not much different from some studies that have reported values of 72-80% [6]. Among six expired cases of 16 patients who underwent inappropriate therapy, four patients had active cancer with multiple metastasis, one had acute pulmonary edema due to heart failure and one had traumatic subdural hemorrhage with coma metal status. Five of six patients had therapy using third-generation cephalosporin or colistin and one patient had no antimicrobials given. Generally, in our hospital, monotherapy of third-generation cephalosporin or piperacillin-tazobactam is recommended as the intial therapy when systemic infection is suspected, and carbapenam or vancomycin is considered in severe infections. AB is not empirically treated at the initial time of infection, but metronidazole is administered in clinically suspicious circumstances or when prior antimicrobial therapy has failed.
Several factors have been suggested to have an association with a greater likelihood of inappropriate antimicrobial therapy, such as presence of multiple infecting organisms, antibiotic-resistant bacteria, and prolonged length of stay [23]. In this study, however, there was no case of polymicrobial infection or use of antimicrobial agents that the isolate showed resistance in the inappropriately treated groups, and length of hospital stay was longer in the appropriately treated group compared with the inappropriately treated group (30 vs. 17.6 days). We assume that impression of patients with risk of AB is important in the clinical outcome as observed in our study that inappropriately treated groups showed worse survival. In addition, our findings regarding patient characteristics may guide physicians with clinical clues.
The limitations of the present study are that it was a restrospective review and was done with a small sample size. Despite these limitations, in conclusion, AB was frequently noted in patients having maliganancy and the mortality rate in patients with clinically significant AB was 21%. B. fragilis was the most frequently recovered organism with predictable susceptibility patterns. The survival rate of AB was significantly worse in patients who received inappropriate therapy compared with those underwent appropriate therapy and choice of proper initial antimicrobial is fundamental in clinical outcome of AB.
Acknowledgments
This research was supported by a CMB-Yuhan research grant of Yonsei University College of Medicine for 2012 (6-2012-0048).
Footnotes
Conflicts of Interest: No conflicts of interest.
References
- 1.Washington JA., 2nd Comparison of two commercially available media for detection of bacteremia. Appl Microbiol. 1971;22:604–607. doi: 10.1128/am.22.4.604-607.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arzese A, Trevisan R, Menozzi MG. Anaerobe-induced bacteremia in Italy: a nationwide survey. The Italian Anaerobe Study Group. Clin Infect Dis. 1995;20(Suppl 2):S230–S232. doi: 10.1093/clinids/20.supplement_2.s230. [DOI] [PubMed] [Google Scholar]
- 3.Peraino VA, Cross SA, Goldstein EJ. Incidence and clinical significance of anaerobic bacteremia in a community hospital. Clin Infect Dis. 1993;16(Suppl 4):S288–S291. doi: 10.1093/clinids/16.supplement_4.s288. [DOI] [PubMed] [Google Scholar]
- 4.Vazquez F, Mendez FJ, Perez F, Mendoza MC. Anaerobic bacteremia in a general hospital: retrospective five-year analysis. Rev Infect Dis. 1987;9:1038–1043. doi: 10.1093/clinids/9.5.1038. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JR, Limaye AP. Risk factors for mortality in patients with anaerobic bacteremia. Eur J Clin Microbiol Infect Dis. 2004;23:310–316. doi: 10.1007/s10096-004-1111-y. [DOI] [PubMed] [Google Scholar]
- 6.Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26:1413–1417. doi: 10.1086/516355. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CW, Lin HS, Ye JJ, Yang CC, Chiang PC, Wu TS, Lee MH. Clinical significance of and outcomes for Bacteroides fragilis bacteremia. J Microbiol Immunol Infect. 2009;42:243–250. [PubMed] [Google Scholar]
- 8.Summanen P, Baron EJ, Citron DM, Strong CA, Wexler HM, Finegold SM. Wadsworth anaerobic bacteriology manual. 5th ed. Belmont, CA: Star Publishing Co.; 1993. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute (CLSI) Methods for antimicrobial susceptibility testing of anaerobic bacteria : approved standard. 8th ed. Wayne, PA: CLSI; 2012. [Google Scholar]
- 10.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 11.Lenz R, Leal JR, Church DL, Gregson DB, Ross T, Laupland KB. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis. 2012;12:85. doi: 10.1186/1471-2334-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 13.Brook I. Treatment of anaerobic infection. Expert Rev Anti Infect Ther. 2007;5:991–1006. doi: 10.1586/14787210.5.6.991. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen MH, Yu VL, Morris AJ, McDermott L, Wagener MW, Harrell L, Snydman DR. Antimicrobial resistance and clinical outcome of Bacteroides bacteremia: findings of a multicenter prospective observational trial. Clin Infect Dis. 2000;30:870–876. doi: 10.1086/313805. [DOI] [PubMed] [Google Scholar]
- 15.Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44:895–900. doi: 10.1086/512197. [DOI] [PubMed] [Google Scholar]
- 16.Kim HO, Kang CG, Chong Y, Lee SY. Organisms isolated from blood at the Yonsei medical center, 1974-1983. Korean J Infect Dis. 1985;17:15–32. [Google Scholar]
- 17.Koh EM, Lee SG, Kim CK, Kim M, Yong D, Lee K, Kim JM, Kim DS, Chong Y. Microorganisms isolated from blood cultures and their antimicrobial susceptibility patterns at a university hospital during 1994-2003. Korean J Lab Med. 2007;27:265–275. doi: 10.3343/kjlm.2007.27.4.265. [DOI] [PubMed] [Google Scholar]
- 18.Park Y, Lee Y, Kim M, Choi JY, Yong D, Jeong SH, Kim JM, Lee K, Chong Y. Recent trends of anaerobic bacteria isolated from clinical specimens and clinical characteristics of anaerobic bacteremia. Infect Chemother. 2009;41:216–223. [Google Scholar]
- 19.Do SR. Changing trends in Korean patients' use of health facilities. [Accessed 12 February 2016]. Available at: https://www.kihasa.re.kr/web/publication/periodical/issue_view.do?menuId=50&tid=38&bid=21&searchForm=Y&keyField=myear&search-Stat=2009&key=&aid=8&ano=1.
- 20.Yang CC, Hsu PC, Chang HJ, Cheng CW, Lee MH. Clinical significance and outcomes of Clostridium perfringens bacteremia--a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17:e955–e960. doi: 10.1016/j.ijid.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Kornowski R, Schwartz D, Averbuch M, Levo Y, Berger S, Giladi M. Anaerobic bacteremia: a retrospective four-year analysis in general medicine and cancer-patients. Infection. 1993;21:241–244. doi: 10.1007/BF01728898. [DOI] [PubMed] [Google Scholar]
- 22.Robert R, Deraignac A, Le Moal G, Ragot S, Grollier G. Prognostic factors and impact of antibiotherapy in 117 cases of anaerobic bacteraemia. Eur J Clin Microbiol Infect Dis. 2008;27:671–678. doi: 10.1007/s10096-008-0487-5. [DOI] [PubMed] [Google Scholar]
- 23.Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31(Suppl 4):S131–S138. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]