Abstract
Cardiovascular disease is a leading cause of death worldwide and there is a pressing need for new therapeutic strategies to treat such conditions. The risk of developing cardiovascular disease increases dramatically with age, yet the majority of experimental research is executed using young animals. The cardiac extracellular matrix (ECM), consisting predominantly of fibrillar collagen, preserves myocardial integrity, provides a means of force transmission and supports myocyte geometry. Disruptions to the finely balanced control of collagen synthesis, post-synthetic deposition, post-translational modification and degradation may have detrimental effects on myocardial functionality. It is now well established that the aged heart is characterized by fibrotic remodelling, but the mechanisms responsible for this are incompletely understood. Furthermore, studies using aged animal models suggest that interstitial remodelling with disease may be age-dependent. Thus with the identification of new therapeutic strategies targeting fibrotic remodelling, it may be necessary to consider age-dependent mechanisms. In this review, we discuss remodelling of the cardiac collagen matrix as a function of age, whilst highlighting potential novel mediators of age-dependent fibrotic pathways.
Keywords: Aging, Extracellular matrix, Collagen, Fibrosis, Heart failure
Highlights
-
•
Aging is associated with alterations to myocardial collagen and cardiac function.
-
•
Collagen remodelling with disease is age-dependent.
-
•
Collagen remodelling mediators may become targets for disease treatment in elderly.
1. Introduction
It is now apparent that aging is a critical factor to consider in the study of cardiac remodelling. The majority of patients suffering from the most debilitating cardiovascular diseases, including heart failure (HF), are from the aging population. For example the prevalence of HF in persons aged > 75 years is ~ 8.4% compared with ~ 0.7% in those aged 45–54 [1]. Yet this represents a dichotomy, as aged animal models and elderly patients (the latter arbitrarily defined as those aged ≥ 65 years [2]) are poorly represented in basic and clinical research, respectively [3], [4], [5]. For the appropriate translation of research findings, a thorough understanding of aging in both the physiological and pathological setting is required.
Age-associated changes in cardiac physiology occur at the cellular, extracellular and whole-heart levels. Apoptotic or necrotic pathways may be responsible for the progressive loss of myocytes with age [6], [7]. Decreased peripheral vascular compliance and augmented afterload leads to increased oxygen consumption, energy deficits and oxidative stress [8], [9], [10]. In an attempt to normalize left ventricular (LV) wall stress [11], [12], both myocyte death and altered loading conditions lead to hypertrophy of remaining myocytes [13], [14], proliferation of cardiac fibroblasts (CFs) [15], [16], [17] and interstitial fibrosis [18], [19], [20]. Consequently these changes may manifest as LV hypertrophy, impaired ventricular relaxation and diastolic dysfunction [21]. Although systolic function remains relatively preserved in the elderly, contractility may become impaired during exercise [22], [23]. It is thought that these morphological and functional changes contribute to the prevalence of HF with preserved ejection fraction (HFpEF) in the aging population [24], [25].
Although a primary contributor to these changes, peripheral vascular stiffening and/or adaptive responses to co-morbidities may not be the only instigator of age-related myocardial remodelling. Mounting evidence suggests that chronological aging alone may lead to intrinsic changes in the myocardium [26], [27], [28]. In particular alterations to the cardiac extracellular matrix (ECM), once thought of merely as a static myocyte support network, are imperative to the development of cardiac dysfunction with age. As such, consideration of fibrotic pathways may be key to the development of future pharmacological strategies for the treatment of HF; for which there is currently no cure. This highlights the need for the identification of new therapeutic targets, and perhaps, the importance of tailored intervention that accounts for patient variability, including that brought about as a result of age-related remodelling.
In the present manuscript we will review the role of the collagen matrix in cardiac remodelling with age. In addition, recent evidence indicating novel mediators of fibrotic remodelling will be highlighted, and their potential role in age-related cardiac disease discussed.
2. Components and roles of the cardiac extracellular matrix
The ECM consists of a complex lattice-like network of proteins, molecules and non-myocyte cells, embedded in a glycosaminoglycan and proteoglycan hydrogel [26], [29], [30]. The myocytes are surrounded by a network of basement membrane proteins, including lattice networks of collagen type IV and laminin (linked by the proteoglycan perlecan), and the glycoprotein fibronectin which collectively mediate collagen fibril attachment to the sarcolemma [31], [32]. The myocyte's actin cytoskeleton is in contact with the surrounding fibrillar collagen network via integrins — signal mechano-transducers involved in cell signalling, proliferation, migration, excitation and differentiation [33], [34], [35], [36], [37]. Therefore these matrix proteins perform a variety of roles in addition to providing structural integrity. Although less abundant than collagen, alterations to these proteins in disease and indeed aging may contribute to cardiac dysfunction. However the purpose of the present review is to focus on the collagen matrix, and the role of other ECM proteins in aging has been covered elsewhere [26], [38], [39].
Although most plentiful by volume, cardiac myocytes are greatly outnumbered by non-myocyte cells, the latter constituting approximately ~ 70% of all myocardial cells, of which ~ 90% are CFs [40]. CFs are the primary cell type responsible for maintaining ECM homeostasis, and do so by sensing and responding to mechanical, electrical and neurohormonal cues [16], [41], [42], [43], [44], [45]. However following cardiac stress or injury, CFs may differentiate into myofibroblasts; which, by expressing the contractile protein α-smooth muscle actin (αSMA), may contract and migrate, and are particularly sensitive to the molecular signals that are characteristic of the diseased myocardium (reviewed in [16], [26], [44]).
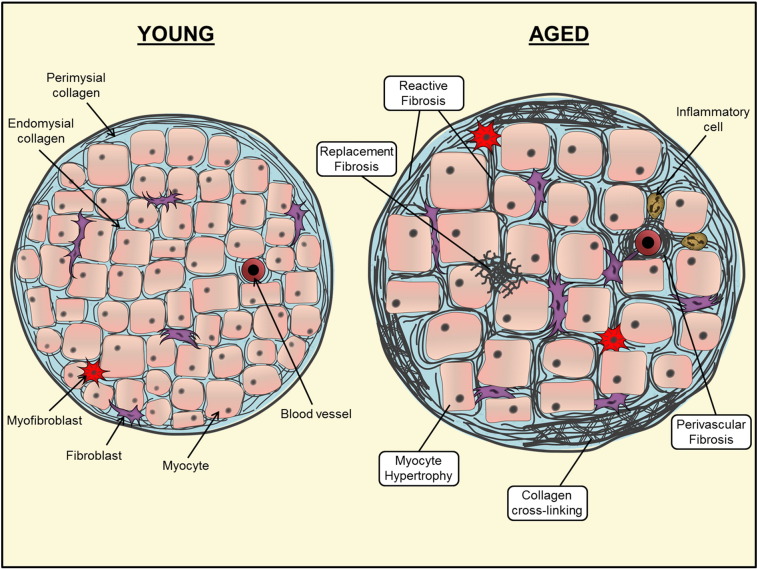
The most abundant protein of the cardiac ECM is fibrillar collagen (elastic fibres are present to a lesser extent in the ventricular myocardium [46], [47], [48], [49]). Most myocardial collagen fibres consist of collagen types I and III, which, (depending on species), account for approximately 80% and 10% of collagen in the healthy heart, respectively [50], [51]. Organization of collagen fibres is intricate and occurs at the level of the myocyte and myofibrillar bundles (see Fig. 1). The endomysial collagen network connects individual myocytes via Z-band-integrin connections, and prevents ventricular dilatation by maintaining myocyte alignment [52], [53]. The perimysial collagen surrounds entire myofibrillar bundles; often in a weave-like structure that provides tensile strength [54]. Thus the primary role of collagen in the heart is to provide a structural framework to the cardiac myocytes, impart stiffness to the myocardial wall and aid force transmission [55], [56]. It is therefore understandable why collagen synthesis, post-translational modification and degradation are highly regulated processes, and even slight variations to the collagen matrix may have drastic effects on myocardial force development [57], relaxation and diastolic stiffness [58] and conduction properties leading to arrhythmogenesis [59]. However, modulation of the collagen matrix may also play a reparative role, for example in the case of scar formation following injury which prevents wall rupture [60], [61].
Fig. 1.
Schematic representation of age-related alterations to the cardiac collagen matrix. Both cellular and interstitial remodelling occur as a result of aging in the myocardium. Myocyte loss occurs through apoptotic and/or necrotic pathways. This results in hypertrophy of remaining myocytes and replacement fibrosis. Perivascular and reactive fibrosis occurs by accumulation of collagen in the interstitial space. Post-translational modification of collagen, including enhanced cross-linking is also present.
Collagen is synthesized by CFs as a procollagen molecule containing N- and C-terminal propeptide regions. In order for collagen to be deposited as a mature fibril, a sequence of post-synthetic processing steps is carried out within the extracellular space. This involves cleavage of the propeptides by specific enzymes [62], [63], [64], [65], [66], association with matricellular proteins [67], [68] and self-assembly of collagen molecules into staggered fibrils [69], [70]. Collagen may be further stabilized by cross-link addition that occurs by at least two known mechanisms: (i), lysyl oxidase (LOX)-mediated aldehyde formation between lysine or hydroxylysine residues [71], [72]; and (ii), advanced glycation end product (AGE) formation between amino groups by reducing sugars [73]. Enhanced collagen cross-linking has been associated with augmented myocardial stiffness [74], [75].
In addition to synthesizing collagen, CFs are also the primary source of the matrix metalloproteinases (MMPs) — a group of endopeptidases responsible for matrix protein degradation. Of the 25 MMP family members identified to date, a subset is present in the myocardium [26], [76], [77]. Collectively the MMPs display activity towards both traditional matrix proteins, as well as non-structural and non-matrix substrates; including those involved in collagen deposition and pro-fibrotic signalling [78], [79], [80], [81] (see Section 4.2). Perhaps as an indication of their importance to myocardial function [77], [82], [83], [84], the MMPs are inhibited by an endogenous group of inhibitors known as the tissue inhibitor of metalloproteinases (TIMPs). All four known TIMP family members are expressed in the myocardium, predominantly by CFs but also a variety of other cell types [85]. Like the MMPs, evidence suggests a cause and effect relationship between TIMP expression and myocardial function [86], [87], [88]. The roles of MMPs and TIMPs in myocardial remodelling with disease have been discussed in detail elsewhere ([76], [85], [89], [90]).
3. Collagen synthesis, deposition and modification in aging
3.1. Age-related alterations to myocardial collagen content
It is now generally accepted that aging is associated with collagen accumulation in several organs including the heart (Fig. 1). Studies in rodents demonstrate that collagen content of the LV increases progressively with age and is associated with increased wall stress and contractile dysfunction [19], [28], [91]. Importantly it has been shown that LV fibrosis may occur without changes to systolic or diastolic blood pressure [91], suggesting that age-related fibrosis is not necessarily a consequence of underlying hypertension. Similar findings of age-related LV fibrosis have also been found in large animal models, including the sheep [20] and the dog [92].
Is this also the case in terms of the human aging? It is often argued that age-related fibrotic remodelling may occur as a result of underlying pathology rather than chronological aging. Nowhere is this more difficult resolve than with human samples — where the difficulty of obtaining non-diseased “control” tissue, and the potential anti/pro-fibrotic effects of therapeutic agents, are likely to hinder the separation of “healthy aging” and subclinical disease. Nevertheless, there are some studies that have quantified LV collagen levels in the aged human heart. Using picrosirius red staining and polarized light microscopy, Debessa et al. reported an increase in collagen content with age (~ 5.9% vs. ~ 3.9%) in human hearts obtained from autopsy with no previous pathologies (age range 67–87 vs. 20–25) [18]. More recently, clinical studies have employed imaging technologies to estimate areas of interstitial fibrosis, scar size and extracellular volume in vivo, as an alternative to the invasive collection of ventricular biopsies [93]. Liu et al. studied over 1200 patients in the age range of 54–93 years using cardiac magnetic resonance (CMR) imaging, late gadolinium enhancement and T1 mapping [94]. The authors found that older age was associated with augmented indices indicative of cardiac fibrosis, including extracellular volume fraction (ECV), but this varied with patient gender depending on multivariable adjustments [94]. ECV increased with age in men both before and following adjustment for markers of subclinical disease (including hypertension, body weight, heart rate, diabetes and LV mass:volume ratio), whereas these differences were only present in women following these adjustments [94]. Neilan et al. also observed a gradual augmentation of ECV with age in patient groups divided as follows: (i) < 40 years, (ii) 40–60 years and (iii) > 60 years [95]. Additionally, this study showed that age was the strongest independent predictor of ECV [95]. Therefore as in the studies using animal models, aging in humans appears to be characterized by myocardial collagen accumulation.
3.2. Alterations to collagen synthesis with age
Although reports of aging-associated myocardial fibrosis are plentiful, the mechanisms responsible for this are less clear. If disease models are used as a paradigm, one could assume that an important instigator would be increased collagen synthesis. For example, in both humans and animal models of cardiovascular disease, elevated levels of collagen mRNA are reported in addition to collagen accumulation [96], [97]. That said, evidence suggests that elevated myocardial collagen in aging is most likely due to post-synthetic or degradative processes. Generally speaking, collagen types I and III mRNA levels either decrease or do not change with age in the heart [20], [98], [99], [100]. Indeed a study in rats has shown that collagen synthesis rates in vivo were at least 10-fold less in the hearts of animals aged 24 months compared to those aged 1 month [101]. Confirming this, another study has shown that both hydroxyproline content and histological quantification of LV collagen increased with age in rats, yet mRNA levels of both procollagen types I and III decreased [102].
3.3. Alterations to collagen post-translational modifications with age
Cross-linking of collagen has proven to significantly alter myocardial stiffness without changes to total collagen content and the degree of myocardial collagen cross-linking increases with age [103]. In particular, glucose-mediated formation of AGEs accumulate with age [104], by modifying the structure of proteins with low turnover rates [73]. Furthermore studies have shown that interruption of AGE formation in the senescent heart can improve myocardial function. Treatment of aged dogs with an AGE cross-link breaker decreased age-associated chamber stiffening and diastolic dysfunction compared to non-treated age-matched animals [105]. In another study, induction of diabetes in older dogs with alloxan monohydrate (a glucose analogue which is toxic to insulin-producing β cells [106]) caused upregulation of LV collagen types I and III, increased LV mass and decreased ejection fraction [107]. However treatment of aged, diabetic animals with an AGE cross-link breaker normalized these parameters without affecting blood glucose level [107]. Additionally the increase in LV collagen solubility following treatment with the cross-link breaker suggests that the mechanism of action was by decreasing collagen cross-linking. Others have suggested that exercise training may reduce age-related augmentation of collagen cross-linking and decrease collagen type I and III mRNA synthesis without affecting total collagen levels [99]. Therefore decreasing collagen cross-linking may in turn affect total collagen content or synthesis of collagen mRNA.
In addition to collagen cross-linking, the matricellular protein secreted protein acidic and rich in cysteine (SPARC) facilitates post-translational processing and thus deposition of mature collagen in the myocardium [108]. Studies in mice have demonstrated that the age-associated increase in myocardial collagen is blunted in SPARC-null animals [109], [110]. Furthermore SPARC deletion reduced the relative proportion of insoluble collagen and decreased papillary muscle stiffness in aged mice [109]. Therefore SPARC is likely an important mediator of collagen deposition and myocardial stiffness in aging, and is discussed in detail by others in this Special Issue [111].
3.4. Patterns of myocardial fibrosis in the aging heart
Generally there are two “types” of fibrotic remodelling: (i) reactive fibrosis (also known as diffuse fibrosis) which describes the expansion of existing collagen fibres without a significant loss of myocytes; and (ii) replacement/reparative fibrosis or “scar” formation (focal fibrosis) which occurs when collagen is newly deposited in place of necrotic/apoptotic myocytes (see Fig. 1) [15], [93], [112], [113]. Both histological and contrast-enhanced CMR imaging modalities provide visual evidence suggesting that diffuse, reactive fibrosis is common in the aged heart [94], [114]. However as it has been suggested that myocyte apoptosis and necrosis increases with age [14], it is plausible to assume that replacement fibrosis may also occur — a hypothesis which has been suggested in some animal models of aging [91]. Conversely others have shown that neither reactive nor reparative fibrosis correlates with age in patients with idiopathic dilated cardiomyopathy [115], although this study was conducted in patients with a disease background rather than observing the isolated effects of aging.
The significance of these fibrotic patterns are likely diverse, as the nature or quality of the collagen network may differentially impact myocardial stiffness or signal propagation. Although it is known that reactive fibrosis increases LV stiffness [116], and electrical mapping studies suggest that the architecture of fibrotic remodelling in disease may differentially impact electrical propagation and conduction delay [117], [118], there is little evidence that directly compares the functional consequences of reactive vs. reparative fibrosis, particularly in the setting of aging.
Additionally, perivascular fibrosis, or accumulation of collagen surrounding blood vessels in the heart, may precede or act as an extension of reactive fibrosis [119]. It has been suggested that perivascular fibrosis increases in the aged heart. For example accumulation of perivascular and interstitial collagen occurred in advanced-aged rhesus macaques (1.5%) compared to young animals (0.33%) [120]. In patients with non-ischemic HF, although perivascular fibrosis was independent of cardiac dysfunction, it was associated with decreased coronary flow of the left anterior descending artery [121]. The authors concluded that perivascular fibrosis may lead to impaired coronary blood flow, which has been demonstrated to correlate with elevated LV wall stress in HF patients [122]. Thus if perivascular fibrosis occurs in aging, this may have local or paracrine effects on the surrounding myocardium which in turn could contribute to cardiac dysfunction in elderly subjects.
3.5. Age-related fibrosis and diastolic dysfunction
As discussed previously, impaired relaxation and therefore diastolic dysfunction is a common characteristic of the aged heart, and it is becoming apparent that myocardial fibrosis may be a contributing factor leading to this phenotype. Interventional studies in disease models suggest that it is excess collagen, and not myocyte hypertrophy that causes myocardial stiffness. For example, following angiotensin II (AngII) receptor antagonism with candesartan in rats with hypertension-induced diastolic HF, collagen deposition, hypertrophic remodelling and myocardial stiffness were all abrogated [123]. Conversely, treatment with a calcenurin inhibitor (which will inhibit some, non-physiological hypertrophic pathways by blocking intracellular calcium signalling), had no effect on cardiac fibrosis and stiffness despite limiting compensated hypertrophy [123]. Furthermore in a clinical study of hypertensive patients, those presenting with diastolic HF were older, and had higher levels of serum markers of collagen turnover [124]. However, others have found no correlation between diastolic dysfunction and collagen content in the aged heart [104]. Further work is required to characterize the role of myocardial fibrosis and diastolic dysfunction specifically in an aged cohort.
4. Role of MMPs and TIMPs in the aged heart
4.1. MMP/TIMP levels in the aged heart
Alterations to myocardial MMPs and TIMPs have been described in animal models of aging. Interestingly whether MMP and TIMP expression is found to increase or decrease seems to depend greatly on the species, ages chosen and soluble vs. insoluble (matrix-bound) localization [125], [126]. Additionally, such inconsistencies in the literature are likely a result of complexity in the roles of MMPs and TIMPs which have been described in detail elsewhere [76], [85], [89], [127], [128]. For example, it is now apparent that MMPs may act in a pro-fibrotic manner in addition to their matrix-degrading capabilities [129] (discussed below). Nevertheless, the majority of available data concerning MMPs and TIMPs in human aging has been limited to circulating rather than cardiac-specific expression. In the absence of cardiovascular disease, circulating MMP-2, MMP-7, TIMP-1 and TIMP-2 increased with age, whereas MMP-9 decreased in patients aged 20–90 years [130]. Furthermore circulating MMP-7, TIMP-1 and TIMP-2 inversely correlated with decreased E/A ratio [130]. Thus enhancement of both MMP and TIMP protein levels are associated with diastolic dysfunction in elderly, non-diseased humans. However studies such as these must be interpreted with caution and further work is required not only to identify which specific MMPs and TIMPs are involved in age-related matrix remodelling, but also to elucidate how increased MMP levels/activity may result in LV fibrosis with age.
4.2. Novel actions of MMPs leading to fibrotic remodelling in the aged heart–non-matrix-degrading roles
Gene manipulation, specifically in aged mice, has begun to address the pro-fibrotic actions of certain MMPs in the aged heart [26]. Global deletion of MMP-9 in aged mice blunted the aged-associated LV fibrosis and diastolic dysfunction present in aged wildtype mice, suggesting that MMP-9 is pro-fibrotic in the aged mouse heart [100]. In this study, the authors found that MMP-9 deletion attenuated the age-associated increase in transforming growth factor-β (TGF-β)-induced protein and phosphorylated Smad2, as well as mRNA expression of pro-fibrotic periostin and connective tissue growth factor (CTGF) [100]. Furthermore others have demonstrated that MMP-9 can cleave latent TGF-β, leading to activation in vitro [131]. Finally MMP-9 knockout resulted in a compensatory increase in MMP-8 levels only in the aged mice, which could contribute to the decrease in age-associated fibrosis in the knockout animals [100]. Thus in the aged myocardium MMP-9 may be pro-fibrotic by increasing availability of active TGF-β, enhancing periostin and CTGF expression and therefore potentiating collagen deposition. However, the age-associated increase in circulating levels of MMP-9 in the mouse [132] is in contrast to the human study mentioned previously [130], highlighting the complexity of investigating the role of the MMPs in aging.
Nevertheless, TGF-β-related signalling may be a common pathway influencing the pro-fibrotic nature of some MMPs. In a study comparing young (3 month) and “middle-aged” (14 month) mice, cardiac-specific over-expression of membrane type MMP-1 (MT1-MMP) not only caused LV fibrosis in the young, but also potentiated the age-related increase in LV collagen (more than 2-fold), LV dilatation and decreased ejection fraction [133]. Low molecular weight latency-associated TGF-binding protein (LTBP-1) increased with age (consistent with increased processing to active TGF-β), but to a greater extent in the aged, MT1-MMP over-expressing mice. Protein levels of TGF-β receptor I (TGF-βRI) and phosphorylated Smad2 were also highest in aged, transgenics [133]. Finally in silico mapping predicted an MT1-MMP binding site on full-length LTBP-1, and a cleavage product equating to the approximate weight of the processed, low molecular weight protein [133]. This was confirmed by in vitro experiments, where wildtype myocardial extracts incubated with recombinant MT1-MMP, resulted in increased levels of the low molecular weight protein following immunoblotting for LTBP-1 [133]. Thus again, this data suggests that certain MMPs may exhibit pro-fibrotic behaviour through TGF-β signalling in the aged heart.
5. Impact of age-related collagen remodelling on cardiac disease in the elderly
The studies described thus far provide evidence for the role of age-related collagen remodelling in the heart. However they also pose an important question — if the aged myocardium is phenotypically and functionally different from the young, does the aged heart undergo a different course of remodelling with injury or disease? Several studies reviewed previously suggest that this may be the case [26]. Generally speaking, investigations that compare aged animal models of disease to young, find that global remodelling and dysfunction is exacerbated with age [20], [92], [134], [135], [136]. In a mouse model of ischaemia–reperfusion (IR), aging led to a suppressed inflammatory response and reduced collagen deposition in the infarct region compared to young animals, alongside worse LV function after injury [134]. Similarly, others show that older rats exhibited blunted myocyte hypertrophy and myocardial fibrosis following MI-induced HF [137]. In a canine model of reperfused MI, markers of matrix turnover were increased in the infarct region with age [92], [135]. Furthermore we find that following tachypacing-induced HF in the sheep, LV collagen is decreased in the aged heart and is associated with greater contractile dysfunction, whereas interstitial fibrosis occurred in the young following tachypacing [20]. Therefore if age is a factor influencing the way in which the heart remodels with injury, this will likely impact the development of therapeutic strategies aimed to treat cardiovascular disease. As an example, in the canine model, the beneficial effects of candesartan (an AngII receptor antagonist) on post-infarct injury, apoptosis and systolic dysfunction were impaired in aged animals compared to young [92].
6. Novel mediators of cardiac fibrosis and their potential role in aging
As aging leads to alterations to the cardiac interstitium, which may alter the course of remodelling with disease, it seems necessary that aging is considered an important factor in the identification of new therapeutic targets for the treatment of cardiac remodelling. In the following sections, we highlight a selection of novel mediators of fibrotic remodelling in disease, and discuss their potential role in age-related cardiac remodelling (see Table 1). We have chosen these particular mediators for discussion based on three lines of evidence: (i), recent clinical studies/trials implicating a potential role in the pathogenesis or treatment of HF; (ii), potential mechanism of action through the collagen matrix; and (iii), potential for aging to further influence this collagen-mediated mechanism of action. We do however acknowledge that several other, important bioactive molecules will be therapeutically relevant for the treatment of HF in the elderly; however these have been reviewed in detail elsewhere (for example TGF-β signalling [138], [139] and renin–angiotensin–aldosterone (RAAS)/natriuretic peptide systems [140], [141], [142], and are therefore not considered in detail here).
Table 1.
Novel mediators of cardiac fibrosis and their potential role in aging.
| Mediator | Type | Extracellular matrix roles | Evidence for role in aging |
|---|---|---|---|
| Relaxin | Hormone | ↓ established fibrosis [149], [151]. ↓ TGF-β/AngII-mediated collagen synthesis, CF proliferation and differentiation [151]. ↑ MMP levels [151], [199]. |
Male relaxin−/− mice ↑ age-related progression cardiac fibrosis, diastolic dysfunction [152]. |
| Galectin-3 | Lectin | ↑ collagen synthesis, deposition and LV fibrosis at baseline and with disease [161], [200]. | Circulating galectin-3 ↑ with age [166]. Conflicting evidence for association between circulating galectin-3 and LV/fibrotic remodelling in the elderly [167], [168]. |
| Cardiotrophin-1 | Cytokine | ↑ procollagen types I and III synthesis [174], [201]. ↑ interstitial and perivascular fibrosis [201]. ↑ MMP-2, MMP-13:TIMP-1, osteopontin and periostin [201]. ↑ fibroblast proliferation and differentiation [174], [202]. |
Aged cardiotrophin-1−/− mice ↓ arterial fibrosis and stiffness, ↑ longevity [175]. |
| miRNAs | Non-coding RNAs | ||
| miR-22 | ↑ miR-22 in aged myocardium [183]. ↑ CF senescence with pre-miR-22 transfection [183]. |
||
| miR-17-92 cluster | ↓ miR-17 with age in several cell types [188]. ↓ cardiac tissue/cellular senescence in miR-17 transgenic mice [187]. ↓ miR-18a, -19a, -19b associated with ↑ CTGF and TSP-1 in aged, HF mice [186]. ↑ CTGF, TSP-1, collagen types I and III mRNA following cardiomyocyte transfection with antagomirs to miR-18a or -19b [186]. |
||
| miR-34a | ↑ miR-34a aged myocardium and vasculature [184], [185]. ↓ age-related myocyte death, age-related cardiac dysfunction and scar formation following MI in miR-34a−/− mice [184]. ↑ senescence and pro-inflammatory factor secretion in VSMCs overexpressing miR34a [185]. |
||
| Osteopontin | Cytokine/matricellular protein | ↑ recruitment of inflammatory cells post-injury [203]. ↑ collagen deposition post-injury [192]. ↑ fibroblast-myofibroblast differentiation [191]. Proteolytically processed by MMPs [204] |
↑ osteopontin in aged rat aorta [195]. ↑ osteopontin mRNA in cardiac biopsies from elderly patients with cardiac fibrosis [196]. Alterations to osteopontin levels following IR injury are age-dependent [92], [134], [135]. |
↑, increase; ↓, decrease; TGF-β, transforming growth factor-β; AngII, angiotensin II; CF, cardiac fibroblast; −/−, genetic deletion; MMP, matrix metalloproteinase; LV, left ventricle; TIMP, tissue inhibitor of metalloproteinase; miRNA, microRNA; CTGF, connective tissue growth factor; TSP-1, thrombospondin-1; VSMC, vascular smooth muscle cell; IR, ischaemia reperfusion.
6.1. Relaxin
Relaxin is a vasoactive peptide hormone encoded by the human relaxin genes RLN1 and RLN2 (producing H1 and H2 relaxin, respectively). By binding to the relaxin family peptide receptor (RXFP), relaxin exerts its actions through G-protein-coupled signalling and downstream mediators including cyclic AMP (cAMP), mitogen activated protein kinases (MAPKs) and nitric oxide [143]. Relaxin has pleiotropic actions on the cardiovascular system [143], and recent clinical trials using serelaxin (a human recombinant form of relaxin-2) suggest that treatment improved dyspnoea and 180-day mortality in patients with acute HF [144], [145]. Notably, it has been demonstrated that relaxin has direct effects on the collagen matrix, and has been shown to decrease collagen accumulation in several fibrotic models of disease [146], [147], [148]. In a recent study using a mouse model of isoprenaline-induced LV fibrosis, serelaxin was a more effective anti-fibrotic agent than the angiotensin converting enzyme (ACE) inhibitor enalapril [149]. Here, the authors show that both treatments attenuated isoprenaline-induced LV fibrosis, TGF-β1 and phosphorylated Smad2 immunoreactivity, but improvement was greatest with serelaxin treatment [149]. Furthermore although combined treatment improved injury-induced remodelling, it was to no greater extent than with serelaxin alone. Others show that relaxin treatment reverses atrial fibrosis and decreases mRNA expression of collagen type I, collagen type III, MMP-2 and MMP-9, and decreases AF vulnerability in the spontaneously hypertensive rat [150]. Collectively these data suggest that relaxin exhibits anti-fibrotic effects following myocardial injury. In vitro models further support this. Treatment of CFs in culture with human recombinant relaxin-2 abrogated the TGF-β or AngII-mediated increase in collagen type I and III secretion, collagen deposition, proliferation, fibroblast-myofibroblast differentiation and increased MMP-2 expression — despite having no effect on baseline levels [151]. Furthermore treatment of β2-adrecoreceptor overexpressing mice with relaxin over a 14 day period significantly reduced LV collagen content in this model of established fibrosis [151].
Characterization of the relaxin knockout mouse suggests that relaxin may also play a role in fibrotic remodelling in the aged heart. Although restricted to male animals, relaxin-null mice exhibited age-related progression of fibrosis in several organs including the myocardium [152]. By 12–24 months of age, relaxin-null mice had a 40–50% increase in cardiac collagen whilst exhibiting increased LV stiffness and decreased diastolic filling although systolic function was maintained. Moreover, treatment of relaxin knockout mice with human recombinant relaxin reversed the established fibrosis [152]. Therefore relaxin may play a protective role in limiting age-related cardiac fibrosis and development of diastolic dysfunction.
6.2. Galectin-3
Galectin-3 is a β-galactoside-binding lectin protein that is secreted from both inflammatory cells and fibroblasts in several organs including the heart [153]. Galectin-3 can bind to several ECM proteins [154], and as it's protein structure contains collagen-like domains, it is a substrate for cleavage by MMPs-2, -7 and -9 [155], [156]. Recent clinical studies suggest that increased levels of circulating galectin-3 are associated with risk and severity of HF, re-hospitalization and all-cause mortality [157], [158], [159]. Evidence from studies using animal models suggests that galectin-3 is involved in fibrotic remodelling with disease. For example, Ren-2 rats (rats expressing the mouse gene for submandibular gland renin [160]) with HF had higher galectin-3 protein levels, and galectin-3 mRNA expression was greater in human heart biopsies from aortic stenosis patients with reduced ejection fraction compared to those with preserved ejection fraction [161]. Intrapericardial infusion of recombinant murine galectin-3 in healthy rats for 4 weeks decreased LV ejection fraction and caused LV collagen accumulation [161]. Finally stimulation of neonatal rat CFs with recombinant galectin-3 increased cell proliferation and collagen production [161]. Others have shown that cardiac fibrosis induced by 3-week aldosterone treatment is abrogated in galectin-3 knockout mice [162], and pharmacological inhibition of galectin-3 with modified citrus pectin improved indices of cardiac fibrosis and inflammation in the spontaneously hypertensive rat without modifying blood pressure [163]. In humans, circulating levels of galectin-3 correlate with diffuse myocardial fibrosis estimated by late gadolinium enhancement CMR and T1 mapping [164], as well as predicting incidence of HF and mortality [165]. Interestingly in the latter study, patients with higher plasma galectin-3 levels tended to be older [165], suggesting that increased galectin-3 levels with age may play a role in cardiac remodelling.
To this end, there is little evidence directly correlating a role for galectin-3 in collagen remodelling with age. Some clinical studies report a correlation between circulating galectin-3 levels and advancing age in the general population [166], and an association has been found between circulating galectin-3 and LV remodelling in HF patients with a mean age of ~ 71 years [167]. However others suggest that age-related increases in galectin-3 are not associated with either fibrosis or clinical characteristics of HFpEF [168]. Clearly there is a need for further studies (perhaps using aged animal models), to elucidate the potential role of cardiac galectin-3 and fibrotic remodelling with age.
6.3. Cardiotrophin-1
As a member of the IL-6 superfamily of cytokines, cardiotrophin-1 is secreted from cardiac myocytes and fibroblasts in response to both stretch [169] and neurohormonal activations [153]. Recent clinical studies suggest that circulating cardiotrophin-1 levels are higher in both hypertensive patients and those with diastolic HF compared to controls [170], [171]. Initially, the role of cardiotrophin-1 was described as potentiating myocyte hypertrophy and promotion of myocyte survival by inhibition of apoptosis [172], [173]. However, further studies have now characterized a role for cardiotrophin-1 in fibrotic remodelling in disease. For example, cardiotrophin-1 protein levels were increased in endomyocardial biopsies from hypertensive patients with HF compared to control cardiac tissue [174]. Additionally, cardiotrophin-1 levels correlated with collagen type I and III protein, and stimulation of isolated human CFs with cardiotrophin-1 increased mRNA expression of αSMA and procollagen types I and III [174]. With regards to aging, the effect of cardiotrophin-1 deletion on age-dependent arterial remodelling has been investigated. In this study, cardiotrophin-1 knockout mice aged 29 months exhibited less arterial fibrosis, decreased arterial stiffness and survived on average 5 months longer than wildtype mice [175]. Thus cardiotrophin-1 may be an important mediator of age-associated vascular remodelling. Further work is required to elucidate its role in the myocardium with age.
6.4. miRNAs
MicroRNAs (miRNAs) are small (approximately 22 nucleotides in length), non-coding RNAs that can post-transcriptionally modify gene expression. miRNAs have complementary binding sequences to mRNA, leading to either degradation or translational repression of their target mRNA [176], [177]. Although miRNAs are likely to take part in all cellular processes, several specific miRNAs have been identified as critical mediators of fibrotic remodelling in disease. This subject has been reviewed elsewhere [176], [178], [179]. However it is of note that the properties of miRNAs mean the potential to exploit or target specific miRNAs therapeutically in the treatment of HF is great [180], [181], [182]. In contrast to these studies of miRNA roles in disease, relatively little information exists depicting which miRNAs are involved in cardiac remodelling with age. Herein we describe a few examples from the literature of miRNAs which may play a role in age-related cardiac remodelling.
Jazbutyte et al. identified miR-22 and its target mimecan (also known as osteoglycin) as a regulator of CF senescence [183]. In this study, normotensive mice (neonatal to 19 months of age) were characterized by LV fibrosis and increased miR-22 expression; the latter inversely correlating with mimecan expression [183]. Furthermore transfection of neonatal rat CFs with either the precursor to miR-22 (pre-miR-22) or siRNA targeted to mimecan, increased β-galactosidase expression (a marker of cellular senescence) [183]. Others demonstrate a critical role for elevated miR-34a expression in cardiac aging in both mice and humans [184]. In a cardiac myocyte-mediated role, miR-34a deletion in the mouse offered protection against age-related myocyte death, contractile dysfunction and scar formation following myocardial infarction [184]. This finding of increased miR-34a in aging has been confirmed by others with regards to vascular smooth muscle cell senescence and age-related inflammation [185]. Thus miRNA involvement in cardiac aging may encompass both cardiac myocytes as well as fibroblasts. Other important miRNAs involved in aging include members of the miR-17-92 cluster. Van Almen et al. found that age-associated increases in the matricellular proteins CTGF and thrombospondin-1 (TSP-1) were associated with decreased expression of miR-18a, -19a and -19b in a mouse model of age-related HF [186]. Additionally in vitro transfection using mimics of miR-18a or 19b decreased CTGF and TSP-1 protein and collagen type I and III mRNA, whereas transfection with antagomirs resulted in increased CTGF and TSP-1 and collagen mRNA [186]. Interestingly however these effects were limited to myocytes and not fibroblasts — signifying the importance of myocyte-mediated miRNA-induced ECM remodelling with age. That said, both CFs transfected with miR-17 expression constructs, and miR-17 transgenic mice exhibit blunted senescence at the cellular and tissue level, respectively, as well as increased CF viability [187]. This is further supported by evidence that miR-17 is downregulated with age in several cell types [188]. Thus members of the miR-17-92 cluster may be important in post-transcriptional regulation of ECM remodelling with age, although direct experimental evidence to this effect is currently lacking.
6.5. Osteopontin
Osteopontin is an acidic, matricellular cytokine involved in numerous tissue-remodelling processes including ECM turnover, post-injury recruitment of inflammatory cells and fibroblast–myofibroblast differentiation [189], [190], [191]. Classical pro-fibrotic and pro-inflammatory mediators stimulate osteopontin expression [190], and as a typical matricellular protein, osteopontin is upregulated following injury despite low basal level expression. Specifically with regards to collagen remodelling, osteopontin may be minimally significant in normal hearts, as osteopontin null mice exhibit normal cardiac structure and function [192]. Conversely, MI-induced upregulation of collagen type I mRNA and protein was dramatically attenuated in osteopontin knockout mice [192]. Furthermore osteopontin levels were predictive of all-cause mortality in patients with acute congestive HF, and HF patients had higher osteopontin levels than control subjects [193], [194]. These data suggest a critical role for this cytokine in collagen remodelling following injury.
There is little direct evidence supporting a role for osteopontin in cardiac remodelling with age, although increased osteopontin expression has been observed in aged rat aorta [195]. However as discussed in previous sections of this review, it is known that MI-induced remodelling may be influenced by age [92], [134], [135]. Therefore could osteopontin-mediated collagen remodelling following injury be affected further in senescent subjects? Aged mice subjected to IR injury displayed decreased osteopontin expression in the infarct compared to young IR hearts [134]. Conversely in a canine model, osteopontin expression was increased in the ischemic region compared to young [92], [135]. The explanation for this apparent discrepancy is unknown, however it may reflect species differences. Nevertheless, collectively this data suggest that osteopontin may play a role in age-related remodelling following ischemic injury. Interestingly, a recent study elegantly demonstrates a relationship between osteopontin and miR-21 in fibrotic remodelling with disease [196]. Here, the authors show that both osteopontin mRNA and miR-21 are increased in cardiac biopsies from patients with aortic stenosis, whereas AngII receptor blockade was associated with decreased osteopontin expression [196]. Furthermore whilst miR-21 was increased in the hearts of wildtype mice following AngII infusion via minipump, this did not occur in osteopontin null mice. Finally AngII-mediated fibrosis was augmented further following cardiotropic AAV9-mediated overexpression of osteopontin — an effect abolished with further treatment with locked nucleic acids (LNA) targeted to miR-21 [196]. Although not an aging study per se, it is noteworthy that mean age of the patients in this study was ~ 78 years [196]. Moreover, in addition to the recognized, critical role of miR-21 in myocardial fibrotic remodelling with disease [197], miR-21 has also been implicated in age-associated skeletal muscle fibrosis [198]. Therefore a role may exist for osteopontin and miR-21 in age-dependent collagen remodelling with disease.
7. Conclusions
The myocardium undergoes fibrotic remodelling as a function of age resulting in decreased myocardial compliance and altered functionality. Although the precise mechanisms resulting in the age-dependent accumulation of collagen have yet to be fully identified, it is becoming apparent that enhanced collagen synthesis is not (or at least not solely) responsible. The emerging roles for collagen cross-linkers and matricellular proteins in post-synthetic collagen modulation, and MMPs as pro-fibrotic mediators afford further complexity to the process of “fibrosis” in aging. Our understanding of these processes will undoubtedly increase, aided by the use of aged animal models in research, and imaging technologies such as late gadolinium enhancement CMR imaging. In doing so, novel mediators of collagen remodelling may become future therapeutic targets in the aged, diseased heart.
Acknowledgements
The authors wish to acknowledge funding from the British Heart Foundation FS/12/57/29717.
References
- 1.Redfield M.M., Jacobsen S.J., Burnett J.C., Jr., Mahoney D.W., Bailey K.R., Rodeheffer R.J. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. J. Am. Med. Assoc. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Jugdutt B.I. Aging and heart failure: changing demographics and implications for therapy in the elderly. Heart Fail. Rev. 2010;15:401–405. doi: 10.1007/s10741-010-9164-8. [DOI] [PubMed] [Google Scholar]
- 3.Cherubini A., Oristrell J., Pla X., Ruggiero C., Ferretti R., Diestre G. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch. Intern. Med. 2011;171:550–556. doi: 10.1001/archinternmed.2011.31. [DOI] [PubMed] [Google Scholar]
- 4.Herrera A.P., Snipes S.A., King D.W., Torres-Vigil I., Goldberg D.S., Weinberg A.D. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am. J. Public Health. 2010;100(Suppl. 1) doi: 10.2105/AJPH.2009.162982. (S105-S12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konrat C., Boutron I., Trinquart L., Auleley G.R., Ricordeau P., Ravaud P. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajstura J., Cheng W., Sarangarajan R., Li P., Li B., Nitahara J.A. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am. J. Phys. 1996;271:H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- 7.Kung G., Konstantinidis K., Kitsis R.N. Programmed necrosis, not apoptosis, in the heart. Circ. Res. 2011;108:1017–1036. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 8.Wohlgemuth S.E., Calvani R., Marzetti E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J. Mol. Cell. Cardiol. 2014;71:62–70. doi: 10.1016/j.yjmcc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Dai D.F., Chiao Y.A., Marcinek D.J., Szeto H.H., Rabinovitch P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toprak A., Reddy J., Chen W., Srinivasan S., Berenson G. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the Bogalusa Heart Study) Am. J. Cardiol. 2009;103:978–984. doi: 10.1016/j.amjcard.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Lorell B.H., Carabello B.A. Left ventricular hypertrophy. Pathogenesis, detection and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 12.Samarel A.M. Mechanotransduction in cardiomyocyte hypertrophy. In: Walsh R.A., editor. Molecular Mechanisms of Cardiac Hypertrophy and Failure. Taylor & Francis; Abingdon: 2005. pp. 89–100. [Google Scholar]
- 13.Anversa P., Hiler B., Ricci R., Guideri G., Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. J. Am. Coll. Cardiol. 1986;8:1441–1448. doi: 10.1016/s0735-1097(86)80321-7. [DOI] [PubMed] [Google Scholar]
- 14.Olivetti G., Melissari M., Capasso J.M., Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ. Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 15.Biernacka A., Frangogiannis N.G. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 16.Porter K.E., Turner N.A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Camelliti P., Borg T.K., Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Debessa C.R.G., Maifrino L.B.M., de Souza R.R. Age related changes of the collagen network of the human heart. Mech. Aging Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 19.Eghbali M., Eghbali M., Robinson T.F., Seifter S., Blumenfeld O.O. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc. Res. 1989;23:723–729. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- 20.Horn M.A., Graham H.K., Richards M.A., Clarke J.D., Greensmith D.J., Briston S.J. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J. Mol. Cell. Cardiol. 2012;53:82–90. doi: 10.1016/j.yjmcc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Nichols W.W., O'Rourke M.F. 5th ed. Oxford University Press Inc.; New York: 2005. Aging. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. [Google Scholar]
- 22.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 23.Stratton J.R., Levy W.C., Cerqueira M.D., Schwartz R.S., Abrass I.B. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- 24.Kitzman D.W., Gardin J.M., Gottdiener J.S., Arnold A., Boineau R., Aurigemma G. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am. J. Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 25.Arbab-Zadeh A., Dijk E., Prasad A., Fu Q., Torres P., Zhang R. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 26.Horn M.A. Cardiac physiology of aging: extracellular considerations. Compr. Physiol. 2015;5:1069–1121. doi: 10.1002/cphy.c140063. [DOI] [PubMed] [Google Scholar]
- 27.Reed A.L., Tanaka A., Sorescu D., Liu H., Jeong E.M., Sturdy M. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am. J. Physiol. Heart Circ. Physiol. 2011;301 doi: 10.1152/ajpheart.00407.2010. (H824-H31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capasso J.M., Palackal T., Olivetti G., Anversa P. Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Am. J. Physiol. Heart. Circ. Physiol. 1990;259 doi: 10.1152/ajpheart.1990.259.4.H1086. (H1086-H96) [DOI] [PubMed] [Google Scholar]
- 29.Graham H.K., Horn M., Trafford A.W. Extracellular matrix profiles in the progression to heart failure. Acta Physiol. 2008;194:3–21. doi: 10.1111/j.1748-1716.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 30.Hein S., Schaper J. The extracellular matrix in normal and diseased myocardium. J. Nucl. Cardiol. 2001;8:188–196. doi: 10.1067/mnc.2001.113331. [DOI] [PubMed] [Google Scholar]
- 31.Behrens D.T., Villone D., Koch M., Brunner G., Sorokin L., Robenek H. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem. 2012;287:18700–18709. doi: 10.1074/jbc.M111.336073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jane-Lise S., Corda S., Chassagne C., Rappaport L. The extracellular matrix and the cytoskeleton in heart hypertrophy and failure. Heart Fail. Rev. 2000;5:239–250. doi: 10.1023/A:1009857403356. [DOI] [PubMed] [Google Scholar]
- 33.Ross R.S., Borg T.K. Integrins and the myocardium. Circ. Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 34.Rueckschloss U., Isenberg G. Contraction augments L-type Ca2 + currents in adherent guinea-pig cardiomyocytes. J. Physiol. 2004;560:403–411. doi: 10.1113/jphysiol.2004.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y.G., Samarel A.M., Lipsius S.L. Laminin acts via beta 1 integrin signalling to alter cholinergic regulation of L-type Ca(2 +) current in cat atrial myocytes. J. Physiol. 2000;526(Pt 1):57–68. doi: 10.1111/j.1469-7793.2000.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto S., Teramoto H., Gutkind J.S., Yamada K.M. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C., Li R., Ross R.S., Manso A.M. Integrins and integrin-related proteins in cardiac fibrosis. J. Mol. Cell. Cardiol. 2016;93:162–174. doi: 10.1016/j.yjmcc.2015.11.010. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen N., Yabluchanskiy A., de Castro Bras L.E., Jin Y.F., Lindsey M.L. Aging-related changes in extracellular matrix: implications for ventricular remodeling following myocardial infarction. In: Jugdutt B.I., editor. Aging and Heart Failure: Mechanisms and Management. Springer; New York, NY: 2014. pp. 377–389. [Google Scholar]
- 39.Daniel L., Joyner W.L., Singh M., Singh K. Integrins: implications for aging in heart failure therapy. In: Jugdutt B.I., editor. Aging and Heart Failure: Mechanisms and Management. Springer; New York, NY: 2014. pp. 401–410. [Google Scholar]
- 40.Jugdutt B.I. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 41.Eghbali M., Czaja M.J., Zeydel M., Weiner F.R., Zern M.A., Seifter S. Collagen chain mRNAs in isolated heart cells from young and adult rats. J. Mol. Cell. Cardiol. 1988;20:267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- 42.Eghbali M., Blumenfeld O.O., Seifter S., Buttrick P.M., Leinwand L.A., Robinson T.F. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J. Mol. Cell. Cardiol. 1989;21:103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- 43.Kohl P., Gourdie R.G. Fibroblast–myocyte electrotonic coupling: does it occur in native cardiac tissue? J. Mol. Cell. Cardiol. 2014;70:37–46. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ. Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 45.Carver W., Nagpal M.L., Nachtigal M., Borg T.K., Terracio L. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ. Res. 1991;69:116–122. doi: 10.1161/01.res.69.1.116. [DOI] [PubMed] [Google Scholar]
- 46.Robinson T.F., Geraci M.A., Sonnenblick E.H., Factor S.M. Coiled perimysial fibers of papillary muscle in rat heart: morphology, distribution, and changes in configuration. Circ. Res. 1988;63:577–592. doi: 10.1161/01.res.63.3.577. [DOI] [PubMed] [Google Scholar]
- 47.Wang B., Tedder M., Perez C., Wang G., Jongh Curry A., To F. Structural and biomechanical characterizations of porcine myocardial extracellular matrix. J. Mater. Sci. Mater. Med. 2012;23:1835–1847. doi: 10.1007/s10856-012-4660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizuno T., Yau T.M., Weisel R.D., Kiani C.G., Li R.K. Elastin stabilizes an infarct and preserves ventricular function. Circulation. 2005;112:I–81. doi: 10.1161/01.CIRCULATIONAHA.105.523795. [DOI] [PubMed] [Google Scholar]
- 49.Neuman R.E., Logan M.A. The determination of collagen and elastin in tissues. J. Biol. Chem. 1950;186:549–556. [PubMed] [Google Scholar]
- 50.Bashey R.I., Martinez-Hernandez A., Jimenez S.A. Isolation, characterization, and localization of cardiac collagen type VI. Associations with other extracellular matrix components. Circ. Res. 1992;70:1006–1017. doi: 10.1161/01.res.70.5.1006. [DOI] [PubMed] [Google Scholar]
- 51.Medugorac I., Jacob R. Characterisation of left ventricular collagen in the rat. Cardiovasc. Res. 1983;17:15–21. doi: 10.1093/cvr/17.1.15. [DOI] [PubMed] [Google Scholar]
- 52.Caulfield J.B., Norton P., Weaver R.D. Cardiac dilatation associated with collagen alterations. Mol. Cell. Biochem. 1992;118:171–179. doi: 10.1007/BF00299396. [DOI] [PubMed] [Google Scholar]
- 53.Robinson T.F., Factor S.M., Capasso J.M., Wittenberg B.A., Blumenfeld O.O., Seifter S. Morphology, composition, and function of struts between cardiac myocytes of rat and hamster. Cell Tissue Res. 1987;249:247–255. doi: 10.1007/BF00215507. [DOI] [PubMed] [Google Scholar]
- 54.Weber K.T., Sun Y., Bhattacharya S.K., Ahokas R.A., Gerling I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 55.Factor S.M., Robinson T.F., Dominitz R., Cho S. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture. A preliminary report. Am. J. Cardiovasc. Pathol. 1986;1:91–97. [PubMed] [Google Scholar]
- 56.Jalil J.E., Doering C.W., Janicki J.S., Pick R., Shroff S.G., Weber K.T. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ. Res. 1989;64:1041–1050. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- 57.Baicu C.F., Stroud J.D., Livesay V.A., Hapke E., Holder J., Spinale F.G. Changes in extracellular collagen matrix alter myocardial systolic performance. Am. J. Physiol. Heart Circ. Physiol. 2003;284 doi: 10.1152/ajpheart.00233.2002. (H122-H32) [DOI] [PubMed] [Google Scholar]
- 58.Kato S., Spinale F.G., Tanaka R., Johnson W., Cooper G., Zile M.R. Inhibition of collagen cross-linking: effects on fibrillar collagen and ventricular diastolic function. Am. J. Phys. 1995;269:H863–H868. doi: 10.1152/ajpheart.1995.269.3.H863. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen D.T., Ding C., Wilson E., Marcus G.M., Olgin J.E. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm. 2010;7:1438–1445. doi: 10.1016/j.hrthm.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 60.Schellings M.W., Vanhoutte D., Swinnen M., Cleutjens J.P., Debets J., van Leeuwen R.E. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J. Exp. Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroen B., Heymans S., Sharma U., Blankesteijn W.M., Pokharel S., Cleutjens J.P. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ. Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 62.Baicu C.F., Zhang Y., Van Laer A.O., Renaud L., Zile M.R., Bradshaw A.D. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H234–H240. doi: 10.1152/ajpheart.00227.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldsmith E.C., Bradshaw A.D., Spinale F.G. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am. J. Physiol. Cell Physiol. 2013;304:C393–C402. doi: 10.1152/ajpcell.00347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi K., Luo M., Zhang Y., Wilkes D.C., Ge G., Grieskamp T. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat. Cell Biol. 2009;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadler K.E., Hill A., Canty-Laird E.G. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kessler E., Takahara K., Biniaminov L., Brusel M., Greenspan D.S. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 67.Rentz T.J., Poobalarahi F., Bornstein P., Sage E.H., Bradshaw A.D. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J. Biol. Chem. 2007;282:22062–22071. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- 68.Norris R.A., Damon B., Mironov V., Kasyanov V., Ramamurthi A., Moreno-Rodriguez R. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadler K.E., Holmes D.F., Trotter J.A., Chapman J.A. Collagen fibril formation. Biochem. J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kadler K.E., Hojima Y., Prockop D.J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J. Biol. Chem. 1987;262:15696–15701. [PubMed] [Google Scholar]
- 71.Eyre D.R., Paz M.A., Gallop P.M. Cross-linking in collagen and elastin. Annu. Rev. Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 72.Lopez B., Gonzalez A., Hermida N., Valencia F., de Teresa E., Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1–H9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 73.Hartog J.W.L., Voors A.A., Bakker S.J.L., Smit A.J., van Veldhuisen D.J. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur. J. Heart Fail. 2007;9:1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Lopez B., Querejeta R., Gonzalez A., Beaumont J., Larman M., Diez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension. 2009;53:236–242. doi: 10.1161/HYPERTENSIONAHA.108.125278. [DOI] [PubMed] [Google Scholar]
- 75.Herum K.M., Lunde I.G., Skrbic B., Louch W.E., Hasic A., Boye S. Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart. Cardiovasc. Res. 2015;106:217–226. doi: 10.1093/cvr/cvv002. [DOI] [PubMed] [Google Scholar]
- 76.Spinale F.G. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 77.Ma Y., Halade G.V., Zhang J., Ramirez T.A., Levin D., Voorhees A. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ. Res. 2013;112:675–688. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sasaki T., Gohring W., Mann K., Maurer P., Hohenester E., Knauper V. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J. Biol. Chem. 1997;272:9237–9243. doi: 10.1074/jbc.272.14.9237. [DOI] [PubMed] [Google Scholar]
- 79.Maurer P., Gohring W., Sasaki T., Mann K., Timpl R., Nischt R. Recombinant and tissue-derived mouse BM-40 bind to several collagen types and have increased affinities after proteolytic activation. Cell. Mol. Life Sci. 1997;53:478–484. doi: 10.1007/s000180050059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karsdal M.A., Larsen L., Engsig M.T., Lou H., Ferreras M., Lochter A. Matrix metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 2002;277:44061–44067. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 81.Zile M.R., Baicu C.F., Stroud R.E., Van Laer A.O., Jones J.A., Patel R. Mechanistic relationship between membrane type-1 matrix metalloproteinase and the myocardial response to pressure overload. Circ. Heart Fail. 2014;7:340–350. doi: 10.1161/CIRCHEARTFAILURE.113.000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ducharme A., Frantz S., Aikawa M., Rabkin E., Lindsey M., Rohde L.E. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayashidani S., Tsutsui H., Ikeuchi M., Shiomi T., Matsusaka H., Kubota T. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2003;285 doi: 10.1152/ajpheart.00207.2003. (H1229-H35) [DOI] [PubMed] [Google Scholar]
- 84.Spinale F.G., Mukherjee R., Zavadzkas J.A., Koval C.N., Bouges S., Stroud R.E. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J. Biol. Chem. 2010;285:30316–30327. doi: 10.1074/jbc.M110.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanhoutte D., Heymans S. TIMPs and cardiac remodeling: ‘embracing the MMP-independent-side of the family’. J. Mol. Cell. Cardiol. 2010;48:445–453. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 86.Ikonomidis J.S., Hendrick J.W., Parkhurst A.M., Herron A.R., Escobar P.G., Dowdy K.B. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am. J. Physiol. Heart Circ. Physiol. 2005;288 doi: 10.1152/ajpheart.00370.2004. (H149-H58) [DOI] [PubMed] [Google Scholar]
- 87.Kandalam V., Basu R., Abraham T., Wang X., Soloway P.D., Jaworski D.M. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ. Res. 2010;106:796–808. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 88.Fan D., Takawale A., Basu R., Patel V., Lee J., Kandalam V. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc. Res. 2014;103:268–280. doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 89.Iyer R.P., de Castro Brás L.E., Jin Y.-F., Lindsey M.L. Translating Koch's postulates to identify matrix metalloproteinase roles in postmyocardial infarction remodeling: cardiac metalloproteinase actions (CarMA) postulates. Circ. Res. 2014;114:860–871. doi: 10.1161/CIRCRESAHA.114.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spinale F.G. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ. Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 91.Lin J., Lopez E.F., Jin Y., Van Remmen H., Bauch T., Han H.C. Age-related cardiac muscle sarcopenia: combining experimental and mathematical modeling to identify mechanisms. Exp. Gerontol. 2008;43:296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jugdutt B.I., Jelani A., Palaniyappan A., Idikio H., Uweira R.E., Menon V. Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation. 2010;122:341–351. doi: 10.1161/CIRCULATIONAHA.110.948190. [DOI] [PubMed] [Google Scholar]
- 93.Mewton N., Liu C.Y., Croisille P., Bluemke D., Lima J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu C.Y., Liu Y.C., Wu C., Armstrong A., Volpe G.J., van der Geest R.J. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neilan T.G., Coelho-Filho O.R., Shah R.V., Abbasi S.A., Heydari B., Watanabe E. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. J. Am. Coll. Cardiol. Img. 2013;6:672–683. doi: 10.1016/j.jcmg.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boluyt M.O., O'Neill L., Meredith A.L., Bing O.H.L., Brooks W.W., Conrad C.H. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Circ. Res. 1994;75:23–32. doi: 10.1161/01.res.75.1.23. [DOI] [PubMed] [Google Scholar]
- 97.Querejeta R., Lopez B., Gonzalez A., Sanchez E., Larman M., Martinez Ubago J.L. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 98.Annoni G., Luvarà G., Arosio B., Gagliano N., Fiordaliso F., Santambrogio D. Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mech. Aging Dev. 1998;101:57–72. doi: 10.1016/s0047-6374(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 99.Thomas D.P., Zimmerman S.D., Hansen T.R., Martin D.T., McCormick R.J. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J. Appl. Physiol. 2000;89:1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- 100.Chiao Y.A., Ramirez T.A., Zamilpa R., Okoronkwo S.M., Dai Q., Zhang J. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in aging mice. Cardiovasc. Res. 2012;96:444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mays P.K., McAnulty R.J., Campa J.S., Lauquin G.J. Age-related changes in collagen synthesis and degradation in rat tissues: importance of degradation of newly synthesized collagen in regulating collagen production. Biochem. J. 1991;276:307–313. doi: 10.1042/bj2760307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Besse S., Robert V., Assayag P., Delcayre C., Swynghedauw B. Nonsynchronous changes in myocardial collagen mRNA and protein during aging: effect of DOCA-salt hypertension. Am. J. Phys. 1994;267 doi: 10.1152/ajpheart.1994.267.6.H2237. (H2237-H44) [DOI] [PubMed] [Google Scholar]
- 103.Thomas D.P., McCormick R.J., Zimmerman S.D., Vadlamudi R.K., Gosselin L.E. Aging- and training-induced alterations in collagen characteristics of rat left ventricle and papillary muscle. Am. J. Physiol. Heart Circ. Physiol. 1992;263 doi: 10.1152/ajpheart.1992.263.3.H778. (H778-H83) [DOI] [PubMed] [Google Scholar]
- 104.Campbell D.J., Somaratne J.B., Jenkins A.J., Prior D.L., Yii M., Kenny J.F. Diastolic dysfunction of aging is independent of myocardial structure but associated with plasma advanced glycation end-product levels. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Asif M., Egan J., Vasan S., Jyothirmayi G.N., Masurekar M.R., Lopez S. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 107.Liu J., Masurekar M.R., Vatner D.E., Jyothirmayi G.N., Regan T.J., Vatner S.F. Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2587–H2591. doi: 10.1152/ajpheart.00516.2003. [DOI] [PubMed] [Google Scholar]
- 108.McCurdy S., Baicu C.F., Heymans S., Bradshaw A.D. Cardiac extracellular matrix remodeling: fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC) J. Mol. Cell. Cardiol. 2010;48:544–549. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bradshaw A.D., Baicu C.F., Rentz T.J., Van Laer A.O., Bonnema D.D., Zile M.R. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am. J. Physiol. Heart Circ. Physiol. 2010;298 doi: 10.1152/ajpheart.00474.2009. (H614-H22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Castro Bras L.E., Toba H., Baicu C.F., Zile M.R., Weintraub S.T., Lindsey M.L. Age and SPARC change the extracellular matrix composition of the left ventricle. Biomed. Res. Int. 2014;2014:810562. doi: 10.1155/2014/810562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bradshaw A.D. The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: Does expression of SPARC by macrophages influence outcomes? J. Mol. Cell. Cardiol. 2016;93:156–161. doi: 10.1016/j.yjmcc.2015.11.014. (in this issue) [DOI] [PubMed] [Google Scholar]
- 112.Burstein B., Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 113.Silver M.A., Pick R., Brilla C.G., Jalil J.E., Janicki J.S., Weber K.T. Reactive and reparative fibrillar collagen remodelling in the hypertrophied rat left ventricle: two experimental models of myocardial fibrosis. Cardiovasc. Res. 1990;24:741–747. doi: 10.1093/cvr/24.9.741. [DOI] [PubMed] [Google Scholar]
- 114.Jellis C., Martin J., Narula J., Marwick T.H. Assessment of nonischemic myocardial fibrosis. J. Am. Coll. Cardiol. 2010;56:89–97. doi: 10.1016/j.jacc.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 115.Schalla S., Bekkers S.C., Dennert R., van Suylen R.J., Waltenberger J., Leiner T. Replacement and reactive myocardial fibrosis in idiopathic dilated cardiomyopathy: comparison of magnetic resonance imaging with right ventricular biopsy. Eur. J. Heart Fail. 2010;12:227–231. doi: 10.1093/eurjhf/hfq004. [DOI] [PubMed] [Google Scholar]
- 116.Creemers E.E., Pinto Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011;89:265–272. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 117.Comtois P., Nattel S. Interactions between cardiac fibrosis spatial pattern and ionic remodeling on electrical wave propagation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011;2011:4669–4672. doi: 10.1109/IEMBS.2011.6091156. [DOI] [PubMed] [Google Scholar]
- 118.Kawara T., Derksen R., de Groot J.R., Coronel R., Tasseron S., Linnenbank A.C. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104:3069–3075. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
- 119.Nicoletti A., Michel J.B. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc. Res. 1999;41:532–543. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 120.Macri S.C., Bailey C.C., de Oca N.M., Silva N.A., Rosene D.L., Mansfield K.G. Immunophenotypic alterations in resident immune cells and myocardial fibrosis in the aging rhesus macaque (Macaca mulatta) heart. Toxicol. Pathol. 2012;40:637–646. doi: 10.1177/0192623311436177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dai Z., Aoki T., Fukumoto Y., Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J. Cardiol. 2012;60:416–421. doi: 10.1016/j.jjcc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 122.van den Heuvel A.F., van Veldhuisen D.J., van der Wall E.E., Blanksma P.K., Siebelink H.M., Vaalburg W.M. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2000;35:19–28. doi: 10.1016/s0735-1097(99)00499-4. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto K., Masuyama T., Sakata Y., Nishikawa N., Mano T., Yoshida J. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc. Res. 2002;55:76–82. doi: 10.1016/s0008-6363(02)00341-3. [DOI] [PubMed] [Google Scholar]
- 124.Martos R., Baugh J., Ledwidge M., O'Loughlin C., Conlon C., Patle A. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 125.Lindsey M.L., Goshorn D.K., Squires C.E., Escobar G.P., Hendrick J.W., Mingoia J.T. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc. Res. 2005;66:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 126.Robert V., Besse S., Sabri A., Silvestre J.S., Assayag P., Nguyen V.T. Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab. Investig. 1997;76:729–738. [PubMed] [Google Scholar]
- 127.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 128.Lindsey M.L., Iyer R.P., Jung M., DeLeon-Pennell K., Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. J. Mol. Cell. Cardiol. 2016;91:134–140. doi: 10.1016/j.yjmcc.2015.12.018. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giannandrea M., Parks W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonnema D.D., Webb C.S., Pennington W.R., Stroud R.E., Leonardi A.E., Clark L.L. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) J. Card. Fail. 2007;13:530–540. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu Q., Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 132.Chiao Y.A., Dai Q., Zhang J., Lin J., Lopez E.F., Ahuja S.S. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ. Cardiovasc. Genet. 2011;4:455–462. doi: 10.1161/CIRCGENETICS.111.959981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spinale F.G., Escobar G.P., Mukherjee R., Zavadzkas J.A., Saunders S.M., Jeffords L.B. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling with aging. Circ. Heart Fail. 2009;2:351–360. doi: 10.1161/CIRCHEARTFAILURE.108.844845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bujak M., Kweon H.J., Chatila K., Li N., Taffet G., Frangogiannis N.G. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J. Am. Coll. Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jugdutt B.I., Palaniyappan A., Uwiera R.R., Idikio H. Role of healing-specific-matricellular proteins and matrix metalloproteinases in age-related enhanced early remodeling after reperfused STEMI in dogs. Mol. Cell. Biochem. 2009;322:25–36. doi: 10.1007/s11010-008-9936-9. [DOI] [PubMed] [Google Scholar]
- 136.Yabluchanskiy A., Ma Y., DeLeon-Pennell K.Y., Altara R., Halade G.V., Voorhees A.P. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J. Gerontol. A Biol. Sci. Med. Sci. 2015 doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raya T.E., Gaballa M., Anderson P., Goldman S. Left ventricular function and remodeling after myocardial infarction in aging rats. Am. J. Physiol. Heart Circ. Physiol. 1997;273 doi: 10.1152/ajpheart.1997.273.6.H2652. (H2652-H8) [DOI] [PubMed] [Google Scholar]
- 138.Edgley A.J., Krum H., Kelly D.J. Targeting fibrosis for the treatment of heart failure: a role for transforming growth factor-beta. Cardiovasc. Ther. 2012;30:e30–e40. doi: 10.1111/j.1755-5922.2010.00228.x. [DOI] [PubMed] [Google Scholar]
- 139.Dobaczewski M., Chen W., Frangogiannis N.G. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Macdonald P.S. Combined angiotensin receptor/neprilysin inhibitors: a review of the new paradigm in the management of chronic heart failure. Clin. Ther. 2015 doi: 10.1016/j.clinthera.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 141.von Lueder T.G., Sangaralingham S.J., Wang B.H., Kompa A.R., Atar D., Burnett J.C. Renin–angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ. Heart Fail. 2013;6:594–605. doi: 10.1161/CIRCHEARTFAILURE.112.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lang C.C., Struthers A.D. Targeting the renin–angiotensin–aldosterone system in heart failure. Nat. Rev. Cardiol. 2013;10:125–134. doi: 10.1038/nrcardio.2012.196. [DOI] [PubMed] [Google Scholar]
- 143.Du X.J., Bathgate R.A., Samuel C.S., Dart A.M., Summers R.J. Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat. Rev. Cardiol. 2010;7:48–58. doi: 10.1038/nrcardio.2009.198. [DOI] [PubMed] [Google Scholar]
- 144.Teerlink J.R., Cotter G., Davison B.A., Felker G.M., Filippatos G., Greenberg B.H. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 145.Filippatos G., Teerlink J.R., Farmakis D., Cotter G., Davison B.A., Felker G.M. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur. Heart J. 2014;35:1041–1050. doi: 10.1093/eurheartj/eht497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Unemori E.N., Pickford L.B., Salles A.L., Piercy C.E., Grove B.H., Erikson M.E. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J. Clin. Invest. 1996;98:2739–2745. doi: 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Williams E.J., Benyon R.C., Trim N., Hadwin R., Grove B.H., Arthur M.J. Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut. 2001;49:577–583. doi: 10.1136/gut.49.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Samuel C.S., Cendrawan S., Gao X.M., Ming Z., Zhao C., Kiriazis H. Relaxin remodels fibrotic healing following myocardial infarction. Lab. Investig. 2011;91:675–690. doi: 10.1038/labinvest.2010.198. [DOI] [PubMed] [Google Scholar]
- 149.Samuel C.S., Bodaragama H., Chew J.Y., Widdop R.E., Royce S.G., Hewitson T.D. Serelaxin is a more efficacious antifibrotic than enalapril in an experimental model of heart disease. Hypertension. 2014;64:315–322. doi: 10.1161/HYPERTENSIONAHA.114.03594. [DOI] [PubMed] [Google Scholar]
- 150.Parikh A., Patel D.R., CF M.T., Xiang W., Haney J., Yang L. Relaxin suppresses atrial fibrillation by reversing fibrosis and myocyte hypertrophy, and increasing conduction velocity and sodium current in spontaneously hypertensive rat hearts. Circ. Res. 2013 doi: 10.1161/CIRCRESAHA.113.301646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Samuel C.S., Unemori E.N., Mookerjee I., Bathgate R.A., Layfield S.L., Mak J. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology. 2004;145:4125–4133. doi: 10.1210/en.2004-0209. [DOI] [PubMed] [Google Scholar]
- 152.Samuel C.S., Zhao C., Bathgate R.A., Du X.J., Summers R.J., Amento E.P. The relaxin gene-knockout mouse: a model of progressive fibrosis. Ann. N. Y. Acad. Sci. 2005;1041:173–181. doi: 10.1196/annals.1282.025. [DOI] [PubMed] [Google Scholar]
- 153.Heymans S., Gonzalez A., Pizard A., Papageorgiou A.P., Lopez-Andres N., Jaisser F. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur. J. Heart Fail. 2015;17:764–771. doi: 10.1002/ejhf.312. [DOI] [PubMed] [Google Scholar]
- 154.Ochieng J., Furtak V., Lukyanov P. Extracellular functions of galectin-3. Glycoconj. J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 155.Puthenedam M., Wu F., Shetye A., Michaels A., Rhee K.J., Kwon J.H. Matrilysin-1 (MMP7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm. Bowel Dis. 2011;17:260–267. doi: 10.1002/ibd.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ochieng J., Fridman R., Nangia-Makker P., Kleiner D.E., Liotta L.A., Stetler-Stevenson W.G. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 157.Felker G.M., Fiuzat M., Shaw L.K., Clare R., Whellan D.J., Bettari L. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ. Heart Fail. 2012;5:72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Edelmann F., Holzendorf V., Wachter R., Nolte K., Schmidt A.G., Kraigher-Krainer E. Galectin-3 in patients with heart failure with preserved ejection fraction: results from the Aldo-DHF trial. Eur. J. Heart Fail. 2015;17:214–223. doi: 10.1002/ejhf.203. [DOI] [PubMed] [Google Scholar]
- 159.Meijers W.C., Januzzi J.L., de Filippi C., Adourian A.S., Shah S.J., van Veldhuisen D.J. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: a pooled analysis of 3 clinical trials. Am. Heart J. 2014;167:853–860. doi: 10.1016/j.ahj.2014.02.011. e4. [DOI] [PubMed] [Google Scholar]
- 160.Moriguchi A., Brosnihan K.B., Kumagai H., Ganten D., Ferrario C.M. Mechanisms of hypertension in transgenic rats expressing the mouse Ren-2 gene. Am. J. Phys. 1994;266:R1273–R1279. doi: 10.1152/ajpregu.1994.266.4.R1273. [DOI] [PubMed] [Google Scholar]
- 161.Sharma U.C., Pokharel S., van Brakel T.J., van Berlo J.H., Cleutjens J.P.M., Schroen B. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 162.Calvier L., Martinez-Martinez E., Miana M., Cachofeiro V., Rousseau E., Sadaba J.R. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59–67. doi: 10.1016/j.jchf.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 163.Martínez-Martínez E., Calvier L., Fernández-Celis A., Rousseau E., Jurado-López R., Rossoni L.V. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.115.05876. [DOI] [PubMed] [Google Scholar]
- 164.Lepojarvi E.S., Piira O.P., Paakko E., Lammentausta E., Risteli J., Miettinen J.A. Serum PINP, PIIINP, galectin-3, and ST2 as surrogates of myocardial fibrosis and echocardiographic left venticular diastolic filling properties. Front. Physiol. 2015;6:200. doi: 10.3389/fphys.2015.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ho J.E., Liu C., Lyass A., Courchesne P., Pencina M.J., Vasan R.S. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Boer R.A., van Veldhuisen D.J., Gansevoort R.T., Muller Kobold A.C., van Gilst W.H., Hillege H.L. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012;272:55–64. doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 167.Lok D.J., Lok S.I., de la Porte PW B.-A., Badings E., Lipsic E., van Wijngaarden J. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin. Res. Cardiol. 2013;102:103–110. doi: 10.1007/s00392-012-0500-y. [DOI] [PubMed] [Google Scholar]
- 168.AbouEzzeddine O.F., Haines P., Stevens S., Nativi-Nicolau J., Felker G.M., Borlaug B.A. Galectin-3 in heart failure with preserved ejection fraction. A RELAX trial substudy (phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure) JACC Heart Fail. 2015;3:245–252. doi: 10.1016/j.jchf.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pan J., Fukuda K., Saito M., Matsuzaki J., Kodama H., Sano M. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ. Res. 1999;84:1127–1136. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- 170.Celik A., Sahin S., Koc F., Karayakali M., Sahin M., Benli I. Cardiotrophin-1 plasma levels are increased in patients with diastolic heart failure. Med. Sci. Monit. 2012;18:CR25–CR31. doi: 10.12659/MSM.882197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Song K., Wang S., Huang B., Luciano A., Srivastava R., Mani A. Plasma cardiotrophin-1 levels are associated with hypertensive heart disease: a meta-analysis. J. Clin. Hypertens. (Greenwich) 2014;16:686–692. doi: 10.1111/jch.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bristow M.R., Long C.S. Cardiotrophin-1 in heart failure. Circulation. 2002;106:1430–1432. doi: 10.1161/01.cir.0000034024.61382.42. [DOI] [PubMed] [Google Scholar]
- 173.Sheng Z., Knowlton K., Chen J., Hoshijima M., Brown J.H., Chien K.R. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J. Biol. Chem. 1997;272:5783–5791. doi: 10.1074/jbc.272.9.5783. [DOI] [PubMed] [Google Scholar]
- 174.López B., González A., Querejeta R., Larman M., Rábago G., Díez J. Association of cardiotrophin-1 with myocardial fibrosis in hypertensive patients with heart failure. Hypertension. 2014;63:483–489. doi: 10.1161/HYPERTENSIONAHA.113.02654. [DOI] [PubMed] [Google Scholar]
- 175.López-Andrés N., Calvier L., Labat C., Fay R., Díez J., Benetos A. Absence of cardiotrophin 1 is associated with decreased age-dependent arterial stiffness and increased longevity in mice. Hypertension. 2013;61:120–129. doi: 10.1161/HYPERTENSIONAHA.112.201699. [DOI] [PubMed] [Google Scholar]
- 176.Thum T. Noncoding RNAs and myocardial fibrosis. Nat. Rev. Cardiol. 2014;11:655–663. doi: 10.1038/nrcardio.2014.125. [DOI] [PubMed] [Google Scholar]
- 177.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 178.Boon R.A., Dimmeler S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 179.Wijnen W.J., Pinto Y.M., Creemers E.E. The therapeutic potential of miRNAs in cardiac fibrosis: where do we stand? J. Cardiovasc. Transl. Res. 2013;6:899–908. doi: 10.1007/s12265-013-9483-y. [DOI] [PubMed] [Google Scholar]
- 180.van Rooij E., Olson E.N. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat. Rev. Drug Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van Rooij E., Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol. Med. 2012;4:3–14. doi: 10.1002/emmm.201100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jazbutyte V., Fiedler J., Kneitz S., Galuppo P., Just A., Holzmann A. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age. 2013;35:747–762. doi: 10.1007/s11357-012-9407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boon R.A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S. MicroRNA-34a regulates cardiac aging and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 185.Badi I., Burba I., Ruggeri C., Zeni F., Bertolotti M., Scopece A. MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated pro-inflammatory secretory factors. J. Gerontol. A Biol. Sci. Med. Sci. 2014 doi: 10.1093/gerona/glu180. [DOI] [PubMed] [Google Scholar]
- 186.van Almen G.C., Verhesen W., van Leeuwen R.E., van de Vrie M., Eurlings C., Schellings M.W. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Du W.W., Li X., Li T., Li H., Khorshidi A., Liu F. The microRNA miR-17-3p inhibits mouse cardiac fibroblast senescence by targeting Par4. J. Cell Sci. 2015;128:293–304. doi: 10.1242/jcs.158360. [DOI] [PubMed] [Google Scholar]
- 188.Hackl M., Brunner S., Fortschegger K., Schreiner C., Micutkova L., Muck C. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9:291–296. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh M., Foster C.R., Dalal S., Singh K. Role of osteopontin in heart failure associated with aging. Heart Fail. Rev. 2010;15:487–494. doi: 10.1007/s10741-010-9158-6. [DOI] [PubMed] [Google Scholar]
- 190.Frangogiannis N.G. Matricellular proteins in cardiac adaptation and disease. Physiol. Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lenga Y., Koh A., Perera A.S., McCulloch C.A., Sodek J., Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ. Res. 2008;102:319–327. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 192.Trueblood N.A., Xie Z., Communal C., Sam F., Ngoy S., Liaw L. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ. Res. 2001;88:1080–1087. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 193.Behnes M., Brueckmann M., Lang S., Espeter F., Weiss C., Neumaier M. Diagnostic and prognostic value of osteopontin in patients with acute congestive heart failure. Eur. J. Heart Fail. 2013;15:1390–1400. doi: 10.1093/eurjhf/hft112. [DOI] [PubMed] [Google Scholar]
- 194.Rosenberg M., Zugck C., Nelles M., Juenger C., Frank D., Remppis A. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circ. Heart Fail. 2008;1:43–49. doi: 10.1161/CIRCHEARTFAILURE.107.746172. [DOI] [PubMed] [Google Scholar]
- 195.Miller S.J., Watson W.C., Kerr K.A., Labarrere C.A., Chen N.X., Deeg M.A. Development of progressive aortic vasculopathy in a rat model of aging. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2634–H2643. doi: 10.1152/ajpheart.00397.2007. [DOI] [PubMed] [Google Scholar]
- 196.Lorenzen J.M., Schauerte C., Hubner A., Kolling M., Martino F., Scherf K. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur. Heart J. 2015;36:2184–2196. doi: 10.1093/eurheartj/ehv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 198.Ardite E., Perdiguero E., Vidal B., Gutarra S., Serrano A.L., Munoz-Canoves P. PAI-1-regulated miR-21 defines a novel age-associated fibrogenic pathway in muscular dystrophy. J. Cell Biol. 2012;196:163–175. doi: 10.1083/jcb.201105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Unemori E.N., Amento E.P. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J. Biol. Chem. 1990;265:10681–10685. [PubMed] [Google Scholar]
- 200.Yu L., Ruifrok W.P.T., Meissner M., Bos E.M., van Goor H., Sanjabi B. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail. 2013;6:107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 201.Lopez-Andres N., Rousseau A., Akhtar R., Calvier L., Inigo C., Labat C. Cardiotrophin 1 is involved in cardiac, vascular, and renal fibrosis and dysfunction. Hypertension. 2012;60:563–573. doi: 10.1161/HYPERTENSIONAHA.112.194407. [DOI] [PubMed] [Google Scholar]
- 202.Drobic V., Cunnington R.H., Bedosky K.M., Raizman J.E., Elimban V.V., Rattan S.G. Differential and combined effects of cardiotrophin-1 and TGF-beta1 on cardiac myofibroblast proliferation and contraction. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1053–H1064. doi: 10.1152/ajpheart.00935.2006. [DOI] [PubMed] [Google Scholar]
- 203.Dobaczewski M., Bujak M., Zymek P., Ren G., Entman M.L., Frangogiannis N.G. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 204.Lindsey M.L., Zouein F.A., Tian Y., Padmanabhan Iyer R., de Castro Bras L.E. Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can. J. Physiol. Pharmacol. 2015;1-8 doi: 10.1139/cjpp-2015-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]