Abstract
BACKGROUND
Neurologic complications (NCs) are the major adverse events after left ventricular assist device (LVAD) surgery. Pre-operative and post-operative factors associated with NCs in patients with LVADs were investigated.
METHODS
We reviewed 307 consecutive patients undergoing LVAD surgery (167 HeartMate I and 140 HeartMate II devices) at Columbia University Medical Center between November 2000 and December 2010. Clinical characteristics and hemodynamic and laboratory indexes were analyzed. NC was defined according to the Interagency Registry for Mechanically Assisted Circulatory Support definition of neurologic dysfunction, including transient ischemic attack (TIA) and ischemic or hemorrhagic cerebrovascular accident (CVA).
RESULTS
NCs developed in 43 patients (14.0%) at 91.8 ± 116.3 days post-operatively. The frequency of NC development was similar in HeartMate I and II patients. Patients with NC showed a higher frequency of pre-LVAD CVA history (27.9% vs 15.5%, p = 0.046), lower pre-operative sodium (129.0 ± 7.0 vs 132.1 ± 8.1 mg/dl, p = 0.018) and albumin concentrations (3.5 ± 0.7 vs 3.7 ± 0.6 mg/dl, p = 0.049), lower post-operative hematocrit (34.9% ± 5.1% vs 37.8% ± 6.1%, p = 0.0034), sodium (131.6 ± 7.7 vs 134.4 ± 6.4 mg/dl, p = 0.010) and albumin concentrations (3.7 ± 0.5 vs 3.9 ± 0.5 mg/dl, p = 0.0016), and higher frequency of post-operative infection (39.5% vs 19.3%, p = 0.003) than those without NC. Multiple regression analysis revealed that CVA history (odds ratio, 2.37, 95% confidence interval, 1.24 –5.29; p = 0.011) and post-operative infection (odds ratio, 2.99, 95% confidence interval, 1.16 –10.49; p = 0.011) were highly associated with NC development. The combination of CVA history, pre-operative and post-operative sodium and albumin, and post-operative hematocrit and infection could discriminate patients developing NCs with a probability of 76.6%.
CONCLUSIONS
Previous stroke, persistent malnutrition and inflammation, severity of heart failure, and post-LVAD infections are key factors associated with development of NCs after LVAD implantation.
Keywords: ventricular assistventricular assist device, neurologic complications, heart failure, risk factor
Cardiac transplantation provides considerable survival benefits for patients with end-stage heart failure (HF); however, its use is severely limited due to donor shortage.1,2 A growing number of heart transplant candidates require long-term support by a left ventricular assist device (LVAD) while they await cardiac transplantation. LVAD therapy has evolved into a standard therapy for patients with advanced HF,3–5 not only as a bridge to cardiac transplantation but also as a destination therapy or a bridge to myocardial recovery.6,7
Long-term LVAD support, however, can result in serious complications such as cerebrovascular accidents (CVAs), hemorrhage, and infection.4,8 CVA remains the leading cause of death and the primary reason for withdrawal from transplant eligibility in LVAD-supported patients. In addition, transplant recipients with a history of CVA face tremendous difficulties in their post-operative course, including higher morbidity and mortality and problems to reintegrate into society, often for years after transplant.4,5,8,9 An incidence of ischemic and hemorrhagic CVAs after LVAD placement of 8% to 25% has been reported.5,10,11 This study was initiated to assess the pre-operative and post-operative factors associated with the development of neurologic complications (NCs) in patients undergoing LVAD placement, and we investigated factors associated with NCs after LVAD surgery in our single-center experience.
Methods
Patients and study design
We reviewed 307 consecutive patients who underwent HeartMate I or II (Thoratec Corp, Pleasanton, CA) LVAD placement at Columbia University Medical Center between November 2000 and December 2010. Patients who underwent other types of LVAD surgery were excluded. Definition of NC was based on the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) definition of neurologic dysfunction: any new, temporary or permanent, focal or global neurologic deficit, including transient ischemic attack (TIA) that resolves within 24 hours, and ischemic or hemorrhagic intracranial CVA that persists beyond 24 hours or less than 24 hours with infarction on an image study.8,12 In patients with multiple episodes of NC, the first episode of CVA was used for the analysis for patients developing CVA, and the first TIA episode was used for the analysis of patients with only TIA and not progressing to CVA.
Aspirin (81 mg daily) was initiated early post-operatively in all patients who received a HeartMate I device; however, warfarin was not initiated for the first month, even if patients had a history of atrial fibrillation. The anti-coagulation protocol for patients with HeartMate II device included heparin, warfarin, and anti-platelet agents, such as aspirin and/or dipyridamole, except for those with contraindication to the medication or/and active bleeding. Heparin was used as a bridge therapy until patients taking warfarin reached a therapeutic international normalized ratio (INR). A target INR was 1.8 to 2.5 for the studied patients. Various anti-coagulation treatments were optimized according to tailored management to each patient’s clinical condition.
We performed 2 different analyses. First, univariate and multivariate analyses were performed on 43 patients to define pre-operative and post-operative factors associated with NCs, including TIA and CVA. Second, after excluding the 10 patients who only developed TIA but not CVA, we analyzed factors associated with CVA. All patients were first divided into 2 groups: those who developed NC at any time after LVAD placement (Group NC) and those who did not develop any NC throughout the post-operative period (Group non-NC). After excluding patients with only TIA from those in Group NC, patients who developed CVA were classified as Group CVA.
Clinical characteristics, pre-operative hemodynamic data, and laboratory examinations were compared between patients with and without NCs as well as between patients with CVA and without CVA. In addition, pre-operative LV end-diastolic diameters and ejection fractions derived from echocardiograms were assessed by biplane Simpson’s method and compared among the groups.
Post-operative laboratory examinations, infection, and warfarin or/and aspirin administration were also compared among the groups. Pre-operative variables were obtained within 7 days before surgery. Post-operative laboratory data for patients with NC or CVA were collected within 7 days before the events, and data for patients without NC were collected within 7 days from the end of observation or device removal due to transplant, recovery, or death. A post-operative infection was defined as >2 positive cultures when the patient developed any symptom of an infection. Urinary tract infection was defined as >2 positive urine cultures with >105 colonies/ml with signs of urinary tract infection.
Statistical analysis
Data are presented as means ± standard deviation. Normality was evaluated for each variable on the basis of normal distribution plots and histograms and by the Kolmogorov-Smirnov test. Clinical characteristics, hemodynamic, and laboratory data were compared among groups using Student’s unpaired two-tailed t-test or chi-square analysis. Univariate logistic regression analysis was used to select factors associated with NC or CVA for inclusion in subsequent multivariate analysis. A stepwise forward selection method was used to select variables that discriminated patients with NC or CVA from those without any episodes of NC. The partial F value of 0.2 was used for selection criteria. The discriminant score and discriminant probability were calculated using a discriminant function test. All statistical analyses were performed using JMP 7.0 software (SAS Institute, Cary, NC).
Results
The clinical course of 307 patients (167 patients with HeartMate I device and 140 patients with HeartMate II device) was retrospectively analyzed (Figure 1). Patients were a mean age of 54 ± 14 years at the time of surgery, and the mean post-operative observation period was 259 ± 304 days. The mean observation periods were 138 ± 224 days (range 3–1,434 days) for HeartMate I patients and 277 ± 333 days (range, 3–2,069 days) for HeartMate II patients.
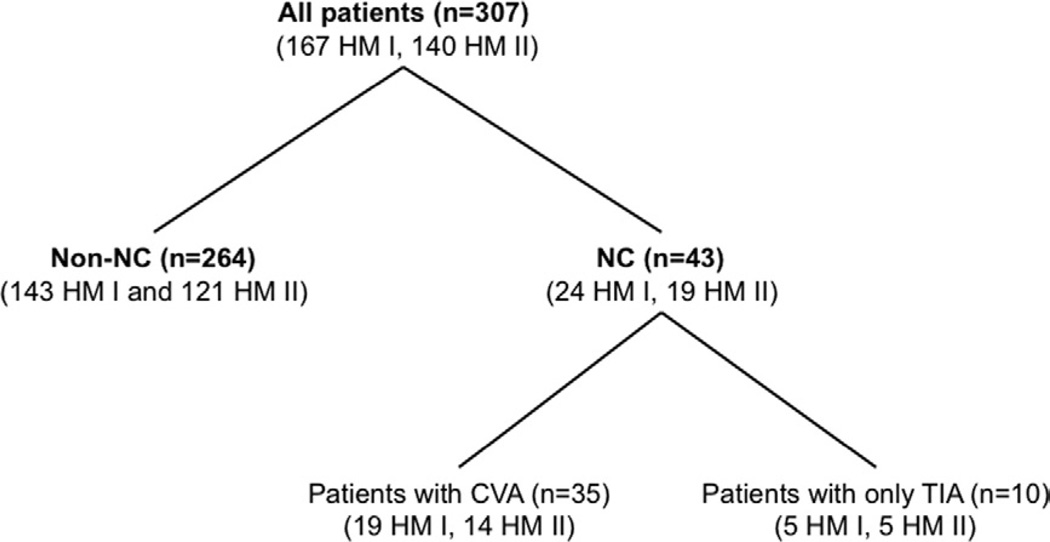
Figure 1.
Patients who underwent placement of a HeartMate I (HM I) or HeartMate II (HM II) device were divided into 2 groups: those with any neurologic complication (NC), including transient ischemic attack (TIA; Group NC), and those who did not develop NC after the surgery (Group non-NC). After excluding patients with only TIA episodes, patients with ischemic or hemorrhagic cerebrovascular accident (CVA) were classified as Group CVA. The analysis was performed between Group NC and Group non-NC, and between Group CVA between Group non-NC.
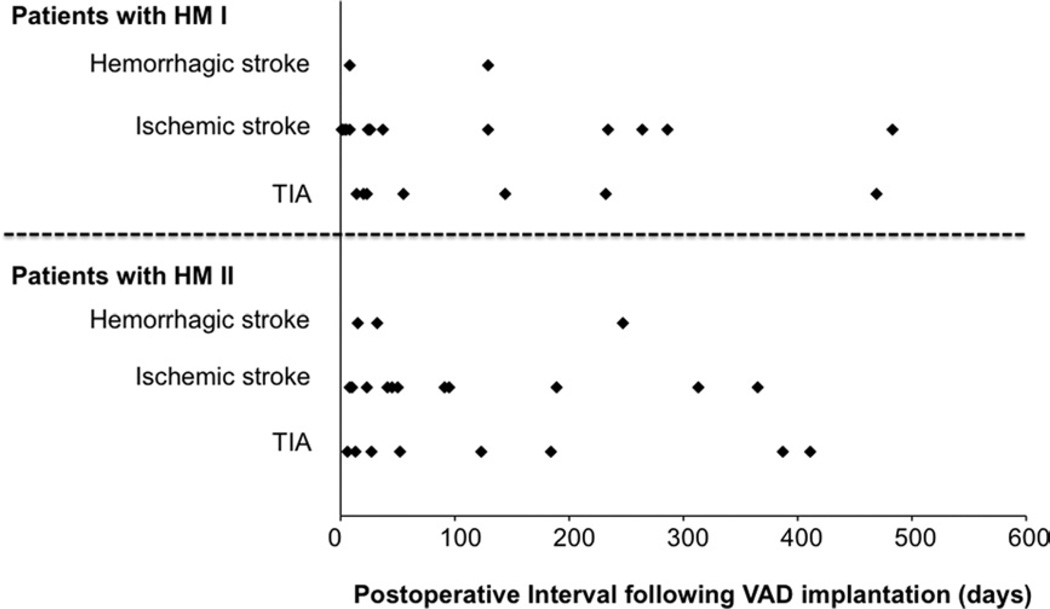
A total of 51 NC events occurred in 43 patients (14.0%, 0.23 events/patient per year) after a mean of 92 ± 116 days after LVAD surgery, consisting of 27 events in 24 patients (14.4%) with HeartMate I and 24 events in 19 patients (13.6%) with HeartMate II. These 43 patients were classified as those in Group NC. A total of 39 CVA events occurred in 33 patients (10.7%, 0.18 events/patient per year) at 80 ± 103 days after the surgery, consisting of 22 events in 19 patients (11.4%, 0.34 events/patient per year) with HeartMate I and 17 events in 14 patients (10.0%, 0.16 events/patient per year) with HeartMate II. They were considered as Group CVA. The duration from the LVAD surgery to all NC events is shown in Figure 2, which revealed that 37 of 51 events (72.5%) occurred within 6 months after LVAD surgery.
Figure 2.
Complications during the post-operative observation period after placement of a HeartMate I (HM I) or HeartMate II (HM II) ventricular assist device (VAD). The study cohort sustained 51 events. TIA, transient ischemic attack.
Multiple NCs occurred in 6 patients (2.0%); however, analysis was performed based on 1 event/patient using the first episode of CVA in patients with CVA or the first episodes of TIA in patients with only TIA to avoid double- or triple-counting of those patients’ clinical data and to purely discriminate patients with NC or CVA from those who remained free of NC.
Comparison of variables in patients with NC (CVA and TIA) and those without NC
Clinical characteristics of all patients are summarized in Table 1. Age, sex, body surface area, baseline heart disease, and type of LVAD were not significantly different between Group NC and Group non-NC. The proportion of patients with a history of CVA was higher in Group NC than in Group non-NC. Other factors of patients’ previous medical histories, including atrial fibrillation, were not significantly different among the groups. There was no difference among the patients in simultaneous surgical procedures at the time of LVAD implantation such as patent foramen ovale closure, tricuspid reconstructions, or left atrial exclusion.
Table 1.
Clinical Characteristics
| Variablea | Group non-NC (n = 264) |
Group NC (n = 43) |
p-value (Non-NC vs NC) |
Group CVA (n = 35) |
p-value (Non-NC vs CVA) |
|---|---|---|---|---|---|
| Age, years | 53.6 ± 12.6 | 54.4 ± 13.1 | 0.701 | 54.1 ± 15.6 | 0.830 |
| Male sex | 216 (79.1) | 33 (76.7) | 0.126 | 29 (82.9) | 0.266 |
| Body surface area, m2 | 1.96 ± 0.24 | 1.91 ± 0.21 | 0.578 | 1.93 ± 0.22 | 0.484 |
| Medical history | |||||
| Stroke | 41 (15.5) | 12 (27.9) | 0.046 | 10 (28.6) | 0.054 |
| Diabetes mellitus | 77 (29.1) | 12 (27.9) | 0.866 | 10 (28.6) | 0.941 |
| Hypertension | 120 (47.0) | 18 (40.9) | 0.660 | 18 (51.4) | 0.505 |
| Hyperlipidemia | 84 (31.8) | 13 (30.2) | 0.836 | 13 (37.4) | 0.527 |
| PVD | 29 (11.0) | 8 (18.6) | 0.225 | 7 (20.0) | 0.124 |
| Renal failure | 76 (28.8) | 11(25.0) | 0.665 | 9 (25.7) | 0.704 |
| Atrial fibrillation | 134 (50.8) | 25 (58.1) | 0.369 | 23 (62.8) | 0.096 |
| Etiology of heart disease | |||||
| Ischemic | 207(78.8) | 28 (65.1) | 0.056 | 25 (71.4) | 0.352 |
| Non-ischemic | 57(21.2) | 15 (34.9) | 0.056 | 10 (28.6) | 0.352 |
| Type of LVAD | |||||
| HeartMate I | 143 (54.2) | 24 (55.8) | 0.804 | 19 (54.3) | 0.989 |
| HeartMate II | 121 (55.8) | 19 (44.2) | 0.804 | 16 (45.7) | 0.989 |
CVA, cerebrovascular accident; LVAD, left ventricular assist device; NC, neurologic complication; PVD, peripheral vascular disease.
Continuous data are presented as mean ± standard deviation; categoric data as number (%).
Table 2 summarizes pre-LVAD hemodynamic, laboratory, and echocardiographic parameters within 7 days before surgery. Hemodynamic variables did not differ significantly between Group NC and Group non-NC. Pre-operative serum sodium and albumin concentrations were lower in Group NC than in Group non-NC. The LV end-diastolic diameters and ejection fractions were not significantly different between the groups.
Table 2.
Results of Pre-operative Hemodynamic and Laboratory Examinations
| Variablea | Group non-NC (n = 264) |
Group NC (n = 43) |
p-value (Non-NC vs NC) |
Group CVA (n = 35) |
p-value (Non-NC vs CVA) |
|---|---|---|---|---|---|
| Hemodynamic variables | |||||
| Cardiac index, liters/min/m2 | 1.8 ± 0.4 | 1.7 ± 0.3 | 0.118 | 1.7 ± 0.5 | 0.179 |
| PAWP, mm Hg | 28.4 ± 8.1 | 2.3 ± 8.9 | 0.268 | 28.8 ± 10.0 | 0.790 |
| Mean pressure, mm Hg | |||||
| Pulmonary artery | 36.2 ± 9.4 | 38.5 ± 9.7 | 0.140 | 37.2 ± 12.0 | 0.568 |
| Right atrial | 12.9 ± 7.9 | 13.2 ± 7.8 | 0.817 | 12.6 ± 8.0 | 0.833 |
| Atrial | 79.1 ± 12.8 | 76.3 ± 9.6 | 0.171 | 77.1 ± 10.0 | 0.375 |
| Vascular resistance, WU | |||||
| Peripheral | 3.8 ± 2.2 | 3.9 ± 2.4 | 0.785 | 3.7 ± 2.5 | 0.804 |
| Systemic | 23.1 ± 6.3 | 23.8 ± 9.2 | 0.530 | 23.8 ± 7.9 | 0.550 |
| Laboratory examinations | |||||
| White cell count, ×103/µl | 8.2 ± 2.3 | 9.0 ± 3.9 | 0.060 | 9.0 ± 3.8 | 0.078 |
| Lymphocytes, % | 11.4 ± 5.2 | 10.9 ± 3.7 | 0.545 | 10.3 ± 4.6 | 0.234 |
| Hematocrit, % | 33.2 ± 5.9 | 32.4 ± 6.2 | 0.304 | 32.1 ± 6.3 | 0.414 |
| Platelets, ×103/µl | 191 ± 86 | 190 ± 80 | 0.898 | 189 ± 85 | 0.876 |
| Bilirubin, mg/dl | |||||
| Total | 1.7 ± 1.3 | 1.8 ± 1.5 | 0.678 | 1.6 ± 1.6 | 0.375 |
| Direct | 0.6 ± 0.5 | 0.7 ± 0.7 | 0.254 | 0.7 ± 0.9 | 0.322 |
| Sodium, mEq/L | 132.1 ± 8.1 | 129.0 ± 7.0 | 0.018 | 129.1 ± 7.1 | 0.038 |
| Potassium, mEq/L | 4.3 ± 0.5 | 4.4 ± 0.5 | 0.225 | 4.4 ± 0.4 | 0.257 |
| Blood urea nitrogen, mg/dl | 37 ± 18 | 35 ± 19 | 0.460 | 34 ± 18 | 0.442 |
| Creatinine, mg/dl | 1.6 ± 0.4 | 1.5 ± 0.9 | 0.224 | 1.5 ± 0.7 | 0.212 |
| Albumin, mg/dl | 3.7 ± 0.6 | 3.5 ± 0.7 | 0.049 | 3.5 ± 0.7 | 0.030 |
| ALT, IU/liter | 99 ± 100 | 88 ± 96 | 0.509 | 91 ± 91 | 0.661 |
| AST, IU/liter | 72 ± 86 | 55 ± 77 | 0.231 | 60 ± 77 | 0.428 |
| BNP, pg/ml | 1835 ± 1117 | 2101 ± 1046 | 0.145 | 1921 ± 946 | 0.663 |
| International normalized ratio | 1.4 ± 0.5 | 1.3 ± 0.4 | 0.213 | 1.3 ± 0.3 | 0.249 |
| Echocardiographic parameters | |||||
| LVEDD, mm | 69.5 ± 12.1 | 71.9 ± 12.8 | 0.240 | 70.7 ± 12.5 | 0.588 |
| LVEF, % | 18.4 ± 10.0 | 20.0 ± 12.4 | 0.353 | 19.1 ± 10.8 | 0.713 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; CVA, cerebrovascular accident; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; NC, neurologic complication; PAWP, pulmonary arterial wedge pressure.
Data are presented as mean ± standard deviation.
Table 3 summarizes post-LVAD warfarin and aspirin administration and laboratory examinations in all patients. The proportion of patients with warfarin or/and aspirin administration was not significantly different between the NC and non-NC groups. Post-operative hematocrit was lower, and again, serum sodium and albumin concentrations were lower in Group NC than in Group non-NC.
Table 3.
of Post-operative Medication and Laboratory Examinations in All Patients
| Variablea | Group non-NC (n = 264) |
Group NC (n = 43) |
p-value (Non-NC vs NC) |
Group CVA (n = 35) |
p-value (Non-NC vs CVA) |
|---|---|---|---|---|---|
| Medication | |||||
| Warfarin | 195 (73.8) | 36 (83.7) | 0.165 | 29 (82.9) | 0.249 |
| Aspirin | 238 (90.1) | 38 (88.4) | 0.828 | 32 (91.4) | 0.721 |
| Laboratory examinations | |||||
| White cell count, ×103/µL | 7.9 ± 2.3 | 8.5 ± 3.4 | 0.142 | 8.2 ± 3.5 | 0.499 |
| Lymphocytes, % | 13.0 ± 6.2 | 12.1 ± 4.9 | 0.365 | 12.3 ± 4.6 | 0.520 |
| Hematocrit, % | 37.8 ± 6.1 | 34.9 ± 5.1 | 0.003 | 34.7 ± 6.3 | 0.005 |
| Platelets, ×103/µl | 189 ± 90 | 201 ± 82 | 0.402 | 199 ± 85 | 0.54 |
| Bilirubin, mg/dl | |||||
| Total | 1.6 ± 2.9 | 1.9 ± 3.1 | 0.534 | 1.7 ± 2.6 | 0.846 |
| Direct | 0.8 ± 2.2 | 1.0 ± 1.8 | 0.572 | 0.8 ± 0.9 | 0.937 |
| Sodium, mEq/L | 134.4 ± 6.4 | 131.6 ± 7.7 | 0.010 | 131.9 ± 7.1 | 0.033 |
| Potassium, mEq/L | 4.3 ± 0.6 | 4.4 ± 0.4 | 0.292 | 4.3 ± 0.4 | 0.848 |
| Blood urea nitrogen, mg/dl | 33 ± 14 | 32 ± 18 | 0.866 | 33 ± 17 | 0.755 |
| Creatinine, mg/dl | 1.5 ± 0.6 | 1.5 ± 0.7 | 0.623 | 1.4 ± 0.7 | 0.717 |
| Albumin, mg/dl | 3.9 ± 0.5 | 3.7 ± 0.5 | 0.016 | 3.7 ± 0.7 | 0.036 |
| ALT, IU/liter | 67 ± 99 | 66 ± 38 | 0.921 | 61 ± 51 | 0.716 |
| AST, IU/liter | 79 ± 86 | 58 ± 80 | 0.140 | 60 ± 77 | 0.222 |
| BNP, pg/ml | 802 ± 894 | 673 ± 685 | 0.571 | 712 ± 746 | 0.663 |
| International normalized ratio | 1.5 ± 0.9 | 1.6 ± 0.7 | 0.532 | 1.5 ± 0.3 | 0.845 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; CVA, cerebrovascular accident; NC, neurologic complication.
Categoric data are presented as number (%), and continuous data as mean ± standard deviation.
Table 4 summarizes the comparison of post-LVAD infection between the groups. The infections analyzed were sepsis, LVAD-related infection, including driveline, pocket, and/or wound infection, and urinary tract, respiratory, or other infections, including gastrointestinal, and/or pressure ulcer infection. The frequency of LVAD-related infections alone was significantly higher in patients in Group NC than those in Group non-NC; however, the aggregate end point of all types of infection was significantly higher in patients in Group NC as well as in Group CVA than those in Group non-NC (Table 4).
Table 4.
Post-operative Infection Data in All Patients
| Infection typea | Group non-NC (n = 264) |
Group NC (n = 43) |
p-value (non-NC vs NC) |
Group CVA (n = 35) |
p-value (non-NC vs CVA) |
|---|---|---|---|---|---|
| All forms | 51 (19.3) | 17 (39.5) | 0.003 | 13 (37.1) | 0.016 |
| Sepsis | 41 (15.1) | 9 (20.9) | 0.377 | 7 (20.0) | 0.498 |
| LVAD relatedb | 30 (11.4) | 10 (23.3) | 0.031 | 7 (20.0) | 0.145 |
| Urinary tract | 45 (17.0) | 11 (25.6) | 0.179 | 9 (25.7) | 0.082 |
| Respiratory | 30 (11.4) | 6 (14.0) | 0.624 | 5 (14.2) | 0.613 |
| Othersc | 14 (5.3) | 4 (9.3) | 0.874 | 2 (5.7) | 0.577 |
CVA, cerebrovascular accident; NC, neurologic complications.
Data are presented as number (%).
Left ventricular assist device-related infection of the driveline, pocket, or/and wound infections.
Includes gastrointestinal, genital, otorhinolaryngologic and/or pressure ulcer infections.
Comparison of variables in patients with only CVA and those without any episodes of NC
After excluding patients with only TIAs, the comparison between patients with CVA and patients without any NC did not show any significant differences in clinical characteristics (Table 1), pre-LVAD hemodynamic data (Table 2), and post-operative warfarin and aspirin administration (Table 3). Pre-operative and post-operative sodium and albumin concentrations were lower in patients with CVA than in non-NC patients (Tables 2 and 3). The proportion of patients who developed infections was also higher in Group CVA than in Group non-NC (Table 4).
Multivariate analysis of factors associated with NC and CVA after LVAD surgery
As a result of this comparative analysis between Group NC and Group non-NC, history of CVA, pre-operative sodium and albumin, post-operative sodium, hematocrit, and albumin, and post-operative infection were selected for inclusion in a subsequent multivariate analysis.
Stepwise forward selection analysis revealed that history of CVA and post-operative infection were independently associated with the development of NCs after LVAD surgery (Table 5). A discriminant function test revealed that a discriminant score (Z), defined by using the following equation, yielded a discriminant probability of 76.6%:
A result of Z > 0 indicates patients developing NC; Z < 0 indicates patients not developing NC after LVAD.
Table 5.
Stepwise Forward Selection Analysis of Factors Associated With Neurologic Complication and Cerebrovascular Accident After Left Ventricular Assist Device Placement
| Factors | OR (95% CI) | p-value |
|---|---|---|
| Associated with overall NC development |
||
| History of CVA | 2.37 (1.24–5.29) | 0.011 |
| Pre-operative factor | ||
| Sodium | 0.93 (0.90–1.12) | 0.208 |
| Albumin | 0.51 (0.21–1.37) | 0.079 |
| Post-operative factor | ||
| Hematocrit | 0.96 (0.71–1.22) | 0.184 |
| Sodium | 0.84 (0.68–1.21) | 0.075 |
| Albumin | 0.71 (0.46–2.42) | 0.143 |
| Infection | 2.99 (1.16–10.49) | 0.011 |
| Associated with CVA development |
||
| Pre-operative factor | ||
| Sodium | 0.95 (0.92–1.01) | 0.057 |
| Post-operative factor | ||
| Sodium | 0.92 (0.90–1.02) | 0.060 |
| Albumin | 0.43 (0.23–0.98) | 0.050 |
| Infection | 4.24 (1.69–14.58) | 0.0005 |
CI, confidence interval; CVA, cerebrovascular accident; NC, neurologic complication; OR, odds ratio.
Multiple stepwise forward selection analysis for CVA development after excluding patients with only TIA revealed that pre-operative sodium, and post-operative sodium and albumin levels, and infection were discriminant factors for development of CVA. Among those variables, only post-operative infection was independently associated with CVA (Table 4).
Discussion
In the present study, we have demonstrated that:
overall frequency of NC including TIA was 14.0% after LVAD placement and that the frequency of ischemic/hemorrhagic CVA was 11.4%;
the frequency of NC was not different between patients with HeartMate I vs HeartMate II devices;
history of CVA and post-operative infection were factors independently associated with development of NCs after LVAD placement;
the combination of prior CVA, pre-operative sodium and albumin, post-operative sodium, hematocrit and albumin, and post-operative infection could discriminate patients who develop NCs with a discriminant probability of 76.6%; and
an analysis done for CVA patients after excluding patients with only TIA yielded similar results.
NC is a devastating adverse event after LVAD placement.4–9 The incidence of CVA after LVAD placement was reported to be 8% to 25%,5,10,11 The Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure (REMATCH) trial showed that sepsis was the leading cause of death and CVA was the third leading cause of death (9.0%) after LVAD placement.4 An analysis of the INTERMACS database, which includes pulsatile-flow and continuous-flow devices, also reported that NC was one of the leading causes of death.13 NCs affect not only the device outcome, but also a patient’s quality of life, even after transplantation.4,5,8,9 Therefore, discrimination of patients who are at high risk for NCs is key to achieving an acceptable short-term and long-term outcome after LVAD placement. A reliable system to distinguish patients at high risk for NC would allow special attention to be given to these patients to prevent NCs and potentially even initiate preventive interventions to avoid the development of NCs.
It is noteworthy that post-operative infection was the single factor independently associated with both NC and CVA development. Association between infection and atherosclerotic coronary artery disease has been reported previously.14,15 We speculate that infection causes changes in the microvascular structure, reactivity, and overall function as well as coagulation abnormalities that altogether result in a higher risk of NC development.16,17 Nakajima et al9 reported that longstanding HF with right heart dysfunction before LVAD placement and infection after LVAD placement was associated with CVA development after LVAD placement.
Patients with biventricular failure likely require a long duration of inotropic support before and after LVAD, which may lead to line infections and systemic infection. Owing to the retrospective nature of this study, we could not include right ventricular variables derived from pre-operative echocardiography, because not all patients with severe HF requiring LVAD surgery could provide good right ventricular images that permit a quantitative assessment. The relationship between pre-LVAD biventricular failure and post-LVAD complications requires additional investigation.
Furthermore, in the present study, hypoalbuminemia, hyponatremia, and post-LVAD anemia were also discriminant factors for NC development. Hypoalbuminemia has been often described in patients with severe HF and is associated with poor outcome.18,19 Hyponatremia is also a common problem in patients with HF, indicates activation of the renin-angiotensin-aldosterone system, and also predicts poor prognosis.20,21 In addition, anemia was also reported to be related to adverse outcomes in patients with LVAD support.22
Our findings indicate that malnutrition and inflammation, pre-LVAD and post-LVAD factors that are known to be associated with severity of HF, are also associated with the development of major complications after LVAD placement such as NC and infection. Thus, major complications after LVAD placement, such as NC and infection, may also have a cause-and-effect relationship with each other. Also, patients who were severely ill pre-operatively with deterioration of general condition would likely develop complications after LVAD implantation. Our observation may support the findings that the INTERMACS levels identified patients at risk for developing complications after mechanical circulatory support.23,24 Of note, we evaluated a number of variables and showed that not a single comorbidity, but the combination of variables, could predict NC development. Further investigation is required to investigate the mechanism underlying these interactions.
In the present study, the anti-coagulation status reflected by INR, as well as history of atrial fibrillation, were not significantly different among patients with NC or CVA and those without NC. We did not perform an analysis by dividing patients with ischemic events and hemorrhagic events due to the small number of events in the sub-groups. Also, several patients developed multiple NCs, with both ischemic and hemorrhagic events, with varying degrees of anti-coagulation. In fact, some of those patients developed subsequent events within 24 to 48 hours after the first event regardless of the intense or less intense anti-coagulation condition. We speculate that a patient who is prone to develop an ischemic NC is also prone to develop a hemorrhagic NC, and vice versa, although the anti-coagulation state remains within a therapeutic range. We will further review the anti-coagulation status of patients with NC, focusing on serial changes of INR levels before the events. In addition, more detailed observations, such as pre-operative transesophageal echocardiograms and post-operative evaluation for device-related thrombus formation by the use of speed ramp studies, would be helpful for further prospective analysis.
Previous studies reported that the event rate of NC was considerably reduced in continuous-flow devices compared with pulsatile-flow devices.5,13 In the present study, the overall frequency of developing NC was not significantly different between patients supported with HeartMate I vs II devices. Because the observation period of HeartMate II patients was significantly longer than that in HeartMate I patients (p = 0.0057) in the present study and because most events occurred in the early post-operative period in both devices (Figure 2), we could not simply compare the event rate/patient per year in both devices by the patient’s cohort. Further investigation would be required to investigate actual events rate associated with the different devices in a different study design.
In conclusion, we demonstrated that previous CVA, persistent malnutrition, persistent inflammation, severity of HF, and post-LVAD infections are key factors associated with NC as well as CVA development after LVAD implantation. Our study did not reveal differences in frequency of NC development between devices in different generation. These findings provide helpful guidance for risk stratification and clinical management strategies of patients with advanced HF receiving LVAD support.
Acknowledgments
The authors thank Norio Sugimoto (Sugimoto Data Analysis Service) for his statistical review of the manuscript and Dr Hirokazu Akashi for his help in database coordination.
Dr Naka reports receiving consulting fees from Thoratec and Terumo Heart. Dr Jorde reports receiving consulting fees from Thoratec and Jarvik Heart.
Footnotes
Disclosure statement
None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report—2010. J Heart Lung Transplant. 2010;29:1089–1103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Jaski BE, Kim JC, Naftel DC, et al. Cardiac Transplant Research Database Research Group. Cardiac transplant outcome of patients supported on left ventricular assist device vs intravenous inotropic therapy. J Heart and Lung Transplant. 2001;20:449–456. doi: 10.1016/s1053-2498(00)00246-1. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–1533. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Birks EJ, Yacoub MH, Banner NR, Khaghani A. The role of bridge to transplantation: should LVAD patients be transplanted? Curr Opin Cardiol. 2004;19:148–153. doi: 10.1097/00001573-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 8.Holman WL, Kormos RL, Naftel DC, et al. Predictors of death and transplant in patients with a mechanical circulatory support device: a multi-institutional study. J Heart Lung Transplant. 2009;28:44–50. doi: 10.1016/j.healun.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima I, Kato TS, Komamura K, et al. Pre- and post-operative risk factors associated with cerebrovascular accidents in patients supported by left ventricular assist device. Circ J. 2011;25:1138–1146. doi: 10.1253/circj.cj-10-0986. [DOI] [PubMed] [Google Scholar]
- 10.John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227–1234. doi: 10.1016/j.athoracsur.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Tsukui H, Abla A, Teuteberg JJ, et al. Cerebrovascular accidents in patients with a ventricular assist device. J Thorac Cardiovasc Surg. 2007;134:114–123. doi: 10.1016/j.jtcvs.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27:1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998;31:1217–1225. doi: 10.1016/s0735-1097(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 15.Frishman WH, Ismail AA. Role of infection in atherosclerosis and coronary artery disease: a new therapeutic target? Cardiol Rev. 2002;10:199–210. doi: 10.1097/00045415-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Syrjänen J. Infection as a risk factor for cerebral infarction. Eur Heart J. 1993;14(Suppl K):17–19. [PubMed] [Google Scholar]
- 17.Kannoth S, Iyer R, Thomas SV, et al. Intracranial infectious aneurysm: presentation, management and outcome. J Neurol Sci. 2007;256:3–9. doi: 10.1016/j.jns.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Uthamalingam S, Kandala J, Daley M, et al. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J. 2010;160:1149–1155. doi: 10.1016/j.ahj.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–889. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Blair JE, Zannad F, Konstam MA, et al. EVEREST Investigators. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol. 2008;52:1640–1648. doi: 10.1016/j.jacc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 21.Gheorghiade M, Abraham WT, Albert NM, et al. OPTIMIZE-HF Investigators and Coordinators. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 22.Vrtovec B, Radovancevic R, Delgado RM, et al. Significance of anaemia in patients with advanced heart failure receiving long-term mechanical circulatory support. Eur J Heart Fail. 2009;11:1000–1004. doi: 10.1093/eurjhf/hfp110. [DOI] [PubMed] [Google Scholar]
- 23.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant. 2009;28:827–833. doi: 10.1016/j.healun.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–407. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]