Abstract
We describe a 75-year-old female patient with nonvalvular atrial fibrillation who presented with acute ischemic stroke during treatment with dabigatran 2 × 110 mg per day. After informed consent, we reversed the anticoagulant effects of dabigatran using idarucizumab and applied an intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (off-label use). An intracerebral hemorrhage was excluded after systemic thrombolysis. Despite the IVT, the patient's clinical condition deteriorated and she developed an ischemic lesion in the right pons, the right thalamus and right cerebellum. To date, the literature lacks data concerning the thrombolytic treatment of acute ischemic stroke in patients after specific reversal of the non-vitamin K oral anticoagulant dabigatran using idarucizumab. Given the rapid and sustainable efficacy of idarucizumab, the reversal of dabigatran followed by thrombolysis seems to be safe, but further studies and register data are still needed to confirm our preliminary observation, especially to provide additional data concerning the risk-benefit evaluation.
Keywords: Atrial fibrillation, Embolic stroke, Non-vitamin K oral anticoagulants, Dabigatran, Idarucizumab, Intravenous thrombolysis
Introduction
Non-vitamin K oral anticoagulants (NOACs) are widely used in the primary and secondary prevention of embolic stroke in patients with atrial fibrillation and risk factors according to the CHA2DS2-VASc-Score [1]. Idarucizumab is the first available specific reversal agent for dabigatran in the group of NOACs at this time [2]. In this case report, we describe a 75-year-old female with atrial fibrillation on dabigatran who developed symptoms of an acute ischemic stroke and was treated with idarucizumab before intravenous thrombolysis (IVT). To the best of our knowledge, this is the first description of this therapeutic strategy in the literature.
Case Presentation
A 75-year-old female with a history of nonvalvular atrial fibrillation, hypertensive heart disease due to chronic arterial hypertension and colitis ulcerosa presented with acute left hemiparesis, gaze deviation to the left and homonymous hemianopia to the right side that started about 60 min before admission to our hospital. The patient was treated with dabigatran (2 × 110 mg daily). On admission, blood pressure was 160/60 mm Hg and the NIHSS score equaled 7. Blood tests directly run at admission showed a significantly elevated thrombin time (>150 s; normal 14–21 s), a normal partial thromboplastin time (35.5 s; normal 23–36 s) and a normal international normalized ratio (1.10; normal 0.85–1.18). The dabigatran concentration was 90 ng/ml, i.e. dabigatran has regularly been ingested according to an elimination half time of 12–17 h. Cranial computed tomography (CT) showed no early signs of cerebral ischemia or intracerebral hemorrhage (ICH) (fig. 1a). CT angiography revealed an occluded left posterior cerebral artery (PCA) (fig. 1b) and a hypoplastic right vertebral artery (not shown). The remaining large brain vessels were unaffected, so endovascular therapy was not considered. After informed consent, we reversed the anticoagulant effects of dabigatran using idarucizumab (according to the protocol of [2]) and the patient received IVT with recombinant tissue plasminogen activator (rt-PA) at 0.9 mg/kg (total dose 67 mg) 120 min after the stroke symptoms started. After the IVT, follow-up cranial CT scans ruled out an ICH or infarction in the PCA territory, but revealed an ischemic lesion in the right pons, the right thalamus and right cerebellum (fig. 1c). Due to a pacemaker, the patient was not eligible for MRI. Ultrasound of the cerebral vasculature showed a resistance profile in the hypoplastic right vertebral artery. Because of insufficient temporal acoustic windows, a transcranial examination was not possible. The patient developed pneumonia due to aspiration and was treated with antibiotics (ceftriaxon 2 g i.v. per day). Ventilation was not necessary. Despite the IVT, the NIHSS score deteriorated from 7 at admission to 18 at discharge, mainly due to the pontine and thalamic infarction. Noteworthy, also in another control CT scan, there was no infarct development in the left PCA territory, making a reperfusion of the left PCA very likely. The patient was transferred to a rehabilitation facility. We recommended restarting oral anticoagulation with another NOAC (i.e. rivaroxaban) 3–4 weeks after the occurrence of the stroke. In the meantime, the patient was treated with aspirin 100 mg per day. Three months after the stroke, the patient has markedly improved but was still dependent on care.
Fig. 1.
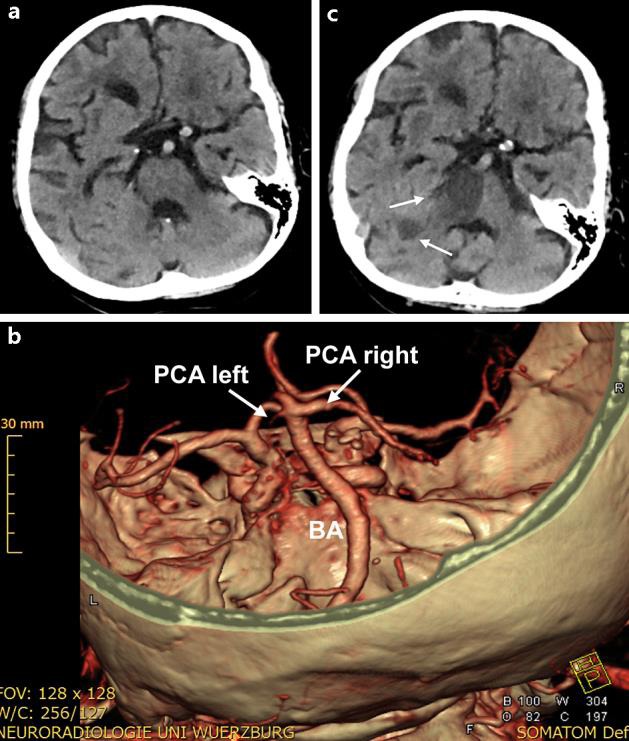
Cranial CT scans including intracranial CT angiography. a CT scan at admission without ICH or early signs of acute ischemic stroke. b Thee-dimensional visualization of the basilar artery (BA) and the PCAs at admission. The left PCA is occluded (arrow). c CT scan 6 days after admission with infarction of the right pons and right cerebellum (arrows). No PCA infarction developed, indicative for successful recanalization of the initially occluded left PCA.
Discussion
NOACs are increasingly used in the primary and secondary prevention of embolic stroke in patients with atrial fibrillation [1]. An improved safety and efficacy profile of dabigatran in comparison to warfarin was proven in the RE-LY trial [3]. However, ICH still occurs under treatment with NOACs; therefore, there is an urgent need for specific reversal agents in case of major bleedings and before emergency surgery. Since its approval in the United States in October 2015, idarucizumab is the first available specific antidote for dabigatran [2, 4]. In the phase III REVERSE AD trial, patients under dabigatran who suffered a serious bleeding complication or required an urgent procedure were recruited. Idarucizumab completely reversed the dilute thrombin time and the ecarin clotting time within minutes. The effect of idarucizumab lasted for several hours (concentrations of unbound dabigatran below 20 ng/ml at 24 h in 79% of the patients) [4]. This sustained pharmacokinetic effect might be an important advantage for safety reasons in the context of IVT as early antithrombotic therapy after IVT led to increased bleeding complications [5].
To the best of our knowledge, this is the first case report to describe a patient with acute ischemic stroke undergoing IVT after reversing the anticoagulant effects of dabigatran by application of idarucizumab. In this scenario, the IVT was safe, i.e. no ICH occurred after IVT, and most likely effective to thrombolyse the left PCA occlusion. The most likely cause of the multiple infarctions in the vertebrobasilar circulation is a transient basilar artery occlusion. Spontaneous reperfusion and transmission of (a part of) the clot in the left PCA could explain the PCA occlusion at admission. We cannot completely exclude a prothrombotic effect of idarucizumab in our patient or a negative impact of idarucizumab on rt-PA effectiveness. Nevertheless, this seems to be unlikely as treatment most likely led to PCA reperfusion on the left side and an in vitro study showed no effects of idarucizumab on the action of rt-PA [6].
Conclusion
IVT was not clinically effective but safe (no ICH after IVT) after antagonization of dabigatran with idarucizumab in a patient with atrial fibrillation and ischemic stroke. Further data from controlled trials and register studies are needed to figure out the best emergency management of acute ischemic stroke in patients with atrial fibrillation under treatment with dabigatran and other anticoagulants [e.g. the Registry of Acute Stroke Under Novel Oral Anticoagulants – Prime (RASUNOA-Prime), ClinicalTrials.gov: NCT02533960].
Statement of Ethics
Informed consent was obtained from the patient.
Disclosure Statement
W.K. received travel and educational support from Genzyme, TEVA, Allergan and Grifols. P.K. received consulting honoraria, speakers’ honoraria, travel support or research support from Boehringer Ingelheim, Bayer HealthCare, BMS Pfizer and Daiichi Sankyo. The sponsors had no influence on the manuscript.
Acknowledgement
The authors thank L. Solymosi for providing the CT scans.
References
- 1.Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 2.Pollack CV, Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 4.Pollack CV, Jr, et al. Design and rationale for RE-VERSE AD: a phase 3 study of idarucizumab, a specific reversal agent for dabigatran. Thromb Haemost. 2015;114:198–205. doi: 10.1160/TH15-03-0192. [DOI] [PubMed] [Google Scholar]
- 5.Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380:731–737. doi: 10.1016/S0140-6736(12)60949-0. [DOI] [PubMed] [Google Scholar]
- 6.Van Ryn J, Schurer J, Fischer D. No influence of dabigatran or its specific reversal agent, idarucizumab, on rTPA-induced thrombolysis of clots in human plasma: an in vitro study. Stroke. 2016;47:AWP73. [Google Scholar]


